Abstract
PPARγ (peroxisome proliferator-activated receptor γ) is a ligand-activated transcription factor that responds to 15dPGJ2 (15-deoxy-Δ12,14-prostglandin J2). 15dPGJ2, in vitro, halts neuroblastoma cell growth, but reported mechanisms vary. Here we evaluated the modulatory effects of endogenous serum lipid mitogens upon the extent of 15dPGJ2-induced growth inhibition and on the precise cellular responses of neuroblastoma cells to PPARγ activation. We show that 15dPGJ2 specifically inhibited cell growth in both complete and delipidated media. 15dPGJ2-induced growth inhibition was accompanied by decreased cell viability, although the effect was far more marked in delipidated medium than in complete medium. Incubation with 15dPGJ2 in complete medium resulted in cytoplasmic changes characteristic of type II programmed cell death (autophagy), while prior serum lipid removal resulted in cell death via an apoptotic mechanism. These distinct, serum lipid-dependent cellular responses to 15dPGJ2 were accompanied by increases in the expression of a reporter gene construct containing a PPAR response element of 2.3-fold in complete medium, but of 4.8-fold in delipidated medium. Restoration of the serum lysolipid LPA (lysophosphatidic acid) to cells in delipidated medium reduced 15dPGJ2-mediated PPARγ activation, growth inhibition and cell death; following addition of S1P (sphingosine 1-phosphate), decreases were apparent but more marginal. Further, while the effects of LPA in delipidated medium were mediated through a Gi/phosphoinositide 3-kinase/MAPK (mitogen-activated protein kinase) pathway, those of S1P did not involve the MAPK component. These data suggest that the serum lysolipid LPA modulates the degree of PPARγ activation and the precise cellular response to 15dPGJ2 via activation of a Gi/phosphoinositide 3-kinase/MAPK pathway.
Keywords: apoptosis, autophagy, lysophosphatidic acid (LPA), neuroblastoma, peroxisome proliferator-activated receptor (PPAR), sphingosine 1-phosphate
Abbreviations: 15dPGJ2, 15-deoxyΔ12,14-prostglandin J2; ERK, extracellular-signal-regulated kinase; FCS, fetal calf serum; LPA, lysophosphatidic acid; MAPK, mitogen-activated protein kinase; MDC, monodansylcadaverine; MEK, MAPK/ERK kinase; PARP, poly(ADP-ribose) polymerase; PI3K, phosphoinositide 3-kinase; PPARγ, peroxisome proliferator-activated receptor γ; PPRE, PPAR response element; PTX, pertussis toxin; RT-PCR, reverse transcription–PCR; S1P, sphingosine 1-phosphate
INTRODUCTION
Mammalian cell cycle progression is regulated by numerous stimulatory or inhibitory signals, orchestrated in part by a complex interplay of extracellular peptide and lipid growth factors. One antiproliferative strategy for selected tumours has been targeting of the signalling network that acts through PPARγ (peroxisome proliferator-activated receptor γ) ligation as a locus for inhibition of cell growth. PPARγ, along with other nuclear receptors including those for oestrogen, thyroid hormone and retinoic acid, is a ligand-activated transcription factor. PPARγ regulates gene expression by binding as a heterodimer with the retinoid X receptor to specific response elements [PPREs (PPAR response elements)] of target promoters [1]. In humans, there are three PPAR isoforms, α, β and γ [2–4], which respond to a discrete set of ligands. For PPARα, fatty acids and the fibrate class of hypolipidaemic drugs are agonists, while long-chain fatty acids activate PPARβ [5–7]. Polyunsaturated fatty acids, synthetic thiazolidinedione insulin sensitizers, 15dPGJ2 (15-deoxyΔ12,14-prostglandin J2), oxidized phospholipids and LPA (lysophosphatidic acid) bind to and activate PPARγ [8–11].
PPARs show variable tissue expression patterns attributable to their distinct functions. PPARα is expressed at high levels in liver, where it regulates the expression of genes involved in β-oxidation [12]. In contrast, PPARβ is expressed ubiquitously, with reported roles in regulating PPARγ activity [13] and in embryo implantation [14]. PPARγ is highly expressed in adipose tissue, where it plays a critical role in adipocyte differentiation [15,16]. However, lower-level expression has been recorded in other cells [17] where, for example, it modulates placenta and heart tissue differentiation [18], macrophage activation [19] and T-lymphocyte apoptosis [20]. Furthermore, PPARγ activation by both natural and synthetic ligands induces growth arrest in many cancer cell lines [21–24]. Mechanistically, PPARγ ligand-mediated growth arrest occurs through differentiation [21,22] or by activation of apoptotic pathways [23,24]. Activation of PPARγ by 15dPGJ2 likewise inhibits human neuroblastoma cell growth in culture, although reported mechanisms vary with ligand concentration [25,26]. PPARγ-mediated attenuation of neuroblastoma cell growth in vitro suggests that its targeted activation may be an effective therapeutic strategy for neuroblastoma in vivo. However, before any clinical potential of PPARγ activators can be examined, their interaction with other circulating factors that may influence PPARγ activation and neuroblastoma growth need to be established.
Studies by Monard and co-workers have shown that circulating lipids may inhibit the differentiation of neuroblastoma cells. The delipidation of FCS (fetal calf serum), which removes known serum lipid mitogens including LPA [27] and S1P (sphingosine 1-phosphate) [28], leads to the spontaneous differentiation of the mouse neuroblastoma NB2A [29]. Interestingly, the serum lipid LPA has also been shown to act as a potent PPARγ ligand [11]. Natural biological variations in the lysolipid content of serum may therefore exert confounding effects upon both the degree of 15dPGJ2-induced growth inhibition and the precise cellular response. Here we have investigated the effects of serum lipid mitogens on these parameters. We show that 15dPGJ2-induced growth inhibition in the human neuroblastoma cell line IMR-32 occurs through neither differentiation nor apoptosis, but rather through programmed cell death type II or autophagy. Moreover, removal of serum lipid enhances the degree of 15dPGJ2-induced growth inhibition and the level of PPARγ activation, and alters the specific cellular response. In addition, we establish that the serum lyso-lipid LPA, acting through a Gi/PI3K (phosphoinositide 3-kinase)/MAPK (mitogen-activated protein kinase) signalling pathway, is responsible for modulating the cellular response of neuroblastoma cells to 15dPGJ2.
EXPERIMENTAL
Materials
15dPGJ2 (11-oxoprosta-5Z,9,12E,14Z-tetraen-1-oic acid) was obtained from Cayman Chemicals (Ann Arbour, MI, U.S.A.) supplied in methyl acetate. The solvent was removed by evaporation under N2, and 15dPGJ2 was resuspended in 100 μl of DMSO and used at 5 μM (EC50). WY-14643 {[4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid; pirinixic acid} was obtained from Sigma, dissolved in DMSO at 40 mg/ml and used at 5 μM (dose–response experiments showed no effect of WY-14643 on cell growth or viability at concentrations from 0.1 to 200 μM). PTX (pertussis toxin) was obtained from Sigma, PD980559 and LY294002 were obtained from Promega, and wortmannin, rottlerin and Y27632 were from Calbiochem. Antibodies against PARP [poly(ADP-ribose) polymerase] and phospho-MEK1/2 [phospho-MAPK/ERK (extracellular-signal-regulated kinase) kinase 1/2] were obtained from New England Biolabs. The reporter plasmid PPRE-Luc was a gift from R. M. Evans (Salk Institute for Biological Studies and Howard Hughes Medical Institute, La Jolla, CA, U.S.A.).
Cell culture
The human neuroblastoma IMR-32 cell line was cultured in Dulbecco's modified Eagle's medium containing 10% (v/v) FCS and 100 mM glutamine, supplemented with 0.1 mg/ml penicillin/streptomycin and fungizone® (2.5 μg/ml amphotericin B).
Lysolipid supplements
Stock solutions of S1P (Sigma-Aldrich; 500 μM) and oleoyl LPA (Sigma-Aldrich; 500 μM) were prepared by binding to fatty acid-free BSA (100 mg/ml) in Hanks balanced salt solution without Ca2+/Mg2+ (InVitrogen), adjusted to pH 7.2 and filter sterilized through 0.45 μm filters.
RT-PCR (reverse transcription–PCR)
Total RNA was isolated from cells using TRIZOL® reagent (InVitrogen), and 0.1 μg was used as a template to prepare cDNA using 100 units of M-MLV (Moloney murine leukaemia virus) reverse transcriptase. cDNA was amplified using primers specific for the housekeeping gene cyclophilin, which was used as an internal standard to ensure that equivalent amounts of cDNA were amplified in each reaction (5′-TTGGGTCGCGTCTGCTTCGA-3′ and 5′-GCCAGGACCTGTATGCTTCA-3′), for PPARα (5′-GCCTCAGGCTATCATTACG-3′ and 5′-CTTCTATGTCATGTTCACAG-3′) and for PPARγ (5′-TGCAGATTACAAGTATGAC-3′ and 5′-TCGATATCACTGGAGATC-3′). All PCR products were verified by restriction mapping and by hybridization with PPARα and PPARγ probes.
Delipidation of FCS
FCS (500 ml) was mixed with 1 litre of butan-1-ol/di-isopropyl ether (40:60, v/v) and vortexed overnight, in a variation of the technique of Cham and Knowles [30], which effectively delipidates protein solutions in a non-denaturing environment. The lower aqueous fraction was decanted and centrifuged at 1000 g for 10 min, and the lower aqueous layer decanted again. Finally, prior to use, it was dialysed in batches of 50 ml against two changes of Hanks balanced salt solution to remove excess organic solvent and filter-sterilized through a 0.45 μm filter.
Cell growth and viability assays
IMR-32 cells were seeded at 2×104 cells/well into six-well plates and supplemented daily with 2 ml of Dulbecco's modified Eagle's medium containing DMSO (vehicle control), 5 μM 15dPGJ2 or 5 μM WY-14643. Cells were harvested and cell pellets resuspended in 25 μl of PBS and 25 μl of 0.4% Trypan Blue. For cell growth experiments, four counts of viable cells were made per treatment per day and an average calculated. For cell viability assays, 500 cells were counted per sample, and cell death was expressed as a percentage of viable cells. For S1P and LPA replacement experiments, S1P and LPA were added at the stated concentrations to the cells in delipidated medium after overnight attachment.
Western blotting for PARP cleavage
Adherent and non-adherent cells were harvested and pooled. Nuclear extracts were prepared using the method of Dignam et al. [31]. A 5 μg portion of the extract was then electrophoresed on an SDS/10%-PAGE gel and transferred to a PVDF membrane (Amersham Pharmacia Biotech). The membrane was blocked for 1 h at room temperature in 5% (w/v) skimmed milk powder/PBS and incubated overnight at 4 °C with anti-PARP antibody (1:1000; New England Biolabs) or an anti-phospho-MEK1/2 antibody (1:2000; New England Biolabs) in 5% (w/v) skimmed milk powder/PBS/0.05% Tween-20. After washing (3×5 min in PBS/0.05% Tween-20), the blot was incubated at room temperature with a horseradish peroxidase-conjugated secondary anti-rabbit antibody (1:10000; Santa Cruz). PARP fragments and phospho-MEK1/2 were detected using Super-Signal® West Dura Extended Duration Substrate (Pierce, Rockford, IL, U.S.A.) and autoradiography.
Caspase-3 assay
Adherent and non-adherent cells were harvested and pooled. Cell pellets were resuspended in 60 μl of caspase assay lysis buffer [50 μM Hepes, 100 μM NaCl, 0.01% (v/v) Nonidet P-40, 10 μM dithiothreitol, 0.1% (v/v) glycerol, 1 μM EDTA] and put on ice for 1 h. Cell debris was pelleted at 13000 g for 20 min, and 20 μl of lysate was incubated with the caspase-3 substrate N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide to give a final substrate concentration of 90 μM. Reactions were set up in 96-well plates and incubated at 37 °C overnight. The absorbance of the reactions was measured at 410 nm.
Staining with MDC (monodansylcadaverine)
IMR-32 cells were grown on coverslips for 24 h in either complete or delipidated medium in the presence or absence of 15dPGJ2. At 1 h prior to analysis, 0.05 M MDC was added to the medium. Cells were then washed three times with PBS and analysed for fluorescence by digital microscopy (Zeiss digital microscope). Twenty randomly selected fields at ×40 magnification were counted.
Electron microscopy
IMR-32 cells were grown on Thermenox coverslips (Miles Scientific, Naperville, IL, U.S.A.) treated with fibronectin. Cells were fixed in 3% (v/v) glutaraldehyde (Agar Scientific, Stanstead, U.K.), 4% (v/v) formaldehyde and Pipes buffer (5 M NaOH and 0.1 M Pipes, pH 7.2). Cells were post-fixed with 1% (w/v) osmium tetroxide (Oxkem, Oxford, U.K.) in Pipes buffer (pH 7.2). Cells were dehydrated and embedded in 100% TAAB resin (TAAB Laboratories, Aldermaston, U.K.). Silver sections (60–90 nm) were cut with an Ultracut E microtome (Leica Instruments, Milton Keynes, U.K.) and stained with saturated uranyl acetate solution in 50% (v/v) ethanol (Agar Scientific). Sections were washed with fresh double-distilled water, and subsequently stained with Reynolds' lead citrate stain and washed as before. Cells were observed using a Hitachi H7000 transmission electron microscope (Hitachi Instruments, Finchampstead, U.K.) at 75 kV.
Transfections and luciferase assays
For the decoy DNA transfections, 5×105 IMR-32 cells were transfected in 96 mm2 dishes with 0, 10 or 20 μg of plasmid containing either a PPRE binding site (5′-CGAAAGCTTCAAAAGGGGACCAGGACAAAGGTCACGTTCGGGATCCGCC-3′) or a mutant PPRE sequence (5′-CGAAAGCTTCAAAAGGGGACCATTACAAAGCACACGTTCGGGATCCGCC-3′), as described previously [32]. After the calcium precipitates were removed, the cells were incubated in growth medium either with DMSO as a vehicle control or with 15dPGJ2 (5 μM) for 72 h, and the effects on cell viability determined.
The level of PPAR activation was assessed by transfecting IMR-32 cells (2×105) in 56 mm2 dishes with the reporter plasmid PPRE-Luc (2 μg), along with pGL3Con (2 μg) as an internal control. After the calcium precipitates were removed, the cells were incubated in complete or delipidated medium for 24 h with either DMSO (vehicle control) or 5 μM 15dPGJ2. Luciferase assays were performed using the Dual-Luciferase® Assay System (Promega) and quantified using a Turner luminometer.
Statistical analysis
Statistical analysis was carried out using Student's t-test, and a significant difference was assumed at P<0.05.
RESULTS
Expression of PPARγ in IMR-32 cells
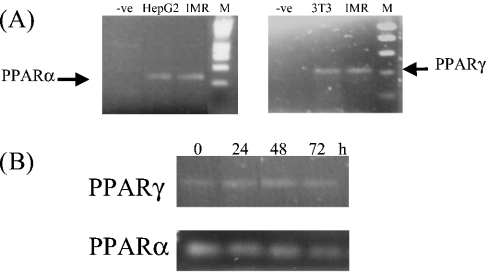
Expression of the PPAR isoforms PPARα and PPARγ in the IMR-32 human neuroblastoma cell line was examined using an RT-PCR assay. Total RNA was prepared from IMR-32 cells, reverse transcribed into cDNA and amplified with primers specific for PPARα and PPARγ. cDNA was also prepared from HepG2 and Li-adipocytes as positive controls for PPARα and PPARγ respectively. Single bands of the predicted sizes for PPARα and PPARγ of 225 and 385 bp respectively were detected in IMR-32 cells (Figure 1A), demonstrating that IMR-32 cells express both α and γ isoforms. Furthermore, to determine whether expression of the PPAR isoforms was affected by serum delipidation, levels of PPARα or PPARγ mRNA were also analysed in IMR-32 cells cultured in delipidated medium over a 72 h period. However, we found no change in the level of expression of either PPARα or PPARγ mRNA upon serum delipidation (Figure 1B).
Figure 1. RT-PCR analysis of PPARα and PPARγ mRNA expression in IMR-32 cells.
(A) cDNA was prepared from IMR-32 (IMR), HepG2 and 3T3-Li (3T3) cells and amplified with primers specific for PPARα and PPARγ. For the negative control (−ve), water was added to the cDNA reaction instead of RNA. Lane M contains size markers. (B) cDNA prepared from IMR-32 cells cultured in delipidated medium for 0, 24, 48 or 72 h was amplified with primers specific for PPARα and PPARγ.
The PPARγ ligand 15dPGJ2 inhibits IMR-32 cell growth
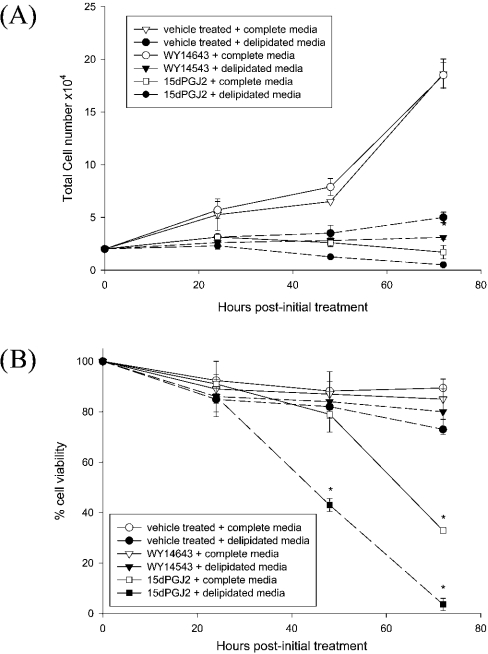
The effects of specific PPARα and PPARγ ligands on IMR-32 cell growth were assessed in the presence or absence of serum lipid mitogens. While vehicle-treated cells in complete medium showed a steady increase in cell number over 72 h, cell growth over the same period in delipidated medium was inhibited (Figure 2A). A concentration of 5 μM WY-14643, an activator of PPARα, did not alter cell growth in either medium, but the same concentration of 15dPGJ2, a PPARγ ligand, substantially inhibited cell growth in both complete and delipidated media (Figure 2A).
Figure 2. Ligands of PPARγ, but not of PPARα, inhibit IMR-32 cell growth and reduce cell viability.
IMR-32 cells were grown in the presence of a vehicle control (DMSO), WY-14643 (5 μM) or 15dPGJ2 (5 μM) over a 3-day period in complete or delipidated medium, and effects on cell growth (A) and cell viability (B) were assessed. Values represent means±S.E.M. for four independent experiments. *P<0.05 compared with untreated control cells in complete medium.
The effects of 15dPGJ2 supplementation on cell viability were assessed using the Trypan Blue exclusion assay (Figure 2B). In complete or delipidated medium, in either the presence or the absence of WY-14643, little cell death was observed (Figure 2B). Similarly, 15dPGJ2 elicited minimal cell death over the first 48 h of treatment in complete medium. However, subsequently there was a large decrease in cell viability, such that, by 72 h, 68.4±2.3% of cells stained positive for Trypan Blue (Figure 2B). In delipidated medium, 15dPGJ2 resulted in 57.8±7.1% of cells staining positive for Trypan Blue at 48 h, and by 72 h few viable cells remained. These data suggest that the timing and extent of cell death induced by 15dPGJ2 is influenced by the presence of lipid-extractable serum components.
Effects of delipidation on the cellular response of IMR-32 cells to 15dPGJ2
The mode of 15dPGJ2-induced cell death was probed by measuring two characteristic features of apoptosis: caspase-3 activation and PARP cleavage. Neither phenomenon was seen in control or 15dPGJ2-treated cells grown in complete medium (Table 1, Figure 3A), suggesting that 15dPGJ2 did not induce cell death through a classical apoptotic pathway. In contrast, 15dPGJ2 treatment of cells in delipidated medium led to both caspase-3 activation and PARP cleavage (Table 1, Figure 3A), consistent with the activation of apoptosis.
Table 1. 15dPGJ2 induces caspase-3 activation in IMR-32 cells in delipidated medium.
IMR-32 cells were treated with vehicle control or 15dPGJ2 in complete or delipidated medium for 72 h, and cleavage of the caspase-3 substrate N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide was measured at 410 nm. Values are means±S.E.M. for three independent experiments.
| Culture conditions | Caspase-3 activity (A410 units) |
|---|---|
| Complete medium+vehicle control | 6.04±0.8 |
| Complete medium+15dPGJ2 | 7.05±0.7 |
| Delipidated medium+vehicle control | 10.03±1.1 |
| Delipidated medium+15dPGJ2 | 49.8±4.6 |
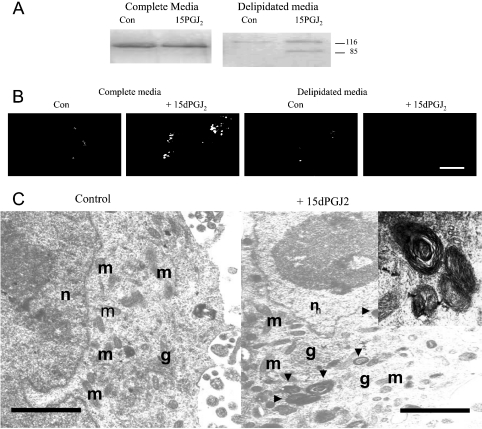
Figure 3. 15dPGJ2 induces apoptosis in delipidated medium, but autophagy in complete medium.
(A) Nuclear extracts prepared from IMR-32 cells were treated with either a vehicle control (Con) or 5 μM 15dPGJ2 in complete or delipidated medium for 72 h and analysed by Western blot analysis with an anti-PARP antibody. (B) IMR-32 cells grown in the presence of a vehicle control (Con) or 5 μM 15dPGJ2 in complete or delipidated medium were stained with MDC and visualized by digital microscopy. Scale bar=2 μm. (C) Transmission electron micrographs showing morphological changes in IMR-32 cells treated with a vehicle control or 5 μM 15dPGJ2 in complete medium. n, nucleus; g, Golgi complexes; m, mitochrondria; autophagic vesicles are indicated by black arrowheads. An example of an autophagic vesicle is shown in the inset. Scale bar=1 μm (100 nm in inset).
In the prostate cancer cell lines LNCaP, DU145 and PC-3, 15dPGJ2-induced cell death occurs through a type II mechanism (autophagy) [33]. We assessed whether an autophagic mechanism was induced by 15dPGJ2 in IMR-32 cells in complete medium by staining cells with MDC, a fluorescent dye that specifically stains autophagic vesicles [34]. Counting the average number of vesicles staining positive for MDC per cell section provided a semi-quantitative assessment of autophagy (Figure 3B, Table 2). Little autophagic activity was apparent in cells grown in complete medium (0.74±0.24), or in cells grown in delipidated medium with or without 15dPGJ2. In contrast, addition of 15dPGJ2 to cells in complete medium increased autophagic activity, and an average of 14.7±0.95 autophagic vesicles were observed per cell section. The presence of autophagic vesicles was verified by visualization by transmission electron microscopy. Using this technique, these vesicles appear as whorls of membranes or membrane-bound vesicles, containing cellular components. Vehicle-treated control cells had normal cellular ultrastructure without autophagic vesicles (Figure 3C). However, IMR-32 cells treated with 15dPGJ2 in complete medium for 72 h possessed both whorled vesicles and single membrane-bound vesicles, which appeared to contain cellular components, possibly organelles (Figure 3C). No chromatin condensation or nuclear fragmentation, characteristic of apoptotic cell death, was seen. Furthermore, the organelles within 15dPGJ2-treated cells appeared intact, without the swelling characteristic of necrosis. Together, these findings indicate that 15dPGJ2 induces autophagic cell death in IMR-32 cells in complete medium, but apoptosis in delipidated medium.
Table 2. Number of autophagic vesicles per cell section in 15dPGJ2-treated IMR-32 cells.
IMR-32 cells were grown in either complete or delipidated medium in the presence of a vehicle control or 5 μM 15dPGJ2, and then stained with MDC and visualized by digital microscopy. The numbers of autophagic vesicles per cell section in 20 randomly selected fields at ×40 magnification were counted.
| Treatment | Autophagic vesicles per cell (n=50) |
|---|---|
| Complete medium+vehicle control | 0.74±0.24 |
| Complete medium+15dPGJ2 | 14.70±0.95 |
| Delipidated medium+vehicle control | 1.96±1.14 |
| Delipidated medium+15dPGJ2 | 2.14±0.87 |
Involvement of PPARγ in 15dPGJ2-induced cell death
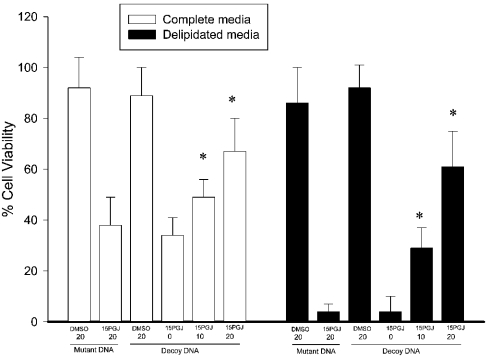
A double-stranded decoy DNA approach [35] was used to exclude the possibility that 15dPGJ2 exerts its effects independently of its receptor PPARγ [36]. The strategy of transient transfection of IMR-32 cells with increasing concentrations of a decoy DNA containing the PPRE sequence allows competition with the PPRE sequences within the promoters of PPARγ target genes for the activated receptor, thereby blocking PPAR-dependent transcriptional responses. Cells were transiently transfected with increasing concentrations of plasmid DNA containing either the decoy PPRE sequence or a mutant PPRE sequence. After calcium precipitate removal and treatment with 15dPGJ2 or DMSO in complete or delipidated medium, the effect on cell viability was determined. Introduction of increasing concentrations of PPRE decoy DNA, but not the mutant PPRE DNA, inhibited 15dPGJ2-induced cell death in both complete and delipidated media in a dose-dependent manner (Figure 4), suggesting that PPARγ-induced transcription is required for 15dPGJ2-induced cell death in both complete and delipidated media.
Figure 4. Transfection of increasing concentrations of decoy DNA abolishes 15dPGJ2-induced cell death in both complete (□) and delipidated (▪) medium.
IMR-32 cells were transfected with increasing concentrations of DNA (0, 10 or 20 μg) containing either a consensus PPRE binding site (decoy DNA) or a mutant PPRE sequence (mutant DNA) and treated with either the vehicle control (DMSO) or 5 μM 15dPGJ2 for 72 h. Values represent means±S.E.M. of three independent experiments; *P<0.05 for increased cell viability in 15dPGJ2-treated cells treated with 10 or 20 μg of decoy DNA compared with 15dPGJ2-treated cells transfected with 0 μg of decoy DNA.
Effects of serum lipids on PPARγ activation
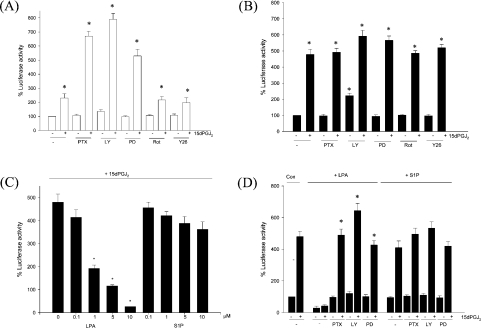
The mechanism of modulation of 15dPGJ2-induced cell responses by serum lipids was next examined at the level of PPARγ activation. IMR-32 cells were transiently transfected with the luciferase reporter PPRE-Luc, in which three PPREs had been cloned upstream of the thymidine kinase promoter, and treated with either 15dPGJ2 or DMSO in complete or delipidated medium for 24 h. We found that basal PPRE-Luc activity was 1.5-fold higher in cells incubated in delipidated medium than in cells in complete medium. However, the addition of 15dPGJ2 led to an increase in PPRE-Luc activity in cells grown in either medium, although the magnitude of the increased activity varied: addition of 15dPGJ2 to delipidated medium elicited a 4.8-fold increase (Figure 5A), but addition to complete medium elicited only a 2.3-fold increase (Figure 5B). Interestingly, the degree of PPARγ activation in 15dPGJ2-supplemented cells in complete medium rose significantly upon the addition of PTX (an inhibitor of the G-protein Gαi), LY294002 (a PI3K inhibitor) or PD98059 (an inhibitor of MEK1, which signals to the MAPKs p42mapk/ERK1 and p44mapk/ERK2) (Figure 5A). In contrast, the protein kinase C inhibitor rottlerin and the Rho kinase inhibitor Y27632 were without effect upon PPARγ activation. These data suggest that serum lipids attenuate the degree of PPARγ activation via a Gi/PI3K/MEK signalling pathway. Expression of a reporter construct lacking the three PPREs remained unchanged upon 15dPGJ2 treatment in either medium (results not shown).
Figure 5. Addition of 15dPGJ2 to IMR-32 cells in either complete or delipidated medium leads to the transcriptional activation of a PPRE-containing reporter gene construct.
IMR-32 cells were transiently transfected with PPRE-Luc (2 μg) together with pGL3Con (2 μg). After the calcium precipitates were removed, the cells were incubated in the presence or absence of 5 μM 15dPGJ2 in complete (A) or delipidated (B) medium for 24 h with PTX (100 ng/ml), LY294002 (LY; 50 μM), PD98059 (PD; 50 μM), rottlerin (Rot; 10 μM) or Y27632 (Y26; 10 μM). *P<0.05 compared with untreated cells. (C) IMR-32 cells transfected with PPRE-Luc (2 μg) and pGL3Con (2 μg) were incubated for 24 h in delipidated medium with 5 μM 15dPGJ2 and increasing concentrations of LPA or S1P (0, 0.1, 1, 5 or 10 μM). *P<0.05 compared with cells treated with 15dPGJ2 alone. (D) IMR-32 cells transfected with PPRE-Luc (2 μg) and pGL3Con (2 μg) were incubated for 24 h in delipidated medium with 5 μM 15dPGJ2 and either 10 μM LPA or 10 μM S1P in the absence or presence of PTX (100 ng/ml), LY294002 (LY; 50 μM) or PD98059 (PD; 50 μM). *P<0.05 denotes significantly reduced LPA attenuation of 15dPGJ2-induced PPRE-Luc activity in 15dPGJ2/LPA co-cultures treated with PTX, LY or PD compared with cells treated with 15dPGJ2 and LPA only. Values represent means±S.E.M. for three independent experiments.
To determine which serum factor may be responsible for attenuating the degree of PPARγ activation, the serum lipid mitogens LPA and S1P were added individually to 15dPGJ2-treated cells in delipidated medium and their effects on PPARγ activation determined. The addition of increasing concentrations of LPA to 15dPGJ2-treated cells in delipidated medium resulted in a dramatic, dose-dependent decrease in PPARγ activation (Figure 5C). However, the ability of LPA to attenuate the degree of PPARγ activation was blocked by PTX, LY294002 and PD98059 (Figure 5D). In contrast, a second lipid-extractable serum factor, S1P, only marginally decreased the degree of PPARγ activation induced by 15dPGJ2 (Figure 5C); while PTX and LY294002 blocked this effect, the MEK inhibitor PD98059 did not (Figure 5D).
LPA attenuates the cytotoxic effects of 15dPGJ2
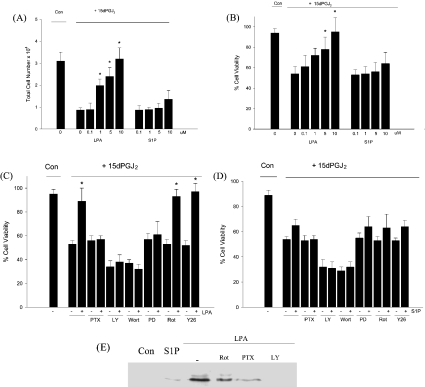
The cellular response to 15dPGJ2 treatment was also altered in the presence of LPA. LPA at 10 μM, the concentration that totally blocked 15dPGJ2-induced PPARγ activation, similarly completely abolished the growth inhibition (Figure 6A) and cell death (Figure 6B) resulting from 15dPGJ2 treatment. In contrast, S1P altered 15dPGJ2-induced growth inhibition and cell death only minimally. The effects of LPA on 15dPGJ2-induced cell death were abolished in the presence of PTX, PD98059 or LY294002 (Figure 6C), while attenuation due to S1P was abolished in the presence of PTX and LY204002, recapitulating the effects upon PPARγ activation (Figure 6D). This suggests that the presence of LPA modulates both the degree of PPARγ activation and the cellular response to 15dPGJ2 via the Gi/PI3K/MEK pathway, while S1P acts through a Gi/PI3K-dependent but MEK-independent pathway. Furthermore, to confirm that supplementation with LPA, but not S1P, leads to MEK activation in IMR-32 cells, we used Western analyses with antibodies raised against phosphorylated MEK1/2. Our results show that while LPA activated MEK, S1P addition to cells in delipidated medium did not (Figure 6E). Moreover LPA activation of MEK was abolished by the presence of PTX and LY294002.
Figure 6. LPA attenuates 15dPGJ2-induced growth inhibition and cell death.
IMR-32 cells were treated with 5 μM 15dPGJ2 together with increasing concentrations of LPA or S1P for 24 h in delipidated medium, and effects on cell growth (A) and cell viability (B) were assessed. (C, D) IMR-32 cells were treated with 5 μM 15dPGJ2 in the presence of 10 μM LPA (C) or 10 μM S1P (D) with PTX (100 ng/ml), PD98059 (PD; 50 μM), LY294002 (LY; 50 μM), wortmannin (Wort; 200 nM), rottlerin (Rot; 10 μM) or Y27632 (Y26; 10 μM) for 24 h, and effects on cell viability assessed. All values represent means±S.E.M. for three independent experiments; *P<0.05 compared with 15dPGJ2-treated cells. (E) Western blot analysis of IMR-32 cell extracts prepared from cells cultured in delipidated medium either alone (Con) or with S1P (10 μM), LPA (10 μM), LPA+rottlerin (Rot; 10 μM), LPA+PTX (100 ng/ml) or LPA+LY294002 (LY; 50 μM) for 24 h.
DISCUSSION
In order to evaluate the antiproliferative potential of targeting PPARγ-mediated signalling in neuroblastoma, we initially needed to confirm its involvement in altering the growth and/or viability of neuroblastoma cells. In human IMR-32 neuroblastoma cells, despite the expression of both PPARα and PPARγ, only the PPARγ ligand 15dPGJ2 attenuated cell growth/viability, supporting the concept that activation of the PPARγ signalling pathway has antiproliferative effects. Indeed, different effects of PPARα and PPARγ ligands in IMR-32 cells are consistent with numerous reports that these receptor isoforms play distinct cellular roles.
Serum delipidation induces the spontaneous morphological differentiation of neuroblastoma cells [29], implying that lipid components within serum suppress the differentiation of neuroblastoma cells. Consequently, we were keen to establish whether the extent of 15dPGJ2-induced growth inhibition might be enhanced in the absence of serum lipids. In agreement with this hypothesis, an additive effect of prior lipid removal in combination with 15dPGJ2 upon cell viability was seen at both 48 h and 72 h. Serum lipid removal clearly rendered the subsequent 15dPGJ2-mediated decreases in cell growth and viability more marked. Mechanistically, while others have repored that 15dPGJ2 treatment of the neuroblastoma cell line SHSY5Y led to the halting of growth via apoptosis [26], we found no evidence of 15dPGJ2-induced apoptosis or necrosis in cells in complete medium. Instead, our investigation using MDC staining and transmission electron microscopy of 15dPGJ2-treated IMR-32 cells revealed changes consistent with the operation of an autophagic mechanism. This phenomenon, common to both embryogenesis and the regression of hormone-dependent tumours, has been suggested previously to be responsible for the spontaneous regression of neuroblastoma in vivo [37]. In contrast, however, the addition of 15dPGJ2 to cells in delipidated medium led to caspase-3 activation and PARP cleavage, suggestive of the operation of a classical apoptotic pathway and implying that the lipophilic components of FCS can modulate the observed pattern of cell death.
With little understanding of the complexity underlying the instruction and regulation of autophagy, its relationship with apoptosis and any link to PPARγ-mediated signalling remain unclear. It has been suggested that abundant overlap exists between autophagy and apoptosis [38], and therefore it is perhaps unsurprising that 15dPGJ2-induced cell death in IMR-32 cells, whether in the presence or the absence of lipid, requires the activation of the PPARγ transcriptional pathway. However, in delipidated medium, 5 μM 15dPGJ2 induced a 4.8-fold increase in PPARγ activity, whereas in complete medium only a 2.3-fold increase in PPARγ activity was observed. Moreover, the use of signalling pathway inhibitors suggested that serum lipids limit the degree of PPARγ activity through the activation of a Giα/PI3K/MEK pathway. Interestingly, we found that the addition of LPA alone to IMR-32 cells in delipidated medium attenuated the degree of PPARγ activation induced by 15dPGJ2, suggesting that the presence of this lysolipid within serum normally acts to limit PPARγ activation by 15dPGJ2. LPA addition also attenuated the growth inhibitory and cytotoxic effects of 15dPGJ2. In contrast with LPA, the related serum lysolipid S1P only marginally attenuated 15dPGJ2-mediated PPARγ activation, growth inhibition and cell death. Although S1P and LPA are structurally similar, they have been shown to act independently through related but distinct receptors – LPA acts through three receptors (LPA1–LPA3) and S1P acts through five (S1P1–S1P5) [39]. The lysolipid receptors are coupled to several heterotrimeric G-proteins. Gi is sensitive to PTX and activates Ras through its βγ subunits to stimulate MAPK phosphorylation and PI3K activation. Gq activates phospholipase C directly, with subsequent alteration in intracellular Ca2+. LPA and S1P also directly activate Rho downstream of G13. In our experiments, the antagonistic activity of LPA was blocked in the presence of PTX, LY294002 or PD98059, consistent with LPA inhibiting PPARγ through a Gi/PI3K/MEK/MAPK signalling pathway. Interestingly, LPA has recently been reported to function as a potent transmembrane PPARγ agonist: McIntyre et al. [11] showed that LPA, but not S1P, displaced the drug rosiglitazone from the ligand binding pocket of PPARγ. Moreover, LPA supplementation of the RAW264.7 macrophage cell line led to the stimulation of a reporter gene construct containing a PPRE. In IMR-32 cells, however, LPA antagonized PPARγ indirectly via activation of the Gi/PI3K/MEK/MAPK pathway. This difference in LPA action is likely to reflect differential transport of LPA and/or differences in intracellular metabolism between neuroblastoma cells and macrophages.
The ability of LPA to limit the degree of 15dPGJ2-induced PPARγ activation in IMR-32 cells via activation of the MEK/MAPK pathway would be consistent with the reported role of MAPK as a powerful inhibitor of the transcriptional transactivation ability of PPARγ in adipocytes [40,41]. MAPK-mediated phosphorylation at Ser-84 of PPARγ greatly reduced transcriptional activity and impaired the ability to promote differentiation. Elsewhere, differential PPARγ activation has been reported to govern the cellular response to 15dPGJ2 in the breast cancer cell line MDA-MB-231 [42]. If the extent of PPARγ activation likewise dictates the cellular response to 15dPGJ2 in neuroblastoma, then the modulatory effects of LPA may explain the differences in cellular responses observed between cells in complete and delipidated media. However, it is interesting to note that while 5 μM 15dPGJ2 induced autophagy in IMR-32 cells in complete medium and apoptosis in delipidated medium in the present study, 10 μM 15dPGJ2 has been reported to induce differentiation of LAN-5 cells [25], and 20 μM 15dPGJ2 has been observed to induce apoptosis of SHSY5Y cells [26], although in the latter case serum-free media were used [26]. Given our findings, it will be important to determine whether the degree of PPARγ activation correlates with a precise cellular response to 15dPGJ2 in these neuroblastoma cells, or whether differences in the precise genotypes of the neuroblastoma cells also play a role in modulating the cellular response to 15dPGJ2.
Finally, our data point to a common but largely unrecognized and uncontrolled variable in cell culture systems used to evaluate the regulation of cell growth by morphogens, namely the presence in FCS of lipid mitogens capable of modulating agonist responses. The implications of this are extensive since, even if these substances are controlled or removed, data obtained in vitro may not provide an accurate guide to the effectiveness of candidate morphogens in vivo, where tumour cells are likely to encounter a different balance of circulating lysolipids. Future work will need to take greater account of the potential role of lysolipids in disease manifestation.
Acknowledgments
This work was funded by the Wessex Cancer Trust.
References
- 1.Issemann I., Green S. Activation of a member of the steroid hormone receptor super-family by peroxisome proliferators. Nature (London) 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal β oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 3.Sher T., Yi H. F., McBrie O. W., Gonzalez F. J. cDNA cloning, chromosomal mapping and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry. 1993;32:5598–5604. doi: 10.1021/bi00072a015. [DOI] [PubMed] [Google Scholar]
- 4.Greene M. E., Blumberg B., McBride O. W., Yi H. F., Kronquist K., Kwan K., Hsieh L., Greene G., Nimer S. D. Isolation of the human PPARγ cDNA: expression in haemopoietic cells and chromosomal mapping. Gene Expression. 1995;4:281–299. [PMC free article] [PubMed] [Google Scholar]
- 5.Krey G., Braissant O., L'Horset F., Kalkhoven E., Perroud M., Parker M. G., Wahli W. Fatty acids, eicosanoids and hypolipidaemic agents identified as ligands of PPARs by co-activator dependent receptor ligand assay. Mol. Endrocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 6.Forman B. M., Chen J., Evans R. M. Hypolipidaemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for PPARα and δ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely B. G., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., Lehmann J. M. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., Evans R. M. 15-DeoxyΔ12,14-prostglandin J2 is a ligand for the adipocyte determination factor, PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 9.Kliewer S. A., Lenhard J. M., Willson T. M., Patel I., Morris D. C., Lehmann J. M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 10.Davies S. S., Pontsler A. V., Marathe G. K., Harrison K. A., Murphy R. C., Hinshaw J. C., Prestwich G. D., Hilaire A. S., Prescott S. M., Zimmerman G. A., McIntyre T. M. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J. Biol. Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre T. M., Pontsler A. V., Silva A. R., St Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., Prestwich G. D. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. U.S.A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. Fatty acids and retinoids control lipid metabolism through the activation of PPAR/retinoid-X-receptor heterodimers. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jow L., Mukherjee R. The human PPAR subtype NUC1 represses the activation of hPPARα and the thyroid hormone receptors. J. Biol. Chem. 1995;270:3836–3840. doi: 10.1074/jbc.270.8.3836. [DOI] [PubMed] [Google Scholar]
- 14.Lim H., Dey S. K. PPARδ functions as a prostacyclin receptor in blastocyst implantation. Trends Endocrinol. Metab. 1999;11:137–142. doi: 10.1016/s1043-2760(00)00243-5. [DOI] [PubMed] [Google Scholar]
- 15.Chawla A., Schwarz E. J., Dimaculangan D. D., Lazar M. A. PPARγ: adipose predominant expression and induction in early adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P., Hu E., Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee R., Jow L., Croston G. E., Paterniti J. R., Jr Identification, characterization and tissue distribution of human PPAR isoforms PPARγ2 versus PPARγ1 and activation with retinoid X receptor agonists and antagonists. J. Biol. Chem. 1997;272:8071–8076. doi: 10.1074/jbc.272.12.8071. [DOI] [PubMed] [Google Scholar]
- 18.Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. PPARγ is required for placental, cardiac and adipose tissue development. Mol. Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 19.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. The peroxisome proliferator activated receptor gamma is a negative regulator of macrophage activation. Nature (London) 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 20.Harris S. G., Phipps R. T. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. Eur. J. Immunol. 2001;31:1098–1105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Tontonoz P., Singer S., Forman B. M., Sarraf P., Fletcher J. A., Fletcher C. D. M., Brun R. P., Mueller E. M., Altiok S., Oppenheim H., et al. Terminal differentiation of human liposarcoma cells induced by ligands for PPAR and the retinoid-X receptor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarraf P., Mueller E., Jones D., King F. J., DeAngelo D. J., Partridge J. B., Holden S. A., Chen L. B., Singer S., Fletcher C., Spiegelman B. M. Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat. Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 23.Elstner E., Muller C., Koshizuka K., Williamson E. A., Park D., Asou H., Shintaku P., Said J. W., Heber D., Koeffler H. P. Ligands for PPARγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang T.-H., Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Res. 2000;60:1129–1138. [PubMed] [Google Scholar]
- 25.Han S. W., Greene M. E., Pitts J., Wada R. K., Sidell N. Novel expression and function of peroxisome proliferator activated receptor gamma (PPARγ) in human neuroblastoma cells. Clin. Cancer Res. 2001;7:98–104. [PubMed] [Google Scholar]
- 26.Rohn T. T., Wong S. M., Cotman C. W., Cribbs D. H. 15 DeoxyΔ12,14 prostaglandin J2, a specific ligand for peroxisome proliferator activated receptor γ induces neuronal apoptosis. Mol. Neurosci. 2001;12:839–843. doi: 10.1097/00001756-200103260-00043. [DOI] [PubMed] [Google Scholar]
- 27.Eichholtz T., Jalink K., Fahrenfort L., Moolenaar W. H. The bioactive phospholipid lysophosphatidicacid is released from activated platelets. Biochem. J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yatomi Y., Igarashi Y., Yang L., Hisano N., Qi R., Asazuma N., Satoh K., Ozaki Y., Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 29.Monard D., Rentsch M., Schuerch-Rathce Y., Lindsay R. M. Morphological differentiation of neuroblastoma cells in medium supplemented with delipidated serum. Proc. Natl. Acad. Sci. U.S.A. 1997;74:3893–3897. doi: 10.1073/pnas.74.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J. Lipid Res. 1976;17:176–181. [PubMed] [Google Scholar]
- 31.Dignam J. D., Lebobitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdge G. C., Rodway H. A., Kohler J. A., Lillycrop K. A. Effect of fatty acid supplementation upon growth and differentiation of human IMR-32 neuroblastoma cells in vitro. J. Cell. Biochem. 2001;80:266–273. doi: 10.1002/1097-4644(20010201)80:2<266::aid-jcb160>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Butler R., Mitchell S. H., Tindall D. J., Young C. Y. F. Non-apoptotic cell death associated with S-phase arrest of prostrate cancer cells via the peroxisome proliferator-activated receptor γ ligand, 15-deoxyΔ12,14-prostglandin J2. Cell Growth Differ. 2000;11:49–61. [PubMed] [Google Scholar]
- 34.Biederbeck A., Kern H. F., Elsasser H. P. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- 35.Bishop-Bailey D., Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxyΔ12,14-prostglandin J2. J. Biol. Chem. 1999;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 36.Straus D. S., Pascual G., Li M., Welch J. S., Ricote M., Hsiang C. H., Sengchanthalangsy L. L., Ghosh G., Glass C. K. 15 DeoxyΔ12,14 prostaglandin J2 inhibits multiple steps in the NF-κB signalling pathway. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitanaka C., Kato K., Ijiri R., Sakurada K., Tomiyama A., Noguchi K., Nagashima Y., Nakagawara A., Momoi T., Toyoda Y., et al. Increased Ras expression and caspase-independent neuroblastoma cell death: possible mechanism of spontaneous neuroblastoma regression. Int. Natl. Cancer Inst. 2002;94:358–368. doi: 10.1093/jnci/94.5.358. [DOI] [PubMed] [Google Scholar]
- 38.Zackeri Z., Bursch W., Tenniswood M., Lockshin R. A. Cell death: programmed, apoptosis, necrosis, or other? Cell Death Differ. 1995;2:87–96. [PubMed] [Google Scholar]
- 39.Lynch K. R. Lysophospholipid receptor nomeclature. Biochim. Biophys. Acta. 2002;1582:70–71. doi: 10.1016/s1388-1981(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 40.Hu E., Kim J. B., Sarraf P., Spiegelman B. M. Inhibition of adipogenesis through MAP kinase mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 41.Adams M., Reginato M. J., Shao D., Lazar M. A., Chatterjee V. K. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen activated protein kinase site. J. Biol. Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 42.Clay C. E., Namen A. M., Atsumi G. I., Willingham M. C., High K. P., Kute T. E., Trimboli A. J., Fonteh A. N., Dawson P. A., Chilton F. H. Influence of J series prostaglandins on apoptosis and tumourigenesis of breast cancer cells. Carcinogenesis. 1999;20:1905–1911. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]