Abstract
Fructosamine 3-kinase (FN3K), an enzyme initially identified in erythrocytes, catalyses the phosphorylation of fructosamines on their third carbon, leading to their destabilization and their removal from protein. We show that human erythrocytes also contain FN3K-related protein (FN3K-RP), an enzyme that phosphorylates psicosamines and ribulosamines, but not fructosamines, on the third carbon of their sugar moiety. Protein-bound psicosamine 3-phosphates and ribulosamine 3-phosphates are unstable, decomposing at pH 7.1 and 37 °C with half-lives of 8.8 h and 25 min respectively, as compared with 7 h for fructosamine 3-phosphates. NMR analysis indicated that 1-deoxy-1-morpholinopsicose (DMP, a substrate for FN3K and FN3K-RP), like 1-deoxy-1-morpholinofructose (DMF, a substrate of FN3K), penetrated erythrocytes and was converted into the corresponding 3-phospho-derivative. Incubation of erythrocytes with 50 mM allose, 200 mM glucose or 10 mM ribose for 24 h resulted in the accumulation of glycated haemoglobin, and this accumulation was approx. 1.9–2.6-fold higher if DMP, a competitive inhibitor of both FN3K and FN3K-RP, was present in the incubation medium. Incubation with 50 mM allose or 200 mM glucose also caused the accumulation of ketoamine 3-phosphates, which was inhibited by DMP. By contrast, DMF, a specific inhibitor of FN3K, only affected the glucose-dependent accumulation of glycated haemoglobin and ketoamine 3-phosphates. These data indicate that FN3K-RP can phosphorylate intracellular, protein-bound psicosamines and ribulosamines, thus leading to deglycation.
Keywords: fructosamine, glycation, protein repair, psicosamine, ribose, ribulosamine
Abbreviations: DMF, 1-deoxy-1-morpholinofructose; DMP, 1-deoxy-1-morpholinopsicose; FN3K, fructosamine 3-kinase; FN3K-RP, fructosamine 3-kinase-related protein
INTRODUCTION
The objective of the present work was to get a better understanding of the potential physiological role of fructosamine 3-kinase-related protein (FN3K-RP). Fructosamine 3-kinase (FN3K) is a recently identified enzyme that phosphorylates both low-molecular-mass and protein-bound fructosamines [1,2]. These are formed through a spontaneous reaction of glucose with primary amines, followed by an Amadori rearrangement [3,4]. Fructosamine 3-phosphates are unstable, breaking down spontaneously to 3-deoxyglucosone, inorganic phosphate (Pi) and the amino compound that originally reacted with glucose [2]. Therefore, FN3K is a protein-repair enzyme. Evidence has been provided [5], with the help of DMF (1-deoxy-1-morpholinofructose), a cell permeable substrate and competitive inhibitor of FN3K [6], that this enzyme does remove fructosamine residues from haemoglobin in erythrocytes, a cell type in which FN3K is particularly active and from which it was first isolated [1,2].
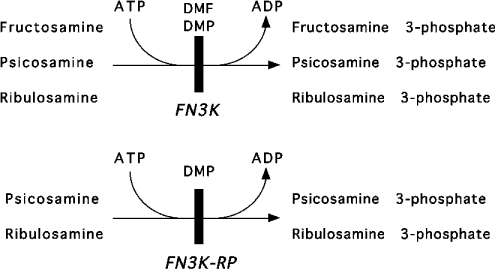
FN3K-RP, which shares approx. 65% sequence identity with FN3K, catalyses the phosphorylation of low-molecular-mass and protein-bound ribulosamines and psicosamines, but not fructosamines [7]. By contrast, FN3K was found to catalyse the phosphorylation of the three types of substrates (Scheme 1). These findings are particularly intriguing, since allose and free ribose, in vitro precursors of psicosamines and ribulosamines, are not expected to be present in significant amount in mammalian cells.
Scheme 1. Specificity of FN3K and FN3K-RP.
Both enzymes phosphorylate ribulosamines and psicosamines, including DMP. The latter can therefore be used as a competitive inhibitor for both enzymes. Only FN3K can phosphorylate fructosamines, including DMF, which serves therefore as a specific inhibitor for FN3K.
The main goals of the present work were: (1) to check for the presence of FN3K-RP in human erythrocytes (a cellular model that is suitable to study glycation and deglycation, since it is available in large amounts and can be incubated ex vivo with appropriate sugars and effectors for prolonged periods of time); (2) to test if DMP (1-deoxy-1-morpholinopsicose), a low-molecular-mass substrate of both FN3K and FN3K-RP (Scheme 1), can penetrate erythrocytes and be phosphorylated by these enzymes, therefore being able to act as a competitive inhibitor; and (3) to check if FN3K-RP can participate in the deglycation of ribulosamine and psicosamine residues that form when erythrocytes are incubated with elevated concentrations of ribose or allose, and if a substrate for FN3K-RP forms when these cells are incubated with an elevated concentration of glucose.
EXPERIMENTAL
Reagents
DEAE–Sepharose, SP–Sepharose, Blue Sepharose, Blue Trisacryl, Sephacryl S-200 and radiochemicals were from Amersham Biosciences (Little Chalfont, Bucks., U.K.), and Biogel P2 from Bio-Rad Laboratories (Hercules, CA, U.S.A.). DMF and D-ribose were from Sigma, and D-allose from ICN (Irvine, CA, U.S.A.). DMP was synthesized and purified as described previously [7].
Assay of ketoamine kinases
Psicosamine kinase activity was assayed at 30 °C as described previously [7] in a mixture (100 μl) containing 25 mM Tris, pH 7.8, 1 mM EGTA, 1 mM MgCl2, 1 mM dithiothreitol, 50 μM ATP-Mg, 500000 c.p.m. [γ-32P]ATP, 2.1 mg/ml lysozyme glycated with allose (bearing psicosamine residues). Phosphorylation of DMF and DMP was assayed as described previously [7] using [γ-32P]ATP as radioactive substrate.
Purification of FN3K-RP
A frozen pellet of washed erythrocytes (150 ml) was thawed and diluted with 600 ml of a buffer containing 25 mM Hepes, pH 7.1, 2 mM EDTA, 1 mM dithiothreitol, 1 μg/ml leupeptin and 1 μg/ml antipain (buffer A). The resulting lysate was centrifuged for 1 h at 4 °C and 11000 g. The supernatant (600 ml) was gently mixed for 1 h at 4 °C with 125 ml of wet Blue Sepharose (equilibrated in the same buffer). The gel was collected by centrifugation, resuspended in 125 ml buffer A and packed in a column containing 50 ml of Blue Sepharose. The gel was washed with 200 ml of buffer A. A NaCl gradient (0–750 mM in 2×150 ml of buffer A) and 100 ml 1 M NaCl in buffer A were then successively applied. Two peaks of psicosamine kinase activity were eluted with the salt gradient. The first one, at 400 mM NaCl, was insensitive to DMF, whereas the second one, which eluted at approx. 700 mM NaCl, was completely inhibited with 5 mM DMF.
The fractions containing DMF-insensitive psicosamine kinase activity from two such columns (180 ml; approx. 1 g of protein) were pooled, and the buffer was replaced by buffer B (as buffer A but with 1 mM MgCl2 instead of EDTA) through two rounds of concentration (to 50 ml) and dilution in a 200 ml Amicon cell equipped with a YM-10 filter. The sample (200 ml) was applied on a 30 ml Blue-Trisacryl column, equilibrated with buffer B. The column was washed with 100 ml of buffer B and protein was eluted with a 0–2 M NaCl gradient in 2×75 ml of buffer B. A large peak of DMF-insensitive psicosamine kinase activity preceded a smaller peak of DMF-sensitive activity. Fractions of the larger peak that did not contain any detectable DMF-kinase activity were pooled (42 ml), and concentrated to 2.5 ml on a YM-10 membrane in an Amicon cell. Further purification was achieved by loading 1.25 ml aliquots of this preparation on a 1.8 cm×55 cm Sephacryl S-200 column equilibrated with 25 mM Hepes, pH 7.1, 100 mM NaCl. In this step, psicosamine kinase co-eluted with an approx. 32 kDa polypeptide and was nearly homogeneous as estimated by SDS/PAGE (results not shown). It was confirmed that this preparation did not phosphorylate DMF or protein-bound fructosamines (lysozyme glycated with glucose). The procedure allowed the preparation of approx. 9 m-units of FN3K-RP with an approx. 3% yield.
Stability of ketoamine 3-phosphates
Lysozyme (2.1 mg/ml) bearing fructosamines (1.35 mol/mol), psicosamines (1.67 mol/mol) or ribulosamines (4 mol/mol), prepared as described previously [7], was incubated at 30 °C in a reaction mixture (0.4 ml) containing 25 mM Hepes, pH 7.1, 1 mM EGTA, 1 mM MgCl2, 10 μM ATP-Mg, 1×107 c.p.m. [γ-32P]-ATP and 75 μg of recombinant FN3K [1]. EDTA was added to a final concentration of 10 mM after 20 min (ribulosamines) or 40 min (fructosamines or psicosamines) to stop the reaction. The incubation medium was diluted with 3 ml of ice-cold 25 mM Hepes, pH 7.1, containing 1 μg/ml leupeptin and 1 μg/ml antipain (buffer C), and loaded on to a 1 ml SP–Sepharose column equilibrated with the same buffer and maintained at 4 °C. The column was washed with 4 ml of buffer C, the flow-through and the washing fractions were discarded and the protein-bound radioactivity was eluted with 450 mM NaCl; fractions of 1 ml were then collected. The most radioactive fraction was diluted with 2 ml buffer A (to reach a nearly physiological concentration of salt), and incubated at 37 °C. Samples of 40 μl were spotted at the indicated times on to 4 cm2 P81 papers (Whatman Biosystems Ltd., Maidstone, Kent, U.K.). Papers were washed and counted for radioactivity as described previously [1].
31P-NMR experiments
Packed human erythrocytes (2 ml) were incubated at 37 °C in a final volume of 10 ml of Krebs–Henseleit bicarbonate buffer [8], equilibrated with a gaseous mixture of O2/CO2 (19:1) and containing 1 mg/ml BSA, 100 units/ml penicillin/streptomycin, 10 mM glucose and the indicated concentrations of DMF or DMP. The incubations were stopped after 18 h and the samples were centrifuged for 10 min at 1000 g and 4 °C. The erythrocyte pellets were mixed with 6 ml of ice-cold 10% (v/v) HClO4 and centrifuged for 20 min at 16000 g and 4 °C. The supernatant was brought to approx. pH 6 by mixing with 50 ml of a tris-N-octylamine/chloroform (1:3.6) mixture. The aqueous phase was collected and concentrated under vacuum to 1.5 ml. Mes, pH 6.0, was added to a final concentration of 25 mM and the sample was freeze-dried.
For the 31P-NMR measurements the samples were redissolved in 1.35 ml of distilled water and 0.15 ml of 2H2O (for the deuterium locking of the spectrometer) to which 20 mg of Na2EDTA was added per ml of sample. Partially saturated 31P-NMR spectra were acquired at room temperature in an AMX 360 (8.4 Tesla) spectrometer (Bruker, Karlsruhe) with a 10 mm probe at a 31P frequency of 145.79 MHz using a 60° pulse. Spectroscopic parameter settings were repetition time=1.5 s, number of scans=24576 and the WALTZ-16 proton decoupling using sequence during the 0.82 s acquisition period. Before Fourier-transformation, a 1 Hz-line broadening exponential filter was used upon the time-domain signals. Chemical shifts are expressed with reference to the frequency of the β-phosphate of NTP.
Incubation of erythrocytes
Human erythrocytes were collected, washed and incubated at a 1/20 dilution in a final volume of 10 ml as described previously [5], using the indicated concentrations of glucose, allose or ribose, and inhibitors (DMF, DMP). For the incubations in the presence of allose or ribose, glucose was added to a concentration of 5 mM. After 24 h, the cells were harvested by centrifugation (10 min at 1000 g and 4 °C), washed twice with 5 ml of ice-cold 150 mM NaCl containing 1 mg/ml BSA, and once with 150 mM NaCl. For the incubation with 200 mM glucose, the NaCl concentration was brought to 200 mM to avoid cell breakage. The erythrocyte pellet was kept at −70 °C until use. In the case of the 4-day incubations, cells were collected by centrifugation and resuspended in fresh medium after 48 h to avoid glucose depletion and lactate accumulation.
Total glycated haemoglobin was measured by retention on affinity columns (boronate columns; PerkinElmer Life Sciences, Norton, OH, U.S.A.) and elution with salts, according to the manufacturer's instructions, except that 25 μl of packed washed erythrocytes were used instead of 50 μl of fresh total blood. Measurement of protein-bound, alkali-labile phosphate was performed as described previously [5].
Phosphorylation of haemoglobin with purified human FN3K-RP
Haemoglobin was partially purified by anion exchange chromatography [5], and incubated at a concentration of 1.3 mg/ml in a mixture (150 μl) containing 50 mM Tris, pH 7.8, 5 mM dithiothreitol, 1 mM MgCl2, 1 mM EGTA, 0.2 mM ATP-Mg, 2×106 c.p.m. [γ-32P]ATP and 0.17 m-units/ml FN3K-RP. Aliquots (40 μl) of the reaction mixture were spotted after various times on 4 cm2 P81 papers. These were washed and counted for radioactivity as described previously [1].
Glycation of lysozyme with glucose in the presence of Pi
Solutions containing 60 mg/ml hen egg lysozyme, 25 mM Hepes, pH 7.1, and 0, 20 or 200 mM sodium phosphate, pH 7.1, were filtered on a 0.22 μm pore size membrane and incubated at 37 °C. At the indicated times, aliquots of 2.5 ml were taken and purified by gel filtration on a Biogel P2 column equilibrated with water [1], to separate glycated lysozyme from glucose and Pi. Protein was assayed [9] to estimate the concentration of lysozyme and the mean substitution level of this protein was estimated by electrospray mass spectrometry [7].
RESULTS AND DISCUSSION
Presence of FN3K-RP in erythrocytes
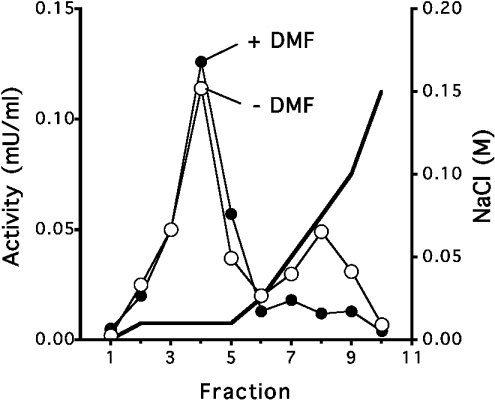
To test for the presence of FN3K-RP in erythrocytes, human erythrocyte lysates were chromatographed on DEAE–Sepharose. Two peaks of psicosamine 3-kinase activity were observed (Figure 1): the first, in the washing fractions, was not inhibited by DMF and therefore corresponded to FN3K-RP, whereas the second, eluting with the salt gradient, was inhibited by DMF, thus representing FN3K. From such experiments, it was concluded that FN3K-RP is indeed present in erythrocytes, where its activity amounts to 4.7 m-units/g of protein and accounts for approx. 75% of the total psicosamine 3-kinase activity. Like the enzyme overexpressed in human embryonic kidney cells [7], the erythrocyte enzyme phosphorylated DMP but not DMF (results not shown), further confirming that it was FN3K-RP. A procedure for the purification of FN3K-RP from erythrocytes to homogeneity is mentioned in the Experimental section. The presence of FN3K-RP in erythrocytes agrees with previous Northern blots [7] showing high levels of the corresponding mRNA in the bone marrow of mice.
Figure 1. Evidence for the presence of FN3K-RP in erythrocyte extracts.
A 2.5 ml sample of a human haemolysate (1:12 in 20 mM Hepes, pH 7.1, 1 mM dithiothreitol, 1 μg/ml leupeptin/antipain) was loaded on to a 1 ml DEAE–Sepharose column equilibrated with the same buffer. The flow-through was collected, and the column was washed with 4 ml of 10 mM NaCl, and then with a stepwise NaCl gradient in the same buffer; 1 ml fractions were collected. Psicosamine 3-kinase activity was measured in 50 μl of each fraction in the absence (open symbols) or in the presence (closed symbols) of 5 mM DMF. One representative experiment is shown.
Stability of ketoamine 3-phosphates
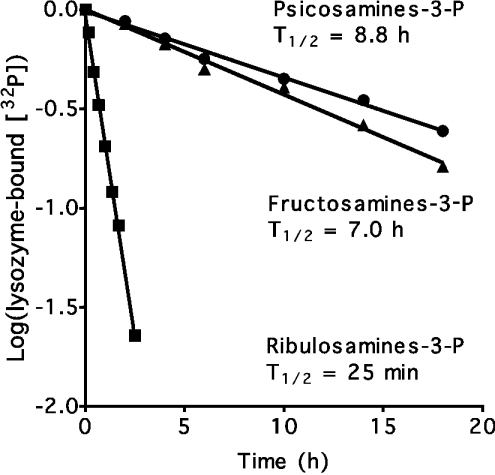
The instability of fructosamine 3-phosphates, which spontaneously degrade to an amine, 3-deoxyglucosone and Pi [2], means that FN3K is a deglycating enzyme. It was therefore of interest to test if psicosamine 3-phosphates and ribulosamine 3-phosphates are also unstable. For this purpose, lysozyme glycated with glucose, allose or ribose, was phosphorylated with FN3K and [γ-32P]ATP. The phosphorylated protein was purified by chromatography on SP–Sepharose and further incubated at neutral pH and 37 °C. At various times, protein-bound [32P]phosphate was estimated. An exponential decrease in protein-bound radioactivity was observed in all three cases (Figure 2). Half-lives of 7 h, 8.8 h, and 25 min were calculated for fructosamine, psicosamine and ribulosamine 3-phosphates respectively. It was confirmed that the liberated radioactivity corresponded to Pi [10] (results not shown).
Figure 2. Stability of ketoamine 3-phosphates.
Glycated lysozyme was phosphorylated with FN3K and [γ-32P]ATP, purified and incubated for the indicated times at 37 °C and pH 7.1. The results are expressed as the log of the ratio of protein-bound radioactivity at the indicated times to protein-bound radioactivity at 0 min.
The much lower stability of ribulosamine 3-phosphates compared with psicosamine 3-phosphates and fructosamine 3-phosphates is presumably due to the fact that they are much less stabilized by hemiketalization. Accordingly, approx. 20% of ribulose exists in solution as the acyclic oxo form [11], whereas the linear forms of psicose and fructose are undetectable, being probably less than 1% [12,13]. It is indeed likely that the spontaneous degradation of the ketoamine 3-phosphates proceeds by way of their acyclic form, which tautomerizes to a 1,2-enolamine 3-phosphate, before elimination of the phosphate group.
Phosphorylation of DMP in erythrocytes
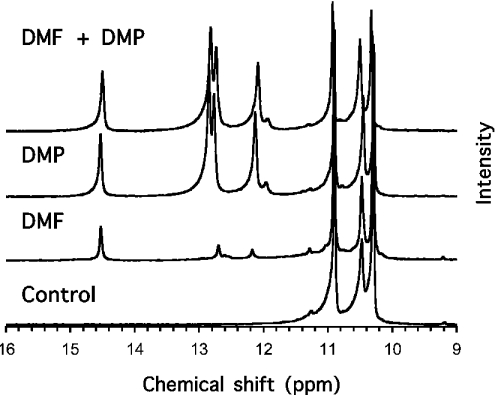
Previous experiments showed that DMF penetrates erythrocytes, where it can be phosphorylated by FN3K, and thereby act as a competitive inhibitor for this enzyme [6]. To test if the same was true for its C3 epimer, erythrocytes were incubated for 18 h in the presence of 5 mM glucose together with 5 mM DMF, DMP or both. Perchloric acid extracts were prepared and analysed by 31P-NMR. As reported previously [6], three distinct additional peaks were observed with erythrocytes incubated with DMF, corresponding most probably to the β-pyranose and α- and β-furanose forms of DMF 3-phosphate (Figure 3). Four distinct peaks were observed in extracts of erythrocytes incubated with DMP, most likely corresponding to the four hemiketalic forms of DMP 3-phosphate (α- and β-furanose; α- and β-pyranose) [7]. Integration of the peaks indicated that the amount of DMP 3-phosphate was 10-fold higher than that of DMF 3-phosphate. Little, if any, effect of DMF on the accumulation of DMP 3-phosphate was observed, suggesting that FN3K played only a minor role in the phosphorylation of DMP, which therefore was largely catalysed by FN3K-RP. Due to peak overlapping it was not possible to assess whether the phosphorylation of DMF was reciprocally inhibited by DMP. From these experiments, it was concluded that DMP gets access to FN3K and FN3K-RP in erythrocytes and that it can therefore be used to inhibit the action of these enzymes on other substrates.
Figure 3. 31P-NMR spectra of extracts of erythrocytes incubated with DMF, DMP or both.
Freshly isolated human erythrocytes were incubated for 18 h with 5 mM glucose in the absence or in the presence of 5 mM DMF, 5 mM DMP or a combination of both. Perchloric acid extracts were prepared, neutralized, enriched with 50 mM EDTA and analysed as described previously [6].
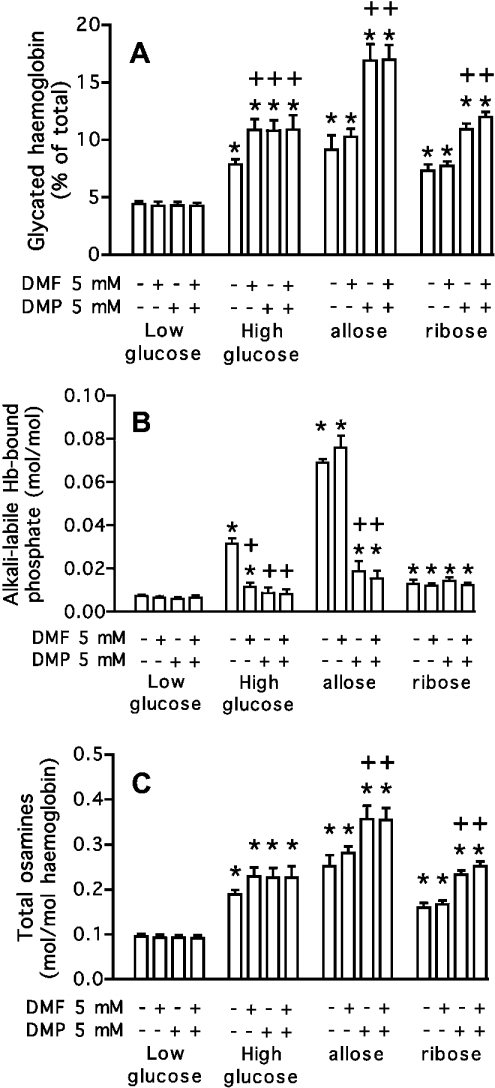
Effect of DMF and DMP on the accumulation of glycated haemoglobin and ketoamine 3-phosphates in erythrocytes
If FN3K-RP is involved in a protein deglycation pathway, an inhibitor of this enzyme such as DMP (Scheme 1) should enhance the accumulation of glycated haemoglobin when erythrocytes are incubated with aldoses (allose, ribose) that are precursors for its substrates (psicosamines and ribulosamines respectively). As DMP also inhibits FN3K, DMF was used to monitor the effect of FN3K inhibition. Incubations were carried out also with an elevated concentration (200 mM) of glucose to facilitate comparison with previous work [5]. As allose and ribose are more potent glycating agents than glucose [14], they were tested at lower concentrations (50 and 10 mM respectively).
As shown in Figure 4(A), incubation of erythrocytes for 24 h under these conditions led to the accumulation of glycated haemoglobin (as assessed by affinity-chromatography on boronate columns), whereas no significant change was observed in cells incubated with 5 mM glucose. With 200 mM glucose, both DMF and DMP independently increased the accumulation of glycated haemoglobin, whereas only DMP had a significant effect in the presence of allose or ribose. In all cases, the increment of glycated haemoglobin was 1.9–2.6-fold higher in the presence of the inhibitors than in their absence, in agreement with previous results obtained with glucose and DMF [5]. DMF and DMP did not cause any change in glycated haemoglobin in the presence of 5 mM glucose, indicating that they did not act as glycating agents. These results indicated that FN3K and FN3K-RP phosphorylated intracellular protein-bound osamines.
Figure 4. Glycated haemoglobin (A), alkali-labile, protein-bound phosphate (B) and total haemoglobin-bound osamines (C) in erythrocytes incubated with glucose, allose or ribose.
Erythrocytes were incubated for 24 h at 37 °C in the presence of 5 mM glucose (low glucose), 200 mM glucose (high glucose), 5 mM glucose+50 mM allose (allose) and 5 mM glucose+10 mM ribose (ribose), with or without 5 mM DMF and DMP. The total osamine values were calculated by taking into account the fact that haemoglobin is retained as a dimer on boronate affinity columns [15]. The values are the means±S.E.M. for four subjects. *, significantly different (P< 0.01; Student's t test) from ‘low glucose’; +, significantly different from the corresponding incubation without DMF and DMP.
Assays of ketoamine 3-phosphates (Figure 4B) agreed with this interpretation. About twice as much protein-bound, alkali-labile phosphate accumulated in the presence of 50 mM allose than in the presence of 200 mM glucose, consistent with the higher accumulation of glycated haemoglobin in the former condition. Both DMP and DMF decreased protein-bound alkali-labile phosphate when fructosamines were formed, whereas only DMP acted when psicosamines were formed. The accumulation of alkali-labile phosphate observed in erythrocytes incubated with ribose was much smaller than in the other two conditions (Figure 4B), presumably because of the much shorter half-life of ribulosamine 3-phosphates as compared with fructosamine 3-phosphates or psicosamine 3-phosphates. No significant effect of DMP was observed in this case, possibly because the inhibition of FN3K-RP by DMP was progressively overcome with time by accumulating ribulosamines. It is worthwhile recalling here that FN3K-RP and FN3K display an approx. 50-fold higher affinity for ribulosamines than for psicosamines [7]. Therefore, the effect of a competitive inhibitor (DMP) is expected to be more readily alleviated when protein-bound ribulosamines accumulate than when protein-bound psicosamines accumulate.
The total amount of haemoglobin-bound osamines (sum of haemoglobin-bound sugar and osamine 3-phosphates; Figure 4C) was significantly lower in samples incubated in the absence of DMP (in the case of fructosamines, psicosamines and ribulosamines) or of DMF (in the case of fructosamines) than in their presence. Taken together, the results presented in this section indicated that FN3K, which is sensitive to both DMF and DMP, causes deglycation of fructosamine residues, whereas FN3K-RP, which is only sensitive to DMP, is mainly responsible for the deglycation of psicosamine and ribulosamine residues.
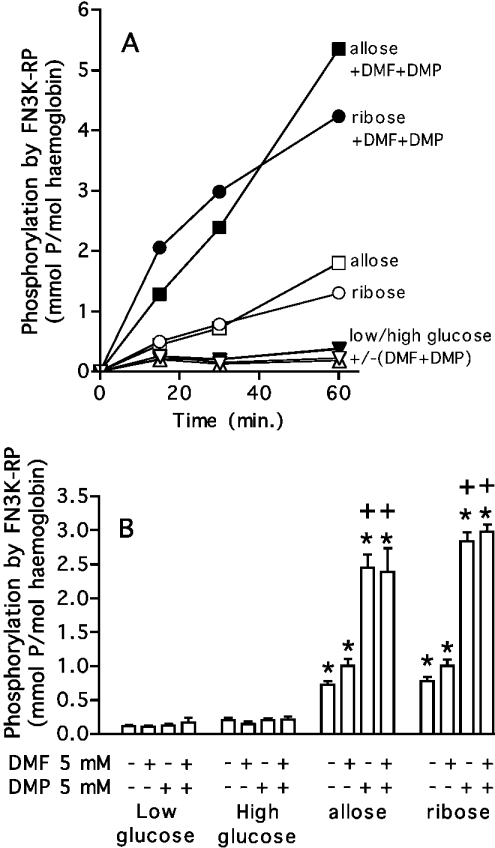
Formation of substrates for FN3K-RP in erythrocytes
In order to verify that substrates of FN3K-RP were indeed formed under the conditions described above, haemoglobin was partially purified from erythrocyte lysates and submitted to phosphorylation by FN3K-RP in the presence of [γ-32P]ATP. As shown in Figure 5, FN3K-RP indeed progressively phosphorylated haemoglobin from erythrocytes that had been incubated with allose or ribose, and this phosphorylation was increased approx. 3-fold if glycation had proceeded in the presence of DMP or the combination of DMF and DMP. Incorporation of phosphate was linear as a function of time with haemoglobin glycated with allose, but tended to level off after 30 min with haemoglobin glycated with ribose, presumably because of the short half-life of ribulosamine 3-phosphates.
Figure 5. Phosphorylation of glycated haemoglobin by FN3K-RP.
The experimental details are as in Figure 4. Haemoglobin was partially purified from the various samples and phosphorylated with FN3K-RP for the indicated times (A). (B) shows the phosphorylation stoichiometry after 30 min. Results shown are the means±S.E.M. for four subjects. Statistical analysis is shown in (B). *, significantly different (P<0.01; Student's t test) from ‘low glucose’; +, significantly different from the corresponding incubation without DMF and DMP.
A modest increase in the amount of substrate for FN3K-RP was observed in cells incubated for 24 h with 200 mM glucose (Figure 5B). As this might represent a slow formation of psicosamines or ribulosamines, we tested the effect of a 4-day incubation in the presence of elevated concentrations of this hexose. DMF and DMP had no effect in cells incubated with 5 mM glucose, but both caused a statistically significant (P<0.01) increase in the amount of FN3K-RP substrate in cells incubated with 200 mM glucose (phosphorylation stoichioimetry after 30 min incubation with FN3K-RP: 0.16±0.03 mol% in the absence of inhibitor; 0.28±0.02 mol% and 0.35±0.05 mol% in the presence of 5 mM DMF and 5 mM DMP respectively, as compared with 2.45±0.19 mol% for erythrocytes incubated with 50 mM allose, 5 mM DMP for 24 h). The half-life of the phosphorylation product, determined after stopping the FN3K-RP reaction with EDTA, amounted to approx. 6.5 h, and was therefore consistent with that of psicosamine 3-phosphates. Taken together, these data indicated a low rate of psicosamine formation from glucose in intact erythrocytes.
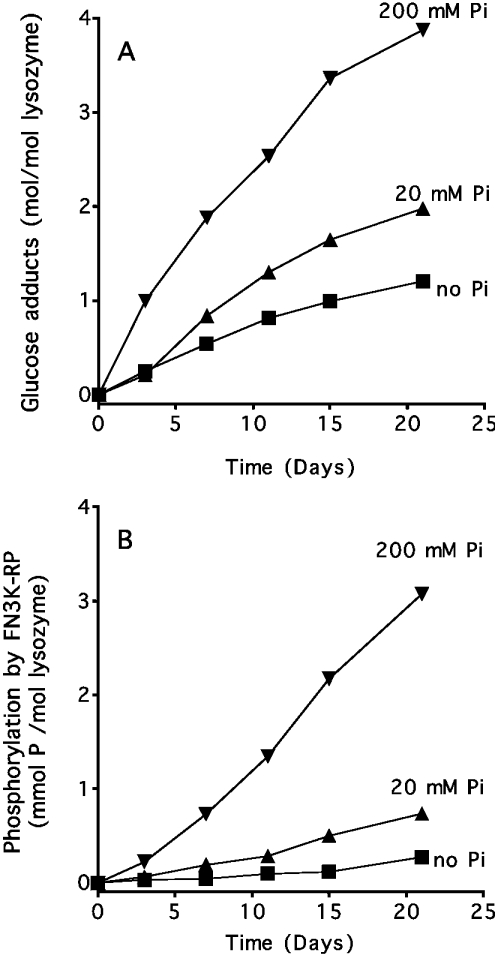
Mechanism of the formation of psicosamines from glucose
As Pi is known to facilitate the formation of fructosamines [16], we tested the possibility that it could also give rise to the formation of psicosamines (or at least, substrates of FN3K-RP) when proteins are incubated with glucose. As shown in Figure 6(A), Pi increased the rate of glycation of lysozyme with glucose, estimated by mass spectrometry, by approx. 50% and 250% at 20 and 200 mM respectively. Figure 6(B) shows that the presence of Pi also allowed the formation of a substrate for FN3K-RP. The rate of phosphorylation of lysozyme glycated under these conditions was compared with that observed with lysozyme containing various amounts of psicosamines (obtained by incubation of lysozyme with allose in the absence of Pi). Such titration experiments indicated that lysozyme glycated for 21 days with glucose in the presence of Pi contained 0.64 mol/mol of psicosamines (results not shown), which represented approx. 16% of total protein-bound sugar.
Figure 6. Formation of a substrate for FN3K-RP upon incubation of lysozyme with glucose in the presence of Pi.
Lysozyme (60 mg/ml) was incubated for the indicated times with 1 M glucose and the indicated concentrations of Pi. (A) The mean glycation level expressed as hexose moiety per lysozyme molecule as estimated by MS. (B) The phosphorylation reached after 15 min of incubation with FN3K-RP under the conditions described in the Experimental section.
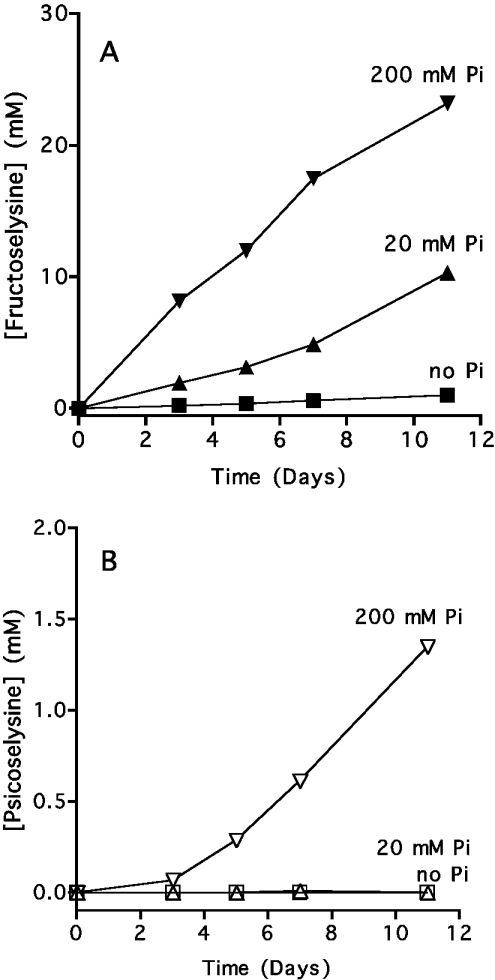
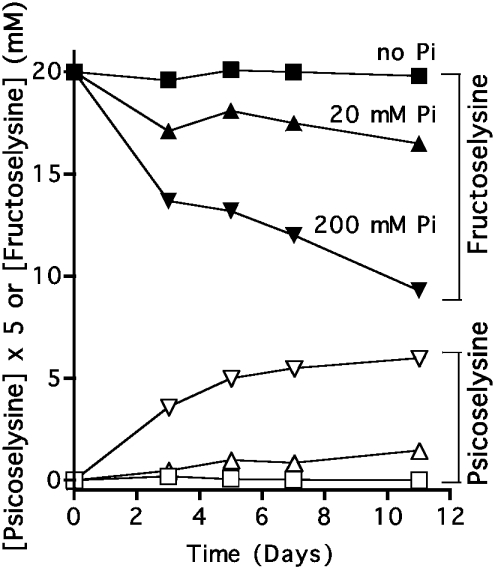
To confirm that Pi indeed favoured the formation of fructosamines and psicosamines from glucose, we performed similar incubations with free lysine and measured the formation of psicose-ε-lysine and fructose-ε-lysine with specific enzymic assays based on Escherichia coli fructoselysine 6-kinase and fructoselysine 3-epimerase [17,18]. As shown in Figure 7(A), Pi stimulated the formation of fructosamines by up to 30-fold at 200 mM. Furthermore, the highest concentration of Pi used (200 mM) led to the progressive appearance of psicose-ε-lysine, which accelerated with time (Figure 7B). A ratio of psicose-ε-lysine to fructose-ε-lysine of 0.07 was observed after 11 days.
Figure 7. Effect of Pi on the formation of fructose-ε-lysine and psicose-ε-lysine from glucose and lysine.
L-Lysine (0.2 M) was incubated at 37 °C at pH 7.0 with 0.2 M glucose, and 0, 20 and 200 mM sodium phosphate under sterile conditions. Fructose-ε-lysine and psicose-ε-lysine were assayed spectrophotometrically using fructoselysine 6-kinase and fructoselysine 3-epimerase [17,18]. Results are the means of two experiments.
The progressive acceleration in the formation of psicose-ε-lysine suggested that it proceeded via fructose-ε-lysine. This is not unexpected, since this formation presumably involves a 2,3-enediol intermediate. We therefore investigated the conversion of fructose-ε-lysine into psicose-ε-lysine in the presence of different concentrations of Pi. This anion stimulated the disappearance of fructose-ε-lysine and its conversion into psicose-ε-lysine (Figure 8). The rate constant calculated for this conversion amounted to approx. 0.015/day, which readily accounts for the formation of psicose-ε-lysine observed in Figure 7. Of note is the fact that psicose-ε-lysine only accounted for part of the disappearance of fructose-ε-lysine, indicating that Pi stimulated other reactions.
Figure 8. Rate of conversion of frutoselysine into psicoselysine in the presence or in the absence of phosphate.
Fructose-ε-lysine (20 mM) was incubated at 37 °C and pH 7.0 in the presence of 50 mM Hepes and the indicated concentrations of Pi under sterile conditions. Fructose-ε-lysine and psicose-ε-lysine were assayed at the indicated times. Results are the means of two experiments.
Taken together, these data indicate that protein-bound fructosamines formed from glucose may slowly isomerize to psicosamines in the presence of Pi and potentially of other intracellular chemical catalysts.
Conclusion
Our work indicates that FN3K-RP is present in erythrocytes and displays an activity that is several-fold higher than FN3K. Although the conditions that we have used are artificial, involving non-physiological concentration of sugars, our experiments show that FN3K-RP can act inside erythrocytes as a deglycating enzyme by converting ketoamines into unstable phosphate esters. Potential physiological substrates are psicosamines, which appear to be formed slowly from fructosamines. However, it seems to us that this slow formation does not ‘justify’ the presence of an enzyme that is several-fold more active than FN3K, and that ribulosamines are more likely to be the important physiological substrates of this enzyme. How these are formed, from lysines glycated with ribose 5-phosphate, ADP-ribose or another unknown compound, remains to be determined. DMP may provide an interesting tool in this search.
Acknowledgments
We thank Mrs Geneviève Berghenouse for her help in experimental work and Dr Maria Veiga-da-Cunha, for her thoughtful comments. This work was supported by the Concerted Research Action Program of the Communauté Française de Belgique; the Interuniversity Attraction Poles Program-Belgian Science Policy, the Belgian Scientific Fund for Medical Research (FRSM); the European Foundation for the Study of Diabetes and by a grant from the European Community (Euroglycan network, QLG1-CT-2000-00047). F.C., E.W. and J.F. are fellows of the Fonds pour l’Encouragement à la Recherche dans l’Industrie et dans l’Agriculture, and G.D., chargé de recherche of the Belgian FNRS.
References
- 1.Delpierre G., Rider M. H., Collard F., Stroobant V., Vanstapel F., Santos H., Van Schaftingen E. Identification, cloning, and heterologous expression of a mammalian fructosamine 3-kinase. Diabetes. 2000;49:1627–1634. doi: 10.2337/diabetes.49.10.1627. [DOI] [PubMed] [Google Scholar]
- 2.Szwergold B. S., Howell S., Beisswenger P. J. Human fructosamine 3-kinase: purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes. 2001;50:2139–2147. doi: 10.2337/diabetes.50.9.2139. [DOI] [PubMed] [Google Scholar]
- 3.Hodge J. E. The Amadori rearrangement. Adv. Carbohydr. Chem. 1955;10:169–205. doi: 10.1016/s0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]
- 4.Baynes J. W., Watkins N. G., Fisher C. I., Hull C. J., Patrick J. S., Ahmed M. U., Dunn J. A., Thorpe S. R. The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res. 1989;304:43–67. [PubMed] [Google Scholar]
- 5.Delpierre G., Collard F., Fortpied J., Van Schaftingen E. Fructosamine 3-kinase is involved in an intracellular deglycation pathway. Biochem. J. 2002;365:801–808. doi: 10.1042/BJ20020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delpierre G., Vanstapel F., Stroobant V., Van Schaftingen E. Conversion of a synthetic fructosamine into its 3-phospho derivative in human erythrocytes. Biochem. J. 2000;352:835–839. [PMC free article] [PubMed] [Google Scholar]
- 7.Collard F., Delpierre G., Stroobant V., Matthijs G., Van Schaftingen E. A mammalian protein homologous to fructosamine 3-kinase is a ketosamine-3-kinase acting on psicosamines and ribulosamines but not on fructosamines. Diabetes. 2003;52:2888–2895. doi: 10.2337/diabetes.52.12.2888. [DOI] [PubMed] [Google Scholar]
- 8.Krebs H., Henseleit K. Untersuchungen über die Harnstoffbildung in Tierkörper. Hoppe-Seyler's Z. Physiol. Chem. 1932;210:33–60. [Google Scholar]
- 9.Lowry O. H., Ronsenbrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 10.McClard R. W. Synthesis and purification of [1-32P]fructose 1,6-bisphosphate with high specific radioactivity. Anal. Biochem. 1979;96:500–503. doi: 10.1016/0003-2697(79)90612-2. [DOI] [PubMed] [Google Scholar]
- 11.Wu J., Serianni A. S., Vuorinen T. Furanose ring anomerization: kinetic and thermodynamic studies of the D-2-pentuloses by 13C-NMR spectroscopy. Carbohydr. Res. 1990;206:1–12. doi: 10.1016/0008-6215(90)84001-b. [DOI] [PubMed] [Google Scholar]
- 12.Swenson C. A., Barker R. Proportion of keto and aldehydo forms in solutions of sugars and sugar phosphates. Biochemistry. 1971;10:3151–3154. doi: 10.1021/bi00792a026. [DOI] [PubMed] [Google Scholar]
- 13.Que L., Gray G. R. 13C nuclear magnetic resonance spectra and the tautomeric equilibria of ketohexoses in solution. Biochemistry. 1974;13:146–153. doi: 10.1021/bi00698a023. [DOI] [PubMed] [Google Scholar]
- 14.Bunn H. F., Higgins P. J. Reaction of monosaccharides with proteins: possible evolutionary significance. Science (Washington, D.C.) 1981;213:222–224. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Medzihradszky K. F., Cunningham J., Lee P. D., Rognerud C. L., Ou C. N., Harmatz P., Witkowska H. E. Characterization of glycated hemoglobin in diabetic patients: usefulness of electrospray mass spectrometry in monitoring the extent and distribution of glycation. J. Chromatogr. B. Biomed. Sci. Appl. 2001;759:1–15. doi: 10.1016/s0378-4347(01)00196-7. [DOI] [PubMed] [Google Scholar]
- 16.Watkins N. G., Neglia-Fisher C. I., Dyer D. G., Thorpe S. R., Baynes J. W. Effect of phosphate on the kinetics and specificity of glycation of protein. J. Biol. Chem. 1987;262:7207–7212. [PubMed] [Google Scholar]
- 17.Wiame E., Delpierre G., Collard F., Van Schaftingen E. Identification of a pathway for the utilization of the Amadori product fructoselysine in Escherichia coli. J. Biol. Chem. 2002;277:42523–42529. doi: 10.1074/jbc.M200863200. [DOI] [PubMed] [Google Scholar]
- 18.Wiame E., Van Schaftingen E. Fructoselysine 3-epimerase, an enzyme involved in the metabolism of the unusual Amadori compound psicoselysine in Escherichia coli. Biochem. J. 2004;378:1047–1052. doi: 10.1042/BJ20031527. [DOI] [PMC free article] [PubMed] [Google Scholar]