Abstract
Plasmodium vivax is the second most common species of malaria parasite and causes up to 80 million episodes of infection each year. New drug targets are urgently needed because of emerging resistance to current treatments. To study new potential targets, we have functionally characterized two natural variants of the hexose transporter of P. vivax (PvHT) after heterologous expression in Xenopus oocytes. We show that PvHT transports both glucose and fructose. Differences in the affinity for fructose between the two variants of PvHT establishes that sequence variation is associated with phenotypic plasticity. Mutation of a single glutamine residue, Gln167, predicted to lie in transmembrane helix 5, abolishes fructose transport by PvHT, although glucose uptake is preserved. In contrast, the exofacial site located between predicted helices 5 and 6 of PvHT is not an important determinant of substrate specificity, despite exhibiting sequence polymorphisms between hexose transporters of different Plasmodium spp. Indeed, replacement of twelve residues located within this region of PvHT by those found in the orthologous Plasmodium falciparum sequence (PfHT) is functionally silent with respect to affinity for hexoses. All PvHT variants are inhibited by compound 3361, a long-chain O-3 derivative of D-glucose effective against PfHT. Furthermore, compound 3361 kills short term cultures of P. vivax isolated from patients. These data provide unique insights into the function of hexose transporters of Plasmodium spp. as well as further evidence that they could be targeted by drugs.
Keywords: glucose, malaria, oocyte, transport, Xenopus
Abbreviations: 2,5-AHM, 2,5-anhydro-D-mannitol; DOG, deoxy-D-glucose; 3-OMG, 3-O-methyl-D-glucose; PfHT, Plasmodium falciparum hexose transporter; PvHT, Plasmodium vivax hexose transporter
INTRODUCTION
Malaria is a major global health problem, despite substantial efforts at control conducted over several decades [1]. Four Plasmodium spp. are responsible for most human infections. Plasmodium falciparum infects 300–500 million people, and kills more than 1 million individuals annually. Plasmodium vivax is the second most common pathogen, causing up to 80 million cases of malaria each year. Most malaria morbidity outside Africa is due to P. vivax and occurs in Southeast Asia and the Americas. The recent recognition of chloroquine [2] and antifolate [3] resistance, in the asexual stages of P. vivax in some geographical areas, is an ominous signal of the need to identify new drug targets for this parasite as urgently as those being sought for multidrug resistant P. falciparum. The repertoire of therapies for P. vivax is limited further by developing resistance to 8-aminoquinolines, the only class of drug used to treat the latent hepatic stages of disease, responsible for relapse after cure of the asexual stages of infection [4].
Intraerythrocytic stages of P. falciparum and P. vivax are wholly dependent upon host glucose for energy. We have hypothesized that PfHT, the P. falciparum hexose transporter, may be an attractive new drug target, as parasites do not contain energy stores and require a continuous supply of glucose for energy and growth [5]. We characterized PfHT functionally using Xenopus laevis oocytes for heterologous expression [6,7]. PfHT transports both fructose and glucose (unlike mammalian orthologues GLUT1 and GLUT5 which mainly transport glucose and fructose respectively) [8]. We compared directly the interaction of substrates with PfHT and mammalian GLUTs and identified important differences between host and parasite transporters. 3-O-methyl derivatives of glucose proved particularly useful discriminators between mammalian transporters and PfHT, and were further exploited by synthesis of a long-chain O-3 hexose derivative [9]. This undecyl O-3 glucose derivative potently and selectively inhibits PfHT expressed in oocytes, and kills parasites in culture as well as in a rodent model of infection.
In this study we extend our studies on PfHT to include PvHT, the hexose transporter of P. vivax. We compare results from site-mutagen studies on PvHT with those obtained for PfHT, where a single amino acid residue (Gln169) is responsible for selectivity of glucose versus fructose [8]. We also examine the inhibitory activity of compound 3361 (an O-3 glucose derivative with a long aliphatic chain) against natural and mutagenized variants of PvHT expressed in oocytes. Finally, we present results of the inhibitory activity of 3361 in short term cultures of P. vivax isolated from patients. These data provide the first detailed experimental evidence that it is worth exploiting the hexose transporter of P. vivax as a new drug target.
MATERIALS AND METHODS
Cloning and site-directed mutations of PvHT mutants
Sequence encoding PvHT was isolated from two P. vivax variants. Firstly, genomic DNA from the strain Sal1 (isolated from a naturally acquired infection of a patient from the La Paz region of El Salvador) was used as PCR template to generate the full-length pvhtsal sequence for expression studies (there is no intron in pvht). A second pvht sequence was isolated from an infected patient returning from India (pvhtind; EMBL accession number AJ549815, [9,10]). Primers containing BglII restriction sites and, for the 5′ end, a strong eukaryotic Kozak consensus before the underlined start codon (CACCATG) were designed for each hexose transporter.
PvHTind mutants were obtained by site-directed mutagenesis using complementary primers according to the ‘splicing by overlap extension’ method as described previously [11]. Complementary internal forward (5′-GGGGTCCTCCACAATCTGTTCATCACCTTTGGG-3′) and reverse (5′-CCCAAAGGTGATGAACAGATTGTGGAGGACCCC-3′) primers were designed to introduce a single point-mutated construct of amino acid Gln167 resulting in a Q167N substitution (using single letter amino acid coding). Other complementary internal forward (5′-GCTGATTCGACTGAGCCATTAACTTCGTTTCAGAAGATATGGTGGAGACTC-3′) and reverse (5′-TGGCTCAGTCGAATCAGCCTTAGGACCCTCCCCCATGGCCATGCCCAA-3′) primers were designed to replace twelve amino acids from the interhelix region between predicted helices 5 and 6 (187APDAQKAASLEE198) by amino acids present in the equivalent PfHT loop (189GPKADSTEPLTS200) (see Figure 1). Full-length products of PvHT Q167N and PvHT mutants with the interhelix 5/6 loop replaced, were generated as before.
Figure 1. Sequence alignment of predicted helix 5 region from different P. vivax hexose transporters.
Alignment in the predicted helix 5 region of amino acid sequences from different natural variants of PvHT was generated by the ClustalW program. Non-conserved residues are highlighted in black boxes. Assignment of helix 5 is based on predictions determined with the Tmpred program. PvHTbelem, PvHTsal and PvHTind were identified from the Belem strain, Salvador 1 strain and from an Indian isolate respectively. Site-directed mutants generated in this study are also shown. PvHT Q167N corresponds to the replacement of the glutamine by an asparagine in position 167. PvHTchim corresponds to a PvHT chimera in which amino acids located at the exofacial site between predicted helices 5 and 6 have been replaced by those of PfHT (see Materials and methods).
All PCR was for 35 cycles as follows: 30 s at 94 °C, 45 s at 53 °C and 3 min at 68 °C, using platinum Pfx DNA polymerase (Invitrogen Ltd., Paisley, U.K.). Products were ligated into pSP64 which contains 5′ and 3′ untranslated Xenopus β-globin sequences, and were verified by sequence analysis.
Heterologous expression in Xenopus oocytes
Xenopus oocytes were assayed as described previously in detail [6]. cRNAs for each transporter were transcribed (MEGAscript™ SP6, Ambion, Austin, TX, U.S.A.) from XbaI linearized plasmids and micro-injected into oocytes (approx. 10 ng per oocyte). RNase-free water-injected oocytes acted as controls. Uptake assays were performed at 22 °C for 30 min, 48–72 h after injection, on groups of 8 oocytes in Barth's medium containing permeant D-[U-14C]glucose (310 mCi·mmol−1) from Amersham International (Little Chalfont, Bucks., U.K.). All uptakes were linear for the times used in these assays and each result was confirmed by at least 3 independent experiments. Estimations of kinetic parameters by non linear regression analysis used a Michaelis–Menten model (PRISM V3, Graphpad, San Diego, U.S.A.).
Competition by hexose analogues was studied 48–72 h after injection in Barth's medium containing radiolabelled D-glucose (2.69 μM, 323 mCi·mmol−1 D-[U-14C]glucose and 35 μM unlabelled D-glucose, with varying amounts of competitor) (all from Sigma except compound 3361). All solutions were allowed to equilibrate for at least 4 h prior to competition experiments. A one-site competition model was fitted to results (PRISM).
Antimalarial drug sensitivity assay
Studies were conducted in the Hospital for Tropical Diseases, Bangkok and were approved by the Ethical and Scientific Review Committee of the Research Committee, Ministry of Public Health, Royal Government of Thailand. Blood (5 ml) was collected in heparinized tubes from patients, and thick and thin blood films were prepared using standard procedures. Previous undisclosed self-medication with quinine or mefloquine was detected using a semi-quantitative dipstick technique, and these samples were excluded. Blood samples from patients with no history of anti-malarial drug treatment, single species P. vivax malaria (confirmed by PCR), tightly synchronized ring-stage infected red cells over 0.5% parasitaemia, and with no antimalarial drug detected in plasma were chosen for this study.
P. vivax-infected blood was centrifuged (600 g, 5 °C, 5 min). After plasma and buffy coat were discarded, packed red cells were washed (3 times) and resuspended (3% cell suspension) in media containing 4 mM (low extracellular) glucose and 6.6 mM (high extracellular) glucose. Cell suspension (50 μl) was added to triplicate wells of a microtitre plate. Compound 3361 was prepared with a 2-fold dilution concentration range from 1 μM to 100 μM. 3-O-ethyl-D-glucose (over a concentration range of 1 to 10 mM, [9]) was used as a negative control in parallel studies, as this compound does not affect PfHT or PvHT activity. Parasites were incubated (37 °C, 5% CO2) for 40–44 h, depending on the initial stages of parasite development, and thick and thin blood films were made from each well. Wells without drugs were included as controls. For parasite inhibition studies, the proportion of parasites maturing to schizonts containing >8 nuclei (counted in 3000 red cells) at each drug concentration was determined. Drug concentrations inhibiting 50% and 90% of parasites maturing to schizonts were calculated using a sigmoid Emax model and gave IC50 and IC90 values respectively. Statistical comparisons between groups were carried out with Student's t test or ANOVA, as appropriate.
RESULTS
Sequence analysis
Pvht has been cloned from two P. vivax natural variants. 97.6% of the deduced amino acids are conserved between PvHTsal (from the SalI strain; accession number AJ629308) and from a parasite acquired in India (PvHTind; accession number AJ549815 [10]) (Figure 1). By contrast, only 93.3% amino acid residues are conserved between PvHTind and PvHTbelem (from the Belem strain; accession number AJ488939). Most of the mismatches are concentrated in three regions: amino acids 191–197, 381–387 and 455–468 of PvHTind sequence respectively, located at the exofacial sites predicted between helices 5 and 6, 9 and 10 and inside predicted helix 12. Interestingly, these regions were also found to be highly divergent between hexose transporters from different Plasmodium spp. such as P. falciparum (PfHT), P. knowlesi and P. yoelii (see Figure 1 in [12]). PvHT is relatively polymorphic compared with PfHT because no variation in PfHT was seen in derived amino acid sequences of eight laboratory isolates and more than twenty parasites isolated from patients infected with P. falciparum [13].
Functional characterization of P. vivax hexose transporter
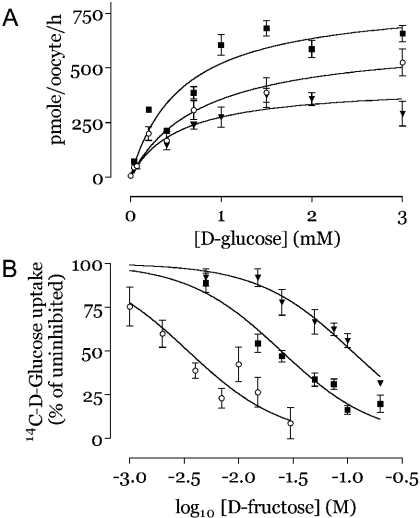
We investigated hexose transport by PvHT in the Xenopus laevis heterologous expression system. Both cRNAs encoding PvHTind and PvHTsal induced a large increase in D-glucose uptake when injected into oocytes, typically 5- to 10-fold compared with water-injected controls. The Km for D-glucose was not significantly different between PvHTsal (0.52±0.13 mM) and PvHTind (0.77±0.18 mM; see Table 1). Results from a representative experiment assessing substrate saturation are shown in Figure 2. This affinity for D-glucose does not differ significantly from its closest orthologue PkHT (the hexose transporter of P. knowlesi, 84.9% identity in amino acids sequence, Km=0.67 mM, [12]) or from PfHT (Km=1 mM, [8]). As with all other Plasmodium hexose transporters that have been functionally characterized [12], the two PvHT variants mediate uptake of D-fructose as well as D-glucose. However, the Ki for D-fructose is significantly different between PvHTsal (5.91±1.29 mM, see Figure 2 and Table 1) and PvHTind (19.03±4.4 mM). To confirm this difference in the handling of D-fructose, we used 2,5-anhydro-D-mannitol (2,5-AHM), a fixed fructofuranose deoxy analogue, as a competitor for D-glucose uptake. As expected, Ki values for 2,5-AHM competing for D-glucose uptake are also different for PvHTsal (1.48±0.45 mM) and PvHTind (6.33±0.94 mM).
Table 1. Substrate Km and active analogue Ki studies on native variants and mutants of PvHT.
Values (means±S.E.M.) are expressed in mM and are based on results of at least three independent replicates. Ki values of active analogues are based on inhibition of D-glucose uptake.
| Substrate or competitor | PfHT | PvHTind | PvHTind Q167N | PvHTind chimaera | PvHTsal |
|---|---|---|---|---|---|
| D-glucose | 1.0±0.2 (Km)* | 0.77±0.18 (Km)* | 0.60±0.12 (Km) | 0.65±0.24 (Km) | 0.52±0.13 (Km) |
| D-fructose | 11.5±1.6 (Km)* | 19.03±4.4 (Ki) | 93.6±14.85 (Ki) | 17.05±2.76 (Ki) | 5.91±1.29 (Ki) |
| 2,5-AHM | 1.4±0.3 (Ki)* | 6.33±0.94 (Ki) | 10.19±0.89 (Ki) | 5.31±0.89 (Ki) | 1.48±0.45 (Ki) |
| Compound 3361 | 0.053±0.02 (Ki)* | 0.12±0.01 (Ki)* | 0.52±0.09 (Ki) | 0.18±0.05 (Ki) | 0.13±0.01 (Ki) |
| 2-DOG | 1.3±0.3 (Km)* | 2.1±0.4 (Ki) | 1.81±0.2 (Ki) | 1.45±0.12 (Ki) | 1.24±0.24 (Ki) |
| 6-DOG | 2.2±0.9 (Ki)* | 11.74±3.04 (Ki) | 8.03±1.3 (Ki) | 10.52±2.43 (Ki) | 3.61±0.83 (Ki) |
Figure 2. Transport properties of PvHTsal, PvHTind and Q167N mutant PvHTind in Xenopus laevis oocytes.
(A) Initial mean uptake rates (eight oocytes per concentration; mean±S.E.M.) of D-glucose are shown against concentration of substrate (representative of three experiments; see Table 1). (B) D-fructose used to compete for D-glucose transport. Uptake assays (mean±S.E.M. of 8 oocytes per condition) were carried out with D-[14C]glucose. Percentage of D-glucose uptake at various fructose concentration was compared with uptake in uncompeted oocytes (control). PvHTind (▪); PvHTind Q167N (▾); PvHTsal (○).
Mutational analysis of PvHT
The effects of changing Gln167 (a residue in predicted helix 5 which is highly phylogenetically conserved in hexose transporters of different Plasmodium spp.) of PvHTind to an asparagine residue (Q167N) were examined in Xenopus oocytes. Similarly, the effects of replacing twelve amino acids predicted to be at the exofacial region between helices 5 and 6 in PvHT (highly divergent in hexose transporters of different Plasmodium spp.), by amino acids present in interhelix region of PfHT were also studied. The affinity of glucose for both mutants is not significantly different from that of native PvHT (Table 1; P>0.5). In contrast, the Q167N mutation strongly impaired fructose transport and therefore increased the Ki for fructose inhibition of glucose transport from 19.03±4.4 to 93.6±14.9 mM (>4-fold increase; Table 1 and Figure 2; P=0.009). As is the case for D-glucose, the affinity for D-fructose of the PvHT exofacial site substituent is not significantly different to that for native PvHT, showing that this exofacial region is not of any functional relevance in the handling of substrate.
Substrate analogue studies on PvHT and its mutants
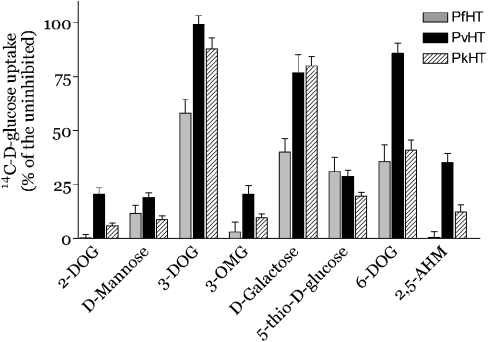
We studied the structural requirements for ligand interactions between PvHTind and hexoses by using a variety of competitors (Figure 3). Comparison in oocytes of transport properties between PvHT and the previously described PfHT and PkHT can be used to map critical positions in hexoses that interact in the same way or differently with those transporters. Deoxy-D-glucose (DOG) analogues provide insight into the interactions of different possible hydrogen bonding sites on glucose with PvHT. These potential hydrogen-bonding sites are interrupted by removal of the hydroxyl group (on carbons 1–4 and 6) and by replacing the oxygen on C-5 with a sulphur atom. 2-Deoxy-D-glucose (2-DOG), mannose (the 2-epimer of glucose), and 5-thio-D-glucose (all at 10 mM) all inhibit D-glucose uptake in PvHT relatively effectively, suggesting that hydroxyl groups in C-2 and C-5 positions are of minor importance in high affinity interactions with the transporter.
Figure 3. Active analogue studies on PvHTInd in Xenopus laevis oocytes.
Uptake assays (mean±S.E.M. of 8 oocytes per condition) were carried out with D-[14C]glucose. Percentage of D-glucose uptake for each condition was compared with uptake in uncompeted oocytes (control). All compounds were used at 10 mM. Grey bars, PfHT; black bars, PvHTind; hatched bars, PkHT.
In contrast, the hydroxyl groups at the C-3, C-4 and C-6 positions in glucose are important in uptake by PvHT as an excess of 3-DOG, D-galactose (the 4-epimer of glucose) or 6-DOG (all at 10 mM) compete poorly when glucose is used as permeant. The effects of aldose analogues in C-3, C-4 and C-6 positions on PvHT are very similar to those obtained with its phylogenetically most closely related orthologue, PkHT. As for PfHT and PkHT, the oxygen atom at the C-3 hydroxyl moiety is likely to be a hydrogen bond recipient, because 3-OMG (3-O-methyl-D-glucose) restored the capacity to inhibit transport of glucose.
This analysis allows comparison between PfHT and PvHTind and shows that the C-6 position in glucose may be more important in the handling of substrate by PvHTind than by PfHT. The Ki of 6-DOG for D-glucose uptake by PvHTind (Ki=11.74±3.04 mM, Table 1) was closer to that for PkHT (9.48±2.7 mM) compared with the Ki for PfHT (2.2±0.9 mM). In contrast to PfHT, 2-DOG did not completely inhibit glucose uptake by PvHTind, suggesting that the C-2 position may also be partially involved in substrate recognition. However, the Ki of 2-DOG for D-glucose uptake by PvHTind was not significantly higher (Ki=2.1±0.4 mM, Table 1) than for PfHT (Ki=1.3±0.3 mM; P>0.5), consistent with the small magnitude of this difference. Results for competition with 2.5-AHM (10 mM) also did not completely abolish D-glucose uptake by PvHTind, and this fixed fructofuranose derivative competes for transport by PvHT with a lower affinity (Ki=6.33±0.94 mM) than it does with PfHT (Ki= 1.4±0.3 mM). Interestingly, properties of PvHTind in substrate handling are not fully shared by PvHTsal, as, for this latter variant, the Ki values for 6-DOG and 2-DOG in competition for D-glucose uptake are closer to those for PfHT than for PkHT. These results highlight the high degree of functional diversity of hexose transporters of different P. vivax strains.
Specific inhibition of PvHT
We have shown previously that a specific inhibitor of PfHT, compound 3361, also inhibited D-glucose uptake mediated by P. knowlesi, P. yoelii and P. vivax hexose transporters [9]. In this report, we confirm that compound 3361 is active against both PvHTind and PvHTsal in the same concentration range (Table 1). Interestingly, the Q167N mutant is significantly less susceptible to inhibition by compound 3361 (by about 5-fold) a finding also observed with PfHTQ169N [9].
Inhibition of P. vivax development
Identification of a specific inhibitor of PvHT will only validate this transporter as a drug target if it also kills parasites. Culture of P. vivax is usually only for one cycle because of marked preference of this species for reticulocytes, which are difficult to obtain regularly [14]. We therefore cultured P. vivax-infected erythrocytes directly from patients who had not received antimalarials, in order to confirm that inhibition of PvHT by compound 3361 in Xenopus oocytes translates to parasitocidal activity in short-term culture. Two independent patient isolates were cultured and parasite maturation in the absence of inhibitors was observed in all control wells (mean±S.D.=99%±0.5). Drug concentrations inhibiting schizont maturation by 50% and 90% (IC50 and IC90 values respectively) are summarized in Table 2. As predicted from results in oocytes, the negative control 3-O-ethyl-D-glucose does not impair parasite development in concentrations between 1–10 mM (results not shown). By contrast, trophozoites develop pyknotic nuclei and parasites do not progress to the schizont stage in the presence of compound 3361.
Table 2. IC50 and IC90 (means±S.E.M.) values for inhibition of P. vivax maturation by compound 3361.
Pv1, Pv2, isolates from patients 1 and 2 respectively.
| Compound 3361 | ||||
|---|---|---|---|---|
| Low glucose (4 mM) | High glucose (6 mM) | |||
| Isolate… | Pv1 | Pv2 | Pv1 | Pv2 |
| IC50 (μM) | 9±0.04 | 6±0.1 | 11±0.03 | 9±0.04 |
| IC90 (μM) | 24±1.36 | 7±0.1 | 58±2 | 24±1.8 |
DISCUSSION
The SalI strain, isolated from a naturally acquired infection of a patient from the La Paz region of El Salvador [15] is often regarded as the reference strain for P. vivax and has been chosen for genome sequence analysis [16]. However, in contrast to P. falciparum, there is considerable genotypic and phenotypic variation within species classified as P. vivax [17]. The diversity of P. vivax is apparent in comparisons of morphology, genetic diversity, biochemistry and drug resistance. In this report, we show that sequence divergence between several protein orthologues of the hexose transporter of different isolates of P. vivax translates to functional diversity.
The hexose transporters of Plasmodium spp. share many structural and functional characteristics. Rodent and primate parasite transporters have >70% identity in primary amino acid sequences, they all transport both glucose and fructose with consistently higher affinities for glucose, and all are inhibited by compound 3361, a long-chain O-3 glucose derivative [9,12]. However, detailed analysis of PvHT, and comparisons between natural variants exposes unexpected differences. There is considerable polymorphism in primary sequence from different P. vivax isolates, which can affect up to 6% of amino acids (e.g. between PvHTind and PvHTbelem). This contrasts with the invariance of primary amino acid sequences of PfHT obtained from a variety of laboratory strains and patients isolates [13]. Furthermore, the sequence variation in PvHT also alters the affinity of the transporter for D-fructose, but not to a significant degree for D-glucose. PvHTsal has an approximately 3-fold higher affinity for fructose compared with PvHTind. The importance of a single residue in predicted helix 5 in determining fructose specificity has been confirmed by mutation of position Gln167 to an asparagine. An analogous experiment in PfHT (Q169N) abolished fructose but not glucose uptake and decreased susceptibility to inhibition by compound 3361, as is the case for PvHTQ167N [8,9]. Although the segment between predicted transmembrane helices 5 and 6 is one of the most polymorphic in comparisons made between hexose transporters of Plasmodium spp., introducing the sequence in this region from PfHT to PvHT did not change affinities for hexoses. This suggests that polymorphisms located in this interhelical segment are functionally silent with respect to affinity for hexoses. There is, nevertheless, a significant decrease in inhibition by compound 3361 in the resulting exofacial site mutant, implicating this region as a possible site of interaction with inhibitor. Overall, the affinity for fructose seems to be correlated better with susceptibility to compound 3361 than Ki values for C4 and C6 derivatives of D-glucose. 6-DOG inhibits D-glucose uptake by PvHT relatively poorly compared with PfHT, implying that C6 hydrogen bonding is more important for handling D-glucose in PvHT compared with PfHT.
P. vivax genetic and phenotypic diversity is of practical significance since the structure of parasite populations may influence the usefulness of a particular drug or affect the rate at which newly selected drug-resistant mutants spread. In this context, the fact that compound 3361 is similarly active against PvHT from two natural variants is helpful in the validation of this transporter as a novel drug target. Moreover, we have shown that this compound is also active in killing cultured parasites which have been freshly isolated from patients in Thailand (strains other than SalI and Belem). Inhibition of schizont maturation occurred at similar concentrations (IC50 range 9–58 μM) to those required to inhibit maturation of P. falciparum (IC50 range 12–75 μM) [5]. The range of IC50 values reflects the fact that increasing D-glucose concentrations in culture increases IC50 values. This is the case for P. falciparum, confirming that inhibition of PvHT by compound 3361 is also partially competitive in nature.
As well as providing the most detailed comparisons of a critical transport protein studied in different Plasmodium spp., these findings establish that if inhibitors of hexose transport are developed as novel antimalarials, they will be effective against both P. falciparum and P. vivax.
Acknowledgments
This work was funded by grant number G9800300 from the Medical Research Council (U.K.) to SK. We thank Dr John W. Barnwell (CDC, Parasitic Diseases, Atalanta, GA, U.S.A.) for the gift of genomic DNA from P. vivax SalI strain.
References
- 1.Greenwood B., Mutabingwa T. Malaria in 2002. Nature (London) 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 2.Ruebush T. K., 2nd, Zegarra J., Cairo J., Andersen E. M., Green M., Pillai D. R., Marquino W., Huilca M., Arevalo E., Garcia C., et al. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 2003;69:548–552. [PubMed] [Google Scholar]
- 3.Imwong M., Pukrittayakamee S., Renia L., Letourneur F., Charlieu J. P., Leartsakulpanich U., Looareesuwan S., White N. J., Snounou G. Novel point mutations in the dihydrofolate reductase gene of Plasmodium vivax: evidence for sequential selection by drug pressure. Antimicrob. Agents Chemother. 2003;47:1514–1521. doi: 10.1128/AAC.47.5.1514-1521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilairatana P., Silachamroon U., Krudsood S., Singhasivanon P., Treeprasertsuk S., Bussaratid V., Phumratanaprapin W., Srivilirit S., Looareesuwan S. Efficacy of primaquine regimens for primaquine-resistant Plasmodium vivax malaria in Thailand. Am. J. Trop. Med. Hyg. 1999;61:973–977. doi: 10.4269/ajtmh.1999.61.973. [DOI] [PubMed] [Google Scholar]
- 5.Joet T., Morin C., Fischbarg J., Louw A. I., Eckstein-Ludwig U., Woodrow C., Krishna S. Why is the Plasmodium falciparum hexose transporter a promising new drug target? Expert Opin. Ther. Targets. 2003;7:593–602. doi: 10.1517/14728222.7.5.593. [DOI] [PubMed] [Google Scholar]
- 6.Woodrow C. J., Penny J. I., Krishna S. Intraerythrocytic Plasmodium falciparum expresses a high-affinity facilitative hexose transporter. J. Biol. Chem. 1999;274:7272–7277. doi: 10.1074/jbc.274.11.7272. [DOI] [PubMed] [Google Scholar]
- 7.Krishna S., Woodrow C. J., Burchmore R. J., Saliba K. J., Kirk K. Hexose transport in asexual stages of Plasmodium falciparum and kinetoplastidae. Parasitology Today. 2000;16:516–521. doi: 10.1016/s0169-4758(00)01762-2. [DOI] [PubMed] [Google Scholar]
- 8.Woodrow C. J., Burchmore R. J. S., Krishna S. Hexose permeation pathways in Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9931–9936. doi: 10.1073/pnas.170153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joet T., Eckstein-Ludwig U., Morin C., Krishna S. Validation of the hexose transporter of Plasmodium falciparum as a novel drug target. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7476–7479. doi: 10.1073/pnas.1330865100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn S., Krishna S., Jarvis J., Joet T., Macallan D. Pernicious complications of benign tertian malaria. Trans. R. Soc. Trop. Med. Hyg. 2004 doi: 10.1016/s0035-9203(03)80024-x. (in the press) [DOI] [PubMed] [Google Scholar]
- 11.Manning S. K., Woodrow C., Zuniga F. A., Iserovich P., Fischbarg J., Louw A. I., Krishna S. Mutational analysis of the hexose transporter of Plasmodium falciparum and development of a three-dimensional model. J. Biol. Chem. 2002;277:30942–30949. doi: 10.1074/jbc.M204337200. [DOI] [PubMed] [Google Scholar]
- 12.Joet T., Holterman L., Stedman T. T., Kocken C. H., Van Der Wel A., Thomas A. W., Krishna S. Comparative characterization of hexose transporters of Plasmodium knowlesi Plasmodium yoelii and Toxoplasma gondii highlights functional differences within the apicomplexan family. Biochem. J. 2002;368:923–929. doi: 10.1042/BJ20021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishna S., Eckstein-Ludwig U., Joët T., Uhlemann A.-C., Morin C., Webb R., Woodrow C. J., Kün J., Kremsner P. Transport processes in Plasmodium falciparum – infected erythrocytes: potential as new drug targets. Int. J. Parasitol. 2002;32:1567–1573. doi: 10.1016/s0020-7519(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 14.Russell B. M., Udomsangpetch R., Rieckmann K. H., Kotecka B. M., Coleman R. E., Sattabongkot J. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 2003;47:170–173. doi: 10.1128/AAC.47.1.170-173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins W. E., Contacos P. G., Krotoski W. A., Howard W. A. Transmission of four Central American strains of Plasmodium vivax from monkey to man. J. Parasitol. 1972;58:332–335. [PubMed] [Google Scholar]
- 16.Carlton J. The Plasmodium vivax genome sequencing project. Trends Parasitol. 2003;19:227–231. doi: 10.1016/s1471-4922(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 17.Cui L., Escalante A. A., Imwong M., Snounou G. The genetic diversity of Plasmodium vivax populations. Trends Parasitol. 2003;19:220–226. doi: 10.1016/s1471-4922(03)00085-0. [DOI] [PubMed] [Google Scholar]