We present three cases of rosette-forming glioneuronal tumours (RGNT), diagnosed through DNA-methylation profiling, without the classical FGFR1 alteration commonly reported in this entity.
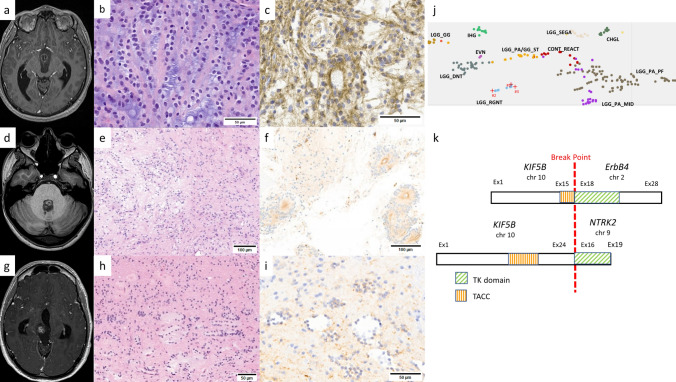
Case 1 involves a 29-year-old man with no significant medical history, presenting with two episodes of severe headaches, occurring 1 year apart, which resolved spontaneously. Ophthalmological examination revealed metamorphopsia in the bitemporal hemifields, also resolving spontaneously. MRI revealed a lesion in the third ventricle with central enhancement, obstructing the interventricular foramina, invading the basal cisterns and making contact with the pituitary stalk and optic chiasm (Fig. 1a). Biopsy during ventriculocysternostomy (VCS) revealed a proliferation forming numerous neurocytic rosettes, occasionally longitudinally sectioned and resembling columns, accompanied by a myxoid background (Fig. 1b). There were no histological signs of aggressiveness. The cells strongly expressed glial markers and synaptophysin (Fig. 1c). Monitoring was decided upon and the patient has been asymptomatic for 6 months.
Fig. 1.
MRI on the T1 sequence revealed well-circumscribed lesions with heterogeneous gadolinium enhancement located in the third ventricle obstructing the two interventricular foramens and invading the basal cisterns in case 1 (a), in the fourth ventricle in case 2 (d) and in the right mesencephalon in case 3 (g). Haematoxylin and eosin (HE) staining showed characteristic neurocytic rosettes viewed longitudinally, with a columnar arrangement in this small biopsy in case 1 (b), a biphasic proliferation with neurocytic rosettes (left) and piloid features (right) in case 2 (e) and rosettes with various size and shapes in case 3 (h). Cell staining for synaptophysin was diffuse and strong in case 1 (c), more heterogeneous in cases 2 (f) and 3 (i). Zoom on t-SNE using idat files freely available on GEO (publication PMID: 29539639) enhanced with personal data on extraventricular neurocytomas (EVN) (j). Schematic representation of KIF5B-ERBB4 fusions in case 1 and in the lung adenocarcinoma described by Guenzi et al. [6] (top) compared to the KIF5B-NTRK2 fusion reported in extraventricular neurocytoma by Gubbiotti et al. [5] (bottom) (k)
Case 2 concerns a 27-year-old woman who had been suffering from subacute headaches of increasing intensity for 2 years. Ophthalmological examination showed bilateral early papilledema. MRI revealed a lesion centred on the fourth ventricle with FLAIR hyperintensity and spontaneous T1 peripheral hyperintensity, without significant gadolinium enhancement. The lesion had infiltrated the cerebellar vermis and extended into the beginning of the foramina of Luschka (Fig. 1d). Histological analysis of surgical resection revealed a biphasic proliferation alternating oligo-like or piloid areas with areas containing neurocytic and perivascular rosettes (Fig. 1e). There were no histological signs of aggressiveness. The cells exhibited strong expression of glial markers and heterogeneous synaptophysin expression (Fig. 1f). The patient remained clinically and radiologically free of relapse at the last follow-up which took place 14 months post-surgery.
Case 3 concerns a 33-year-old man with migraines since childhood, with recent onset of photophobia and binocular diplopia accompanying the headaches as well as Parinaud's syndrome. MRI revealed a lesion in the right mesencephalon with heterogeneous enhancement after gadolinium injection, extending into the quadrigeminal cistern, pineal region and thalamus (Fig. 1g). Surgical resection showed a biphasic proliferation alternating oligo-like or piloid areas with Rosenthal fibres and areas containing rosette formations centred on amorphous, hyaline material (Fig. 1h). There were no histological signs of aggressiveness. Immunohistochemistry revealed strong Olig2 expression and weaker, focal expression of GFAP and synaptophysin (Fig. 1i). Last follow-up MRI, which took place six years post-surgery, showed no tumour recurrence.
For case 1, DNA-methylation profiling using v2 chip revealed diagnostic scores of 0.98693 for MC RGNT in v12.8 and 0.71257 in v12.5. Cases 2 and 3, analysed using v1 chips, showed diagnostic scores of 0.99851 and 0.78276 in v12.5 and 0.99951 and 0.49826 in v12.8, respectively. t-SNE analysis localised the cases within the MC RGNT cluster (Fig. 1j). Copy number variation (CNV) analysis showed a flat profile for all three cases.
All three cases underwent next-generation sequencing (NGS) molecular analysis with large panel targeting the FGFR1 gene, with no alterations of this latter found: DRAGON (SureSelect CD Curie CGP) panel was used for cases 1 and 2 and DNA panel from Toulouse University Hospital for case 3 (Supplementary Tables 1 and 2, online resource). For case 1, no molecular alteration was identified. It is noteworthy that this case could not undergo RNA sequencing due to the limited amount of biopsy material available. Case 2 underwent RNA sequencing using the RNA Illumina TSO500 panel and panel from Toulouse University Hospital while case 3 utilised the Illumina TruSight RNA Fusion panel (Supplementary Tables 3, 4 and 5, online resource).
Case 2 exhibited an AKT2 E17K mutation, previously reported in thyroid and breast cancers [7, 9]. AKT2 is a serine/threonine kinase playing a role in the PTEN/PI3K/AKT pathway, including numerous oncogenes, in particular PIK3CA, which is frequently mutated in RGNT [1, 8]. The E17K hotspot mutation occurs at the beginning of exon 3 within the protein's functional domain. A similar mutation has been described in breast, colorectal and ovarian carcinomas in the AKT1 gene, with experimental studies demonstrating the oncogenic nature of this mutation through overactivation of the protein's kinase activity, thereby setting in motion the cell survival pathway [2]. The significant similarity between the AKT1 and AKT2 genes suggests the oncogenic potential of the E17K mutation can be extended to AKT2.
Case 3 showed a KIF5::ERBB4 fusion which was structurally identical to that recently reported in lung adenocarcinoma (Fig. 1k) [6]. The KIF5B protein has already been observed in several oncogenic fusions with other tyrosine kinases [4]. The coiled-coil portion of KIF5B leads to dimerisation, followed by transphosphorylation and activation of its partner's tyrosine kinase domain. The ERBB4 protein belongs to the MAPK signalling pathway. In the brain, no ERBB4 fusion has yet been reported. However, a fusion between the KIF5B gene and NTRK2, coding another protein with tyrosine kinase activity, has been described in an extraventricular neurocytoma (Fig. 1k) [5].
RGNT are defined in the 5th edition of the World Health Organization (WHO) classification of central nervous system tumours as biphasic tumours comprising both glial and neurocytic components, with neurocytic rosette or perivascular pseudorosette formations associated with synaptophysin expression. Desirable criteria include the presence of a FGFR1 mutation with coexisting PIK3CA and/or NF1 mutation. Several teams have reported the constant presence of FGFR1 mutation in their RGNT series, suggesting that this criterion should appear as essential in the next classification [1, 8]. Nevertheless, our three cases of RGNT proven by DNA-methylation profiling show that the absence of FGFR1 alteration does not eliminate this diagnosis. Furthermore, two of our cases which underwent exhaustive molecular investigations (NGS and RNA sequencing with a large panel) presented only a single oncogenic event. On the other hand, our observations concur with previous studies on the exclusive localisation of these tumours in the midline which could be a significant diagnostic aid given the lack of specificity of current WHO diagnostic criteria [1].
This study extends the spectrum of RGNT by including tumours with genetic abnormalities other than FGFR1 mutation. Our finding is important from a therapeutic point of view since there are AKT inhibitors involved in clinical trials as well as second-generation pan-ErbB tyrosine kinase inhibitors [3, 10].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the “111 des Arts” Association for their support. Samples were retrieved from Toulouse University Hospital Centre Tumour Bank BB-0033-00014, Montpellier University Hospital Centre Tumour Bank BB-0033-00031 and Bordeaux University Hospital Centre Tumour Bank BB- 0033-00036.
Funding
This article is funded by the association “111 des Arts”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Appay R, Bielle F, Sievers P, Barets D, Fina F, Boutonnat J et al (2022) Rosette-forming glioneuronal tumours are midline, FGFR1-mutated tumours. Neuropathol Appl Neurobiol 48(5):e12813. 10.1111/nan.12813 10.1111/nan.12813 [DOI] [PubMed] [Google Scholar]
- 2.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM et al (2007) A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448(7152):439–344. 10.1038/nature05933 10.1038/nature05933 [DOI] [PubMed] [Google Scholar]
- 3.Davies BR, Guan N, Logie A, Crafter C, Hanson L, Jacobs V et al (2015) Tumors with AKT1E17K mutations are rational targets for single agent or combination therapy with AKT inhibitors. Mol Cancer Ther 14(11):2441–2451. 10.1158/1535-7163.MCT-15-0230 10.1158/1535-7163.MCT-15-0230 [DOI] [PubMed] [Google Scholar]
- 4.Gow CH, Liu YN, Li HY, Hsieh MS, Chang SH, Luo SC et al (2018) Oncogenic function of a KIF5B-MET fusion variant in non-small cell lung cancer. Neoplasia 20(8):838–847. 10.1016/j.neo.2018.06.007 10.1016/j.neo.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubbiotti MA, Santi M, Storm PB, Li M, Xu F, Abdullaev Z et al (2023) First-time identification of a KIF5B-NTRK2 fusion in extraventricular neurocytoma. J Neuropathol Exp Neurol 82(3):272–275. 10.1093/jnen/nlad002 10.1093/jnen/nlad002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenzi E, Pluvy J, Guyard A, Nguenang M, Rebah K, Benrahmoune Z et al (2021) A new KIF5B-ERBB4 gene fusion in a lung adenocarcinoma patient. ERJ Open Res 7(1):00582–02020. 10.1183/23120541.00582-2020 10.1183/23120541.00582-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S et al (2018) Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 24(13):3059–3668. 10.1158/1078-0432.CCR-18-0373 10.1158/1078-0432.CCR-18-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sievers P, Appay R, Schrimpf D, Stichel D, Reuss DE, Wefers AK et al (2019) Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol 138(3):497–504. 10.1007/s00401-019-02038-4 10.1007/s00401-019-02038-4 [DOI] [PubMed] [Google Scholar]
- 9.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC et al (2012) The landscape of cancer genes and mutational processes in breast cancer. Nature 486(7403):400–404. 10.1038/nature11017 10.1038/nature11017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Li J (2019) Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer. Onco Targets Ther 12:6535–6548. 10.2147/OTT.S198945 10.2147/OTT.S198945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.