Abstract
We have previously shown that CD32A (or FcγRIIA), one of the main opsonin receptors, was rapidly insolubilized and degraded in intact neutrophils after its cross-linking. In view of these experimental difficulties, the early signalling steps in response to CD32A activation were studied in purified plasma membranes of neutrophils. After CD32A cross-linking in these fractions, the tyrosine phosphorylation of two major substrates, the receptor itself and the tyrosine kinase Syk, was observed. Phosphorylation of these two proteins was observed only in the presence of orthovanadate, indicating the presence, in the membranes, of one or more tyrosine phosphatases that maintain CD32A dephosphorylation. The tyrosine phosphorylation of these two proteins was inhibited by the Src kinase inhibitor, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2). The ligation of CD32A led to its recruitment to a previously uncharacterized subset of high-density flotillin-1-positive DRMs (detergent-resistant membranes). The changes in the solubility properties of CD32A were observed in the absence of added ATP; therefore, they were probably not secondary to the tyrosine phosphorylation of the receptor, rather they preceded it. Src kinases as well as Syk were constitutively present in DRMs of high and low density and no evident changes in their distribution were detected after cross-linking of CD32A. Pretreatment of plasma membranes with methyl-β-cyclodextrin did not inhibit the recruitment of CD32A to DRMs, although it led to the loss of the Src kinase Lyn from these fractions. In addition, methyl-β-cyclodextrin inhibited the tyrosine phosphorylation of CD32A and Syk induced by cross-linking of CD32A. This membrane model allowed us to observe a movement of CD32A from detergent-soluble regions of the membranes to DRMs, where it joined Src kinases and Syk and became tyrosine-phosphorylated.
Keywords: CD32A signalling, detergent-resistant membrane domain, Fc receptor, neutrophil, Src kinase, tyrosine phosphorylation
Abbreviations: iPr2P-F, di-isopropyl fluorophosphate; DRM, detergent-resistant membrane; GAM, goat F(ab′)2 anti-mouse IgG; ITAM, immunoreceptor tyrosine-based activation motif; M-β-CD, methyl-β-cyclodextrin; NP40, Nonidet P40; OGD, N-octyl-β-D-glucoside; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; RLB, relaxation buffer
INTRODUCTION
Human neutrophils constitutively express two receptors of the FcγR family, namely CD32A (or FcγRIIA) and CD16B (FcγRIIIB), which participate, probably in a non-redundant manner, in the phagocytic function of the neutrophils [1–4]. CD32A is a single-transmembrane receptor of 40 kDa, which possesses two conserved tyrosine residues arranged in a non-canonical ITAM (immunoreceptor tyrosine-based activation motif). Although it has never been directly shown in fresh isolated neutrophils, results obtained in other cells indicate that phosphorylation of tyrosine residues in the ITAM during activation of the receptor is one of the earliest steps leading to the tyrosine phosphorylation of downstream proteins, reorganization of actin filaments and, finally, phagocytosis [5–7]. Depending on the model used (transfected cells, cell lines or primary cells), the tyrosine kinases suggested to be involved in the phosphorylation of CD32A are the Src kinases Lyn, Fgr and Hck [8–10]. This suggests that CD32A interacts with multiple Src-related protein tyrosine kinases in a cell-type-specific manner. Knockout models [11] as well as pharmacological manipulations [12] indicate that the tyrosine kinase Syk also plays a critical role in the control of the phagocytic process. Syk is generally supposed to be recruited from the cytosol to the tyrosine-phosphorylated ITAM of CD32A [13].
Several immunoreceptors, including the T-cell receptor [14], B-cell receptor [15] and several Fc receptors [16], are believed to initiate their signalling cascade by associating with specialized lipid regions of the membrane, which are also (albeit somewhat loosely) referred to as rafts, detergent-insoluble glycolipid-enriched domains or DRMs (detergent-resistant membranes) [17]. However, most, if not all, the studies on the role of membrane rafts in signal transduction through Fc receptors have been performed on cell lines. In neutrophils, experiments in the presence of cholesterol-scavenging or -binding molecules suggest a role for these membrane domains in phagocytosis [18], superoxide generation [19] and cell polarization [20]. It is noteworthy, however, that M-β-CD (methyl-β-cyclodextrin) and nystatin enhanced rather than inhibited the tyrosine phosphorylation and calcium responses observed subsequent to CD32A cross-linking in human neutrophils [21].
We have shown recently that CD32A is rapidly tyrosine-phosphorylated and it translocated to a non-ionic detergent-insoluble fraction different from the low-density lipid raft fraction after its cross-linking in human neutrophils [22]. Immediately after this translocation (<2 min), a large amount of the receptor appears to be degraded in a tyrosine phosphorylation-dependent manner. These two sets of observations (the rapid insolubilization of CD32A and its subsequent degradation) render biochemical investigations of the signalling pathways associated with CD32A in intact human neutrophils particularly difficult. In addition, they cast serious doubts about the physiological relevance of the results obtained using lysates derived from stimulated cells. This led us to develop a novel approach to study the earliest steps of signal transduction downstream of CD32A in human neutrophils. Using both cytoplasts and purified plasma membranes, we observed that the basic signalling unit associated with CD32A was preserved in purified plasma membranes, in which cross-linking stimulated the tyrosine phosphorylation of various substrates (including CD32A itself and the tyrosine kinase Syk) as well as its insolubility in non-ionic detergents. Cross-linked CD32A was also shown to translocate to detergent-resistant fractions of a density higher than that classically associated with lipid rafts.
MATERIALS AND METHODS
Reagents
iPr2P-F (di-isopropyl fluorophosphate) was obtained from Helixx Technologies (Scarborough, ON, Canada) and NP40 (Nonidet P40) and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (referred to as PP2) were obtained from Calbiochem (San Diego, CA, U.S.A.). Sodium orthovanadate (Na2VO4), Optiprep [60% (w/v) stock solution] and M-β-CD (methyl-β-cyclodextrin) were obtained from Sigma–Aldrich Canada (Oakville, ON, Canada). Dextran T-500, Percoll and Protein A–Sepharose were purchased from Amersham Biosciences (Baie d'Urfé, QC, Canada). Aprotinin, leupeptin and OGD (N-octyl-β-D-glucoside) were purchased from ICN Biomedicals (Aurora, OH, U.S.A.) and the chemiluminescence kit was from NEN Life Science (Boston, MA, U.S.A.). Ficoll Paque was obtained from Wisent (St-Bruno, QC, Canada).
Antibodies
The anti-CD32A monoclonal antibody IV.3 was purified from ascites of mice inoculated with hybridoma HB 217 obtained from the A.T.C.C. (Manassas, VA, U.S.A.). CT10 is an IgG-purified fraction of a polyclonal rabbit antiserum against the cytoplasmic domain of CD32A raised against the synthetic peptide whose sequence was published by Ibarrola et al. [8]. Goat anti-mouse F(ab′)2 was used for cross-linking [anti-Fc, catalogue no. 115-006-071 or anti-F(ab′)2, catalogue no. 115-006-072], and horseradish peroxidase-labelled donkey anti-rabbit IgG (catalogue no. 711-035-152) were purchased from Jackson Immuno-research Laboratories (West Grove, PA, U.S.A.). Horseradish peroxidase-labelled sheep anti-mouse IgGs (catalogue no. NXA931) were obtained from Amersham Biosciences. 4G10 was purchased from Upstate Biotechnology (Lake Placid, NY, U.S.A.). Anti-Lyn, anti-Fgr and anti-Hck polyclonal antibodies (catalogue nos. sc-15, sc-130 and sc-72 respectively) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), and monoclonal anti-Syk (catalogue no. MAB88906) was from Chemicon (Temecula, CA, U.S.A.). Monoclonal anti-flotillin-1 (catalogue no. F65020-050) was purchased from BD Biosciences (Mississauga, ON, Canada).
Preparation of U-cytoplasts
U-cytoplasts are granule-poor anucleate fragments released from neutrophils. They were obtained essentially as described previously [23] by centrifugation of untreated neutrophils on discontinuous gradients of Ficoll. They retain locomotion, chemotactic responsiveness, killing of phagocytized bacteria and stimulatable oxidase activity even after storage at −80 °C.
Isolation of neutrophil membranes
Neutrophils were obtained from healthy adult volunteers as described previously [24]. Briefly, they were purified under sterile conditions by 2% (w/v) dextran sedimentation, followed by centrifugation on a Ficoll cushion. Contaminating red blood cells were removed by hypo-osmotic lysis. Isolated neutrophils were then disrupted by nitrogen cavitation as described by Kjeldsen et al. [25] with some modifications. The cells were resuspended at 40–60×106 cells/ml in modified RLB [relaxation buffer; 100 mM KCl, 3 mM NaCl, 10 mM Hepes (pH 7.4), 3 mM iPr2P-F, 10 μg/ml aprotinin and 10 μg/ml leupeptin]. Cells were pressurized under nitrogen for 5 min at 2622 kPa in a nitrogen bomb. The cavitae were then collected in drops, and nuclei and intact cells were pelleted by centrifugation at 300 g for 3 min. Postnuclear supernatants (6 ml) were applied slowly on top of a two-step Percoll gradient composed of 9 ml of a 1.05 g/ml solution layered above 9 ml of a 1.12 g/ml solution. The gradients were centrifuged at 37000 g for 30 min in a fixed-angle rotor. The upper band, with the clear cytosol at its top, contained the plasma membranes. These were collected and centrifuged at 100000 g for 45 min to remove Percoll. After the centrifugation, the biological material visible as a disc above the pelleted Percoll was collected and stored in aliquots at −80 °C. The amount of membranes was determined by comparison with fixed concentrations of whole cells immunoblotted with CT10.
Stimulation of membrane receptor
Neutrophil membranes were resuspended in RLB at 20–25×106 cell equivalents/ml. They were then incubated with 4 μg/ml of IV.3 antibody for 10 min at 4 °C. A kinase mixture composed of 2 mM Na2-ATP and 400 μM MnCl2 (final concentrations) was added at room temperature (23 °C), 3 min before the addition of 2 mM Na2VO4 and 50 μg/ml goat F(ab′)2 directed against mouse Fc; the reaction mixtures were then incubated for 5 min or as indicated. The reactions were stopped by direct transfer of aliquots to the same volume of 2× boiling Laemmli's sample buffer [composition of 1×: 62.5 mM Tris/HCl (pH 6.8), 4% (w/v) SDS, 5% (v/v) 2-mercaptoethanol, 8.5% (v/v) glycerol, 2.5 mM orthovanadate, 10 mM para-nitrophenylphosphate, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 0.025% Bromophenol Blue].
For CD32A immunoprecipitation, IV.3 antibody was cross-linked with a goat F(ab′)2 directed against the F(ab′)2 portion of mouse IgG to allow the IV.3 Fc portion to be free for Protein A binding.
For Syk immunoprecipitation, CD32A was stimulated with 2 μg/ml F(ab′)2 fragment of IV.3 and cross-linking was performed as described above.
Isolation of DRMs
After CD32A cross-linking, plasma membranes were chilled on ice for 5 min and 1% NP40 (final concentration) was added for 20 min on ice. Solubilized membranes were adjusted to 40% (v/v) Optiprep with a stock solution (59.4% Optiprep/10 mM Hepes, pH 7.4). Then, 700 μl was transferred to 4 ml centrifuge tubes and overlaid with 700 μl of ice-cold solutions of 35, 30, 25, 20 and 0% Optiprep in RLB containing 2 mM Na2VO4. The gradients were centrifuged at 380000 g for 3 h at 4 °C in a TLA 100.4 rotor and, subsequently, 13 fractions of 300 μl were collected from the top of the gradients. Their densities were measured with a refractometer in 50 μl of each fraction. Proteins were chloroform/methanol-precipitated as described previously [26]. The precipitates were resuspended in 60 μl of 2× Laemmli's sample buffer.
Immunoprecipitation
After solubilization in 100 mM OGD at 4 °C, the membranes were centrifuged for 10 min at 15000 g. For CD32A immunoprecipitation, 50 μl of 33% (v/v) reconstituted Protein A–Sepharose slurry was added to the supernatants. For Syk immunoprecipitation, 2 μg of an anti-Syk antibody previously bound to Protein A–Sepharose was added to the supernatants. After a 90 min incubation, immunoprecipitates were washed three times in RLB containing 2 mM Na2VO4 and 100 mM OGD and solubilized in boiling Laemmli's sample buffer.
Electrophoresis and immunoblotting
Proteins were analysed on SDS/PAGE (7.5–20% gel) gradients, except for Optiprep fractions, which were analysed by SDS/PAGE (12% gel). After electrophoresis, proteins were transferred to Immobilon-P membranes (Millipore, Bedford, MA, U.S.A.). Immunoblotting was performed using 7% (w/v) Blotto as a blocking agent for CT10 and anti-flotillin-1 antibodies, and using 2% (w/v) gelatin as a blocking agent for the other antibodies used in the present study. Dilution of the antibodies was as follows: anti-flotillin-1, 1:500; CT10 (anti-C32A), anti-Lyn, anti-Fgr and anti-Hck, 1:1000; 4G10, 1:4000. The blocking agents and antibodies were diluted in TBS-Tween (25 mM Tris/HCl, pH 7.8/190 mM NaCl/0.15% Tween 20). The horseradish peroxidase-labelled secondary antibody was diluted to 1:20000 in TBS-Tween and a chemiluminescence reagent was used for detection. Development time periods were never longer than 5 min.
RESULTS
CD32A cross-linking in U-cytoplasts and in purified plasma membranes
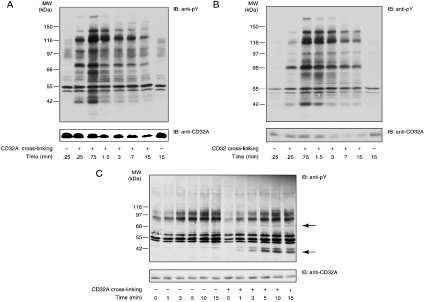
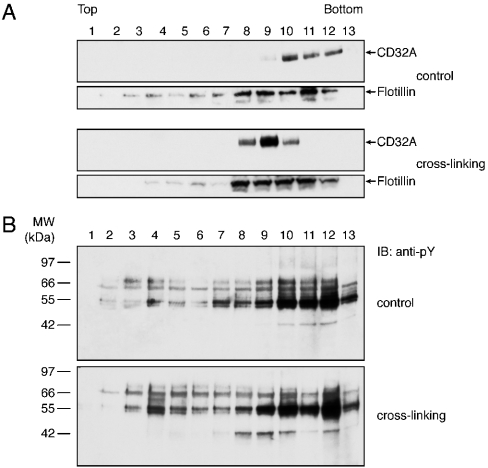
In an attempt to focus on the earliest steps of CD32A signalling and owing to the difficulties associated with the investigation of the CD32A signalling pathways in whole neutrophils (see the Introduction section), we opted for the use of simpler cellular or subcellular models. The first of these models used U-cytoplasts, which represent multifunctional enucleated neutrophil fragments that are largely depleted in intracellular organelles [23,27]. CD32A was also cross-linked in whole cells (Figure 1A) for the sake of comparison. As described previously, in intact neutrophils, a rapid and transient pattern of tyrosine phosphorylation was induced after CD32A cross-linking, and a decrease in the amount of CD32A was observed after 1.5 min of stimulation (Figure 1A). In U-cytoplasts, the pattern of tyrosine phosphorylation was similar to that observed in whole cells. Moreover, a decrease in the amount of CD32A was also observed (Figure 1B). These results provide additional evidence for the functional integrity of U-cytoplasts; however, they indicate that use of U-cytoplasts will not help circumvent the technical problems alluded to above. Therefore we turned next to purified plasma membranes from neutrophils in which CD32A was directly cross-linked, as described in the Materials and methods section. Two major tyrosine-phosphorylated substrates of molecular masses 40 and 72 kDa were detected in response to CD32A cross-linking in these membranes (Figure 1C, indicated by arrows). Moreover, in contrast with what was observed in intact cells (Figure 1A) and U-cytoplasts (Figure 1B), the tyrosine phosphorylation levels and immunoreactivity of CD32A remained constant for up to 15 min after CD32A cross-linking. These last observations prompted us to characterize further this model, since CD32A degradation represents a major difficulty in the study of CD32A signalling in neutrophils.
Figure 1. Tyrosine phosphorylation and receptor degradation induced after CD32A cross-linking in neutrophils, U-cytoplasts and isolated plasma membranes.
After a 10 min incubation with 1 mM iPr2P-F, CD32A was cross-linked on human neutrophils (A) or cytoplasts (B) at 20×106 cells/ml. The cells or the cytoplasts were incubated with IV.3 (2 μg/ml) for 5 min at room temperature, followed by the addition of goat F(ab′)2 anti-mouse IgG (20 μg/ml) at 37 °C for the time periods indicated. Samples were probed with an anti-phosphotyrosine (anti-pY) or anti-CD32A antibody. (C) CD32A was cross-linked on neutrophil membranes as described in the Materials and methods section for the time periods indicated. Samples were probed with an anti-pY or anti-CD32A antibody. IB, immunoblot. The two major substrates whose tyrosine phosphorylations are enhanced are indicated with arrows.
Characterization of the plasma-membrane model
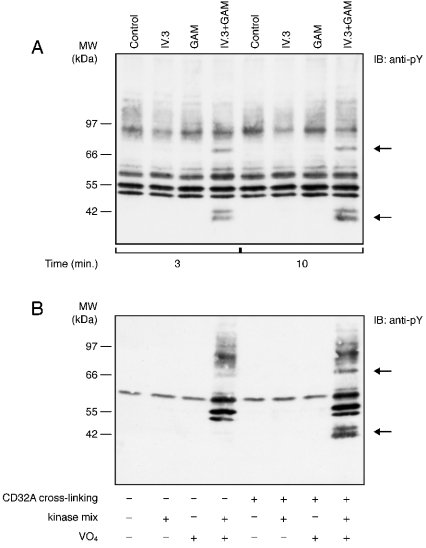
First, we wanted to confirm the specificity of the tyrosine phosphorylation response described in Figure 1(C). As shown in Figure 2(A), neither the anti-CD32A (IV.3) nor the secondary antibody [goat F(ab′)2 anti-mouse IgG, referred to as GAM] alone induced a detectable tyrosine phosphorylation response. On the other hand, a combination of these two antibodies (IV.3+GAM) led to the rapid tyrosine phosphorylation of the 40 and 72 kDa substrates. The addition of ATP and MnCl2 (a cofactor for tyrosine kinases) was needed for detecting a phosphotyrosine response, since the membranes were stimulated in the absence of cytoplasm. Orthovanadate was also found to be required, indicating that tyrosine phosphatases were present and active in the purified neutrophil membranes (Figure 2B). A similar pattern of tyrosine phosphorylation was observed if MgCl2 was used instead of MnCl2 (results not shown).
Figure 2. Kinetics and specificity of the tyrosine phosphorylation responses to CD32A cross-linking on neutrophil membranes.
(A) Neutrophil membranes were incubated with IV.3 alone, GAM alone or both for the time periods indicated, as indicated in the Materials and methods section. Samples were probed with an anti-pY. (B) CD32A was cross-linked on neutrophil membranes for 10 min in the absence (−) or presence (+) of ATP and MnCl2 (kinase mix) and orthovanadate (VO4). Samples were probed with an anti-pY. The two major substrates whose tyrosine phosphorylations are enhanced are indicated by arrows.
CD32A and Syk are specifically tyrosine-phosphorylated in response to CD32A cross-linking in plasma membranes
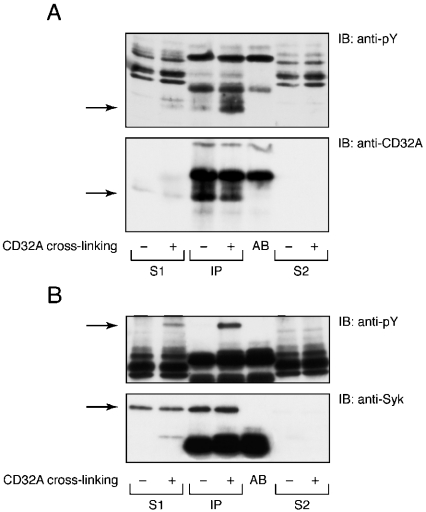
The molecular masses of the two tyrosine-phosphorylated substrates of 40 and 72 kDa suggest that they probably correspond to the receptor CD32A and Syk respectively. Immunoprecipitations were performed to test these hypotheses and the results are shown in Figure 3. After CD32A cross-linking, membranes were solubilized in 100 mM OGD, and CD32A and Syk were immunoprecipitated. The specific immunoblots indicated that Syk and the receptor itself were tyrosine-phosphorylated in response to CD32A cross-linking. The amount of CD32A immunoprecipitated after cross-linking was lower than that in controls because of the goat anti-mouse secondary antibody, which prevented complete binding of Protein A to the Fc portion of IV.3. These results do not exclude the possibility that other tyrosine-phosphorylated proteins may also be present in the cross-linked membranes.
Figure 3. Tyrosine phosphorylation of Syk and CD32A on neutrophil membranes after CD32A cross-linking.
Syk (A) and CD32A (B) were immunoprecipitated from neutrophil membranes after CD32A cross-linking, as described in the Materials and methods section. An aliquot of the 15000 g supernatant (S1) and an aliquot of the supernatant after immunoprecipitation (S2) were analysed alongside the immunoprecipitates (IP). AB, antibody alone. Samples were subsequently analysed by SDS/PAGE and immunoblotted with an anti-pY or CT10 antibody (anti-CD32A).
CD32A resists detergent extraction after cross-linking
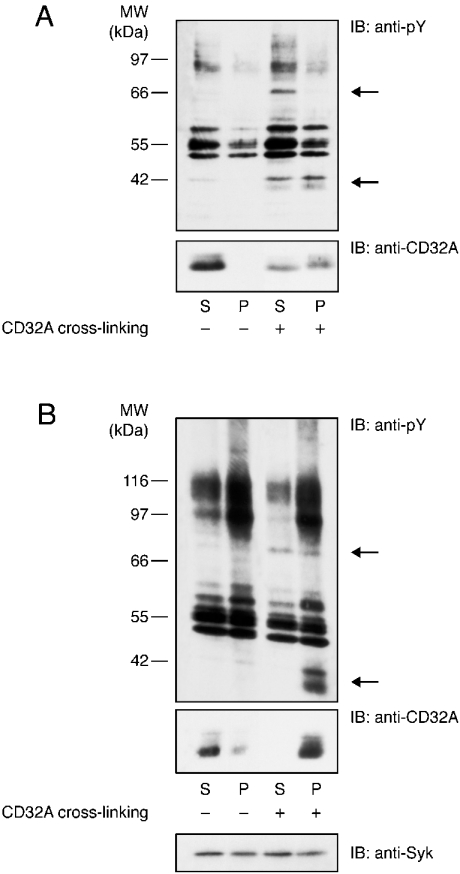
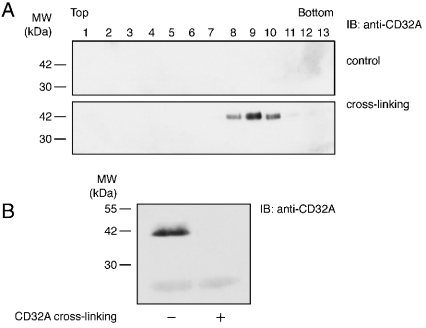
We next examined whether, similar to other immunoreceptors [14–16], CD32A became insoluble in non-ionic detergents after cross-linking in isolated plasma membranes. The results presented in Figure 4(A) indicate that CD32A was solubilized in 1% NP40 under resting conditions, whereas in the pellet of a 10 min centrifugation at 15000 g, approx. 50% of the receptor was found after CD32A cross-linking. Tyrosine-phosphorylated CD32A was equally present in the pellet and supernatant of the 15000 g centrifugation (Figure 4A, upper panel). Since a more rigorous definition of a soluble protein is that it is still present in the supernatant after a 1 h centrifugation at 100000 g, the same experiment was repeated under these conditions. The results shown in Figure 4(B) indicate that, under resting conditions, most of the CD32A was present in the supernatant of the 100000 g centrifugation, whereas only a small amount was detected in the pellet. In contrast, after its cross-linking, essentially all of the CD32A translocated to the pellets. Moreover, the differences observed between these two centrifugations indicate that, even in the presence of detergent, the 15000 g supernatants contained more than soluble proteins. These results illustrate that, irrespective of the centrifugation speed, CD32A partitioning between the supernatants and pellets changed drastically after receptor cross-linking. In contrast, Syk was equally distributed in the supernatants and pellets and this partitioning remained unchanged after CD32A cross-linking (Figure 4B, lower panel).
Figure 4. Cross-linking-induced insolubility of CD32A in non-ionic detergents on neutrophil membranes.
CD32A was cross-linked on neutrophil membranes as described in the Materials and methods section and membranes were solubilized in 1% NP40 and centrifuged at 15000 g for 10 min (A) or at 100000 g for 1 h (B). The supernatant (S) and pellet (P) were subsequently analysed by SDS/PAGE and immunoblotted with an anti-pY or CT10 antibody (anti-CD32A). The two major substrates whose tyrosine phosphorylation is enhanced are indicated with arrows.
CD32A is recruited to high-density DRMs after its cross-linking
The results described above suggested that CD32A was recruited to DRMs of the plasma membrane after its cross-linking. DRMs, because of their high lipid content, can be purified by density-gradient ultracentrifugation. Previous studies using whole cells provided evidence that cross-linked CD32A did not migrate to classical low-density buoyant DRMs, but was instead recovered in the pellet of the sucrose-gradient ultracentrifugation [22]. In the present study, we decided to use an Optiprep gradient, which was recently used for the isolation of neutrophil-membrane DRMs [28]. Various gradient conditions (number of steps, Optiprep concentrations in each step etc.) were tested to distinguish the highdensity DRMs from the insoluble material present in the pellet. After CD32A cross-linking, membranes were solubilized in 1% NP40 as described in the Materials and methods section. An Optiprep step gradient was overlaid on to 700 μl of the solubilized membranes, which were then ultracentrifuged as described in the Materials and methods section. Analysis of each fraction of the gradients derived from resting and cross-linked membranes is presented in Figure 5. These results indicate that CD32A was detected in the last fractions [10–12] of the gradient representing the soluble proteins in the absence of cross-linking (Figure 5A). After its cross-linking, a shift of the receptor to lighter-density fractions (8–10) was observed. Flotillin-1, a structural raft component [29,30], was enriched in fractions 8–12 and its distribution remained the same before and after CD32A crosslinking (Figure 5A). Immunoblotting with an anti-phosphotyrosine antibody (Figure 5B) shows that a 40 kDa tyrosine-phosphorylated substrate corresponding to CD32A was present in the same fractions (8–10).
Figure 5. Cross-linking of CD32A in isolated plasma membranes induces its recruitment to high-density DRMs.
CD32A was cross-linked on neutrophil membranes as described in the Materials and methods section. After NP40 solubilization, the membranes were subjected to ultracentrifugation on Optiprep gradients. Then, 13 fractions were collected and the proteins were precipitated and analysed by SDS/PAGE. (A) Membranes were immunoblotted with a CT10 (anti-CD32A) or anti-flotillin-1 antibody. (B) Membranes were immunoblotted with an anti-pY antibody.
After its cross-linking, phosphorylated CD32A was detected in a region of the gradients with a density of 1.18–1.2 g/ml (fractions 8–10), which could correspond not only to soluble proteins but also to DRMs of high density, as described by Nebl et al. [28]. To distinguish between these two possibilities, the soluble fractions were eliminated by performing a 100000 g ultracentrifugation before the Optiprep-gradient ultracentrifugation. Under these conditions (Figure 6A), cross-linked CD32A was detected in fractions 8–10, where flotillin-1 was also present (see Figure 5A), whereas before cross-linking, it was essentially completely soluble (Figure 6B). These results indicate that fractions 8–10 of the Optiprep gradient represent high-density DRMs.
Figure 6. Insolube CD32A translocates to high-density DRMs.
CD32A was cross-linked on neutrophil membranes as described in the Materials and methods section. After NP40 solubilization, membranes (600 μl) were ultracentrifuged for 1 h at 100000 g on 100 μl of a 48% Optiprep cushion. (A) The pellets were diluted to 40% Optiprep and subjected to Optiprep-gradient ultracentrifugation as in Figure 5. (B) The supernatants were immunoblotted with a CT10 antibody (anti-CD32A).
Effect of PP2 on the tyrosine phosphorylation response to CD32A cross-linking
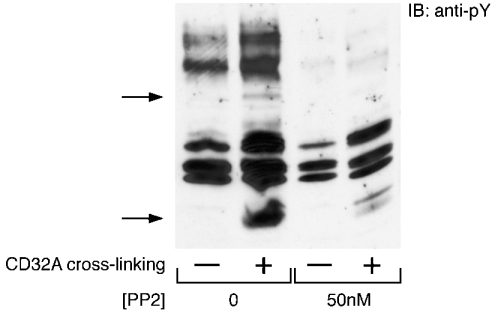
Although several lines of evidence support the idea that an Src kinase promotes the initial tyrosine phosphorylation of the receptor, co-immunoprecipitation experiments in neutrophils have so far yielded controversial results (see the Introduction section). Ambiguities in the interpretation of the latter set of results probably originate from the stimulated changes in the solubility of CD32A and its rapid degradation in intact neutrophils. To investigate further the link between Src kinases and the activation of CD32A, we tested the effect of PP2, a well-described Src kinase inhibitor [31], on the tyrosine phosphorylation response to CD32A cross-linking in purified plasma membranes. As shown in Figure 7, this response was strongly inhibited after a 10 min incubation with 50 nM PP2. This concentration is 100-fold lower compared with the concentration used in whole cells. This result confirms the involvement of Src kinases in the early steps of CD32A signalling.
Figure 7. Inhibition by PP2 of the tyrosine phosphorylation response induced in plasma membranes after cross-linking of CD32A.
Plasma membranes were preincubated for 10 min in the absence or presence of 50 nM PP2. CD32A was then cross-linked as indicated in the Materials and methods section. Samples were probed with an anti-pY antibody. The two major substrates whose tyrosine phosphorylations are enhanced are indicated with arrows.
Solubility of the Src kinases after CD32A cross-linking
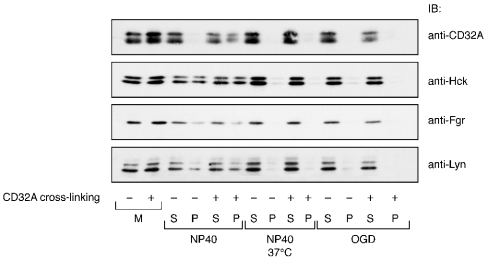
Since we observed the detergent insolubilization of CD32A after its cross-linking, we next compared the partitioning of the three Src kinases and of CD32A between the 15000 g pellets and supernatants derived from resting and cross-linked membranes solubilized under various conditions. Because of their tight lipid packing, DRMs are relatively resistant to solubilization at a low temperature (4 °C) by detergents such as NP40 or Triton X-100; on the other hand, DRMs are solubilized at 37 °C. However, DRMs can be readily solubilized in detergents such as OGD even at 4 °C [32]. The results of these experiments are shown in Figure 8. Samples of the same experiments were blotted independently with antibodies recognizing Lyn, Fgr and Hck and CD32A. In unlabelled NP40-solubilized membranes, the three Src were present in both soluble and insoluble fractions in about equal amounts, whereas CD32A was mainly detected only in the supernatants (solubilized fraction) as shown in Figure 4. CD32A cross-linking did not change the partitioning of the three Src kinases, whereas a fraction of CD32A moved to the insoluble pellet. These results do not support the hypothesis that CD32A and (any of) the Src kinases jointly move to DRMs after cross-linking of the receptor.
Figure 8. Solubilization profiles of CD32A and Src kinases on neutrophil membranes after cross-linking of CD32A.
CD32A was cross-linked on neutrophil membranes as described in the Materials and methods section. Membranes were then solubilized for 20 min in 1% NP40 on ice, 1% NP40 at 37 °C or 100 mM OGD on ice. After centrifugation (15000 g, 10 min), the supernatants (S) and pellets (P) were analysed by SDS/PAGE and probed for CD32A, Hck, Lyn or Fgr as described in the Materials and methods section. M, whole membranes before solubilization in detergents.
After its cross-linking, CD32A could be extracted from the membranes with NP40 at high temperatures and with OGD. The three Src kinases were also fully extracted from the membranes under these two sets of conditions. These results provide pharmacological evidence that the fraction of CD32A that becomes resistant to extraction by non-ionic detergents after cross-linking indeed represents DRMs. They also indicate that a fraction of the three Src kinases are constitutively present in these fractions.
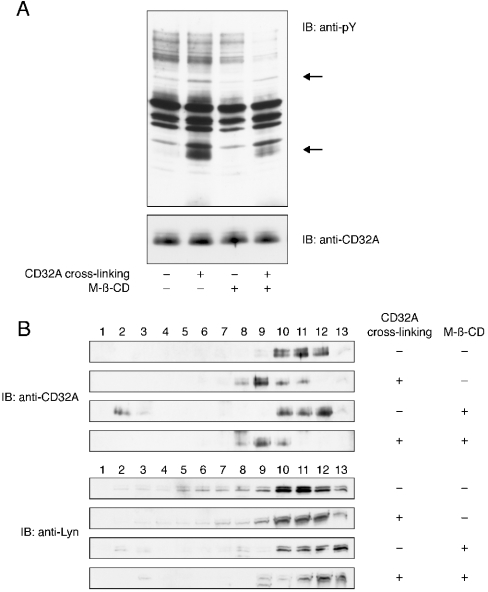
M-β-CD decreases the tyrosine phosphorylation response without affecting the translocation of CD32A
To confirm the re-localization of CD32A to DRMs after its cross-linking, we studied the effect of the cholesterol-chelating agent M-β-CD. As shown in Figure 9(A), incubation of membranes with 20 mM M-β-CD before CD32A cross-linking decreased the tyrosine phosphorylation of the two major substrates Syk and CD32A. However, distribution of the receptor inside the membrane, as analysed by an Optiprep gradient, was not modified, since CD32A is still detected in fractions 8–10 after treatment with M-β-CD (Figure 9B). Since the decrease in the tyrosine phosphorylation pattern observed in Figure 9(A) can be explained by an alteration in the distribution of the tyrosine kinase(s) involved, we also examined the presence of Lyn in the Optiprep gradient fractions after M-β-CD treatment. We observed that Lyn was released from the insoluble portion of the gradient (fractions 2–10) after incubation with M-β-CD.
Figure 9. Effect of M-β-CD on tyrosine phosphorylation and translocation of CD32A to DRMs.
Plasma membranes were preincubated for 20 min in the absence or presence of 20 mM M-β-CD. CD32A was then cross-linked as indicated in the Materials and methods section. (A) Samples were probed with an anti-pY or anti-CD32A antibody. (B) After NP40 solubilization, the membranes were subjected to ultracentrifugation on Optiprep gradients as in Figure 5. Samples were immunoblotted (IB) with a CT10 (anti-CD32A) or anti-Lyn antibody. The two major substrates whose tyrosine phosphorylation is enhanced are indicated with arrows.
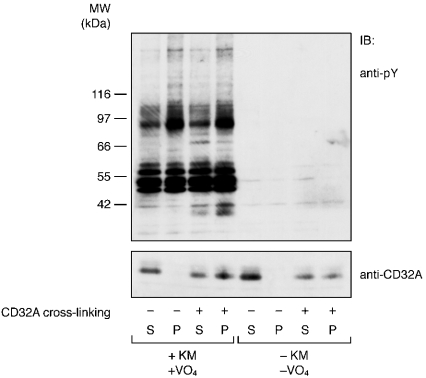
Tyrosine phosphorylation is not essential for the recruitment of CD32A to DRMs
As tyrosine phosphorylation represents a widely described event after CD32A cross-linking in neutrophils, we examined its link to the recruitment of CD32A to DRMs. Our membrane model is well suited to examine this point, since CD32A can be cross-linked without promoting tyrosine phosphorylation events if orthovanadate and/or the kinase mixture are omitted (see Figure 2). The results shown in Figure 10 indicate that translocation of CD32A to DRMs occurred in the absence of tyrosine phosphorylation events, since a similar level of insolubilization of CD32A was observed in membranes in which CD32A was cross-linked in the presence or absence of the kinase mixture and orthovanadate. This result was confirmed with Optiprep gradients analysis, in which we also observed a shift of CD32A to lighter-density fractions after its cross-liking in the absence of the kinase mixture and orthovanadate (results not shown). This is in accord with the lack of effect of the Src kinase inhibitor, 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (referred to as PP1) on the insolubility of CD32A in whole cells [22].
Figure 10. Cross-linking-induced insolubility of CD32A occurs in the absence of tyrosine phosphorylation.
CD32A was cross-linked on neutrophil membranes in the presence (+) or absence (−) of the kinase mixture (KM) and VO4. Membranes were solubilized with 1% NP40 and after centrifugation (15000 g, 10 min), the supernatants (S) and pellets (P) were probed for an anti-pY or anti-CD32A antibody as described in the Materials and methods section.
DISCUSSION
Although phagocytosis is one of the main functions of neutrophils, the early steps of signal transduction leading to particle ingestion and inactivation are still poorly characterized and only rarely investigated directly in this primary cell. We wanted to understand better the molecular and cellular basis of two of the earliest biochemical events observed in response to CD32A activation in neutrophils, namely its tyrosine phosphorylation and insolubilization [22], the study of which in intact neutrophils was limited by the loss of immunoreactivity of the receptor after its activation.
The approach we adopted consisted in the use of successively simpler subcellular systems (granule-poor U-cytoplasts and purified plasma membranes), in which we monitored the tyrosine phosphorylation response as well as the preservation of the immunoreactivity of CD32A. Our results demonstrate that a robust tyrosine phosphorylation response was preserved in both preparations. However, the immunoreactivity of CD32A was maintained only in plasma membranes. After CD32A cross-linking in purified plasma membranes, the tyrosine phosphorylation of the receptor and Syk was observed, and we showed that cross-linked CD32A is recruited from detergent-soluble regions to DRMs of high density. On the other hand, the Src kinases Lyn, Fgr and Hck were constitutively present in detergent-soluble and -insoluble regions of the plasma membrane and this repartition was not modified after CD32A cross-linking.
U-cytoplasts are granule-poor, enucleated neutrophil fragments capable of chemotactic, oxidative and phagocytic responses [33]. Our results showing essentially intact biochemical responses to the cross-linking of CD32A are consistent with the retention of the phagocytic potential by the U-cytoplasts [34]. Our results also demonstrate that the as yet uncharacterized mechanism responsible for the degradation (loss of immunoreactivity) of CD32A was present and functional in these cell fragments and was probably independent of either nuclear signalling or the presence of granule components. Although U-cytoplasts indeed represent a simplified cellular system (compared with intact cells), they did not allow us to bypass the experimental difficulties caused by the stimulated degradation of CD32A. To achieve this goal, we went one step further and designed a ‘semi in vitro system’ that consisted of purified plasma membranes of neutrophils in which we cross-linked CD32A. Our membrane preparation was based on a previously described procedure [25]. Although we did not fully characterize the membrane fractions, gross contamination with cytosol can be ruled out owing to the fact that neither Cbl, a cytosolic protein, nor Tec, a cytosolic tyrosine kinase, were detected in our membrane preparations (results not shown).
The basic findings resulting from our investigations were that purified membranes retained the mechanism for cross-linking-enhanced CD32A insolubilization and tyrosine phosphorylation, but did not retain degradation. Thus plasma membranes contain the basic signalling unit associated with CD32A. As such, this preparation is probably significantly more amenable to immunobiochemical studies when compared with intact cells. To our knowledge, this is the first time that neutrophil membranes have been used to study transduction signals downstream of this receptor. A similar approach could be used to study other neutrophil receptors. It is relevant in this respect to note that aggregation of FcεRI was shown to trigger the tyrosine phosphorylation of the receptor itself in purified plasma membranes of rat basophilic leukaemia RBL-2H3 cells [35].
First, the results obtained with purified membranes allowed us to establish that the tyrosine kinase(s) that phosphorylates the receptor is present in the membrane preparation and, secondly, that no cytoplasmic recruitment of the receptor itself or of other proteins is required for this tyrosine phosphorylation event. In this context, as a control of the validity of our system, we evaluated the ability of the membranes to respond to the chemoattractant fMet-Leu-Phe (N-formylmethionyl-leucylphenylalanine). No tyrosine phosphorylation was observed after the stimulation of membranes with fMet-Leu-Phe (results not shown). This suggests that the receptors for this chemotactic factor, in contrast with CD32A, rely on the recruitment of cytosolic proteins for linking to and activating the tyrosine phosphorylation signalling cascades it is associated with.
Tyrosine phosphorylation reflects the result of tyrosine kinase and phosphatase activities. In our model, since orthovanadate was required to detect the tyrosine phosphorylation of the receptor, we can conclude that one or more tyrosine phosphatase(s) present in the membrane maintained CD32A in a dephosphorylated state. CD45 and CD148 are transmembrane tyrosine phosphatases abundantly present in neutrophils. They have been proposed to regulate neutrophil responses downstream of CD32A and CD16B [36,37], perhaps by regulating Src kinase activities [38]. CD45 has been detected in DRMs in HL-60 cells [39]. The presence of orthovanadate in our assay also explains that the tyrosine phosphorylation level is maintained for up to 15 min. Further experiments to characterize the phosphatase activity responsible for the termination of the response can be performed with our model. Membranes as well as cytosolic phosphatases are probably involved, since SHP-1 (SH2-containing tyrosine phosphatase 1, where SH2 stands for Src homology 2) has been proposed to be involved in the regulation of Fcγ receptor-mediated phagocytosis [40].
We were surprised to find detectable amounts of Syk in the plasma membrane since it is essentially described as a cytosolic protein. However, in monocytic THP-1 cells, which express CD32A just as neutrophils do, Syk was found to be associated with this receptor [41]; on the other hand, in neutrophils, Syk has also been proposed to be associated with CD32A after its cross-linking [42]. In NP40-solubilized membrane, Syk is present in both the soluble and insoluble fractions. Our results differ somewhat from those of Katsumata et al. [19], who could not detect Syk in low-density fractions of HL-60 cells. We should stress that we have focused on a specific subset of DRMs of relatively high density that are distinct from the low-density DRMs commonly investigated. We are currently planning to compare the composition and responsiveness of these two DRM populations. Since Piceatannol, an Syk inhibitor, has little effect on the overall pattern of tyrosine phosphorylation induced after CD32A cross-linking in neutrophils [43], the role of Syk in the signal transduction pathways associated with this receptor remains to be clarified. Because of its SH2 domains and phosphotyrosine residues, the membrane fraction of Syk might serve as a docking protein to initiate the signalling cascade, whereas the cytosolic fraction of Syk might amplify the response.
In contrast, the Src kinase inhibitor PP1 [31] strongly inhibited the tyrosine phosphorylation response observed after CD32A engagement [22], and we observed the same results with the purified membranes preincubated in the presence of PP2 (Figure 7). These and other results [8–10] support a prominent role for Src kinases in the initiation of the tyrosine phosphorylation response induced by CD32A cross-linking. In the present study, we show that the three Src kinases Lyn, Fgr and Hck were present in both the soluble and insoluble fractions of NP40-solubilized membranes and this distribution was not modified after CD32A cross-linking. Hence, a fraction of the three Src kinases was constitutively present in neutrophil DRMs. We confirmed this observation using the Optiprep gradient preparation (Figure 9B).
In contrast, the results of the present study demonstrate that CD32A was recruited from detergent-soluble regions to high-density DRMs after its cross-linking. The step gradient we chose coupled with the 100000 g centrifugation (Figure 6) allowed us to distinguish these high-density DRMs from the soluble proteins despite the similarity between their density values (1.18– 1.2 g/ml). This distinction cannot be made in the sucrose gradients (5–35%) classically used to analyse DRMs because of the narrowness of the density range. It should also be pointed out that the high-density DRMs recovered after the Optiprep ultracentrifugation performed in the present study sediment in the pellet of the classical sucrose-gradient ultracentrifugation [22]. Furthermore, in NP40-solubilized cells, a large amount of cytoskeletal proteins are insoluble and they are recovered in the pellet of the sucrose gradient. These may retain DRMs that non-specifically associate with cytoskeletal components [44]. A recent report [28] showed that high-density DRMs in neutrophils contain membrane skeletal proteins, which increase the ratio of proteins to lipids. In the context of phagocytosis, cytoskeletal proteins present in high-density DRMs with CD32A might be involved in the cytoskeletal reorganization that underlies the phagocytic process.
CD32A was recruited to DRMs after its cross-linking in HL-60 cells [19] just as in U937 cells [45] and neutrophils (the present study). However, we could not detect any change in the distribution of Src kinases in the same membranes under the experimental conditions tested. This contrasts with the results obtained in U937 cells where Lyn became more abundant in DRMs after CD32A cross-linking [45]. These differences may be due to the specific characteristics of DRMs, which probably depend on the function of the cell or its state of differentiation and activation. For example, the composition of gangliosides present in DRMs is markedly different for THP-1 cells and neutrophils, GM1 being very abundant in THP-1 cells and barely detectable in neutrophils [42].
Furthermore, comparison between the results obtained in different investigations is complicated by the fact that the specific experimental conditions utilized (type and concentration of detergent, density-gradient conditions) strongly influence the composition of DRMs [46] and can lead to different interpretations such as the presence or absence of a protein in the DRMs or the existence of protein–protein associations [47]. Furthermore, the results of the experiments illustrated in Figure 4 indicate that a 15000 g supernatant may still contain DRMs that do not sediment at this speed. Consequently, protein–protein associations described in 15000 g supernatants may reflect a co-localization in DRMs rather than a true association.
Since DRMs are enriched in cholesterol, alterations in the cholesterol content of the plasma membrane could lead to their destabilization and/or disruption. We observed that the translocation of CD32A into DRMs was not modified after M-β-CD treatment, whereas the tyrosine phosphorylation response was decreased. The observations reported in the literature concerning the effect of M-β-CD are controversial [48]. Depending on the cell type and the proteins examined, detergent-resistant microdomains have been described to be either disrupted or resistant to M-β-CD. In neutrophils, a flotillin-rich buoyant fraction can still be isolated after M-β-CD treatment [29], and M-β-CD has been shown to enhance the stimulation of the pattern of tyrosine phosphorylation elicited by fMet-Leu-Phe [21]. Our results indicate differences in M-β-CD sensitivity between a protein constitutively present in DRMs (Lyn) and a protein that is translocated to DRMs after activation (CD32A). In the U937 cell line, Kwiatkowska et al. [52] showed that Lyn was completely displaced to the fractions containing the detergent-soluble proteins, whereas CD32A was displaced to intermediate fractions; this result is in accordance with our results and indicates a difference in the M-β-CD sensitivity between these two proteins. The effect of M-β-CD on Lyn (or other Src kinases) may explain the decrease in tyrosine phosphorylation observed in Figure 9(A), since Src kinases are involved in the CD32A signalling pathway. Furthermore, our results underline the diversity of the interactions of proteins with DRMs. Cholesterol is a common component of all DRMs; however, the latter are heterogeneous in terms of their dependence on or interaction with cholesterol. Results may also depend on the cholesterol-modifying agent utilized, since filipin, a cholesterol-sequestering antibiotic, slightly increased the pattern of tyrosine phosphorylation observed in response to CD32A cross-linking in whole neutrophils [22].
In mouse thymocytes, Montixi et al. [49] showed that Src family kinases are required for the recruitment of T-cell receptor to lipid rafts. However, other reports indicate that tyrosine phosphorylation is not required for the association of CD32B with the detergent-insoluble raft fraction in macrophages [50] and for the association of Fcεreceptor with DRMs in RBL-2H3 cells [51]. Concerning CD32A, tyrosine phosphorylation was not required for its recruitment to DRMs in HL-60 cells [19]; however, it was necessary for actin rearrangement and receptor capping in U937 cells [52]. We show in the present study that, in neutrophils, the translocation of CD32A to DRMs was independent of Src kinase activity, since it occurred equally well in the absence of tyrosine phosphorylation (Figure 10). This indicates that recruitment of CD32A to DRMs precedes its tyrosine phosphorylation.
Recent reports [17], including the present one, indicate that several kinds of membrane rafts exist in a single cell, which differ in their lipid/protein ratio (density value) and lipid and protein compositions. CD32A after cross-linking was concentrated in high-density DRMs, whereas Src kinases were present in high- as well as low-density DRMs. We cannot rule out the possibility that a small amount of Src kinases shifts from one kind of DRM to another after CD32A cross-linking. This raises the question of differences in Src kinase activities in the different DRMs. After its cross-linking, CD32A moves to high-density DRMs where it joins Src kinases; therefore Src kinase activities may be stronger in these DRMs than in low-density DRMs. Further experiments focusing on each of the three Src kinases will be performed to elucidate this point.
To our knowledge, this is the first time that the presence of CD32A in DRMs and, more specifically, in high-density DRMs has been demonstrated in human neutrophils. Moreover, the membrane system used in the present study clearly shows the recruitment of CD32A, after its cross-linking, from the NP40-soluble region of the membrane to the DRMs where the receptor is tyrosine-phosphorylated. More and more transmembrane proteins are now described in DRMs. They are mostly palmitoylated molecules, such as the co-receptors CD4 and CD8. It is at present not clear which signal or modification of CD32A targets this protein specifically to DRMs [53,54]. It has been proposed that, after receptor cross-linking, the clustered constituents have a stronger association with raft lipid domains [55].
Further investigations focusing on the characterization of the tyrosine phosphatase(s), the identification of the Src kinase(s) involved and the composition of the DRMs containing CD32A will be necessary to understand fully the mechanism of CD32A activation in neutrophils.
Acknowledgments
This work was partially supported by grants from the Canadian Institutes for Health Research. P. H. N. is a recipient of the Canada Research Chair on the Molecular Physiopathology of the Neutrophil.
References
- 1.Ravetch J. V. Fc receptors: rubor redux. Cell (Cambridge, Mass.) 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 2.Daëron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 3.Ravetch J. V., Bolland S. IgG Fc receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Garcia E., Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 2002;72:1092–1108. [PubMed] [Google Scholar]
- 5.Mitchell M. A., Huang M. M., Chien P., Indik Z. K., Pan X. Q., Schreiber A. D. Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor FcγRIIA: effect on receptor tyrosine phosphorylation and phagocytosis. Blood. 1994;84:1753–1759. [PubMed] [Google Scholar]
- 6.Hunter S., Kamoun M., Schreiber A. D. Transfection of an Fcγ receptor cDNA induces T cells to become phagocytic. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10232–10236. doi: 10.1073/pnas.91.21.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg S. Modular components of phagocytosis. J. Leukoc. Biol. 1999;66:712–717. doi: 10.1002/jlb.66.5.712. [DOI] [PubMed] [Google Scholar]
- 8.Ibarrola I., Vossebeld P. J., Homburg C. H., Thelen M., Roos D., Verhoeven A. J. Influence of tyrosine phosphorylation on protein interaction with FcγRIIA. Biochim. Biophys. Acta. 1997;1357:348–358. doi: 10.1016/s0167-4889(97)00034-7. [DOI] [PubMed] [Google Scholar]
- 9.Ghazizadeh S., Bolen J. B., Fleit H. B. Physical and functional association of Src-related protein tyrosine kinases with FcγRII in monocyte THP-1 cells. J. Biol. Chem. 1994;269:8878–8884. [PubMed] [Google Scholar]
- 10.Hamada F., Aoki M., Akiyama T., Toyoshima K. Association of immunoglobulin G Fc receptor II with Src-like protein-tyrosine kinase Fgr in neutrophils. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6305–6309. doi: 10.1073/pnas.90.13.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley M. T., Costello P. S., Fitzer-Attas C. J., Turner M., Meng F., Lowell C., Tybulewicz V. L., DeFranco A. L. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J. Exp. Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raeder E. M., Mansfield P. J., Hinkovska-Galcheva V., Shayman J. A., Boxer L. A. Syk activation initiates downstream signaling events during human polymorphonuclear leukocyte phagocytosis. J. Immunol. 1999;163:6785–6793. [PubMed] [Google Scholar]
- 13.Chacko G. W., Brandt J. T., Coggeshall K. M., Anderson C. L. Phosphoinositide 3-kinase and p72syk noncovalently associate with the low affinity Fcγ receptor on human platelets through an immunoreceptor tyrosine-based activation motif. Reconstitution with synthetic phosphopeptides. J. Biol. Chem. 1996;271:10775–10781. doi: 10.1074/jbc.271.18.10775. [DOI] [PubMed] [Google Scholar]
- 14.Horejsi V. The roles of membrane microdomains (rafts) in T cell activation. Immunol. Rev. 2003;191:148–164. doi: 10.1034/j.1600-065x.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta N., DeFranco A. L. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol. Biol. Cell. 2003;14:432–444. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field K. A., Holowka D., Baird B. Structural aspects of the association of FcεRI with detergent-resistant membranes. J. Biol. Chem. 1999;274:1753–1758. doi: 10.1074/jbc.274.3.1753. [DOI] [PubMed] [Google Scholar]
- 17.Horejsi V. Membrane rafts in immunoreceptor signaling: new doubts, new proofs? Trends Immunol. 2002;23:562–564. doi: 10.1016/s1471-4906(02)02330-x. [DOI] [PubMed] [Google Scholar]
- 18.Peyron P., Bordier C., N’Diaye E.-N., Maridonneau-Parini I. Nonopsonic phagocytosis of Mycobacterium kansaii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J. Immunol. 2000;165:5186–5191. doi: 10.4049/jimmunol.165.9.5186. [DOI] [PubMed] [Google Scholar]
- 19.Katsumata O., Hara-Yokoyama M., Sautès-Friedman C., Nagatsuka Y., Katada T., Hirabayashi Y., Shimizu K., Fujita-Yoshigaki J., Sugiya H., Furuyama S. Association of FcγRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 2001;167:5814–5823. doi: 10.4049/jimmunol.167.10.5814. [DOI] [PubMed] [Google Scholar]
- 20.Pierini L. M., Eddy R. J., Fuortes M., Seveau S., Casulo C., Maxfield F. R. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 21.Barabé F., Paré G., Fernandes M. J. G., Bourgoin S. G., Naccache P. H. Cholesterol-modulating agents selectively inhibit calcium influx induced by chemoattractants in human neutrophils. J. Biol. Chem. 2002;277:13473–13478. doi: 10.1074/jbc.M112149200. [DOI] [PubMed] [Google Scholar]
- 22.Barabé F., Rollet-Labelle E., Gilbert C., Fernandes M. J. G., Naccache S., Naccache P. H. Early events in the activation of FcγRIIA in human neutrophils: stimulated insolubilization, translocation to detergent-resistant domains, and degradation of FcγRIIA. J. Immunol. 2002;168:4042–4049. doi: 10.4049/jimmunol.168.8.4042. [DOI] [PubMed] [Google Scholar]
- 23.Malawista S. E., Van Blaricom G. Cytoplasts made from human blood polymorphonuclear leukocytes with or without heat: preservation of both motile function and respiratory burst oxidase activity. Proc. Natl. Acad. Sci. U.S.A. 1987;84:454–458. doi: 10.1073/pnas.84.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollet E., Caon A. C., Roberge C. J., Liao N. W., Malawista S. E., McColl S. R., Naccache P. H. Tyrosine phosphorylation in activated human neutrophils. Comparison of the effects of different classes of agonists and identification of the signaling pathways involved. J. Immunol. 1994;153:353–363. [PubMed] [Google Scholar]
- 25.Kjeldsen L., Sengelov H., Borregaard N. Subcellular fractionation of human neutrophils on Percoll density gradients. J. Immunol. Methods. 1999;232:131–143. doi: 10.1016/s0022-1759(99)00171-4. [DOI] [PubMed] [Google Scholar]
- 26.Wessel D., Flugge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 27.Malawista S. E., Van Blaricom G., Chevance de Boisfleury A. Cytokineplasts and U-cytoplasts from human polymorphonuclear leukocytes: role of granule-poor motile fragments in the analysis of cell physiology. Blood Cells. 1993;19:63–80. [PubMed] [Google Scholar]
- 28.Nebl T., Pestonjamasp K. N., Leszyk J. D., Crowley J. L., Oh S. W., Luna E. J. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membrane. J. Biol. Chem. 2002;277:43399–43409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 29.Shao D., Segal A. W., Dekker L. V. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 30.Slaughter N., Laux I., Tu X., Whitelegge J., Zhu X., Effros R., Bickel P., Nel A. The flotillins are integral membrane proteins in lipid rafts that contain TCR-associated signaling components: implications for T-cell activation. Clin. Immunol. 2003;108:138–151. doi: 10.1016/s1521-6616(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 31.Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 32.Cain T. J., Liu Y., Takizawa T., Robinson J. M. Solubilization of glycosylphosphatidylinositol-anchored proteins in quiescent and stimulated neutrophils. Biochim. Biophys. Acta. 1995;1235:69–78. doi: 10.1016/0005-2736(94)00308-c. [DOI] [PubMed] [Google Scholar]
- 33.Malawista S. E., van Blaricom G., Breittenstein M. G. Cryopreservable neutrophil surrogates: stored cytoplasts from human polymorphonuclear leukocytes retain chemotactic, phagocytic, and microbicidal function. J. Clin. Invest. 1989;83:728–732. doi: 10.1172/JCI113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malawista S. E., van Blaricom G. Phagocytic capacity of cytokineplasts from human blood polymorphonuclear leukocytes. Blood Cells. 1986;12:167–177. [PubMed] [Google Scholar]
- 35.Priluba V. S., Metzger H. Transmembrane signaling by the high-affinity IgE receptor on membrane preparations. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11446–11450. doi: 10.1073/pnas.89.23.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hundt M., Schmid R. E. Functional characterization of receptor-type protein tyrosine phosphatase CD148 (HPTPη/DEP-1) in Fcγ receptor IIa signal transduction of human neutrophils. Eur. J. Immunol. 1997;27:3532–3535. doi: 10.1002/eji.1830271255. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmeyer F., Witte K., Gebhardt U., Schmidt R. E. The low affinity FcγRIIa and FcγRIIIb on polymorphonuclear neutrophils are differentially regulated by CD45 phosphatase. J. Immunol. 1995;155:4016–4023. [PubMed] [Google Scholar]
- 38.Thomas M. L., Brown E. J. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol. Today. 1999;20:406–411. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 39.Parolini I., Sargiacomo M., Lisanti M. P., Peschle C. Signal transduction and glycophosphatidylinositol-linked proteins (Lyn, Lck, CD4, CD45, G-proteins and CD55) selectively localize in triton-insoluble plasma membrane domains of human leukemic cell lines and normal granulocytes. Blood. 1996;87:3783–3794. [PubMed] [Google Scholar]
- 40.Kant A. M., De P., Peng X., Yi T., Rawlings D. J., Kim J. S., Durden D. L. SHP-1 regulates Fcγ receptor-mediated phagocytosis and the activation of RAC. Blood. 2002;100:1852–1859. [PubMed] [Google Scholar]
- 41.Ghazizadeh S., Bolen J. B., Fleit H. B. Tyrosine phosphorylation and association of Syk with FcγRII in monocytic THP-1 cells. Biochem. J. 1995;305:669–674. doi: 10.1042/bj3050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbert C., Rollet-Labelle E., Caon A. C., Naccache P. H. Immunoblotting and sequential lysis protocols for the analysis of tyrosine phosphorylation-dependent signaling. J. Immunol. Methods. 2002;271:185–201. doi: 10.1016/s0022-1759(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 43.Desaulniers P., Fernandes M. J. G., Gilbert C., Bourgoin S. G., Naccache P. H. Crystal-induced neutrophil activation. VII. Involvement of Syk in the responses to monosodium urate crystals. J. Leukoc. Biol. 2001;70:659–668. [PubMed] [Google Scholar]
- 44.Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell (Cambridge, Mass.) 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 45.Kwiatkowska K., Sobota A. The clustered Fcγ receptor II is recruited to Lyn-containing membrane domain and undergoes phosphorylation in a cholesterol-dependent manner. Eur. J. Immunol. 2001;31:989–998. doi: 10.1002/1521-4141(200104)31:4<989::aid-immu989>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 46.Schuck S., Honsho M., Ekroos K., Shevchenko A., Simons K. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korzeniowski M., Kwiatkowska K., Sobota A. Insights into the association of FcγRII and TCR with detergent-resistant membrane domains: isolation of the domains in detergent-free density gradients facilitates membrane fragment reconstitution. Biochemistry. 2003;42:5358–5367. doi: 10.1021/bi027135x. [DOI] [PubMed] [Google Scholar]
- 48.Rajendran L., Masilamani M., Solomon S., Tikkanen R., Stuermer C. A. O., Plattner H., Illges H. Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8241–8246. doi: 10.1073/pnas.1331629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montixi C., Langlet C., Bernard A. M., Thimonier J., Dubois C., Wurbel M. A., Chauvin J. P., Pierres M., He H. T. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kono H., Suzuki T., Yamamoto K., Okada M., Yamamoto T., Honda Z. Spatial raft coalescence represents an initial step in FcγR signaling. J. Immunol. 2002;169:193–203. doi: 10.4049/jimmunol.169.1.193. [DOI] [PubMed] [Google Scholar]
- 51.Field K. A., Holowka D., Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J. Biol. Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- 52.Kwiatkowska K., Frey J., Sobota A. Phosphorylation of FcγRIIA is required for the receptor-induced actin rearrangement and capping: the role of membrane rafts. J. Cell Sci. 2003;116:537–550. doi: 10.1242/jcs.00254. [DOI] [PubMed] [Google Scholar]
- 53.Kenworthy A. Peering inside lipid rafts and caveolae. Trends Biochem. Sci. 2002;27:435–438. doi: 10.1016/s0968-0004(02)02178-3. [DOI] [PubMed] [Google Scholar]
- 54.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 55.Harder T., Scheiffele P., Verkade P., Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]