Abstract
Background
Nitric oxide (NO) is a strong vasodilator, selectively directed on pulmonary circulation through inhaled administration. In adult intensive care units (ICU), it is mainly used for refractory hypoxemia in mechanically ventilated patients. Several medical delivery devices have been developed to deliver inhaled nitric oxide (iNO). The main purpose of those devices is to guarantee an accurate inspiratory NO concentration, whatever the ventilator used, with NO2 concentrations lower than 0.3 ppm. We hypothesized that the performances of the different available iNO delivery systems could depend on their working principle and could be influenced by the ventilator settings. The objective of this study was to assess the accuracy of seven different iNO-devices combined with different ICU ventilators’ flow-by to reach inspiratory NO concentration targets and to evaluate their potential risk of toxicity.
Methods
We tested seven iNO-devices on a test-lung connected to distinct ICU ventilators offering four different levels of flow-by. We measured the flow in the inspiratory limb of the patient circuit and the airway pressure. The nitric oxide/nitrogen (NO/N2) flow was measured on the administration line of the iNO-devices. NO and NO2 concentrations were measured in the test-lung using an electrochemical analyzer.
Results
We identified three iNO-device generations based on the way they deliver NO flow: “Continuous”, “Sequential to inspiratory phase” (I-Sequential) and “Proportional to inspiratory and expiratory ventilator flow” (Proportional). Median accuracy of iNO concentration measured in the test lung was 2% (interquartile range, IQR -19; 36), -23% (IQR -29; -17) and 0% (IQR -2; 0) with Continuous, I-Sequential and Proportional devices, respectively. Increased ventilator flow-by resulted in decreased iNO concentration in the test-lung with Continuous and I-Sequential devices, but not with Proportional ones. NO2 formation measured to assess potential risks of toxicity never exceeded the predefined safety target of 0.5 ppm. However, NO2 concentrations higher than or equal to 0.3 ppm, a concentration that can cause bronchoconstriction, were observed in 19% of the different configurations.
Conclusion
We identified three different generations of iNO-devices, based on their gas administration modalities, that were associated with highly variable iNO concentrations’ accuracy. Ventilator’s flow by significantly impacted iNO concentration. Only the Proportional devices permitted to accurately deliver iNO whatever the conditions and the ventilators tested.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01351-w.
Keywords: Acute respiratory distress syndrome, Artificial lung, Bias flow, byflow, ICU ventilators, Inhaled nitric oxide, iNO delivery systems, Mechanical ventilation, Ventilator flow-by
Background
Nitric oxide (NO) is a free radical with a low molecular weight and a high affinity for oxygen (O2), whose strong vasodilator properties were described in the 80's [1, 2]. Because of a very short biologic half-life, inhaled nitric oxide (iNO) can be restricted to the pulmonary circulation preferentially in well-ventilated lungs areas. Thanks to this specific delivery administration, iNO may permit to improve gas exchange in case of ventilation/perfusion mismatch and/or limit right ventricular afterload in case of pulmonary hypertension without deleterious systemic vasodilation effect [3].
In adult intensive care units (ICU), inhaled vasodilators like NO are provided in roughly 8% of patients with acute respiratory distress syndrome (ARDS) and 13% when ARDS is severe [4], despite the lack of strong evidence-based guidelines [5–9]. A recent retrospective analysis of a large series of patients with COVID-19 associated ARDS reported an increased use of iNO (almost 20% of the patients) [10] with a possible benefit in the most severe patients. iNO improves oxygenation in 50% to 70% of mechanically ventilated patients [5, 11–18].
Several iNO-devices have been successively developed to administrate iNO, since the 80’s. The delivery could take place directly into the ventilator air inlet or into the inspiratory limb of the patient circuit [19–21]. New technologies developed by manufacturers were essentially driven by the need to obtain a stable and accurate concentration of iNO inside the inspiratory circuit [21–24] and to limit the risk of toxicity. Regarding the risk, both the formation of nitrogen dioxide (NO2) by contact of O2 with NO and the formation of methaemoglobin are potentially deleterious [25].
In this experimental in vitro study, after classifying the different iNO-devices commonly used nowadays based on their different working principles, we assessed their accuracy and potential risk of toxicity.
We hypothesized that the technical differences in iNO-devices and ventilator characteristics (as the flow-by that is highly variable from one ICU ventilator to another) could substantially affect the accuracy of iNO administration.
Methods
Lung Model
Bench settings
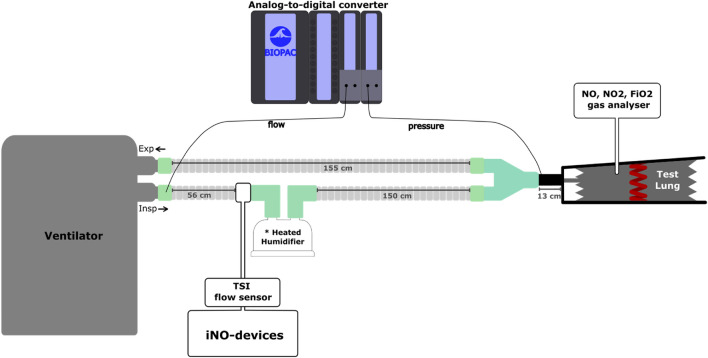
A Michigan test-lung (Michigan Dual Adult Test Lung, Michigan Instruments, Grand Rapids, Michigan, USA) was used to reproduce mechanical ventilation in ARDS condition, with a respiratory system compliance of 30 mL/cmH2O and resistances of 20cmH2O/L/s. The experimental bench setting is described in Fig. 1. The ventilator tested was connected to the test-lung via a double limb circuit with a heated humidifier. iNO-devices were implemented to provide NO in the inspiratory limb of the patient circuit.
Fig. 1.
Description of the experimental bench model. The figure presents the bench set-up. The ventilators were connected to a Michigan test lung simulating mechanical ventilation in an ARDS patient. The patient circuit included a heated humidifier and the administration line of the iNO-device was connected to the humidifier inlet. The heated humidifier (MR290, Fisher and Paykel, Auckland, New-Zealand), with a volume of 280 mL, was full and at ambient temperature. A precise flow sensor was inserted on the administration line to record the NO flow delivered by each device. A pressure transducer was used to measure the airway pressure at the Y piece. A pneumotachometer was used to record the flow through the inspiratory limb of the patient circuit. The same specific breathing circuit, for heated humidifier, was used for each iNO-device and each ventilator (RT380, Fisher and Paykel, Auckland, New-Zealand). A majority of experiments has been done in the Vent’Lab (Angers university hospital) while some of them have been performed in the medical intensive care unit of the university hospital of Amiens
iNO-devices and ventilators tested
We tested seven iNO-devices, the characteristics of which are shown in Table 1.
Table 1.
Characteristics of iNO-devices tested
| Manufacturer | Pressure or flow sensor or cable | Electrochemical NO and NO2 analyzer available | Response time of the analyzer | |
|---|---|---|---|---|
| INOmax DSIR | Mallinckrodt Pharmaceuticals, Dublin, Ireland | Flow sensor | Yes | 30 s |
| Just Press | ITC, Madrid, Spain | NA | No | NA |
| MiniKINOX | Cahouet, Montreuil, France | NA | No | NA |
| NO-A | EKU Elektronik GmbH, Leiningen, Germany | Cable | Yes | 10 s |
| NOX-tec | ITC, Madrid, Spain | Flow sensor | Yes | 10 s |
| OptiKINOX | ALMS, Antony, France | Pressure sensor | No | NA |
| SoKINOX | iNOsystems, Antony, France | Flow sensor | Yes | 10 s |
NA Not applicable
Those iNO-devices were assessed with different ventilators offering different flow-by: Servo 900C (Siemens-Elema AB, Solna, Sweden) and Evita 4 (Dräger, Lübeck, Germany) without flow-by, SERVO-i (Getinge, Göteborg, Sweden) and Evita Infinity V500 (Dräger, Lübeck, Germany) with a flow-by of 2L/min, Engström Carestation and Carescape R860 (GE Healthcare, Madison, USA) with a flow-by of 10L/min, Elisa 500 (Löwenstein, Steinbach, Germany) with flow-by of 10 and 30L/min. The flow-by is a specific flow not participating in patient minute ventilation, through the patient circuit during the expiration phase, necessary to generate triggering and to improve non-invasive ventilation performances. Values of flow-by for various ventilators are detailed in the Additional data 1.
Data recording and analysis
As illustrated in Fig. 1, a pneumotachometer (3700 Series, Hans Rudolph Inc., Shawnee, USA) recorded the flow in the inspiratory limb of the patient circuit. A pressure transducer was connected at the Y-piece to measure the airway pressure. The NO/N2 flow administrated was measured directly from the device with a high accuracy flowmeter (Mass flowmeter 4140, TSI Incorporated, Shorview, USA).
All the signals (ventilator’s flow, airway pressure, NO/N2 flow) were converted to digital signals using an analog-to-digital converter (MP 150, Biopac systems Inc., Goleta, California, USA) and analyzed by a dedicated software (Acqknowledge version 5, Biopac systems Inc.) (Fig. 1).
NO and NO2 concentrations were measured directly in the test-lung, by the electrochemical analyzer of the SoKINOX (the analyzer has a response time of 10 s and the screen has a display refresh of 7.8 Hz). Supplementary tests have been performed using a chemiluminescent sensor (with a response time of 1 s) to validate the measurements performed using the electrochemical technique (see Additional data 2).
Protocol
The ventilators were set in volume mode, with a tidal volume of 450 mL (corresponding to 6 mL/kg predicted body weight for an adult male, 180 cm tall), constant inspiratory flow of 60L/min, respiratory rate of 25 cycles/min, plateau time of 200 ms, positive end-expiratory pressure of 10cmH2O (to mimic end-expiratory lung volume), trigger sensitivity was set at the default value (so there was no auto-triggering). FiO2 was set at 100% to maximize the risk of NO2 formation.
Four iNO concentrations were tested: 5, 10, 14 and 20 ppm for each couple of iNO-device/ventilator. The settings for each iNO-device were performed based on the recommendations of the user manual with some additional tests when required (see Additional data 3 for details). The iNO administration site was similar whatever the iNO-device, see Fig. 1. When several working modes were available, the most advanced mode was chosen. However, the hot-wire flow sensor was not used for the NO-A.
All tests were performed at two initial cylinder concentrations of NO/N2 gas mixture: 450 and 800 ppm.
Statistical analysis
The relative iNO concentration error (ΔNO) was defined as the relative difference between the effective iNO concentration measured in the test lung and the targeted iNO concentration in percentage. ΔNO was expressed as median and interquartile ranges (IQR) over four tested iNO concentrations (5, 10, 14 and 20 ppm) and two tested cylinder concentrations (450 and 800 ppm) according to the data availability expressed in the Additional data 4.
Performances of iNO-devices were considered as acceptable when ΔNO was within ± 20%, as indicated by the FDA guidance [26].
Results
Description of three different generations of iNO-devices
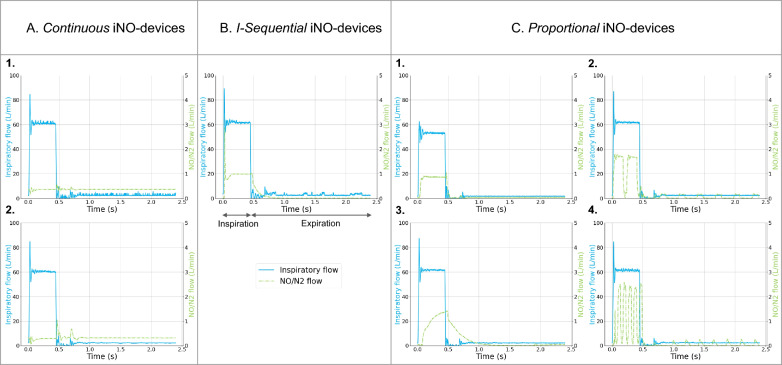
Based on the different patterns of iNO delivery among the seven devices tested, we identified three generations of iNO-devices (Fig. 2).
Fig. 2.
Classification of iNO-devices. Three generations of iNO-devices are described. For each type, one respiratory cycle is illustrated including inspiration and expiration. The blue curve is the inspiratory flow recorded through the inspiratory limb of the patient circuit. The dotted green curve is the NO/N2 flow recorded through the administration line. From the left to the right: A Continuous delivery: constant NO/N2 flow during both inspiratory and expiratory phases, the A.1 is Just Press and A.2 is MiniKINOX; B I-Sequential delivery: the NO/N2 flow is null during expiratory phase, the panel is OptiKINOX; C Proportional delivery: the NO/N2 flow increases during inspiratory phase and decreases but stays positive during expiratory phase, the C.1 is INOmax, C.2 is NO-A, C.3 is NOXtec and C.4 is SoKINOX
A. Continuous iNO-devices (Just Press, MiniKINOX): based on a simple flowmeter, this technology provides a constant and continuous NO/N2 flow during both inspiratory and expiratory. The NO/N2 flow is manually set on the flowmeter based on predefined tables taking into account the targeted iNO concentration, the patient minute-ventilation, the cylinder concentration and possibly the inspiratory time (Ti).
B. Sequential to inspiration or I-Sequential iNO-devices (OptiKINOX): this technology allows to administer a NO/N2 flow only during the inspiratory phase, whereas the NO administration is stopped during the expiratory phase. The NO flow is synchronized with the inspiratory phase by the detection of pressure variations inside the inspiratory limb of the patient circuit. The targeted iNO concentration, patient minute ventilation and Ti are set on the device by the clinician.
C. Proportional to inspiratory and expiratory ventilator flow or Proportional iNO-devices (INOmax, NO-A, NOXtec and SoKINOX): this technology allows to administer a NO/N2 flow proportional to ventilator flow going through the inspiratory limb of the patient circuit during both the inspiratory and expiratory phases. As a result, iNO delivery to the patient circuit differs during the inspiratory and expiratory phases. This adaptation of NO flow necessitates a continuous measurement or estimation of the minute ventilation either by a direct measurement on the inspiratory limb of the patient circuit or by electronic interface with the ventilator.
Accuracy of iNO delivery according to the different iNO-device generations
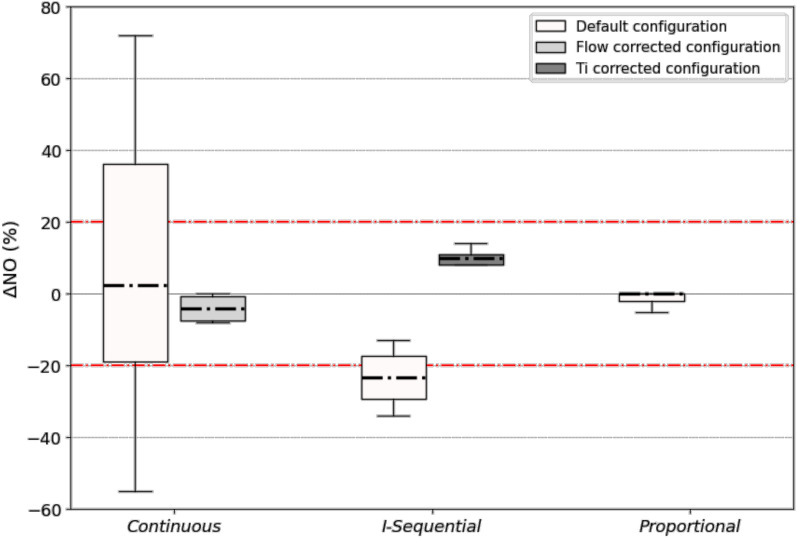
The accuracy of iNO delivery for the three different generations of iNO-devices described above with a ventilator providing a flow-by of 2L/min is displayed in Fig. 3 and detailed in the additional data 5 by targeted concentrations.
Fig. 3.
Relative iNO concentration error (ΔNO) with the three generations of iNO-devices. Box-plots represent median, interquartile, maximal and minimal values of ΔNO, for each generation of iNO-devices. The dotted red line represents the acceptable target range of iNO concentration error in relative percentage. Each box-plot includes four targeted iNO concentrations (5, 10, 14, 20 ppm) and two NO/N2 cylinder concentrations (450 and 800 ppm) when available. For default configuration, n = 16 for continuous devices, n = 12 for I-Sequential devices, and n = 32 for proportional devices. *Flow corrected configuration means adjustment of NO/N2 flow by a precise flow sensor to the calculated value of flow for continuous devices. *Ti corrected configuration means adjustment of Ti/Ttot ratio to the insufflation time for I-Sequential devices
ΔNO for continuous devices was low but a high dispersion was recorded (2%, IQR -19; 36%, n = 20). Actual iNO concentrations’ accuracies were significantly improved when the NO/N2 flow was adjusted using an external flow sensor that was more precise than the pointer of the iNO-devices (flow corrected configuration, Fig. 3). The adjusting method of the continuous iNO-devices can also impact delivery accuracy, as detailed in Additional data 6 and 7.
I-Sequential iNO-devices systematically resulted in an under-administration of iNO (ΔNO -23%, IQR -29; -17%, n = 12). The accuracy was improved when plateau time was excluded from the Ti set on the device (Ti corrected configuration, Fig. 3), as explained in the Additional data 8.
With proportional iNO-devices, ΔNO was systematically in the ± 20% error range (0%, IQR -2; 0%, n = 32).
NO2 formation never exceeded the predefined safety target of 0.5 ppm whatever the device and condition tested. Of note, we measured NO2 concentrations higher than or equal to 0.3 ppm in 19% of our configurations, which could cause bronchoconstriction [27].
The cylinder concentration had no significant impact on ΔNO (data not shown).
Impact of the flow-by on iNO delivery
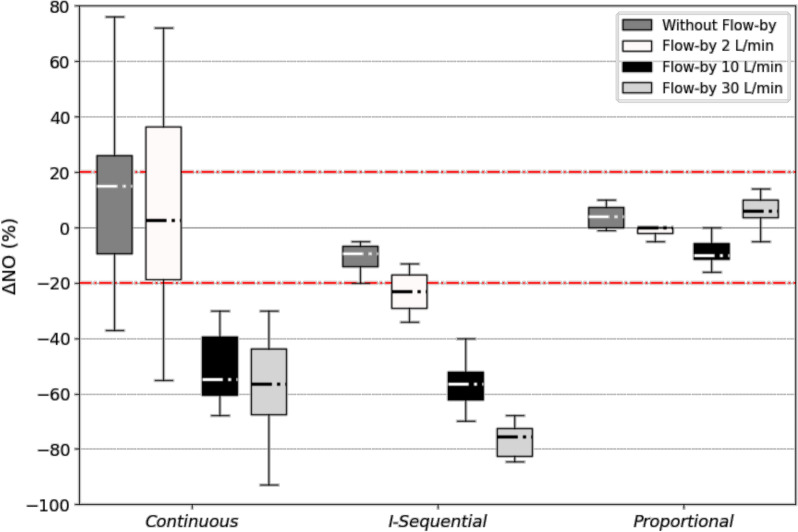
Among the four flow-by tested (0, 2, 10 and 30L/min), higher was the flow-by, higher was the under-delivery of iNO, except for the proportional iNO-devices (Fig. 4). Proportional devices were the only ones able to maintain iNO concentration within the target when flow-by increased.
Fig. 4.
Impact of flow-by on relative iNO concentration error (ΔNO) in the three generations of iNO-devices. Box plots represent median, interquartile range, maximal and minimal values of ΔNO, for each generation of iNO-devices. Four flow-by values (0, 2, 10 and 30 L/min) were tested for each generation. The dotted red line represents the acceptable target range of iNO concentration error in relative percentage. Each box-plot includes four targeted iNO concentrations (5, 10, 14, 20 ppm) and two NO/N2 cylinder concentrations (450 and 800 ppm) when available. For continuous devices, n = 8 without flow-by, n = 16 for flow-by of 2 L/min, n = 16 for flow-by of 10 L/min and n = 12 for flow-by of 30 L/min. For I-Sequential devices, n = 4 without flow-by, n = 12 for flow-by of 2 and 10 L/min and n = 8 for flow-by of 30 L/min. For Proportional devices, n = 8 without flow-by, n = 32 for flow-by of 2 L/min, n = 20 for flow-by of 10 L/min and n = 12 for flow-by of 30 L/min
Of note, the impact of iNO administration site on iNO delivery has been assessed in Additional data 9.
Discussion
Present experimental in-vitro findings could be summarized as follows:
We proposed herein to classify commercially available iNO-devices in three technological generations (Continuous, I-Sequential, Proportional).
Their performances to deliver the targeted iNO concentrations in the test-lung were highly variable, depending on their working principle.
The adjunction of a flow-by (highly variable and often blind on ICU ventilators) resulted in a systematic and significant under-delivery of iNO with Continuous and I-Sequential generations of iNO-devices.
Only the Proportional devices allowed to maintain the NO concentration in the test lung within the predefined ± 20% error margins for all conditions tested including different ICU ventilators flow-by.
Classification of iNO-devices
To our knowledge, a classification including the latest commercially available iNO-devices and taking into account the interactions with different ventilators has never been proposed. The classification that we propose herein describes and differentiates three different iNO administration patterns that variably affect iNO delivery accuracy. In addition, this classification is valuable for understanding the substantial impact of ventilators flow-by on iNO delivery. This concern has never been systematically investigated but deserves to be addressed because of the increasing variety and heterogeneity of ICU ventilators [28].
Heterogeneity of accuracy performances among the different generations of iNO-devices
Continuous administration iNO-devices
iNO actually delivered and resulted iNO concentration reached with continuous devices were highly variable. This heterogeneity might be explained by the type of table and by the precision of the flowmeter used to adjust the NO/N2 flow.
Two types of tables are currently used to determine the NO/N2 flow necessary to reach the targeted iNO concentration (see Additional data 3). The “dilution method” takes into account the minute ventilation to estimate the dilution of NO/N2 flow. Differently, the “Ti method” integrates the Ti, considering that the gas delivered during the expiratory phase will not reach the lungs. The calculated NO flow is systematically higher with the “Ti method” than with the “dilution method” because iNO has to be administered over a shorter period of time; in return, a higher dose of iNO is also delivered during the expiratory phase in the inspiratory limb of the patient circuit. This results in significantly higher iNO concentrations and higher NO2 formation.
The insufficient precision of the flowmeter may also explain the heterogeneity of the results observed with continuous administration as shown by the improved iNO delivery performances when using an additional more sensitive flow sensor.
I-Sequential administration iNO-devices
I-Sequential devices resulted in a systematic under-administration of iNO. This observation is mainly explained by the integration in the iNO-device of the Ti (ventilator setting including the insufflation and the end-inspiratory occlusion times) instead of the insufflation time only, to estimate the NO-flow to deliver. I-Sequential devices deliver NO-flow whose value is calculated assuming a Ti (including the end-inspiratory occlusion time) equal to the insufflation time while the NO flow is stopped during the occlusion, leading to an under-administration of iNO, as illustrated in Fig. 3 (see Ti-corrected configuration) and Additional data 8. To manage this limitation, it is possible to set on the device the insufflation time instead of the Ti. This apparently unimportant detail complicates the management of severe patients in routine practice since an end-inspiratory occlusion permitting to continuously monitor plateau pressure is often used.
Additionally, with Continuous and I-Sequential systems, any direct or indirect change in the minute ventilation (due to changes in settings by the clinician or to patient spontaneous triggering) may impact the accuracy of iNO delivery.
Proportional administration iNO-devices
Based on our results, proportional devices appear to be the most precise systems. This observation is perfectly expected in the light of their specific working principle that continuously adapt the NO/N2 flow delivered in the patient circuit as a proportion of the flow actually delivered by the ventilator. This generation automatically adapts iNO delivery to any change in ventilator patterns, (either controlled or assisted ventilation) thus maintaining constant the iNO concentration in the ventilator circuit during both inspiratory and expiratory phases.
Noticeably, this last generation of iNO-devices required a complex calibration process before starting the treatment that may complicate their use in emergency situations.
Impact of ventilator flow-by on iNO delivery
In most recent ICU ventilators, a flow-by is present, often variable, but usually blind and misunderstood by the clinicians (see Additional data 1). Our results demonstrate that the additional flow related to the flow-by directly affects the NO delivered close to the humidifier resulting in a systematic and significant washout of NO present in the inspiratory limb during the expiratory phase. This washout is directly proportional to the level of flow-by. As a result, NO concentration reached in the test-lung significantly decreased when the flow-by increased except with the last generation devices that measure the flow-by and automatically compensate it to maintain optimal iNO concentrations.
Interestingly, positioning iNO administration close to the Y-piece, on the inspiratory limb, prevents flow-by washout effect but is in turn associated with a systematic iNO under-delivery (see Additional data 9). This site of administration available only with continuous and I-Sequential iNO-devices permits to theoretically reduce the risk of NO2 formation This configuration requires higher NO flows to obtain the target concentration depending on the inspiratory flow set on the ventilator.
Bolus effect
A phenomenon called “bolus effect” has been well described with continuous iNO-devices [23, 24]. It is defined as an over-administration of NO due to NO accumulation in the circuit during the expiratory phase and delivered to the patient at the beginning of the next inspiration. This accumulation may be more pronounced for continuous iNO-devices, as they continue to deliver NO flow in the circuit during the expiratory phase, which is not the case for I-Sequential iNO-devices. Interestingly, ventilators tested in these studies did not have flow-by. The substantial wash-out induced by the flow-by may explain, at least in part, that this bolus effect was not observed in our study. It should also be noted that the bolus effect may differ depending on the site of iNO administration. Similarly, the addition of a mixing chamber on the inspiratory limb of the circuit (such as a heated humidifier) may also have reduced this phenomenon [29].
Limitations of the bench model
Our model has some limitations. First, it is an experimental inert model, without the ability to simulate NO uptake. This model has the benefit to be reproducible, whatever the iNO-devices tested and without temporal bias. Other models have been proposed to simulate NO uptake but present other limitations [24, 28]. As the tests were carried out with a heated humidifier in favor of iNO mixing, these results may not be generalized to a setting with a heat and moisture exchanger filter. iNO dosages in the present study were limited from 5 to 20 ppm since it represents the most commonly used dosages to treat ARDS patients. Different results could be observed when setting higher dosages and when using different ventilators in neonatology or during anesthesia. We chose to monitor NO and NO2 concentrations inside the test-lung to attempt to approach the most accurate concentrations we could observe inside a lung, but the experimental design of the study may have affected these measurements. Additionally, the technology used to monitor iNO in the present study was electrochemical analysis while chemiluminescence characterized by a higher sampling rate has also been widely used for this purpose. Importantly, additional tests confirmed the consistency of the NO concentration measured with chemiluminescence and electrochemical techniques. Finally, pressure regulated modes have not been specifically tested.
Conclusion
Based on the present bench experiment, we describe three different generations of iNO-devices that exhibit heterogeneous results regarding iNO concentrations accuracy. The presence of flow-by is probably one of the most common and unrecognized causes of misadministration. Last generation of proportional devices are the only ones able to accurately deliver iNO whatever the conditions and ventilator settings tested.
Ethics approval and consent to participate
Not applicable.
Consent for publications
Not applicable.
Competing interests
ML is PhD student attached to the Mitovasc Lab and the Vent'Lab (Angers University Hospital) and co-financed by Air Liquide Medical Systems. AV, CB, YZ, JMF and MLT declare no competing interest. AL is employed by Air Liquide Medical Systems. JCR received a part-time salary from Air Liquide Medical Systems as scientific director of the MedLab. NP is employed by iNOsystems. MS is employed by Air Liquide Santé International. AM received consulting fees from Air Liquide Medical Systems. FB reports personal fees from Löwenstein and Air Liquide Medical Systems and research support from GE healthcare, outside this work.
Supplementary Information
Acknowledgements
The authors wish to acknowledge Pr. Björn Jonson and his indirect contribution to this work as an inventor of the Servo 900C ventilator.
Abbreviations
- NO
Nitric oxide
- ICU
Intensive care units
- iNO
Inhaled nitric oxide
- O2
Dioxygen
- NO/N2
Nitric oxide in nitrogen gas mixture
- NO2
Nitrogen dioxide
- I-Sequential
Sequential to inspiratory phase
- Proportional
Proportional to inspiratory and expiratory ventilator flow
- IQR
Interquartile range
- ARDS
Acute respiratory distress syndrome
- FiO2
Inspired fraction of oxygen
- ppm
Parts per million
- FDA
Food and Drug Administration
- Ti
Inspiratory time
Author contributions
AV and ML have equally contributed to the design of the work, the acquisition, analysis and interpretation of data. They have written the draft. NP has contributed to the design of the work, the acquisition, analysis and interpretation of the data. MS has contributed to the acquisition and the analysis of the data. CB, YZ, JMF have contributed to the acquisition of data. AM has contributed to the design of the work and interpretation of data. AL has contributed to the analysis of the data and to write the draft. FB and JCR have contributed to the design of the work, the interpretation of data and contributed to write the draft. All the authors have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
AV received a 1‐year research fellowship grant from the University Hospital of Angers, France.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alice Vuillermoz and Mathilde Lefranc contribute equally to this work.
Jean-Christophe Richard and François Beloncle contribute equally to this work.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. 10.1038/288373a0 [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9. 10.1073/pnas.84.24.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puybasset L, Rouby JJ, Mourgeon E, Stewart TE, Cluzel P, Arthaud M, et al. Inhaled nitric oxide in acute respiratory failure: dose-response curves. Intensive Care Med. 1994;20:319–27. 10.1007/BF01720903 [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 5.Germann P, Braschi A, Della Rocca G, Dinh-Xuan AT, Falke K, Frostell C, et al. Inhaled nitric oxide therapy in adults: European expert recommendations. Intensive Care Med. 2005;31:1029–41. 10.1007/s00134-005-2675-4 [DOI] [PubMed] [Google Scholar]
- 6.Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6: e000420. 10.1136/bmjresp-2019-000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papazian L, Aubron C, Brochard L, Chiche J-D, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. 10.1186/s13613-019-0540-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49:727–59. 10.1007/s00134-023-07050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–63. 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 10.Mekontso Dessap A, Papazian L, Schaller M, Nseir S, Megarbane B, Haudebourg L, et al. Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes. Ann Intensive Care. 2023;13:57. 10.1186/s13613-023-01150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–9. 10.1001/jama.291.13.1603 [DOI] [PubMed] [Google Scholar]
- 12.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999;25:911–9. 10.1007/s001340050982 [DOI] [PubMed] [Google Scholar]
- 13.Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, et al. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med. 1998;157:1372–80. 10.1164/ajrccm.157.5.96-10089 [DOI] [PubMed] [Google Scholar]
- 14.Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998;26:15–23. 10.1097/00003246-199801000-00011 [DOI] [PubMed] [Google Scholar]
- 15.Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483–8. 10.1164/ajrccm.157.5.9707090 [DOI] [PubMed] [Google Scholar]
- 16.Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;2016:CD002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagate F, Tuffet S, Masi P, Perier F, Razazi K, de Prost N, et al. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann Intensive Care. 2020;10:151. 10.1186/s13613-020-00769-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spina S, Marrazzo F, Morais CCA, Victor M, Forlini C, Guarnieri M, et al. Modulation of pulmonary blood flow in patients with acute respiratory failure. Nitric Oxide. 2023;136–137:1–7. 10.1016/j.niox.2023.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Lu Q, Mourgeon E, Law-Koune JD, Roche S, Vézinet C, Abdennour L, et al. Dose-response curves of inhaled nitric oxide with and without intravenous almitrine in nitric oxide-responding patients with acute respiratory distress syndrome. Anesthesiology. 1995;83:929–43. 10.1097/00000542-199511000-00005 [DOI] [PubMed] [Google Scholar]
- 20.Stenqvist O, Kjelltoft B, Lundin S. Evaluation of a new system for ventilatory administration of nitric oxide. Acta Anaesthesiol Scand. 1993;37:687–91. 10.1111/j.1399-6576.1993.tb03790.x [DOI] [PubMed] [Google Scholar]
- 21.Puybasset L, Rouby JJ. Pulmonary uptake and modes of administration of inhaled nitric oxide in mechanically-ventilated patients. Crit Care. 1998;2:9–17. 10.1186/cc118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JD. A universal nitric oxide delivery system. Br J Anaesth. 1994;73:700–2. 10.1093/bja/73.5.700 [DOI] [PubMed] [Google Scholar]
- 23.Mourgeon E, Gallart L, Rao GSU, Lu Q, Law-Koune JD, Puybasset L, et al. Distribution of inhaled nitric oxide during sequential and continuous administration into the inspiratory limb of the ventilator. Intensive Care Med. 1997;23:849–58. 10.1007/s001340050421 [DOI] [PubMed] [Google Scholar]
- 24.Imanaka H, Hess D, Kirmse M, Bigatello LM, Kacmarek RM, Steudel W, et al. Inaccuracies of nitric oxide delivery systems during adult mechanical ventilation. Anesthesiology. 1997;86:676–88. 10.1097/00000542-199703000-00021 [DOI] [PubMed] [Google Scholar]
- 25.Foubert L, Fleming B, Latimer R, Jonas M, Oduro A, Borland C, et al. Safety guidelines for use of nitric oxide. The Lancet. 1992;339:1615–6. 10.1016/0140-6736(92)91886-D [DOI] [PubMed] [Google Scholar]
- 26.Health C for D and R. Guidance Document for Premarket Notification Submissions for Nitric Oxide Delivery Apparatus, Nitric Oxide Analyzer and Nitrogen Dioxide Analyzer. FDA; 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-document-premarket-notification-submissions-nitric-oxide-delivery-apparatus-nitric-oxide. Accessed 22 Sep 2023.
- 27.Petit PC, Fine DH, Vásquez GB, Gamero L, Slaughter MS, Dasse KA. The pathophysiology of nitrogen dioxide during inhaled nitric oxide therapy. ASAIO J. 2017;63:7–13. 10.1097/MAT.0000000000000425 [DOI] [PubMed] [Google Scholar]
- 28.Pickerodt PA, Hofferberth MBT, Busch T, Russ M, Taher M, Boemke W, et al. In vitro validation and characterization of pulsed inhaled nitric oxide administration during early inspiration. J Clin Monit Comput. 2022;36:637–48. 10.1007/s10877-021-00689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sydow M, Bristow F, Zinserling J, Allen SJ. Variation of nitric oxide concentration during inspiration. Crit Care Med. 1997;25:365–71. 10.1097/00003246-199702000-00028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.