Abstract
Agonists that deplete intracellular Ca2+ stores also activate Ca2+ entry, although the mechanism by which store release and Ca2+ influx are linked is unclear. A potential mechanism involves ‘store-operated channels’ that respond to depletion of the intracellular Ca2+ pool. Although SOCE (store-operated Ca2+ entry) has been considered to be the principal route for Ca2+ entry during hormonal stimulation of non-electrically excitable cells, recent evidence has suggested that alternative pathways activated by metabolites such as arachidonic acid are responsible for physiological Ca2+ influx. It is not clear whether such messenger-activated pathways exist in all cells, whether they are truly distinct from SOCE and which metabolites are involved. In the present study, we demonstrate that HeLa cells express two pharmacologically and mechanistically distinct Ca2+ entry pathways. One is the ubiquitous SOCE route and the other is an arachidonate-sensitive non-SOCE. We show that both these Ca2+ entry pathways can provide long-lasting Ca2+ elevations, but that the channels are not the same, based on their differential sensitivity to 2-aminoethoxydiphenyl borate, LOE-908 {(R,S)-(3,4-dihydro-6,7-dimethoxy-isochinolin-1-yl)-2-phenyl-N,N-di[2-(2,3,4-trimethoxyphenyl)ethyl]acetamid mesylate} and gadolinium. In addition, non-SOCE and not SOCE was permeable to strontium. Furthermore, unlike SOCE, the non-SOCE pathway did not require store depletion and was not sensitive to displacement of the endoplasmic reticulum from the plasma membrane using jasplakinolide or ionomycin pretreatment. These pathways did not conduct Ca2+ simultaneously due to the dominant effect of arachidonate, which rapidly curtails SOCE and promotes Ca2+ influx via non-SOCE. Although non-SOCE could be activated by exogenous application of arachidonate, the most robust method for stimulation of this pathway was application of the widely used calmodulin antagonist calmidazolium, due to its ability to activate phospholipase A2.
Keywords: arachidonic acid, calcium, calmidazolium, calmodulin, inositol, phospholipase
Abbreviations: AA, arachidonic acid; ACA, N-(p-amylcinnamoyl) anthranillic acid; 2-APB, 2-aminoethoxydiphenyl borate; CaM, calmodulin; DAG, diacylglycerol; GFP, green fluorescent protein; EGFP, enhanced GFP; EM, extracellular medium; ER, endoplasmic reticulum; Ins(1,4,5)P3R, inositol 1,4,5-trisphosphate receptor; LOE-908, (R,S)-(3,4-dihydro-6,7-dimethoxy-isochinolin-1-yl)-2-phenyl-N,N-di[2-(2,3,4-trimethoxyphenyl)ethyl]acetamid mesylate; PKC, protein kinase C; PLA2, phospholipase A2; PLC, phospholipase C; RyR, ryanodine receptor; SOCE, store-operated Ca2+ entry; TFP, trifluoperazine; TRPC, canonical transient receptor potential; W-7, N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide
INTRODUCTION
Calcium (Ca2+) is a universal and versatile intracellular messenger that regulates a diverse range of cellular processes [1]. Cells have access to two sources of calcium, finite stores located in intracellular organelles and a more substantial pool of extracellular Ca2+. In many cells types, release of Ca2+ from intracellular stores leads to the activation of a Ca2+ influx pathway denoted as SOCE (store-operated Ca2+ entry) [2]. This mechanism for promoting Ca2+ entry is responsible for replenishing depleted intracellular stores and prolonging cellular Ca2+ signals [3]. The molecular identity of the channels responsible for SOCE and their precise mechanism of activation are unclear. The probable candidates are members of the TRPC (canonical transient receptor potential) family [3,4]. Several studies support this hypothesis and have shown that the expression or ablation of various TRPC isoforms can modulate SOCE responses (see e.g. [5–7]).
A prominent model for the stimulation of TRPs during SOCE activation proposes a direct interaction between these plasma membrane channels and Ca2+ release channels on the intracellular stores. The latter would serve to sense a decrease in luminal Ca2+ concentration, and via a conformational change transmit this information to promote SOCE channel opening [2,3,8,9]. A direct interaction between TRPs and two families of intracellular Ca2+ release channel, Ins(1,4,5)P3Rs (inositol 1,4,5-trisphosphate receptors) and RyRs (ryanodine receptors) has been demonstrated [10,11] (see [3,12] for reviews). When the Ca2+ concentration on the luminal domain of Ins(1,4,5)P3Rs or RyRs is sufficiently low, this interaction can lead to TRP channel activation. Interventions that prevent the coupling of intracellular channels with TRPs, using either peptides that prevent the interaction (see e.g. [13]) or formation of a subplasmalemmal actin barrier [14,15], inhibit SOCE activation.
SOCE can be readily demonstrated in almost all non-electrically excitable cells and in some excitable cells after hormonal/neurotransmitter stimulation or depletion of intracellular Ca2+ stores using SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) inhibitors, such as thapsigargin [2,3,9,12]. The broad expression of the SOCE pathway has led to it being considered a prominent mechanism for Ca2+ entry into cells after Ca2+ pool discharge. However, it is becoming increasingly apparent that stimulation of cells with hormones that invoke the production of Ca2+-releasing messengers not only activates SOCE, but can also promote additional Ca2+ entry pathways [16,17].
When Ins(1,4,5)P3 is produced after phosphoinositide hydrolysis, there is a concomitant production of DAG (diacylglycerol). Unlike water-soluble Ins(1,4,5)P3, DAG stays in the plane of the plasma membrane where it can activate PKC (protein kinase C) or is metabolized further. Both PKC and DAG (or membrane-permeant analogues) have been demonstrated to cause Ca2+ influx distinct from SOCE in some cell types [18,19]. Furthermore, other messengers resulting from DAG metabolism, including AA (arachidonic acid) and leukotrienes, activate non-SOCE Ca2+ influx [20–22]. The molecular target of these messengers is not established, although TRPC isoforms have again been implicated. Although there are an increasing number of reports demonstrating significant Ca2+ influx via non-SOCE, at present, it is not clear whether all cells employ a non-SOCE mechanism, and if so, in response to which messenger. Probably, the best-characterized non-SOCE activator is AA. Studies from both the Shuttleworth [21] and Taylor [20] labs have demonstrated pathways for AA-stimulated Ca2+ entry that are clearly distinct from SOCE.
CaM (calmodulin) plays a critical role in transducing the effects of cytosolic Ca2+ signals on cellular processes. It is also involved in the regulation of many channels that generate Ca2+ signals. For example, two of the most prominent mechanisms for releasing intracellular Ca2+ stores, via Ins(1,4,5)P3Rs and RyRs, have been demonstrated to be regulated by CaM [23,24]. For Ins(1,4,5)P3Rs this is a largely negative effect, whereas for RyRs CaM can mediate both inhibition and activation (see [25,26] for reviews). CaM has also been demonstrated to modulate negatively Ca2+ influx through SOCE and TRP channels [27–30].
A common approach in investigating the action of CaM has been to use membrane-permeant pharmacological antagonists such as calmidazolium, W-7 [N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide] and TFP (trifluoperazine). However, these agents have been shown to cause robust Ca2+ signals albeit through unknown mechanisms. Calmidazolium, for example, has been shown to cause Ca2+ release and Ca2+ entry in Dictyostelium [31], platelets [32], thyroid FRTL-5 cells [33], Madin–Darby canine kidney cells [34] and HL-60 cells [35]. These responses could be consistent with CaM causing a tonic inhibition of Ca2+ channels, which is relieved during activation or application of CaM antagonists.
We investigated the mechanism by which agents such as calmidazolium triggered Ca2+ increases using HeLa cells. Calmidazolium was capable of evoking Ca2+ signals that fully mimicked those stimulated by Ins(1,4,5)P3-generating agonists in all respects except for the pathway through which Ca2+ influx occurred, i.e. via non-SOCE and not via SOCE.
MATERIALS AND METHODS
HeLa cell culture
HeLa cell culture was performed as described previously [36]. All experimental procedures were performed at room temperature (20–22 °C). Before imaging, the culture medium was replaced with an EM (extracellular medium) containing (mM): NaCl, 121; KCl, 5.4; MgCl2, 0.8; CaCl2, 1.8; NaHCO3, 6; D-glucose, 5.5; Hepes, 25 (pH 7.3). Histamine, calmidazolium, U73122 and thapsigargin were obtained from Sigma. Fura 2 was obtained from Molecular Probes (Eugene, OR, U.S.A.). Statistics were performed using Student's t test (GraphPad Prism, San Diego, CA, U.S.A.).
Video imaging
Measurement of cytosolic Ca2+ in HeLa cells was performed by monitoring fura 2 fluorescence of cells adhered to glass cover-slips using either a Spex or a PerkinElmer imaging system. Fura 2 was loaded into the cells by incubation with 2 μM fura 2 acetoxymethyl ester (30 min incubation followed by a 30 min period for de-esterification). For the Spex system, cells on coverslips were mounted on a Nikon Diaphot inverted epi-fluorescence microscope. Fluorescent images were obtained by alternate 40 ms excitations at 340 and 380 nm using twin xenon arc lamps each coupled with a spex monochrometer (Spex Industries, Edison, NJ, U.S.A.), with the wavelengths being switched by a rotating chopper mirror (Glen Creston Instruments, Stanmore, U.K.). Emission signals at 510 nm were collected using an intensified charge-coupled device video camera (Photonics Science, Robertson, U.K.) and filtered with a 200 ms time constant (Spex system) before off-line storage for analysis using an Imagine image processing system (Synoptics, Cambridge, U.K.).
With the PerkinElmer system, a single glass coverslip with adherent cells was mounted on the stage of a Nikon Diaphot 300 inverted epi-fluorescence microscope coupled with a xenon arc lamp (Nikon, Tokyo, Japan) light source. Fluorescence images were obtained with alternate excitations at 340 and 380 nm, selected for using either a Sutter filter wheel (340HT15 and 380HT15; Sutter Industries, Novarta, CA, U.S.A.) or a Spectramaster II monochrometer. Emitted light was filtered at 510 nm and collected by a cooled Astrocam digital camera. The acquired images were stored and subsequently processed off-line with Ultraview software (PerkinElmer LifeSciences, Great Shelford, Cambridge, U.K.).
Mn2+ entry was measured indirectly by recording the quench of fura 2 fluorescence when excited at 360 nm. To minimize the effect of contaminating Ca2+, Mn2+-containing Hepes buffer was supplemented with 1 mM EGTA and the Mn2+ concentration was adjusted to avoid chelation (2 mM Mn2+ and free Mn2+ ∼1 mM). MaxChelator (http://www.stanford.edu/~cpatton/maxc.html) was used to match free and total chelator and metals in all solutions containing Mn2+, Gd3+ and Sr2+.
Results are expressed as means±S.E.M. Representative traces are shown for most of the experiments. These depict the most consistent pattern of response from multiple cells imaged in at least three independent experiments, with experiments repeated on different days. Statistical significance was calculated using Student's t test.
Expression of type 1 Ins(1,4,5)P3 5′-phosphatase
The cDNA encoding the type 1 Ins(1,4,5)P3 5′-phosphatase was amplified by PCR from a cDNA clone kindly provided by Professor C. Erneux (Université Libre de Bruxelles, Brussels, Belgium) and subcloned into pdc515-EGFP-C1 (where EGFP stands for enhanced green fluorescent protein). All constructs were transiently transfected using GeneJuice™ (Novagen, Nottingham, U.K.), according to the manufacturer's instructions. Briefly, 24 h before transfection, cells were seeded on to 22 mm glass coverslips at 50–80% confluency. Cells were incubated for 24 h post-transfection at 37 °C, in 5% CO2 atmosphere with saturated humidity to allow expression of the construct. GFP-tagged actin was transfected into cells using the same procedure.
Jasplakinolide treatment
To stimulate cortical actin polymerization, cells were incubated for 30 min at 22 °C, with 10 μM jasplakinolide in nominally Ca2+ free solution. To confirm the effect of jasplakinolide on actin rearrangement, images of cells expressing GFP-tagged actin were acquired using a Bio-Rad MRC1024 confocal laser-scanning microscope (×60 objective; 1.4 NA). GFP was excited using a 488 nm laser line and emission was collected through a 505 nm long-pass filter. Optical sections were taken every 0.4 μm. Image analysis and processing were performed with the public domain software ImageJ (NIH, http://rsb.info.nih.gov.ij).
Preparation and storage of AA
Porcine liver AA (Na+ salt; Calbiochem) was dissolved in MilliQ water as a concentrated stock solution before being dispensed in aliquots; they were then frozen and stored at −20 °C in a light-resistant container. Before use, an aliquot of AA was diluted to the required concentration by addition of EM containing 0.1% DMSO (to aid membrane permeability). All samples of AA were kept in the dark and on ice to suppress oxidation.
Use of Ba2+ as a Ca2+ surrogate
For some experiments, Ba2+ was used to monitor cation entry in place of Ca2+. Ba2+ had been used in previous studies as a Ca2+ surrogate to provide a measure of unidirectional cation flux, since it was poorly sequestered by intracellular Ca2+ pumps or extruded from cells. Increases in cytosolic Ba2+ were monitored using fura 2 as described above, using the same excitation and emission settings as for Ca2+. The results are presented as uncalibrated ratio (emission at 340 nm/excitation at 380 nm) units.
For the experiments shown in Figure 8, Ba2+ was employed simply because we could not use calcium with ionomycin-treated cells, since it would be impossible to identify Ca2+ that entered cells through the ionophore or via SOCE/non-SOCE. In our experiments, Ba2+ did not permeate into cells via ionomycin.
Figure 8. Cortical actin deposition or ionomycin-induced ER fragmentation blocks SOCE, but not calmidazolium-evoked non-SOCE.
HeLa cells were treated with jasplakinolide as described in the Materials and methods section to induce the redistribution of actin from the cell centre to the periphery. The effect of jasplakinolide treatment is shown in (A). The left- and right-hand sides of the panel depict cells before and after jasplakinolide treatment respectively. Two-dimensional images and axial projections are shown to indicate the redistribution of actin. The axial projection was taken across the portion of the image between the white arrowheads. (B) Effect of pretreating cells with jasplakinolide or ionomycin on SOCE and their ineffectiveness on non-SOCE. After incubation with jasplakinolide or control buffer, the cells were stimulated with 1 μM thapsigargin in Ca2+-free medium for 5 min to activate SOCE. For the ionomycin-treated cells, thapsigargin was not added since the ionophore by itself should have been sufficient to deplete the Ca2+ stores and activate SOCE.
RESULTS
CaM antagonist calmidazolium evokes calcium release and activates a non-SOCE
CaM has been proposed to suppress the activation of both Ins(1,4,5)P3Rs and SOCE channels. A common method of antagonizing the action of CaM is to use membrane-permeant pharmacological antagonists, such as calmidazolium, W-7 and TFP. Consistent with the idea that CaM antagonists relieve CaM-dependent inhibition of Ca2+ channels, these compounds have all been demonstrated to cause Ca2+ signals within intact cells. However, it has not been established whether their cellular target is CaM or other processes involved in Ca2+ signal transduction. We therefore determined whether there was a direct effect of CaM antagonists on the activity of Ins(1,4,5)P3Rs and SOCE channels. We initially concentrated on the action of the imidazole compound calmidazolium, since this has been widely used to abrogate effects of CaM and has also been shown to activate Ca2+ mobilization and Ca2+ entry.
When applied in Ca2+-free medium, calmidazolium evoked a rapid transient increase in cytosolic Ca2+ (Figure 1A; average peak response 462±44 nM; n=126 cells), which was followed by robust calcium entry when extracellular calcium was replaced (average response 935±71 nM; n=126 cells). Effects of calmidazolium were reversible, since pulsatile application caused repetitive Ca2+ responses (Figure 1B). Calmidazolium evoked concentration-dependent Ca2+ increases (Figure 1C), with all cells responding to doses of calmidazolium >2 μM (n>600 cells from 20 independent experiments). At concentrations ≤1 μM calmidazolium, global Ca2+ responses were not observed. Instead, subcellular Ca2+ transients that we, and others, have described as ‘Ca2+ puffs’ [37] were evoked (results not shown).
Figure 1. Characterization of calmidazolium-evoked Ca2+ transients.
(A) Ca2+ mobilization and entry induced by calmidazolium in HeLa cells are depicted. Cells were perfused with 10 μM calmidazolium in a nominally Ca2+-free medium before being perfused with 10 μM calmidazolium in the presence of extracellular Ca2+. (B) Reversibility of cytosolic Ca2+ increase activated by a maximal calmidazolium concentration (2 min application followed by a 12 min continuous wash) is shown. The concentration–response relationship for calmidazolium-induced Ca2+ signalling is depicted in (C). To distinguish the concentration dependence for calmidazolium-mediated SOCE activation versus stimulation of the non-SOCE pathway, 100 μM 2-APB was added as shown in the key. In the presence of 2-APB and absence of extracellular Ca2+ (denoted Ca2+o in the Figure), calmidazolium had a negligible effect on cellular Ca2+. Results indicate means±S.E.M. for three independent experiments with at least ten cells analysed per experiment for each condition. (D, E) Overlap between the histamine- and calmidazolium-releasable Ca2+ pool. The traces in (A, B, D and E) were obtained from single HeLa cells and are typical of at least 20 cells analysed in three independent experiments.
The Ca2+ pool mobilized by calmidazolium overlapped largely with the Ins(1,4,5)P3-sensitive Ca2+ store. Application of a maximal histamine concentration (100 μM) in Ca2+-free medium to deplete the Ins(1,4,5)P3-sensitive Ca2+ pools decreased the response to a subsequent addition of calmidazolium (10 μM) usually to a single low-amplitude Ca2+ transient (Figure 1D). In the reciprocal experiment, application of a maximal calmidazolium concentration (10 μM) in Ca2+-free medium substantially reduced the magnitude of response observed with an ensuing histamine challenge (Figure 1E). When applied to naive cells, 100 μM histamine evoked an average peak Ca2+ increase in 763±12 nM (n=49 cells). After calmidazolium treatment, histamine responses were reduced to 283±16 nM (n=37 cells). With cells in Ca2+-free medium, but not Ca2+-containing medium, calmidazolium frequently triggered Ca2+ oscillations that were similar in rise time and duration to those observed during hormonal stimulation (cf. Figures 1D and 1E).
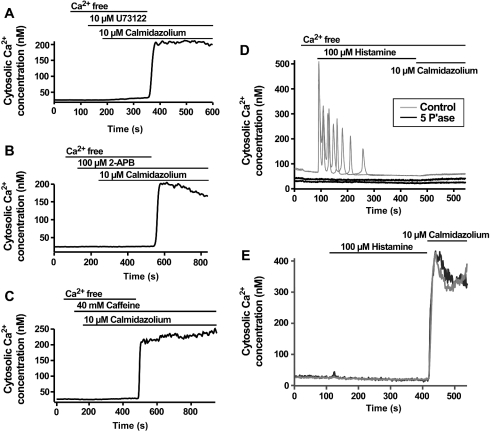
Calmidazolium-activated Ca2+ signals described above were comparable in almost all respects with typical responses triggered by hormonal stimulation of HeLa cells [38]. Similar to hormonal agonists, it appeared that calmidazolium was capable of releasing the Ins(1,4,5)P3-sensitive Ca2+ store and triggering Ca2+ entry. To determine the mechanism by which calmidazolium could activate Ca2+ release, we examined the effects of well-known inhibitors of PLC (phospholipase C) and Ins(1,4,5)P3Rs. Application of the PLC inhibitor U73122 at a concentration that blocks agonist-evoked Ca2+ signalling in HeLa cells [39] completely inhibited Ca2+ mobilization by calmidazolium (Figure 2A). Whereas all cells responded to 10 μM calmidazolium under control conditions (see above), no cells displayed Ca2+ mobilization in response to 10 μM calmidazolium in the presence of U73122. The inactive analogue U73343 did not prevent Ca2+ release activated by 10 μM calmidazolium (n=60 cells; results not shown). 2-APB (2-aminoethoxydiphenyl borate), which we have demonstrated previously to inhibit Ins(1,4,5)P3-induced Ca2+ release in HeLa cells [40], also prevented Ca2+ mobilization by calmidazolium (Figure 2B). Caffeine, which acts as a combined blocker of PLC and Ins(1,4,5)P3Rs, also prevented calmidazolium-evoked Ca2+ release (Figure 2C). Heterologous expression of the 5′-phosphatase enzyme, which degrades Ins(1,4,5)P3 to the non-Ca2+ releasing metabolite Ins(1,4)P2, prevented Ca2+ mobilization in response to either a maximal histamine concentration (100 μM) or a maximal calmidazolium dose (10 μM; Figure 2D). Since calmidazolium has been shown previously to activate PLC activity [34], the simplest interpretation of the results presented above is that calmidazolium mobilizes Ca2+ stores by activation of PLC. Therefore, with respect to Ca2+ release from Ins(1,4,5)P3Rs, calmidazolium mimics an Ins(1,4,5)P3-generating agonist.
Figure 2. Activation of Ca2+ entry by calmidazolium does not require Ca2+ store release.
(A–C) Lack of Ca2+ release after calmidazolium application if Ins(1,4,5)P3 production or Ins(1,4,5)P3Rs are inhibited using pharmacological agents. (D, E) A similar inhibition of calmidazolium-induced Ca2+ release was observed in cells overexpressing the Ins(1,4,5)P3 metabolizing 5′-phosphatase enzyme (denoted 5 P′ase in the Figure). Note that the control traces in (D) have been shifted upwards by 50 nM to allow clearer visualization of the responding control and non-responding test traces. There was no difference in the basal Ca2+ levels between control and 5′-phosphatase-expressing cells. Representative traces from two individual cells are shown for both the control and 5′-phosphatase-expressing cells. Although overexpression of the 5′-phosphatase enzyme blocks calmidazolium-induced Ca2+ release, the traces in (E) show that calmidazolium could still activate Ca2+ influx. The responses of two representative 5′-phosphatase-expressing cells are shown. Histamine was added during the experiment to confirm the abolition of Ins(1,4,5)P3-induced Ca2+ signals by the 5′-phosphatase expression. The traces in (A–C) were obtained from single HeLa cells and are typical of at least 20 cells analysed in three independent experiments.
Although the mobilization of Ins(1,4,5)P3-sensitive Ca2+ stores by calmidazolium can be ascribed to activation of PLC, the entry of Ca2+ in calmidazolium-treated cells appears to occur via a distinct mechanism. None of the agents that blocked calmidazoliuminduced Ca2+ mobilization (Figures 2A–2C) inhibited Ca2+ entry when Ca2+ was reapplied to cells. In addition, expression of the 5′-phosphatase enzyme to levels that completely abrogated histamine-evoked Ca2+ signals and prevented calmidazolium causing Ca2+ release (Figure 2D), did not inhibit calmidazolium-induced Ca2+ entry (Figure 2E). Furthermore, similar to previous results [41], we have demonstrated that 2-APB completely inhibited SOCE in HeLa cells [40], yet it did not prevent calmidazolium-induced Ca2+ influx (Figure 2B). These results indicate that calmidazolium activated a Ca2+ entry pathway that was distinct from that triggered by store depletion.
Calmidazolium-activated non-SOCE is pharmacologically distinct from SOCE
Calmidazolium induced an additional increase in the plateau Ca2+ level after full SOCE activation by application of a maximal concentration of thapsigargin (2 μM; Figure 3A), which was completely blocked by the cation channel inhibitor LOE-908 {(R,S)-(3,4-dihydro-6,7-dimethoxy-isochinolin-1-yl)-2-phenyl-N,N-di[2-(2,3,4-trimethoxyphenyl)ethyl]acetamid mesylate} (Figure 3B). In contrast, LOE-908 gave only a modest inhibition of Ca2+ entry stimulated by thapsigargin (Figures 3C and 3D).
Figure 3. LOE-908 inhibits calmidazolium-induced Ca2+ entry, but not SOCE.
(A–D) The responses of individual HeLa cells to the agents are denoted by the bars. For each panel, the response is typical of at least 20 cells analysed in three independent experiments.
Gadolinium (Gd3+) has been used previously as a high-affinity irreversible inhibitor of SOCE (see e.g. [42]). We similarly observed that 1 μM Gd3+ completely blocked Ca2+ influx evoked by application of thapsigargin (Figure 4A). However, Gd3+ had only a partial effect on calmidazolium-induced Ca2+ entry when applied at concentrations ≤10 μM (Figure 4B). The weak effect of Gd3+ on calmidazolium-evoked bivalent cation entry was also observed by substituting extracellular Ca2+ with manganese (Mn2+) and by following the quenching of fura 2 fluorescence as Mn2+ entered the cells. Calmidazolium evoked a significant increase in the rate of Mn2+ entry (Figure 4C), which was unaffected by the presence of Gd3+ (Figure 4D). A further difference in cation sensitivity of the two influx pathways was observed when Ca2+ in the EM was substituted by strontium (Sr2+). The SOCE pathway in HeLa cells did not appreciably conduct Sr2+, whereas Sr2+ entry was readily detectable after calmidazolium treatment (Figure 4E). A summary of the pharmacological distinction between SOCE and the Ca2+ entry pathway activated by calmidazolium is shown in Figure 4(F).
Figure 4. Characteristics of calmidazolium-activated non-SOCE.
(A) Complete inhibitory effect of 1 μM Gd3+ on SOCE, with little effect on calmidazolium-evoked non-SOCE is illustrated. The concentration–response relationship for the effect of Gd3+ on SOCE and non-SOCE-mediated Ca2+ entry is shown in (B). The solid line depicts the effect of Gd3+ on thapsigargin (TG)-evoked SOCE, whereas the broken line illustrates the corresponding effect on calmidazolium-activated non-SOCE. (C, D) Calmidazolium activated entry of Mn2+ into HeLa cells, despite the presence of Gd3+ at a concentration that completely inhibits SOCE. (E) SOCE pathway in HeLa cells is not permeable to Sr2+, but entry of this cation can be achieved by application of calmidazolium. A summary of the pharmacological profiles of the SOCE and non-SOCE cation entry pathways is depicted in (F). The traces shown in this Figure were obtained from individual HeLa cells and are indicative of similar responses in at least 20 cells from three independent experiments.
During prolonged activation, SOCE-mediated Ca2+ influx diminishes despite intracellular Ca2+ stores remaining empty (see e.g. [43,44]; Figure 5A). This has been demonstrated to be due to progressive inhibition of the Ca2+ entry pathway and acceleration of Ca2+ sequestration [44]. Approx. 20 min after stimulation with thapsigargin, the cytosolic Ca2+ concentration had returned to the resting level. Subsequent application of calmidazolium provoked a steep increase in Ca2+ (Figure 5A), which was abolished by LOE-908 (results not shown). By itself, LOE-908 had little effect on the time course of thapsigargin-evoked SOCE (results not shown). Although the amplitude of both SOCE- and calmidazolium-evoked Ca2+ entries varied between cells, the latter pathway generally produced larger Ca2+ signals, as illustrated in Figure 5(B). For the experiment depicted in Figure 5(B), the mean peak amplitudes for Ca2+ entry through SOCE- and calmidazolium-sensitive pathway were 117±33 and 275±36 nM respectively (n=30; P<0.002).
Figure 5. Calmidazolium can activate Ca2+ influx after run-down of SOCE.
Prolonged activation of SOCE leads to a progressive decrease in Ca2+ to basal levels. (A) Application of calmidazolium activated Ca2+ entry despite run-down of SOCE is shown. (B) Method for estimating the relative amplitude of SOCE- and non-SOCE-mediated Ca2+ signals.
In a recent study, Bolotina and co-workers [45] utilized calmidazolium to activate SOCE in smooth-muscle cells. They demonstrated that low concentrations of calmidazolium specifically activated SOCE, as judged by complete abolition of Ca2+ influx with 2-APB. At higher concentrations of calmidazolium, they found that the Ca2+ entry was insensitive to 2-APB. We observed a similar concentration-dependent action of calmidazolium. 2-APB suppressed responses to low (≤2.5 μM), but not high (≥5 μM), calmidazolium concentrations (Figure 1C). Similar to Bolotina and co-workers, we interpret these results as indicating that calmidazolium has distinct effects at low and high concentrations. Low concentrations activate SOCE, whereas higher doses switch the Ca2+ entry to 2-APB-insensitive non-SOCE.
Bolotina and co-workers [45] suggested that low calmidazolium concentrations activated SOCE in the absence of calcium release. In contrast, using real-time confocal microscopy, we observed that Ca2+ release was the earliest response of the cells to calmidazolium. As described above, concentrations of ≤1 μM calmidazolium triggered Ca2+ puffs, which can lead to Ca2+ waves [37]. Therefore the most sensitive response of cells to calmidazolium is Ca2+ release, not Ca2+ entry.
Calmidazolium activates the same non-SOCE pathway as AA in HeLa cells
Various messengers and metabolites have been shown to activate Ca2+ influx independently of Ca2+ store release, including PKC, DAG and AA. We therefore used established pharmacological agents to probe the contribution of these moieties in calmidazolium-activated Ca2+ entry.
Treatment of cells with the PKC activators PMA (1 μM; n=50 cells) or 1-oleoyl-2-acetyl-sn-glycerol (1 μM; n=90 cells) neither caused Ca2+ increase nor prevented Ca2+ response to calmidazolium when perfused on to cells for up to 30 min. The DAG lipase inhibitor, RHC-80267, has been used in previous studies (see e.g. [20]) to abrogate the release of AA from DAG and thus prevent the subsequent activation of an AA-sensitive Ca2+ influx. However, pretreatment of HeLa cells with RHC-80267 (50 μM; 15 min preincubation; n=25 cells) did not affect the amplitude or extent of calmidazolium-induced Ca2+ entry.
Mammalian cells express a diverse array of PLA2 (phospholipase A2) subtypes [46]. In the present study, three PLA2 inhibitors, ACA [N-(p-amylcinnamoyl) anthranillic acid], isotetrandrine and AACOCF3 (arachidonyltrifluoromethyl ketone), were used to investigate the role of PLA2 in the activation of Ca2+ entry by calmidazolium in HeLa cells. Isotetrandrine, an inhibitor of G-protein-linked PLA2 (10 μM; 20 min preincubation; [21]) had no effect on calmidazolium-induced Ca2+ signals. Similarly, AACOCF3, a selective inhibitor of cytosolic (85 kDa) PLA2 (100 μM; 20 min preincubation) did not affect Ca2+ signals evoked by calmidazolium. In contrast, 10 μM ACA, which blocks Ca2+-independent PLA2 activity [47], rapidly and reversibly inhibited both Ca2+ mobilization and Ca2+ entry evoked by calmidazolium (Figure 6A). The ability of ACA to prevent calmidazolium-induced Ca2+ signals implicates AA or one of its metabolites as the critical messenger(s) underlying the activation of the non-SOCE Ca2+ influx pathway described above. Consistent with the notion that calmidazolium stimulates PLA2 and that the resultant AA production activates Ca2+ signalling, direct application of 10 μM AA to cells in the presence of ACA evoked both Ca2+ mobilization and Ca2+ entry (Figure 6B).
Figure 6. Calmidazolium-induced Ca2+ signalling is inhibited by the Ca2+-independent PLA2 inhibitor ACA.
(A) The reversible inhibition of calmidazolium-evoked Ca2+ release and influx by ACA is depicted. (B) That AA could still release Ca2+ and activate non-SOCE in the presence of ACA, indicating that the PLA2 inhibitor was not blocking non-SOCE channels is illustrated. The traces are taken from individual cells and are typical of at least 30 cells from three independent experiments.
To confirm that AA and calmidazolium were activating the same Ca2+ influx pathway, we examined the pharmacological profile and cation sensitivity of the Ca2+ signals evoked by direct AA application. Similar to calmidazolium, Ca2+ entry stimulated by 10 μM AA was not inhibited by 100 μM 2-APB (Figure 7A) or 10 μM U73122 (Figure 7B) or sensitive to expression of the 5′-phosphatase enzyme (n=10 cells). Similar to the calmidazolium-provoked Ca2+ entry after run-down of SOCE (Figure 5A), AA evoked a substantial Ca2+ entry after the decrease in SOCE (Figure 7C), and this AA-induced Ca2+ entry was completely inhibited by LOE-908 (30 μM; n=35 cells). The AA-activated pathway was also permeable to Sr2+ (Figure 7D) and not blocked by acute addition of 1 μM Gd3+ (n=120 cells). In all respects, the Ca2+ influx pathway activated by AA resembled that observed with calmidazolium stimulation. Neither linolenic acid (n=60 cells) nor linoleic acid (n=32 cells) activated Ca2+ influx.
Figure 7. AA activates a non-SOCE pathway in HeLa cells.
The panels in this Figure depict the action of AA in evoking Ca2+ influx with a similar pharmacology to calmidazolium-induced non-SOCE. The traces in (A–D) are taken from individual cells and are representative of at least 30 cells from three independent experiments for each panel.
Although the experiments presented above indicate that AA production can stimulate the non-SOCE pathway, they do not implicate AA itself as the sole activator since it can be readily metabolized. We therefore examined the effect of inhibiting pathways responsible for AA metabolism. Lipoxygenase and cyclo-oxygenase enzymes convert AA into leukotrienes and prostaglandins, prostacyclins and thromboxanes respectively. Incubation of cells with the cyclo-oxygenase inhibitor indomethacin (10 μM; 25–90 min incubation in different experiments) did not alter basal Ca2+ levels or prevent Ca2+ signals evoked by calmidazolium (n=45 cells). Similarly, the lipoxygenase inhibitor aspirin (100 μM; 30–90 min incubation in different experiments) had no effect (n=30 cells). Finally, metabolism of AA via mono-oxygenases was prevented by using 100 μM metyrapone, which also did not alter Ca2+ mobilization or Ca2+ influx in response to calmidazolium (n=20 cells). Calmidazolium belongs to a family of substituted imidazole compounds including econazole and miconazole. These compounds have a similar structure to calmidazolium, and may also affect CaM, although they are more commonly employed as inhibitors of cytochrome P450 mono-oxygenases. As with calmidazolium, 20 μM econazole and 20 μM miconazole evoked Ca2+ mobilization and Ca2+ entry. Co-application of maximal concentrations (10 μM calmidazolium, 20 μM econazole and 20 μM miconazole) of these imidazole compounds indicated that their Ca2+ responses were not additive (results not shown), suggesting that they activated the same Ca2+ influx pathway.
Mechanistic differences between SOCE- and calmidazolium-activated non-SOCE
To distinguish further between SOCE and the AA-activated Ca2+ entry pathway in HeLa cells, we examined the effect of remodelling the cytoskeleton to produce a cortical actin deposition. This technique was used previously to inhibit SOCE activation [14,15,48]. Reorganization of the actin cytoskeleton was achieved by incubating HeLa cells with jasplakinolide (10 μM; 30 min preincubation), and monitored by confocal imaging of EGFP–actin-transfected cells. In control cells, there was a largely homogeneous pool of fluorescence, reflecting monomeric proteins, but with fluorescent stress fibres also visible. After treatment with jasplakinolide, the EGFP-tagged actin redistributed to the subplasmalemmal region of cells (Figure 8A). This treatment abolished the entry of barium (Ba2+), which was used as a slowly permeant surrogate for Ca2+ through the SOCE pathway (Figure 8B). However, it did not prevent Ba2+ entry stimulated by calmidazolium (Figure 8B).
Similar to previous results [49], we have observed that incubation of cells with ionomycin causes retraction of the ER (endoplasmic reticulum) from the plasma membrane and fragmentation into discrete vesicles [50]. In Ca2+-containing medium, such ionomycin-induced ER fragmentation and retraction occurs within approx. 5 min to 100% of cells examined (n>500). Since the entry of Ca2+ itself cannot be measured in cells where ionomycin is present, we examined the effect of ER disruption on Ca2+ entry using Ba2+. Despite the fact that ionomycin discharges the ER Ca2+ store and thus should activate SOCE, Ba2+ did not appear to permeate into ionomycin-treated cells by any detectable level. In contrast, addition of 10 μM calmidazolium stimulated Ba2+ entry significantly. These results indicate that the status and proximity of the ER to the plasma membrane can modulate Ca2+ entry through SOCE, but not the Ca2+ influx pathway activated by calmidazolium.
Effects of W-7 and TFP on Ca2+ signalling
We examined the effects of other structurally unrelated CaM antagonists to explore the possibility that activation of the non-SOCE pathway was somehow linked to their common action of CaM inhibition. In the absence of extracellular Ca2+, both 300 μM W-7 and 10 μM TFP induced Ca2+ release from intracellular stores, which was followed by Ca2+ entry when extracellular Ca2+ was restored (results not shown). Unlike calmidazolium, W-7 was capable of releasing Ca2+ stores despite the presence of 100 μM 2-APB and activate Ca2+ entry in the presence of either 2-APB or LOE-908. TFP activated an LOE-908-sensitive Ca2+ influx, but unlike the substantial and robust Ca2+ influx signals evoked by calmidazolium, TFP triggered highly variable modest Ca2+ changes (results not shown). The distinct characteristics of the Ca2+ signals evoked by the CaM antagonists suggests that they have different modes of action, and that their ability to increase cytosolic Ca2+ is not due to their common ability to antagonize CaM.
DISCUSSION
Previous studies have demonstrated that application of CaM antagonists elicit Ca2+ signals, and proposed that this is due to relief of CaM-dependent Ca2+ channel inhibition. We similarly observed that calmidazolium evoked robust Ca2+ signals, but found that this action was due to stimulation of PLC and PLA2. Calmidazolium-evoked responses in HeLa cells closely mimicked the profile of Ca2+ signals evoked by a PLC-coupled agonist (Figure 1). The release of Ca2+ from intracellular stores was blocked by U73122 and caffeine (Figure 2), established antagonists of PLC-mediated Ins(1,4,5)P3 production. Furthermore, calmidazolium released Ca2+ from the same pool as histamine (Figures 1D and 1E), in an Ins(1,4,5)P3-dependent manner (Figure 2D). Activation of PLC and production of Ins(1,4,5)P3 therefore appears to underlie the release of intracellular Ca2+ stores by calmidazolium.
In addition to stimulation of PLC, calmidazolium activated a non-SOCE pathway. This Ca2+ entry mechanism was demonstrably different from SOCE based on its pharmacological profile (Figures 2A–2C, 3B and 4A–4D), ion selectivity (Figure 4E), lack of sensitivity to remodelling actin in a cortical ring (Figure 8B) or ionomycin-induced fragmentation and retraction of the ER (Figure 8B). The activation of non-SOCE by calmidazolium appeared to be due to stimulation of PLA2 since inhibition of this enzyme completely prevented Ca2+ influx in response to calmidazolium. Furthermore, the product of PLA2 activation, AA, was capable of stimulating a non-SOCE pathway with exactly the same pharmacological profile (Figures 7A and 7B) and ion selectivity (Figure 7D) as calmidazolium itself. Inhibition of lipoxygenase, cyclo-oxygenase and mono-oxygenase pathways did not alter the ability of calmidazolium to trigger Ca2+ influx, suggesting that AA is the messenger responsible for the activation of non-SOCE.
Of all the metabolites demonstrated to activate non-SOCE, possibly the most commonly effective moiety is AA. This polyunsaturated fatty acid has been demonstrated to activate Ca2+ influx in a variety of different cell types [7,20–22,51,52]. In the present study, we observed that addition of AA to cells rapidly inhibited SOCE and switched the mode of Ca2+ influx to LOE-908-sensitive non-SOCE (Figure 7C). Calmidazolium also switched the pathway of Ca2+ influx from SOCE to LOE-908-sensitive non-SOCE (Figure 3B), although it caused a more rapid changeover when compared with AA. These results suggest that AA is a dominant Ca2+ influx effector over the SOCE mechanism, and that the pathways do not function in an additive manner. We found that both calmidazolium and AA could activate Ca2+ entry despite an ongoing SOCE response (e.g. Figures 3A and 7C). Addition of either of the compounds at the peak or plateau phase of a thapsigargin response, triggered non-SOCE, suggesting that this mechanism is not particularly sensitive to ambient Ca2+ levels. It therefore appears to us that the exclusivity of these Ca2+ influx pathways reflects the dominance of AA, it shuts off SOCE and promotes non-SOCE.
Our results have some similarity to the recent study of Bolotina and co-workers [45], who demonstrated that low concentrations of calmidazolium stimulated 2-APB-sensitive SOCE, whereas higher doses of calmidazolium invoked a 2-APB-insensitive Ca2+ influx. Similar to the results of the present study, Bolotina and co-workers suggested that the key target of calmidazolium was PLA2. They focused on the effects of low calmidazolium concentrations and suggested that the lysophospholipids produced by PLA2 caused SOCE activation. We have characterized the Ca2+ entry generated by higher concentrations of calmidazolium and found that AA probably plays a significant role. Calmidazolium therefore appears to be capable of activating both SOCE and non-SOCE depending on its concentration. Both effects stem from the activation of PLA2, but may depend on the balance between lysophospholipids and AA.
Although they are widely used, cellular actions of CaM antagonists are not fully resolved. The consistent observation that they increase cytosolic Ca2+ is problematic considering that they are generally used to antagonize downstream actions of Ca2+. Concentration-dependent Ca2+ increases have been reported using various cell types in response to CaM antagonists including TFP, W-7, fendiline, chlorpromazine as well as calmidazolium. For most of these agents, the mechanism by which they cause Ca2+ signals is not known. Calmidazolium is perhaps one of the best understood and is known to affect Ins(1,4,5)P3Rs [53], SERCA1 Ca2+ pumps [54] and L-type voltage-operated Ca2+ channels [55]. Consistent with our observations, calmidazolium has been demonstrated to cause Ins(1,4,5)P3 production through activation of PLC in several cell types (see e.g. [34,56]). Furthermore, calmidazolium activated Ca2+ release and Ca2+ entry in Madin–Darby canine kidney cells, with the effects being abolished by the PLA2 inhibitor aristolochic acid [34].
Accumulating evidence suggests that CaM is a significant regulator of SOCE activity. CaM may prevent Ca2+ entry by interfering with the physical coupling of Ins(1,4,5)P3Rs and SOCE channels. C-terminal portions of different TRPCs have been demonstrated to bind CaM or an N-terminal sequence of Ins(1,4,5)P3Rs in a mutually exclusive manner [27,57,58]. Displacement of CaM by short peptides corresponding to the competing region of Ins(1,4,5)P3Rs leads to activation of TRP channels [30]. CaM therefore appears to act as a barrier in the activation of Ca2+ entry by occluding the site through which TRPs and Ins(1,4,5)P3Rs interact. Consistent with this notion, application of CaM antagonists (W-7 and TFP) significantly reduced the delay in development of SOCE after store depletion, whereas increasing CaM concentration caused the converse [28]. Furthermore, calmidazolium was capable of enhancing TRP activity by displacing CaM from the site where it prevented interaction with Ins(1,4,5)P3Rs [30,57]. For Ins(1,4,5)P3Rs, CaM can bind to an N-terminal region and decrease the ability of Ins(1,4,5)P3 to activate the channels [24,59]. In addition, CaM may be responsible for the negative feedback effects of increased cytosolic Ca2+ on Ins(1,4,5)P3R activation [23]. CaM therefore can provide both a tonic and a dynamic inhibitory influence on Ca2+ release and entry in non-electrically excitable cells.
Although we suppose that the ability of calmidazolium to release Ca2+ and activate non-SOCE was not due to CaM antagonism, we did observe an effect of calmidazolium on SOCE, which is consistent with the proposed inhibitory action of CaM. Activation of SOCE by thapsigargin allows the entry of Ca2+ or Ba2+ into HeLa cells. Ba2+ is a useful surrogate, since it is poorly transported by cellular Ca2+ pumps and therefore provides a unidirectional measure of cation entry. In the presence of ACA to prevent activation of non-SOCE, calmidazolium increased the rate of thapsigargin-stimulated Ba2+ entry into cells by approx. 7-fold. In the absence of calmidazolium, the initial rate of Ba2+ entry was 0.19±0.04 ratio units/min (n=5; measured using fura 2; see the Materials and methods section). In the presence of 10 μM calmidazolium, the rate of Ba2+ entry was 1.26±0.21 ratio units/min (n=6). It therefore appears that calmidazolium does potentiate SOCE by working as a conventional CaM antagonist.
In summary, we have demonstrated that HeLa cells express two distinct Ca2+ entry pathways, one regulated by store depletion and the other by AA. These pathways do not operate simultaneously due to the dominant effect of AA, which inhibits SOCE and promotes non-SOCE. The non-SOCE pathway can be directly activated by exogenous AA application, but in HeLa cells it is also robustly stimulated by calmidazolium. The effect of calmidazolium in promoting Ca2+ release and non-SOCE is not due to CaM antagonism, but rather due to activation of PLC and PLA2. At present the function of the non-SOCE pathway in HeLa cells is not clear. Our attempts to find cellular agonists that couple with this pathway have so far proved negative. Histamine, ATP and serum all strongly activate Ca2+ release in HeLa cells, which is followed by Ca2+ entry. However, the Ca2+ influx was sensitive to 1 μM Gd3+, which is indicative of SOCE. In addition, LOE-908 does not affect prolonged Ca2+ oscillations activated by histamine (results not shown). Calmidazolium can stimulate Ca2+ oscillations, but only in Ca2+-free medium when the non-SOCE pathway is not transporting Ca2+ into the cells. Therefore, rather than supporting Ca2+ oscillations, the non-SOCE channel appears to compromise the ability of HeLa cells to display repetitive Ca2+ spikes.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (Swindon, U.K.). M.D.B. gratefully acknowledges the support of a Royal Society Fellowship.
References
- 1.Berridge M. J., Bootman M. D., Roderick H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Putney J. W., Jr, Broad L. M., Braun F. J., Lievremont J. P., Bird G. S. Mechanisms of capacitative calcium entry. J. Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 4.Nilius B. From TRPs to SOCs, CCEs, and CRACs: consensus and controversies. Cell Calcium. 2003;33:293–298. doi: 10.1016/s0143-4160(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 5.Freichel M., Suh S. H., Pfeifer A., Schweig U., Trost C., Weissgerber P., Biel M., Philipp S., Freise D., Droogmans G., et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat. Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 6.Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ. Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 7.Wu X., Babnigg G., Zagranichnaya T., Villereal M. L. The role of endogenous human Trp4 in regulating carbachol-induced calcium oscillations in HEK-293 cells. J. Biol. Chem. 2002;277:13597–13608. doi: 10.1074/jbc.M110881200. [DOI] [PubMed] [Google Scholar]
- 8.Irvine R. F. ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates – a possible mechanism. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 9.Berridge M. J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiselyov K. I., Shin D. M., Wang Y., Pessah I. N., Allen P. D., Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol. Cell. 2000;6:421–431. doi: 10.1016/s1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 11.Kiselyov K., Xu X., Mozhayeva G., Kuo T., Pessah I., Mignery G., Zhu X., Birnbaumer L., Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature (London) 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaumer L., Boulay G., Brown D., Jiang M., Dietrich A., Mikoshiba K., Zhu X., Qin N. Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog. Horm. Res. 2000;55:127–161. discussion 161–162. [PubMed] [Google Scholar]
- 13.Boulay G., Brown D. M., Qin N., Jiang M., Dietrich A., Zhu M. X., Chen Z., Birnbaumer M., Mikoshiba K., Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson R. L., van Rossum D. B., Gill D. L. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell (Cambridge, Mass.) 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- 15.Rosado J. A., Sage S. O. Activation of store-mediated calcium entry by secretion-like coupling between the inositol 1,4,5-trisphosphate receptor type II and human transient receptor potential (hTrp1) channels in human platelets. Biochem. J. 2001;356:191–198. doi: 10.1042/0264-6021:3560191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bootman M. D., Berridge M. J., Roderick H. L. Calcium signalling: more messengers, more channels, more complexity. Curr. Biol. 2002;12:R563–R565. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 17.Shuttleworth T. J. What drives calcium entry during [Ca2+]i oscillations? Challenging the capacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- 18.Rosado J. A., Sage S. O. Protein kinase C activates non-capacitative calcium entry in human platelets. J. Physiol. (Cambridge U.K.) 2000;529:159–169. doi: 10.1111/j.1469-7793.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tesfai Y., Brereton H. M., Barritt G. J. A diacylglycerol-activated Ca2+ channel in PC12 cells (an adrenal chromaffin cell line) correlates with expression of the TRP-6 (transient receptor potential) protein. Biochem. J. 2001;358:717–726. doi: 10.1042/0264-6021:3580717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moneer Z., Taylor C. W. Reciprocal regulation of capacitative and non-capacitative Ca2+ entry in A7r5 vascular smooth muscle cells: only the latter operates during receptor activation. Biochem. J. 2002;362:13–21. doi: 10.1042/0264-6021:3620013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mignen O., Thompson J. L., Shuttleworth T. J. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J. Biol. Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 22.Peppelenbosch M. P., Tertoolen L. G., den Hertog J., de Laat S. W. Epidermal growth factor activates calcium channels by phospholipase A2/5-lipoxygenase-mediated leukotriene C4 production. Cell (Cambridge, Mass.) 1992;69:295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- 23.Missiaen L., Parys J. B., Weidema A. F., Sipma H., Vanlingen S., De Smet P., Callewaert G., De Smedt H. The bell-shaped Ca2+ dependence of the inositol 1,4,5-trisphosphate-induced Ca2+ release is modulated by Ca2+/calmodulin. J. Biol. Chem. 1999;274:13748–13751. doi: 10.1074/jbc.274.20.13748. [DOI] [PubMed] [Google Scholar]
- 24.Michikawa T., Hirota J., Kawano S., Hiraoka M., Yamada M., Furuichi T., Mikoshiba K. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 25.Nadif Kasri N., Bultynck G., Sienaert I., Callewaert G., Erneux C., Missiaen L., Parys J. B., De Smedt H. The role of calmodulin for inositol 1,4,5-trisphosphate receptor function. Biochim. Biophys. Acta. 2002;1600:19–31. doi: 10.1016/s1570-9639(02)00440-5. [DOI] [PubMed] [Google Scholar]
- 26.Taylor C. W., Laude A. J. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32:321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- 27.Singh B. B., Liu X., Tang J., Zhu M. X., Ambudkar I. S. Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Mol. Cell. 2002;9:739–750. doi: 10.1016/s1097-2765(02)00506-3. [DOI] [PubMed] [Google Scholar]
- 28.Vaca L., Sampieri A. Calmodulin modulates the delay period between release of calcium from internal stores and activation of calcium influx via endogenous TRP1 channels. J. Biol. Chem. 2002;277:42178–42187. doi: 10.1074/jbc.M204531200. [DOI] [PubMed] [Google Scholar]
- 29.Boulay G. Ca2+-calmodulin regulates receptor-operated Ca2+ entry activity of TRPC6 in HEK-293 cells. Cell Calcium. 2002;32:201–207. doi: 10.1016/s0143416002001550. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., Tang J., Tikunova S., Johnson J. D., Chen Z., Qin N., Dietrich A., Stefani E., Birnbaumer L., Zhu M. X. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlatterer C., Schaloske R. Calmidazolium leads to an increase in the cytosolic Ca2+ concentration in Dictyostelium discoideum by induction of Ca2+ release from intracellular stores and influx of extracellular Ca2+ Biochem. J. 1996;313:661–667. doi: 10.1042/bj3130661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luckhoff A., Bohnert M., Busse R. Effects of the calmodulin antagonists fendiline and calmidazolium on aggregation, secretion of ATP, and internal calcium in washed human platelets. Naunyn Schmiedebergs Arch. Pharmacol. 1991;343:96–101. doi: 10.1007/BF00180683. [DOI] [PubMed] [Google Scholar]
- 33.Tornquist K., Ekokoski E. Inhibition of agonist-mediated calcium entry by calmodulin antagonists and by the Ca2+/calmodulin kinase II inhibitor KN-62. Studies with thyroid FRTL-5 cells. J. Endocrinol. 1996;148:131–138. doi: 10.1677/joe.0.1480131. [DOI] [PubMed] [Google Scholar]
- 34.Jan C. R., Tseng C. J. Calmidazolium-induced rises in cytosolic calcium concentrations in Madin–Darby canine kidney cells. Toxicol. Appl. Pharmacol. 2000;162:142–150. doi: 10.1006/taap.1999.8844. [DOI] [PubMed] [Google Scholar]
- 35.Harper J. L., Daly J. W. Effect of calmidazolium analogs on calcium influx in HL-60 cells. Biochem. Pharmacol. 2000;60:317–324. doi: 10.1016/s0006-2952(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 36.Bootman M. D., Taylor C. W., Berridge M. J. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1992;267:25113–25119. [PubMed] [Google Scholar]
- 37.Bootman M. D., Lipp P., Berridge M. J. The organisation and functions of local Ca2+ signals. J. Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 38.Bootman M. D., Berridge M. J. Subcellular Ca2+ signals underlying waves and graded responses in HeLa cells. Curr. Biol. 1996;6:855–865. doi: 10.1016/s0960-9822(02)00609-7. [DOI] [PubMed] [Google Scholar]
- 39.Bobanovic F., Bootman M. D., Berridge M. J., Parkinson N. A., Lipp P. Elementary [Ca2+]i signals generated by electroporation functionally mimic those evoked by hormonal stimulation. FASEB J. 1999;13:365–376. doi: 10.1096/fasebj.13.2.365. [DOI] [PubMed] [Google Scholar]
- 40.Peppiatt C. M., Collins T. J., Mackenzie L., Conway S. J., Holmes A. B., Bootman M. D., Berridge M. J., Seo J. T., Roderick H. L. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium. 2003;34:97–108. doi: 10.1016/s0143-4160(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 41.Bootman M. D., Collins T. J., Mackenzie L., Roderick H. L., Berridge M. J., Peppiatt C. M. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 42.Broad L. M., Cannon T. R., Taylor C. W. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J. Physiol. (Cambridge U.K.) 1999;517:121–134. doi: 10.1111/j.1469-7793.1999.0121z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett D. L., Bootman M. D., Berridge M. J., Cheek T. R. Ca2+ entry into PC12 cells initiated by ryanodine receptors or inositol 1,4,5-trisphosphate receptors. Biochem. J. 1998;329:349–357. doi: 10.1042/bj3290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madge L., Marshall I. C., Taylor C. W. Delayed autoregulation of the Ca2+ signals resulting from capacitative Ca2+ entry in bovine pulmonary artery endothelial cells. J. Physiol. (Cambridge U.K.) 1997;498:351–369. doi: 10.1113/jphysiol.1997.sp021863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smani T., Zakharov S. I., Csutora P., Leno E., Trepakova E. S., Bolotina V. M. A novel mechanism for the store-operated calcium influx pathway. Nat. Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 46.Balsinde J., Winstead M. V., Dennis E. A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 47.Simonsson E., Karlsson S., Ahren B. Ca2+-independent phospholipase A2 contributes to the insulinotropic action of cholecystokinin-8 in rat islets: dissociation from the mechanism of carbachol. Diabetes. 1998;47:1436–1443. doi: 10.2337/diabetes.47.9.1436. [DOI] [PubMed] [Google Scholar]
- 48.Ma H. T., Patterson R. L., van Rossum D. B., Birnbaumer L., Mikoshiba K., Gill D. L. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 49.Pedrosa Ribeiro C. M., McKay R. R., Hosoki E., Bird G. S., Putney J. W., Jr Effects of elevated cytoplasmic calcium and protein kinase C on endoplasmic reticulum structure and function in HEK293 cells. Cell Calcium. 2000;27:175–185. doi: 10.1054/ceca.2000.0108. [DOI] [PubMed] [Google Scholar]
- 50.Roderick H. L., Collins T. J., Berridge M. J., Bootman M. D. Elevated cytosolic calcium leads to remodelling of mitochondria and the endoplasmic reticulum. J. Physiol. (Cambridge U.K.) 2003;547P:PC33. [Google Scholar]
- 51.Shuttleworth T. J., Thompson J. L. Discriminating between capacitative and arachidonate-activated Ca2+ entry pathways in HEK293 cells. J. Biol. Chem. 1999;274:31174–31178. doi: 10.1074/jbc.274.44.31174. [DOI] [PubMed] [Google Scholar]
- 52.Chyb S., Raghu P., Hardie R. C. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature (London) 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 53.Khan S. Z., Dyer J. L., Michelangeli F. Inhibition of the type 1 inositol 1,4,5-trisphosphate-sensitive Ca2+ channel by calmodulin antagonists. Cell. Signal. 2001;13:57–63. doi: 10.1016/s0898-6568(00)00140-6. [DOI] [PubMed] [Google Scholar]
- 54.Khan S. Z., Longland C. L., Michelangeli F. The effects of phenothiazines and other calmodulin antagonists on the sarcoplasmic and endoplasmic reticulum Ca2+ pumps. Biochem. Pharmacol. 2000;60:1797–1806. doi: 10.1016/s0006-2952(00)00505-0. [DOI] [PubMed] [Google Scholar]
- 55.Sunagawa M., Yokoshiki H., Seki T., Nakamura M., Laber P., Sperelakis N. Direct block of Ca2+ channels by calmidazolium in cultured vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 1999;34:488–496. doi: 10.1097/00005344-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Lin W. W. Potentiation of stimulus-induced phosphoinositide breakdown by calmodulin antagonists in C6 glioma cells. Naunyn Schmiedebergs Arch. Pharmacol. 1995;352:679–684. doi: 10.1007/BF00171328. [DOI] [PubMed] [Google Scholar]
- 57.Tang J., Lin Y., Zhang Z., Tikunova S., Birnbaumer L., Zhu M. X. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J. Biol. Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trost C., Bergs C., Himmerkus N., Flockerzi V. The transient receptor potential, TRP4, cation channel is a novel member of the family of calmodulin binding proteins. Biochem. J. 2001;355:663–670. doi: 10.1042/bj3550663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel S., Morris S. A., Adkins C. E., O'Beirne G., Taylor C. W. Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulin: redistribution of calmodulin as a possible means of regulating Ca2+ mobilization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11627–11632. doi: 10.1073/pnas.94.21.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]