Abstract
Cortactin was first identified over a decade ago, and its initial characterization as both an F-actin binding protein and v-Src substrate suggested that it was likely to be a key regulator of actin rearrangements in response to tyrosine kinase signalling. The recent discovery that cortactin binds and activates the actin related protein (Arp)2/3 complex, and thus regulates the formation of branched actin networks, together with the identification of multiple protein targets of the cortactin SH3 domain, have revealed diverse cellular roles for this protein. This article reviews current knowledge regarding the role of cortactin in signalling to the actin cytoskeleton in the context of these developments.
Keywords: actin-related protein (Arp2/3) complex, cell motility, endocytosis, Src, suppressor of cAMP receptor (SCAR)/WASP family verprolin homologous (WAVE), Wiskott–Aldrich syndrome protein (WASP)
Abbreviations: Abp1, actin-binding protein 1; Arp, actin-related protein; ADF-H, actin depolymerizing factor homology; CCND1, cell cycle regulatory protein cyclin D1; CD2AP, CD2-associated protein; CortBP1, cortactin binding protein 1; EC MLCK, endothelial cell myosin light-chain kinase; EGF, epidermal growth factor; EHEC, enterohaemorrhagic Escherichia coli; EPEC, enteropathogenic E. coli; EVH1, Ena VASP homology 1; Fgd1, faciogenital dysplasia 1; GBD, GTPase binding domain; GEF, guanine nucleotide exchange factor; GST, glutathione S-transferase; GK(AP), guanylate kinase (associated protein); HGF, hepatocyte growth factor; Hip1R, Huntingtin-interacting protein 1-related; HP, helical and proline-rich (region); HS1, haematopoietic lineage cell-specific protein 1; MEK, MAP kinase/ERK kinase; NMDA, N-methyl-D-aspartate; NPF, nucleation promoting factor; NTA, N-terminal acidic (domain); PAK, p21-activated kinase; PDGF, platelet-derived growth factor; PDZ, PSD-95/Dlg/ZO-1; PIP2, phosphatidylinositol 4,5 bisphosphate; PSD, post-synaptic density; SCAR, suppressor of cAMP receptor; SH, Src homology; WASP, Wiskott–Aldrich syndrome protein; N-WASP, neural WASP; WAVE, WASP family verprolin homologous; WCA, WH2-central–acidic region; WH, WASP homology; WIP, WASP-interacting protein; ZO-1, zonnula occludens 1
INTRODUCTION
Dynamic actin networks perform a critical role in a variety of cellular processes requiring plasma membrane remodelling or intracellular movement of vesicles or particles [1–4]. For example, lamellipodia are thin, sheet-like protrusive structures formed at the leading edge of migrating cells, and consist of a lattice-like meshwork of actin filaments. Membrane ruffles are closely related structures which form on the dorsal surface of cells or if non-adherent lamellipodia fold back over the dorsal surface. Filopodia are thin, cylindrical projections consisting of bundled, crosslinked actin filaments. Formation of these structures at the leading edge of cells, together with coordinated retraction of the tail-end, are fundamental to cell motility [1,2]. The underlying cytoskeletal reorganization is regulated by Rho family GTPases, with the Rac subfamily promoting membrane ruffling and the formation of lamellipodia, Cdc42-like GTPases stimulating the extension of filopodia, and Rho proteins regulating actomyosin contractility [2,5]. In addition, the actin cytoskeleton is implicated in the membrane remodelling and vesicle movement involved in endocytosis [3], and specific pathogens utilize branched actin networks to form ‘comet tails’ which drive intracellular motility [4].
Actin polymerization can be initiated by the uncapping of actin filaments to expose their barbed ends, severing of filaments to create new barbed ends or de novo nucleation by the actin related protein (Arp)2/3 complex [6]. The latter plays a major role in the rapid induction of cortical actin polymerization involved in plasma membrane remodelling, as well as the formation of actin structures that regulate vesicle and pathogen motility. The activity of the Arp2/3 complex can be stimulated by actin nucleation promoting factors (NPFs). Two important classes of NPFs are WASP (Wiskott–Aldrich syndrome protein) and SCAR/WAVE (suppressor of cAMP receptor/WASP family verprolin homologous) proteins, which provide a link between Cdc42 and Rac activation respectively, and stimulation of actin nucleation [7]. As described in this review, cortactin also possesses NPF activity and serves to link a variety of cellular proteins to the Arp2/3 complex, and hence dynamic actin networks.
CORTACTIN CHARACTERISTICS: AN OVERVIEW
Structure
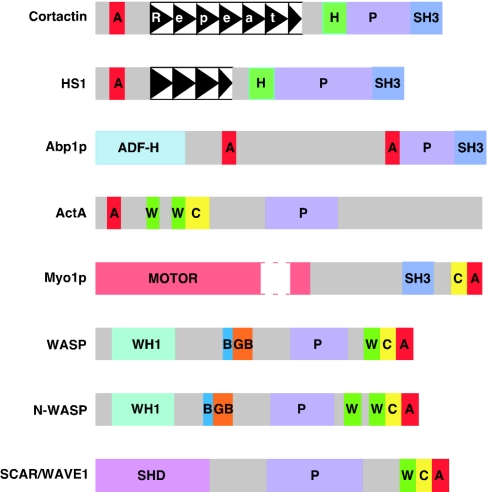
Human, rodent and avian cortactins exhibit a conserved molecular architecture and high sequence similarity [8–11]. The N-terminal region of approx. 90 amino acids lacks clear structural determinants, but harbours a stretch of 21–22 amino acids rich in acidic residues, and is designated the N-terminal acidic domain (NTA) (Figure 1) [12]. As described in more detail later, the NTA contains a conserved tryptophan-containing motif (DDW in cortactin), which is characteristic of NPFs such as WASP, N-WASP (neural WASP), SCAR/WAVE proteins, yeast myosin-I homologues and Listeria ActA that interact with the Arp2/3 complex [7,12]. This is followed by 6.5 tandem copies of a 37 amino acid repeat. Secondary structure predictions suggest that these tandem repeats exhibit a helix–turn–helix structure. In the rat, two additional isoforms of cortactin exist, cortactin B (lacking the sixth repeat) and cortactin C (lacking the fifth and sixth repeats) [11]. Corresponding human isoforms (SV1-cortactin and SV2-cortactin respectively) have recently been characterized; these are derived by alternative mRNA splicing of exons 10 and 11 of the human gene encoding cortactin, designated EMS1 [13]. Although the cortactin-type repeats are conserved in invertebrate cortactins, Drosophila cortactin only contains 4 tandem copies [14,15]. The repeats are followed by an α-helical domain of approx. 50 amino acids, a less well-conserved region which is rich in proline, serine and threonine residues, and a C-terminal Src homology (SH3) domain.
Figure 1. Structure of cortactin and other nucleation promoting factors.
Schematic representation of the domain structure of mammalian cortactin, HS1, WASP, N-WASP and SCAR/WAVE1, yeast Abp1p and Myo1p and Listeria Act A, drawn approximately to scale. A gap is inserted into the catalytic motor domain of Myo1p (white box). A, acidic domain; B, basic region; C, central region; GB, GTPase binding domain; H, helical domain; P, proline-rich domain; SHD, SCAR homology domain; W, WASP homology 2 region.
Hydrodynamic and electron microscopy studies have determined that cortactin is a monomeric, asymmetric, elongated molecule with a length of 220–290 Å (1 Å=0.1 nm) [16]. However, both vertebrate and invertebrate cortactins migrate as a doublet separated by 5 kDa upon SDS/PAGE, and exhibit a marked retardation in electrophoretic mobility relative to the molecular masses predicted from their primary sequence, which is likely to be due to the presence of the proline-rich domain. For example, vertebrate cortactins, which have predicted relative molecular masses of 61–63 kDa, migrate as 80 and 85 kDa bands, often designated as p80 and p85 [8,10]. The two bands are likely to represent different conformational states, as a single band is observed when SDS/PAGE is performed in the presence of 5 M urea [17]. Deletion of the α-helical and proline-rich domains, but not the SH3 domain, results in migration as a single band, indicating that the structural determinants contributing to these two states reside in the former two regions [18].
Two other proteins exhibit related domain arrangements to cortactin (Figure 1). HS1 (haematopoietic lineage cell-specific protein 1) is composed of the same types of protein modules, but contains only 3.5 cortactin-type repeats [19]. HS1 also exhibits close sequence similarity in the NTA, repeat and SH3 regions (68%, 69% and 77% amino acid similarity respectively, for the murine proteins). In contrast, murine actin-binding protein 1 (mAbp)1/SH3P7 lacks cortactin-type repeats, possessing an ADF-H (actin depolymerizing factor homology) domain at its N-terminus, but contains the C-terminal succession of α-helical, proline-rich and SH3 domains [20]. Notably, the SH3 domains of murine cortactin and mAbp1 are closely related (68% amino acid similarity).
Tissue expression profile
In the mouse, cortactin mRNA transcripts are detected in a wide range of tissues, but not in the whole spleen, splenic B cells or plasma cells [21]; however, murine megakaryocytes and platelets contain high levels of cortactin protein [22], and cortactin expression is also increased upon differentiation of chicken monocytes into osteoclasts [23]. Immunohistochemical studies of human tissues suggest a role for cortactin in the formation and regulation of actin-based structures. Strong cortactin staining is detected in the apical surfaces of polarized epithelium, such as the terminal web of small bowel epithelium, in other cell types with actin-rich microvilli, and in cells with actin-based contractile activity, such as the smooth muscle of the blood vessels and visceral walls [24]. In contrast with cortactin, HS1 is expressed only in haematopoietic lineages [19].
Importantly, increased expression of cortactin occurs in several types of human cancer and in tumour-derived cell lines. The human gene encoding cortactin, EMS1, localizes to chromosome 11q13, a region commonly amplified in squamous carcinomas of the head and neck, lung and oesophagus, and carcinomas of the liver, bladder and breast [25,26]. In primary breast, and head and neck cancers, and in cell lines derived from these malignancies, overexpression of EMS1 is generally linked to gene amplification [27–30]. However, increased expression of EMS1/cortactin mRNA in the absence of corresponding gene amplification has been reported in bladder tumours [31] and murine plasmacytoma cells [21]. Interestingly, 15% of breast cancer patients exhibit a humoral immune response against cortactin [32], which is consistent with the frequency of EMS1 amplification, and hence cortactin overexpression, in this cancer type [33].
Protein isoforms generated by alternative mRNA splicing often display different tissue expression profiles, and this is also the case for the human cortactin variants [13]. Cortactin SV2 is only expressed at significant levels in frontal cerebrum, but SV1 cortactin transcripts are detectable in a range of carcinoma cell lines and normal tissues. In testis, for example, SV1 transcripts predominate over those encoding full-length cortactin.
Molecular interactions
The NTA region is sufficient to directly bind the Arp2/3 complex [12], and mutation of both acidic residues, or the tryptophan, in the DDW motif abolishes Arp2/3 binding [34]. Chemical crosslinking studies have demonstrated that the NTA region binds the Arp3 subunit of the complex [16]. Binding studies using a recombinant NTA region and purified Arp2/3, determined a Kd of approx. 1 μM, indicating an affinity 5-fold lower than the Arp2/3-interacting region [WASP homology (WH)2-central–acidic region (WCA); Figure 1] from the well-characterized NPF N-WASP [12,34].
Early studies revealed subcellular co-localization of cortactin with F-actin in cortical structures, and treatment of cells with the microfilament-disrupting agent cytochalasin D led to co-aggregation of these molecules [8,35]. Co-sedimentation assays confirmed that cortactin was indeed an F-actin-binding protein, with a Kd of 0.4 μM, and localized this activity to the repeat region [35]. Importantly, unlike certain other NPFs (e.g. N-WASP), cortactin does not bind G-actin [35]. Removal of repeat 4 from full-length cortactin eliminates F-actin binding, identifying a requirement for this sub-domain, but the presence of a single adjacent repeat in N-terminal or C-terminal deletion mutants (i.e. repeat 3 or repeat 5) is required for efficient binding [12]. Supporting a role for flanking repeats in this interaction, rat cortactins B and C, and human cortactin SV2, bind F-actin with a lower affinity [11,13]. Cortactin binds along the sides of F-actin filaments [11].
The ability of cortactin to crosslink actin is controversial. Huang et al. [17] reported that incubation of recombinant cortactin with F-actin markedly enhanced sedimentation of the latter upon centrifugation, and resulted in the formation of bundle-like structures, as determined by electron microscopy. Determination of the crosslinking activity of human cortactin variants revealed that SV1 has relatively low activity, while SV2 has none [13]. In contrast, while rat cortactin can crosslink F-actin, both the B and C isoforms lack this activity [11]. However, the mechanism whereby cortactin might perform this function is unclear, as it is a monomeric protein [16], and only contains one F-actin binding site [12]. Furthermore, other workers have failed to detect F-actin crosslinking or bundling activity for cortactin [15,36].
The fourth repeat of cortactin also contains a cluster of basic amino acid residues which represent a putative binding site for PIP2 (phosphatidylinositol 4,5 bisphosphate) [37]. Pre-incubation of cortactin with PIP2 inhibited the ability of cortactin to crosslink actin filaments, which may be related to competition for F-actin binding. Interestingly, HS1 contains a putative PIP2-binding site in each of its 3 repeats, and binds PIP2 with a higher affinity [37].
Considering the C-terminal half of the molecule, the role of the cortactin helical domain is unknown, whereas, as described in the next section, one function of the proline-rich region is to mediate interaction with specific tyrosine kinases. As with the NTA region, our understanding of the role of the most C-terminal protein module found in cortactin, the SH3 domain, has progressed greatly in recent years. Screening of a peptide phage display library identified the consensus binding motif for the cortactin SH3 domain as +PPΨPXKPXWL (where + and Ψ denote basic and aliphatic residues respectively) [38]. Multiple naturally occurring cortactin SH3 domain targets have now been identified, and are summarized, along with their molecular characteristics and functional roles, in Table 1. Reflecting the close similarity of the cortactin and Abp1 SH3 regions, several cortactin SH3 targets [dynamin 2, faciogenital dysplasia 1 (Fgd1) and cortactin binding protein 1 (CortBP1)/Shank 2] also bind the mAbp1 SH3 domain [39–41]. The cellular functions of particular cortactin-containing protein complexes are discussed in more detail later in this review.
Table 1. Characteristics of cortactin SH3 domain-binding proteins.
The ‘Evidence’ column lists the experimental approaches used to confirm the interaction. Amino acids in the binding site that match the cortactin SH3 binding consensus are underlined. Two binding sites were identified on Dynamin 2 and CD2AP. In the case of the latter, the second site at amino acid 392 is a lower affinity site. The binding motifs on CortBP1 and CBP90 are predicted based upon deletion analysis and similarity to the consensus sequence. CBP90, cortactin-binding protein of 90 kDa; Co-IP, co-immunoprecipitation; DZO-1, Drosophila zonnula occludens 1; N.D., not determined; Y2H, yeast two hybrid analysis.
| Protein | Class | Evidence | Cellular role | Binding site (consensus+PPΨPXKP) | Reference |
|---|---|---|---|---|---|
| CortBP1/Shank2 | Scaffold | Y2H | Organization of transmembrane protein complexes, particularly at PSD | 946 KPPVPPKP | 80 |
| GST pull-down | |||||
| Co-IP | |||||
| Co-localization | |||||
| CBP90 | Unknown | GST overlay and pull-down | Unknown | 539 PPPIPPKKP | 11 |
| DZO-1 | Scaffold | Y2H | Organization of transmembrane protein complexes at cellular tight junctions | 1194 KPVPPPKP* | 14 |
| Co-IP | |||||
| Co-localization | |||||
| Dynamin 2 | Mechano-chemical | GST overlay and pull-down | Vesicle fission in receptor-mediated endocytosis Cortical actin rearrangement | 826 PPQIPSRP† | 86 |
| GTPase | Co-IP | 849 APAAPSRP† | |||
| Co-localization | |||||
| CD2AP | Adaptor/scaffold | GST pull-down and overlay | Endocytosis and downregulation of growth factor receptors | 381 KPAAPQVP* | 79 |
| Co-IP | 392 KPTAPTKA* | ||||
| Co-localization | Organization of transmembrane receptor complexes | ||||
| Fgd1 | Cdc42 GEF | Y2H | Cortical actin organization | 158 KPQVPPKP* | 40 |
| GST overlay | Mutated in faciogenital dysplasia | ||||
| Co-localization | |||||
| Co-IP | |||||
| N-WASP | NPF | GST pull-down and overlay | Effector in Cdc42-regulated actin nucleation | N.D. | 85 |
| Co-IP | Filopodia formation | ||||
| Co-localization | |||||
| WIP | Adaptor | Y2H | Regulates N-WASP NPF activity | N.D. | 73 |
| GST pull-down | Yeast homologue Vrp1p regulates actin dynamics | ||||
| Co-IP | |||||
| Co-localization |
* Binding site confirmed by site-directed mutagenesis.
† Binding site determined by phosphopeptide competition.
Tyrosine phosphorylation of cortactin
Cortactin was first identified by virtue of its tyrosine phosphorylation in v-Src-transformed cells [8], and a large body of subsequent work implicated c-Src in tyrosine phosphorylation of this protein in response to a variety of physiological or pathogenic stimuli, including FGF-1 stimulation [9,42], integrin-mediated cell adhesion and spreading [43], and invasion of epithelial cells by the bacterium Shigella flexneri [44]. This area has been reviewed in detail previously [15]. An elegant demonstration of the role of c-Src in cortactin tyrosine phosphorylation was provided by studies on fibroblasts deficient for Csk, a negative regulator of Src family kinases. Cortactin is hyperphosphorylated on tyrosine residues in Csk−/− cells, but normal phosphorylation is restored in cells deficient for both Csk and Src [45]. In support of Src phosphorylating cortactin directly, the two proteins co-immunoprecipitate in a variety of cellular systems [42,46,47], and Src phosphorylates murine cortactin in vitro on three sites (Tyr421, Tyr466 and Tyr482), which are also the major sites of phosphorylation in v-Src transformed cells [48]. In a recent study, use of phosphospecific antibodies has demonstrated that mutation of Tyr421 to phenylalanine prevents the phosphorylation of Tyr466 [49]. This suggests a processive phosphorylation model in which phosphorylation of Tyr421 by Src creates a binding site for the Src SH2 domain. Binding of Src to cortactin is then followed by phosphorylation of Tyr466 and possibly other sites.
Three other kinases strongly implicated in cortactin tyrosine phosphorylation also belong to the non-receptor-type subgroup. These are Fyn (during melanoma cell migration) [50], Syk (in platelet shape change) [51] and Fer (in PDGF-stimulated fibroblasts) [52]. Furthermore, a signalling pathway involving Fer activation downstream of Fyn results in cortactin phosphorylation in response to osmotic shock [53], and this pathway is also activated by F-actin depolymerization in response to treatment of cells with latrunculin B [54]. Activation of Fer in response to hypertonicity or actin depolymerization leads to phosphorylation of the same cortactin tyrosine residues regulated by Src [53,54]. Finally, tyrosine phosphorylation of cortactin in response to hepatocyte growth factor (HGF) stimulation of A431 cells may be directly mediated by the HGF/MET receptor [55].
Cortactin tyrosine phosphorylation may serve a variety of functions. One role is to provide binding sites for specific signalling proteins with SH2 domains, e.g. Src family kinases and Nck [46], which may regulate the cellular functions performed by cortactin, or act as effectors in this regard. Also, tyrosine phosphorylation may alter the conformational state of the protein, since the major Src phosphorylation sites reside within the proline-rich region, which may act as a ‘hinge’ separating the repeat region and SH3 domain. This may then modify interactions with other proteins. This is supported by two observations. First, digestion of cortactin by calpain in vitro is markedly enhanced by prior phosphorylation by Src, suggesting that Src-mediated phosphorylation induces conformational changes that increase the efficiency of proteolytic cleavage [56]. Second, interaction of cortactin with EC MLCK (endothelial cell myosin light-chain kinase) is enhanced by prior incubation of cortactin with Src [57]. A further role for cortactin phosphorylation is provided by the observation of Zhan and co-workers that this modification modestly inhibits the F-actin binding activity of cortactin and strongly reduces its F-actin crosslinking activity [17]. A similar effect of Src on the latter parameter was also reported by Bourguignon et al. [58]. This suggests a negative regulatory role which may regulate the flexibility and/or turnover of actin networks. However, the ability of cortactin to crosslink actin filaments has been disputed [15,36].
Serine/threonine phosphorylation of cortactin
Cortactin isolated from a variety of cell types exhibits phosphorylation on serine and threonine residues, and this can be enhanced by EGF (epidermal growth factor) or vanadate treatment [8,18,47]. Increases in phosphorylation on these residues accompanies a mobility shift of cortactin upon SDS/PAGE, from p80 to p85 [18,47]. In HEK293 cells, conversion of p80 into p85 upon EGF treatment or cell detachment is blocked by the MEK (MAP kinase/ERK kinase) inhibitor PD98059, and expression of constitutively active MEK is sufficient to stimulate the conversion, indicating that kinases downstream of MEK regulate this process [18]. However, the basal levels of p85 in serum-starved cells are insensitive to MEK inhibition, indicating that MEK-independent pathways also contribute to cortactin phosphorylation. Deletion of the combined helical and proline-rich (HP) regions prevented the mobility shift and cortactin phosphorylation, and tryptic phosphopeptide mapping highlighted the ERKs as potential HP region kinases, with two consensus ERK phosphorylation sites (Ser405 and Ser418) as likely targets [18]. Phosphorylation of the latter residue in cortactin has been confirmed by mass spectrometry (D. K. Lynch, S. Cordwell and R. J. Daly, unpublished results).
Studies on thrombin-induced lamellipodial spreading in platelets have implicated a second serine/threonine kinase in cortactin regulation, the Rac/Cdc42 effector PAK (p21-activated kinase) [59]. Thrombin stimulation of platelets leads to activation of Rac/Cdc42 and PAK, and co-localization of Rac and cortactin in cortical regions. Interestingly, cortactin and PAK co-immunoprecipitate from resting platelets but dissociate upon thrombin stimulation, which may contribute to the observed redistribution of cortactin. PAK can also phosphorylate recombinant cortactin in vitro, although the regulated sites are not known. Finally, although cortactin binds directly to EC MLCK, it has not been reported whether it acts as a substrate for this enzyme [57].
THE ROLE OF CORTACTIN IN DENDRITIC ACTIN NUCLEATION BY THE Arp2/3 COMPLEX
Regulation of actin polymerization by the Arp2/3 complex
Since the formation of new actin filaments from pure actin monomers is unfavourable, cells require a molecular apparatus to drive de novo actin nucleation. This function is provided by the Arp2/3 complex, an evolutionarily-conserved assembly of seven sub-units: two actin-related proteins, Arp2 and Arp3, and five other polypeptides, designated ARPC1-5 [7]. In isolation, the Arp2/3 complex has a low actin-nucleating activity, but, as described in the next section, this can be enhanced by binding to specific NPFs. Importantly, the activity of an NPF–Arp2/3 assembly can be stimulated further by binding to the side of an existing or ‘mother’ actin filament, so that nucleation is coupled to branching. This results in the formation of actin networks exhibiting characteristic ‘Y-branches’ at an angle of 70°; a process termed dendritic actin nucleation [7,60].
Regulation of the Arp2/3 complex by NPFs
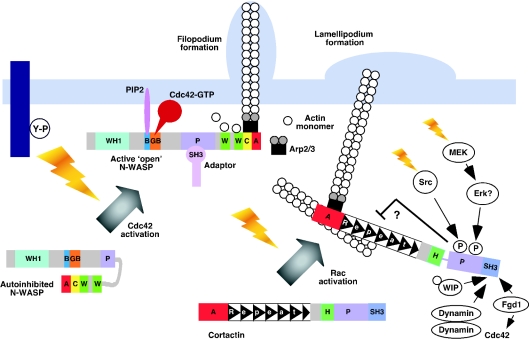
The structure of a selection of Arp2/3 activators is shown schematically in Figure 1. All of these proteins contain an acidic (A) region which contributes to binding and activating the Arp2/3 complex and harbours a conserved tryptophan-containing motif with the consensus sequence DDW [7,61]. In the WASP/SCAR family, the A region is preceded by one or two WH2 motifs which bind monomeric actin, and a central (C) region. The latter also contributes to Arp2/3 complex interaction and activation. A WASP/SCAR fragment, consisting of the WCA regions, is sufficient to activate the Arp2/3 complex, with maximal activation occurring when both the Arp2/3 complex and an actin monomer are bound. Actin filaments enhance binding of WCA to Arp2/3 complex and actin nucleation, thus favouring the formation of branched actin networks [7]. Although related in certain structural characteristics, the mode of regulation of WASP/N-WASP and SCAR proteins differs, being based upon auto-inhibition and the presence of associated proteins (trans-inhibition) respectively. The observation that N-WASP requires both GTP–Cdc42 and PIP2 for full activation [7] has led to a model in which auto-inhibition is mediated by binding of the C and A regions to the GBD and basic domain respectively. Binding of GTP–Cdc42 to the GBD and PIP2 to the basic region then induces release from the inactive conformation (Figure 2) [7,62]. Tyrosine phosphorylation within the GBD and serine phosphorylation at the C/A region border also regulate WASP NPF activity [63,64]. In contrast with WASP/N-WASP, SCAR/WAVE proteins are not auto-inhibited, and although they are activated downstream of Rac, they do not appear to interact directly with this G-protein, as predicted by the absence of a GBD (GTPase binding domain) [7]. Evidence for a trans-inhibition model for SCAR/WAVE regulation is that WAVE1 can be purified in an inactive complex with four other proteins, PIR121/Sra1, Nck-associated protein (NAP)125, HSPC300 and Abl-interactor 2 (Abi2) [65]. Incubation of the complex with either GTP–Rac or Nck results in disassembly and release of active WAVE1/HSPC300. However, an alternative mode of activation has been proposed whereby the adaptor IRSp53 directly links activated Rac to WAVE proteins [66].
Figure 2. Comparison of N-WASP and cortactin regulation in response to growth factor receptor activation.
Upon receptor activation (gold jagged arrows), the auto-inhibited conformation of N-WASP is released by binding of Cdc42–GTP and PIP2 to the GTPase binding and basic regions respectively, allowing Arp2/3 complex activation. Binding of specific SH3 domain-containing adaptors (e.g. Grb2) can further stimulate NPF activity. In contrast, cortactin is not auto-inhibited in the basal state, and is translocated in a Rac-dependent manner to the cell periphery upon growth factor stimulation, where it functions to either co-activate Arp2/3 with other NPFs or to stabilize actin branchpoints formed following the transient association of NPFs with the Arp2/3 complex. The activity of cortactin towards the Arp2/3 complex can be modulated by binding of specific proteins to its SH3 domain. For example, dynamin 2 and WIP enhance NPF activity by inducing dimerization, or by providing actin monomers respectively. Recruitment of Fgd1 provides a potential mechanism for crosstalk with Cdc42/N-WASP. Phosphorylation of cortactin on tyrosine and/or serine/threonine residues may induce a conformational change and regulate intermolecular interactions. In the case of tyrosine phosphorylation, this may inhibit F-actin binding or crosslinking, and hence the rigidity or turnover of actin networks.
Interestingly, WASP/SCAR proteins can directly recruit regulators of specific Rho family GTPases. For example, WAVE-1 recruits WRP, a Rac GTPase-activating protein [67], and binding of N-WASP to the endocytic protein intersectin-1 enhances the GEF (guanine nucleotide exchange factor) activity of the latter protein towards Cdc42 [68]. Consequently, this scaffolding function of WASP/SCAR proteins is used to generate multiprotein complexes with built-in capacity for enhancement or termination of NPF activity. As described later in this review, an interesting parallel is the recruitment of the Cdc42 GEF Fgd1 by cortactin [40]. Since Cdc42 does not regulate cortactin directly, this suggests that crosstalk may exist between different classes of NPFs.
Regulation of actin nucleation and branching by cortactin
Initial attempts to detect effects of cortactin on actin polymerization failed, as these assays were performed with a relatively low concentration of Arp2/3 complex (10 nM) [12]. However, when a 10-fold higher concentration of Arp2/3 complex was used, cortactin decreased the half-time to reach a steady state of actin polymerization and also enhanced the rate of actin polymerization [34,36]. Although these effects were relatively weak when compared with a WCA fragment from N-WASP, cortactin could further enhance WCA-stimulated actin polymerization. Weaver et al. [36] reported a synergistic effect on the formation of new actin barbed ends. Also, cortactin increased actin filament branching in the presence of Arp2/3 complex, and cortactin and WCA enhanced this process in a synergistic manner. Importantly, stimulation of actin polymerization by cortactin required the presence of both the NTA and repeat regions required for binding Arp2/3 complex and F-actin respectively. Consistent with this, cortactin SV2, which binds F-actin more weakly than SV1, exhibits lower activity towards the Arp2/3 complex in actin polymerization assays [13]. Uruno et al. [34] further demonstrated that mutation of either the acidic residues or the tryptophan in the DDW motif, both required for Arp2/3 complex binding, abrogated cortactin NPF activity. This indicated a novel mode for activation of actin polymerization; in contrast with WASP/SCAR proteins, cortactin binds F-actin rather than G-actin, and the affinity of this interaction is approx. 20-fold higher than that for Arp2/3-F-actin binding [34]. Since association with F-actin is required for optimal activation of Arp2/3 complex [7], a key aspect of the mechanism whereby cortactin acts as an NPF is its ability to promote the association of Arp2/3 complex with actin filaments. A similar mechanism has been proposed for Abp1p, which binds to actin filaments via its ADF-H domain [69]. However, an additional aspect of cortactin function towards actin networks is that it stabilizes filament branch points and hence inhibits debranching and disassembly [36].
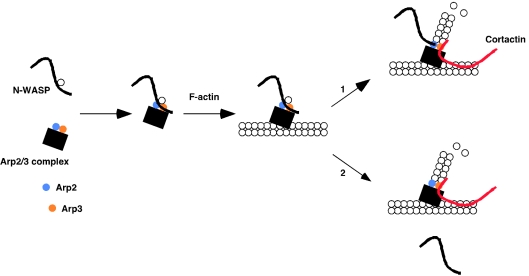
How could additive or synergistic enhancement of Arp2/3 activation be observed with a combination of cortactin and N-WASP WCA, if both activators bind the Arp2/3 complex, and a single acidic region in both cortactin and WCA is implicated in binding? One group have addressed this question by a chemical crosslinking approach [16]. This revealed that the cortactin NTA interacts with the Arp3 subunit of the Arp2/3 complex, while WCA associates with Arp3, Arp2 and ARPC1, and that a ternary complex of Arp2/3-WCA–NTA could form. Furthermore, saturating amounts of NTA (which in the absence of the repeat region binds, but does not activate Arp2/3) did not inhibit Arp2/3 activation by WCA. These studies suggest that cortactin and N-WASP can act in concert to regulate the Arp2/3 complex, with cortactin bridging Arp3 and F-actin, and N-WASP interacting with Arp2 and ARPC1 (Figure 3). However, it has recently been reported that full-length cortactin can stimulate actin nucleation in the presence of a saturating concentration of glutathione S-transferase (GST)–WCA, with a half maximal stimulation observed at 20 nM cortactin [70]. Similar concentrations of cortactin were also able to displace recombinant N-WASP WCA from the Arp2/3 complex, but only when the assay was performed in the presence of actin polymerization. These results have led to a model in which cortactin acquires a higher affinity for Arp2/3 by virtue of an interaction with both Arp2/3 and F-actin at the branching site [70], and sequential binding of N-WASP and cortactin to the Arp2/3 complex occurs (Figure 3). This model is more consistent with the distinct localization of N-WASP and cortactin in the actin comet tail induced by intracellular pathogens. Here, N-WASP is situated at the junction of the pathogen and the tail, where actin assembly is initiated, while cortactin is distributed throughout the comet tail and hence the established actin network [70,71]. A similar differential distribution of the two proteins is also observed in actin tails associated with intracellular vesicle-like particles [72].
Figure 3. Different models for Arp2/3 complex activation by N-WASP and cortactin.
Association with N-WASP and the side of an actin filament activates the Arp2/3 complex. For simplicity, WCA is shown binding to Arp2 and Arp3, although it also contacts ARPC1. Further stimulation of actin nucleation has been proposed to occur in one of two modes. (1) Co-activation by N-WASP and cortactin. WCA remains in contact with Arp2 (and ARPC1), but the cortactin NTA displaces WCA and binds Arp3. (2) A sequential model. N-WASP is displaced by cortactin which has acquired a higher affinity for the activated Arp2/3 complex at the branch site. This results in the formation of a stable complex for dendritic nucleation.
Unlike WASP/SCAR proteins, the NPF activity of cortactin does not appear to be inhibited by intra- or inter-molecular interactions, and although phosphorylation represents a likely modulatory mechanism, this has yet to be demonstrated. However, cortactin NPF activity can be regulated by the recruitment of SH3 domain-binding partners (Figure 2). The addition of WIP (WASP-interacting protein) to pyrene actin polymerization assays increases actin nucleation in the presence of cortactin and Arp2/3 complex, while it has no effect on the ability of cortactin to inhibit debranching [73]. Since WIP contains a G-actin binding WH2 module [74], while cortactin does not bind actin monomers [35], WIP could enhance actin polymerization by cortactin–Arp2/3 complex by provision of G-actin for incorporation into new filaments. Additionally, WIP could stabilize filaments against depolymerization [74]. Furthermore, dynamin 2 regulates actin polymerization by cortactin-Arp 2/3 complex in a biphasic manner, with a modest enhancement of actin nucleation occurring at molar ratios of dynamin:cortactin of <1:1, and inhibition at higher molar ratios [75]. The recent finding that dimerization of cortactin markedly enhances its NPF activity [76] may provide an explanation for these observations. Since dynamin multimerizes at low concentrations [77], this is likely to promote cortactin dimerization. However, the formation of higher-order oligomers with increasing dynamin 2 concentrations may ultimately inhibit cortactin activity towards the Arp2/3 complex. In support of this model, the addition of dynamin 2 to actin polymerization assays containing cortactin and Arp2/3 complex, induces bundling of actin filaments [75]. This mechanism for regulation of cortactin activity may be relevant to other binding proteins that dimerize or oligomerize e.g. CD2-associated protein (CD2AP) [78,79] and CortBP1/Shank 2 [80,81]. Finally, the cortactin-interacting protein EC MLCK markedly inhibits the ability of cortactin to promote Arp2/3-stimulated actin polymerization, although the underlying mechanism is unclear [57].
CELLULAR ROLES OF CORTACTIN IN NORMAL AND DISEASE STATES
Cortical actin organization
Strong immunostaining for cortactin is observed in membrane ruffles and lamellipodia in a variety of adherent cell types, including fibroblasts, aortic smooth muscle cells, endothelial cells and human carcinoma cells of different origins [10,35,79,82]. Cortactin is also implicated in cortical actin arrangements in neuronal cells, since it localizes to the growth cones of developing hippocampal neurons [80] and is required for the formation of dendritic spines [83]. In addition to these protrusive structures, certain cell types also form podosomes, which are specialized cell-substratum adhesion sites consisting of tubular invaginations of the plasma membrane surrounded by a columnar sheath of actin cytoskeleton [84]. Cortactin co-localizes with F-actin at these structures in Src-transformed cells [8,84,85] and in carcinoma cells with EMS1 gene amplification [10]. Microinjection of the cortactin SH3 domain inhibits podosome formation in Src-transformed cells [85], which may reflect its interaction with N-WASP [85] or dynamin 2 [84,86].
In Swiss 3T3 fibroblasts, activation of Rac is required for platelet-derived growth factor (PDGF)- or PMA-induced translocation of cortactin from the cytoplasm to the cell cortex, and is also sufficient to drive this process [82]. Importantly, both the NTA region and the fourth cortactin repeat, and hence the ability to bind to both the Arp2/3 complex and F-actin, are required for Rac-induced cortical localization of this protein [12]. Similar findings were reported for Rac-dependent translocation of cortactin to the cell periphery in response to osmotic stress [87], and Rac also stimulates cortical accumulation of the related protein mAbp1 [20]. Interestingly, tyrosine phosphorylation of the major Src phosphorylation sites in cortactin requires cortical targeting, and is also dependent on Rac activation [49]. Since activation of Rac directs Src to membrane ruffles and lamellipodia [88], this GTPase regulates the coordinated movement of both cortactin and a tyrosine kinase intimately involved in its regulation to specific sites of dynamic cortical actin reorganization.
Cortactin, and its associated proteins, perform several functions at the cell cortex relating to remodelling of the plasma membrane and the underlying actin cytoskeleton. As described previously, one role of cortactin within lamellipodia/ruffles is presumably to enhance the formation of branched actin networks (via interaction with Arp2/3 complex) and/or stabilize those formed by the activity of the Rac-stimulated NPF SCAR. However, cortactin also recruits specific binding proteins to cortical regions of the cell via its SH3 domain. For example, cortactin mediates PDGF-stimulated translocation of dynamin 2 to membrane ruffles [86] and these proteins also co-localize in transient ‘waves’ on the dorsal surface of growth-factor-stimulated cells, which, via disassembly of F-actin networks, provide more pliable regions of the cell cortex which facilitate lamellipodial protrusion [89]. However, although dynamin 2 binding regulates the NPF activity of cortactin, and the dynamin 2-cortactin complex alters the organization of actin filaments at a lipid interface [75], the exact role(s) of this assembly in wave formation and membrane protrusion is unclear [90]. Also, binding to the cortactin SH3 domain may contribute to the localization of both Fgd1 [40] and WIP [73] to the cell cortex. Both of these proteins play a positive role in Cdc42-dependent filopodia formation, the former by virtue of its GEF activity towards Cdc42 [91], the latter due to its interaction with N-WASP [74]. Consequently, these proteins may function together with cortactin to regulate cortical actin rearrangements, which is supported by the observation that co-expression of cortactin and WIP in cells enhances the formation of membrane protrusions [73].
Importantly, the formation of Rac-induced lamellipodia is required for cell motility [2]. Consistent with this, cortactin overexpression enhances migration of both fibroblasts [92] and endothelial cells [48] in vitro, and the first genetic analysis of cortactin function, performed in Drosophila, has identified a role for this protein in border cell migration during oogenesis [93]. Importantly, the ability of cortactin to promote migration of human endothelial cells requires the presence of the major sites of Src-mediated tyrosine phosphorylation [48], and a cortactin phosphorylation-deficient mutant can function in a dominant-negative manner to inhibit motility [48,55]. Therefore, Src family kinases may contribute to cell migration by phosphorylating cortactin. In support of this model, enhanced cell motility correlates with activation of this signalling pathway in a variety of cell culture systems, including FGF-1-stimulated fibroblasts [94], hyaluronic acid-treated ovarian cancer cells [58], and murine melanoma cell lines with different metastatic potential [50]. Src-cortactin signalling is also implicated in N-syndecan-regulated neurite formation in N18 neuroblastoma cells [95] and in the formation of actin-rich structures termed ring canals in Drosophila [93].
Association with transmembrane protein complexes
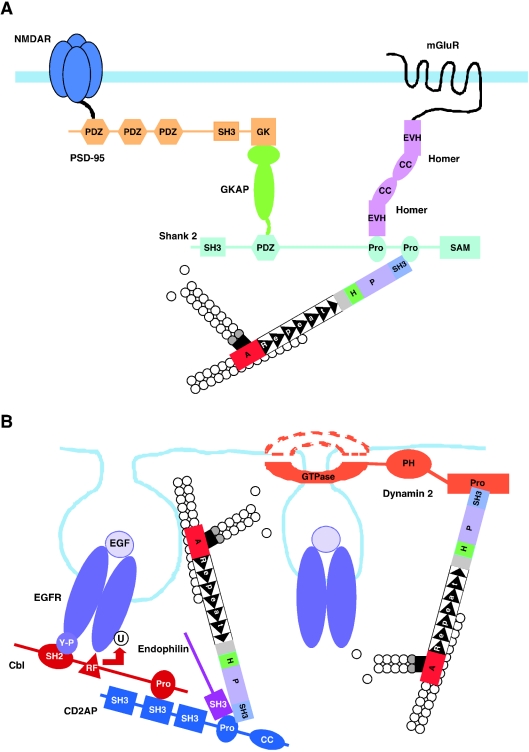
Cortactin also provides an indirect link between specific transmembrane receptors and the underlying actin cytoskeleton. This often occurs via ligation of the cortactin SH3 domain to submembranous scaffolding proteins (Figure 4). The first cortactin SH3 target identified, CortBP1 [80], was subsequently characterized as a splice variant of Shank 2, a member of a protein family which also contains Shank 1 and Shank 3 [81]. The cortactin SH3 module binds to a region of Shank 2 harbouring a proline-rich motif which bears close similarity to the consensus-binding motif for this SH3 domain [80] (Table 1). This motif is also present in Shank 3 but not Shank 1 [81]. Shank 2 is predominantly expressed in brain, while Shank 3 is widely expressed [81].
Figure 4. Cellular roles for cortactin.
Several roles are consistent with a model in which cortactin binds specific protein complexes via its SH3 domain and links them to the Arp2/3 complex and hence dynamic actin networks. (A) Organization of transmembrane receptor complexes at the postsynaptic density (PSD), via binding to Shank 2. CC, coiled-coil domain; Pro, proline-rich region; SAM, sterile α-motif domain. (B) Receptor-mediated endocytosis via binding to dynamin 2 and CD2AP. PH, pleckstrin homology domain; RF, ring finger domain; U, ubiquitin.
The best-characterized role for Shank proteins is in the assembly of receptor clusters at the postsynaptic density (PSD) of excitatory synapses [81,96]. Shank PDZ (PSD-95/Dlg/ZO-1) domains bind to specific C-terminal sequence motifs in guanylate kinase associated proteins (GKAPs), which in turn bind the GK domain of PSD-95 (Figure 4). The latter tethers the complex to specific transmembrane proteins. For example, the first two PDZ domains can bind the C-termini of the NMDA (N-methyl-D-aspartate) receptor. In addition, the Shank PDZ domains can also bind directly to particular receptors e.g. the type 2 somatostatin receptor, and the group 1 metabotropic receptors mGluR1a and mGluR5. An additional Shank-binding partner is Homer, which can bind via its EVH1 (Ena VASP homology 1) domain to one of the proline-rich regions of Shank proteins. Since the Homer EVH1 domain also binds to mGluR1a and 5, as well as inositol triphosphate receptors, and Homer proteins can dimerize via a C-terminal coiled-coil domain, a Homer dimer can link specific receptors to Shanks. Furthermore, the latter can crosslink other Shank-binding partners (e.g. PSD-95–NMDA receptors) to Homer-associated receptors [81,96] (Figure 4).
The theme of cortactin recruitment to scaffolding protein complexes is further developed by its binding to ZO-1 (zonnula occludens 1), a protein with similar molecular architecture to PSD-95, which localizes to adherens junctions in Drosophila and to epithelial tight junctions in mammals [14,97,98]. The latter are intercellular junctions formed at the apical end of the lateral membrane which restrict the diffusion of molecules between the apical and basolateral membrane compartments and which also regulate epithelial paracellular permeability [98]. A direct interaction between the cortactin SH3 domain and ZO-1 was first demonstrated using the Drosophila proteins [14], and the binding site in Drosophila ZO-1 exhibits similarity to the consensus sequence (Table 1). However, GST pull-down and co-immunoprecipitation experiments indicate that mammalian cortactin and ZO-1 also interact [14] (D. K. Lynch, S. C. Winata and R. J. Daly, unpublished results). The association of human cortactin and ZO-1 at tight junctions is also consistent with the immunohistochemical localization of cortactin to the terminal web in human small bowel epithelium [24]. As with Shank proteins, ZO-1 can interact directly with transmembrane receptors. Thus, the GK and acidic regions of ZO-1 bind to occludin [99] while the first PDZ domain binds the C-termini of claudins [100]. Occludin and claudins both contain four transmembrane regions and assemble into heteropolymers, acting as critical determinants of tight junction structure and as regulators of paracellular diffusion [98]. The role of the ZO-1–cortactin interaction in structural organization of tight junctions is unclear.
Interestingly, cortactin contributes to the formation of another type of cell–cell junction, the adherens junction, formed upon homophilic binding of E-cadherin molecules on juxtaposed cells. This type of cell–cell contact is critical for tissue organization in developing and adult organisms [101]. Nascent E-cadherin contacts are converted into stable zones of adhesion through co-operation between the cadherins and the underlying actin cytoskeleton [102]. In MDCK cell monolayers, cortactin localizes to E-cadherin-based cell–cell contacts, and cortactin is recruited along with the Arp2/3 complex to the extending outer margins of cadherin-based lamellipodia [103]. Importantly, inhibition of cortactin function reduced cadherin-based lamellipodia formation, altered cell morphology and prevented accumulation of E-cadherin and β-catenin at cell–cell contacts. Together, these data indicate that cortactin plays a role in the actin re-organization required for the formation of stable cadherin-based contact zones. Although cortactin and E-cadherin co-immunoprecipitate from cells exhibiting homophilic E-cadherin ligation, the underlying mechanism is not clear. However, given the role of Rac in regulating cortactin localization [82], and in signalling downstream of E-cadherin [104], this GTPase may play a role in regulating complex formation.
Roles in endocytosis and vesicle trafficking
Genetic studies in yeast have demonstrated a functional link between endocytosis and the actin cytoskeleton, and in higher organisms cell biological and biochemical evidence supports a role for actin polymerization in most endocytic pathways [3]. Actin polymerization could regulate a variety of steps during endocytosis, including membrane invagination and fission and the movement of endocytic vesicles via the formation of actin ‘comet’ tails [105].
One link between cortactin and endocytosis is its SH3 domain-mediated binding to dynamin 2, a mechanochemical GTPase with a well-characterized role in membrane severing during endocytic vesicle formation (Figure 4) [86,106]. Electron microscopy has revealed that cortactin distributes in a ring around the base of the clathrin coat in invaginating coated pits, in a similar manner to dynamin 2. Furthermore, microinjection of anti-cortactin antibodies, or expression of the cortactin SH3 domain in cells, demonstrated that cortactin is required for efficient receptor-mediated, but not fluid-phase, endocytosis [107]. Interestingly, addition of dynamin 2 to a mixture of cortactin, Arp2/3 complex and PIP2 vesicles led to vesicle aggregation and the association of these aggregates with loose bundles of actin filaments, and addition of GTP altered the organization of actin filaments associated with the lipid aggregates [75]. Thus a cortactin–dynamin 2 complex can regulate the organization of actin filaments associated with membranes. It should be noted that Abp1, which is structurally similar to cortactin, also binds to dynamin, and co-localizes with dynamin and endocytic coat components in punctate spots at the cortex of growth factor-stimulated fibroblasts [39]. Furthermore, overexpression of the Abp1 SH3 domain blocks transferrin uptake, indicating similar roles for Abp1 and cortactin in regulating receptor-mediated endocytosis.
A second mechanism whereby cortactin is recruited to the endocytic machinery is via binding to the scaffolding protein CD2AP (Figure 4) [79]. Upon EGF stimulation of cells, cortactin is transiently recruited into a molecular complex containing the EGFR, c-Cbl, CD2AP and endophilin, and co-localization of the EGFR, CD2AP and cortactin can be demonstrated in membrane ruffles. In a similar complex formed around the CD2AP-related protein CIN85 (Cbl-interacting protein of 85 kDa), endophilin is thought to promote membrane invagination, while c-Cbl ubiquitylates the receptor, providing a signal for lysosomal sorting and receptor degradation [108]. Whether cortactin binds CD2AP in other cellular contexts, for example in T-cells, where CD2AP associates with the CD2 adhesion molecule [109], or in podocytes, where it binds nephrin and podocin [110,111], is currently unknown.
Recently, studies on the role of Hip1R (Huntingtin-interacting protein 1-related), which links clathrin cages to actin filaments [112], have revealed an unsuspected interplay between Hip1R and cortactin during clathrin-mediated endocytosis. In cells in which Hip1R expression was suppressed by RNAi (RNA interference), unusual actin structures, such as tails and rings, accumulated at the cell cortex, and these structures were enriched for components of both the actin polymerization and endocytic machineries [113]. Electron microscopy studies revealed that these structures were tethered to the cell membrane. Thus Hip1R depletion results in the accumulation of actin filaments at coated pits that do not allow vesicle release. Importantly, concomitant suppression of cortactin inhibited the formation of these cortical structures. This provides further evidence for a functional role for cortactin-stimulated actin polymerization in receptor-mediated endocytosis, but indicates that Hip1R may somehow negatively regulate this process to ensure a transient and productive interaction between coated pits and actin during vesicle internalization.
In addition to playing a role during vesicle internalization, cortactin has also been localized to actin tails associated with both endosomes [114] and with vesicle-like structures, possibly fluid-phase pinosomes, induced by expression of activated ADP-ribosylation factor, ARF6 [72]. These actin structures presumably contribute to vesicle propulsion in a similar manner to the actin comet tails associated with certain intracellular pathogens. Cortactin is localized throughout the tail, whereas N-WASP is situated at the junction of the tail and vesicle, and cortactin does not appear to be tyrosine phosphorylated within these structures [72,115].
Roles in cellular entry and cytoplasmic movement of pathogens
Cortactin contributes to two mechanisms underlying cellular infection by specific pathogens. First, the reorganization of the cortical actin cytoskeleton that occurs upon pathogen attachment and entry. For example, cortactin is recruited to the F-actin-rich ‘pedestals’ formed upon attachment of enteropathogenic and enterohaemorrhagic Escherichia coli (EPEC and EHEC respectively), and upon EPEC infection, co-immunoprecipitates with the intimin receptor (Tir), a virulence factor translocated into the host cell by the bacteria [116,117]. A functional role for cortactin in the formation of these structures was demonstrated by the inhibition of F-actin accumulation at the bacterial adherence site in cells expressing a truncated cortactin lacking the NTA and repeat regions [117]. Cortactin also localizes to the cellular protrusions which form the ‘entry structure’ responsible for Shigella flexneri uptake by cells [44] and to the interaction site between Cryptosporidium parvum and host cells [118]. The requirement for cortactin tyrosine phosphorylation varies depending on the pathogen, probably reflecting the different modes of cellular infection and hence types of actin reorganization involved. Tyrosine phosphorylation of cortactin occurs upon infection of cells with Shigella [44,119], vaccinia virus [120] and Cryptosporidium [118]. Inhibition of Src activity, and hence cortactin tyrosine phosphorylation, decreases Shigella and Cryptosporidium invasion, implicating a Src-cortactin pathway in this infectious process [118,119]. In the case of Cryptosporidium infection, this was confirmed by expressing a tyrosine phosphorylation-defective cortactin mutant in host cells, which reduced parasite invasion [118]. In contrast, cortactin tyrosine phosphorylation does not occur upon EPEC or EHEC infection, and accumulation of cortactin at the bacterial adherence sites is unaffected by the Src family kinase inhibitor PP1 [116]. Also, although cortactin localizes to the bacterial vacuole formed upon infection of epithelial cells with Chlamydia trachomatis, enhanced tyrosine phosphorylation of cortactin in infected cells is not detected [121].
The second mechanism whereby cortactin contributes to infection is in the formation of actin tails which are used by pathogens for movement within and between cells. Thus cortactin has been localized to the ‘rocketing’ or ‘comet’ tails produced by intracellular Listeria monocytogenes [15], Shigella [70] and vaccinia virus particles [71]. Actin networks in these tails have a similar dendritic organization to those in lamellipodia, with the Arp2/3 complex localized to Y-junctions [122]. Cortactin localizes with the Arp2/3 complex throughout the tail, whereas N-WASP is restricted to the pathogen-tail interface [70,71,123]. Interestingly, vaccinia-induced tails do not stain with anti-phosphotyrosine antibodies, suggesting that cortactin is not tyrosine phosphorylated in this setting [124].
Roles in tumour progression
As described earlier in this review, cortactin is overexpressed in several human cancers, usually due to gene amplification; however, it is important to note that the 11q13 amplicon, where the cortactin-encoding EMS1 gene localizes, is large and contains several known or potential oncogenes [26]. Consequently, EMS1 is not the only target of 11q13 amplifications. In particular, CCND1, encoding the cell cycle regulatory protein cyclin D1, is situated within this chromosomal region. The contribution of particular 11q13 subregions has been investigated in several large studies on breast cancer patients which indicate that there are four ‘cores’ of gene amplification within the 11q13 locus, and that CCND1 and EMS1 are the best candidates to ‘drive’ amplification of their respective cores [26]. In support of independent roles for EMS1 and CCND1 in breast cancer, a study of 961 breast cancer patients revealed that approx. 7% of patients exhibited EMS1 amplification in the absence of increases in CCND1 copy number, and that EMS1 and CCND1 amplification were not correlated [33]. Furthermore, in contrast with CCND1, which appeared to play a role in oestrogen receptor-positive disease, EMS1 amplification was associated with an increased risk of relapse and death in patients with oestrogen receptor-negative tumours. More recently, EMS1 amplification, unlike that of CCND1, was determined to be an independent predictor of death from head and neck cancer [125], suggesting that cortactin overexpression also contributes to disease progression in this cancer type.
How might cortactin promote tumour progression? The role of cortactin in regulating the formation of actin-based structures intimately associated with cell motility [35,82], its localization to podosome-like structures in transformed cells [8,10,84,85], and the enhancement of cell migration upon cortactin overexpression [48,92] are all consistent with a role in cancer cell invasion and metastasis. Two studies on the breast cancer cell line MDA-MB-231, which exhibits a motile, invasive phenotype in vitro, support this hypothesis. In these cells, cortactin localizes to invadopodia, filopodia-like protrusions into the extracellular matrix which are associated with matrix-degrading proteolytic activity [126]. A role for cortactin in the formation of these structures is supported by the inhibition of matrix degradation at cell-substratum contact sites by microinjection of an anti-cortactin antibody. Moreover, overexpression of cortactin in these cells increases transendothelial invasion in vitro and the formation of bone metastases following intracardiac injection into nude mice, in a manner dependent upon cortactin tyrosine phosphorylation [127]. Consequently, there is strong evidence that cortactin functions in the relatively late stages of disease progression to promote tumour cell dissemination. However, the presence of EMS1 gene amplification in primary tumours may indicate that cortactin overexpression provides a proliferative advantage in the early stages of tumour development [128]. Although such an effect was not observed in studies on cultured NIH3T3 fibroblasts [92] or on MDA-MB-231 breast cancer cells grown in vitro or as tumours in nude mice [127], this possibility should not be discounted, given cortactin's multiple cellular roles.
SUMMARY AND PERSPECTIVES
It is now clear that the modular nature of cortactin enables coupling of two key biochemical properties, the ability to nucleate and stabilize Arp2/3-generated dendritic actin networks and SH3 domain-mediated targeting to specific protein complexes. This has been exploited by the cell to link a variety of proteins with diverse functions to dynamic actin assemblies. However, more insight is required into how cortactin functions in combination with other NPFs to regulate the Arp2/3 complex in vivo, and how this leads to the generation of different cytoskeletal structures. Also, despite the marked tyrosine phosphorylation of cortactin in response to specific stimuli, the role of this modification is still unclear, while from both a mechanistic and functional standpoint, cortactin serine/threonine phosphorylation is even more poorly understood. Finally, the roles of cortactin during tumour progression, and how these are affected by other signalling pathways upregulated in particular cancers (e.g. those initiated by specific receptor tyrosine kinases) are likely to prove fruitful avenues of research.
Acknowledgments
R.J.D. is supported by the National Health and Medical Research Council of Australia and the Cancer Council New South Wales.
References
- 1.Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell (Cambridge, Mass.) 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Ridley A. J. Rho GTPases and cell migration. J. Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 3.Engqvist-Goldstein A. E. Y., Drubin D. G. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 4.Fehrenbacher K., Huckaba T., Yang H.-C., Boldogh I., Pon L. Actin comet tails, endosomes and endosymbionts. J. Exp. Biol. 2003;206:1977–1984. doi: 10.1242/jeb.00240. [DOI] [PubMed] [Google Scholar]
- 5.Burridge K., Wennerberg K. Rho and Rac take center stage. Cell (Cambridge, Mass.) 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 6.Pollard T., Blanchoin L., Mullins R. D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Ann. Rev. Biophys. Biomol. Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 7.Higgs H. N., Pollard T. D. Regulation of actin filament network formation through Arp2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 8.Wu H., Reynolds A. B., Kanner S. B., Vines R. R., Parsons J. T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan X., Hu X., Hampton B., Burgess W. H., Friesel R., Maciag T. Murine cortactin is phosphorylated in response to fibroblast growth factor-1 on tyrosine residues late in the G1 phase of the BALB/c 3T3 cell cycle. J. Biol. Chem. 1993;268:24427–24431. [PubMed] [Google Scholar]
- 10.Schuuring E., Vernoeven E., Litvinov S., Michalides R. J. A. M. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol. Cell. Biol. 1993;13:2891–2898. doi: 10.1128/mcb.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohoka Y., Takai Y. Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes to Cells. 1998;3:603–612. doi: 10.1046/j.1365-2443.1998.00216.x. [DOI] [PubMed] [Google Scholar]
- 12.Weed S. A., Karginov A., Schafer D. A., Weaver A. M., Kinley A. W., Cooper J. A., Parsons J. T. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rossum A. G. S. H., de Graaf J. H., Schuuring-Scholtes E., Kluin P. M., Fan Y.-X., Zhan X., Moolenaar W. H., Schuuring E. Alternative splicing of the actin binding domain of human cortactin affects cell migration. J. Biol. Chem. 2003;278:45672–45679. doi: 10.1074/jbc.M306688200. [DOI] [PubMed] [Google Scholar]
- 14.Katsube T., Takahisa M., Ueda R., Hashimoto N., Kobayashi M., Togashi S. Cortactin associates with the cell–cell junction protein ZO-1 in both Drosophila and mouse. J. Biol. Chem. 1998;273:29672–29677. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- 15.Weed S. A., Parsons J. T. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 16.Weaver A. M., Heuser J. E., Karginov A. V., Lee W.-L., Parsons J. T., Cooper J. A. Interaction of cortactin and N-WASP with Arp2/3 complex. Curr. Biol. 2002;12:1270–1278. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang C., Ni Y., Wang T., Gao Y., Haudenschild C. C., Zhan X. Down-regulation of the filamentous actin cross-linking activity of cortactin by src-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:13911–13915. doi: 10.1074/jbc.272.21.13911. [DOI] [PubMed] [Google Scholar]
- 18.Campbell D. H., Sutherland R. L., Daly R. J. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 1999;59:5376–5385. [PubMed] [Google Scholar]
- 19.Kitamura D., Kaneko H., Miyagoe Y., Ariyasu T., Watanabe T. Isolation and characterization of a novel human gene expressed specifically in the cells of the hematopoietic lineage. Nucleic Acids Res. 1989;17:9367–9379. [PMC free article] [PubMed] [Google Scholar]
- 20.Kessels M. M., Engqvist-Goldstein A. E. Y., Drubin D. G. Association of mouse actin-binding protein 1 (mAbp1/SH3P7), a src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol. Biol. Cell. 2000;11:393–412. doi: 10.1091/mbc.11.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miglarese M. R., Mannion-Henderson J., Wu H., Parsons J. T., Bender T. P. The protein tyrosine kinase substrate cortactin is differentially expressed in murine B lymphoid tumors. Oncogene. 1994;9:1989–1997. [PubMed] [Google Scholar]
- 22.Zhan X., Haudenschild C. C., Ni Y., Smith E., Huang C. Upregulation of cortactin expression during the maturation of megakaryocytes. Blood. 1997;89:457–464. [PubMed] [Google Scholar]
- 23.Hiura K., Lim S., Little S. P., Lin S., Sato M. Differentiation dependent expression of tensin and cortactin in chicken osteoclasts. Cell Motil. Cytoskeleton. 1995;30:272–284. doi: 10.1002/cm.970300405. [DOI] [PubMed] [Google Scholar]
- 24.Wu H., Montone K. T. Cortactin localization in actin-containing adult and fetal tissues. J. Histochem. Cytochem. 1998;46:1189–1191. doi: 10.1177/002215549804601011. [DOI] [PubMed] [Google Scholar]
- 25.Schuuring E. The involvement of the 11q13 region in human malignancies: cyclin D1 and EMS1 are two candidate oncogenes – a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- 26.Ormandy C. J., Musgrove E. A., Hui R., Daly R. J., Sutherland R. L. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 2003;78:323–335. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- 27.Schuuring E., Verhoeven E., Mooi W., Michalides R. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1 within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–361. [PubMed] [Google Scholar]
- 28.Patel A. M., Incognito L. S., Schechter G. L., Wasilenko W. J., Somers K. D. Amplification and expression of EMS-1 (cortactin) in head and neck squamous cell carcinoma cell lines. Oncogene. 1996;12:31–35. [PubMed] [Google Scholar]
- 29.Campbell D., deFazio A., Sutherland R., Daly R. Expression and tyrosine phosphorylation of EMS1 in human breast cancer cell lines. Int. J. Cancer. 1996;68:485–492. doi: 10.1002/(SICI)1097-0215(19961115)68:4<485::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Hui R., Ball J. R., Macmillan R. D., Kenny F. S., Prall O. W. J., Campbell D. H., Cornish A. L., McClelland R. A., Daly R. J., Forbes J. F., Blamey R. W., Musgrove E. A., Robertson J. F. R., Nicholson R. I., Sutherland R. L. EMS1 gene expression in primary breast cancer: relationship to cyclin D1 and oestrogen receptor expression and patient survival. Oncogene. 1998;17:1053–1059. doi: 10.1038/sj.onc.1202023. [DOI] [PubMed] [Google Scholar]
- 31.Bringuier P. P., Tamimi Y., Schuuring E., Schalken J. Expression of cyclin D1 and EMS1 in bladder tumours; relationship with chromosome 11q13 amplification. Oncogene. 1996;12:1747–1753. [PubMed] [Google Scholar]
- 32.Lagarkova M. A., Boitchenko V. E., Mescheryakov A. A., Kashkarova U. A., Nedospasov S. A. Human cortactin as a putative cancer antigen. Oncogene. 2000;19:5204–5207. doi: 10.1038/sj.onc.1203826. [DOI] [PubMed] [Google Scholar]
- 33.Hui R., Campbell D. H., Lee C. S. L., McCaul K., Horsfall D. J., Musgrove E. A., Daly R. J., Seshadri R., Sutherland R. L. EMS1 amplification can occur independently of CCND1 or INT2 amplification at 11q13 and may identify different phenotypes in primary breast cancer. Oncogene. 1997;15:1617–1623. doi: 10.1038/sj.onc.1201311. [DOI] [PubMed] [Google Scholar]
- 34.Uruno T., Liu J., Zhang P., Fan Y.-X., Egile C., Li R., Mueller S. C., Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 35.Wu H., Parsons J. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver A. M., Karginov A. V., Kinley A. W., Weed S. A., Li Y., Parsons J. T., Cooper J. A. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Current Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 37.He H., Watanabe T., Zhan X., Huang C., Schuuring E., Fukami K., Takenawa T., Kumar C. C., Simpson R. J., Maruta H. Role of phosphatidylinositol 4,5-bisphosphate in Ras/Rac-induced disruption of the cortactin-actomyosin II complex and malignant transformation. Mol. Cell. Biol. 1998;18:3829–3837. doi: 10.1128/mcb.18.7.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparks A. B., Rider J. E., Hoffman N. G., Fowlkes D. M., Quilliam L. A., Kay B. K. Distinct ligand preferences of Src homology 3 domains from Src, Yes, Abl, Cortactin, p53bp2, PLCγ, Crk and Grb2. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1540–1544. doi: 10.1073/pnas.93.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessels M. M., Engqvist-Goldstein A. E. Y., Drubin D. G., Qualmann B. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J. Cell Biol. 2001;153:351–366. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou P., Estrada L., Kinley A. W., Parsons J. T., Votjek A. B., Gorski J. L. Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum. Mol. Genet. 2003;12:1981–1993. doi: 10.1093/hmg/ddg209. [DOI] [PubMed] [Google Scholar]
- 41.Qualmann B., Boeckers T. M., Jeromin M., Gundelfinger E. D., Kessels M. M. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J. Neurosci. 2004;24:2481–2495. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhan X., Plourde C., Hu X., Friesel R., Maciag T. Association of fibroblast growth factor receptor-1 with c-Src correlates with association between c-Src and cortactin. J. Biol. Chem. 1994;269:20221–20224. [PubMed] [Google Scholar]
- 43.Vuori K., Ruoslahti E. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 44.Dehio C., Prevost M. C., Sansonetti P. J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas S. M., Soriano P., Imamoto A. Specific and redundant roles of src and Fyn in organizing the cytoskeleton. Nature (London) 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- 46.Okamura H., Resh M. D. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J. Biol. Chem. 1995;270:26613–26618. doi: 10.1074/jbc.270.44.26613. [DOI] [PubMed] [Google Scholar]
- 47.van Damme H., Brok H., Schuuring-Scholtes E., Schuuring E. The redistribution of cortactin into cell-matrix contact sites in human carcinoma cells with 11q13 amplification is associated with both overexpression and post-translational modification. J. Biol. Chem. 1997;272:7374–7380. doi: 10.1074/jbc.272.11.7374. [DOI] [PubMed] [Google Scholar]
- 48.Huang C., Liu J. L., Haudenschild C. C., Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 49.Head J. A., Jiang D., Li M., Zorn L. J., Schaefer E. M., Parsons J. T., Weed S. A. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol. Biol. Cell. 2003;14:3216–3229. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J., Asawa T., Takato T., Sakai R. Cooperative roles of Fyn and cortactin in cell migration of metastatic murine melanoma. J. Biol. Chem. 2003;278:48367–48376. doi: 10.1074/jbc.M308213200. [DOI] [PubMed] [Google Scholar]
- 51.Gallet C., Rosa J. P., Habib A., Lebret M., Levy-Toledano S., Maclouf J. Tyrosine phosphorylation of cortactin associated with Syk accompanies thromboxane analogue-induced platelet shape change. J. Biol. Chem. 1999;274:23610–23616. doi: 10.1074/jbc.274.33.23610. [DOI] [PubMed] [Google Scholar]
- 52.Craig A. W. B., Zirngibl R., Williams K., Cole L.-A., Greer P. A. Mice devoid of Fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell Biol. 2001;21:603–613. doi: 10.1128/MCB.21.2.603-613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapus A., Di Ciano C., Sun J., Zhan X., Kim L., Wong T. W., Rotstein O. D. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell–cell contact sites. The role of Fyn and Fer kinases. J. Biol. Chem. 2000;275:32289–32298. doi: 10.1074/jbc.M003172200. [DOI] [PubMed] [Google Scholar]
- 54.Fan L., Di Ciano-Oliveira C., Weed S. A., Craig A. W. B., Greer P. A., Rotstein O. D., Kapus A. Actin depolymerization-induced tyrosine phosphorylation of cortactin: the role of Fer kinase. Biochem. J. 2004;380:581–591. doi: 10.1042/BJ20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crostella L., Lidder S., Williams R., Skouteris G. G. Hepatocyte growth factor/scatter factor induces phosphorylation of cortactin in A431 cells in a Src kinase-independent manner. Oncogene. 2001;20:3735–3745. doi: 10.1038/sj.onc.1204474. [DOI] [PubMed] [Google Scholar]
- 56.Huang C., Tandon N., Greco N., Ni Y., Wang T., Zhan X. Proteolysis of platelet cortactin by calpain. J. Biol. Chem. 1997;272:19248–19252. doi: 10.1074/jbc.272.31.19248. [DOI] [PubMed] [Google Scholar]
- 57.Dudek S. M., Birukov K. G., Zhan X., Garcia J. G. N. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 2002;298:511–519. doi: 10.1016/s0006-291x(02)02492-0. [DOI] [PubMed] [Google Scholar]
- 58.Bourguignon L. Y. W., Zhu H., Shao L., Chen Y.-W. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J. Biol. Chem. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- 59.Vidal C., Geny B., Melle J., Jandrot-Perrus M., Fontenay-Roupie M. Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood. 2002;100:4462–4469. doi: 10.1182/blood.V100.13.4462. [DOI] [PubMed] [Google Scholar]
- 60.Machesky L. M., Mullins R. D., Higgs H. N., Kaiser D. A., Blanchoin L., May R. C., Hall M. E., Pollard T. D. SCAR, A WASP-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver A. M., Young M. E., Lee W.-L., Cooper J. A. Integration of signals to the Arp2/3 complex. Curr. Opinion Cell Biol. 2003;15:23–30. doi: 10.1016/s0955-0674(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 62.Caron E. Regulation of Wiskott–Aldrich syndrome protein and related molecules. Curr. Opin. Cell Biol. 2002;14:82–87. doi: 10.1016/s0955-0674(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 63.Torres E., Rosen M. K. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol. Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 64.Cory G. O. C., Cramer R., Blanchoin L., Ridley A. J. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell. 2003;11:1229–1239. doi: 10.1016/s1097-2765(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 65.Eden S., Rohatgi R., Podtelejnikov A. V., Mann M., Kirschner M. W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature (London) 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 66.Miki H., Yamaguchi H., Suetsugu S., Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature (London) 2000;408:732–735. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- 67.Soderling S. H., Binns K. L., Wayman G. A., Davee S. M., Ong S. H., Pawson T., Scott J. D. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat. Cell Biol. 2002;4:970–975. doi: 10.1038/ncb886. [DOI] [PubMed] [Google Scholar]
- 68.Hussain N. K., Jenna S., Glogauer M., Quinn C. C., Wasiak S., Guipponi M., Antonarakis S. E., Kay B. K., Stossel T. P., Lamarche-Vane N., McPherson P. S. Endocytic protein intersectin-1 regulates actin assembly via Cdc42 and N-WASP. Nat. Cell Biol. 2001;3:927–932. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- 69.Goode B. L., Rodal A. A., Barnes G., Drubin D. G. Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J. Cell Biol. 2001;153:627–634. doi: 10.1083/jcb.153.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uruno T., Liu J., Li Y., Smith N., Zhan X. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with Neural Wiscott–Aldrich syndrome Protein (N-WASP) and cortactin during branched actin filament network formation. J. Biol. Chem. 2003;278:26086–26093. doi: 10.1074/jbc.M301997200. [DOI] [PubMed] [Google Scholar]
- 71.Zettl M., Way M. New tricks for an old dog? Nat. Cell. Biol. 2001;3:E74–E75. doi: 10.1038/35060152. [DOI] [PubMed] [Google Scholar]
- 72.Schafer D. A., D'souza-Schorey C., Cooper J. A. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1:892–903. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- 73.Kinley A. W., Weed S. A., Weaver A. M., Karginov A. V., Bissonette E., Cooper J. A., Parsons J. T. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 2003;13:384–393. doi: 10.1016/s0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Quiles N., Rohatgi R., Anton I. M., Medina M., Saville S. P., Miki H., Yamaguchi H., Takenawa T., Hartwig J. H., Geha R. S., Rarnesh N. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat. Cell Biol. 2001;3:484–491. doi: 10.1038/35074551. [DOI] [PubMed] [Google Scholar]
- 75.Schafer D. A., Weed S. A., Binns D., Karginov A. V., Parsons J. T., Cooper J. A. Dynamin 2 and cortactin regulate actin assembly and filament organization. Current Biol. 2002;12:1852–1857. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim K., Hou P., Gorski J. L., Cooper J. A. Effect of Fgd1 on cortactin in Arp2/3 complex-mediated actin assembly. Biochemistry. 2004;43:2422–2427. doi: 10.1021/bi036173t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muhlberg A. B., Warnock D. E., Schmid S. L. Domain structure and intramolecular regulation of dynamin GTPase. EMBO J. 1997;16:6676–6683. doi: 10.1093/emboj/16.22.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirsch K. H., Georgescu M.-M., Ishimaru S., Hanafusa H. CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6211–6216. doi: 10.1073/pnas.96.11.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lynch D. K., Winata S. C., Lyons R. J., Hughes W. E., Lehrbach G. M., Wasinger V., Corthals G., Cordwell S., Daly R. J. A cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 2003;278:21805–21813. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 80.Du Y., Weed S. A., Xiong W.-C., Marshall T. D., Parsons J. T. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol. Cell. Biol. 1998;18:5838–5851. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boeckers T. M., Bockmann J., Kreutz M. R., Gundelfinger E. D. ProSAP/Shank proteins – a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 2002;81:903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 82.Weed S. A., Du Y., Parsons J. T. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 1998;111:2433–2443. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- 83.Hering H., Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J. Neurosci. 2003;23:11759–11769. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ochoa G.-C., Slepnev V. I., Neff L., Ringstad N., Takei K., Daniell L., Kim W., Cao H., McNiven M., Baron R., De Camilli P. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 2000;150:377–389. doi: 10.1083/jcb.150.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizutani K., Miki H., He H., Maruta H., Takenawa T. Essential role of neural Wiskott-Aldrich Syndrome Protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 86.McNiven M. A., Kim L., Krueger E. W., Orth J. D., Cao H., Wong T. W. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell. Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Ciano C., Nie Z., Szaszi K., Lweis A., Urono T., Zhan X., Rotstein O. D., Mak A., Kapus A. Osmotic stress-induced remodeling of the cortical cytoskeleton. Am. J. Physiol. Cell Physiol. 2002;283:C850–C865. doi: 10.1152/ajpcell.00018.2002. [DOI] [PubMed] [Google Scholar]
- 88.Timpson P., Jones G. E., Frame M. C., Brunton V. G. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 89.Krueger E. W., Orth J. D., Cao H., McNiven M. A. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell. 2003;14:1085–1096. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orth J. D., McNiven M. A. Dynamin at the actin-membrane interface. Curr. Opin. Cell Biol. 2003;15:31–39. doi: 10.1016/s0955-0674(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 91.Nagata K.-I., Driessens M., Lamarche N., Gorski J. L., Hall A. Activation of G1 progression, JNK mitogen-activated protein kinase, and actin filament assembly by the exchange factor FGD1. J. Biol. Chem. 1998;273:15453–15457. doi: 10.1074/jbc.273.25.15453. [DOI] [PubMed] [Google Scholar]
- 92.Patel A. S., Schechter G. L., Wasilenko W. J., Somers K. D. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- 93.Somogyi K., Rorth P. Cortactin modulates cell migration and ring canal morphogenesis during Drosophila oogenesis. Mech. Dev. 2004;121:57–64. doi: 10.1016/j.mod.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Liu J., Huang C., Zhan X. Src is required for cell migration and shape changes induced by fibroblast growth factor 1. Oncogene. 1999;18:6700–6706. doi: 10.1038/sj.onc.1203050. [DOI] [PubMed] [Google Scholar]
- 95.Kinnunen T., Kaksonen M., Saarinen J., Kalkkinen N., Peng H. B., Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- 96.Kreienkamp H.-J. Organization of G-protein-coupled receptor signalling complexes by scaffolding proteins. Curr. Opin. Pharmacol. 2002;2:581–586. doi: 10.1016/s1471-4892(02)00203-5. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez-Mariscal L., Betanzos A., Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Sem. Cell Devel. Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- 98.Matter K., Balda M. S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 99.Fanning A. S., Jameson B. J., Jesaitis L. A., Anderson J. M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 100.Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2 and ZO-3, with the C-termini of claudins. J. Cell. Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yap A., Brieher W. M., Gumbiner B. M. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 102.Vasioukhin V., Bauer C., Yin M., Fuchs E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell (Cambridge, Mass.) 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 103.Helwani F. M., Kovacs E. M., Paterson A. D., Verma S., Ali R. G., Fanning A. S., Weed S. A., Yap A. S. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kovacs E. M., Ali R. G., McCormack A. J., Yap A. S. E-cadherin homophilic ligation directly signals through Rac and PI3-kinase to regulate adhesive contacts. J. Biol. Chem. 2002;277:6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- 105.Qualmann B., Kessels M. M., Kelly R. B. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 2000;150:F111–F116. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McNiven M., Cao H., Pitts K. R., Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 107.Cao H., Orth J. D., Chen J., Weller S. G., Heuser J. E., McNiven M. A. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marmor M. D., Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 109.Dustin M. L., Olszowy M. W., Holdorf A. D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P. A., Allen P. M., Shaw A. S. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell (Cambridge, Mass.) 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 110.Shih N.-Y., Li J., Cotran R., Mundel P., Miner J. H., Shaw A. S. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am. J. Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schwarz K., Simons M., Reiser J., Saleem M. A., Faul C., Kriz W., Shaw A. S., Holzman L. B., Mundel P. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J. Clin. Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Engqvist-Goldstein A. E. Y., Warren R. A., Kessels M. M., Keen J. H., Heuser J., Drubin D. G. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 2001;154:1209–1223. doi: 10.1083/jcb.200106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engqvist-Goldstein A. E. Y., Zhang C. X., Carreno S., Barroso C., Heuser J. E., Drubin D. G. RNAi-mediated Hip1R silencing resukts in stable association between the endocytic machinery and the actin assembly machinery. Mol. Biol. Cell. 2004;15:1666–1679. doi: 10.1091/mbc.E03-09-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaksonen M., Peng H. B., Rauvala H. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J. Cell Sci. 2000;113:4421–4426. doi: 10.1242/jcs.113.24.4421. [DOI] [PubMed] [Google Scholar]
- 115.Taunton J., Rowning B. A., Coughlin M. L., Wu M., Moon R. T., Mitchison T. J., Larabell C. A. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cantarelli V. V., Takahashi A., Akeda Y., Nagayama K., Honda T. Interaction of enteropathogenic or enterohemorrhagic Escherichia coli with HeLa cells results in translocation of cortactin to the bacterial adherence site. Infect. Immun. 2000;68:382–386. doi: 10.1128/iai.68.1.382-386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cantarelli V. V., Takahashi A., Yanagihara I., Akeda Y., Imura K., Kodama T., Kono G., Sato Y., Iida T., Honda T. Cortactin is necessary for F-actin accumulation in pedestal structures induced by enteropathogenic Escherichia coli infection. Infect. Immun. 2002;70:2206–2209. doi: 10.1128/IAI.70.4.2206-2209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen X. M., Huang B. Q., Splinter P. L., Cao H., Zhu G., McNiven M. A., LaRusso N. F. Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-Src. Gastroenterol. 2003;125:216–228. doi: 10.1016/s0016-5085(03)00662-0. [DOI] [PubMed] [Google Scholar]
- 119.Dumenil G., Olivo J. C., Pellegrini S., Fellous M., Sansonetti P. J., Tran Van Nhieu G. Interferon α inhibits a Src-mediated pathway necessary for Shigella-induced cytoskeletal rearrangements in epithelial cells. J. Cell Biol. 1998;143:1003–1012. doi: 10.1083/jcb.143.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frischknecht F., Moreau V., Rottger S., Gonfioni S., Reckmann I., Superti-Furga G., Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature (London) 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 121.Fawaz F. S., van Ooij C., Homola E., Mutka S. C., Engel J. N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect. Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cameron L. A., Svitkina T. M., Vignjevic D., Theriot J. A., Borisy G. G. Dendritic organization of actin comet tails. Curr. Biol. 2001;11:130–135. doi: 10.1016/s0960-9822(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 123.Egile C., Loisel T. P., Laurent V., Li R., Pantaloni D., Sansonetti P. J., Carlier M. F. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frischknecht F., Cudmore S., Moreau V., Reckmann I., Rottger S., Way M. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr. Biol. 1999;9:89–92. doi: 10.1016/s0960-9822(99)80020-7. [DOI] [PubMed] [Google Scholar]
- 125.Rodrigo J. P., Garcia L. A., Ramos S., Lazo P. S., Suarez C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2000;6:3177–3182. [PubMed] [Google Scholar]
- 126.Bowden E. T., Barth M., Thomas D., Glazer R. I., Mueller S. C. An invasion-related complex of cortactin, paxillin and PKCμ associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 127.Li Y., Tondravi M., Liu J., Smith E., Haudenschild C. C., Kaczmarek M., Zhan X. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–6911. [PubMed] [Google Scholar]
- 128.Bernards R., Weinberg R. A. Metastasis genes: a progression puzzle. Nature (London) 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]