Abstract
FB_MR5 is a nucleotide-binding domain and leucine-rich repeat protein identified from wild apple species Malus × robusta 5 conferring disease resistance to bacterial fire blight. FB_MR5 (hereafter MrMR5) recognizes the cysteine protease effector EaAvrRpt2 secreted from the causal agent of bacterial fire blight, Erwinia amylovora. We previously reported that MrMR5 is activated by the C-terminal cleavage product (ACP3) of Malus domestica RIN4 (MdRIN4) produced by EaAvrRpt2-directed proteolysis. We show that MbMR5 from a wild apple species Malus baccata shares 99.4% amino acid sequence identity with MrMR5. Surprisingly, transient expression of MbMR5 in Nicotiana benthamiana showed autoactivity in contrast to MrMR5. Domain swap and mutational analyses revealed that 1 amino acid polymorphism in the MbMR5 CC domain is critical in enhancing autoactivity. We further demonstrated that MrMR5 carrying 7 amino acid polymorphisms present in MbMR5 is not activated by MdRIN4 ACP3 but recognizes AvrRpt2 without MdRIN4 in N. benthamiana. Our findings indicate that naturally occurring polymorphisms of MR5 natural variants can confer its cell death-inducing activity and the effector recognition mechanism likely due to altered compatibility with RIN4.
Keywords: Disease resistance gene, Fire blight, Nucleotide-binding domain and leucine-rich repeat, Pathogen effector
INTRODUCTION
Plant pathogens secrete small molecules known as effectors into plant cells to promote virulence or manipulate host physiology, thereby creating more favorable conditions for microbial proliferation (Jones et al., 2024, Kim et al., 2023). In turn, plants have developed a complex innate immune system that recognizes phytopathogenic microbes through cell surface and intracellular receptors (Jones and Dangl, 2006). Nucleotide-binding domain and leucine-rich repeat (NLR) protein family members constitute one of the major classes of plant immune receptors (Ngou et al., 2022). NLRs often indirectly recognize effectors by monitoring the effector-mediated modifications of host targets, thus acting as “guardees” or “decoys” (van der Hoorn and Kamoun, 2008). Recognition of effectors by NLRs leads to effector-triggered immunity, which is often accompanied by a localized programmed cell death known as a hypersensitive response (HR) (Saur et al., 2021).
Erwinia amylovora is the causal agent of fire blight, which is among the most destructive plant diseases affecting Rosaceae species such as pear (Pyrus sp.) and apple (Malus sp.). FB_MR5 is a fire blight resistance gene encoding an NLR protein from Malus × robusta 5, a hybrid between Malus baccata and Malus prunifolia (Fahrentrapp et al., 2013). FB_MR5 (hereafter MrMR5) recognizes the type III secretion system effector EaAvrRpt2 secreted from E. amylovora (Vogt et al., 2013). In Arabidopsis (Arabidopsis thaliana), resistant to Pseudomonas syringae 2 (RPS2) recognizes an ortholog of EaAvrRpt2 secreted from P. syringae (PsAvrRpt2) (Kunkel et al., 1993). Pseudomonas tomato race 1 (Ptr1) is another NLR recognizing PsAvrRpt2 in the wild Solanaceae species Solanum lycopersicoides and Nicotiana benthamiana (Ahn et al., 2023, Mazo-Molina et al., 2019). Notably, MrMR5, RPS2, and Ptr1 share little amino acid sequence identity, indicating the convergent evolution of these NLRs in distantly related plant species to recognize AvrRpt2.
Plant NLRs share structurally similar domains, such as the nucleotide-binding adaptor, APAF-1, R proteins, and CED-4 (NB-ARC) domain, and leucine-rich repeat (LRR) domain. Coiled-coil (CC), Toll/interleukin-1 receptor, and resistance to powdery mildew 8-like are the most common domains identified at the N-terminus of plant NLRs (Duxbury et al., 2021, Steele et al., 2019, Wang and Chai, 2020). MrMR5, RPS2, and Ptr1 carry a CC domain that is involved in molecular functions, such as cell death or self-association (Bentham et al., 2018). Notably, the CC domain of HopZ-activated resistance 1 (ZAR1) was recently shown to form a funnel-shaped structure upon pentamerization of ZAR1, functioning as a plasma membrane–localized cation channel during ZAR1-mediated cell death (Wang et al., 2019). However, it is not clear how the CC domains of RIN4-interacting CC-type NLRs function in mediating immune responses.
RPS2 and MrMR5 have distinct molecular mechanisms underlying AvrRpt2 recognition (Prokchorchik et al., 2020). RPS2 is an autoactive NLR that induces HR when transiently expressed in N. benthamiana leaf cells; moreover, coexpression with Arabidopsis RPM1-interacting protein 4 (AtRIN4) suppresses RPS2 autoactivity (Day et al., 2005). AvrRpt2 specifically recognizes 2 RIN4 cleavage sites (RCS1 and RCS2) on nitrate-induced (NOI) domain and processes AtRIN4 into 3 cleavage products (activated by the C-terminal cleavage product (ACP)1, ACP2, and ACP3) (Chisholm et al., 2005). Cleavage of AtRIN4 interferes with the suppression of RPS2 autoactivity resulting in elevated defense responses (Day et al., 2005). In apple, 2 RIN4 homologs have been reported, Malus domestica RIN4-1 (hereafter MdRIN4) and MdRIN4-2, sharing 91% amino acid sequence identity (Vogt et al., 2013). In contrast to RPS2, MrMR5 alone does not induce HR when transiently expressed in N. benthamiana (Prokchorchik et al., 2020). However, MdRIN4 ACP3 generated upon EaAvrRpt2-directed cleavage is sufficient to activate MrMR5 (Prokchorchik et al., 2020). Notably, AtRIN4 and MdRIN4 specifically regulate RPS2 and MrMR5, respectively. AtRIN4, but not MdRIN4, suppresses RPS2 autoactivity, whereas ACP3 of MdRIN4, but not that of AtRIN4, activates MrMR5 (Prokchorchik et al., 2020). Two naturally occurring sequence polymorphisms in RIN4 determine the regulatory specificity between RIN4 and corresponding NLRs (Kim et al., 2022, Prokchorchik et al., 2020). However, it remains unclear whether natural polymorphisms in NLRs also alter their regulatory specificity with RIN4. In this study, we identified that a few natural sequence polymorphisms present in M. baccata ortholog of MrMR5 result in enhanced autoactivity and altered RIN4 regulatory specificity.
MATERIALS AND METHODS
Cloning MbMR5 and MrRIN4 Variants
To clone MrMR5 homologs from M. baccata, a set of primers targeting the 5′ and 3′ ends of full-length MrMR5 (primer sequences are indicated in Table S1) were used to amplify DNA fragments from genomic DNA extracted from wild M. baccata accession “KW-JB-4.” gDNA of M. baccata accession KW-JB-4 was provided from Rural Development Administration Apple Research Institute. Subsequently, the full-length sequence of the DNA fragment was confirmed by Sanger sequencing and cloned into binary vector pICH86988 using the Golden Gate cloning as described in previous reports (Engler et al., 2008, Prokchorchik et al., 2020). To clone RIN4 variants from M. robusta, a set of primers targeting 5′ and 3′ ends of full-length RIN4 (primer sequences are indicated in Table S1) were used to amplify DNA fragments from cDNA extracted from wild M. robusta. cDNA of M. robusta was provided by Rural Development Administration Apple Research Institute. Subsequently, the DNA fragment was cloned into a binary vector as described above. To generate chimeric MR5 variants, DNA fragments each containing CC-NB-ARC or LRR domain of MR5 were amplified from full-length MrMR5 and MbMR5, respectively, using 2 primer sets named “MR5 pt1” or “MR5 pt2” (Table S1) and subcloned into pICH41021 as Golden Gate compatible modules. Subsequently, chimeric MR5 variants were assembled into the binary vector pICH86988 using desired combinations of modules by Golden Gate cloning. To generate mutant variants of MR5, sets of primers each inducing single amino acid polymorphism (Table S1) were used to amplify the DNA fragments containing desired mutations. Subsequently, the DNA fragments were subcloned into pICH41021 as Golden Gate compatible modules and assembled into the binary vector pICH86988.
Agrobacterium-Mediated Transient Expression
The binary vectors assembled with genes of interest were transformed into Agrobacterium tumefaciens strain AGL1 by electroporation and grown at 28°C on Luria-Bertani (LB) agar plates supplemented with 100 μg/ml of carbenicillin and 50 μg/ml of kanamycin. Subsequently, cells were grown in LB liquid media overnight in a 28°C shaking incubator and resuspended in 10 mM MgCl2. OD600 was adjusted as indicated in each figure and infiltrated by a needless syringe into fully expanded leaves of 4- to 5-week-old N. benthamiana grown under long-day conditions (16 hours of light per day).
Measurement and Quantification of Cell Death
Cell death was measured at 4 days after infiltration on an 8-point scale from no cell death (0) to full cell death (7) as previously reported (Ahn et al., 2023). For each measurement, 2 to 3 biological replicates from different generations of plants each including 3 to 4 technical repeats tested on different leaves were performed. The number of repeats and raw value of cell death scores are listed in Table S2. The cell death score was visualized as violin plots with individual data points (red dots) and statistical significance using Prism version 10.2.0 (GraphPad).
Immunoblot
To extract total protein, leaf disks were harvested from Agrobacterium-infiltrated leaves and ground using a liquid nitrogen-chilled pestle. Subsequently, SDS protein loading buffer (250 mM Tris-HCl, 8% SDS, 40% glycerol, 100 mM DTT, 0.1% bromophenol blue) was added and samples were boiled at 95°C for 5 minutes. Finally, boiled samples were loaded for SDS-PAGE and probed with appropriate antibodies (Anti-FLAG, Sigma-Aldrich, 1:5,000 dilution; Anti-MYC, Cell Signaling Technology, 1:5,000 dilution; Anti-HA, 1:5000 dilution) and HRP-conjugated antimouse antibodies (Sigma-Aldrich; 1:20,000 dilution).
RESULTS
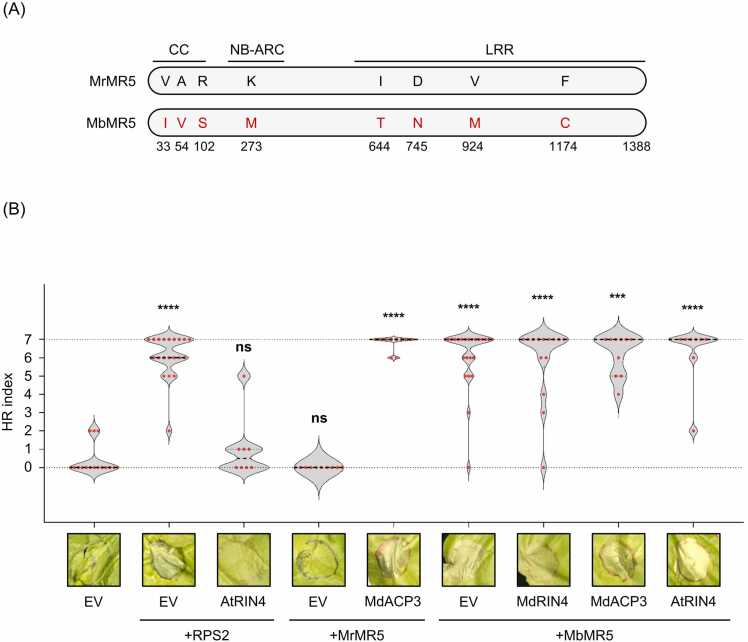
To test if the natural polymorphisms in NLR alter the regulatory specificity with RIN4, we cloned a natural variant of MrMR5 from M. baccata. The amino acid sequence of M. baccata MrMR5 homolog (hereafter MbMR5) was 99.4% identical to MrMR5 and only differs by 8 amino acids (Fig. 1A). Three substitutions (V33I, A54V, and R102S) are located in the CC domain, 1 (K273M) in the NB-ARC domain, and the remaining 4 (I644T, D745N, V924M, and F1174C) in the LRR domain (Fig. 1A). To test the function of MbMR5, we transiently coexpressed MbMR5 with an empty vector (EV) control or with MdRIN4, MdRIN4 ACP3, or AtRIN4 using Agrobacterium-mediated transient transformation in N. benthamiana (Fig. 1B). Consistent with previous observations (Prokchorchik et al., 2020), RPS2, but not MrMR5, showed robust autoactivity and coexpression of AtRIN4 suppressed RPS2-induced HR, while coexpression of MdRIN4 ACP3-activated MrMR5 (Fig. 1B). Unlike MrMR5, transient expression of MbMR5 in N. benthamiana leaves resulted in strong cell death comparable to that induced by RPS2 (Fig. 1B). In addition, coexpressing MbMR5 with AtRIN4, MdRIN4, or MdRIN4 ACP3 failed to suppress MbMR5-induced HR (Fig. 1b). Next, we tested if RIN4 homologs from M. robusta suppress MbMR5-induced HR. To this end, we cloned 2 RIN4 homologs from M. robusta (hereafter MrRIN4-1 and MrRIN4-2) which only had 1 and 2 polymorphic residues compared to MdRIN4-2, respectively (Fig. S1A). Coexpression of MrRIN4-1 or MrRIN4-2 with MrMR5 in the presence or absence of AvrRpt2 showed that MrRIN4 homologs regulate MrMR5 similar to MdRIN4 (Fig. S1B). Also, coexpression of MrRIN4 homologs failed to suppress MbMR5-induced HR (Fig. S1B). An immunoblot analysis demonstrated that AtRIN4, MdRIN4, MdRIN4 ACP3, MrRIN4-1, and MrRIN4-2 protein accumulated to similar level and that AvrRpt2 protein was stably accumulated (Fig. S2), indicating the lack of suppression of MbMR5-induced HR was not due to protein instability. These results indicate that MbMR5 has enhanced autoactivity compared to MrMR5, and MbMR5-induced HR is differently regulated than RPS2-induced HR.
Fig. 1.
Malus baccata MR5 shows enhanced autoactivity when transiently expressed in Nicotiana benthamiana. (A) Amino acid sequences of MrMR5 and MbMR5 were aligned using the ClustalW within Geneious 7.1.9. Black and red letters indicate the polymorphic amino acid residues between MrMR5 and MbMR5, respectively. Numbers indicate the position of residues and the end of the sequences. Predicted CC, NB-ARC, and LRR domains are indicated. (B) MbMR5 shows autoactivity that is not suppressed by MdRIN4. The indicated NLRs and RIN4 variants were coexpressed in N. benthamiana leaves by Agrobacterium-mediated transient transformation. The Agrobacterium inoculum densities (OD600) were as follows: empty vector (EV) = 0.4; RIN4 variants = 0.4; MdRIN4 ACP3 = 0.4; RPS2 = 0.1; MrMR5 = 0.4; and MbMR5 = 0.4. Representative cell death scores (top) and photographs (bottom) are shown. Agrobacterium carrying construct encoding virus-derived suppressor of gene silencing P19 (OD600 = 0.1) was added in each inoculum. Photographs were taken at 4 days after infiltration. Cell death was scored on an 8-point scale from no cell death (0) to full cell death (7) as previously reported (Ahn et al., 2023) and presented as violin plots with individual data points (red dots). Raw cell death score data are listed in Table S2. Statistical significance compared to the reference (leaves infiltrated with EV alone) was assessed by 1-way ANOVA followed by Dunn’s multiple comparisons test using GraphPad Prism version 10.2.0 (ns, not significant; ****, P < .0001).
We set out to identify the polymorphic residues responsible for the enhanced autoactivity of MbMR5. To this end, we constructed chimeras using intact MrMR5 and MbMR5. MrMR5Mb-LRR carries 4 polymorphic residues from MbMR5 in the LRR domain whereas MrMR5Mb-CN harbors 4 polymorphic residues from the CC and NB-ARC domains (hereafter CN domain) (Fig. 2A). MrMR5Mb-CN but not MrMR5Mb-LRR-induced HR comparable to MbMR5 when transiently expressed in N. benthamiana (Fig. 2A). Therefore, the introduction of the 4 polymorphic residues in the CN domain of MbMR5 in MrMR5 (V33I, A54V, R102S, and K273M) is sufficient to confer autoactivity. To identify the minimum requirement for MbMR5 autoactivity, we repeated the assay with MrMR5 mutant variants, substitution of a single amino acid in MrMR5 with its corresponding residue from MbMR5 in the CN domain (V33I, A54V, R102S, or K273M) (Fig. 2B) (primer sequences for site-directed mutagenesis are indicated in Table S1). Only the MrMR5 A54V showed enhanced autoactivity in N. benthamiana (Fig. 2B), indicating that the V54 residue is critical for the enhanced autoactivity of MbMR5. However, MrMR5 A54V-induced HR was weaker than MbMR5-induced HR, indicating additional residues might be required for full autoactivity of MbMR5 (Fig. 2B). We thus tested pairs of substitutions: A54V together with V33I, R102S, or K273M in MrMR5 (Fig. 2B). Only the MrMR5 A54V K273M double mutant showed autoactivity comparable to that of MbMR5 (Fig. 2B), indicating that the M273 residue also contributes to enhanced autoactivity of MbMR5. To further assess the role of V54 and M273 in MbMR5 autoactivity, we generated 2 MbMR5 mutant variants, MbMR5 V54A, MbMR5 M273K, and MbMR5 V54A M273K (Fig. 2B) (primer sequences for mutagenesis were indicated in Table S1). In line with the mutation analysis of MrMR5, MbMR5 V54A and MbMR5 V54A M273K showed significantly lower autoactivity when its encoding construct was transiently expressed in N. benthamiana (Fig. 2B). Importantly, wild-type and mutant variants of MrMR5 protein accumulated to similar levels, indicating that lack of autoactivity is not due to protein instability (Fig. S3). Taken together, these results demonstrate that a single polymorphic residue in the CC domain of MbMR5, V54, plays a major role in enhanced autoactivity, while another substitution in the NB-ARC domain, M273, partially contributes to the enhanced autoactivity.
Fig. 2.
The N-terminal region is critical for MrMR5 autoactivity in Nicotiana benthamiana. (A) MbMR5 CN domain is important for autoactivity. Diagrams of MrMR5, MbMR5, and 2 chimeras (MrMR5Mb-LRR and MrMR5Mb-CN) and the predicted CC, NB-ARC, and LRR domains are shown. MrMR5Mb-LRR carries the 4 polymorphic residues of MbMR5 in the LRR domain. MrMR5Mb-CN carries the 4 polymorphic residues of MbMR5 in the CC and NB-ARC domains. Black and red letters indicate the residues in MrMR5 and MbMR5, respectively. MrMR5, MbMR5, or chimeric variants were expressed in N. benthamiana leaves by Agrobacterium-mediated transient transformation. The Agrobacterium inoculum densities (OD600) used were 0.4. Agrobacterium carrying construct encoding virus-derived suppressor of gene silencing P19 (OD600 = 0.1) was added in each inoculum. Representative cell death scores and photographs are shown. Photographs were taken at 4 days after infiltration. Cell death was scored as described in Figure 1. Statistical significance compared to reference (leaves infiltrated with wild-type MrMR5) was assessed by 1-way ANOVA followed by Dunn’s multiple comparisons test using GraphPad Prism version 10.2.0 (ns, not significant; ****, P < .0001). Raw cell death score data are listed in Table S2. (B) The V54 residue in the CC domain of MbMR5 plays a critical role in the enhanced autoactivity of MbMR5. Wild-type or mutant variants of MrMR5 were expressed in N. benthamiana leaves by Agrobacterium-mediated transient transformation. Black and red letters indicate the residues in MrMR5 and MbMR5, respectively. Numbers indicate the position of residues. The Agrobacterium inoculum densities (OD600) used were 0.4. Agrobacterium carrying construct encoding virus-derived suppressor of gene silencing P19 (OD600 = 0.1) was added in each inoculum. Photographs were taken at 4 days after infiltration. Cell death was scored as described in Figure 1. Raw cell death score data are listed in Table S2. Statistical significance compared to the reference (leaves infiltrated with wild-type MrMR5) was assessed by 1-way ANOVA followed by Dunn’s multiple comparisons test using GraphPad Prism version 10.2.0 (ns, not significant; **, P < .01; ****, P < .0001).
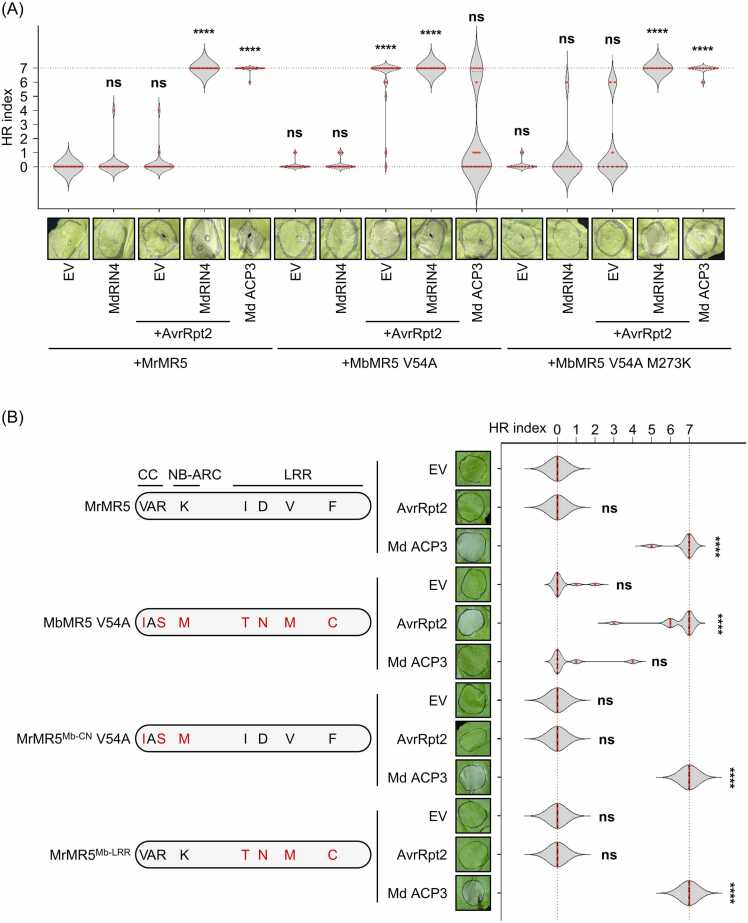
MbMR5 V54A and MbMR5 V54A M273K showed significantly reduced autoactivity when transiently expressed in N. benthamiana (Fig. 2B). We tested if these 2 mutant variants can be fully activated in a mechanism similar to MrMR5 by coexpressing MrMR5, MbMR5 V54A, or MbMR5 V54A M273K with EV, MdRIN4, AvrRpt2, or MdRIN4 ACP3 (Fig. 3A). To avoid HR resulted from AvrRpt2 recognition by N. benthamiana Ptr1 (NbPtr1), we performed the HR assay in an N. benthamiana mutant line knocked out for NbPtr1 (Nb1 ptr1) by gene editing (Ahn et al., 2023). Surprisingly, unlike MrMR5 or MbMR5 V54A M273K, coexpression of AvrRpt2 and MbMR5 V54A−induced HR without coexpression of MdRIN4 (Fig. 3A). Moreover, coexpression with MdRIN4 ACP3 only weakly activated MbMR5 V54A (Fig. 3A). To identify the residues responsible for the altered mode of activation shown by MbMR5 V54A, we generated 2 additional chimeric variants of MrMR5 by introducing polymorphic residues from MbMR5 V54A in the CN domain (MrMR5Mb-CN V54A, Fig. 3B) or in the LRR domain (MrMR5Mb-LRR, Figs. 2A and 3B). We then coexpressed MrMR5, MbMR5 V54A, MrMR5Mb-CN V54A, or MrMR5Mb-LRR together with EV, AvrRpt2, or MdRIN4 ACP3 (Fig. 3B). Interestingly, both MrMR5Mb-CN V54A and MrMR5Mb-LRR displayed activation upon coexpression with MdRIN4 ACP3 but not in the presence of AvrRpt2 alone (Fig. 3B). An immunoblot analysis demonstrated that MrMR5, MbMR5 V54A, MrMR5Mb-CN V54A, and MrMR5Mb-LRR accumulate to similar levels, indicating that lack of activation by AvrRpt2 or MdRIN4 ACP3 was not due to low protein abundance (Fig. S4). Taken together, these results indicate that the altered mode of activation shown by MbMR5 V54A requires additional polymorphic residues in both the CN and LRR domains.
Fig. 3.
MbMR5 shows altered RIN4 compatibility compared to MrMR5. (A) MdRIN4 is not required for the activation of MbMR5 V54A. Constructs encoding MrMR5, MbMR5 V54A, or MbMR5 V54A M273K were coinfiltrated in N. benthamiana (Nb1 ptr1) leaves with empty vector (EV) or constructs encoding MdRIN4, AvrRpt2, or MdRIN4 ACP3 by Agrobacterium-mediated infiltration. The Agrobacterium inoculum densities (OD600) used were as follows: EV = 0.4; MdRIN4 = 0.4; MdRIN4 ACP3 = 0.4; all MR5 variants = 0.4; and AvrRpt2 = 0.1. Agrobacterium carrying construct encoding virus-derived suppressor of gene silencing P19 (OD600 = 0.1) was added in each inoculum. Representative cell death scores and photographs are shown. Photographs were taken at 4 days after infiltration. Cell death was scored as described in Figure 1. Raw cell death score data are listed in Table S2. Statistical significance compared to the reference (leaves infiltrated with MrMR5 with EV) was assessed by 1-way ANOVA followed by Dunn’s multiple comparisons test using GraphPad Prism version 10.2.0 (ns, not significant; ****, P < .0001). (B) The CN and LRR domains are both responsible for the altered RIN4 compatibility of MbMR5 V54A. Diagrams of MrMR5, MbMR5 V54A, and 2 chimeras (MrMR5Mb-CN V54A and MrMR5Mb-LRR). The predicted CC, NB-ARC, and LRR domains are shown. MrMR5Mb-CN V54A carries 3 polymorphic residues from the CN domain of MbMR5. MrMR5Mb-LRR carries 4 polymorphic residues from the LRR domain of MbMR5. Black and red letters indicate residues from MrMR5 and MbMR5, respectively. Constructs encoding indicated MrMR5 variants were coinfiltrated with EV or constructs encoding AvrRpt2 or MdRIN4 ACP3 in N. benthamiana leaves by Agrobacterium-mediated infiltration. The Agrobacterium inoculum densities (OD600) used were the same as above. Agrobacterium carrying construct encoding virus-derived suppressor of gene silencing P19 (OD600 = 0.1) was added in each inoculum. Cell death was scored as described in Figure 1. Raw cell death score data are listed in Table S2. Statistical significance compared to the reference (leaves infiltrated with wild-type MrMR5) was assessed by 1-way ANOVA followed by Dunn’s multiple comparisons test using GraphPad Prism version 10.2.0 (ns, not significant; ****, P < .0001).
N. benthamiana contains 3 endogenous RIN4 homologs (hereafter NbRIN4-1/2/3) which fail to activate MrMR5 in the presence of AvrRpt2 (Prokchorchik et al., 2020). To rule out the possibility that the cleavage of NbRIN4-1/2/3 by AvrRpt2 activates MbMR5 V54A, we silenced NbRIN4-1/2/3 using virus-induced gene silence as previously reported (Ahn et al., 2023). Semiquantitative PCR showed the reduced expression of NbRIN4-1/2/3 (Fig. S5A). However, coexpression of MrMR5 or MbMR5 V54A with EV, MdRIN4, AvrRpt2, or MdRIN4 ACP3 showed that the activation of MrMR5 or MbMR5 V54A is not abolished by the silencing of NbRIN4-1/2/3 (Fig. S5B). Taken together, these results indicate that the activation of MbMR5 V54A by AvrRpt2 does not require NbRIN4-1/2/3.
DISCUSSION
In this study, we identified MbMR5, a homolog of MrMR5 originating from M. baccata. Despite their high sequence identity, MbMR5 showed significantly enhanced autoactivity (Fig. 1B). Two natural polymorphisms located in the CC and NB-ARC domains were responsible for this autoactivity (Fig. 2B). A54V is located in the second most N-terminal α-helix (α2) of the CC domain. Previous studies of the ZAR1 CC domain showed that in its active state, ZAR1 α2 forms the outer ring of the helical barrel structure and establishes oligomerization surfaces with other adjacent α-helixes of the CC domain (Wang et al., 2019). Previous reports on Arabidopsis NLR resistance to Pseudomonas syringae pv Maculicola 1 (RPM1) showed that hydrophobic and conserved residues located in the RPM1 CC domain corresponding to ZAR1 α2 contribute to self-association and interaction with AtRIN4 (El Kasmi et al., 2017). However, whether the CC domain of MrMR5 also contributes to the formation of a resistosome or association with other proteins is unknown. The other polymorphism in MbMR5, K273M, is located near the Walker B motif of the MrMR5 NB-ARC domain (Fahrentrapp et al., 2013). Walker B motifs participate in ATP hydrolysis by making direct contact with nucleotides and contain the consensus sequence hhhhD, where “h” denotes hydrophobic residues and “D” denotes aspartate (Sandall et al., 2020). The K273M polymorphism is located at 2 amino acids upstream of the first hydrophobic residue of the Walker B motif (Fig. S6). At this position, lysine (K) and arginine (R) are highly conserved among plant NLRs (list of studied plant NLRs was retrieved from (Kapos et al., 2019)), including RPM1, RPS2, and ZAR1 (Fig. S6). One of the few NLRs carrying other residues at this position is N. benthamiana NLR N requirement gene 1 (Fig. S6), belonging to NLRs carrying resistance to powdery mildew 8-like domains at their N-terminal end (RNLs) and functioning as a helper NLR (Castel et al., 2019). Notably, proline (P) is most frequently present at this position among predicted RNLs (Martin et al., 2022). The molecular function of the residues corresponding to MrMR5 K273 in other NLRs is unclear; yet further investigation of A54V, K273M, and their synergistic effect on enhancing MrMR5 autoactivity could help us better understand NLR activation mechanisms in the future.
We showed that MrMR5 with 7 naturally occurring polymorphisms from MbMR5 (with A54 only remaining) is only weakly activated by MdRIN4 ACP3 (Fig. 3A). Surprisingly, MbMR5 V54A also showed the gain of activation by AvrRpt2 in the absence of MdRIN4 or NbRIN4-1/2/3 (Fig. 3A and Fig. S5B). One possible explanation for this unexpected result is that MbMR5 V54A is no longer compatible with MdRIN4 but gained compatibility with novel N. benthamiana proteins, such as NOI proteins (Fig. 4). In this scenario, transient expression of AvrRpt2 would produce cleavage products of NOI and subsequently activate MbMR5 V54A (Fig. 4). Combined with the previous observation that 2 substitutions in RIN4 natural variants lead to the gain of MrMR5 activation (Kim et al., 2022, Prokchorchik et al., 2020), our findings indicate that small polymorphisms either on RIN4 or RIN4-interacting NLRs may recruit new interacting partners (Fig. 4). However, it remains unclear if MbMR5 V54A gained compatibility with N. benthamiana NOI proteins or if such flexible interaction between MdRIN4 and MrMR5 can also be found in other RIN4-interacting NLRs, such as RPS2, RPM1, or Ptr1. Further comparative investigation of MrMR5 and MbMR5 would unveil the molecular basis of the altered mode of activation shown by MbMR5 V54A.
Fig. 4.
Schematic representation of interaction between different RIN4 and MR5 alleles upon AvrRpt2-induced cleavage. Blue and red ellipses indicate full length or ACP (AvrRpt2 cleavage product) of Arabidopsis and apple RIN4, respectively. Green ellipse indicates N. benthamiana NOI protein. Purple shape and arrow indicate AvrRpt2 and AvrRpt2-induced cleavage, respectively. Black solid line indicates regulation of NLR. Gray dashed line indicates the loss or absence of NLR regulation.
Author Contributions
Kee Hoon Sohn: Writing – review and editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Conceptualization. Jong Taek Park: Resources, Investigation. Minseon Kim: Visualization, Validation, Investigation, Formal analysis, Data curation. Jieun Kim: Visualization, Validation, Investigation, Formal analysis, Data curation. Haseong Kim: Writing – review and editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The author is an Editorial Board Member/Editor-in-Chief/Associate Editor/Guest Editor for Molecules and Cells and was not involved in the editorial review or the decision to publish this article.
Acknowledgments
This research was supported by the Cooperative Research Program for Agricultural Science and Technology Development, Rural Development Administration, Korea (PJ015301042021), the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Agriculture and Food Convergence Technologies Program for Research Manpower Development, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. RS-2024-00398300) and New Faculty Startup Fund from Seoul National University.
ORCID
Haseong Kim: https://orcid.org/0000-0001-8800-7992.
Jieun Kim: https://orcid.org/0009-0006-0299-6587.
Minseon Kim: https://orcid.org/0009-0006-6416-5412.
Jong Taek Park: https://orcid.org/0000-0001-6486-739X.
Kee Hoon Sohn: https://orcid.org/0000-0002-9021-8649.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.mocell.2024.100094.
Contributor Information
Haseong Kim, Email: haseongkim@snu.ac.kr.
Jieun Kim, Email: jieun.kim21@snu.ac.kr.
Minseon Kim, Email: tjsl1011@gmail.com.
Jong Taek Park, Email: jongtaek@korea.kr.
Kee Hoon Sohn, Email: keehoon.sohn@snu.ac.kr.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- Ahn Y.J., Kim H., Choi S., Mazo-Molina C., Prokchorchik M., Zhang N., Kim B., Mang H., Koehler N., Kim J., et al. Ptr1 and ZAR1 immune receptors confer overlapping and distinct bacterial pathogen effector specificities. New Phytol. 2023;239:1935–1953. doi: 10.1111/nph.19073. [DOI] [PubMed] [Google Scholar]
- Bentham A.R., Zdrzałek R., De la Concepcion J.C., Banfield M.J. Uncoiling CNLs: structure/function approaches to understanding CC domain function in plant NLRs. Plant Cell Physiol. 2018;59:2398–2408. doi: 10.1093/pcp/pcy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel B., Ngou P.-M., Cevik V., Redkar A., Kim D.-S., Yang Y., Ding P., Jones J.D.G. Diverse NLR immune receptors activate defence via the RPW8-NLR NRG1. New Phytol. 2019;222:966–980. doi: 10.1111/nph.15659. [DOI] [PubMed] [Google Scholar]
- Chisholm S.T., Dahlbeck D., Krishnamurthy N., Day B., Sjolander K., Staskawicz B.J. Molecular characterization of proteolytic cleavage sites of the Pseudomonas syringae effector AvrRpt2. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2087–2092. doi: 10.1073/pnas.0409468102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B., Dahlbeck D., Huang J., Chisholm S.T., Li D., Staskawicz B.J. Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell. 2005;17:1292–1305. doi: 10.1105/tpc.104.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury Z., Wu C.H., Ding P. A comparative overview of the intracellular guardians of plants and animals: NLRs in innate immunity and beyond. Annu. Rev. Plant Biol. 2021;72:155–184. doi: 10.1146/annurev-arplant-080620-104948. [DOI] [PubMed] [Google Scholar]
- El Kasmi F., Chung E.H., Anderson R.G., Li J., Wan L., Eitas T.K., Gao Z., Dangl J.L. Signaling from the plasma-membrane localized plant immune receptor RPM1 requires self-association of the full-length protein. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E7385–e7394. doi: 10.1073/pnas.1708288114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C., Kandzia R., Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrentrapp J., Broggini G.A.L., Kellerhals M., Peil A., Richter K., Zini E., Gessler C. A candidate gene for fire blight resistance in Malus × robusta 5 is coding for a CC–NBS–LRR. Tree Genet. Genomes. 2013;9:237–251. [Google Scholar]
- Jones J.D.G., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jones J.D.G., Staskawicz B.J., Dangl J.L. The plant immune system: from discovery to deployment. Cell. 2024;187:2095–2116. doi: 10.1016/j.cell.2024.03.045. [DOI] [PubMed] [Google Scholar]
- Kapos P., Devendrakumar K.T., Li X. Plant NLRs: from discovery to application. Plant Sci. 2019;279:3–18. doi: 10.1016/j.plantsci.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Kim W., Jeon H., Lee H., Sohn K.H., Segonzac C. The Ralstonia pseudosolanacearum type III effector RipL delays flowering and promotes susceptibility to Pseudomonas syringae in Arabidopsis thaliana. Mol. Cells. 2023;46:710–724. doi: 10.14348/molcells.2023.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Prokchorchik M., Sohn K.H. Investigation of natural RIN4 variants reveals a motif crucial for function and provides an opportunity to broaden NLR regulation specificity. Plant J. 2022;110:58–70. doi: 10.1111/tpj.15653. [DOI] [PubMed] [Google Scholar]
- Kunkel B.N., Bent A.F., Dahlbeck D., Innes R.W., Staskawicz B.J. RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2. Plant Cell. 1993;5:865–875. doi: 10.1105/tpc.5.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.C., Spiridon L., Goverse A., Petrescu A.J. NLRexpress-a bundle of machine learning motif predictors-reveals motif stability underlying plant Nod-like receptors diversity. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.975888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo-Molina C., Mainiero S., Hind S.R., Kraus C.M., Vachev M., Maviane-Macia F., Lindeberg M., Saha S., Strickler S.R., Feder A. The Ptr1 Locus of Solanum lycopersicoides Confers Resistance to Race 1 Strains of Pseudomonas syringae pv. tomato and to Ralstonia pseudosolanacearum by Recognizing the Type III Effectors AvrRpt2 and RipBN. Mol. Plant Microbe Interact. 2019;32(8):949–960. doi: 10.1094/MPMI-01-19-0018-R. Aug. [DOI] [PubMed] [Google Scholar]
- Ngou B.P.M., Ding P., Jones J.D.G. Thirty years of resistance: zig-zag through the plant immune system. Plant Cell. 2022;34:1447–1478. doi: 10.1093/plcell/koac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokchorchik M., Choi S., Chung E.H., Won K., Dangl J.L., Sohn K.H. A host target of a bacterial cysteine protease virulence effector plays a key role in convergent evolution of plant innate immune system receptors. New Phytol. 2020;225:1327–1342. doi: 10.1111/nph.16218. [DOI] [PubMed] [Google Scholar]
- Sandall C.F., Ziehr B.K., MacDonald J.A. ATP-binding and hydrolysis in inflammasome activation. Molecules. 2020;25:4572. doi: 10.3390/molecules25194572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur I.M.L., Panstruga R., Schulze-Lefert P. NOD-like receptor-mediated plant immunity: from structure to cell death. Nat. Rev. Immunol. 2021;21:305–318. doi: 10.1038/s41577-020-00473-z. [DOI] [PubMed] [Google Scholar]
- Steele J.F.C., Hughes R.K., Banfield M.J. Structural and biochemical studies of an NB-ARC domain from a plant NLR immune receptor. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn R.A., Kamoun S. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell. 2008;20:2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt I., Wöhner T., Richter K., Flachowsky H., Sundin G.W., Wensing A., Savory E.A., Geider K., Day B., Hanke M.V., et al. Gene-for-gene relationship in the host-pathogen system Malus × robusta 5-Erwinia amylovora. New Phytol. 2013;197:1262–1275. doi: 10.1111/nph.12094. [DOI] [PubMed] [Google Scholar]
- Wang J., Chai J. Molecular actions of NLR immune receptors in plants and animals. Sci. China Life Sci. 2020;63:1303–1316. doi: 10.1007/s11427-019-1687-6. [DOI] [PubMed] [Google Scholar]
- Wang J., Hu M., Wang J., Qi J., Han Z., Wang G., Qi Y., Wang H.W., Zhou J.M., Chai J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science. 2019;364:eaav5870. doi: 10.1126/science.aav5870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material