Abstract
ACK2 (activated Cdc42-associated tyrosine kinase 2) is a specific downstream effector for Cdc42, a member of the Rho family of small G-proteins. ACK2 interacts with clathrin, an endocytic vesicle coating protein, and SH3PX1, a sorting nexin, and is involved in clathrin-mediated endocytosis. While searching for proteins that interact with ACK2, we found that HSP90 (heat-shock protein 90) binds to ACK2. Analysis of a series of truncation mutants of ACK2 has defined the regions within the kinase domain of ACK2 that are required for binding to HSP90. The binding of HSP90 to ACK2 is blocked upon treatment with geldanamycin, an HSP90-specific ATPase inhibitor, and is required for the in vivo kinase activity of ACK2 and its association with Cdc42. Overall, our data suggest a novel mechanism of regulation in which HSP90 serves as a regulatory component in an ACK2 functional complex and plays a role in sustaining its kinase activity.
Keywords: activated Cdc42-associated tyrosine kinase 2 (ACK2), Cdc42, geldanamycin, heat-shock protein 90 (HSP90)-binding region, Rho G-protein, tyrosine kinase
Abbreviations: ACK, activated Cdc42-associated tyrosine kinase; ACK-PCC, peptide containing the first 100 amino acid residues of the N-terminus of ACK2, including the positively charged cluster (residues 64–83); EGF, epidermal growth factor; GST, glutathione S-transferase; HA, haemagglutinin; HSP90, heat-shock protein 90; MOK, MAPK (mitogen-activated protein kinase)/MAK (male germ cell-associated kinase)/MRK (MAK-related kinase) overlapping kinase; PCC, positively charged cluster; SH3, Src homology 3
INTRODUCTION
The ACKs (activated Cdc42-associated kinases), which include ACK1 and ACK2, comprise a family of non-receptor tyrosine kinases that serve as specific targets for the Rho-related GTP-binding protein Cdc42 [1,2]. We have cloned and biochemically characterized ACK2 [2–6], and shown that the tyrosine kinase activity of ACK2 is stimulated by EGF (epidermal growth factor), bradykinin and cell adhesion [2,3,7]. Overexpression of ACK2 has been shown to regulate cell growth, morphogenesis and cellular spreading, and to dissolve actin stress fibres [4,7]. Biochemical studies have shown that ACK2 associates with focal adhesion complexes and binds to the SH3 (Src homology 3) domain of the c-Src kinase; furthermore, upon its overexpression in NIH3T3 cells, ACK2 causes inhibition of the tyrosine kinase activity of p125FAK, thus suggesting that ACK2 plays a regulatory role in integrin-mediated cell adhesion signalling [4].
More recently, we have found that ACK2 plays an important role in clathrin-mediated endocytosis [5]. Both ACK1 and ACK2 possess a highly conserved clathrin-binding motif and have been shown to interact with clathrin [5,8]. Overexpression of ACK2 severely impairs transferrin receptor endocytosis, causes aberrant localization of AP-2 (adaptor protein-2), and induces changes in clathrin assembly [5]. Furthermore, ACK2 interacts with SH3PX1, a member of sorting nexin family, via its proline-rich domain 1 region, and phosphorylates SH3PX1 to facilitate the degradation of EGF receptors [6]. These data suggest that ACK2 is a clathrin-interacting protein and functions as a regulatory protein in clathrin-mediated receptor endocytosis.
While searching for additional ACK2-interacting proteins, we have found that ACK2 binds to HSP90 (heat-shock protein 90), a chaperone protein that is known to influence the molecular structure and function of many signalling proteins, including steroid hormone receptors, the EGF receptor, p53, Raf, Src, Wee1, the protein kinase CK2 and Akt [9–16]. In the present study, we have characterized the interaction between ACK2 and HSP90 and defined the HSP90-binding region in ACK2. Furthermore, we have demonstrated that the interaction with HSP90 is required both for the binding of ACK2 to Cdc42 and for the tyrosine kinase activity of ACK2, indicating that HSP90 plays an important regulatory role in ACK2 function.
MATERIALS AND METHODS
Materials
Glutathione–agarose beads, Protein A–Sepharose beads and geldanamycin were purchased from Sigma; anti-HSP90 and anti-(clathrin heavy chain) antibodies were obtained from Transduction Laboratory; anti-Myc and anti-HA (haemagglutinin) antibodies were purchased from Covance; anti-phosphotyrosine antibody (4G10) was obtained from UBI. EGF was purchased from Invitrogen. Anti-ACK-PCC antibody was raised in a rabbit using GST (glutathione S-transferase)–ACK-PCC as an antigen, which contains the first 100 amino acid residues of the N-terminus, including the PCC (positively charged cluster; residues 64–83) of ACK2 that is conserved in ACK1 (95% identical).
Construction of ACK2 mutants
The Myc-tagged ACK2 mutants (depicted in Figure 2A as NT, ΔNT, ΔNT1, ΔNT2, ΔNT3, ΔNT5, ΔNT6, ΔNT8, M3, M8, M9, M11, M12, M13 and M14) were constructed using PCR-directed mutagenesis with wild-type ACK2 as a template. The PCR products were cloned into the pCR2.1 vector using the TA Cloning Kit (Invitrogen) according to the manufacturer's protocol. The resulting colonies were screened using the Spin Miniprep Kit (Invitrogen) according to the manufacturer's protocol. The inserts from the positive clones were isolated by digestion with BamHI and EcoRI and then subcloned into the Myc-tagged mammalian expression vector pcDNA3. The mutations were confirmed by DNA sequencing.
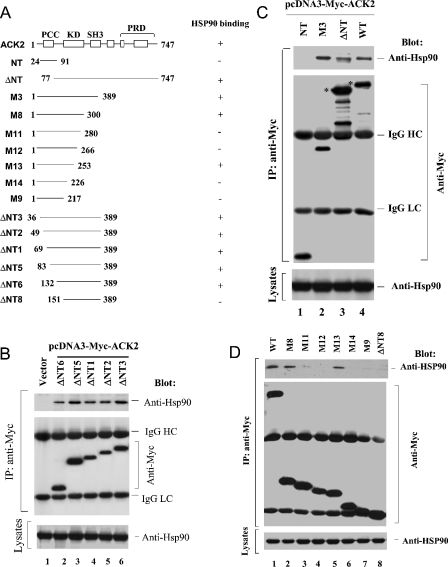
Figure 2. Identification of HSP90-binding regions in the kinase domain of ACK2.
(A) Schematic depiction of the truncation mutants of ACK2. PCC, residues 64–83; KD, kinase domain (residues 132–389); SH3 domain, residues 391–444; PRD, proline-rich domain (residues 512–644). The numbers indicate the positions of the amino acid residues at the ends of the truncation constructs. The HSP90-binding capacity of each truncation mutant is summarized on the right: +, binding; −, no binding. (B)–(D) Mapping of the HSP90-interacting region in ACK2. The truncation mutants were Myc-tagged and transfected into COS7 cells. The expressed truncation mutants were immunoprecipitated with an anti-Myc antibody (middle panel) and the co-immunoprecipitated HSP90 was detected by immnoblotting with an anti-HSP90 antibody (top panel). The level of HSP90 in the lysates is shown in the bottom panels. In (C), the full-length ACK2 wild type (lane 4) and ΔNT (lane 3) proteins are marked by the asterisks. The bands below the full-length proteins are degradation products. The data in these three figures indicate that (i) the PCC domain is not required for binding to HSP90 (B); (ii) the kinase domain is the HSP90-interacting domain (C); and (iii) there are at least two regions in the kinase domain required for binding to HSP90 (D). HC, heavy chain; LC, light chain.
Cell culture
COS7 and Neura-2a cells were grown in Dulbecco's modified Eagle's medium containing 10% (v/v) fetal bovine serum. The cell lines were maintained in an incubator with 5% CO2 at 37 °C.
Transfection
COS7 cells, grown to ∼90% confluency in 60 mm dishes, were transfected with Myc-tagged wild-type ACK2 or ACK2 mutant constructs (3 μg), or with HA-tagged SH3PX1 (2 μg), using lipofectAMINE™ (Gibco/BRL), and then were incubated for 48 h.
Geldanamycin treatment
Geldanamycin stock (10 mM) was made in DMSO. COS7 or Neura-2a cells were serum-starved overnight and then treated with either geldanamycin (final concentration 10 μM) or DMSO (controls) for the desired periods by adding the solutions directly into the culture medium. In order to maintain a constant transfection time (48 h) for all of the samples, the treatment periods were set to be completed exactly at the 48 h transfection time point.
Immunoprecipitation and immunoblot
The COS7 or Neuro-2a cells were rinsed with PBS and then lysed with the cell lysis buffer (40 mM Hepes, pH 7.4, 100 mM NaCl, 1% Triton X-100, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate and 10 μg/ml aprotinin and leupeptin). The lysates were cleared by centrifugation at 12000 g for 10 min at 4 °C, and then incubated with antibodies for 30 min at 4 °C. Protein A beads (20 μl of a 1:1 slurry in mammalian lysis buffer) were added and the suspensions were rotated at 4 °C for 3 h. After washing three times with 0.7 ml of cold mammalian lysis buffer, the beads were resuspended in 20 μl of 2×sample buffer.
Each sample was combined with 2×SDS sample buffer, boiled, and subjected to SDS/PAGE. The proteins were transferred to a PVDF membrane and blocked with TBST (20 mM Tris, pH 7.4, 137 mM glycine and 0.02% Tween 20) containing 1% (w/v) BSA. The membrane was incubated with the primary antibodies diluted in TBST overnight at 4 °C, then washed once with TBST for 10 min. The anti-mouse or anti-rabbit antibodies conjugated to horseradish peroxidase (Amersham Corp.), diluted 1:5000 in TBST, were incubated with the membrane for 1 h at 22 °C, followed by three washes with TBST for 10 min each. The protein bands were detected with an ECL® detection kit (Amersham).
Binding assays with GST–Cdc42(Q61L) fusion proteins
GST–Cdc42(Q61L) fusion proteins were expressed in Escherichia coli (JM109) and purified by affinity purification with glutathione–agarose beads as described previously [5]. The glutathione–agarose-bead-bound GST–Cdc42(Q61L) proteins were incubated with Myc–ACK2-transfected COS7 cell lysates for 3 h at 4 °C. The beads were washed three times with the mammalian cell lysis buffer, and the bound proteins were eluted in 2 ×SDS sample buffer and subjected to SDS/10%-PAGE. The co-precipitated Myc–ACK2 and HSP90 were detected by immunoblotting with anti-ACK-PCC antibody or anti-HSP90 antibody respectively. The GST fusion proteins were visualized by Coomassie Blue staining.
RESULTS
HSP90 is co-immunoprecipitated with ACK2
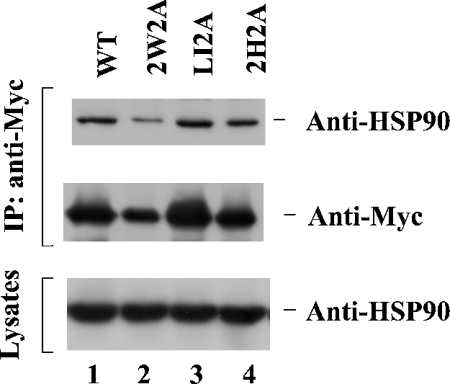
To better understand the function and regulation of ACK2, we have set out to identify binding partners for ACK2 using GST–ACK2 fusion protein pull-down assays. These assays identified clathrin and SH3PX1 as ACK2-interacting proteins [5,6], and more recently showed that the heat-shock protein HSP90 associates with ACK2 (results not shown). To confirm that ACK2 associates with HSP90 in cells, as well as to investigate the regulation of this interaction, we transfected Myc-tagged wild-type ACK2, the SH3 domain-defective mutant ACK2-2W2A, the mutant ACK2-LI2A (defective in clathrin binding), and the mutant ACK2-2H2A (defective in Cdc42 binding) into COS7 cells, and then examined the binding of ACK2 and the different ACK2 mutants to endogenous HSP90 by co-immunoprecipitation assays. As shown in Figure 1, wild-type ACK2 was capable of binding to endogenous HSP90 (lane 1). The SH3 domain-defective mutant 2W2A (lane 2), the clathrin-binding-defective mutant LI2A (lane 3) and the Cdc42-binding-defective mutant 2H2A (lane 4) were all capable of binding to endogenous HSP90, similar to wild-type ACK2, thus indicating that interaction of ACK2 with clathrin, Cdc42 or SH3 domain-associated proteins does not affect its binding to HSP90.
Figure 1. ACK2 interacts with HSP90.
Myc-tagged cDNA of wild-type ACK2, the SH3 domain-defective mutant ACK2-2W2A, the clathrin-binding-defective mutant ACK2-LI2A or the Cdc42-binding-defective mutant ACK2-2H2A was transfected into COS7 cells. The overexpressed ACK2 constructs were immunoprecipitated (IP) with an anti-Myc antibody (middle panel). The co-immunoprecipitated endogenous HSP90 was detected by immunoblotting with an anti-HSP90 antibody (top panel). The levels of HSP90 in the lysates are shown in the bottom panel.
The HSP90-binding region is located in the kinase domain of ACK2
To map the HSP90-binding region in ACK2, we made a series of Myc-tagged truncation mutants of ACK2 and tested their binding to endogenous HSP90 in COS7 cells using co-immunoprecipitation assays (Figure 2A). There is a PCC at the N-terminus of ACK2, and we first examined whether the PCC might contain the binding site for HSP90. To do this, we used the N-terminal-truncation mutants ΔNT1, ΔNT2, ΔNT3, ΔNT5 and ΔNT6 (Figure 2B). The different Myc-tagged truncation mutants were transfected into COS7 cells, and the ability of HSP90 to be co-immunoprecipitated with the ACK2 constructs was determined by immunoblotting. As shown in Figure 2(B), deletion of the N-terminal 131 residues of ACK2 did not affect its binding to HSP90, indicating that the HSP90-binding region is not located within the PCC domain. The truncation mutant ΔNT6, which contains residues 132–389 that is the isolated kinase domain of ACK2 [2], retained the ability to bind to HSP90 (Figure 2B, lane 2), indicating that the HSP90-binding region is within the kinase domain of ACK2. This was confirmed through binding assays using the ACK2 truncation mutants NT, ΔNT and M3, as shown in Figure 2(C). The NT truncation mutant, which includes residues 24–91, did not show any detectable binding to HSP90 (Figure 2C, lane 1), while M3, which contains amino acid residues 1–389, bound to HSP90 in a manner similar to wild-type ACK2 (compare lane 2 with lane 4 in the top panel of Figure 2C). Taken together, the data from Figures 2(B) and 2(C) indicate that the HSP90-binding region of ACK2 resides within the kinase domain that includes amino acid residues 132–389. This is consistent with the HSP90-binding region defined in Akt and MOK [MAPK (mitogen-activated protein kinase)/MAK (male germ cell-associated kinase)/MRK (MAK-related kinase) overlapping kinase] [14,17].
To delineate further the HSP90-binding region within the kinase domain of ACK2, we made additional deletions from the N-terminal end of ΔNT6 as well as from the C-terminal end of the M3 mutant (the kinase domain), thus generating the constructs ΔNT8, M8, M9, M11, M12, M13 and M14 (Figure 2A). We then tested the ability of these ACK2 mutants to bind to HSP90 by co-immunoprecipitation assays. As shown in Figure 2(D), endogenous HSP90 was co-immunoprecipitated with M8 and M13 (lanes 2 and 5), but not with M11, M12, M14, M9 or ΔNT8 (lanes 3, 4, 6–8). These data indicate that the minimum HSP90-binding region is between amino acid residues 132 and 300. However, the interaction of HSP90 with M13 is unexpected. One explanation for the interaction is that the portion comprising residues 253–283 may block the accession of the HSP90-binding region between residues 226 and 253 to HSP90; thus M11 and M12 did not bind to HSP90. These data suggest that there are at least two putative HSP90-binding regions in the kinase domain, located between residues 132–151 and 226–253 in ACK2.
The ATPase activity of HSP90 is required for the interaction with ACK2
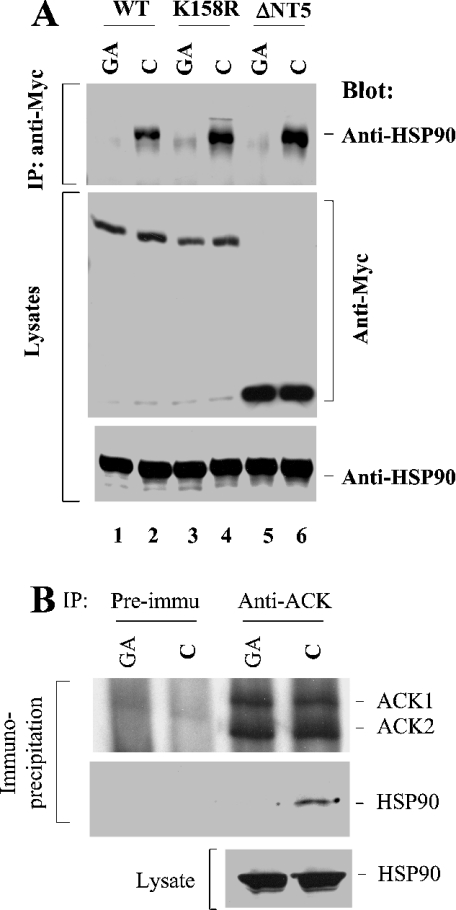
We next examined whether there is a functional consequence of the binding of HSP90 to ACK2. To answer this question, we set out to disrupt the interaction between ACK2 and HSP90 and then assessed how this affected ACK2 activity. Geldanamycin is an HSP90-specific ATPase inhibitor and has been used to block the interaction of HSP90 with its client proteins [18,19]. We transfected wild-type ACK2, the kinase-dead mutant ACK2-(K158R) and the N-terminal truncation mutant ΔNT5 into COS7 cells and then examined the binding of these ACK2 constructs to endogenous HSP90 by co-immunoprecipitation assays, with or without treatment with geldanamycin (Figure 3A). When the cells were treated with geldanamycin for 30 min, the binding of HSP90 to wild-type ACK2, the kinase-dead mutant ACK2-(K158R) or the N-terminal truncation mutant ΔNT5 was diminished (compare lanes 1, 3 and 5 with lanes 2, 4 and 6 in Figure 3A), suggesting that the ATPase activity is essential for HSP90–ACK2 binding interactions.
Figure 3. The ATPase activity of HSP90 is essential for its interaction with ACK2.
(A) COS7 cells were transfected with Myc-tagged wild-type ACK2, the kinase-dead mutant ACK2(K158R) or the N-terminal truncation mutant ΔNT5 for 48 h. Before harvesting the cells, 10 μM of the HSP90-specific ATPase inhibitor geldanamycin (GA) or an equivalent amount of DMSO (solvent for geldanamycin; C) was added into the culture medium for 30 min. The cells were immediately lysed and the expressed ACK2 constructs were immunoprecipitated (IP) with an anti-Myc antibody. The co-immunoprecipitated HSP90 was detected by immunoblotting with an anti-HSP90 antibody (top panel). The immunoprecipitated ACK or its mutant was detected by immunoblotting with an anti-Myc antibody (middle panel). (B) Neura-2a cells were serum-starved overnight, then treated with DMSO (control; C) or 10 μM geldanamycin (GA) for 30 min and lysed with lysis buffer. The lysates (1 mg/sample) were subject to immunoprecipitation with anti-ACK-PCC or pre-immune serum. HSP90 in the lysates (bottom panel) and that co-immunoprecipitated with ACK (middle panel) was detected by immunoblotting with anti-HSP90 antibody. The immunoprecipitated ACK was detected by immunoblotting with anti-ACK-PCC (top panel).
To determine whether the interaction of HSP90 with endogenous ACK is ATPase-dependent, we treated Neuro-2a cells (a neuroblastoma cell line) with geldanamycin, immunoprecipitated endogenous ACK with anti-ACK-PCC (which recognizes both ACK1 and ACK2), and detected HSP90 in the anti-ACK-immunoprecipitated complex by immunoblotting. As shown in Figure 3(B), the controls of immunoprecipitation with pre-immune serum did not precipitate either ACK or HSP90 (lanes 1 and 2). The anti-ACK antibody precipitated both ACK1 and ACK2 (lanes 3 and 4, top panel, Figure 3B). HSP90 was co-immunoprecipitated with endogenous ACK only in the cell lysates that were not treated with geldanamycin (lane 4). The treatment of geldanamycin blocked the interaction of endogenous ACK with HSP90 (lane 3). These data indicate that endogenous ACK interacts with HSP90 in vivo and that the interaction requires the ATPase activity of HSP90.
Interaction with HSP90 is required for the kinase activity and the Cdc42-binding capacity of ACK2
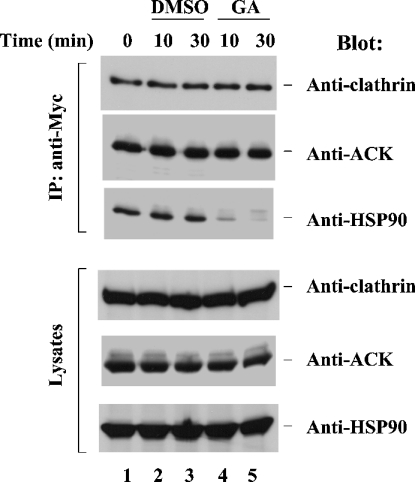
ACK2 is a clathrin-interacting protein and contains a clathrin-binding domain located at its C-terminus [5]. To examine the effect of the interaction of ACK2 with HSP90 on the ability of ACK2 to bind to clathrin, we transfected Myc-tagged ACK2 into COS7 cells, treated the cells with geldanamycin or DMSO (the solvent for geldanamycin) for either 10 or 30 min, and then immunoprecipitated ACK2 with an anti-Myc antibody and detected the co-immunoprecipitated endogenous HSP90 and clathrin by immunoblotting (Figure 4). While treatment with geldanamycin inhibited the binding of HSP90 to ACK2, it did not affect the association of clathrin with ACK2 (compare lanes 4 and 5 with lanes 2 and 3 in Figure 4).
Figure 4. Interaction with HSP90 is not required for clathrin binding by ACK2.
Myc-tagged ACK2 was transfected into COS7 cells for 48 h. The cells were then treated with geldanamycin (GA) or DMSO for either 10 or 30 min. The expressed ACK2 was immunoprecipitated (IP) with an anti-Myc antibody. The co-immunoprecipitated clathrin heavy chain and HSP90 were detected by immunoblotting with an anti-(clathrin heavy chain) antibody (top panel) and an anti-HSP90 antibody (third panel from the top) respectively. The immunoprecipitated ACK2 was detected by immunoblotting with an anti-ACK antibody (second panel from the top). The amounts of clathrin, ACK and HSP90 in the lysates are shown in the bottom three panels. The zero-time point sample represents cells without any treatment.
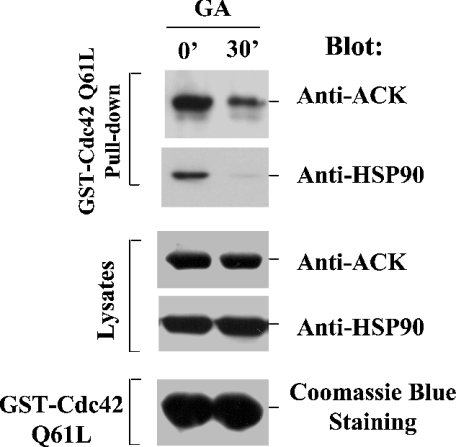
ACK2 is a specific downstream target of Cdc42 [2], and so it was of interest to see whether the interaction of ACK2 with HSP90 affected the ability of ACK2 to bind to active Cdc42. Glutathione–agarose-bead-immobilized GST–Cdc42(Q61L), a GTPase-defective mutant, was incubated with geldanamycin-treated or untreated cell lysates that were transfected with Myc–ACK2. The amount of Myc–ACK2 that was co-precipitated by GST–Cdc42-(Q61L) was detected by immunoblotting with an anti-Myc antibody. As shown in Figure 5, the amount of ACK2 that co-precipitated with GST–Cdc42(Q61L) from geldanamycin-treated cell lysates (lane 2, top panel) was much lower than that from control cell lysates (lane 1, top panel), suggesting that the interaction between ACK2 and HSP90 is required for ACK2 to bind to Cdc42.
Figure 5. Binding to HSP90 is required for ACK2 to interact with Cdc42.
COS7 cells were transfected with Myc-tagged ACK2 for 48 h and then treated with 10 μM geldanamycin (GA) for 0 or 30 min. The cell lysates were incubated with GST–Cdc42(Q61L)-bound glutathione–agarose beads [approx. 30 μg of GST–Cdc42(Q61L) fusion protein] at 4 °C for 3 h with rotation. The co-precipitated ACK2 and HSP90 were detected by immunoblotting with anti-ACK-PCC and anti-HSP90 antibodies (top and second panels respectively). The amounts of ACK2 and HSP90 in the lysates are shown in the third and fourth panels. The amount of GST–Cdc42(Q61L) is shown in the bottom panel by Coomassie Blue staining.
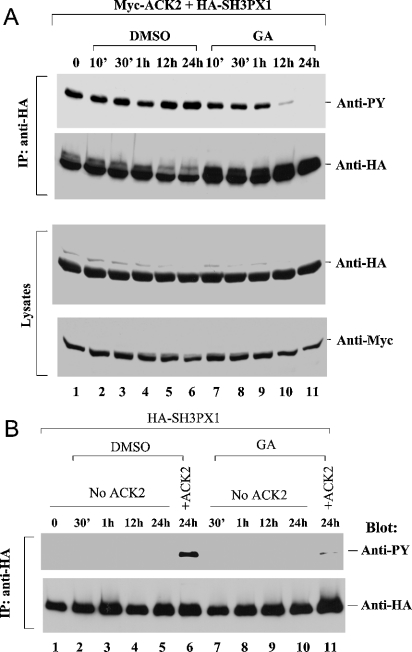
We have identified SH3PX1, a member of the sorting nexin family, as an in vivo phosphorylation substrate for ACK2 [6]. To determine the effect of the interaction with HSP90 on the tyrosine kinase activity of ACK2, we used the in vivo phosphorylation of SH3PX1 as a read-out. We co-transfected Myc-tagged ACK2 with HA-tagged SH3PX1 into COS7 cells, treated the cells with geldanamycin or DMSO (control) for up to 24 h, and detected the tyrosine phosphorylation of SH3PX1 by immunoblotting with an anti-phosphotyrosine antibody. Treatment with DMSO for up to 24 h did not cause a significant change in the tyrosine phosphorylation of SH3PX1 (Figure 6A, lanes 2–6). However, treatment with geldanamycin for 12 h strongly decreased the tyrosine phosphorylation of SH3PX1, with phosphorylation being eliminated by a 24 h treatment with geldanamycin (Figure 6A, lanes 10 and 11). To confirm that the decrease in the tyrosine phosphorylation of SH3PX1 due to geldanamycin treatment indeed reflected inhibition of an ACK2-catalysed phosphorylation event, rather than arising from another endogenous tyrosine kinase activity, we transfected HA-tagged SH3PX1 alone into COS7 cells and detected the tyrosine phosphorylation of SH3PX1 upon treatment with geldanamycin. As shown in Figure 6(B), without co-transfection of ACK2, the tyrosine phosphorylation of immunoprecipitated HA–SH3PX1 was undetectable (lanes 1–5 and 7–10), whereas the ACK2-catalysed tyrosine phosphorylation of SH3PX1 was significant (lane 6), suggesting that the tyrosine phosphorylation of SH3PX1 was indeed due to ACK2. In addition, we did not observe degradation of ACK2 or SH3PX1 with up to 24 h of treatment with geldanamycin (see the lysate samples in the two bottom panels in Figure 6A). Therefore the decrease in the levels of tyrosine phosphorylation was not the outcome of a decrease in the protein levels of SH3PX1 or ACK2. Thus we conclude that the interaction between ACK2 and HSP90 is required for the kinase activity of ACK2.
Figure 6. Binding to HSP90 is required for ACK2 tyrosine kinase activity.
(A) COS7 cells were transfected with both Myc-tagged ACK2 and HA-tagged SH3PX1 for 24 h, and then treated with either 10 μM geldanamycin (GA) or DMSO for 24 h, 12 h, 1 h, 30 min or 10 min. The cells were harvested at the time point when the transfection period was 48 h. The expressed SH3PX1 was immunoprecipitated (IP) with an anti-HA antibody. Tyrosine phosphorylation of the immunoprecipitated SH3PX1 was detected by immunoblotting with an anti-phosphotyrosine (anti-PY) antibody (4G10; top panel), and the amount of immunoprecipitated SH3PX1 was determined by immunoblotting with an anti-HA antibody (second panel). The amounts of SH3PX1 and ACK2 in the cell lysates are shown in the bottom panels. (B) The experimental procedures were similar to those in (A), except that SH3PX1 was transfected alone in lanes 1–5 and lanes 7–10.
DISCUSSION
HSP90 has been shown to interact with and regulate many signalling molecules, including steroid hormone receptors, the EGF receptor, v-Src, Raf, Wee1, CK2, p53 and Akt [9–16]. The mechanism underlying such regulation is generally thought to be that HSP90 complexes with its co-chaperones, such as Cdc37 or Cyp-40, to stabilize the conformation and prevent the degradation of the client proteins [20,21]. In our studies, interaction with HSP90 is required for the kinase activity and the Cdc42-binding capacity of ACK2, suggesting that HSP90 determines the functional conformational structure of ACK2. To our knowledge, this is the first report that HSP90 modulates the function of a component in the signalling network of Rho-family small G-proteins.
The HSP90-interacting region in its client kinases has been identified in their kinase domains [14,17]. Residues 8–78 (subdomains I–IV) in the kinase domain of MOK, and residues 229–309 of Akt, have been identified as the HSP90-binding regions [14,17]. Interestingly, the HSP90-binding region identified in MOK matches the first HSP90-binding region (residues 132–151) in ACK2, while the HSP90-binding region identified in Akt matches the second HSP90-binding region (residues 226–253) in ACK2, indicating that HSP90 interacts with its kinase clients through at least two conserved domains. Our data indicate that both regions are required for HSP90 binding. Furthermore, the conservation of HSP90-binding regions in the kinase domain also raises a question: how general is the interaction between HSP90 and kinases? Answering this question will help us to understand the mechanism underlying the HSP90-mediated regulation of kinases.
The apparent requirement that ACK2 must complex with HSP90 in order to acquire Cdc42-binding capability, as well as tyrosine kinase activity (Figures 5 and 6), raises the possibility that ACK2 associates constitutively with HSP90. Notably, HSP90 binds to the non-receptor tyrosine kinase LCK as a nascent protein to stabilize the folding of its kinase domain. In addition, the interaction between LCK and HSP90 is responsible for the localization of LCK to the plasma membrane [22]. The binding of HSP90 to ACK2 may serve a similar function, such that HSP90 may be critical for mediating both the proper folding and activation of the kinase domain of ACK2. As a specific downstream target of Cdc42, ACK2 is involved in cell adhesion signalling and clathrin-mediated endocytosis [3,5]. Sustaining the kinase activity of ACK2 by interaction with HSP90 ensures the cellular function of ACK2 in addition to its regulation by Cdc42.
Our data have shown that the binding of ACK2 to clathrin does not require association of the kinase with HSP90, implying that the interaction with HSP90 may not affect the conformation of the C-terminus of ACK2. However, disruption of the interaction with HSP90 interferes with the binding of ACK2 to Cdc42 (Figure 5), suggesting a conformational co-operation between the HSP90-binding domain (the kinase domain) and the Cdc42-binding domain of ACK2. We suspect that the improper folding of the kinase domain will induce the improper structure of the Cdc42-binding domain, thus decreasing the binding affinity for Cdc42. Taken together, the interaction with HSP90 provides an important means of regulating the binding of ACK2 to Cdc42. It will be interesting in future to determine how the interaction of HSP90 with ACK2 plays a role in the regulation of Cdc42 signalling and the in vivo function of ACK2.
Acknowledgments
We thank Dr David Carey (Weis Center for Research) for reading the manuscript and providing his invaluable comments.
References
- 1.Manser E., Leung T., Salihuddin H., Tan L., Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature (London) 1993;363:364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- 2.Yang W., Cerione R. A. Cloning and characterization of a novel Cdc42-associated tyrosine kinase, ACK2, from bovine brain. J. Biol. Chem. 1997;272:24819–24824. doi: 10.1074/jbc.272.40.24819. [DOI] [PubMed] [Google Scholar]
- 3.Yang W., Lin Q., Guan J. L., Cerione R. A. Activation of the Cdc42-associated tyrosine kinase-2 (ACK2) by cell adhesion via integrin β1. J. Biol. Chem. 1999;274:8524–8530. doi: 10.1074/jbc.274.13.8524. [DOI] [PubMed] [Google Scholar]
- 4.Yang W., Lin Q., Zhao J., Guan J. L., Cerione R. A. The nonreceptor tyrosine kinase ACK2, a specific target for Cdc42 and a negative regulator of cell growth and focal adhesion complexes. J. Biol. Chem. 2001;276:43987–43993. doi: 10.1074/jbc.M104819200. [DOI] [PubMed] [Google Scholar]
- 5.Yang W., Lo C. G., Despenza T., Cerione R. A. The Cdc42 target ACK2 directly interacts with clathrin and influences clathrin assembly. J. Biol. Chem. 2001;276:17468–17473. doi: 10.1074/jbc.M010893200. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q., Lo C. G., Cerione R. A., Yang W. The Cdc42 target ACK2 interacts with sorting nexin 9 (SH3PX1) to regulate epidermal growth factor receptor degradation. J. Biol. Chem. 2002;277:10134–10138. doi: 10.1074/jbc.M110329200. [DOI] [PubMed] [Google Scholar]
- 7.Coon M., Herrera R. Modulation of HeLa cell spreading by the non-receptor tyrosine kinase ACK-2. J. Cell. Biochem. 2002;84:655–665. doi: 10.1002/jcb.10078. [DOI] [PubMed] [Google Scholar]
- 8.Teo M., Tan L., Lim L., Manser E. The tyrosine kinase ACK1 associates with clathrin-coated vesicles through a binding motif shared by arrestin and other adaptors. J. Biol. Chem. 2001;276:18392–18398. doi: 10.1074/jbc.M008795200. [DOI] [PubMed] [Google Scholar]
- 9.Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature (London) 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 10.Blagosklonny M. V., Toretsky J., Bohen S., Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stancato L. F., Chow Y. H., Hutchison K. A., Perdew G. H., Jove R., Pratt W. B. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J. Biol. Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- 12.Nathan D. F., Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- 14.Sato S., Fujita N., Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W., Mimnaugh E., Rosser M. F., Nicchitta C., Marcu M., Yarden Y., Neckers L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 16.Aligue R., Akhavan-Niak H., Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyata Y., Ikawa Y., Shibuya M., Nishida E. Specific association of a set of molecular chaperones including HSP90 and Cdc37 with MOK, a member of the mitogen-activated protein kinase superfamily. J. Biol. Chem. 2001;276:21841–21848. doi: 10.1074/jbc.M010944200. [DOI] [PubMed] [Google Scholar]
- 18.Whitesell L., Mimnaugh E. G., De Costa B., Myers C. E., Neckers L. M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stebbins C. E., Russo A. A., Schneider C., Rosen N., Hartl F. U., Pavletich N. P. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 20.Young J. C., Moarefi I., Hartl F. U. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt W. B., Toft D. O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 22.Bijlmakers M. J., Marsh M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck) Mol. Biol. Cell. 2000;11:1585–1595. doi: 10.1091/mbc.11.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]