Abstract
Insects express arthro-series glycosphingolipids, which contain an α1,4-linked GalNAc residue. To determine the genetic basis for this linkage, we cloned a cDNA (CG17223) from Drosophila melanogaster encoding a protein with homology to mammalian α1,4-glycosyltransferases and expressed it in the yeast Pichia pastoris. Culture supernatants from the transformed yeast were found to display a novel UDP-GalNAc:GalNAcβ1,4GlcNAcβ1-R α-N-acetylgalactosaminyltransferase activity when using either a glycolipid, p-nitrophenylglycoside or an N-glycan carrying one or two terminal β-N-acetylgalactosamine residues. NMR and MS in combination with glycosidase digestion and methylation analysis indicate that the cloned cDNA encodes an α1,4-N-acetylgalactosaminyltransferase. We hypothesize that this enzyme and its orthologues in other insects are required for the biosynthesis of the N5a and subsequent members of the arthro-series of glycolipids as well as of N-glycan receptors for Bacillus thuringiensis crystal toxin Cry1Ac.
Keywords: Drosophila; glycolipid; glycosyltransferase; insect; α1,4-N-acetylgalactosaminyltransferase; N-glycan
Abbreviations: MALDI–TOF-MS, matrix-assisted laser-desorption ionization–time-of-flight mass spectrometry; RT, reverse transcriptase
INTRODUCTION
The sequencing of the complete Drosophila genome has revealed that there are many putative glycosyltransferases in this organism [1]; however, our knowledge regarding the actual biochemical function of most of these proteins is incomplete. In the present study, we have identified two sections of the fly genome, which when translated in silico showed homology to α1,4-glycosyltransferases. In mammals, α1,4-glycosyltransferases, specifically the Gb3/CD77/Pk-synthesizing α1,4-galactosyltransferase [2–6] and an α1,4-N-acetylglucosaminyltransferase involved in the biosynthesis of some O-glycans [7], have been found to be members of the same family of glycosyltransferases (CAZy family 32 [8,9], see http://afmb.cnrs-mrs.fr/CAZY/GT_32.html). Other members of this family include the Och1 α1,6-mannosyltransferases from various yeasts as well as predicted proteins encoded by the genomes of Arabidopsis thaliana and some bacteria. However, the fact that glycoconjugate structures present in mammals may be absent from lower organisms, and vice versa, even though there are homologous glycosyltransferases, means that quite different acceptor/donor combinations have to be considered for members of the same glycosyltransferase family.
To identify the molecular function of Drosophila α1,4-glycosyltransferase homologues, we considered which α1,4-linkages have already been found in insects. Whereas an α1,4-galactosyltransferase activity capable of converting the core 1 O-glycan disaccharide into a Galα1,4Galβ1,3GalNAc structure has been detected in Mamestra brassica cells [10], an α1,4-linked N-acetylgalactosamine residue has been found as part of the glycosphingolipids of some insects, including Drosophila, specifically within the structure GalNAcα1,4GalNAcβ1,4GlcNAcβ1,3Manβ1,4GlcβCer [11,12]. On this basis, we hypothesized that one of the potential genes (CG17223, hereafter called α4GT1) should encode a Drosophila melanogaster UDP-GalNAc:GalNAcβ1,4GlcNAcβ1-R α-N-acetylgalactosaminyltransferase, to prove this, we assayed the recombinant form of the enzyme with N-glycan, p-nitrophenyl and glycolipid substrates.
EXPERIMENTAL
Cloning of Drosophila melanogaster α4GT1 cDNA
BLAST searching using the sequence of the human Gb3/CD77/Pk-synthesizing α1,4-galactosyltransferase resulted in the detection of two possible homologues in the Drosophila melanogaster genome, designated by us as α4GT1 and α4GT2 (reading frame based on the in silico predicted CG5878 gene). The open reading frame for α4GT1 was assembled from a genomic fragment sequence (GenBank® accession number AC007765, mapped to chromosome 2L, region 23C1/23C5). The cDNA encoding α4GT1 was amplified by RT (reverse transcriptase)–PCR using sense (agt1-1) 5′-CCTCTGGTTGCCCATTGC-3′ and antisense (agt1-2) 5′-GAAGTATTCGCCAGCAGC-3′ primers (20 pmol of each primer, annealing at 50 °C, 35 cycles) with Drosophila Canton S cDNA and Hot Start polymerase (Promega); the DNA product was subcloned into the pTOPO-2.1 vector (Invitrogen).
The cDNA encoding the soluble form of putative α4GT1 was obtained by RT–PCR employing sense (agt1-3) 5′-CGCCGA-ATTCTACTTCGGAAAATAAATACCACTC-3′ and antisense (agt1-4) 5′-CGCCGGTACCTCTAGAAGTATTCGCCAGCAGC-3′ primers with an annealing temperature of 55 °C and Pfu polymerase (Stratagene). After A-tailing, the DNA fragment was cloned into the pTOPO-2.1 vector and subsequently cleaved with the restriction enzymes EcoRI and KpnI and re-subcloned into EcoRI–KpnI-digested and dephosphorylated pPICZαC expression vector (Invitrogen). Sequences of DNA constructs were determined using the dideoxy dye-terminator technique and analysed using an Applied Biosystems 373A DNA Sequenator.
Expression of Drosophila α4GT1
After transformation of Pichia pastoris strain GS115 with the linearized expression vector, colonies were selected for expression. Preculturing overnight in MGYC medium [1% (w/v) yeast extract, 2% (w/v) peptone 140, 1% (w/v) casamino acids, 1.34% (w/v) yeast nitrogen base, 4×10−5% (w/v) biotin and 1% (v/v) glycerol] was performed at 30 °C in the presence of zeocin, whereas expression was induced using methanol-containing MMYC medium [composition as for MGYC, except that 1% (v/v) methanol substitutes for glycerol] at 16 or 30 °C [13]. Culture supernatants were concentrated 10- or 20-fold using Ultrafree centrifugal concentration devices (Millipore; cut off Mr 30000) before the determination of enzymic activity. Alternatively, the supernatants from 100 ml cultures were concentrated 10-fold using an Amicon ultrafiltration device, washed with 25 mM Tris/HCl (pH 7), reconcentrated and applied to Affi-Gel Blue-Sepharose (2.5 ml). The column was washed with 25 mM Tris/HCl (pH 7) until the absorbance A280 stabilized, and the enzyme activity was then eluted with the same buffer containing 0.6 M NaCl.
MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight mass spectrometry)-based assay using an N-glycopeptide substrate
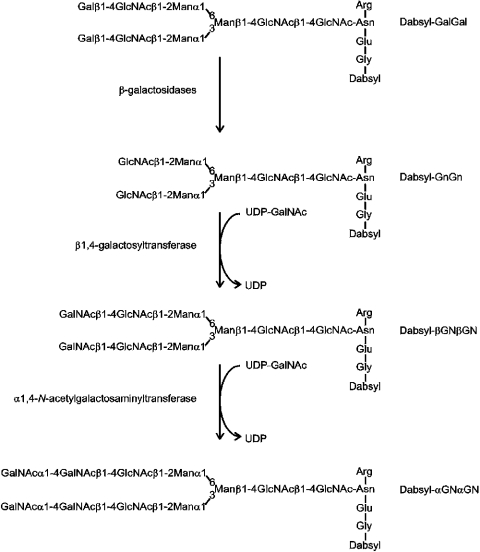
The dabsyl-glycopeptide βGNβGN (carrying two LacDiNAc-modified antennae) was derived by enzymic modification of a dabsylated desialylated fibrin glycopeptide (see Figure 1). Bovine fibrin was digested with pronase to yield a glycopeptide with the sequence GENR; the peptide was then desialylated, dabsylated and purified as described previously [14]. Thereafter, 300 nmol of the resulting dabsyl-GalGal was degalactosylated using Aspergillus oryzae and bovine testes β-galactosidases to remove respectively all β1,4- and β1,3-linked galactose residues (approx. 90% of the galactose residues are sensitive to the Aspergillus enzyme). The resulting dabsyl-GnGn was then purified by reversed phase-HPLC (ODS Hypersil 5 μm, 0.4 cm×25 cm) at room temperature (23 °C) using a gradient of 15–50% B over 20 min (buffer A: 100 mM ammonium acetate, pH 6; buffer B: buffer A/propan-2-ol/acetonitrile in the ratio 30:10:60); A500 was monitored. The collected dabsyl-GnGn was freeze-dried, dissolved in water and incubated for 65 h at 37 °C with 0.6 unit of bovine β1,4-galactosyltransferase (Fluka, Buchs, Switzerland) in the presence of 9 mM UDP-GalNAc, 80 mM Tris (pH 7.5) and 10 mM MnCl2 (exploiting the ability of bovine milk β1,4-galactosyltransferase to also utilize UDP-GalNAc as a donor [15]). The thereby-generated dabsyl-βGNβGN (92.5% conversion) was again purified by reversed-phase HPLC as above and freeze-dried. Degalactosylation and ‘β-GalNAcylation’ were monitored by MALDI–TOF MS. The amounts of glycopeptide were estimated by reversed-phase HPLC peak height comparison with the original dabsyl-GalGal glycopeptide whose amino sugar content had been previously determined.
Figure 1. N-glycan remodelling by β-galactosidases and by β- and α-GalNAc transferase activities.
Dabsyl-βGNβGN, dabsyl-GnGn and dabsyl-GalGal (0.1 mM) were assayed as substrates for the Drosophila α4GT1 in mixtures of 100 mM Tris/HCl (pH 8) (or other buffer), 10 mM MnCl2 (or other bivalent cation) with 1 mM UDP-GalNAc (or other tested UDP-sugar) and 0.5 μl of concentrated culture supernatant or 3 μl of Affi-Gel-purified enzyme (final volume of 5 μl). After incubation for the time period and temperature mentioned above, 0.1 μl aliquots were removed and mixed with 0.9 μl of water and 1 μl of 1% (w/v) α-cyano-4-hydroxycinnamic acid in 70% (v/v) acetonitrile. Spectra were collected with a ThermoBioanalysis Dynamo bench-top MALDI–TOF-MS.
HPLC-based assay using a p-nitrophenyl substrate
Assays using p-nitrophenyl-β-N-acetylgalactosaminide as acceptor were performed in PCR tubes in a final volume of 16 μl containing 100 mM Tris/HCl (pH 8), 3.3 mM p-nitrophenyl-β-N-acetylgalactosaminide (stock 50–200 mM in DMSO), 10 mM MnCl2, 5.6 mM UDP-GalNAc and 2.5 μl of 10-fold concentrated culture supernatant. After overnight incubation at 23 or 37 °C, the mixture was injected on to an ODS Hypersil 5 μm reversed-phase HPLC column (0.4 cm×25 cm). Components were eluted using a gradient of 40–88% B [buffer A, 100 mM ammonium formate (pH 4); buffer B, 30% methanol in water] over 20 min at 23 °C at a flow rate of 0.7 ml/min and detected by measuring A245. For the determination of the Km (app) value, the acceptor concentration was varied between 1.6 and 25 mM while keeping the final concentration of DMSO at 12.5% (v/v); the reactions, in this case, were incubated for 4 h at 37 °C in the presence of 5.6 mM UDP-GalNAc. The effect of pH was tested using a series of solutions of 2-amino-2-methyl-1,3-propanediol as buffer.
Exoglycosidase digestion
After incubation of 10 nmol of dabsyl-βGNβGN with α4GT1 and purification by reversed-phase HPLC, the fractions containing predominantly the glycopeptide carrying two terminal α-GalNAc residues (dabsyl-αGNαGN) as judged by MALDI–TOF-MS were pooled and freeze-dried. The purified glycopeptide (0.4 nmol) was then incubated with 5 milliunits of chicken liver N-acetyl-α-galactosaminidase (Sigma) in a final volume of 12.5 μl of 80 mM sodium citrate (pH 4.0) at 37 °C. An aliquot of the incubation, as well as of one containing no enzyme, was removed and analysed by MALDI–TOF-MS. An aliquot of purified p-nitrophenyl disaccharide was also treated in the same manner before reversed-phase HPLC.
NMR analysis
Approx. 400 μg of the HPLC-purified reaction product of p-nitrophenyl-β-N-acetylgalactosaminide with α4GT1 was freeze-dried twice and taken up in 2H2O before NMR analysis. Spectra were recorded at 300 K at 300.13 MHz for 1H and at 75.47 MHz for 13C with a Bruker AVANCE 300 spectrometer equipped with a 5 mm quadrupole nuclear probehead with z-gradients. Data acquisition and processing were performed with the standard XWINNMR software (Bruker, Rheinstetten, Germany). 1H spectra were referenced internally to 2,2-dimethyl-2-silapentane-5-sulphonic acid (δ=0), 13C spectra were referenced externally to 1,4-dioxan (δ=67.40). 1H/13C (HMQC heteronuclear single quantum correlation)- and 1H/13C HMBC (heteronuclear multiple bond correlation) spectra were recorded in the phase-sensitive mode using time-proportional phase increments and pulsed field gradients for coherence selection.
Incubation of a glycolipid substrate with α4GT1
A glycolipid purified from Ascaris suum, modified with an α-galactose and a phosphocholine (P-Cho) moiety (component A; Galα1,3GalNAcβ1,4(P-Cho6)GlcNAcβ1,3Manβ1,4GlcβCer) [16], was treated with hydrofluoric acid and α-galactosidase to yield the structure GalNAcβ1,4GlcNAcβ1,3Manβ1,4GlcβCer. The digestion was verified by MALDI–TOF-MS after butanol extraction of the enzymic degalactosylation. The dried butanol phase containing 10 μg (approx. 7 nmol) of glycolipid was resuspended by sonication in 14 μl of a mixture of 0.2% (w/v) sodium taurodeoxycholate and 50 mM Tris (pH 8). The suspension was aliquoted and assays were performed using 2 nmol of glycolipid and 0.5 μl of a 20-fold concentrated supernatant of yeast expressing α4GT1 with final concentrations of 40 mM Tris (pH 8), 20 mM MnCl2, 2 mM UDP-GalNAc and 0.16% sodium taurodeoxycholate in a volume of 5 μl; incubations were performed at 23 °C. After freeze-drying, the samples were redissolved in butanol and analysed by MALDI–TOF-MS with α-cyano-4-hydroxycinnamic acid as matrix using an Ultraflex TOF–TOF instrument (Bruker-Daltonics, Bremen, Germany).
Methylation analysis
Glycosphingolipids were permethylated and hydrolysed as described previously [17]. After subsequent reduction and peracetylation, the partially methylated alditol acetates obtained were analysed by capillary GLC/MS using the instrumentation and microtechniques described elsewhere [18].
Developmental expression profile
RT–PCR of eight developmental stages and of adult male and female heads and bodies was performed using the OriGene Rapid-Scan™ Drosophila Gene Expression Panel, which consists of dried cDNAs derived from the Canton S strain normalized with respect to the amount of the rp49 transcript. To each well, 12.5 μl of Promega PCR Master Mix and 10 pmol of each primer were added in a final volume of 25 μl. For testing α4GT1 expression, the primers DmαGT1/1 (GCGACGATGTGGCGTAC) and DmαGT1/2 (GTCTGATATGTGCGAGAAC) were used. Before gel electrophoresis, 30 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 2 min were performed.
RESULTS
Cloning of the α4GT1 cDNA
In our theoretical survey of the Drosophila melanogaster genome for glycosyltransferases [1], we noticed the presence of two possible homologues (which we designated α4GT1 and α4GT2) of human α1,4-glycosyltransferases. One of these homologues was encoded by two exons (α4GT1, which corresponds to the CG17223 gene), whereas another theoretical reading frame (α4GT2, the 3′-region of which corresponds to the theoretical reading frame CG5878 on chromosome 3R; see GenBank® accession number AC008209) was found to be interrupted by a 9 kb region containing, as noted by Kaminker et al. [19], a roo transposon. In the initial experiments, the entire reading frame of the former, but no portion of the latter, was isolated by RT–PCR; we, therefore, concentrated on cloning and expression of the α4GT1 cDNA for our subsequent studies. The sequence we obtained from Canton S flies (Figure 2) is not entirely identical with that of the genomic sequence derived from isogenic y; cn bw sp flies. Indeed, the two clones we isolated had seven identical nucleotide substitutions, when compared with the isogenic strain, resulting in four differences at the protein level; one clone had an additional nucleotide substitution resulting in an extra amino acid change. Both cDNA clones, however, were later found to encode proteins with α-N-acetylgalactosaminyltransferase activity.
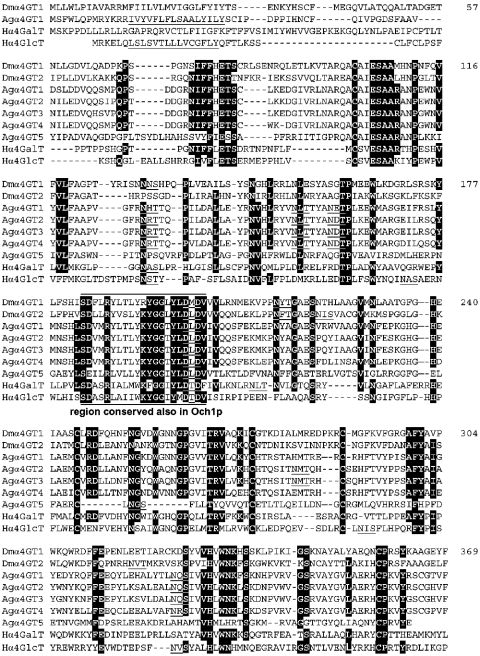
Figure 2. Sequence alignment of animal family 32 α1,4-glycosyltransferases.
The sequences of human Pk α1,4-galactosyltransferase (Hα4GalT), human α1,4-N-acetylglucosaminyltransferase (Hα4GlcT), Drosophila α4GT1, the reconstructed Drosophila α4GT2 and A. gambiae putative α4GT homologues 1–5 were aligned using the Multalin program (prodes.toulouse.inra.fr/multalin/multalin.html). Residues shared by at least one human sequence with at least five of the insect sequences are highlighted, whereas putative transmembrane domains and potential N-glycosylation sites are underlined. The N-terminal regions of Drosophila α4GT2 and A. gambiae putative α4GT homologues 2–5 are not shown for simplicity.
The Drosophila α4GT1 cDNA encodes a protein of 369 amino acids (Figure 2) with 31% identity over 306 residues to the human Gb3/CD77/Pk-synthesizing α1,4-galactosyltransferase and 24% identity over 286 residues to the human α1,4-N-acetylglucosaminyltransferase, both of which are members of CAZy family 32. It has detectable homology neither to the α1,4-N-acetylglucosaminyltransferase domains of the EXT and tout-velu proteins, members of CAZy family 64, involved in heparan sulphate biosynthesis, nor to the Neisseria lgtC α1,4-galactosyltransferase. Furthermore, five tandemly arranged homologues of α4GT1 were detected by database searching of the Anopheles gambiae (mosquito) draft genome (accession number NW045819), of which at least one, Anopheles α4GT1, is trans-cribed as judged by the presence of expressed sequence tag sequences in the databases. Alignments show that all the cysteine residues and the DXD motif, previously noted as being conserved in the C-terminal region of rat Pk α1,4-galactosyltransferase, human α1,4-N-acetylglucosaminyltransferase and Drosophila α4GT1 [4], are also present in the in silico reconstructed Drosophila α4GT2 and all Anopheles homologues (Figure 2). A short region around the DXD motif (consensus DFLRYLXLXXXGGXYXDMD) also exhibits homology with Saccharomyces cerevisiae Och1p α1,6-mannosyltransferase [20], which is also a member of glycosyltransferase family 32. On the basis of studies of other glycosyltransferases, one would assume that this region is associated with acceptor substrate and/or metal-ion binding.
α4GT1 modifies N-glycan substrates
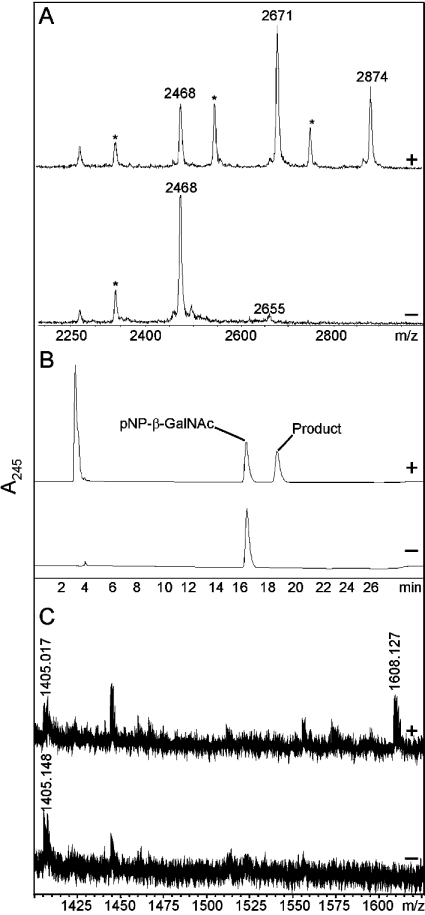
Considering the types of α1,4-linkage known to be present in insects, we expressed α4GT1 in Pichia and tested it with either a core 1 disaccharide or with an N-glycan modified to carry β1,4-linked GalNAc residues. No activity was detected with the core 1 disaccharide Galβ1,3GalNAc and UDP-Gal as substrates (results not shown), whereas the N-glycan substrate, dabsyl-βGNβGN (m/z 2468), proved to be an efficient acceptor. Specifically, assays of Pichia-expressed α4GT1, either in crude supernatants or after partial purification on Affi-Gel Blue-Sepharose, indicated that products with m/z values 203 and 406 greater than that of the original substrate were formed in the presence of Mn(II) and UDP-GalNAc (Figure 3A, upper panel) regardless of whether the enzyme was expressed at 16 or 30 °C. Transferase assays with Pichia-expressed α4GT1 in the absence of UDP-GalNAc (Figure 3A, lower panel) and of Pichia transformed with pPICZαC containing no insert but in the presence of UDP-GalNAc revealed no elongation of the substrate. To test whether the GalNAc transferred by α4GT1 was indeed in an α-linkage, an aliquot of the putative dabsyl-αGNαGN product was purified by reversed-phase HPLC and incubated with chicken liver N-acetyl-α-galactosaminidase, an enzyme previously used in the analysis of Drosophila glycolipids [11]. The subsequent MALDI–TOF-MS analysis showed that overnight incubation resulted in complete removal of all putative α1,4-GalNAc residues (results not shown).
Figure 3. Characterization of the reaction products of Drosophila α4GT1.
(A) MALDI–TOF-MS of dabsyl-βGNβGN (m/z 2468) with Affi-Gel-enriched α4GT1, incubated either for 2 h in the presence of UDP-GalNAc (upper panel) or overnight in the absence of UDP-GalNAc (lower panel). Note that all dabsyl-glycopeptides were subjected to partial laser-induced removal of the distal ring giving rise to peaks (*) m/z 132 smaller than the main substrate or product peak; otherwise the detected peaks correspond to molecular ions of the form [M+H]+. (B) Reversed-phase HPLC analysis of p-nitrophenyl-β-N-acetylgalactosaminide after incubation overnight with Affi-Gel-enriched α4GT1 in the presence (upper panel) and absence (lower panel) of UDP-GalNAc. (C) MALDI–TOF-MS analysis of a core arthro-glycosphingolipid after incubation in the presence (upper panel) and absence (lower panel) of UDP-GalNAc with culture supernatant of Pichia transformed with α4GT1.
In other tests, a monoantennary pyridylaminated N-glycan with a single terminal β1,4-GalNAc residue, generated by partial hexosaminidase, α-mannosidase and β1,4-galactosyltransferase treatments, was also found to be a substrate for α4GT1 (results not shown). Furthermore, assays using UDP-Gal or UDP-GlcNAc as potential donors showed no sign of conversion of dabsyl-βGNβGN, indicating that α4GT1 has a strict donor specificity; tests of the enzyme's activity towards dabsyl-GalGal and dabsyl-GnGn in the presence of UDP-GalNAc also had negative outcomes (results not shown). The accumulated data therefore suggested that a terminal β-linked GalNAc residue is a prerequisite part of the acceptor moiety and that α4GT1 catalyses the formation of an α-GalNAc linkage.
α4GT1 modifies a p-nitrophenyl-linked substrate
To explore the substrate specificity of α4GT1 further, we tested the activity of the recombinant enzyme using p-nitrophenyl-β-N-acetylgalactosaminide, the enzymic product being analysed by reversed-phase HPLC. Whereas no product was seen using control Pichia supernatant or with α4GT1 in the absence of UDP-GalNAc (Figure 3B, lower panel), in the presence of both enzyme and donor this substrate was converted into one with a longer retention time (Figure 3B, upper panel). The substrate and product fractions were collected and analysed by MALDI–TOF; the product fraction contained a species with m/z 203 larger than that of the substrate. Furthermore, after incubation with chicken liver N-acetyl-α-galactosaminidase the product was converted back to a species with the same retention time as the substrate. No apparent conversion of p-nitrophenyl-β-N-acetylglucosaminide by αGT1 to a compound of different retention time occurred when using the same elution programme (results not shown).
As a final proof of the linkage and its anomericity, a larger-scale incubation of α4GT1 with p-nitrophenyl-β-N-acetylgal-actosaminide was used to generate sufficient product for NMR analysis (see Table 1). The 300 MHz 1H NMR spectrum of the disaccharide product displayed four protons in the aromatic region (of the 4-nitrophenyl aglycon), two anomeric protons, ten protons in the bulk region and two N-acetyl groups. The signal at 5.32 ppm had a coupling constant of 8.3 Hz and was assigned to the β-N-acetylgalactosaminide moiety. This was further supported by an HMBC correlation of this proton to a carbon signal at 162.6 ppm. The upfield-shifted proton at 4.98 ppm had a coupling constant of 3.7 Hz, which is consistent with the presence of an α-anomeric linkage. 1H, 1H-COSY and 1H, 13C-HMQC spectra allowed for a full assignment of the proton and carbon signals of this unit as a terminal 2-acetamido-2-deoxy-α-galactopyranosyl moiety. The attachment site of the substrate was identified as C-4 due to the glycosylation shift observed for C-4 at 76.46 ppm.
Table 1. NMR data of α-D-GalpNAc-(1→4)-β-D-GalpNAc-(1→OC6H4NO2).
13C-NMR results are based on the HMQC assignments. n.d., not determined.
| Signal (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Atom H/C… | 1 | 2 | 3 | 4 | 5 | 6 | ||
| α-GalNAc-1→ | 1H | 4.98 | 4.23 | 4.07 | 4.09 | 4.43 | 3.74/3.69 | |
| J(Hz) | 3.7 | 11.0 | n.d. | n.d. | 6.5 | 6.8/11.4 | ||
| 13C | 99.08 | 50.87 | 67.61 | 68.87 | 71.44 | 61.14 | ||
| →4)-β-GalNAc | 1H | 5.32 | 4.31 | 3.95 | 4.11 | 3.98 | 3.80/3.74 | |
| J(Hz) | 8.3 | 11.3 | 2.8 | <1.5 | 6.2 | 6.2/n.d. | ||
| 13C | 99.68 | 52.80 | 70.79 | 76.46 | 76.63 | 60.74 | ||
| C6H4NO2 | 1H | − | 7.22 | 8.27 | − | 8.27 | 7.22 | |
| J(Hz) | − | 9.4 | − | 9.4 | ||||
| 13C | n.d. | 117.10 | 127.15 | n.d. | 127.15 | 117.10 | ||
| NHAc | 1H | 2.04/2.01 | ||||||
| 13C | 22.68/22.88 | |||||||
Further enzymic characterization
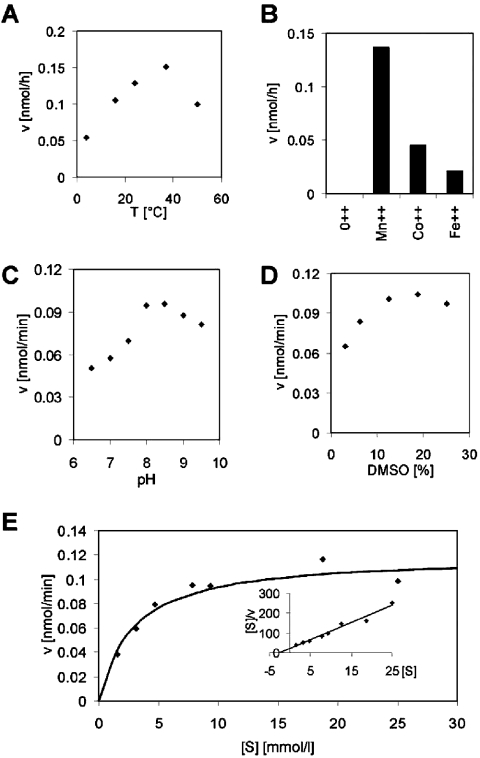
The activity of α4GT1 was measured in buffers with different pH values, at different temperatures and in the presence of different bivalent cations. These assays showed that the enzyme displays optimal activity at 37 °C, in the presence of Mn(II) ions and approx. pH 8 (see Figures 4A–4C respectively). Indeed, the enzyme activity was strictly dependent on the presence of bivalent cations; no activity was detected in the absence of added bivalent cations, in the presence of EDTA or in the presence of Zn(II) ions. The effect of DMSO on the enzyme's activity was also tested since this reagent was used in the stock solution of p-nitrophenyl-β-N-acetylgalactosaminide and may influence the enzyme directly and/or solubility of the substrate during the reaction; the amount of product was maximal in the presence of 10–20% DMSO (Figure 4D) as compared with the 12.5% present during the experiments in which the concentration of substrate was varied. The Km (app) value for p-nitrophenyl-β-N-acetylgalactosaminide was determined by the least-squares and Hanes’ plot methods to be 2.86 and 2.58 mM respectively (Figure 4E). Such a value is comparable with those of other glycosyltransferases, such as the egghead β1,4-mannosyltransferase, with p-nitrophenyl substrates [21].
Figure 4. Enzymic characteristics of Drosophila α4GT1.
Dependency of the enzymic reaction on temperature (A) and bivalent cations (B) was tested with the dabsyl-βGNβGN substrate using the MALDI–TOF-MS method, whereas the effect of pH (C), DMSO concentration (D) and p-nitrophenyl-β-N-acetylgalactosaminide concentration (E) was tested by the HPLC-based method. The Km value was calculated using either the least-squares method with the curve shown in (E) or using the Hanes' plot shown as the inset in (E). Unless otherwise noted, the enzyme-containing yeast culture supernatant was incubated at 37 °C in the presence of 20 mM MnCl2 and Tris/HCl buffer (pH 8) for 2 (A, B) or 4 h (C–E).
α4GT1 modifies a glycolipid substrate
Since the GalNAcα1,4GalNAcβ1,4GlcNAcβ-motif is present in Drosophila glycolipids, it was important to test whether α4GT1 was capable of synthesizing this structure when attached to a lipid. As described in the Experimental section, the substrate used in our study, GalNAcβ1,4GlcNAcβ1,3Manβ1,4Glcβ1,Cer, originated from Ascaris suum [16] and contains the core structure common to both insect and nematode glycolipids. In the presence of UDP-GalNAc, α4GT1 converted this structure (m/z 1405) into a structure of m/z 1608, consistent with the addition of a single GalNAc residue (Figure 3C, upper panel). Such conversion was not seen when the glycolipid was incubated with either α4GT1-containing supernatant in the absence of UDP-GalNAc (Figure 3C, lower panel) or control Pichia supernatant in the presence of UDP-GalNAc (results not shown). Furthermore, methylation analysis of the enzymic reaction product revealed the presence of a 4-substituted N-acetylgalactosaminyl residue, with characteristic electron-impact fragments of 158 and 233, as well as a terminal N-acetylgalactosamine. Thus, in addition to the glycosidase digestion and NMR results, all the results obtained indicated that α4GT1 is indeed an α1,4-N-acetylgalactosaminyltransferase.
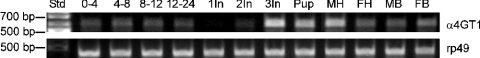
α4GT1 is expressed throughout the Drosophila life cycle
RT–PCR was performed using a Rapid-Scan™ panel with primers whose theoretical product should cross the two splice sites within the α4GT1 gene. The results showed that α4GT1 is expressed from the earliest embryonic stage through to the adult stage (Figure 5), although there appears to be a bias towards expression in some of the later stages. In addition, PCR using either cDNA derived from Schneider S2 cells, BG2-c6 neuronal cells or unsorted Canton S adults resulted in the expected RT–PCR product (theoretical size 586 bp), whereas PCR with genomic DNA generated a larger product consistent with the theoretical size of 705 bp (results not shown). Our results are compatible with the presence in the databanks of the corresponding expressed sequence tag sequences derived from Drosophila embryo, head and Schneider cell cDNA clones.
Figure 5. Expression profile of Drosophila α4GT1.
Semi-quantitative RT–PCR was performed using a Drosophila Rapid-Scan™ panel, which includes cDNAs from 0–4, 4–8, 8–12 and 12–24 h old embryos, first, second and third instar larvae, pupae, male and female heads and male and female bodies. RT–PCR with the α4GT1-specific primers was performed with the 1000×dilution, whereas for rp49-specific primers the 100×dilution was used.
DISCUSSION
The CG17223/α4GT1 gene was identified as a putative α1,4-glycosyltransferase based on its homology to Gb3/CD77/Pk-synthesizing α1,4-galactosyltransferases [2–6], involved in the biosynthesis of mammalian glycolipids, and the α1,4-N-acetylglucosaminyltransferase involved in class III mucin biosynthesis [7]. In theory, the α4GT1 gene could have been required for the biosynthesis of any α1,4-linkage; an enzyme activity synthesizing the O-glycan Galα1,4Galβ1,3GalNAc in Mamestra brassica cells has been described previously [10]. However, according to the admittedly not extensive literature on Drosophila O-glycans, this structure is yet to be found in the fruitfly [22] and the negative result of our assays with the core 1 structure and UDP-Gal would appear to rule out that α4GT1 is an α1,4-galactosyltransferase. On the other hand, GalNAcα1,4-GalNAcβ1,4GlcNAcβ1,3Manβ1,4Glcβ1,Cer is a core structure present in most zwitterionic and acidic glycolipids of Drosophila embryos [11] and our results with both N-glycan and glycolipid substrates (with the synthesized linkage being sensitive to chicken liver N-acetyl-α-galactosaminidase as well as being found to be specifically α1,4 as judged by NMR and methylation analyses) would suggest that α4GT1 is the α1,4-N-acetylgalactosaminyltransferase involved in the extension of Drosophila glycolipids.
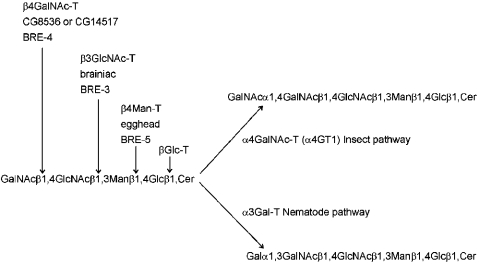
The biosynthesis of glycosphingolipids in insects and nematodes is rather different from that of mammals. Although the modification of ceramide by a glucosyltransferase is a common feature of mammalian and invertebrate glycolipid biosynthesis [23], the subsequent reactions of a β1,4-mannosyltransferase, a β1,3-N-acetylglucosaminyltransferase and of a β1,4-N-acetylgalactosaminyltransferase are specific features of invertebrates and constitute the biosynthesis of the core region of the arthro-series of glycosphingolipids (see Figure 6). Of late, this pathway has received some attention due to the finding that the neurogenic genes egghead and brainiac in Drosophila encode the relevant β1,4-mannosyltransferase and β1,3-N-acetylglucosaminyltransferase [21,24,25] respectively. Furthermore, it appears that abolition of this pathway in Caenorhabditis elegans confers resistance to the Bacillus thuringiensis Cry5B toxin [26], which is one of the pore-forming toxins produced by this bacterium found to be useful as novel pesticides. The Caenorhabditis bre-5 and bre-3 mutants are resistant to Cry5B and have defects in the genes homologous with egghead and brainiac respectively; however, of the four BRE proteins with homology to glycosyltransferases (BRE-2, -3, -4 and -5), only the activity of BRE-4, a β1,4-N-acetylgalactosaminyltransferase [27], has been demonstrated in vitro.
Figure 6. Summary of initial steps of arthro-series glycosphingolipid biosynthesis.
On the basis of structural and genetic information, the first four steps are common to both insects and nematodes. The names of the corresponding enzymes are shown (generic name followed by, for steps 2, 3 and 4, the Drosophila and the Caenorhabditis protein names). The reaction was catalysed by either α4GT1 in Drosophila and a putative α1,3-galactosyltransferase (α3Gal-T) in Caenorhabditis distinguishes the pathways in insects and nematodes. The subsequent steps (not shown) are also not shared by the two phyla. In this scheme, BRE-2, a potential β1,3-galactosyltransferase, would be hypothesized to act after the putative α1,3-galactosyltransferase in the nematode pathway.
The later stages of glycosphingolipid biosynthesis in insects and nematodes, however, diverge (see Figure 6). In Drosophila and Calliphora, the common arthro-core structure can be modified by an α1,4-linked GalNAc residue; further saccharide residues as well as a branching phosphorylethanolamine (P-Etn; aminoethylphosphate) may also be present [11,12,28–30]. The α1,4-linked GalNAc residue was previously shown to be the epitope for the CNF-1 monoclonal antibody immunohistochemically determined to be in most tissues of Drosophila embryos [31]. In contrast, the core of Caenorhabditis glycosphingolipids is modified by α1,3-linked galactose and phosphocholine, the latter being also a component of Caenorhabditis N-glycans and reactive with the TEPC-15 monoclonal IgA [32,33]. In other nematodes, Ascaris suum and Onchocerca volvulus, substitution of the α1,3-galactose by β1,3- and β1,6-linked galactose residues is also observed [16,34,35] and it is possible that traces of these structures are also present in Caenorhabditis. To date, the enzymology and genetic basis for the addition of these variations has not been studied; the action of an α1,3-galactosyltransferase can be assumed to be a feature of nematode glycosphingolipid biosynthesis in general, whereas the subsequent action of a β1,3-galactosyltransferase would be compatible with the homology of Caenorhabditis BRE-2 to β1,3-glycosyltransferases.
Glycolipid structures such as those described are, however, not so easily purified to homogeneity in large quantities and so we decided to utilize an alternative substrate which mimics the non-reducing terminal structure. Indeed, considering that many glycosyltransferases modifying the antennae of glycoconjugates are ‘blind’ to the exact nature of the aglycone, we used a dabsylated N-glycopeptide substrate for most of our studies. Even though recombinant α4GT1 is capable of modifying N-glycans, we do not know whether this is a function of this enzyme in vivo, since neither α-GalNAc-terminating nor LacdiNAc structures have been identified yet in Drosophila; however, a preliminary report suggests that the Manduca sexta midgut aminopeptidase N carries N-glycans with terminal α-N-acetylgalactosamine residues, which act as receptors for B. thuringiensis crystal toxin Cry1Ac [36], whereas N-acetylgalactosamine as a monosaccharide inhibits Cry1Ac binding to Helicoverpa armigera brush-border membrane vesicles [37]. Thus at least some insects must have an α-N-acetylgalactosaminyltransferase that modifies N-glycans in vivo.
The availability of a recombinant LacDiNAc-modifying α-N-acetylgalactosaminyltransferase creates new possibilities in the study of Drosophila glycolipid biosynthesis. One can then synthesize the substrate for the various other elongating enzymes, e.g. the largest glycolipid (Az29) isolated from fruitfly embryos has the structure GlcAβ1,3Galβ1,3GalNAcβ1,4(P-Etn6)GlcNAcβ1,3Galβ1,3GalNAcα1,4GalNAcβ1,4(P-Etn6)GlcNAcβ1,3Manβ1,4Glcβ1,Cer [11]. Thus to complete our knowledge of the biosynthesis of this pathway, perhaps two β1,3-galactosyltransferase, two β1,4-N-acetylgalactosaminyltransferase, one β1,3-N-acetylglucosaminyltransferase and two phosphorylethanolaminyltransferase activities remain to be discovered. The β1,4-N-acetylgalactosaminyltransferases possibly correspond to the two ‘orphan’ β1,4-galactosyltransferase-like genes of the fruitfly (the third such homologue having been defined as galactosyltransferase I of glycosaminoglycan biosynthesis) [38–40]; there are also many putative β1,3-glycosyltransferase genes related to fringe and brainiac, which may also be involved in the genetic basis of fruitfly glycolipid biosynthesis.
It was also of interest to note that other α1,4-glycosyltransferase homologues are present in insect genomes. Indeed, there is a second such possible gene (α4GT2) in Drosophila, but the potential reading frame is interrupted by a roo transposon. One may speculate that this transposon has ‘knocked out’ the core 1-modifying α1,4-galactosyltransferase in the fruitfly. However, such a hypothesis would need to be verified by comparing the sequence with an as yet to be proven α1,4-galactosyltransferase. Whether any of the five homologues present in the draft A. gambiae genome or any of the three homologues present in the draft Drosophila pseudoobscura genome would encode such an enzyme is a matter of speculation at this stage. However, of those so far characterized, the three different types of enzyme belonging to CAZy family 32 transfer either galactose to β1,4-linked galactose, GlcNAc to β1,6-linked galactose or GalNAc to β1,4-linked GalNAc: the common element being use of a UDP-linked sugar as donor and β-linked Gal or GalNAc as acceptor.
The repercussions of identifying α4GT1 are not just confined to creating substrates for other glycosyltransferases. The role of egghead and brainiac in epithelial morphogenesis and oogenesis [41,42] suggests that the glycolipids of Drosophila have important functions, which are presumably mediated by lectins that recognize their oligosaccharide moieties. The subsequent modifications of the arthro-series core, such as that catalysed by α4GT1, result either in blocking such recognition events or in creating new ligands for other lectins. Thus it is possible that the other glycosyltransferases in the biosynthetic pathway also have important roles and reverse genetics approaches become possible with the identification of these genes. The availability of α4GT1 also means that novel ligands for lectin-binding studies or generation of specific antibodies can be synthesized on N-glycan or glycolipid platforms. For instance, it would be of particular interest to have pure glycolipids of defined structures to explore the specificity of gliolectin, since this protein with a role in axon pathfinding binds zwitterionic glycolipids isolated from Drosophila embryos [43,44], or to create specific structures to verify whether α1,4-GalNAc residues indeed mediate binding of B. thuringiensis crystal toxin Cry1Ac to Manduca sexta aminopeptidase N [36,45] and are responsible for ‘non-specific’ binding of Cry1Ac to Schneider S2 cells [46]. Thus, in various ways, the identification of α4GT1 opens up new avenues in the study of invertebrate glycoconjugates.
Acknowledgments
This work was funded in part by a grant from the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (P13810) to I.B.H.W. We thank A. Linder and T. Dalik for help in preparing the dabsyl fibrin glycopeptide, Dr F. Altmann for access to the Dynamo MALDI–TOF-MS and Dr G. Fabini for preparing Drosophila RNA. J.D. was recipient of an ERASMUS studentship.
References
- 1.Altmann F., Fabini G., Ahorn H., Wilson I. B. H. Genetic model organisms in the study of N-glycans. Biochimie. 2001;83:703–712. doi: 10.1016/s0300-9084(01)01297-4. [DOI] [PubMed] [Google Scholar]
- 2.Kojima Y., Fukumoto S., Furukawa K., Okajima T., Wiels J., Yokoyama K., Suzuki Y., Urano T., Ohta M., Furukawa K. Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo series glycosphingolipids. J. Biol. Chem. 2000;275:15152–15156. doi: 10.1074/jbc.M909620199. [DOI] [PubMed] [Google Scholar]
- 3.Steffensen R., Carlier K., Wiels J., Levery S. B., Stroud M., Cedergren B., Sojka B. N., Bennett E. P., Jersild C., Clausen H. Cloning and expression of the histo-blood group Pk UDP-galactose:Galβ1-4Glcβ1-Cer α1,4-galactosyltransferase: molecular basis of the p phenotype. J. Biol. Chem. 2000;275:16723–16729. doi: 10.1074/jbc.M000728200. [DOI] [PubMed] [Google Scholar]
- 4.Keusch J. J., Manzella S. M., Nyame K. A., Cummings R. D., Baenziger J. U. Cloning of Gb3 synthase, the key enzyme in globo-series glycosphingolipid synthesis, predicts a family of α1,4-glycosyltransferases conserved in plants, insects and mammals. J. Biol. Chem. 2000;275:25315–25321. doi: 10.1074/jbc.M002630200. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa K., Iwamura K., Uchikawa M., Sojka B. N., Wiels J., Okajima T., Urano T., Furukawa K. Molecular basis for the p phenotype: identification of distinct and multiple mutations in the α1,4-galactosyltransferase gene in Swedish and Japanese individuals. J. Biol. Chem. 2000;275:37752–37756. doi: 10.1074/jbc.C000625200. [DOI] [PubMed] [Google Scholar]
- 6.Iwamura K., Furukawa K., Uchikawa M., Sojka B. N., Kojima Y., Wiels J., Shiku H., Urano T., Furukawa K. The blood group P1 synthase gene is identical to the Gb3/CD77 synthase gene: a clue to the solution of the P1/P2/p puzzle. J. Biol. Chem. 2003;278:44429–44438. doi: 10.1074/jbc.M301609200. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama J., Yeh J.-C., Misra A. K., Ito S., Katsuyama T., Fukuda M. Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1→4Galβ→R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8991–8996. doi: 10.1073/pnas.96.16.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell J. A., Davies G. J., Bulone V., Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 1997;326:929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho P. M., Deleury E., Davies G. J., Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 10.Lopez M., Gazon M., Juliant S., Plancke Y., Leroy Y., Strecker G., Cartron J. P., Bailly P., Cerutti M., Verbert A., et al. Characterization of a UDP-Gal:Galβ1-3GalNAc α1,4-galactosyltransferase activity in a Mamestra brassicae cell line. J. Biol. Chem. 1998;273:33644–33651. doi: 10.1074/jbc.273.50.33644. [DOI] [PubMed] [Google Scholar]
- 11.Seppo A., Moreland M., Schweingruber H., Tiemeyer M. Zwitterionic and acidic glycosphingolipids of the Drosophila melanogaster embryo. Eur. J. Biochem. 2000;267:3549–3558. doi: 10.1046/j.1432-1327.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- 12.Dennis R. D., Geyer R., Egge H., Peter-Katalinić J., Li S.-C., Stirm S., Wiegandt H. Glycosphingolipids in insects: chemical structures of ceramide tetra-, penta-, hexa-, and heptasaccharides from Calliphora vicina pupae (Insecta: Diptera) J. Biol. Chem. 1985;260:5370–5375. [PubMed] [Google Scholar]
- 13.Bencúrova M., Rendić D., Fabini G., Kopecky E.-M., Altmann F., Wilson I. B. H. Expression of eukaryotic glycosyltransferases in the yeast Pichia pastoris. Biochimie. 2003;85:413–422. doi: 10.1016/s0300-9084(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 14.Altmann F., Schweiszer S., Weber C. Kinetic comparison of peptide: N-glycosidases F and A reveals several differences in substrate specificity. Glycoconj. J. 1995;12:84–93. doi: 10.1007/BF00731873. [DOI] [PubMed] [Google Scholar]
- 15.Chiu M. H., Tamura T., Wadhwa M. S., Rice K. G. In vivo targeting function of N-linked oligosaccharides with terminating galactose and N-acetylgalactosamine residues. J. Biol. Chem. 1994;269:16195–16202. [PubMed] [Google Scholar]
- 16.Lochnit G., Dennis R. D., Ulmer A. J., Geyer R. Structural elucidation and monokine-inducing activity of two biologically active zwitterionic glycosphingolipids derived from the porcine parasitic nematode Ascaris suum. J. Biol. Chem. 1998;273:466–474. doi: 10.1074/jbc.273.1.466. [DOI] [PubMed] [Google Scholar]
- 17.Paz-Parente J., Cardon P., Leroy Y., Montreuil J., Fournet B., Ricard G. A convenient method for methylation of glycoprotein glycans in small amounts by using lithium methyl-sulfinyl carbanion. Carbohydr. Res. 1985;141:41–47. doi: 10.1016/s0008-6215(00)90753-5. [DOI] [PubMed] [Google Scholar]
- 18.Geyer R., Geyer H. Saccharide linkage analysis using methylation and other techniques. Methods Enzymol. 1994;230:86–108. doi: 10.1016/0076-6879(94)30009-7. [DOI] [PubMed] [Google Scholar]
- 19.Kaminker J. S., Bergman C. M., Kronmiller B., Carlson J., Svirskas R., Patel S., Frise E., Wheeler D. A., Lewis S. E., Rubin G. M., et al. The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0084. RESEARCH0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama K., Nagasu T., Shimma Y., Kuromitsu J., Jigami Y. OCH1 encodes a novel membrane bound mannosyltransferase: outer chain elongation of asparagine-linked oligosaccharides. EMBO J. 1992;11:2511–2519. doi: 10.1002/j.1460-2075.1992.tb05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wandall H. H., Pedersen J. W., Park C., Levery S. B., Pizette S., Cohen S. M., Schwientek T., Clausen H. Drosophila egghead encodes a β1,4-mannosyltransferase predicted to form the immediate precursor glycosphingolipid substrate for brainiac. J. Biol. Chem. 2003;278:1411–1414. doi: 10.1074/jbc.C200619200. [DOI] [PubMed] [Google Scholar]
- 22.Kramerov A. A., Arbatsky N. P., Rozovsky Y. M., Mikhaleva E. A., Polesskaya O. O., Gvozdez V. A., Shibaev V. N. Mucin-type glycoprotein from Drosophila melanogaster embryonic cells: characterisation of carbohydrate component. FEBS Lett. 1996;378:213–218. doi: 10.1016/0014-5793(95)01444-6. [DOI] [PubMed] [Google Scholar]
- 23.Leipelt M., Warnecke D., Zähringer U., Ott C., Müller F., Hube B., Heinz E. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants and fungi. J. Biol. Chem. 2001;276:33621–33629. doi: 10.1074/jbc.M104952200. [DOI] [PubMed] [Google Scholar]
- 24.Müller R., Altmann F., Zhou D., Hennet T. The Drosophila melanogaster brainiac protein is a glycolipid-specific β1,3-N-acetylglucosaminyltransferase. J. Biol. Chem. 2002;277:32417–32420. doi: 10.1074/jbc.C200381200. [DOI] [PubMed] [Google Scholar]
- 25.Schwientek T., Keck B., Levery S. B., Jensen M. A., Pedersen J. W., Wandall H. H., Stroud M., Cohen S. M., Amado M., Clausen H. The Drosophila gene brainiac encodes a glycosyltransferase putatively involved in glycosphingolipid synthesis. J. Biol. Chem. 2002;277:32421–32429. doi: 10.1074/jbc.M206213200. [DOI] [PubMed] [Google Scholar]
- 26.Griffitts J. S., Huffman D. L., Whitacre J. L., Barrows B. D., Marroquin L. D., Müller R., Brown J. R., Hennet T., Esko J. D., Aroian R. V. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxin–host interactions. J. Biol. Chem. 2003;278:45594–45602. doi: 10.1074/jbc.M308142200. [DOI] [PubMed] [Google Scholar]
- 27.Kawar Z. S., van Die I., Cummings R. D. Molecular cloning and enzymatic characterization of a UDP-GalNAc:GlcNAcβ-R β1,4-N-acetylgalactosaminyltransferase from Caenorhabditis elegans. J. Biol. Chem. 2002;277:34924–34932. doi: 10.1074/jbc.M206112200. [DOI] [PubMed] [Google Scholar]
- 28.Dabrowski U., Dabrowski J., Helling F., Wiegandt H. Novel phosphorus-containing glycosphingolipids from the blowfly Calliphora vicina Meigen. Structural analysis by 1H and 1H[31P]-edited NMR spectroscopy at 600 and 500 megahertz. J. Biol. Chem. 1990;265:9737–9743. [PubMed] [Google Scholar]
- 29.Weske B., Dennis R. D., Helling F., Keller M., Nores G. A., Peter-Katalinić J., Egge H., Dabrowski U., Wiegandt H. Glycosphingolipids in insects. Chemical structures of two variants of a glucuronic-acid-containing ceramide hexasaccharide from a pupae of Calliphora vicina (Insecta: Diptera), distinguished by a N-acetylglucosamine-bound phosphoethanolamine sidechain. Eur. J. Biochem. 1990;191:379–388. doi: 10.1111/j.1432-1033.1990.tb19133.x. [DOI] [PubMed] [Google Scholar]
- 30.Helling F., Dennis R. D., Weske B., Nores G., Peter-Katalinić J., Dabrowski U., Egge H., Wiegandt H. Glycosphingolipids in insects. The amphoteric moiety, N-acetylglucosamine-linked phosphoethanolamine, distinguishes a group of ceramide oligosaccharides from the pupae of Calliphora vicina (Insecta: Diptera) Eur. J. Biochem. 1991;200:409–421. doi: 10.1111/j.1432-1033.1991.tb16199.x. [DOI] [PubMed] [Google Scholar]
- 31.Keller M., Sorgenfrei B., Dennis R. D., Wiegandt H. Immunochemical analysis of insect carbohydrate antigenic determinants: recognition of a terminal α-linked N-acetylgalactosamine-containing epitope of Calliphora vicina neutral glyco(sphingo)lipids and detection in additional orthopteran and dipteran species. Hybridoma. 1993;12:155–165. doi: 10.1089/hyb.1993.12.155. [DOI] [PubMed] [Google Scholar]
- 32.Gerdt S., Dennis R. D., Borgonie G., Schnabel R., Geyer R. Isolation, characterization and immunolocalization of phosphorylcholine-substituted glycolipids in developmental stages of Caenorhabditis elegans. Eur. J. Biochem. 1999;266:952–963. doi: 10.1046/j.1432-1327.1999.00937.x. [DOI] [PubMed] [Google Scholar]
- 33.Haslam S. M., Gems D., Morris H. R., Dell A. The glycomes of Caenorhabditis elegans and other model organisms. Biochem. Soc. Symp. 2002;69:117–134. [PubMed] [Google Scholar]
- 34.Friedl C. H., Lochnit G., Zähringer U., Bahr U., Geyer R. Structural elucidation of zwitterionic carbohydrates derived from glycosphingolipids of the porcine parasitic nematode Ascaris suum. Biochem. J. 2003;369:89–102. doi: 10.1042/BJ20021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuhrer M., Rickhoff S., Dennis R. D., Lochnit G., Soboslay P. T., Baumeister S., Geyer R. Phosphocholine-containing, zwitterionic glycosphingolipids of adult Onchocerca volvulus as highly conserved antigenic structures of parasitic nematodes. Biochem. J. 2000;348:417–423. [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes-Izquierdo T., Adang M., Alvarez-Manilla G., Pierce J. M. Characterisation of the glycans from the aminopeptidase N1 (APN1) receptor of the Bacillus thuringiensis toxin Cry1Ac. Glycobiology. 2003;13:898. [Google Scholar]
- 37.Estela A., Escriche B., Ferré J. Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae) Appl. Env. Microbiol. 2004;70:1378–1384. doi: 10.1128/AEM.70.3.1378-1384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vadaie N., Hulinsky R. S., Jarvis J. L. Identification and characterisation of a Drosophila melanogaster orthologue of human β1,4-galactosyltransferase VII. Glycobiology. 2002;12:589–597. doi: 10.1093/glycob/cwf074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura Y., Haines N., Chen J., Okajima T., Furukawa K., Urano Y., Stanley P., Irvine K. D., Furukawa K. Identification of a Drosophila gene encoding xylosylprotein β4-galactosyltransferase that is essential for the synthesis of glycosaminoglycans and for morphogenesis. J. Biol. Chem. 2002;277:46280–46288. doi: 10.1074/jbc.M203873200. [DOI] [PubMed] [Google Scholar]
- 40.Takemae H., Ueda R., Okubo R., Nakato H., Izumi S., Saigo K., Nishihara S. Proteoglycan UDP-galactose:β-xylose β1,4-galactosyltransferase I is essential for viability in Drosophila melanogaster. J. Biol. Chem. 2003;278:15571–15578. doi: 10.1074/jbc.M301123200. [DOI] [PubMed] [Google Scholar]
- 41.Goode S., Wright D., Mahowald A. P. The neurogenic locus brainiac cooperates with the Drosophila EGF receptor to establish the ovarian follicle and to determine its dorsal-ventral polarity. Development. 1992;116:177–192. doi: 10.1242/dev.116.1.177. [DOI] [PubMed] [Google Scholar]
- 42.Goode S., Melnick M., Chou T.-Z., Perrimon N. The neurogenic genes egghead and brainiac define a novel signalling pathway essential for epithelial morphogenesis during Drosophila oogenesis. Development. 1996;122:3863–3879. doi: 10.1242/dev.122.12.3863. [DOI] [PubMed] [Google Scholar]
- 43.Tiemeyer M., Goodman C. S. Gliolectin is a novel carbohydrate-binding protein expressed by a subset of glia in the embryonic Drosophila nervous system. Development. 1996;122:925–936. doi: 10.1242/dev.122.3.925. [DOI] [PubMed] [Google Scholar]
- 44.Sharrow M., Tiemeyer M. Gliolectin-mediated carbohydrate binding at the Drosophila midline ensures fidelity of axon pathfinding. Development. 2001;128:4585–4595. doi: 10.1242/dev.128.22.4585. [DOI] [PubMed] [Google Scholar]
- 45.Knight P. K., Carroll J., Ellar D. J. Analysis of glycan structures on the 120 kDa aminopeptidase N of Manduca sexta and their interactions with Bacillus thuringiensis Cry1Ac toxin. Insect Biochem. Mol. Biol. 2004;34:101–112. doi: 10.1016/j.ibmb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Hua G., Jurat-Fuentes J. L., Adang M. J. Fluorescent assays establish Manduca sexta Bt-R1a cadherin as a receptor for multiple Bacillus thuringiensis Cry1A toxins in Drosophila S2 cells. Insect Biochem. Mol. Biol. 2004;34:193–202. doi: 10.1016/j.ibmb.2003.10.006. [DOI] [PubMed] [Google Scholar]