Abstract
The main purpose of the present study was to test the hypothesis that the aging process is associated with a pro-oxidizing shift in the cellular redox state. The amounts of the redox-sensitive free aminothiols (glutathione, cysteine, Cys-Gly and methionine) and protein mixed disulphides were measured at different ages and ambient temperatures in Drosophila melanogaster. GSH/GSSG ratios decreased significantly with increasing age of the flies, due to an increase in GSSG content. Concentrations of Cys-Gly increased and methionine decreased with age. The amounts of protein mixed disulphides, measured as protein-cysteinyl, protein-Cys-Gly and protein-glutathionyl mixed disulphides, increased as a function of age. The pattern of changes in free aminothiol content, glutathione-redox state and protein mixed disulphides varied in proportion to the ambient temperature, which is inversely related to the life expectancy of the flies. Collectively, these results support the idea that the pro-oxidizing shift in the glutathione-redox state, the decrease in methionine content and increase in abundance of protein mixed disulphides are associated with the life expectancy of flies, and are indicative of enhanced oxidative stress during aging.
Keywords: aging, aminothiol, Drosophila, glutathione, oxidative stress, protein thiolation
Abbreviations: ECD, electrochemical detection; MPA, metaphosphoric acid; PrS-SC, PrS-SCG, PrS-SG, protein-cysteinyl, protein-Cys-Gly, protein-glutathionyl mixed disulphides respectively; ROS, reactive oxygen species
INTRODUCTION
A common feature of the life cycle of virtually all multicellular organisms is that there is a progressive and irreversible loss of efficiency of various physiological functions during the post-reproductive phase of life, whereby the ability of the organism to maintain homoeostasis is attenuated, ultimately ending in death. Although many hypotheses have been advanced, the nature of the mechanisms inducing deleterious age-related changes is not well understood. One hypothesis postulates that the progressive accumulation of macromolecular oxidative damage, inflicted by endogenously generated ROS (reactive oxygen species), is a major causal factor underlying senescence [1–3]. Indeed, steady-state amounts of the products of ROS attacks on macromolecules can be detected under physiological conditions even in young and healthy animals, which has been interpreted to suggest that cells exist in a state of chronic imbalance between pro-oxidants and antioxidants, also referred to as ‘oxidative stress’ [4]. Furthermore, an age-associated increase in the amounts of oxidatively damaged macromolecules has been observed in tissues from a variety of animal species [2,5,6]. Whether this increase reflects a steady accumulation of damage under a constant level of oxidative stress or whether the imbalance between pro-oxidant production and antioxidant defences worsens with age remains unclear.
Another major issue that remains unresolved is whether the primary mode of action by which ROS putatively induce age-associated changes is via the infliction of molecular damage or whether ROS play an additional role in the regulation of cellular functions. For instance, an upsurge in ROS production can also cause shifts in the redox state of cells, which is determined by the amounts and ratios of the interconvertible forms of redox couples, such as GSH and GSSG, cysteine, thioredoxin, NAD(P)H and NAD(P)+ [7]. Glutathione is 2–3 orders of magnitude more abundant than any of the other redox-active compounds, and it is therefore widely considered to be the major determinant and indicator of the overall redox state [8–12]. However, the concentration of GSH is dependent on several factors, including the availability of precursors and rates of synthesis and oxidation (Scheme 1). The concentration of free methionine may also affect the redox state of glutathione, since (i) it may undergo direct oxidation to methionine sulphoxide [13] and (ii) it is also a precursor in the biosynthesis of cysteine, which is believed to govern the rate of glutathione biosynthesis [14].
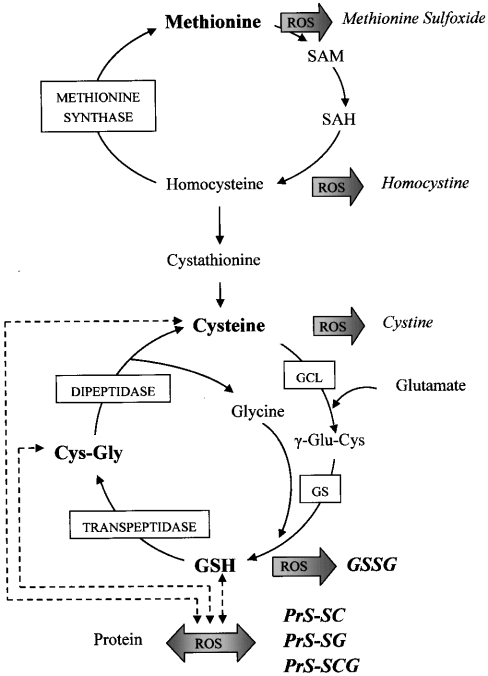
Scheme 1. Schematic representation of aminothiol synthesis and metabolism.
GSH is synthesized from cysteine, glutamate and glycine by the sequential action of GCL (glutamate–cysteine ligase) and GS (glutathione synthase). Intercellular transport of GSH involves its breakdown by the action of γ-glutamyl transpeptidase and dipeptidase, forming Cys-Gly and cysteine respectively. Alternatively, GSH may be oxidized to its disulphide, GSSG, and either GSH or GSSG may interact with protein cysteinyl thiols, forming PrS-SG. Analogously, cysteine may be oxidized to cystine. Cysteine and Cys-Gly may also interact with proteins to form PrS-SC and PrS-SCG. Methionine is the precursor for cysteine synthesis, in which homocysteine and cystathionine are intermediates. Homocysteine is the substrate for the regeneration of methionine by methionine synthase. Methionine and homocysteine may also be oxidized to methionine sulphoxide and homocystine respectively as a result of ROS attacks. SAM (S-adenosylmethionine) and SAH (S-adenosylhomocysteine), which are intermediates in the conversion of methionine into homocysteine, also serve as methyl group donors in anabolic DNA methylation. The metabolites that were measured in the present study are indicated in bold letters.
Oxidation of GSH, cysteine and methionine is among the earliest cellular responses to an increase in ROS production, leading to alterations in the redox potential [7]. An additional consequence of the increase in ROS generation is the reversible oxidation of protein cysteinyl thiols, leading to the formation of PrS-SG (protein-glutathionyl mixed disulphides) with GSH and its metabolites PrS-SC (protein-cysteinyl) and PrS-SCG (protein-Cys-Gly). Thiolation can potentially serve as a regulatable mechanism for the activation or inactivation of proteins involved in a wide variety of functions, such as signal transduction, gene activation and enzyme activities [12,15]. A pro-oxidizing shift in glutathione redox state and increased glutathiolation of proteins have been shown recently to occur in tissues of the C57BL6 mouse [16]. Whether similar changes occur in phylogenetically divergent species, and whether they are correlated with the life expectancy of the animals, remains to be established.
Drosophila melanogaster is an ideal model organism in which we can address questions concerning the relationship between oxidative stress and aging. Tissues of the adult fly are composed of post-mitotic cells; therefore, senescent changes are not diluted by successive cell divisions. Being a poikilotherm, its metabolic rate and life expectancy can be altered simply by varying the ambient temperature [17,18].
In the above context, the main purpose of the present investigation was to determine whether the level of oxidative stress, reflected by the amounts of GSH and GSSG, GSH/GSSG ratio, amounts of cysteine, Cys-Gly, methionine and protein mixed disulphide content, increases during aging. An additional objective was to determine whether life expectancy is related to the severity of the shift in the glutathione redox state.
MATERIALS AND METHODS
Reagents
Cysteine, Cys-Gly, GSH, methionine and GSSG were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.); acetonitrile, MPA (metaphosphoric acid) and 1-octanesulphonic acid were from EM Science (Gibbstown, NJ, U.S.A.). All other chemicals were of HPLC grade or of the highest purity available.
Animals
The y w strain of D. melanogaster was used in these experiments. Male flies were collected in groups of 25 under mild CO2 anaesthesia, approx. 1 day post-eclosion, and subsequently maintained at 25 °C on a cornmeal–sucrose–yeast medium, as described in [19].
Sample preparation
Flies were immobilized on ice for 1–2 min, weighed and homogenized in 10 vol. of freshly prepared ice-cold 5% (w/v) MPA, using 1.5 ml plastic tubes and pestles, obtained from RPI (Mt. Prospect, IL, U.S.A.). The homogenates were incubated for 30 min on ice and centrifuged at 18000 g for 20 min at 4 °C. The supernatants were filtered using 0.45 μm PTFE Acrodisc® CR 4 mm syringe filters obtained from Gelman Laboratory (Ann Arbor, MI, U.S.A.); filtrates were transferred to sampling vials and were either analysed immediately or stored at −80 °C for up to 1 month.
To determine the amounts of protein mixed disulphides, pellets from the acid precipitation were washed three times in 5% MPA to remove the free (non-protein bound) aminothiols. Protein-bound cysteine, Cys-Gly and GSH were subsequently released by incubation of protein pellets in 100 mM phosphate buffer for 1.5 h at 37 °C [20].
It is well known that degradation and oxidation of glutathione can occur during the preparation and storage of tissue samples [9,16,21–23]. Therefore, to validate the methodology used in this study, preliminary experiments were conducted to define the optimal conditions for sample preparation. The technical problems associated with sample preparation from the fruit fly were found to be substantially different from those described previously for whole blood and mammalian tissues [16,22]. Results of these experiments indicated that, in contrast with acidified mammalian tissue homogenates, the oxidation of GSH to GSSG was virtually undetectable during the first several hours of incubation of acidified insect samples on ice (<0.001%). Recovery studies involving different concentrations of MPA (2.5, 5, 10, 20 and 30%) indicated that >10% MPA was detrimental to the recovery due to acid hydrolysis of GSH to its constituent amino acids (as the concentrations of cysteine increased and GSH decreased as a function of acid concentration). Concentrations of MPA <5% were found to be insufficient for the purpose of protein precipitation, but, in contrast with findings in whole blood samples [22], 5% MPA did result in adequate protein precipitation. The purity and source of MPA were found to be of crucial importance for this purpose, as well as for maximal recovery and stability of aminothiols [16]. Specifically, MPA prepared from clear crystals obtained from EM Science gave more satisfactory results than the powdered substance from Sigma. Thus an MPA concentration of 5%, prepared from clear crystals, was found to be optimal for the recovery and quantification of aminothiols. Finally, the presence of a metal chelator (0.5 mM EDTA) in the insect samples was found to have no beneficial effect in terms of prevention of the potential metal-catalysed oxidation of GSH to GSSG; rather, it interfered with cysteine peak separation from the solvent front. Therefore the use of EDTA for sample preparation was avoided. Storage of acidified insect samples at −80 °C for up to 6 months had no effect on the concentrations of aminothiol compounds.
HPLC-coulometric ECD (electrochemical detection)
Aminothiol compounds (cysteine, Cys-Gly, GSH, methionine and GSSG) were separated by HPLC, fitted with a Shimadzu Class VP solvent delivery system, using a reversed-phase C18 Luna (II) column (3 μm; 4.6 mm×150 mm), obtained from Phenomenex (Torrance, CA, U.S.A.). The mobile phase for isocratic elution consisted of 25 mM monobasic sodium phosphate, 0.5 mM of the ion-pairing agent 1-octanesulphonic acid, 1% (v/v) acetonitrile (pH 2.7), adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation was completed in 35 min; GSSG was the last eluted peak, with a retention time of approx. 30 min [16]. Calibration standards of aminothiols were prepared in 5% MPA. Aminothiols were detected with a model 5600 CoulArray® electrochemical detector (ESA, Chelmsford, MA, U.S.A.), equipped with a four-channel analytical cell, using potentials of +400, +600, +750 and +875 mV. Cysteine, GSH and Cys-Gly were detected at +600 mV, whereas methionine and GSSG were detected at +750 mV. Each sample was injected twice and the average of the peak areas was used for quantification.
Statistical analysis
Significances of most of the age- and temperature-dependent trends were determined by linear regression using SYSTAT 10 software (SYSTAT Software, Richmond, CA, U.S.A.). The exceptions were the age-dependent changes in (i) GSH/GSSG ratio and free methionine content, for which non-linear regression was performed (using an exponential model); and (ii) GSSG content, which was assessed by one-factor analysis of variance, using post hoc Tukey tests for comparison of individual age groups.
RESULTS
Life span of flies and cross-sectional sampling of populations
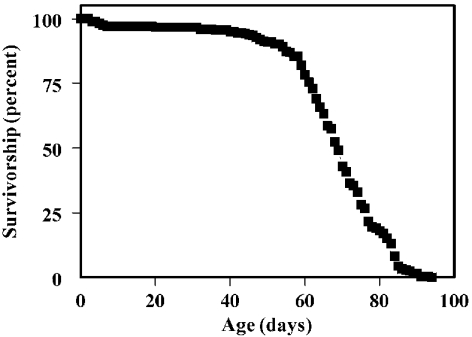
A typical survivorship curve of the y w strain used in this investigation is presented in Figure 1. The mean life span of the flies was 67 days. Biochemical analyses were conducted starting at 10 days of age to avoid complications associated with growth and maturation, which occur during the week following metamorphosis [24]. Cross-sectional sampling was restricted to the period before the onset of rapid mortality, since subsequently survivors have been shown to represent progressively smaller subsets of the population that are undergoing relatively slower rates of aging [25].
Figure 1. Life span of y w D. melanogaster.
Male flies (n=277) were maintained in groups of 25 under constant light at 25.0±0.5 °C. Mortality was recorded and flies were transferred to fresh food vials every 1–2 days.
Age-related changes in the abundance of free aminothiols and glutathione-redox state
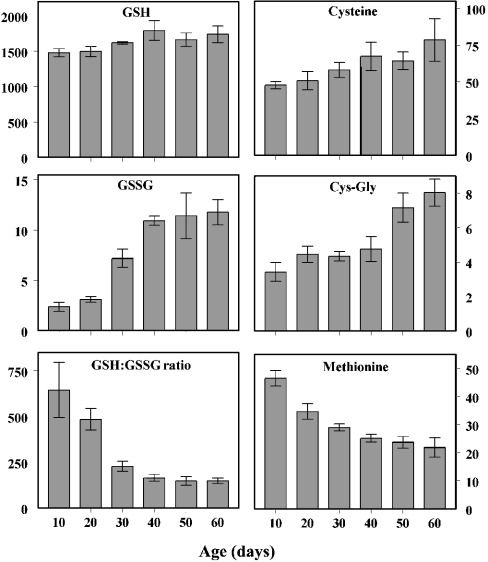
Concentrations of aminothiols were determined in whole-body homogenates of flies at 10, 20, 30, 40, 50 and 60 days of age (Figure 2). The concentrations of free aminothiols in homogenates of 10-day-old flies ranged from 2.5–3.5 pmol/mg of body weight for Cys-Gly and GSSG to 1500 pmol/mg for GSH. The concentrations of cysteine and methionine were approx. 50 pmol/mg.
Figure 2. Age-related changes in GSH, GSSG, GSH/GSSG ratio, cysteine, Cys-Gly and methionine.
The contents of GSH, GSSG, cysteine and Cys-Gly increased significantly as a function of age (P<0.0005 for all comparisons), whereas the GSH/GSSG ratio and methionine content decreased exponentially {GSH/GSSG=971exp [−0.040age (days)] and [methionine]=51.5exp [−0.016age (days)]}. The 95% confidence intervals for the exponential parameters were: [−0.048, −0.032] for GSH/GSSG and [−0.019, −0.014] for methionine. For GSSG content, post hoc analysis revealed no significant differences between the 10 and 20 day age groups or among the 40, 50 and 60 day groups, but there were significant increases from 20 to 30 and from 30 to 40 days of age (P<0.005). Results are means±S.D. for four independent preparations of 25–50 flies at each age and are expressed as pmol/mg of body weight.
The amount of GSH increased slightly but significantly as a function of age (<20%), but there was a proportionally greater age-related increase in the concentrations of the GSH precursors, cysteine and Cys-Gly (by 65 and 135% respectively). The GSSG content also increased, 4-fold, with most of the increase occurring between the ages of 20 and 40 days. Correspondingly, there was an exponential decrease in the GSH/GSSG ratio, amounting to 77% over the 10–60 days age range. A similar exponential pattern of decrease, by 53%, was observed for the free methionine content.
Age-related changes in the amounts of thiolated proteins
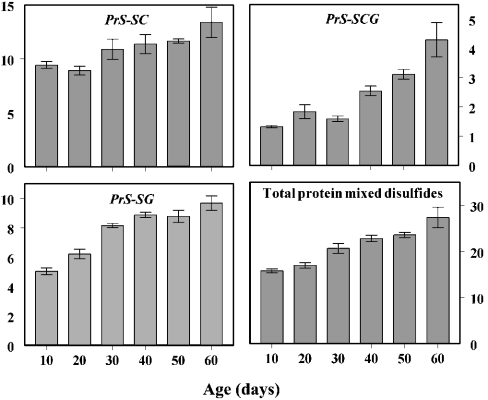
Protein mixed disulphide content was measured as cysteinyl-, cysteinylglycinyl- and glutathionyl-protein disulphides. At 10 days of age, the total content of these disulphides consisted of 60% PrS-SC, 32% PrS-SG and 8% PrS-SCG; thus, a far greater proportion of cysteine and Cys-Gly when compared with that of glutathione was protein-bound. Between 10 and 60 days of age, the amounts of these compounds increased by 20, 100 and 250% respectively (P<0.0005 for all compounds). The combined amounts of these protein mixed disulphides increased linearly as a function of age, nearly doubling between the ages of 10 and 60 days (Figure 3).
Figure 3. Age-related changes in PrS-SC, PrS-SCG, PrS-SG and total protein mixed disulphides.
The concentrations of the individual protein mixed disulphides and of the sum of these compounds increased in a roughly linear fashion, as a function of age (P<0.0005 for each panel). Results are means±S.D. for four independent preparations of 25–50 flies at each age and are expressed as pmol/mg of body weight.
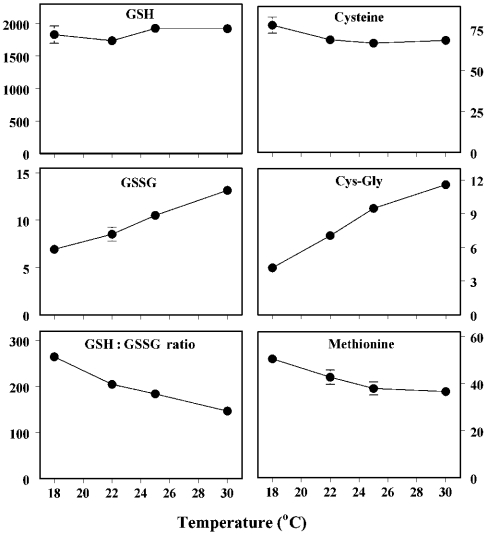
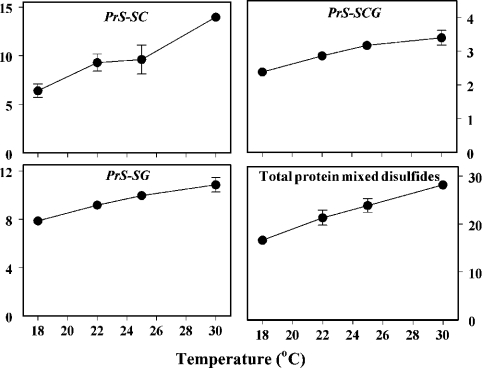
Effects of ambient temperature on aminothiol content, glutathione-redox state and protein thiolation
The life span of Drosophila is inversely related to the ambient temperature and directly related to the rate of oxygen consumption within the physiological range [18]. The hypothesis tested was that changes in aminothiol content, glutathione redox state and protein thiolation would correspond to differences in life expectancy induced by alterations in ambient temperature. Measurements were made in flies housed at 18, 22, 25 and 30 °C (Figure 4). The amounts of GSH and cysteine varied only slightly at different temperatures, whereas the amounts of Cys-Gly and GSSG increased and both the GSH/GSSG ratio and the free methionine content decreased progressively with increasing ambient temperature. The contents of all three forms of thiolated proteins increased in a fairly linear pattern with an increase in temperature (Figure 5). Thus, warmer environments, in which the flies have higher rates of oxygen consumption and shorter life spans, were associated with a more pro-oxidizing glutathione redox state and significantly increased protein thiolation. Additionally, the contents of both free and protein-bound aminothiol compounds reflected the remaining life expectancy of the flies, corresponding to their chronological age or ambient temperature.
Figure 4. Effects of ambient temperature on free GSH, GSSG, GSH/GSSG ratios, cysteine, Cys-Gly and methionine in y w flies.
Flies were maintained at 25 °C for the first 14 days after eclosion and then placed at 18, 22, 25 or 30 °C for the following 21 days. There was no significant difference in GSH and only a small decrease in cysteine content (P<0.02), but concentrations of Cys-Gly and GSSG increased (P<0.0005), whereas methionine content and GSH/GSSG ratios decreased (P<0.0005) as the temperature was increased. Results are means±S.D. for four independent preparations of 25–50 flies at each age and are expressed as pmol/mg of body weight.
Figure 5. Effects of ambient temperature on the amounts of PrS-SC, PrS-SCG, PrS-SG and total protein mixed disulphides.
Flies were maintained at 25 °C for the first 14 days after eclosion and then placed at 18, 22, 25 or 30 °C for the following 21 days. The concentration of each individual mixed disulphide compound increased linearly as a function of ambient temperature, as did the sum of the three compounds (P<0.0005 for all comparisons). Results are means±S.D. for four independent preparations of 25–50 flies at each age and are expressed as pmol/mg of body weight.
DISCUSSION
Results from the present study indicate that the ratio of GSH/GSSG and methionine content decreased and protein mixed disulphide content increased during aging in D. melanogaster. These changes were accelerated or decelerated in response to alterations in the ambient temperature, which is inversely related to the life expectancy of flies.
As discussed extensively in a recent publication [16], the quantification of aminothiols in biological samples can present several specific technical problems associated with sample origin, preparation and handling, as well as the analytical methodology employed for quantification [9,16]. Methods for the quantification of GSH and GSSG in biological samples can be divided into three categories: enzymic, fluorimetric and liquid chromatographic. In general, enzymic and fluorimetric methods have been found to be insensitive, time consuming and inaccurate [9,21,23]. Among the HPLC-based methods for the determination of GSH and GSSG, UV-absorbance and fluorimetric detection are complicated and unreliable because of the requirements of extensive sample manipulation (thiol blocking, acidification, neutralization and derivatization) combined with low sensitivity. In contrast, HPLC-ECD shortens the time required for sample handling, improves the sensitivity of detection and permits simultaneous detection of several aminothiol compounds in one chromatographic separation. As indicated in the Materials and methods section, the procedure used in the present study also resulted in maximal recovery of aminothiol compounds and minimal GSH degradation or oxidation to GSSG. The fact that the ratios of GSH/GSSG reported in the present study are among the highest published values [9,23] suggests that the methodology used in this study did not lead to an artifactual oxidation of GSH to GSSG.
The age-related increase in GSSG content, observed in this study, was not accompanied by any change in GSH levels, which is consistent with earlier results obtained in various organs of the mouse [16] and in other strains of Drosophila [26]. Some earlier reports have claimed that there is a generalized decrease in GSH content during aging [27]. However, as discussed previously [16], the procedures for glutathione quantification employed in the earlier studies lacked the accuracy and sensitivity of the ion-pairing HPLC-ECD methodology used in the present study.
The present finding that the amounts of cysteine and Cys-Gly, required for GSH synthesis [14], increased with age suggests that the tissues require an increased supply of precursors for GSH biosynthesis in older flies. The observed increase in GSSG content and decrease in the GSH/GSSG ratio is also indicative of an increase in oxidative stress, reflecting a widening gap between prooxidant production and antioxidant defences. The age-related decrease in methionine content provides further evidence for an increase in the level of oxidative stress, since it suggests either an increase in the direct oxidation of methionine to methionine sulphoxide by ROS, and/or possibly an increase in methionine consumption for cysteine biosynthesis. All these changes may be underlain by increasing rates of mitochondrial H2O2 generation in aging Drosophila, which have been documented previously [28,29].
The pro-oxidizing shift in glutathione-redox state and increase in protein mixed disulphides has various functional implications, especially in the context of the aging process. For instance, it is consistent with the observation that the age-related accumulation of certain types of oxidatively damaged macromolecules is exponential rather than linear [5,30–32]. Additionally, proteins falling within diverse functional categories have been demonstrated to undergo oxidant-mediated regulation by glutathionylation of functionally sensitive cysteine residues [11,15,23,33–36]. For instance, numerous components of signal transduction pathways have been shown to undergo glutathionylation, including guanyl cyclase, protein tyrosine phosphatase-1B, 5′-lipoxygenase, H-Ras, SoxR, OxyR and several protein kinase C isoenzymes (reviewed in [12,15,37,38]). DNA binding of several transcription factors, such as activator protein (1), nuclear factor κB, p53 and Sp-1, is dependent on the redox state of their cysteinyl residues [39–41]. Activities of metabolic enzymes, including carbonic anhydrase III [42], tyrosine hydroxylase [43], glucose-6-phosphate dehydrogenase [44] and creatine kinase [45], among others, also appear to be subject to regulation by glutathionylation. Protein glutathionylation may even have a direct effect on oxidant production and antioxidant defences, because purified Cu-Zn superoxide dismutase, glutaredoxin and thioredoxin are all susceptible to glutathionylation [46]. Furthermore, there is a reversible increase in the production of O2•− by mitochondrial NADH–ubiquinone oxidoreductase in response to thiolation/dethiolation [47]. However, in counterpoint, it has been suggested that the reaction rates of protein disulphides with GSH may be slow, relative to the rates that would be expected if glutathiolation indeed plays a regulatory role in protein function [48], and a number of examples cited here are based on in vitro rather than in vivo thiolation.
A key question about the significance of any age-related biochemical alteration is whether or not it plays a role in determining the rate of aging or life expectancy of the organism. It can be reasoned that an alteration associated with the life expectancy of the animals is more probably involved in physiological aging than one that correlates only with the chronological age. The life spans of poikilothermic species are inversely related to their metabolic rates, which in turn are directly related to the ambient temperature [17,49]. In the present study, the amounts of GSSG and thiolated proteins were found to be higher in flies kept at warmer temperatures for 3 weeks, beginning 2 weeks after eclosion, than in those kept at cooler temperatures. The GSH/GSSG ratio was also lower in flies kept at relatively warmer temperatures. These responses to changes in ambient temperature are consistent with the idea that the redox state and protein thiolation are associated with the life expectancy of the flies, or the physiological age, and thus might be involved in determining the rate of aging.
In conclusion, the results of the present study suggest that the cellular redox state becomes more pro-oxidizing during aging and is associated with the life expectancy of Drosophila. The results also suggest that the observed changes in glutathione redox state, aminothiol content and protein thiolation may serve as biomarkers of aging in animals.
Acknowledgments
This research was supported by grants RO1 AG17077 to R.S.S. and RO1 AG15122 to W.C.O. from the National Institutes of Health National Institute on Aging.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Beckman K. B., Ames B. N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 3.Sohal R. S., Mockett R. J., Orr W. C. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 4.Sies H. Biochemistry of oxidative stress. Angewandte Chemie. 1986;25:1058–1071. [Google Scholar]
- 5.Stadtman E. R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 6.Sohal R. S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer F. Q., Buettner G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 8.Reed D. J. Regulation of reductive processes by glutathione. Biochem. Pharmacol. 1986;35:7–13. doi: 10.1016/0006-2952(86)90545-9. [DOI] [PubMed] [Google Scholar]
- 9.Jones D. P. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 10.Das K. C., White C. W. Redox systems of the cell: possible links and implications. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9617–9618. doi: 10.1073/pnas.162369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casagrande S., Bonetto V., Fratelli M., Gianazza E., Eberini I., Massignan T., Salmona M., Chang G., Holmgren A., Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9645–9649. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klatt P., Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 13.Moskovitz J., Berlett B. S., Poston J. M., Stadtman E. R. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9585–9589. doi: 10.1073/pnas.94.18.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman H. J., Lui R.-M., Shi M. M. Glutathione synthesis in oxidative stress. In: Packer L., Cadenas E., editors. Biothiols in Health and Disease. New York: Marcel Dekker; 1995. pp. 189–212. [Google Scholar]
- 15.Cotgreave I. A., Gerdes R. G. Recent trends in glutathione biochemistry. Glutathione–protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- 16.Rebrin I., Kamzalov S., Sohal R. S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeb J., Northrop J. H. On the influence of food and temperature upon the duration of life. J. Biol. Chem. 1917;32:103–121. [Google Scholar]
- 18.Miquel J., Lundgren P. R., Bensch K. J., Atlan H. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech. Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 19.Mockett R. J., Orr W. C., Sohal R. S. Overexpression of Cu, ZnSOD and MnSOD in transgenic Drosophila. Methods Enzymol. 2002;349:213–220. doi: 10.1016/s0076-6879(02)49336-6. [DOI] [PubMed] [Google Scholar]
- 20.Rossi R., Cardaioli E., Scaloni A., Amiconi G., Di Simplicio P. Thiol groups in proteins as endogenous reductants to determine glutathione–protein mixed disulfides in biological systems. Biochim. Biophys. Acta. 1995;1243:230–238. doi: 10.1016/0304-4165(94)00133-i. [DOI] [PubMed] [Google Scholar]
- 21.Akerboom T. P. M., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- 22.Stempak D., Dallas S., Klein J., Bendayan R., Koren G., Baruchel S. Glutathione stability in whole blood: effects of various deproteinizing acids. Ther. Drug Monit. 2001;23:542–549. doi: 10.1097/00007691-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler D. M. Role of reversible oxidation–reduction of enzyme thiols-disulfides in metabolic regulation. Annu. Rev. Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 24.Rockstein M. Metachemogenesis postemergence chemical maturation in holometabolous insects. Smithsonian Inst. Misc. Coll. 1959;137:263–286. [Google Scholar]
- 25.Donato H., Hoselton M. A., Sohal R. S. An analysis of the effects of individual variation and selective mortality on population averages in aging populations. Exp. Gerontol. 1979;14:141–147. doi: 10.1016/0531-5565(79)90028-7. [DOI] [PubMed] [Google Scholar]
- 26.Mockett R. J., Sohal R. S., Orr W. C. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 27.Richie J. P., Jr, Lang C. A. A decrease in cysteine levels causes the glutathione deficiency of aging in the mosquito. Proc. Soc. Exp. Biol. Med. 1988;187:235–240. doi: 10.3181/00379727-187-42660. [DOI] [PubMed] [Google Scholar]
- 28.Sohal R. S., Agarwal A., Agarwal S., Orr W. C. Simultaneous overexpression of copper- and zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem. 1995;270:15671–15674. doi: 10.1074/jbc.270.26.15671. [DOI] [PubMed] [Google Scholar]
- 29.Sohal R. S., Arnold L., Orr W. C. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech. Ageing Dev. 1990;56:223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S., Sohal R. S. DNA oxidative damage and life expectancy in houseflies. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskovitz J., Yim M. B., Chock P. B. Free radicals and disease. Arch. Biochem. Biophys. 2002;397:354–359. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 32.Stadtman E. R., Levine R. L. Protein oxidation. Ann. N.Y. Acad. Sci. 2002;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 33.Thomas J. A., Chai Y.-C., Jung C.-H. Protein S-thiolation and dethiolation. Methods Enzymol. 1994;233:385–395. doi: 10.1016/s0076-6879(94)33045-x. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert H. F. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods Enzymol. 1995;251:8–28. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- 35.Hensley K., Robinson K. A., Gabbita S. P., Salsman S., Floyd R. A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 36.Poole L. B., Karplus P. A., Claiborne A. Protein sulfenic acids in redox signaling. Annu. Rev. Pharmacol. Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 37.Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 38.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 39.Galter D., Mihm S., Dröge W. Distinct effects of glutathione disulphide on the nuclear transcription factor κB and the activator protein-1. Eur. J. Biochem. 1994;221:639–648. doi: 10.1111/j.1432-1033.1994.tb18776.x. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y., Oberley L. W. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 41.Morel Y., Barouki R. Repression of gene expression by oxidative stress. Biochem. J. 1999;342:481–496. [PMC free article] [PubMed] [Google Scholar]
- 42.Cabiscol E., Levine R. L. Carbonic anhydrase III. J. Biol. Chem. 1995;270:14742–14747. doi: 10.1074/jbc.270.24.14742. [DOI] [PubMed] [Google Scholar]
- 43.Borges C. R., Geddes T., Watson J. T., Kuhn D. M. Dopamine biosynthesis is regulated by S-glutathionylation. J. Biol. Chem. 2002;277:48295–48302. doi: 10.1074/jbc.M209042200. [DOI] [PubMed] [Google Scholar]
- 44.Grant C. M., Quinn K. A., Dawes I. W. Differential protein S-thiolation of glyceraldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress. Mol. Cell. Biol. 1999;19:2650–2656. doi: 10.1128/mcb.19.4.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy S., Jones A. D., Cross C. E., Wong P. S., van der Vliet A. Inactivation of creatine kinase by S-glutathionylation of the active site cysteine residue. Biochem. J. 2000;347:821–827. [PMC free article] [PubMed] [Google Scholar]
- 46.Klatt P., Pineda-Molina E., Perez-Sala D., Lamas S. Novel application of S-nitrosoglutathione-Sepharose to identify proteins that are potential targets for S-nitrosoglutathione-induced mixed-disulfide formation. Biochem. J. 2000;349:567–578. doi: 10.1042/0264-6021:3490567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor E. R., Hurrel F., Shannon R. J., Lin T. S., Hirst J., Murphy M. P. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 48.Watson W. H., Yang X., Choi Y. E., Jones D. P., Kehrer J. P. Thioredoxin and its role in toxicology. Toxicol. Sci. 2004;78:2–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- 49.Sohal R. S. The rate of living theory: a contemporary interpretation. In: Collatz K. G., Sohal R. S., editors. Insect Aging. Heidelberg: Springer; 1986. pp. 23–44. [Google Scholar]