Abstract
The use of anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs), such as lorlatinib, for the treatment of patients with ALK gene rearrangement (or ALK-positive) non-small cell lung cancer (NSCLC) has been shown to improve the overall survival and quality of life of these patients. However, lorlatinib is not exempt from potential adverse events. Adequate monitoring and management of these adverse events are critical for increasing patient adherence to lorlatinib, thereby maximizing the benefits of treatment and minimizing the risks associated with treatment discontinuation. Considering that the adverse events of lorlatinib can affect different organs and systems, the participation of a multidisciplinary team, including cardiologists, neurologists, internal medicine specialists, and oncology pharmacists, is needed. This article presents specific and pragmatic strategies for identifying and treating the most relevant adverse events associated with lorlatinib in patients with advanced ALK-positive NSCLC based on the clinical experience of a multidisciplinary panel of experts.
Key Points
| Lorlatinib is an anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (TKI) that is highly effective in patients with advanced ALK-positive non-small cell lung cancer (NSCLC). |
| Similar to other treatments, lorlatinib has adverse events that need to be monitored and treated appropriately to maximize drug efficacy and patient safety. |
| Considering that the adverse events of lorlatinib can affect different organs and systems, the participation of a multidisciplinary team, including cardiologists, neurologists, internal medicine specialists, and oncology pharmacists, is needed. |
Introduction
Treatment for advanced non-small cell lung cancer (NSCLC) with anaplastic lymphoma kinase gene rearrangement (ALK+) has evolved significantly since the discovery of crizotinib, a first-generation ALK tyrosine kinase inhibitor (TKI) [1–3]. To date, there are three generations of ALK-TKIs available, and each successive generation has offered increasing potency, improved CNS penetrance, and an enhanced ability to overcome ALK-resistant mutations. Crizotinib and ceritinib demonstrated benefits over chemotherapy for patients with treatment-naïve ALK+ advanced NSCLC, while second-generation ALK-TKIs (i.e., alectinib, brigatinib, and ensartinib) have demonstrated superior efficacy compared with crizotinib [4–11]. More recently, lorlatinib, a third-generation ALK-TKI, was used to treat patients in the Phase 3 CROWN trial; after 5 years of follow-up, the median progression-free survival was not reached, and the landmark 5-year progression-free survival was 60% (95% CI 51–68). Moreover, lorlatinib was associated with an 81% reduction in the risk of death or progression and a higher intracranial response compared to crizotinib (60% vs 11%). However, lorlatinib was also associated with increased rates of grade 3/4 adverse events (AEs) [12].
Most AEs associated with ALK inhibitors are grade 1–2. The most frequent AE associated with lorlatinib is dyslipidemia, which affects approximately 80% of all patients. Serious AEs have been reported in 7.4% of patients receiving lorlatinib, the most frequent of which were cognitive events and pneumonitis [13]. Table 1 shows the frequency and median time to onset of the most relevant lorlatinib-related AEs [13]. As described in the CROWN trial, treatment-related AEs leading to dose reduction were reported in 21% of patients receiving lorlatinib, temporary treatment discontinuation in 39%, and permanent treatment discontinuation in 5% [12]. The most frequent AEs leading to dose reduction were edema and peripheral neuropathy [13–16]. Proper monitoring and management of these AEs are critical for maximizing adherence to lorlatinib, achieving the maximum treatment benefit and minimizing patient risk.
Table 1.
Most relevant lorlatinib-related adverse events [13]
| Adverse event | All grades Frequency (%) |
Grades 3–4 Frequency (%) |
|---|---|---|
| Metabolic and cardiovascular adverse events | ||
| Hypercholesterolemia | 81.1 | 18.3 |
| Hypertriglyceridemia | 67.2 | 19.3 |
| Arterial hypertension | 13.0 | 6.1 |
| Hyperglycemia | 9.2 | 3.2 |
| Atrioventricular block | 0.8 | 0 |
| Neurological adverse events | ||
| Peripheral neuropathy | 43.7 | 2.7 |
| Cognitive events | 27.7 | 2.9 |
| Mood events | 21.0 | 1.5 |
| Speech events | 8.2 | 0.6 |
| Psychotic events | 6.5 | 0.4 |
| Other adverse events | ||
| Edema | 55.7 | 2.7 |
| Weight increase | 30.9 | 10.1 |
| Diarrhea | 22.9 | 1.5 |
| Nausea | 17.6 | 0.6 |
| Constipation | 17.4 | 0.2 |
| Vomiting | 12.0 | < 1 |
| Lipase increase | 12.4 | 6.9 |
| Amylase increase | 11.3 | 2.7 |
| Pneumonitis | 1.9 | 0.6 |
Consensus papers on the management of AEs caused by lorlatinib has already been published, but they address their management from oncologists’ point of view and are not easy to follow from clinical practice [14, 17–21]. However, given the variety of different organs that are affected by lorlatinib-related adverse events, appropriate management requires the involvement of a multidisciplinary group of specialists. This article addresses the need for a multidisciplinary expert consensus on the management of AEs associated with lorlatinib in patients with ALK-positive advanced NSCLC.
The focus of the current consensus is to provide specific recommendations and pragmatic information for clinicians treating these patients on the target organs affected by lorlatinib based on the experience that a multidisciplinary group of specialists (cardiologists, neurologists, internal medicine specialists, and oncology pharmacists) discussed in several meetings. This approach is of particular relevance since lorlatinib is a very effective treatment that can be administered for many years.
Metabolic and Cardiovascular Adverse Events
Regardless of oncological treatments, cancer patients face increased cardiovascular risk. Recent cardiology guidelines recommend managing risk factors and cardiovascular disease (CVD) upon diagnosis. Treatment with ALK inhibitors adds additional risk for cardiovascular and metabolic events [22]. In the case of lorlatinib, the most common conditions (i.e., those with an incidence rate ≥10%) are dyslipidemia, hypertension, and hyperglycemia/diabetes. Sinus bradycardia is a less common event (i.e., incidence rate from 1 to 10%) [22]. Despite these findings, after a 3-year follow-up, no increase in cardiovascular risk was observed with lorlatinib compared to crizotinib [23].
Before starting treatment with lorlatinib, a baseline cardiovascular toxicity risk assessment, including clinical history, physical examination, electrocardiogram, blood pressure assessment, general blood test, glycosylated hemoglobin (HbA1c) (at least in patients with hyperglycemia), and lipid profile, is recommended. Cardiac serum biomarkers and transthoracic echocardiography should be considered in patients with moderate-to-severe preexisting CVD or new abnormal findings during baseline assessment (i.e., abnormal electrocardiogram). For patients taking lorlatinib, a cholesterol profile assessment at 2, 4 and 8 weeks and every 3–6 months should be considered. Additionally, home blood pressure monitoring and an electrocardiogram are recommended at 4 weeks after starting therapy and every 3–6 months in patients with preexisting CVD. In patients who already have or are at significant risk of developing cardiovascular events, a multidisciplinary cardio-oncology team is needed for optimal management [22].
Dyslipidemia
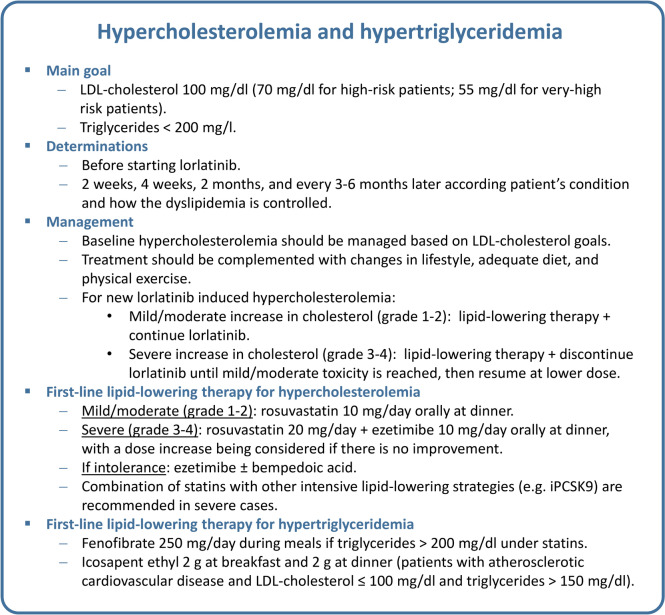
Hypercholesterolemia and hypertriglyceridemia are the most common AEs in patients treated with lorlatinib (incidence rates of 81% and 67%, respectively) (Table 1) [13]. Special care should be taken when patients are previously treated with lipid-lowering agents, have a history of coronary/cardiac/vascular disease, have arterial hypertension, or who are obese.
Comprehensive management of dyslipidemia should be based on the estimated cardiovascular risk and requires baseline and follow-up (at 2 weeks, 4 weeks, and 2 months) assessment of low density lipoprotein (LDL)-cholesterol and triglyceride levels of the patient, and LDL-cholesterol and triglyceride levels should guide specific treatment recommendations (Tables 2 and 3) [24].
Table 2.
LDL-cholesterol and triglyceride level goals according to cardiovascular risk [24]
| Patients without established atherosclerotic cardiovascular disease | High-risk patients* | Very-high risk patients** | |
|---|---|---|---|
| LDL-cholesterol | < 100 mg/dL (< 2.6 mmol/L) | < 70 mg/dL (< 1.8 mmol/L) and ≥ 50 % reduction form baseline | < 55 mg/dL (< 1.4 mmol/L) and ≥ 50 % reduction form baseline |
| Systolic blood pressure | < 140 mm Hg | < 130 mm Hg if tolerated | < 130 mm Hg if tolerated |
| Triglycerides | < 200 mg/dL | < 200 mg/dL | < 200 mg/dL |
LDL low-density lipoprotein
*High-risk patients include
1. Persons without established atherosclerotic cardiovascular disease, diabetes mellitus, chronic kidney disease or familial hypercholesterolemia and a 10-year cardiovascular disease risk estimation using SCORE2 > 7.5 % for patients aged < 50 years or > 10 % for patients aged 50 to 69 years (for patients aged <70 years) or SCORE2-OP > 15 % (for patients aged ≥ 70 years)
2. Patients with familial hypercholesterolemia
3. Patients with type 2 diabetes mellitus without atherosclerotic cardiovascular disease
4. Patients with moderate chronic kidney disease
**Very high-risk patients include
1. Patients with established atherosclerotic cardiovascular disease
2. Patients with type 2 diabetes mellitus with established atherosclerotic cardiovascular disease and/or severe target organ damage
3. Patients with severe chronic kidney disease
Table 3.
| Type | Average LDL-cholesterol reduction (%) | Treatment examples | |
|---|---|---|---|
| Mild/moderate (grade 1–2) | Moderate-intensity statin | 30 | Rosuvastatin 10 mg/day |
| Moderate-intensity statin plus ezetimibe | 40 | Rosuvastatin 10 mg/day + ezetimibe 10 mg/day | |
| Severe (grade 3–4) | High-intensity statin | 50 | Rosuvastatin 20 mg/day |
| High-intensity statin plus ezetimibe | 65 | Rosuvastatin 20 mg/day + ezetimibe 10 mg/day | |
| PCSK9 monoclonal antibodies plus high-intensity statin | 75 | Evolocumab or alirocumab + rosuvastatin 20 mg/day | |
| PCSK9 monoclonal antibodies plus high-intensity statin plus ezetimibe | 85 | Evolocumab or alirocumab + rosuvastatin 20 mg/day + ezetimibe 10 mg/day | |
| siRNA targeting PCSK9 | 53 | Inclisiran 284 mg administered SC on Day 1, Day 90, and every 6 months thereafter + statins | |
| If statin intolerance | Bempedoic acid | 17–25 | Bempedoic acid 180 mg/day |
| Bempedoic acid plus ezetimibe | 38 | Bempedoic acid 180 mg/day + ezetimibe 10 mg/day | |
| Ezetimibe | 15–20 | Ezetimibe 10 mg/day | |
| PCSK9 monoclonal antibodies | 60 |
• Evolocumab 140 mg administered SC once every 2 weeks or 420 mg once a month • Alirocumab 75 mg administered SC once every 2 weeks. Patients requiring greater LDL-cholesterol reduction (> 60 %) may be started on 150 mg once every 2 weeks or 300 mg once every 4 weeks |
|
| Hypertriglyceridemia | Fenofibrate | 29 | 250 mg once a day |
| Icosapent ethyl | 19 | 2 g at breakfast and 2 g at dinner | |
LDL low-density lipoprotein, RNA ribonucleic acid, SC subcutaneously, siRNA small interfering RNA
Hypercholesterolemia management does not usually require interruptions or dose reductions of lorlatinib. Among the treatments used for hypercholesterolemia in patients receiving lorlatinib, the statins pitavastatin, pravastatin, and rosuvastatin have a very low risk of interaction with the cytochrome P450 (CYP) enzymes involved in the metabolism of lorlatinib (Table 4) [24]. Dual therapy combining statins and ezetimibe is usually needed to optimize LDL-cholesterol levels. For patients who do not achieve their goals on a maximum tolerated dose of a statin and ezetimibe, combination therapy including a proprotein convertase subtilisin/kexin type 9 inhibitor (iPCSK9) by monoclonal antibodies (alirocumab and evolocumab), small interfering ribonucleic acid (siRNA) targeting PCSK9 (inclisiran) or bempedoic acid is recommended. Figure 1 summarizes the suggested recommendations for the management of hypercholesterolemia [24].
Table 4.
Practical management of common lorlatinib interactions
| Drug classes | Drug | Resultant of interaction | Practical management |
|---|---|---|---|
| Analgesics | Dipyrone (metamizole) (D) | Decrease in the serum concentration of lorlatinib and may enhance its hepatotoxic effect | This concomitant use should be avoided whenever possible |
|
Acetaminophen (C) Tramadol (C) Codeine (C) |
May decrease the serum concentration of these analgesics |
It is recommended to monitor the analgesic effect, if necessary, the analgesic dose can be increased, without exceeding the maximum recommended dose/day As an alternative, NSAIDs do not have a reduced effect in the presence of lorlatinib |
|
|
Fentanyl (C) Oxycodone (C) Alfentanil (D) |
May decrease the serum concentration of these analgesics |
In the context of the treatment of pain in cancer patients, it is important to bear in mind that this interaction reduces analgesic levels Adjustment of opioid doses might be needed with close monitoring. Lorlatinib does not interact with morphine |
|
| Antibacterial | Rifampicin (X) | Decrease in the serum concentration of lorlatinib and may enhance its hepatotoxic effect |
Rifampicin should be discontinued and an alternative substituted if possible Wait 3 half-lives since discontinuation of the antibacterial before initiating lorlatinib. Rifampicin half-life: 2.5 h, 3–4 h, and 5 h after single administration of 300 mg, 600 mg, and 900 mg, respectively If that is not possible, another ALK inhibitor, such as alectinib, could be used as an alternative, but not brigatinib, as this drug also interacts with rifampicin (X) and clarithromycin (D) Other macrolides, such as azithromycin, do not interact with lorlatinib and could be a suitable alternative If the combination cannot be avoided, reduce the lorlatinib dose from 100 mg once daily to 75 mg once daily |
| Clarithromycin (D) | There is a double interaction through the effect of one drug on the other. The result is an increase in the concentration of lorlatinib and an increase in the active metabolites of clarithromycin | ||
| Telithromycin (D) | Increase in serum concentration of lorlatinib | ||
| Oral anticoagulants |
Dabigatran (X) Edoxaban (D) Apixaban (C) Rivaroxaban (C) Acenocoumarol (C) Warfarin (C) |
The DOACs concentration will be reduced as with warfarin and acenocoumarol, although the mechanism of interaction is different | Avoid dabigatran. A choice should be made between rivaroxaban, apixaban, acenocoumarol and warfarin, based on patient profile and indication, and considering the importance of monitoring anticoagulant efficacy |
| Antidiabetic agents |
Empagliflozin (C) Canagliflozin (C) Dapagliflozin (C) Liraglutide (C) Semaglutide (C) Metformin (C) |
Decrease the therapeutic effect of antidiabetic agents | More intensive blood glucose monitoring is recommended. It may require an increase in the dose of these antidiabetic drugs |
| Antiepileptics |
Carbamazepine (X) Fosphenytoin (X) Phenobarbital (X) Phenytoin (X) Primidone (X) |
Decrease in the serum concentration of lorlatinib and may enhance its hepatotoxic effect |
A change of anticonvulsants is recommended, due to the narrow therapeutic margin of these drugs. A neurologist should supervise this change. There are currently antiepileptics that are not metabolized by CYP3A4 and would not interact with lorlatinib. Some of these are levetiracetam, lamotrigine, and zonisamide Wait 3 half-lives after discontinuation of the antiepileptic before initiating lorlatinib. Although the elimination half-lives of drugs may vary under some conditions, these are the half-lives of the inducing antiepileptics: • Carbamazepine: 12 and 17 h • Fosphenytoin: 15–20 h • Phenobarbital: 50–120 hours • Phenytoin: 22 h • Primidone: 6–14 h In case these antiepileptic drugs cannot be switched to others without CYP3A4 metabolism, the use of another ALK inhibitor, such as alectinib, would be recommended |
| Antiemetics |
Aprepitant (C) Netupitant (C) Rolapitant (C) |
May decrease the serum concentration of neurokinin inhibitors |
Lorlatinib has minimal to low emetic risk (<30 %), based on the NCCN guidelines vs1.2023. Recommended treatment for this level of emesis and the next level include metoclopramide, domperidone or chlorpromazine or 5-HT3 inhibitors such as ondansetron, granisetron or dolasetron. None of these drugs interact with lorlatinib In the case of adding neurokinin inhibitors, it should be noted that they might be less effective in the presence of lorlatinib |
| Corticosteroids |
Dexamethasone (C) Methylprednisolone (C) Prednisolone (C) Prednisone (C) |
May decrease the serum concentration of these corticosteroids |
Dexamethasone prescribing information states that CYP3A4 inducers (lorlatinib) may enhance the metabolism of dexamethasone and that dose increases may be needed. The same applies to the other corticosteroids There are no formal dosage recommendations |
| Deflazacort (X) | Decrease in the serum concentration of deflazacort | The deflazacort active metabolite, 21-desDFZ, was reduced by approximately 95 % following administration of the strong CYP3A4 inducer rifampin. As a result, concurrent use of deflazacort with strong or moderate CYP3A4 (lorlatinib) inducers should be avoided | |
| Antifungals |
Itraconazole (X) Ketoconazole (X) Voriconazole (X) Levoketoconazole (D) Fluconazole (D) |
Increase in the serum concentration of lorlatinib |
Avoid coadministration of strong CYP3A inhibitors (itraconazole, ketoconazole, voriconazole). If concomitant use is unavoidable, reduce the starting dose of lorlatinib from 100 to 75 mg, and from 75 to 50 mg if severe toxicity from lorlatinib is observed Levoketoconazole and fluconazole are moderate CYP3A4 inhibitors. There is no established recommendation although it is advisable to proceed in the same way (reduce the starting dose). Maintain reduction until after 5 half-lives of antifungal elimination after the time of discontinuation of the antifungal agent Some examples: • Itraconazole: Half-life is 16–28 h (single dose) 34–42 h (repeated doses) Time to safe discontinuation of antifungal before starting lorlatinib: 3–6 days (single dose) or 7–8 days (repeated doses) • Ketoconazole: Half-life is biphasic: 2 h (within first 10 h) – 8 h. Time to safe discontinuation of antifungal before starting lorlatinib 2 days • Voriconazole: Half-life is 6–8.5 h or 1.8 h (containing cyclodextrins). Time to safe discontinuation of antifungal drug before starting lorlatinib 1–2 days. If voriconazole contains cyclodextrins 10 h is sufficient • Fluconazole: Half-life is 30 h (46 h in elderly). Time to safely discontinue antifungal before starting lorlatinib: 6.2 days (10 days in the elderly) In cases where loss of efficacy of the antifungal drug could be a major problem, another ALK inhibitor, such as alectinib, should be selected |
| Ibrexafungerp (X) | Decrease in the serum concentration of ibrexafungerp | ||
| Antineoplastic agents |
Tyrosine kinase miscellaneous • Avapritinib (X) • Axitinib (X) • Capmatinib (X) • Bosutinib, (X) • Encorafenib (X) • Entrectinib (X) • Neratinib(X) • Olaparib (X) |
Decrease in the concentrations of some antineoplastic agents, which are metabolized by CYP3A4 | The existence in a patient of synchronous tumors that need to be treated simultaneously is rare. If the antineoplastic to the other tumor cannot be avoided, due to a lack of alternatives, another ALK inhibitor, such as alectinib or brigatinib, could be considered |
|
Breast cancer • Abemaciclib (X) • Palbociclib (C) • Ribociclib (C) |
Decrease in the abemaciclib, palbociclib, and ribociclib AUCs | Association with abemaciclib is contraindicated. Closely monitor for any signs of decreased efficacy of palbociclib or ribociclib when coadministered with lorlatinib. There are no specific guidelines for dose adjustment to prevent loss of effectiveness. It is worth noting that abemaciclib can be safely combined with alectinib, and palbociclib can be safely combined with brigatinib, in terms of their pharmacokinetics | |
|
Prostate cancer • Enzalutamide (X) • Apalutamide (X) • Darolutamide (X) • Abiraterone (C) |
Decrease in serum concentration of lorlatinib and may enhance its hepatotoxic effect Decrease in serum concentration of darolutamide or abiraterone |
Avoid enzalutamide, apalutamide, and darolutamide Abiraterone would be the safest option, as long as the indication allows the change and monitoring the effect of abiraterone. It would be ideal to be monitor plasma levels of abiraterone If enzalutamide or apalutamide are discontinued, wait three half-lives after discontinuation before starting lorlatinib If apalutamide or enzalutamide cannot be avoided, consider the possibility of using alectinib If unable to discontinue darolutamide, monitor the effect of darolutamide |
|
| Antipsychotics |
Clozapine (C) Quetiapine (C) Rabeprazole (C) Lumateperone (X) |
Decrease in antipsychotic levels |
The CNS events associated with lorlatinib generally improved or resolved following dose modifications. These events may be influenced by some factors, like psychiatric conditions and use of neurotropic medications If patients with psychiatric treatment are starting on lorlatinib, their psychiatric symptoms should be closely monitored. If patients are to be treated with these antipsychotics and start lorlatinib, it must be noted that the antipsychotic effect will be reduced Antipsychotics whose levels are less or not at all influenced by the presence of lorlatinib are haloperidol, chlorpromazine, olanzapine or ziprasidone Avoid lumateperone |
| Antivirals | Letermovir (X) | Potential decrease in letermovir plasma concentrations | In this case, there is no therapeutic alternative in its indication. A thorough monitoring of the possible loss of effect of letermovir (CMV reactivation monitoring) is recommended if the risk is manageable, otherwise another ALK inhibitor should be used, such as alectinib) |
|
Dasabuvir (X) Sofosbuvir (X) Velpatasvir (X) Voxilaprevir (X) Asunaprevir (X) Elbasvir (X) Grazoprevir (X) Ledipasvir (X) Simeprevir (X) Daclatasvir (D) |
Decrease in antivirals for hepatitis C treatment |
Product labeling states that use with moderate CYP3A4 inducers (lorlatinib) is contraindicated due to the potential for decreased exposure of each component and the loss of therapeutic efficacy If these antivirals cannot be avoided, it is recommended to start treatment with another ALK inhibitor such as alectinib or brigatinib |
|
| Lenacapavir (X) | Decrease in levels of anti-HIV treatment | There are alternative anti-HIV treatments that do not interact with lorlatinib. Consult with a HIV specialist | |
| Contraceptives and estrogen derivatives | Estrogen derivatives alone or in combination with progestin, topical or systemic | Consider the potential for decreased estrogen derivative efficacy and changes in uterine bleeding if coadministered with lorlatinib. These estrogen derivatives are combined with progestins, the progestin component may be similarly affected and monitoring for decreased progestin efficacy may also be warranted |
The drug data sheet recommends avoiding oral contraceptives during treatment with lorlatinib because of possible loss of efficacy. Other methods of contraception, such as barrier methods, are recommended If the patient is using estrogen derivatives for menopausal symptoms, it should be noted that a worsening of these symptoms might occur when starting lorlatinib |
| Immunosuppressants |
Voclosporin (X) Cyclosporine (C) Tacrolimus (C) Sirolimus (C) |
Decrease these immunosuppressants concentration |
Avoid voclosporin The prescribing information for immunosuppressants states that lorlatinib decreases concentrations and that dose adjustment of the immunosuppressant is essential to achieve the desired immunosuppressant concentration. The lorlatinib data sheet recommends avoiding these combinations Monitoring immunosuppressive drug levels is the most appropriate choice as long as it is feasible. If this is not possible, a noninteracting ALK-blocking alternative should be sought |
| Lipid-Lowering drugs |
Atorvastatin (C) Simvastatin (C) Lovastatin (C) |
Decrease in these statin levels |
Hyperlipidemia, comprising the terms hypercholesterolemia and hypertriglyceridemia, was the most common adverse reaction reported with lorlatinib and usually occurred within the first few weeks of treatment Whether the patient was previously on statin treatment or is due to start statin treatment, it is important to note that there are statins whose metabolism is not affected by lorlatinib, such as pravastatin, rosuvastatin and pitavastatin Consideration should be given to switching to an equivalent dose of these statins or starting one of them, if needed |
| Drugs with cardiovascular activity (not complete list) |
Heart failure: • Finerenone (X) • Ivabradine (X) Hypertrophic myocardia: • Mavacamtem (X) Hypertension: • Nisoldipine (X) • Felodipine (C) • Nifedipine (C) • Nimodipine (C) • Verapamil (C) Antiplatelet: • Vorapaxar (X) Antiarrhythmics: • Digoxin (C) |
Decrease in these cardiovascular drug levels | The patient's cardiovascular risk must be evaluated to assess the importance of these interactions. Referral to the cardio-oncology unit is recommended. In some cases, the medication may be adjusted and in others a change of drugs may be needed, especially in the case of Lexicomp level X interactions |
| Disorders of sexual function |
Erectile dysfunction: • Sildenafil (C) • Avanafil (X) Female sexual desire: • Flibanserin (X) |
Decrease serum levels of these drugs related to erectile dysfunction and female sexual desire |
It is advised to refrain from using avanafil and flibanserin concomitantly with lorlatinib due to potential loss of effectiveness. Furthermore, dose adjustment recommendations for these medications in combination with lorlatinib are not available According to the labeling of sildenafil products for the treatment of pulmonary arterial hypertension, it is stated that higher doses of sildenafil may be necessary when initiating treatment in a patient who is already taking a moderate-to-strong CYP3A inducer, such as lorlatinib. Additionally, when discontinuing treatment with moderate-to-strong CYP3A inducers, the sildenafil dose should be reduced to 20 mg three times a day. However, it is advisable to closely monitor the effectiveness of sildenafil in such combinations, as a potential decrease in efficacy may occur |
| Benzodiazepines |
Short-acting: • Midazolam (C) • Triazolam (C) Intermediate-acting: • Alprazolam (C) • Clonazepam (B) Long-acting: • Diazepam (C) |
Decrease in benzodiazepine levels |
Interestingly, the magnitude and clinical consequences of lorlatinib interaction with midazolam appear to be greater with oral midazolam compared to other routes of midazolam administration Clinically, lorlatinib should be used with caution in patients on diazepam and flunitrazepam There are some benzodiazepines without pharmacokinetic interactions with lorlatinib like the action intermediate benzodiazepines: lorazepam, temazepam, oxazepam or the long-acting benzodiazepines: chlordiazepoxide, and flurazepam |
| Proton pump inhibitors (PPIs), histamine H2 receptor antagonists (anti-H2), and other antacid medications |
Omeprazole, pantoprazole, lansoprazole, esomeprazole Famotidine, ranitidine. Almagate, aluminum hydroxide, magnesium carbonate, magnesium trisilicate, magnesium hydroxide, calcium carbonate, sodium bicarbonate |
There is no evidence of interaction |
The absorption of lorlatinib is not significantly modified by pH change, therefore it is safe to use concomitant PPIs and anti-H2 agents Anti-acids, such as almagate, aluminum hydroxide, magnesium carbonate, magnesium trisilicate, magnesium hydroxide, calcium carbonate sodium bicarbonate are recommended to be administered at least two hours apart from lorlatinib administration |
| Metabolic bone disease agents |
Denosumab Zoledronic acid |
There is no evidence of interaction | These combinations with lorlatinib are safe |
| Drug treatment of edema |
Furosemide Spironolactone |
Concomitant use with lorlatinib is safe | |
| Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers |
Enalapril, ramipril Losartan, candesartan |
There is no evidence of interaction |
Concomitant use with lorlatinib is safe Ramipril has a longer half-life than enalapril, so can better control blood pressure on a 24-hour basis. If patients use enalapril, they may need to take it every 12 hours for better control |
| CNS depressants |
Gabapentin Pregabalin |
There is no evidence of interaction | Concomitant use with lorlatinib is safe |
The descriptions of level C (monitor therapy), level D (consider therapy modification) or level X (avoid combination) of Lexicomp Interactions, revised as of 30 April 2023, were included. Practical management is assessed in the oncology context from the point of view of a patient starting treatment with lorlatinib. The pharmacological alternatives proposed for certain interactions are based entirely on pharmacokinetic criteria [13, 14, 25, 45]
AUC area under the curve, CNS central nervous system, DOAC. direct oral anticoagulant, HIV human immunodeficiency virus, NSAID nonsteroidal anti-inflammatory drug
Fig. 1.
Recommendations for the management of hypercholesterolemia and hypertriglyceridemia. LDL low-density lipoprotein
For some patients, statin intolerance remains an important clinical challenge. We defined statin intolerance as the inability to tolerate at least two statins at any dose or increasing doses not attributable to other known conditions, and statin intolerance was associated with an increased risk of cardiovascular events. Statin intolerance includes muscle symptoms, increased creatine kinase levels, and an association between the onset of symptoms and the initiation of statin treatment. The overall prevalence of statin intolerance ranges from 5.9 to 9.1%. Female sex, hypothyroidism, high statin dose, advanced age, antiarrhythmic therapy, and obesity are the main factors that increase the risk of statin intolerance [26, 27]. For statin intolerance, ezetimibe monotherapy or combination therapy with other non-statin drugs is recommended. These include the use of alirocumab, evolocumab, inclisiran or bempedoic acid either alone or in combination with ezetimibe [28, 29].
Statins are the recommended first-line therapy for patients with hypercholesterolemia and hypertriglyceridemia. For patients taking statins who have triglycerides >200 mg/dL, the addition of fenofibrate 250 mg/day orally during meals is suggested. However, the combination of statins and fibrates should be used with caution due to the risk of rhabdomyolysis. The use of gemfibrozil in combination with statins is not recommended due to the risk of hepatic and renal toxicity [14, 30]. If hypertriglyceridemia cannot be controlled with fibrates, other alternatives, such as icosapent ethyl 2 g twice daily should be considered (Fig. 1) [31, 32].
Arterial Hypertension
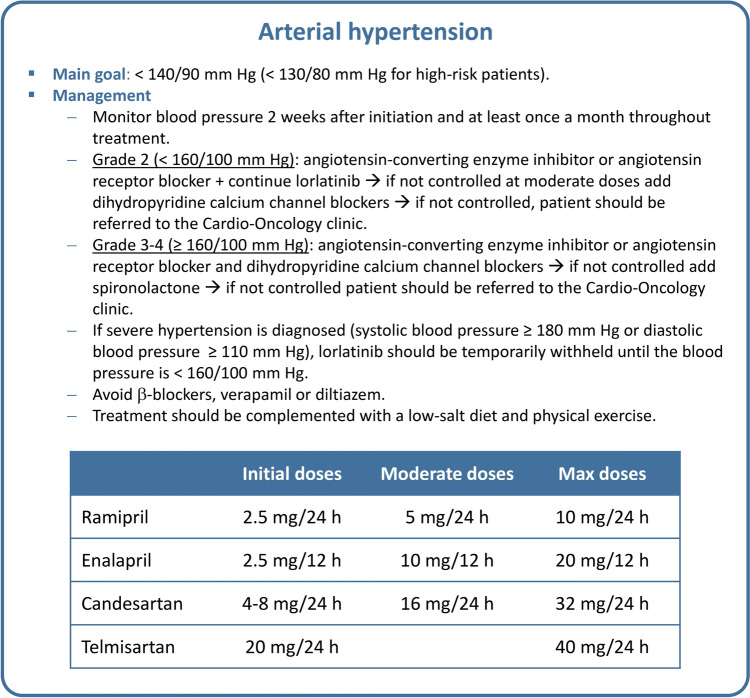
The prevalence of hypertension in patients treated with lorlatinib is 13% (Table 1) [13–15]. The risk is higher in patients with preexisting hypertension or CVDs, advanced age, a history of smoking, hyperlipidemia, and/or obesity. Cardio-oncology guidelines recommend that all cancer patients maintain a blood pressure <140/90 mm Hg, or <130/80 mm Hg for those at very high cardiovascular risk [22]. Blood pressure should be assessed before lorlatinib therapy and monitored 2 weeks after starting therapy and checked at least once a month throughout the treatment period [13].
The first-line treatment for managing hypertension in cancer patients should be an angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin II receptor antagonist (ARA2), as these drugs have a more favorable profile for reducing the risk of diabetic nephropathy or ventricular dysfunction [22]. For patients with a blood pressure <160/100 mm Hg at diagnosis, monotherapy with ACEI or ARA2 is recommended (Fig. 2). For patients with uncontrolled blood pressure (>140/90 mm Hg), combination therapy with dihydropyridine calcium channel blockers (e.g., amlodipine) is advised. If the blood pressure is ≥160/100 mm Hg at diagnosis, dual antihypertensive therapy combining an ACEI or ARA2 with a dihydropyridine calcium channel blocker is recommended as a first-line treatment. If blood pressure remains above the specified thresholds, it is advisable to add aldosterone inhibitors or α-blockers. Given the increased risk of sinus bradycardia, the use of β-blockers, verapamil or diltiazem should be avoided [15]
Fig. 2.
Recommendations for the management of arterial hypertension
Hyperglycemia
The prevalence of hyperglycemia resulting from treatment with lorlatinib is 9% (Table 1) [13–15]. Patients at highest risk were those with a previous history of diabetes, with a history of coronary/cardiac/vascular disease or obesity. There are two groups of drugs that have been shown to control glycemia and reduce cardiovascular risk: sodium/glucose cotransporter 2 inhibitors (iSGLT2s) (empagliflozin, canagliflozin, dapagliflozin) and glucagon-like peptide-1 receptor agonists (GLP1-RAs) (liraglutide, semaglutide) [33]. In patients at high vascular risk with chronic kidney disease or heart failure, iSGLT2s should be considered the first treatment option. If the control is inadequate, metformin can be added. The GLP1-RAs are an interesting therapeutic option (either in association with metformin and/or iSGLT2s or in combination with monotherapy in metformin-intolerant patients) in obese patients with a body mass index ≥30 kg/m2. In patients at low vascular risk, metformin is still considered a valid initial therapy. Figure 3 shows the suggested recommendations for the management of hyperglycemia in patients receiving lorlatinib.
Fig. 3.
Recommendations for the management of hyperglycemia. GLP1-RA glucagon-like peptide-1 receptor agonist, HbA1c glycosylated hemoglobin, iSGLT2 sodium/glucose cotransporter 2 inhibitor
Atrioventricular Block
The PR interval prolongation and atrioventricular (AV) block have been reported in <1% of patients treated with lorlatinib (Table 1) [13, 14]. Patients at higher risk of developing AV blocks are those with a previous history of arrhythmias. Baseline electrocardiogram assessment is recommended for all patients. Electrocardiogram monitoring at 4 weeks and every 3–4 months should be considered in patients with preexisting CVD. In patients who develop AV block, the risks/benefits of lorlatinib should be discussed by a multidisciplinary cardio-oncology team.
Neurological Adverse Events
It has been reported that the neurological or psychiatric AEs related to lorlatinib could be induced by the class effect of ALK-TKIs [34]. Although the mechanism underlying these events is unclear, ALK can play a role in the internalization and regulation of dopamine D2 receptor (D2R), a G protein-coupled receptor expressed in brain regions that control motor function, cognition, and motivation. Its dysregulation may be involved in psychiatric disorders [19, 34]. Nevertheless, lorlatinib has a remarkable ability to enter the brain, mainly due to its low propensity for P-glycoprotein-mediated efflux [35]. Moreover, the mean ratio of cerebral spinal fluid/free plasma concentration for lorlatinib is 0.75 [36]. Hence, there is potential for central nervous system events, including psychotic events and changes in cognitive function, mood, mental status or speech. All of these conditions resolve either by reducing the dose or discontinuing treatment [13, 19].
The overall incidence of these neurological AEs was 35% (62% grade 1, 29% grade 2, 10% grade 3), with a median time to onset of 57 days (1–533 days) and a median duration of 182 days (2–751 days). According to the CROWN study, 62% of patients with such AEs were treated with no specific intervention; 53% of the AEs resolved spontaneously, and only 2% of patients needed permanent discontinuation of lorlatinib [37].
It is important to keep in mind that many of the neurological AEs detected in patients receiving lorlatinib can also be caused by other factors, including brain metastases, brain radiation, brain surgery, preexisting psychiatric disease, and other neurotropic medications, among others [38]. Even more, it is suggested that some AEs are not properly identified in the real-world setting. A recent meta-analysis reported that neurocognitive AEs were more often reported in clinical trials than in retrospective cohort studies [39]. Hence, it is important to establish a differential action protocol both before and after initiating lorlatinib. The patient’s psychiatric history should be explored, and these possible events should be discussed with the patient before initiating treatment with lorlatinib. Figure 4 (Part 1 and Part 2) shows the general recommendations for neurologic AEs in patients receiving lorlatinib, how to adjust lorlatinib, and what supportive treatments are recommended; these recommendations should be used as soon as possible in an attempt to avoid reducing the dose of lorlatinib [13].
Fig. 4.
Recommendations for the management of neurological adverse events. Part 1: Before initiating lorlatinib treatment and how to assess toxicity during treatment. Part 2: After toxicity appears. MRI magnetic resonance imaging
Peripheral Neuropathy
The prevalence of peripheral neuropathy associated with lorlatinib has been found to be 43.7% (Table 1) [13–15]. Given that peripheral neuropathy associated with lorlatinib is different from that caused by platinum or other anticancer neurotoxic drugs and could be confused with metastatic complications, referring to the neurology department is recommended for initial neurological differential diagnosis.
Once neuropathy appears and other causes have been ruled out, the main recommendation is the use of standard neuropathic treatments in cases of painful or disturbing paresthesia, although it should be remembered that such treatments can worsen the patient’s cognitive condition. The use of duloxetine as a first-line treatment is recommended. In addition, as peripheral neuropathy may be associated with peripheral edema, especially in the upper extremities, diuretics may improve symptoms [13, 14]. It is important to note that there is currently no treatment that has been shown to be beneficial for those neuropathies with negative symptoms (such as hypoesthesia/algesia and hypo-paresthesia) or even for nonpainful positive symptoms.
Cognitive Events
The prevalence of cognitive events associated with lorlatinib has been found to be 27.7% (Table 1) [13–15]. This toxicity includes events related to nervous-system disorders according to the organ classification system (amnesia, cognitive impairment, dementia, attention disturbance, memory impairment, mental deterioration) and events related to psychiatric disorders (attention deficit/hyperactivity disorder, confusional state, delirium, disorientation, reading disorder). It is necessary to discuss the possible occurrence of these AEs with patients and caregivers and offer recommendations to minimize their impact on daily life. However, no sustained deterioration in neurocognitive domains has been recorded in the specifically addressed studies [40]. In addition, it is recommended to review potential new medications introduced during treatment and the psychiatric status of the patient to establish a differential action protocol both before and after initiating lorlatinib.
Mood Events
The prevalence of mood events associated with lorlatinib has been found to be 21% (Table 1) [13–15]. These AEs included affective disorders, affective instability, aggressiveness, nervousness, irritability, anxiety, bipolar disorder type I, depressed mood, depression, depressive symptoms, euphoric mood, irritability, mania, altered mood, mood swings, anxiety crisis, personality change, and stress. These mood disorders appear approximately 4–6 weeks after the initiation of lorlatinib [14]. It should be noted that these AEs are reversible.
Several treatments are proposed to treat anxiety and depression that may arise as a consequence of lorlatinib. Benzodiazepines of short or medium half-lives could be useful for reducing anxiety symptoms. Diazepam and flunitrazepam can be used with caution. In any case, it is advisable to use antidepressants with an anxiolytic profile, especially because benzodiazepines have a negative effect on cognition. Table 4 shows the drug interactions. The antidepressants of choice for lorlatinib are duloxetine and agomelatine. Caution should be taken when using paroxetine, fluoxetine, citalopram, escitalopram, sertraline, fluvoxamine, venlafaxine, and mirtazapine. Nonpharmacological treatments include psychotherapy and mindfulness and can be helpful for some patients [17].
Speech Events
The prevalence of speech events associated with lorlatinib has been found to be 8.2% (Table 1) [13–15]. This group includes dysarthria, bradylalia, and speech disorders, which appear approximately 4 to 6 weeks after the initiation of lorlatinib [14]. Before initiating lorlatinib, patients and caregivers should be made aware that these events are reversible after discontinuation or tapering of treatment. Patient management depends on the impact of these interventions on the patient and whether they manifest continuously or intermittently.
Psychotic Events
The most common psychotic events associated with lorlatinib are hallucinations. The prevalence of these AEs is 6.5% (Table 1) [13, 37]. Olanzapine is recommended for treating psychosis/mania/hallucinosis, as quetiapine and ziprasidone can induce interactions with lorlatinib. Risperidone and clozapine should be used with caution (Table 4) [17, 41, 42].
Other Adverse Events
In addition to the main AEs related to metabolic, cardiovascular and psychiatric health, there are others that affect other systems and functions in the same way as other ALK-TKIs. Table 1 shows the frequency of each AE.
Edema
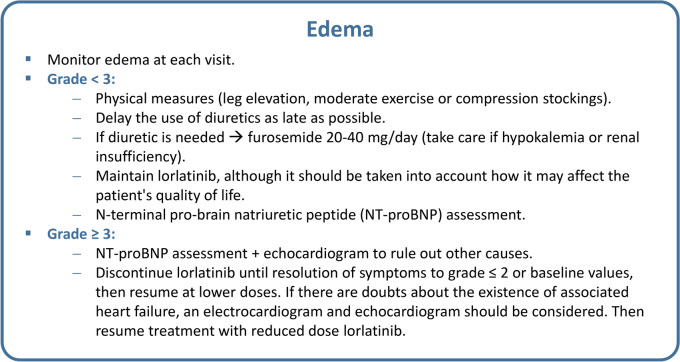
Edema is one of the most common AEs in patients receiving lorlatinib (55.7%) and usually appears after 4 weeks of treatment (Table 1) [13–15]. Figure 5 shows the general recommendations for treating edema associated with lorlatinib. Once treatment with lorlatinib has been initiated, monitoring the occurrence of edema at each visit is suggested, as it is verified that edema does not arise from other causes. N-terminal pro-brain natriuretic peptide (NT-proBNP) assessment is recommended when significant edema occurs in patients with preexisting cardiovascular disease and/or risk factors for heart failure (diabetes, hypertension, obesity, chronic kidney disease). If the NT-proBNP level is higher than normal, the patient should be referred to the cardio-oncology clinic, and transthoracic echocardiography is recommended.
Fig. 5.
Recommendations for the management of edema
In patients without heart failure and in those with grade <3 edema, physical measures, such as leg elevation, moderate exercise or compression stockings, are suggested [14, 17]. Although there is no clear evidence on the role of a low-salt diet or water restriction, these are measures that can be considered. Delaying the use of diuretics as late as possible is recommended since there is no evidence to support their use for lorlatinib-associated edema. If a diuretic is used, furosemide at a dosage of 40 mg/day is recommended; however, the dosage should start at 20 mg/day. Caution should also be taken if the patient has renal injury. In these cases, a higher dose of the diuretic could be used under surveillance by nephrologists or internal medicine specialists [13].
Weight Increase
Mild to moderate weight gain (10–20%) due to increased appetite is another frequent AE associated with lorlatinib [15]. Although there is no specific medical treatment for this weight gain, physical exercise and restriction of food intake are recommended; therefore, it is important to collaborate with a nutritionist or even an endocrinologist if hyperglycemia occurs. If weight gain is associated with edema, treatment of the edema should be managed first. Interruption or reduction of the lorlatinib dose is not usually needed. There are currently no data on the use of semaglutide or other incretins in the management of lorlatinib-induced weight gain [43].
Diarrhea, Nausea, Vomiting, and Constipation
The frequencies of diarrhea, nausea, vomiting, and constipation are shown in Table 1 [13–15]. The primary recommendation for all patients with grade 1–2 disease is to not modify the dose of lorlatinib. For patients with a disease grade ≥3, discontinuation of lorlatinib treatment is recommended until symptoms are resolved to grade ≤2 or baseline values. Treatment can be resumed at a reduced dose [13] (Fig. 6).
Fig. 6.
Recommendations for the management of diarrhea, nausea, vomiting, and constipation. 5-HT3 5-hydroxytryptamine
During the onset of diarrhea and constipation, general measures such as diet adjustment and good hydration are suggested. Loperamide can be used for diarrhea, and lactulose can be used for constipation. Although it is not recommended to modify lorlatinib treatment for grade 2 diarrhea, sustained diarrhea (15–30 days) that interferes with daily life can be treated by reducing the lorlatinib dose.
For the treatment of nausea and vomiting, the recommendations offered by any oncology guide can be used [44, 45]. The recommended treatments for this level of emesis and for the next level include metoclopramide, domperidone, chlorpromazine or 5-HT3 inhibitors such as ondansetron, granisetron or dolasetron (Fig. 6) [44].
Amylase/Lipase Activity Increased
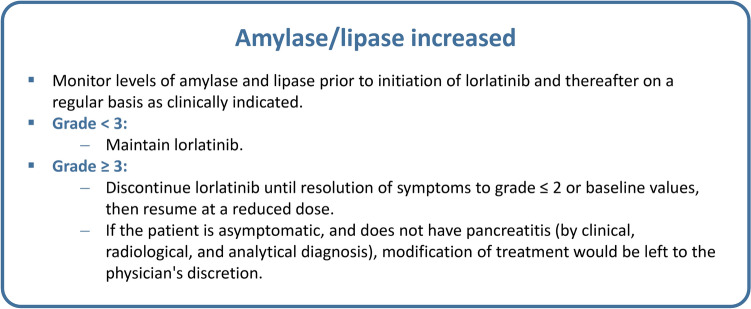
Increases in amylase and/or lipase levels were detected in patients receiving lorlatinib (Table 1). Patients should be monitored for increases in amylase and lipase levels prior to initiation of lorlatinib treatment and thereafter on a regular basis as clinically indicated [14]. If a grade 1–2 increase is detected, the same dose of lorlatinib can be administered at every clinical visit. If a grade 3–4 increase is observed, the main recommendation in clinical trials is to discontinue treatment until amylase/lipase levels return to baseline. Treatment with lorlatinib can be subsequently resumed at a lower dose (Fig. 7) [14]. However, since there is no evidence to support that amylase/lipase elevation leads to symptomatic pancreatitis later in patients with grade 3–4 disease, asymptomatic patients, or those without pancreatitis (according to clinical, radiological, and analytical diagnosis), modification of treatment should be left to the physician's discretion.
Fig. 7.
Recommendations for the management of alterations in amylase or lipase levels
Interstitial Lung Disease/Pneumonitis
Interstitial lung disease, pulmonary opacity, and pneumonitis are rare AEs in patients treated with lorlatinib (Table 1). Neither a radiological pattern nor a temporal pattern of pneumonitis has been described for lorlatinib. There are no data available on the incidence of pneumonitis associated with lorlatinib according to the patient's ethnicity, previous local treatment (radiotherapy, surgery) or previous pulmonary comorbidities. Similarly, there is no evidence on the best radiological technique to be used or how often pneumonitis should be monitored.
For the diagnosis of pneumonitis, a clinical evaluation, imaging tests, and differential diagnosis are recommended, and bronchoscopy is recommended for ruling out other causes of lung damage, such as infection [14]. In clinical practice, biopsy is not usually performed routinely unless there are clinical doubts or unless an infectious process is ruled out. For patients in whom pneumonitis is detected, clinicians should check whether the patient has a history of pulmonary disease, has undergone previous radiotherapy or has received immunotherapy that could explain the development of pneumonitis. Figure 8 shows the main suggested interventions for the treatment and control of pneumonitis. If pneumonitis is caused by a previous treatment, the full dose of lorlatinib can be used [46]. However, patients should be monitored closely because cross-reactivity with other ALK-TKIs has been described [47].
Fig. 8.
Recommendations for the management of interstitial lung disease or pneumonitis. COPD chronic obstructive pulmonary disease, CT computerized tomography, ECOG Eastern Cooperative Oncology Group, ICU intensive care unit, IFN interferon, TNF tumor necrosis factor
To monitor pneumonitis, clinical and radiological monitoring should be performed one week after initiating treatment with lorlatinib until lung toxicity is resolved. For grade 1 or 2 pneumonitis, clinical monitoring by imaging should be performed.
Pharmacological Interactions
As with any treatment, to preserve its efficacy while ensuring its safety, it is necessary to know which concurrent medication the patient is receiving or may receive, either for other diseases or as support to address AEs. Potential interactions between drugs used for the treatment of NSCLC and concomitant medication can be clinically relevant in some patients, decreasing its efficacy or increasing its toxicity [48].
To evaluate possible drug interactions, two aspects must be considered: the pathways by which the drugs are metabolized and the clearance periods. According to pharmacokinetic data, the main metabolic pathways of lorlatinib are oxidation and glucuronidation. In vitro data show that lorlatinib is metabolized primarily by CYP3A4 and UGT1A4, with minor contributions from CYP2C8, CYP2C19, CYP3A5, and UGT1A3 [13, 49]. This is important to keep in mind when administering treatments that involve the same pathway or interfere with inducers or inhibitors of the same pathway, as this could explain the occurrence of certain AEs. For example, drugs that are CYP3A4/5 inducers can decrease the plasma concentration of lorlatinib, thereby decreasing its efficacy. This would be the case with rifampicin, carbamazepine, enzalutamide, mitotane, phenytoin, and even St. John’s wort. For this reason, the use of the potent CYP3A4/5 inducer lorlatinib is contraindicated [13, 17, 50–53]. In addition, it has been reported that the use of potent CYP3A inducers with lorlatinib may increase the risk of severe hepatic toxicity [54].
On the other hand, CYP3A4/5 inhibitors increase the plasma concentration of lorlatinib and could increase its toxicity. This would be the case for some antivirals (boceprevir, ritonavir, and paritaprevir) and antifungals (itraconazole, ketoconazole, voriconazole, levoketoconazole, and fluconazole). Grapefruit products may also increase plasma concentrations of lorlatinib. The main recommendation is to avoid the use of CYP3A4/5 inhibitors or to seek an alternative, but if they are administered, it is recommended that the dose of lorlatinib be reduced [13, 17, 55, 56].
Lorlatinib may also interfere with the metabolism of other drugs by acting as an inhibitor or inducer. Lorlatinib is known to be a moderate inducer of CYP3A, which can result in a decreased concentration of drugs such as midazolam. Therefore, concomitant administration of lorlatinib with CYP3A4/5 substrates, especially those with narrow therapeutic indices, should be avoided [13, 17, 57]. Lorlatinib is also a weak inducer of CYP2B6, CYP2C9, UGT, and P-glycoprotein. In these cases, it is not necessary to modify the dose of drugs metabolized by these pathways, although those with a narrow therapeutic margin should be used with caution to avoid excessively increasing their concentration [13, 17].
Table 4 shows in detail various types of interactions and how to administer many of the drugs used in NSCLC patients treated with lorlatinib.
In addition to the metabolization pathway, the lorlatinib washout period should also be considered to avoid possible drug interactions. This washout period depends on the previous treatment, which is usually five times the elimination half-life of the previous drug. For example, when patients discontinue treatment with lorlatinib, which has a plasma half-life of 23.6 h, it is necessary to wait 5 days (i.e., the lorlatinib washout period) before starting any other treatment. It is very important to take into account this washout period when AEs have developed during the previous treatment [13]. On the other hand, if prior to using lorlatinib an inhibitor of its metabolism was being administered, it is suggested to discontinue this inhibitor at least 14 days (as a general safety period and specifically, it would be sufficient to wait for 5 half-lives of the inhibitor drug in question) before starting to use lorlatinib. However, this approach is not usually feasible in clinical practice, which could explain the greater toxicity of lorlatinib during the first days of treatment.
Renal or Hepatic Impairment
Although renal or hepatic impairments are not AEs associated with lorlatinib, they may result from treatment with lorlatinib. When renal function is normal or there is mild to moderate renal impairment (absolute estimated glomerular filtration rate [eGFR] ≥30 mL/min/1.73 m2), no dose adjustment of lorlatinib is necessary. However, if renal impairment is severe (absolute eGFR <30 mL/min/1.73 m2), the main recommendation is to use a reduced dose of lorlatinib; for example, an initial dose of 75 mg once daily orally. No information is available for patients requiring renal dialysis [13, 58]. In the case of mild hepatic impairment, no dose adjustment of lorlatinib is necessary, but since there is insufficient information in cases of moderate or severe hepatic impairment, treatment with lorlatinib is not recommended [13].
Conclusions
Lorlatinib has been shown to be an effective treatment, but because of its long-term use, it is very important to know how to manage all the possible AEs that may occur during its use to ensure its efficacy and the quality of life of patients. The main recommendation for the management of each of the AEs associated with lorlatinib is to follow the guidelines of the product data sheet. However, there are situations in which it is necessary to approach the management of AEs individually, such as having to reduce the dose of lorlatinib to avoid possible drug interactions with concomitant treatments or to prevent worsening of the patient’s quality of life even when the toxicity is grade 1–2. Similarly, more severe toxicities, that are laboratory findings with no symptomatology (e.g., amylase elevation grade ≥3), may not require discontinuation of treatment or may be left to physician’s discretion.
It is important to note that some AEs have little or questionable evidence. Therefore, both the detection and management of these AEs is necessary in the current clinical context of what is known, and other possible causes must be ruled out.
In addition to all the approaches described above, one of the most important strategies for managing AEs is patient education and direct communication with the patient and family members. It should be discussed with them that lorlatinib is associated with several AEs that are mostly mild and reversible and facilitate the contact with health care professionals to assess these in a timely manner. The ultimate goal is to offer the maximum benefit of lorlatinib for each patient.
Acknowledgments
The authors would like to thank Fernando Sánchez Barbero PhD on behalf of Springer Healthcare for providing medical writing assistance. This assistance was funded by Pfizer.
Declarations
Funding
This project was developed with funding from Pfizer.
Conflict of Interests
Edurne Arriola has received speaking and/or advisory honoraria from Pfizer, Janssen, Sanofi, AstraZeneca, Bristol Myers Squibb, Lilly, Boehringer Ingelheim, Takeda, Roche, and MSD; and travel support from Takeda, Roche, and AstraZeneca. Javier de Castro has received research funding and educational fees from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, and Hoffmann-La Roche; honoraria or held advisory role for AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, GSK, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Hoffmann-La Roche, Sanofi, and Takeda. Rosario García-Campelo has received research funding and grant support from Roche, Pfizer, Merck Serono, and Bristol Myers Squibb and received honoraria or held advisory role for Pfizer, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Sanofi, Lilly, Amgen, Janssen, Boehringer Ingelheim, AstraZeneca, Takeda, Regeneron, and Sanofi; and travel support from Roche, Takeda, Astra Zeneca, Roche, and MSD. Reyes Bernabé has received speaking and/or advisory honoraria from Pfizer, Janssen, Sanofi, AstraZeneca, Bristol Myers Squibb, Roche, Takeda, and Jannsen. Beatriz Bernárdez has received speaking and/or advisory honoraria from Pfizer, Janssen, Astellas, Sanofi, AstraZeneca, Bristol Myers Squibb, Merck, Daiichi Sankyo, Takeda, and MSD. Jordi Bruna has received personal consulting fees or congress travel support from Takeda, Eisai, CSL Behring, Pfizer, and Novocure. Manuel Dómine has received honoraria for advisory board/consultancy from AstraZeneca, Boehringer Ingelheim, Janssen Cilag, MSD, Pfizer, Roche, Sanofi, and Takeda; and honoraria for speaker from AstraZeneca, Bristol Myers Squibb, MSD, Pfizer, Roche, and Takeda. Dolores Isla has received honoraria and held advisory role for Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, F. Hoffmann-La Roche, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Sanofi, and Takeda. Óscar Juan received honoraria and held advisory role for Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genetech, AstraZeneca, Pfizer, Eli Lilly, Takeda, and Janssen; and travel, support from Takeda and AstraZeneca. Teresa López-Fernández has received honoraria for advisory board/speaker from Philips, Pfizer, Bristol Myers Squibb, Janssen, Daichi Sankyo, Myocardial Solutions, AstraZeneca, Beigene, and Bayer. Ernest Nadal has received research funding and grant support from Roche, Pfizer, Merck-Serono, and Bristol Myers Squibb; received honoraria or held advisory role for Pfizer, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Sanofi, Lilly, Amgen, Janssen, Daiichi-Sankyo, Boehringer Ingelheim, AstraZeneca, Qiagen, Pierre Fabre, Takeda, Regeneron, and Sanofi; and travel support from Roche, Takeda, and MSD. Delvys Rodríguez has received personal fees/honoraria for consultancy/advisory role and lectures from Roche/Genentech, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Merck Serono, Eli Lilly, Gilead, Sanofi, Regeneron, Incyte, Pfizer, Takeda, and Novartis; travel expenses from Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Sanofi, Regeneron, and Novartis; and grant support for studies from Bristol Myers Squibb. María Vares has no conflicts of interest. Úrsula Asensio was a Pfizer employee when this manuscript was written. Luis F García is a Pfizer employee. Enriqueta Felip has received personal fees/honoraria for consultancy/advisory roles and speaker bureau from AbbVie, Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, F. Hoffmann-La Roche, Genentech, Gilead, GSK, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peervoice, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Touch Oncology, and Turning Point Therapeutics and has been an independent member of the board for Grifols.
Availability of Data and Material
Not applicable.
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
A main coordinator and other experts developed each section of this article. The metabolic and cardiovascular adverse events section was coordinated by Javier de Castro, with the expertise of Manuel Dómine, Dolores Isla, and Teresa López-Fernández. The neurological adverse events section was coordinated by Enriqueta Felip, with the expertise of Ernest Nadal, Reyes Bernabé, and Jordi Bruna. Other adverse events were coordinated by Rosario García-Campelo with the expertise of Óscar Juan-Vidal, Delvys Rodríguez-Abreu, and María Vares. The pharmacological interactions section was coordinated by Edurne Arriola with the expertise of Beatriz Bernárdez. All the authors participated in the development of the figures and tables.
References
- 1.Wu J, Lin Z. Non-small cell lung cancer targeted therapy: drugs and mechanisms of drug resistance. Int J Mol Sci. 2022;23(23):15056. 10.3390/ijms232315056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YC, Hsieh CC, Lee YL, Li CY. Which should be used first for ALK-positive non-small-cell lung cancer: chemotherapy or targeted therapy? A meta-analysis of five randomized trials. Medicina (Kaunas). 2019;55(2):29. 10.3390/medicina55020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng L, Zhu L, Sun Y, Stebbing J, Selvaggi G, Zhang Y, et al. Targeting ALK rearrangements in NSCLC: current state of the art. Front Oncol. 2022;12: 863461. 10.3389/fonc.2022.863461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 5.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 6.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 7.Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. 10.1016/S0140-6736(17)30565-2 [DOI] [PubMed] [Google Scholar]
- 8.Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–29. 10.1016/S0140-6736(17)30123-X [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Sheng Z, Zhang J, Song J, Teng L, Liu L, et al. Comparison of lorlatinib, alectinib and brigatinib in ALK inhibitor-naive/untreated ALK-positive advanced non-small-cell lung cancer: a systematic review and network meta-analysis. J Chemother. 2022;34(2):87–96. 10.1080/1120009X.2021.1937782 [DOI] [PubMed] [Google Scholar]
- 10.Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):1617–25. 10.1001/jamaoncol.2021.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Pan H, Liu Y, Zhang Y, Hong S, Huang J, et al. Ensartinib in advanced ALK-positive non-small cell lung cancer: a multicenter, open-label, two-staged, phase 1 trial. J Thorac Dis. 2022;14(12):4751–62. 10.21037/jtd-22-1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon BJ, Liu G, Felip E, Mok TSK, Soo RA, Mazieres J, et al. Lorlatinib versus crizotinib in patients with advanced ALK-positive non-small cell lung cancer: 5-year outcomes from the phase III CROWN Study. J Clin Oncol. 2024. 10.1200/JCO2400581. 10.1200/JCO2400581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Product information: Lorviqua® (lorlatinib). Available from: https://www.ema.europa.eu/en/documents/product-information/lorviqua-epar-product-information_en.pdf. Accessed 22 Sep 2023.
- 14.Bauer TM, Felip E, Solomon BJ, Thurm H, Peltz G, Chioda MD, et al. Clinical management of adverse events associated with lorlatinib. Oncologist. 2019;24(8):1103–10. 10.1634/theoncologist.2018-0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–29. 10.1056/NEJMoa2027187 [DOI] [PubMed] [Google Scholar]
- 16.El Darsa H, Abdel-Rahman O, Sangha R. Pharmacological and clinical properties of lorlatinib in the treatment of ALK-rearranged advanced non-small cell lung cancer. Expert Opin Pharmacother. 2020;21(13):1547–54. 10.1080/14656566.2020.1774552 [DOI] [PubMed] [Google Scholar]
- 17.Barata F, Aguiar C, Marques TR, Marques JB, Hespanhol V. Monitoring and managing lorlatinib adverse events in the Portuguese clinical setting: a position paper. Drug Saf. 2021;44(8):825–34. 10.1007/s40264-021-01083-x [DOI] [PubMed] [Google Scholar]
- 18.Reed M, Rosales AS, Chioda MD, Parker L, Devgan G, Kettle J. Consensus recommendations for management and counseling of adverse events associated with lorlatinib: a guide for healthcare practitioners. Adv Ther. 2020;37(6):3019–30. 10.1007/s12325-020-01365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilickap S, Ak S, Dursun OU, Sendur MA, Karadurmus N, Demirci U. Safety of lorlatinib in ALK-positive non-small-cell lung cancer and management of central nervous system adverse events. Future Oncol. 2023;19(29):2003–12. 10.2217/fon-2023-0014 [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Yang Y, Zhang L, Cheng Y, Han B, Lu Y, et al. Expert consensus of management of adverse drug reactions with anaplastic lymphoma kinase tyrosine kinase inhibitors. ESMO Open. 2023;8(3): 101560. 10.1016/j.esmoop.2023.101560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G, Mazieres J, Stratmann J, Ou SI, Mok T, Grizzard M, et al. A pragmatic guide for management of adverse events associated with lorlatinib. Lung Cancer. 2024;191: 107535. 10.1016/j.lungcan.2024.107535 [DOI] [PubMed] [Google Scholar]
- 22.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 23.Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023;11(4):354–66. 10.1016/S2213-2600(22)00437-4 [DOI] [PubMed] [Google Scholar]
- 24.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 25.Waltham MA. Lexicomp Online. UpToDate. Available from: https://online.lexi.com. Accessed 22 Sep 2023.
- 26.Cannon CP. Statin intolerance: how common is it and how do we work with patients to overcome it? Eur Heart J. 2022;43(34):3224–6. 10.1093/eurheartj/ehac156 [DOI] [PubMed] [Google Scholar]
- 27.Bytyci I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43(34):3213–23. 10.1093/eurheartj/ehac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64. 10.1056/NEJMoa2215024 [DOI] [PubMed] [Google Scholar]
- 29.Tokgozoglu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. Eur Heart J. 2022;43(34):3198–208. 10.1093/eurheartj/ehab841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Product information: Lopid® (gemfibrozil). Available from: https://cima.aemps.es/cima/pdfs/es/ft/61026/FT_61026.html.pdf. Accessed 22 Sep 2023.
- 31.Pedro-Botet J, Barrios V, Sánchez-Margalet V, Tamargo J, Arrieta F, Gámez JM, et al. Treatment of hypertriglyceridaemia with icosapent ethyl in patients with high/very high cardiovascular risk. Consensus document of the Sociedad Española de Cardiología [Spanish Society of Cardiology] and the Sociedad Española de Diabetes [Spanish Diabetes Society]. Endocrinol Diabetes Nutr (Engl Ed). 2023;70(Suppl 1):51–62. [DOI] [PubMed]
- 32.Gaba P, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, et al. Prevention of cardiovascular events and mortality with icosapent ethyl in patients with prior myocardial infarction. J Am Coll Cardiol. 2022;79(17):1660–71. 10.1016/j.jacc.2022.02.035 [DOI] [PubMed] [Google Scholar]
- 33.Castro Conde A, Marzal Martín D, Arrarte V, Campuzano R, Dalmau González-Gallarda R, Fernández Olmo MR, et al. Comprehensive approach to the patient with diabetes mellitus and cardiovascular disease or very high cardiovascular risk. REC CardioClinics. 2019;54(3):183–92. 10.1016/j.rccl.2019.04.005 [DOI] [Google Scholar]
- 34.Sisi M, Fusaroli M, De Giglio A, Facchinetti F, Ardizzoni A, Raschi E, et al. Psychiatric adverse reactions to anaplastic lymphoma kinase inhibitors in non-small-cell lung cancer: analysis of spontaneous reports submitted to the FDA adverse event reporting system. Target Oncol. 2022;17(1):43–51. 10.1007/s11523-021-00865-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagasaka M, Ge Y, Sukari A, Kukreja G, Ou SI. A user’s guide to lorlatinib. Crit Rev Oncol Hematol. 2020;151: 102969. 10.1016/j.critrevonc.2020.102969 [DOI] [PubMed] [Google Scholar]
- 36.Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–9. 10.1016/S1470-2045(17)30680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon BJ, Bauer TM, Ignatius Ou SH, Liu G, Hayashi H, Bearz A, et al. Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non-small-cell lung cancer from the phase III CROWN study. J Clin Oncol. 2022;40(31):3593–602. 10.1200/JCO.21.02278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagogo-Jack I, Abbattista A, Murphy JF, Krulewicz S, Do A, Peterson J, et al. Factors associated with developing neurocognitive adverse events in patients receiving lorlatinib after progression on other targeted therapies. J Thorac Oncol. 2023;18(1):67–78. 10.1016/j.jtho.2022.09.219 [DOI] [PubMed] [Google Scholar]
- 39.Prianti JN, Cezar A, Moraes F, Madeira TM, Moisés de Lima Santiago E, Silveira Vilbert M. 1365P Neurocognitive adverse events related to lorlatinib in non-small cell lung cancer: a systematic review and meta-analysis. Ann Oncol. 2023;34(Suppl 2):S784. 10.1016/j.annonc.2023.09.2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenmaekers J, Dijkstra J, van der Wekken A, Paats M, Broen M, Brandts L, et al. In-depth analysis of lorlatinib-related neurocognitive adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2023;25(2):168–74. 10.1016/j.cllc.2023.12.003 [DOI] [PubMed] [Google Scholar]
- 41.Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci. 2003;28(2):99–112. [PMC free article] [PubMed] [Google Scholar]
- 42.Ayano G. Psychotropic medications metabolized by cytochromes P450 (CYP) 3A4 enzyme and relevant drug interactions: review of articles. Austin J Psychiatry Behav Sci. 2016;3(2):1054. [Google Scholar]
- 43.Dahl K, Brooks A, Almazedi F, Hoff ST, Boschini C, Baekdal TA. Oral semaglutide improves postprandial glucose and lipid metabolism, and delays gastric emptying, in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23(7):1594–603. 10.1111/dom.14373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sociedad Español de Oncología Médica. Guía de práctica clínica sobre antieméticos en oncología. Available from: https://www.seom.org/seomcms/images/stories/recursos/infopublico/publicaciones/antiemeticos/capitulo4.pdf. Accessed 22 Sep 2023.
- 45.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Antiemesis - Version 2.2023 - May 24, 2023. Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 22 Sep 2023.
- 46.Pellegrino B, Facchinetti F, Bordi P, Silva M, Gnetti L, Tiseo M. Lung toxicity in non-small-cell lung cancer patients exposed to ALK inhibitors: report of a peculiar case and systematic review of the literature. Clin Lung Cancer. 2018;19(2):e151–61. 10.1016/j.cllc.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 47.Monzonís X, Arriola E. Early onset pulmonary toxicity with lorlatinib in a patient with previous pulmonary toxicity from brigatinib. J Thorac Oncol. 2019;14(11):e247–8. 10.1016/j.jtho.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 48.Fort E, Otero S, Palmero R, Canedo M, Bleda C, Prats J, et al. Interacciones farmacológicas identificadas en pacientes con cáncer de pulmón célula no pequeña avanzado en tratamiento oral. Ecucational Sympisium XI; 2022 June 17-18; Barcelona (Spain).
- 49.Chen J, O’Gorman MT, James LP, Klamerus KJ, Mugundu G, Pithavala YK. Pharmacokinetics of lorlatinib after single and multiple dosing in patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer: results from a global phase I/II study. Clin Pharmacokinet. 2021;60(10):1313–24. 10.1007/s40262-021-01015-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Xu H, Pawlak S, James LP, Peltz G, Lee K, et al. The effect of rifampin on the pharmacokinetics and safety of lorlatinib: Results of a phase one, open-label, crossover study in healthy participants. Adv Ther. 2020;37(2):745–58. 10.1007/s12325-019-01198-9 [DOI] [PubMed] [Google Scholar]
- 51.Hu W, Lettiere D, Tse S, Johnson TR, Biddle KE, Thibault S, et al. Liver toxicity observed with lorlatinib when combined with strong CYP3A inducers: evaluation of cynomolgus monkey as a nonclinical model for assessing the mechanism of combinational toxicity. Toxicol Sci. 2021;182(2):183–94. 10.1093/toxsci/kfab056 [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim SM, Pithavala YK, Vourvahis M, Chen J. A literature review of liver function test elevations in rifampin drug-drug interaction studies. Clin Transl Sci. 2022;15(7):1561–80. 10.1111/cts.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Pithavala YK, Gong J, LaBadie RR, Mfopou JK, Chen J. The effect of modafinil on the safety and pharmacokinetics of lorlatinib: a phase I study in healthy participants. Clin Pharmacokinet. 2021;60(10):1303–12. 10.1007/s40262-021-01026-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Food and Drud Administration. Prescribing information: Lorbrena® (lorlatinib). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210868s004lbl.pdf. Accessed 22 Sep 2023.
- 55.Li W, Sparidans RW, Wang Y, Lebre MC, Beijnen JH, Schinkel AH. Oral coadministration of elacridar and ritonavir enhances brain accumulation and oral availability of the novel ALK/ROS1 inhibitor lorlatinib. Eur J Pharm Biopharm. 2019;136:120–30. 10.1016/j.ejpb.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 56.Patel M, Chen J, McGrory S, O’Gorman M, Nepal S, Ginman K, et al. The effect of itraconazole on the pharmacokinetics of lorlatinib: results of a phase I, open-label, crossover study in healthy participants. Invest New Drugs. 2020;38(1):131–9. 10.1007/s10637-019-00872-7 [DOI] [PubMed] [Google Scholar]
- 57.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38(1):41–57. 10.2165/00003088-200038010-00003 [DOI] [PubMed] [Google Scholar]
- 58.Lin S, Gong J, Canas GC, Winkle P, Pelletier K, LaBadie RR, et al. A phase I study to evaluate the pharmacokinetics and safety of lorlatinib in adults with mild, moderate, and severe renal impairment. Eur J Drug Metab Pharmacokinet. 2022;47(2):235–45. 10.1007/s13318-021-00747-4 [DOI] [PMC free article] [PubMed] [Google Scholar]