Abstract
Middle cerebral artery occlusion (MCAO) is a model for inducing ischemic stroke in rodents, leading to devastating brain damage. Oxidative stress (OS) plays a crucial role in the pathogenesis of ischemia. In this study, the effect of melatonin and N-acetylcysteine on ischemia-reperfusion-induced oxidative stress injury in the cerebral cortex of male rats was investigated. 30 male Wistar rats were divided into sham, ischemic, NAC, melatonin and NAC + melatonin groups. All groups, except the sham group, underwent MCAO on the left side, and the treatment groups received intraperitoneal injections of either 50 mg/kg N-acetylcysteine (NAC) or 5 mg/kg melatonin or a combination of both 24 and 48 hours later. At 24 and 72 hours after surgery, the animals were examined for sensory and motor activity. The cerebral cortex was dissected after sacrificing the rats, infarct volume estimated and the concentrations of glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA) and nuclear factor erythroid-2 related factor 2 (Nrf2) were analyzed by enzyme-linked immunosorbent assay (ELISA). The results indicate that the NAC + melatonin group exhibited elevated sensory-motor activity and a reduced infarct volume rate in comparison to the ischemic group (p≤ 0.05). Compared to the ischemic group, the NAC + melatonin group showed a significant increase in SOD concentration and a significant decrease in MDA (p≤ 0.05). It can therefore be concluded that the simultaneous administration of NAC and melatonin can reduce the cerebral infarction volume, and improve neurological functions by modulating SOD and MDA.
Keywords: Middle cerebral artery occlusion (MCAO), Antioxidant enzymes, Melatonin, N-acetylcysteine (NAC), Cerebral infarction
Graphical Abstract
Highlights
-
•
Ischemic group antioxidants were notably lower than those of sham group and MDA concentration was also significantly higher.
-
•
Sensorimotor activity of the combination group was significantly higher than that of the melatonin, NAC and ischemic group.
-
•
Combination group cerebral infarct volume was significantly lower than that of ischemic group and other treatment groups.
-
•
SOD concentration of the combination, NAC and melatonin group was significantly higher than that of the ischemic group.
-
•
MDA concentration of the combination group was significantly lower than that of the melatonin, NAC and ischemic group.
1. Introduction
Cerebral ischemia is the most common form of stroke and is considered one of the leading causes of death. Middle cerebral artery occlusion (MCAO) is a model for inducing ischemic stroke in rodents, leading to devastating brain damage (Yang, 2019). The cause of ischemia is a sudden interruption of blood flow in the brain, which disrupts the supply of nutrients, oxygen, and glucose to brain cells (Sarkar et al., 2017). Oxidative stress (OS) is considered a key mechanism and plays an important role in the pathogenesis of ischemia (Yang, 2019, Yang et al., 2022). Redox homeostasis is dysregulated in mitochondria after brain injury due to an imbalance between the antioxidant machinery and the high production rate of reactive oxygen species (ROS) (Bhatti et al., 2018). The high level of ROS can attack DNA, destroy membrane structures, and disrupt energy production, ion transport, and cellular functions. OS is particularly damaging to structures that have low antioxidant machinery and high levels of polyunsaturated fatty acids in membranes, as is the case with the blood-brain barrier (BBB) microcirculatory system (Kryl'skii et al., 2019).
The antioxidant defense system consists of several enzymes. Nuclear factor erythroid 2-related factor 2 (Nrf2) is an oxidation-sensitive transcription factor that activates transcription of downstream antioxidant genes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Yang et al., 2022). Studies have shown that decreased expression of Nrf2 during cerebral ischemia increases infarct volume and impairs neurological function, exacerbating brain injury (Sarkar et al., 2017). In addition, after MCAO, the activity of SOD decreased and levels of oxidative stress markers such as malondialdehyde (MDA) increased (Yang et al., 2022). The ischemic penumbra is the region around the infarct core where neurons no longer function but could still be saved if blood flow were quickly restored. The key point is that stroke treatment should focus on saving this tissue (Ermine et al., 2021). One of the most important treatments for ischemia is reperfusion (Bhaskar et al., 2018), but this causes severe damage to the brain, termed ischemia-reperfusion injury (IRI) (Golts & Onaitis, 2021). These include several molecular mechanisms, including oxidative stress, which begins at the time of ischemia and continues until hours or days after the initial time point (Eakin et al., 2014; Feng et al., 2024). The neuronal endogenous antioxidant system is inefficient at the time of an oxidative crisis, as occurs during ischemia (Sarkar et al., 2017). Therefore, to reduce further damage to neuronal subcellular structures, an exogenous antioxidant system may be required (Lee and Lee, 2020, Zhao et al., 2019). Exogenous antioxidants such as melatonin and NAC are known for their broad-spectrum free radical scavenging and antioxidant effects, as well as for enhancing the activity of the endogenous antioxidant system (Mortezaee et al., 2016; Shahripour et al., 2019; Komsiiska, 2019). Melatonin and NAC improve neurological function and reduce infarction rates in cerebral ischemia (Ramos et al. 2017; Watson et al., 2016; Shahripour et al., 2019; Tardiolo et al., 2018). As mentioned earlier, studies have shown the beneficial effects of melatonin and NAC in cerebral ischemia-induced oxidative stress, but to our knowledge, none has investigated the effects of using melatonin and NAC simultaneously. Combining these two antioxidants against oxidative stress during ischemic stroke seems to increase the rate of targeted efficacy and reduce systemic toxicity. In the present study, melatonin and N-acetylcysteine were evaluated concerning ischemia-induced oxidative stress in male rat cerebral cortex.
2. Materials and methods
2.1. Animals and surgical procedures
A total of 30 adult male Wistar rats (age 12 weeks, weight 270–300 g) were obtained from the Pasteur Institute of Iran. The rats received unlimited water and food and were kept under standard conditions (23 ± 2 °C temperature and 12-hour alternation of light and darkness). All procedures were performed according to the guidelines of the Iranian Council and the Ethics Committee of Qazvin University of Medical Sciences (IR.QUMS.REC.1397.195). The following experimental groups were formed (n = 6 for each group): 1) sham that received all surgical procedures except middle cerebral artery occlusion (MCAO) and melatonin and NAC injection, 2) ischemic, 3) NAC, 4) melatonin, and 5) NAC + melatonin (combination) that underwent MCAO surgical procedures. For inducing ischemia, rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) (Alfasan, Netherlands), and the skin on the neck was incised. After exposing the common carotid artery (CCA) and its terminal branches, the external carotid artery and the proximal part of the CCA on the left side were ligated. To induce ischemia, a silicone-coated monofilament (Doccol Corporation, USA) was placed from the distal part of the CCA toward the middle cerebral artery (MCA) and left in this area for 60 minutes, then it was removed (Sabbaghziarani et al., 2017, Sabbaghziarani et al., 2017). Dosages for the treatment were established based on previous studies that showed treatment effectiveness. In the treatment groups, N-acetylcysteine (NAC) (50 mg/kg, Sigma, USA) (Aydin et al., 2023) and melatonin (5 mg/kg, Sigma, USA) (Chen et al., 2006) were intraperitoneally injected 24 and 48 hours post ischemia induction. The sham group received an intraperitoneal injection of normal saline with 5 % DMSO, matching the volume administered to the treatment groups 24 and 48 hours after surgery. Ethical guidelines were followed to minimize animal suffering during and after surgery.
2.2. Neurobehavioral tests
Neurobehavioral tests were performed to assess sensory and motor dysfunction at 24 hours to confirm the ischemia model and at 72 hours to evaluate the efficacy of therapy after MCAO surgery, as described (Dang et al., 2011). Spontaneous activity, body proprioception, climbing, stretching, vibrissae, and walking (each with a score of 0–3) were impaired as a general result of MCAO. The total score in this test for each individual was 18. The sham group always scored 18, whereas the ischemic group scored in the range of 10 or below. It should be noted that the tests were performed in a blinded fashion (Table 1).
Table 1.
Neurobehavioral tests. Assessment of sensory and motor dysfunctions at 24 hours and to compare the efficacy of therapy, 72 hours after ischemic surgery. Spontaneous activity, body proprioception, climbing, stretching, vibrissae, and walking (each with a score of 0–3) are impaired as a general outcome of middle cerebral artery occlusion (MCAO). The total score in this test is 18. Sham-operated animals always score 18, whereas animals that underwent ischemia show scores at the range of 10 or lower.
| Animal activity | Score |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| 1.) Spontaneous activity (in cage for 5 min) (Spontaneous Activity) | No movement □ |
Barely moves □ |
Moves, but does not approach at least three sides of cage □ |
Moves and approaches at least three sides of cage □ |
| 2.) Symmetry of forelimbs (outstretching while held by tail) (Forepaw Outstretching) | Right side: no movement, no outreaching □ |
Right side: slight movement to outreach □ |
Right side:moves and outreaches less than left side □ |
Symmetrical outreach □ |
| 3.) Climbing wall of wire cage (Climbing) | … | Fails to climb □ |
Right side is weak □ |
Normal climbing □ |
| 4.) Reaction to touch on either side of trunk (Body Proprioception) | … | No response on right side □ |
Weak response on right side □ |
Symmetrical response □ |
| 5.) Response to vibrissae touch | … | No response on right side □ |
Weak response on right side □ |
Symmetrical response □ |
| 6.) Behaviour of walk of the rat | Right circling □ |
Right□ | No movement □ |
Straight Ahead □ |
2.3. Triphenyltetrazolium chloride (TTC) and hematoxylin and eosin (H&E) staining and infarct volume evaluation
TTC staining was conducted 24 hours post-MCAO surgery to validate the ischemia model in the left cerebral cortex. It was also performed at 72 hours to evaluate infarct volume and treatment efficacy. After anesthesia and perfusion, the animals' brains were harvested and transferred to an ice-cooled brain matrix (Harvard Apparatus, USA). The brain samples were then cut into 2 mm thick sections, and the sections were immersed in the 2 % TTC solution (Fluka, Germany) at 37 °C for 15 min (Sabbaghziarani et al., 2017, Sabbaghziarani et al., 2017). Images were acquired from the frontal to occipital regions using a digital camera (Olympus E-30, Japan). The infarcted areas were white and showed no staining (penumbra), whereas the healthy areas were stained red. Infarct volume was measured with ImageJ 1.48 v Software using the formula by Ansari et al. in 2011 (Ansari et al., 2011). Additionally, the ischemic group underwent Hematoxylin and eosin (H&E) staining 72 hours after surgery to emphasize lesions and tissue damage in the left hemisphere relative to the right hemisphere.
2.4. Enzyme-linked immunosorbent assay (ELISA)
To examine the impact of melatonin and NAC on cerebral ischemia, the left cerebral cortex was extracted from all groups 72 hours post procedures and preserved at −80 °C for ELISA examination. The concentrations of GPx, SOD, CAT, MDA (Zellbio GmbH, Deutschland, Germany) and Nrf2 (Bioassay Technology Laboratory, China) were measured using ELISA kits according to the manufacturer's instructions.
2.5. Statistical analysis
Values were presented as mean ± SD and analyzed by one-way analysis of variance (ANOVA) using Tukey's or Tamhane's T2 post hoc tests with SPSS 23. Significance between different values was assessed at p ≤ 0.05.
3. Results
3.1. Neurobehavioral tests
The mean value of sensory-motor activity of the NAC + melatonin group (17 ± 1) was approximately 1.2 and 1.8 times higher than that of the melatonin group (14 ± 1), NAC group (13 ± 1), and ischemic group (9 ± 1), respectively (p≤ 0.01, p≤ 0.002, and p≤ 0.001). Meanwhile, there was a significant increase in the melatonin group and NAC group compared to the ischemic group (p≤ 0.001 and p≤ 0.002). The mean value of the ischemic group, melatonin group, and NAC group showed a significant decrease compared to the sham group (18 ± 0) (p≤ 0.001, p≤ 0.002, and p≤ 0.001, respectively). The difference between the melatonin group and NAC group was not significant (Fig. 1).
Fig. 1.
Effects of melatonin in combination with N-acetylcysteine (NAC) on neurobehavioral tests 72 hours after left middle cerebral artery occlusion (MCAO). Data are presented as mean ± SD. a, p≤ 0.01 vs. melatonin; b, p≤ 0.002 vs. NAC; c, p≤ 0.001 vs. ischemic; d, p≤ 0.002 vs. sham treatment; e, p≤ 0.002 vs. ischemic; f, p≤ 0.001 vs. sham treatment; n=6 for each group.
3.2. TTC and H&E staining and infarct volume evaluation
The mean infarct volume in the NAC + Melatonin group (9.32 ± 1.67) decreased significantly compared to the melatonin (18.10 ± 1.30; p≤ 0.02), NAC (24.35 ± 3.07; p≤ 0.001), and ischemic (45.76 ± 5.15; p≤ 0.001) groups. Both the Melatonin and NAC groups showed a significant decrease in infarction volume compared to the ischemic group, with the ischemic group showing a significant increase compared to the sham group (for all p≤ 0.001). There was no significant difference between the melatonin and NAC groups. Tissue damage due to successful MCAO was evident in TTC and H&E staining (Fig. 2, Fig. 3).
Fig. 2.
Triphenyltetrazolium chloride (TTC) staining of five serial coronal sections of brain (a) and infarct volume (b) 72 hours after left middle cerebral artery occlusion (MCAO). (a): In the ischemia and treatment groups, the white (or penumbra) areas (arrow) in the cortex show ischemia induction. In the sham group, the entire surface of the cortex is red. In the Melatonin + NAC group, there was a notable reduction in infarction volume compared to the ischemic group and other treatment groups. (b): Data are presented as mean ± SD. a, p≤ 0.02 vs. Melatonin; b, p≤ 0.001 vs. NAC; c, p≤ 0.001 vs. ischemic; d, p≤ 0.01 vs sham and e, p≤ 0.001 vs. sham treatment; n=6 for each group.
Fig. 3.
Hematoxylin and eosin (H&E) staining 72 hours after left middle cerebral artery occlusion (MCAO) on the ischemic group. The brain sections were examined using both a stereo microscope (a) to show tissue destruction in the left hemisphere marked by a red line, and a light microscope (b & c) to visualize tissue damage in the left hemisphere (c), and the normal tissue composition in the right hemisphere (b).
3.3. ELISA
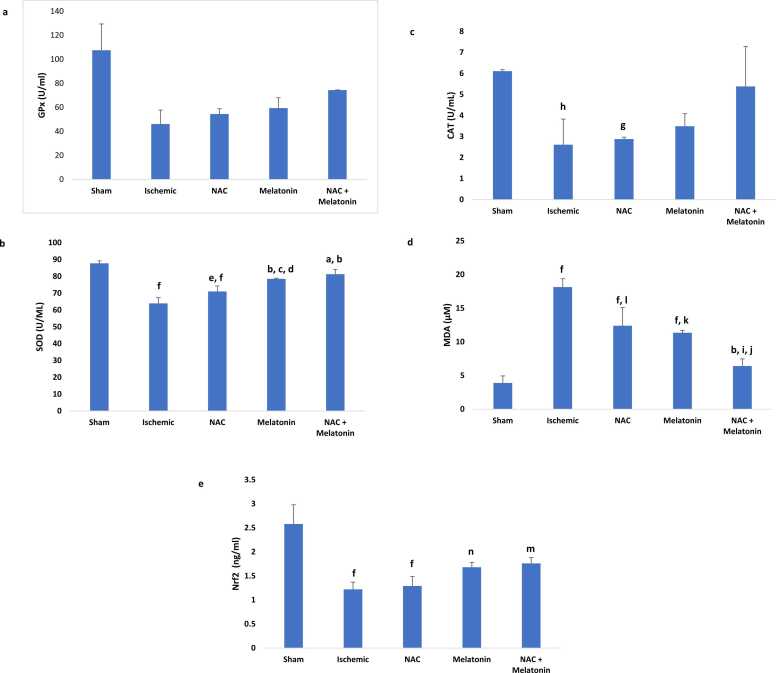
The concentration of GPx enzyme in the ischemic group (46.12 ± 11.60), NAC group (54.44 ± 4.41), melatonin group (59.37 ± 8.61), and NAC + melatonin group (74.35 ± 0.16) showed no significant decrease compared to the sham group (107.53 ± 21.86). Additionally, there was no significant increase in all treatment groups compared to the ischemic group. The difference between all treatment groups was not significant (Fig. 4a).
Fig. 4.
Effects of melatonin in combination with N-acetylcysteine (NAC) on glutathione peroxidase (GPx) (a), superoxide dismutase (SOD) (b), catalase (CAT) (c) malondialdehyde (MDA) (d) and nuclear factor erythroid 2-related factor 2 (Nrf2) (e) level in the brain tissue 72 hours after left middle cerebral artery occlusion (MCAO). Data are presented as mean ± SD. a, p≤ 0.005 vs NAC; b, p≤ 0.001vs ischemic; c, p≤ 0.03 vs NAC; d, p≤ 0.009 vs sham; e, p≤ 0.04 vs ischemic; f, p≤ 0.001 vs sham; g, p≤ 0.02 vs sham; h, p≤ 0.01 vs sham; i, p≤ 0.01 vs melatonin; j, p≤ 0.004 vs NAC; k, p≤ 0.002 vs ischemic; l, p≤ 0.006 vs ischemic; m, p≤ 0.008 vs sham; n, p≤ 0.005 vs sham;. n=6 for each group.
The mean concentration of SOD in the ischemic (63.93 ± 3.41), NAC (71.01 ± 3.30), and melatonin (78.50 ± 0.40) groups showed a significant decrease compared with the sham group (87.73 ± 1.5; p ≤ 0.001 and p ≤ 0.009). The NAC + melatonin (81.28 ± 2.87) and melatonin groups were significantly increased compared to the ischemic (p ≤ 0.001) and NAC groups (p ≤ 0.005; p ≤ 0.03) and also in the NAC group compared to the ischemic group (p ≤ 0.04). The difference in the combined group compared to the melatonin and sham groups was not significant (Fig. 4b).
CAT concentration in the ischemic group (2.61± 1.22) and NAC group (2.88± 0.09) decreased by approximately twofold compared to the sham group (6.11± 0.07; p≤ 0.02 and p≤ 0.01). The difference between all treatment groups and the ischemia group was not significant (Fig. 4c).
Mean MDA concentration showed a significant increase in the ischemic group (18.14 ± 1.24), melatonin group (11.35 ± 0.36), and NAC group (12.40 ± 2.69) compared with the sham group (3.89 ± 1.05; p≤ 0.001). MDA concentration in the combination group (6.41 ± 1.06) decreased two- and threefold, respectively, compared to the melatonin group (11.35 ± 0.36; p≤ 0.01), NAC group (12.40 ± 2.69; p≤ 0.004), and ischemic group (p≤ 0.001). Also, there was a significant decrease in the melatonin and NAC groups compared to the ischemic group (p≤ 0.002; p≤ 0.006). The difference between the NAC and melatonin groups was not significant (Fig. 4d).
Nrf2 concentration in the combination group (1.76 ± 0.12; p≤ 0.008), melatonin group (1.68 ± 0.1; p≤ 0.005), NAC group (1.29 ± 0.2; p≤ 0.001), and ischemic group (1.22 ± 0.15; p≤ 0.001) showed a significant decrease compared to the sham group (2.58 ± 0.4). There was no significant increase in all treatment groups compared to the ischemic group. Also, the difference between all treatment groups was not significant (Fig. 4e).
4. Discussion
OS in rat brain tissue, increases after MCAO, which is due to decreased activity of SOD and increased levels of oxidative stress markers such as MDA (Yang et al., 2022). SOD is the main enzyme for the elimination of ROS, and the consumption of this enzyme during oxidative stress leads to the accumulation of ROS in large amounts, causing damage to the cell. Meanwhile, MDA is produced during lipid peroxidation, indicating the degree of oxidative stress (Feng et al., 2023). In keeping with these studies, the results of the present study showed a decrease and an increase in SOD and MDA, respectively, in all groups compared to the sham group, suggesting post-ischemic injury.
There is no study showing the antioxidant effect of NAC + melatonin in cerebral ischemia, so the results of other studies on the antioxidant effect of melatonin and NAC are presented separately. Many studies have demonstrated the antioxidant benefits of melatonin in cerebral post-ischemic rehabilitation (Azedi et al., 2019), ischemia-reperfusion injury (Sarkar et al., 2017, Yang, 2019, Zhao et al., 2018, Zhao et al., 2019; Watson et al., 2016), and hemorrhagic stroke (Wu et al., 2017). Some studies have shown that cerebral ischemia decreases antioxidant levels and melatonin can induce the upregulation of SOD, CAT, GPx, and Nrf2 (Ramos et al., 2017, Zhao et al., 2018). It is important to emphasize that some compounds, such as melatonin, may regulate Nrf2 expression and ameliorate early brain injury in aneurysmal subarachnoid hemorrhage via the Kelch-like Ech-associated 1 (keap1)-Nrf2- antioxidant response elements (ARE) pathway (Zhang et al., 2023). In addition, some studies have shown the antioxidant effect of NAC in hypoxic-ischemic brain injury (Wang et al., 2007) and traumatic brain injury (Eakin et al., 2014). The results of the Sabetghadam et al. (2020) study in patients with acute cerebral ischemia also showed that NAC decreased MDA and increased serum SOD and GPx (Sabetghadam et al., 2020). NAC affects antioxidant activity possibly by inducing the transcription factor Nrf2, which functions as a central regulator of antioxidant activities (Zhang et al., 2014; Tyuryaeva & Lyublinskaya, 2023). In consistency with these studies, based on the results of the present work, SOD increased and MDA decreased in the cerebral cortex, showing the efficacy of the treatments, especially in the NAC + melatonin group, in improving neurological functions and infarction volume. It has been reported that treatment with the combination of melatonin and NAC leads to their synergistic effects (Sener et al., 2003). However, in the present study, despite the increase in CAT, GPx, and Nrf2 levels in the treatment groups compared to the ischemia group, there was no significant increase. This inconsistency with others may indicate that these two compounds, by signaling pathways other than Nrf2, are involved in the alteration of antioxidants, which, of course, requires further investigation.
It should be noted that in some studies, after treatment with melatonin or NAC, the values of SOD, CAT, and GPx decreased (Danielisová et al., 2005, Kryl'skii et al., 2019), which is not consistent with the results of the present study. The results of a study by Kryl'skii et al. (2019) have shown that the progression of ischemia in mice is associated with an increase in free radical oxidation processes, leading to the formation of an adaptive compensatory response—the activation of the antioxidant defense system and melatonin causes a shift in brain and serum activities of SOD, CAT, and GPX to the corresponding levels of the control group (Kryl'skii et al., 2019). Meanwhile, in many studies, after the simultaneous application of melatonin and NAC in different diseases, an increase or decrease in the levels of the GPx, SOD, and CAT enzymes was observed, despite the reduction of MDA and the improvement of the function of some structures (Adikwu and Bokolo, 2017, Eşrefoğlu et al., 2006). It seems that the reason for these differences lies in dosage, duration, and stages of treatment, as well as the difference in the type of disease, organ, and animal and human studies.
5. Conclusions
The results indicate that the combined use of melatonin and NAC could potentially decrease oxidative stress damage resulting from ischemia-reperfusion, which was associated with higher SOD levels, lower MDA levels, enhanced neurological function, and reduced cerebral cortex infarction volume. Interestingly, no substantial changes were detected in CAT, GPx, and Nrf2 levels. The administration of NAC and melatonin does not appear to activate the Nrf2 pathway, warranting additional research to explore the shared mechanism of these agents, particularly in relation to inflammatory pathways for improving ischemia.
Ethical Approval
The ethical code for this study was IR.QUMS.REC.1397.195 received from Qazvin University of Medical Sciences
Funding
The study was supported by the grant (14003255) received from Qazvin University of Medical Sciences.
CRediT authorship contribution statement
Fatemeh Sabbaghziarani: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Fariba Zafari: Conceptualization, Writing – review & editing. Ehsan Aali: Conceptualization, Writing – review & editing. Pouria Soleimani: Methodology. Farideh Rajabian Eynshikh: Methodology.
Declaration of Generative Artificial Intelligence (AI) and AI-Assisted Technologies in the Writing Process
During the preparation of this work the authors used insta text and cedilla.ai in order to improve readability and language. After using this tool, the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Contributor Information
Fatemeh Sabbaghziarani, Email: saba.ziarani95@gmail.com, f.sabbagh@qums.ac.ir.
Pouria Soleimani, Email: pouriya.soleimani@gmail.com.
Farideh Rajabian Eynshikh, Email: fariderajabian@yahoo.com.
Fariba Zafari, Email: Fariba.zafari@yahoo.com.
Ehsan Aali, Email: en.aali@gmail.com.
References
- Adikwu E., Bokolo B. Prospects of N-acetylcysteine and melatonin as treatments for tramadol-induced renal toxicity in albino rats. Pharm. Sci. 2017;23(3):172–181. doi: 10.15171/apb.2017.044. https:// doi: 10.15171/PS.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari S., Azari H., McConnell D.J., Afzal A., Mocco J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. JoVE. 2011;(51) doi: 10.3791/2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin H., Bulmus O., Korkut O., Altun E., Ulusal A.E. An evaluation of the effectiveness of melatonin and n-acetylcysteine in cerebral ischemia-reperfusion injury in adult rats. Medicina. 2023;59(11):2026. doi: 10.3390/medicina59112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azedi F., Mehrpour M., Talebi S., Zendedel A., Kazemnejad S., Mousavizadeh K., et al. Melatonin regulates neuroinflammation ischemic stroke damage through interactions with microglia in reperfusion phase. Brain Res. 2019;1723 doi: 10.1016/j.brainres.2019.146401. https://doi.org/10.1016/j.brainres.2019.146401. [DOI] [PubMed] [Google Scholar]
- Bhatti J., Nascimento B., Akhtar U., Rhind S.G., Tien H., Nathens A., et al. Systematic review of human and animal studies examining the efficacy and safety of N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) in traumatic brain injury: impact on neurofunctional outcome and biomarkers of oxidative stress and inflammation. Front. Neurol. 2018;8:744. doi: 10.3389/fneur.2017.00744. https:// doi.org/10.3389/fneur.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar S., Stanwell P., Cordato D., Attia J., Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol. 2018;18(1):1–26. doi: 10.1186/s12883-017-1007-y. https://doi:10.1186/s12883-017-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.Y., Chen T.Y., Lee M.Y., et al. Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood–brain barrier permeability after transient focal cerebral ischemia in mice. J. Pineal Res. 2006;41:175–182. doi: 10.1111/j.1600-079X.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- Dang J., Mitkari B., Kipp M., Beyer C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain, Behav., Immun. 2011;25(4):715–726. doi: 10.1016/j.bbi.2011.01.013. https:// doi: 10.1016/j.bbi.2011.01.013. Epub 2011 Jan 28. [DOI] [PubMed] [Google Scholar]
- Danielisová V., Némethová M., Gottlieb M., Burda J. Changes of endogenous antioxidant enzymes during ischemic tolerance acquisition. Neurochem. Res. 2005;30(4):559–565. doi: 10.1007/s11064-005-2690-4. https:// doi.org/10.1007/s11064-005-2690-4. [DOI] [PubMed] [Google Scholar]
- Eakin K., Baratz-Goldstein R., Pick C.G., Zindel O., Balaban C.D., Hoffer M.E., et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PloS one. 2014;9(4) doi: 10.1371/journal.pone.0090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermine C.M., Bivard A., Parsons M.W., Baron J.-C. Vol. 16. 2021. The ischemic penumbra: from concept to reality (2021) pp. 497–509. (International journal of stroke). 0.1177/1747493020975229. [DOI] [PubMed] [Google Scholar]
- Eşrefoğlu M., Gül M., Ateş B., Batçıoğlu K., Selimoğlu M.A. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J. Gastroenterol.: WJG. 2006;12(2):259. doi: 10.3748/wjg.v12.i2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Ling L., Yuan H., Guo Z., Ma J. Ginsenoside Rd: a promising target for ischemia-reperfusion injury therapy (A mini review) Biomed. Pharm. 2024;4(171) doi: 10.1016/j.biopha.2023.116111. [DOI] [PubMed] [Google Scholar]
- Feng S., Yang M., Liu S., He Y., Deng S., Gong Y. Oxidative stress as a bridge between age and stroke: a narrative review. J. Intensive Med. 27. 2023;3(4):313–319. doi: 10.1016/j.jointm.2023.02.002. PMID: 38028635; PMCID: PMC10658045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golts E., Onaitis M. Commentary: Ischemia reperfusion—looking ahead. J. Thorac. Cardiovasc. Surg. 2021;161(2):e124–e125. doi: 10.1016/j.jtcvs.2019.12.010. [DOI] [PubMed] [Google Scholar]
- Komsiiska D., 2019. Oxidative stress and stroke: a review of upstream and downstream antioxidant therapeutic options. Comp Clin Path 1-12. https:// doi.org/10.1007/s00580-019-02940-z.
- Kryl'skii E.D., Popova T.N., Safonova O.A., Stolyarova A.O., Razuvaev G.A., de Carvalho M.A.P. Transcriptional regulation of antioxidant enzymes activity and modulation of oxidative stress by melatonin in rats under cerebral ischemia/reperfusion conditions. Neuroscience. 2019;406:653–666. doi: 10.1016/j.neuroscience.2019.01.046. https:// doi.org/10.1016/j.neuroscience.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Lee Y.-H., Lee S.-R. Neuroprotective effects of N-acetylcysteine via inhibition of matrix metalloproteinase in a mouse model of transient global cerebral ischemia. Brain Res. Bull. 2020;154:142–150. doi: 10.1016/j.brainresbull.2019.10.004. https:// doi.org/10.1016/j.brainresbull.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Mortezaee K., Sabbaghziarani F., Omidi A., Dehpour A.R., Omidi N., Ghasemi S., et al. Therapeutic value of melatonin post-treatment on CCl4-induced fibrotic rat liver. Can. J. Physiol. Pharm. 2016;94(2):119–130. doi: 10.1139/cjpp-2015-0266. https:// doi.org/10.1139/cjpp-2015-0266. [DOI] [PubMed] [Google Scholar]
- Ramos E., Patiño P., Reiter R.J., Gil-Martín E., Marco-Contelles J., Parada E., et al. Ischemic brain injury: new insights on the protective role of melatonin. Free Radic. Biol. Med. 2017;104:32–53. doi: 10.1016/j.freeradbiomed.2017.01.005. https:// doi.org/10.1016/j.freeradbiomed.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Sabbaghziarani F., Mortezaee K., Akbari M., Soleimani M., Moini A., Ataeinejad N., et al. Retinoic acid-pretreated Wharton’s jelly mesenchymal stem cells in combination with triiodothyronine improve expression of neurotrophic factors in the subventricular zone of the rat ischemic brain injury. Met Brain Di. 2017;32(1):185–193. doi: 10.1007/s11011-016-9897-8. https:// DOI 10.1007/s11011-016-9897-8. [DOI] [PubMed] [Google Scholar]
- Sabbaghziarani F., Mortezaee K., Akbari M., Kashani I.R., Soleimani M., Hassanzadeh G., et al. Stimulation of neurotrophic factors and inhibition of proinflammatory cytokines by exogenous application of triiodothyronine in the rat model of ischemic stroke. Cell Biochem. Funct. 2017;35(1):50–55. doi: 10.1002/cbf.3244. https:// doi.org/10.1002/cbf.3244. [DOI] [PubMed] [Google Scholar]
- Sabetghadam M., Mazdeh M., Abolfathi P., Mohammadi Y., Mehrpooya M. Evidence for a beneficial effect of oral N-acetylcysteine on functional outcomes and inflammatory biomarkers in patients with acute ischemic stroke. Neuropsychiatr. Dis. Treat. 2020;16:1265–1278. doi: 10.2147/NDT.S241497. https:// doi: 10.2147/NDT.S241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Mukherjee A., Das N., Swarnakar S. Protective roles of nanomelatonin in cerebral ischemia-reperfusion of aged brain: matrixmetalloproteinases as regulators. Exp. Gerontol. 2017;92:13–22. doi: 10.1016/j.exger.2017.03.009. https:// doi.org/10.1016/j.exger.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Sener G., Tosun O., Sehirli A.O., Kaçmaz A., Arbak S., Ersoy Y., Ayanoğlu-Dülger G. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72(24):2707–2718. doi: 10.1016/s0024-3205(03)00187-5. PMID: 12679188. [DOI] [PubMed] [Google Scholar]
- Shahripour R.B., Maleki A.H.Z., Alexandrov A.V. The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. Springer; 2019. Application of N-Acetylcysteine in Neurological Disorders; pp. 181–202. https:// doi.org/10.1007/978-981-10-5311-5_11. [Google Scholar]
- Tardiolo G., Bramanti P., Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules. 2018;23(12):3305. doi: 10.3390/molecules23123305. https:// doi.org/10.3390/molecules23123305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyuryaeva I., Lyublinskaya O. Expected and unexpected effects of pharmacological antioxidants. Int. J. Mol. Sci. 2023;24(11):9303. doi: 10.3390/ijms24119303. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Svedin P., Nie C., Lapatto R., Zhu C., Gustavsson M., Mallard C. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2007;61(3):263–271. doi: 10.1002/ana.21066. https:// doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- Watson N., Diamandis T., Gonzales-Portillo C., Reyes S., Borlongan C.V. Melatonin as an antioxidant for stroke neuroprotection. Cell Transpl. 2016;25(5):883–891. doi: 10.3727/096368915X689749. https:// doi.org/10.3727/096368915X689749. [DOI] [PubMed] [Google Scholar]
- Wu H.-J., Wu C., Niu H.-J., Wang K., Mo L.-J., Shao A.-W., et al. Neuroprotective mechanisms of melatonin in hemorrhagic stroke. Cell. Mol. Neurobiol. 2017;37(7):1173–1185. doi: 10.1007/s10571-017-0461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc. Res. 2019;123:62–67. doi: 10.1016/j.mvr.2018.12.005. https://doi.org/10.1016/j.mvr.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Yang Y., He B., Zhang X., Yang R., Xia X., Chen L., et al., 2022. Geraniin Protects against Cerebral Ischemia/Reperfusion Injury by Suppressing Oxidative Stress and Neuronal Apoptosis via Regulation of the Nrf2/HO-1 Pathway. Oxidative medicine and cellular longevity. Volume 2022, Article ID 2152746, 13 pages https://doi.org/10.1155/2022/2152746. [DOI] [PMC free article] [PubMed]
- Zhang Q., Zhang J., Mo J. Pharmacological modulations of Nrf2 and therapeutic implications in aneurysmal subarachnoid hemorrhage. Molecules. 2023;28:1747. doi: 10.3390/molecules28041747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu Z., Liu J., Zhu Z., Hu Z. Protective effect of N-acetylcysteine (NAC) on renal ischemia/reperfusion injury through Nrf2 signaling pathway. J. Recept. Signal Transduct. 2014;34(5):396–400. doi: 10.3109/10799893.2014.908916. https:// doi.org/10.3109/10799893.2014.908916. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Lu C., Li T., Wang W., Ye W., Zeng R., et al. The protective effect of melatonin on brain ischemia and reperfusion in rats and humans: In vivo assessment and a randomized controlled trial. J. Pineal Res. 2018;65(4) doi: 10.1111/jpi.12521. https:// doi.org/10.1111/jpi.12521. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang H., Chen W., Chen L., Liu D., Wang X., et al. Melatonin attenuates white matter damage after focal brain ischemia in rats by regulating the TLR4/NF-κB pathway. Brain Res. Bull. 2019;150:168–178. doi: 10.1016/j.brainresbull.2019.05.019. https:// doi.org/10.1016/j.brainresbull.2019.05.019. [DOI] [PubMed] [Google Scholar]