Abstract
Annetocin is structurally related to an OT (oxytocin)/VP (vasopressin) family peptide, which has been isolated from the earthworm Eisenia foetida and has been shown to induce OT-like egg-laying behaviour. We now report the identification of an endogenous AnR (annetocin receptor). The deduced AnR precursor displays high sequence similarity with OT/VP receptors. Genomic analysis of the AnR gene revealed that the intron-inserted position is conserved between the AnR gene and the mammalian OT/VP receptor genes. These results indicate that AnR and mammalian OT/VP receptors share a common ancestor gene. Administration of annetocin to the AnR expressed in Xenopus oocytes induced a calcium-dependent signal transduction. Reverse transcriptase–PCR analysis and in situ hybridization showed that the AnR gene is expressed specifically in the nephridia located in the clitellum region, although the nephridia are distributed throughout the worm body. This result suggests that annetocin induces egg-laying behaviour through its action on the nephridia. This is the first description concerning the functional correlation between an invertebrate OT/VP-related peptide and egg-laying behaviour.
Keywords: annetocin receptor, earthworm, oxytocin, G-protein-coupled receptor, vasopressin
Abbreviations: AnR, annetocin receptor; GPCR, G-protein-coupled receptor; ORF, open reading frame; OT, oxytocin; OTR, OT receptor; RACE, rapid amplification of cDNA ends; RT, reverse transcriptase; VP, vasopressin; V1aR, VP 1a receptor; V2R, VP 2 receptor
INTRODUCTION
Oxytocin (OT) and vasopressin (VP) are homologous mammalian neurohypophysial peptide hormones. Both families are widely distributed in various vertebrate species [1–3]. Peptides of the OT and VP families are classified by the amino acid residue at position 8: VP family peptides contain a basic amino acid and OT family peptides contain a neutral amino acid [1–3]. OT family participates in uterotonic and milk-ejection processes of the reproductive behaviour of mammals, whereas VP is responsible for the control of osmotic balance [1–3].
OT and VP manifest their respective activities through their receptors, which belong to a GPCR (G-protein-coupled receptor) superfamily. To date, one OTR (OT receptor) and three VP receptors [V1aR (VP 1a receptor), V1bR and V2R (VP 2 receptor)] have been identified in mammalian species [4–7]. V2R induces a cAMP-dependent signal transduction pathway [4], whereas activations of V1aR, V1bR and OTR lead to an increase in the intracellular calcium ion [5–7].
In invertebrates, OT/VP-related peptides have also been isolated from several species including snail, octopus, locust, leech and earthworm [8–13]. An annelid structurally OT-related peptide, annetocin, was identified in the earthworm Eisenia foetida [9]. The peptide sequence and precursor organization of annetocin are highly similar to those of OT and VP [9,14], revealing that the OT/VP superfamily peptides are evolutionarily conserved in Annelida. Moreover, injection of annetocin into earthworms induces egg-laying behaviour [15], and annetocin mRNA was localized in a region within the suboesophageal ganglion that is responsible for reproduction [14], confirming that annetocin, similar to members of the vertebrate OT family, is involved in reproduction.
Three OT/VP superfamily peptide receptors have been characterized from molluscs: receptors for Lys-conopressin (LSCPR1 and LSCPR2) from Lymnaea stagnalis [16,17], and a receptor for cephalotocin (CTR) from Octopus vulgaris [18]. Despite the expression of these receptors in the central nervous system and some genital organs, and contractile activity of the vas deferens muscle [17,18], the functional correlations between these peptides and their specific behaviours remain to be elucidated. Annetocin is the only invertebrate OT-related peptide that was found to induce egg-laying behaviour directly as mentioned above [15]. We therefore anticipated that investigation of an AnR (annetocin receptor) would provide crucial clues to a clarification of the biological roles of invertebrate OT/VP-related peptides and evolutionary relationship in the functions between the vertebrate and invertebrate OT/VP families. In the present study, we present the molecular and functional characterization of an AnR, describing the function of OT/VP superfamily peptides in the primitive invertebrate.
EXPERIMENTAL
Animals
Earthworms (E. foetida) were purchased from a fishing-bait shop and kept in wet compost at 16 °C. Frogs (Xenopus laevis) were purchased from Hamamatsu Kyozai (Hamamatsu, Japan) and kept in an aquarium at 18 °C.
Preparation of RNA
Total RNA was prepared from earthworms using TRIzol® reagent (Gibco BRL, Gaithersburg, MD, U.S.A.), and mRNA was purified using Oligotex™-dT 30 (Daiichikagaku, Tokyo, Japan) according to the manufacturer's instructions.
Oligonucleotide primers
All oligonucleotide primers were procured from Kiko-Technology (Osaka, Japan). The oligo(dT) anchor primer and the anchor primer were supplied in a 5′-/3′-RACE (rapid amplification of cDNA ends) kit (Roche Diagnostics, Basel, Switzerland).
Identification of a partial fragment of AnR cDNA
All RT (reverse transcriptase)–PCRs and RACE were performed using TaqEx polymerase (Takara, Kyoto, Japan) and a thermal cycler (model GeneAmp PCR system 9600; PE-Biosystems, Foster City, CA, U.S.A.). The mRNA (0.5 μg) was reverse-transcribed to cDNA at 55 °C for 60 min using the oligo(dT) anchor primer and the AMV RT supplied in the 5′-/3′-RACE kit (Roche). The first-strand cDNA was amplified using the degenerate primers 5′-GTII(G/T)IG(C/T)ITA(C/T)ITIITITG(C/T)TGGI(C/T)ICC-3′ (I represents an inosine residue) and 5′-(A/G)TAIATCCAIGG(A/G)TT(A/G)CA(A/G)C-3′, corresponding to a consensus amino acid sequence at transmembrane domain VI or VII of several OT/VP family peptide receptors respectively. These PCR experiments were performed with five cycles, consisting of 94 °C for 30 s, 45 °C for 30 s and 72 °C for 1 min, followed by 30 cycles, consisting of 94 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min. The first-round PCR products were re-amplified using the degenerate primers 5′-CCITTITTIIIIGTICA(A/G)ATGTGG-3′ and 5′-GGITTICA(A/G)CAIII(A/G)TTIA(A/G)I(C/G)(A/T)IGC-3′ corresponding to the consensus sequence at transmembrane domain VI or VII of the receptors respectively.
The PCR was performed with five cycles of 94 °C for 30 s, 45 °C for 30 s and 72 °C for 1 min, followed by 30 cycles of 94 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min and a final extension at 72 °C for 10 min. The resultant PCR product was purified using the Qiaquick Gel Extraction kit (Qiagen, Valencia, CA, U.S.A.) and subcloned into the pCR2.1 vector using a TA cloning kit (Invitrogen, San Diego, CA, U.S.A.) according to the manufacturer's instructions. Subcloned inserts were sequenced on an ABI PRISMTM 310 Genetic Analyzer (PE Biosystems, Tokyo, Japan) using a Big-Dye sequencing kit (PE Biosystems) and M13 primers.
3′-RACE of AnR cDNA
First-strand cDNA was amplified using the oligo(dT) primer and a gene-specific primer (5′-CCACATGCTCCCTTTGAAGG-3′ complementary to nt 1444–1463) for 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 3 min. The first-round PCR products were re-amplified using the anchor primer and another gene-specific primer (5′-CGGAGATGGTCATAACGCTG-3′ complementary to nt 1466–1485) for 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 3 min (10 min for the last cycle). The products were subcloned and sequenced as described above.
5′-RACE of AnR cDNA
The template cDNA was synthesized using a primer complementary to nt 1785–1804 (5′-GCTACTAAGCACATGGTTCG-3′), followed by dA tailing of the cDNA using dATP and terminal transferase (Roche). The first cDNA was amplified using an oligo(dT) anchor primer and a gene-specific primer (5′-CAGCGTTATGACCATCTCCG-3′ complementary to nt 1466–1485), and the first-round PCR products were amplified using the PCR anchor primer and another gene-specific primer (5′-CCTTCAAAGGGAGCATGTGG-3′ complementary to nt 1444–1463). Each PCR was performed for 30 cycles of 94 °C for 30 s, 50 °C for 30 s and 72 °C for 3 min, followed by 72 °C for 10 min, and the final PCR products were subcloned and sequenced as described above.
Expression of AnR in Xenopus oocytes
The ORF (open reading frame) region of AnR cDNA was amplified and inserted into the Xenopus expression vector pSPUTK (Stratagene, La Jolla, CA, U.S.A.). The plasmid was linearized with HpaI, and cRNA (complementary RNA) was prepared using SP6 RNA polymerase (Ambion, Austin, TX, U.S.A.). cRNA solution (50 nl, 0.05 μg/μl) was injected into Xenopus oocytes. The oocytes were incubated for 2–4 days at 16 °C and transferred to ND96 buffer (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2 and 5 mM Hepes, pH 7.6). The oocytes were voltage-clamped on −70 mV at room temperature (22 °C). The dose–response data and the EC50 values of the experiment were analysed using Origin 6.1 software (OriginLab Corporation, Northampton, MA, U.S.A.).
In situ hybridization
Serial sections (10 μm) of earthworm were prepared as described previously [14]. Labelled AnR antisense probe was synthesized using pSPUTK-AnR plasmid and DIG (digoxigenin) labelling kit according to the manufacturer's instructions, followed by hydrolysis of the synthesized probe with alkaline buffer (40 mM NaHCO3 and 60 mM Na2CO3) for 90 min at 60 °C. Hybridization, washing and staining were performed as described previously [14].
Determination of the exon/intron structure of the AnR gene
The genomic DNA of earthworms was extracted using the MagExtractor (Toyobo, Kyoto, Japan) and the AnR gene was amplified using the genomic PCR with the Expand™ Long Template PCR System (Roche) or TaqEx polymerase (Takara). The genomic PCRs were performed using a primer complementary to nt 877–895 (5′-TCCACCACCGCCCACTACC-3′) or a primer complementary to nt 1671–1694 (5′-AGATGAGCGTGCGTGGGTCGAGTG-3′). Furthermore, 5′-flanking AnR gene was amplified with the earthworm genome using a primer complementary to nt 381–399 (5′-CCGCTCTCCACAAATGGAG-3′) and a primer complementary to nt 1444–1462 (5′-CTTCAAAGGGAGCATGTGG-3′). The amplified products were subcloned and sequenced using several gene-specific primers.
RESULTS
Cloning of AnR cDNA
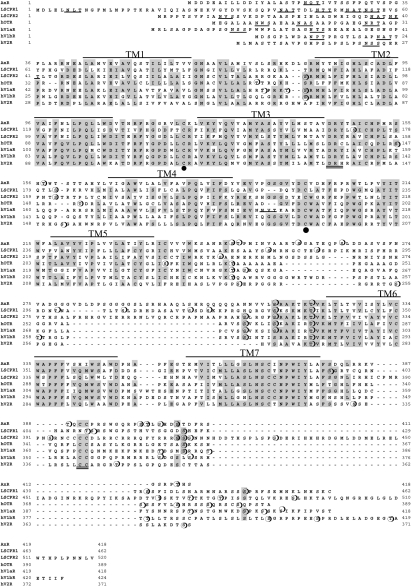
Comparative analysis of amino acid sequences of mammalian OT or VP receptors and Lymnaea Lys-conopressin receptors showed that the sixth and seventh transmembrane domains are highly conserved among all receptors of their superfamily. To identify an AnR, we first performed RT–PCR experiments using degenerative primers corresponding to the conserved regions. An amplified cDNA product of 110 bp was subcloned and sequenced. Moreover, we determined the full-length cDNA sequence encoding the putative GPCR using the 5′-/3′-RACE method. The putative GPCR cDNA was shown to contain a 1260-bp ORF flanked by a 393-bp 5′-untranslated region and a 431-bp 3′-untranslated region. As shown in Figure 1, the deduced protein is composed of 420 amino acid residues. The sequence showed the presence of the seven hydrophobic transmembrane regions, one of the most typical characters of GPCRs. The common cysteine residues (Cys120 and Cys198) responsible for the disulphide bridge between the first and second extracellular loops are found at positions corresponding to those of known OT/VP receptors. An N-linked glycosylation site (Asn-X-Ser/Thr, Asn22) was located at the N-terminal domain. The sequence was found to contain potential sites for phosphorylation by protein kinases including sites for protein kinase A [Arg/Lys-X-(X)-Ser/Thr, Thr54, Thr156, Ser246, Thr247, Ser257, Thr319, Thr387, Ser399 and Thr400], sites for protein kinase C (Ser/Thr-X-Arg/Lys, Ser299) and sites for casein kinase 2 [Ser/Thr-X-(X)-Asp/Glu, Ser264, Ser402 and Ser405] in the intracellular loops and C-terminal region. Other consensus sequences typical of GPCRs such as the Asp/Glu-Arg-Tyr motif (Asp144-Tyr146) in the second intracellular loop and cysteine residues (Cys392-Cys393) utilized as a palmitylation site in the C-terminal region were also present (Figure 1). Furthermore, this putative GPCR displayed high sequence identity with members of the OT/VP receptor family (Figure 1 and Table 1). In addition, the homology search showed no significant similarity of AnR to any other GPCRs. Taken together, these results suggest that the putative GPCR of the earthworm possesses the essential properties of an OT/VP family peptide receptor. Consequently, we conclude that this GPCR is an AnR.
Figure 1. Alignment of the amino acid sequence of OT/VP family peptide receptors.
Three invertebrate receptors (AnR, LSCPR1 and LSCPR2) and four human receptors (hOTR, hV1aR, hV1bR and hV2R) are aligned. Conserved residues are shadowed. Seven putative transmembrane regions (TM1–7) are indicated above the corresponding sequence part. Cysteine residues for a disulphide bridge (Cys120 and Cys198 in AnR sequence) are shown by solid circles. N-glycosylation sites (Asn22 in AnR sequence) are underlined. The Asp-Arg-Tyr sequence and cysteine residues at the C-terminus that are often shown in GPCRs are indicated by double underlines (Asp144−Tyr146 and Cys392−Cys393 in AnR sequence). Potentially phosphorylated serine or threonine residues (Thr54, Thr156, Ser246, Thr247, Ser257, Ser264, Ser299, Thr319, Thr387, Ser399, and Thr400, Ser402 and Ser405 in AnR sequence) are marked by open circles.
Table 1. Identity of the AnR sequence with OT/VP family peptide receptor sequences.
| Receptor | Identity with AnR (%) |
|---|---|
| LSCPR1 | 31 |
| LSCPR2 | 27 |
| CTR | 33 |
| hOTR | 33 |
| hV1aR | 31 |
| hV1bR | 31 |
| hV2R | 30 |
Exon/intron structure of the AnR gene
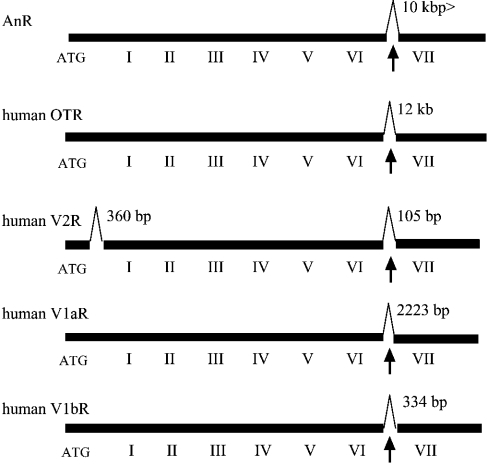
Most GPCR genes lack any introns in their ORFs [19]. However, several GPCR genes including OT/VP receptor genes have been shown to conserve introns at corresponding positions among various species [20–22], and exon/intron structures of such GPCR genes are consistent with their evolutionary relationship. Thus we determined the exon/intron structure of the AnR gene by genomic PCR. The AnR gene is interrupted by an intron at position 1462–1463, which is inserted between transmembrane VI and VII domains (Figure 2). Intriguingly, mammalian OTR, V1aR, V1bR and V2R genes were also found to harbour an intron at the same location [20–22] and, thus, the location of the intron in the AnR gene coincided with those of mammalian OT/VP receptor genes (Figure 2). This comparative analysis of exon/intron structures suggests that the mammalian OT/VP receptor gene and the invertebrate AnR gene were derived from a common ancestral gene.
Figure 2. Exon/intron structures of AnR, OTR and VPRs.
Thick and thin lines indicate exons and introns respectively. Roman numerals indicate the region corresponding to transmembrane domains. The arrowhead points to the common position for intron insertion.
Functional expression of AnR in Xenopus oocytes
It is well established that the binding of peptides to a GPCR coupled with a Gq protein results in the activation of phospholipase C followed by the production of inositol 1,4,5-trisphosphate and increase in the intracellular calcium. In Xenopus oocytes, the interaction of an agonist with its GPCR induces an increase in the intracellular calcium and leads to the activation of a calcium-dependent chloride channel, which can be evaluated by direct observation of the resultant inward chloride current. This system has been employed for functional analyses of OT/VP receptors [5,6,16,18,23]. Thus we examined whether the AnR expressed in Xenopus oocytes was activated by its putative endogenous ligand, annetocin.
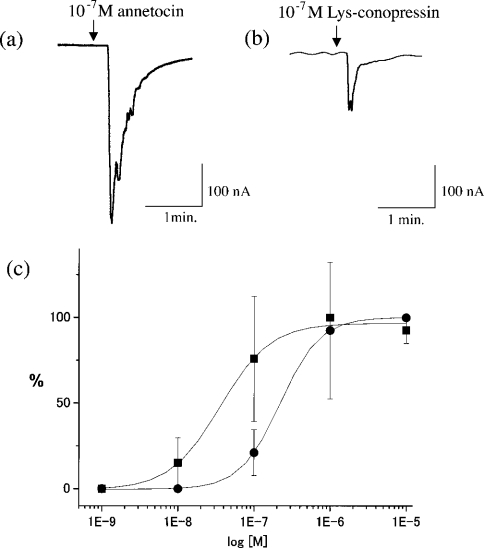
After AnR cRNA was injected into oocytes followed by incubation at 17 °C for 2–4 days, the receptor-expressing oocytes were voltage-clamped at −70 mV. Subsequently, annetocin was added to an oocyte every 20 min at the indicated concentrations to prevent desensitization of the receptor. As shown in Figure 3(a), application of annetocin to AnR-expressing Xenopus oocytes evoked a clear response, whereas no signal was observed in the absence of AnR (results not shown). Furthermore, we examined responses of AnR to several related peptides. Lys-conopressin identified from a landsnail L. stagnalis [17] and a leech Erpbdella octoculata [12] elicited an activity of the AnR (Figure 3b), although the efficiency of Lys-conopressin was somewhat less potent than that of the annetocin. On the other hand, activation of AnR by mammalian OT or VP was not observed even above 10−6 M (results not shown). These results are compatible with the view that Lys-conopressin, but not mammalian OT or VP, is capable of inducing egg-laying behaviour in the earthworm [15]. In addition, a maximal response was observed at more than 400 nM annetocin, and the EC50 values of annetocin and Lys-conopressin were calculated to be approx. 40 and 200 nM by dose–response curves for current shifts (Figure 3c). Altogether, these results provide evidence that annetocin is an endogenous ligand of AnR.
Figure 3. Activation of AnR by annetocin.
Current shift is evoked by adding 10−7 M annetocin (a) and Lys-conopressin (b) for 30 s to the oocytes expressing AnR. (c) Dose–response curve for the assay using annetocin (▪) and Lys-conopressin (•). Maximum membrane currents elicited by the ligand are plotted. The current caused by 10−6 M (1E−6) annetocin or 10−5 M (1E−5) Lys-conopressin was taken as 100%. Error bars denote S.E.M. (n=5).
Localization of the AnR mRNA
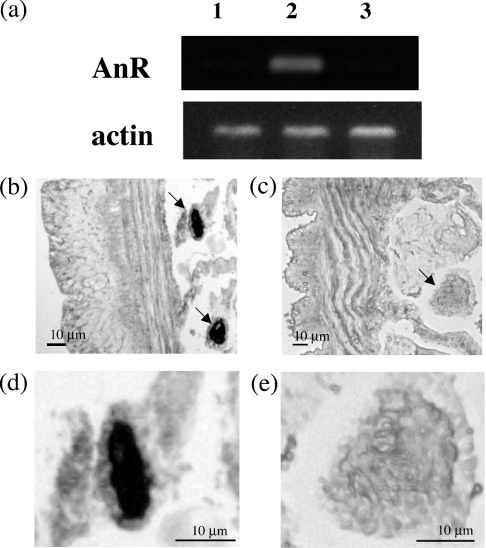
To determine the localization of AnR mRNA, we initially performed an RT–PCR experiment using AnR gene-specific primers. Earthworms were divided into three segments: anterior, central and posterior, followed by extraction of mRNAs from these segments. An amplified signal for AnR mRNA was detected in the central region containing the clitellum (Figure 4a). Expression of AnR mRNA in the clitellum region was then investigated by in situ hybridization. Sections of the earthworm clitellum were hybridized with an AnR antisense cRNA probe. As shown in Figures 4(b) and 4(d), positively stained cells were detected exclusively in the nephridia within the clitellum, whereas other tissues such as reproductive tissue and the nephridia in the posterior regions (Figures 4c and 4e) were not stained. In addition, no positive staining was observed when using the sense probe (results not shown). These results showed that the AnR gene is expressed specifically in the clitellum nephridia.
Figure 4. The AnR mRNA expression in nephridia located in the clitellum.
The AnR RNA and earthworm β-actin RNA expressions were analysed by RT–PCR in anterior (lane 1), central (lane 2) and posterior (lane 3) regions (a), and in situ hybridization of the DIG-labelled AnR RNA probe to fixed 10 μm sections of the earthworm clitellum (b, d) and the posterior region (c, e). In (b) and (c), arrows indicate the nephridia. Scale bar, 10 μm.
DISCUSSION
Although OT/VP-related peptides have been isolated from a variety of invertebrate species, neither the functional mechanisms nor evolutionary relationships of the biological roles between vertebrate and invertebrate OT/VP superfamily peptides is well understood. To address these issues, we characterized a receptor for annetocin, AnR. Comparative sequence analysis showed that AnR displays high similarity to OT/VP receptors (Figure 1 and Table 1) and several characteristic amino acid sequences for mammalian OT/VP receptors are conserved in the AnR (Figure 1). Functional expression analysis clearly demonstrates the activation of AnR by the endogenous ligand, annetocin (Figure 3). Furthermore, the AnR gene was found to be interrupted by an intron between transmembrane VI and VII domains (Figure 2), which is in good agreement with the genomic structures of mammalian OT/VP receptor genes. Thus the structural and biochemical features of the OT/VP family of receptors are conserved in Annelida. These results are also compatible with the hypothesis that the ancestral gene for the OT/VP superfamily was present in the stem group Archaemetazoa, from which invertebrates diverged approx. 600 million years ago [24,25].
AnR was also shown to be activated by Lys-conopressin that possesses a basic residue (Lys) at position 8 similar to VP family peptides, in contrast with annetocin that contains a neutral amino acid (Thr) as do OT family peptides. The Lymnaea Lys-conopressin receptor, LSCPR-1, is responsive to an [Ile8]Lys-conopressin analogue to a similar extent [17]. These findings are consistent with a previous study in which both annetocin and Lys-conopressin induced egg-laying behaviour in earthworm and leech [15]. Given this, invertebrate OT/VP peptides, unlike their vertebrate counterparts, cannot be classified based on the amino acid present at position 8. This is further supported by the fact that only a single OT/VP peptide is present in each invertebrate species aside from octopus [3,13]. Consequently, it can be proposed that the evolutionary lineage of binding modes of invertebrate OT/VP ligand–receptor pairs is distinct from that of vertebrates, although they originated from a common ancestral gene.
A striking feature is that AnR mRNA was detected only in the nephridia situated in the clitellum region (Figures 4a–4e) despite the distribution of nephridia throughout the worm body, and that the expression of the AnR gene was not observed in any reproductive tissue. Since other homologous receptors were not identified, AnR is quite probably the only OT/VP family peptide receptor in the worm. In addition, injection of annetocin into the worm induced egg-laying behaviour [15]. Taken together, these findings strongly support the notion that the AnR expressed in the clitellum nephridia mediates the egg-laying behaviour regulated by annetocin. Furthermore, it can also be presumed that an ancestral OT/VP-related peptide might have participated in egg-laying behaviour through the regulation of the nephridia, and might have diverged into the OT and VP families via gene duplication [3,24,25], followed by acquisition of their respective physiological roles. In keeping with this issue and the specific expression of the AnR gene in the clitellum nephridia, the transcriptional regulation of the AnR and annetocin genes is also an intriguing matter. In mammals, several transcriptional elements were found in the promoter regions of the OT/VP receptor genes [20–23], but the regulatory mechanism remains unclear. On the other hand, the OT and VP genes were shown to be positively regulated by distinct transcriptional factors: the VP gene is up-regulated by transcriptional factors CREB (cAMP-response-element-binding protein) and activator protein-2 activated by cAMP [26–28], whereas oestrogen nuclear receptors, thyroid hormone nuclear receptors, retinoic acid nuclear receptors and chicken ovalbumin upstream promoter-transcription factor 1 enhance the expression of the OT gene [29–35]. Unfortunately, there is no information concerning the transcriptional regulation of invertebrate OT/VP-related peptides and their receptors. However, we found that the promoter encompasses only the VP gene-like transcriptional elements such as CREB and activator protein-2 binding sites (T. Kawada, A. Kanda, H. Minakata, O. Matsushima and H. Satake, unpublished work), implying the possibility that the expression of annetocin gene undergoes a VP gene-like transcriptional regulation and then plays a crucial role in the induction of egg-laying behaviour. Therefore investigation of the transcriptional mechanism for the annetocin and AnR genes will contribute to the understanding of the functional evolution of OT/VP superfamily peptides. The functional promoter analyses of these genes are currently under study.
Expression of annetocin mRNA was restricted to the suboesophageal ganglion [14]. Takahama et al. [36] demonstrated in an immunohistochemical study that annetocin is present in the nerve cord up to the 30th segment, which corresponds to the clitellum region, but is not detected in any tissues posterior to the 31st segment. This suggests that annetocin is transported from the suboesophageal ganglion to the nephridia in the clitellum in a neuroendocrine fashion. Therefore, we presume that annetocin, produced in the suboesophageal ganglion, is transported through the nerve cord as a neuropeptide to the clitellum nephridia, which express the AnR gene and, eventually, induces egg-laying behaviour through activation of AnR.
Egg-laying behaviour of the earthworm is accomplished as follows: eggs of an earthworm are transported to and stored in a jelly egg-bag called a ‘cocoon’ that is formed around the clitellum, and then the cocoon slips forward and leaves the body with eggs contained in it [37]. In addition, the clitellum nephridia are expected to be involved in the production of the cocoon [37]. These findings, combined with the specific expression of AnR in the clitellum nephridia, suggest the involvement of annetocin in egglaying behaviour through the regulation of the production of the cocoon by the clitellum nephridia. This model accounts for the functional correlation of the annetocin-inducing egg-laying behaviour with the expression of AnR gene on the clitellum nephridia, although details of the function of annetocin in the clitellum nephridia have to await further study. The biological activity of annetocin on the clitellum nephridia and transcriptional mechanisms for the annetocin and AnR genes are now being examined.
In conclusion, we identified the structure, genomic organization and the function of a novel invertebrate OT/VP-related peptide receptor, AnR. Our results not only confirmed that the characteristics of AnR are similar to those of members of an invertebrate OT/VP receptor family, but also show the unprecedented functional correlation between an invertebrate OT/VP-related peptide and egg-laying behaviour.
Acknowledgments
We thank Professor H. Takahama for discussions and encouragement.
References
- 1.Acher R. Neurohypophysial peptide systems: processing machinery, hydroosmotic regulation, adaptation and evolution. Regul. Pept. 1993;45:1–13. doi: 10.1016/0167-0115(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 2.Acher R. Molecular evolution of fish neurohypophysial hormones: neutral and selective evolutionary mechanisms. Gen. Comp. Endocrinol. 1996;102:157–172. doi: 10.1006/gcen.1996.0057. [DOI] [PubMed] [Google Scholar]
- 3.Hoyle C. H. Neuropeptide families: evolutionary perspectives. Regul. Pept. 1998;73:1–33. doi: 10.1016/s0167-0115(97)01073-2. [DOI] [PubMed] [Google Scholar]
- 4.Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature (London) 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 5.Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. Structure and expression of a human oxytocin receptor. Nature (London) 1992;356:526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto T., Saito M., Mochizuki S., Watanabe Y., Hashimoto S., Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J. Biol. Chem. 1994;269:27088–27092. [PubMed] [Google Scholar]
- 7.Thibonnier M., Auzan C., Madhun Z., Wilkins P., Berti-Mattera L., Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J. Biol. Chem. 1994;269:3304–3310. [PubMed] [Google Scholar]
- 8.Cruz L. J., de Santos V., Zafaralla G. C., Ramilo C. A., Zeikus R., Gray W. R., Olivera B. M. Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus straitus venoms. J. Biol. Chem. 1987;262:15821–15824. [PubMed] [Google Scholar]
- 9.Oumi T., Ukena K., Matsushima O., Ikeda T., Fujita T., Minakata H., Nomoto K. Annetocin: an oxytocin-related peptide isolated from the earthworm, Eisenia foetida. Biochem. Biophys. Res. Commun. 1994;198:393–399. doi: 10.1006/bbrc.1994.1055. [DOI] [PubMed] [Google Scholar]
- 10.Proux J. P., Miller C. A., Li J. P., Carney R. L., Girardie A., Delaage M., Schooley D. A. Identification of an arginine vasopressin-like diuretic hormone from Locusta migratoria. Biochem. Biophys. Res. Commun. 1987;149:180–186. doi: 10.1016/0006-291x(87)91621-4. [DOI] [PubMed] [Google Scholar]
- 11.Reich G. A new peptide of the oxytocin/vasopressin family isolated from nerves of the cephalopod Octopus vulgaris. Neurosci. Lett. 1992;134:191–194. doi: 10.1016/0304-3940(92)90514-8. [DOI] [PubMed] [Google Scholar]
- 12.Salzet M., Bulet P., Van Dorsselaer A., Malecha J. Isolation, structural characterization and biological function of a lysine-conopressin in the central nervous system of the pharyngobdellid leech Erpobdella octoculata. Eur. J. Biochem. 1993;217:897–903. doi: 10.1111/j.1432-1033.1993.tb18319.x. [DOI] [PubMed] [Google Scholar]
- 13.Takuwa-Kuroda K., Iwakoshi-Ukena E., Kanda A., Minakata H. Octopus, which owns the most advanced brain in invertebrates, has two members of vasopressin/oxytocin superfamily as in vertebrates. Regul. Pept. 2003;115:139–149. doi: 10.1016/s0167-0115(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 14.Satake H., Takuwa K., Minakata H., Matsushima O. Evidence for conservation of the vasopressin/oxytocin superfamily in Annelida. J. Biol. Chem. 1999;274:5605–5611. doi: 10.1074/jbc.274.9.5605. [DOI] [PubMed] [Google Scholar]
- 15.Oumi T., Ukena K., Matsushima O., Ikeda T., Fujita T., Minakata H., Nomoto K. Annetocin, an annelid oxytocin-related peptide, induces egg-laying behavior in the earthworm, Eisenia foetida. J. Exp. Zool. 1996;276:151–156. doi: 10.1002/(SICI)1097-010X(19961001)276:2<151::AID-JEZ8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.van Kesteren R. E., Tensen C. P., Smit A. B., van Minnen J., van Soest P. F., Kits K. S., Meyerhof W., Richter D., van Heerikhuizen H., Vreugdenhil E., et al. A novel G protein-coupled receptor mediating both vasopressin- and oxytocin-like functions of Lys-conopressin in Lymnaea stagnalis. Neuron. 1995;15:897–908. doi: 10.1016/0896-6273(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 17.van Kesteren R. E., Tensen C. P., Smit A. B., van Minnen J., Kolakowski L. F., Meyerhof W., Richter D., van Heerikhuizen H., Vreugdenhil E., Geraerts W. P. Co-evolution of ligand–receptor pairs in the vasopressin/oxytocin superfamily of bioactive peptides. J. Biol. Chem. 1996;271:3619–3626. doi: 10.1074/jbc.271.7.3619. [DOI] [PubMed] [Google Scholar]
- 18.Kanda A., Takuwa-Kuroda K., Iwakoshi-Ukena E., Furukawa Y., Matsushima O., Minakata H. Cloning of Octopus cephalotocin receptor, a member of the oxytocin/vasopressin superfamily. J. Endocrinol. 2003;179:281–291. doi: 10.1677/joe.0.1790281. [DOI] [PubMed] [Google Scholar]
- 19.Bockaert J., Pin J. P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue T., Kimura T., Azuma C., Inazawa J., Takemura M., Kikuchi T., Kubota Y., Ogita K., Saji F. Structural organization of the human oxytocin receptor gene. J. Biol. Chem. 1994;269:32451–32456. [PubMed] [Google Scholar]
- 21.Murasawa S., Matsubara H., Kijima K., Maruyama K., Mori Y., Inada M. Structure of the rat V1a vasopressin receptor gene and characterization of its promoter region and complete cDNA sequence of the 3′-end. J. Biol. Chem. 1995;270:20042–20050. doi: 10.1074/jbc.270.34.20042. [DOI] [PubMed] [Google Scholar]
- 22.Thibonnier M., Graves M. K., Wagner M. S., Auzan C., Clauser E., Willard H. F. Structure, sequence, expression, and chromosomal localization of the human V1a vasopressin receptor gene. Genomics. 1996;31:327–334. doi: 10.1006/geno.1996.0055. [DOI] [PubMed] [Google Scholar]
- 23.Nathanson M. H., Moyer M. S., Burgstahler A. D., O'Carroll A. M., Brownstein M. J., Lolait S. J. Mechanisms of subcellular cytosolic Ca2+ signaling evoked by stimulation of the vasopressin V1a receptor. J. Biol. Chem. 1992;267:23282–23289. [PubMed] [Google Scholar]
- 24.Hoyle C. H. Neuropeptide families and their receptors: evolutionary perspectives. Brain Res. 1999;848:1–25. doi: 10.1016/s0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- 25.van Kesteren R. E., Smit A. B., Dirks R. W., de With N. D., Geraerts W. P., Joosse J. Evolution of the vasopressin/oxytocin superfamily: characterization of a cDNA encoding a vasopressin-related precursor, preproconopressin, from the mollusc Lymnaea stagnalis. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4593–4597. doi: 10.1073/pnas.89.10.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohr E., Richter D. Sequence analysis of the promoter region of the rat vasopressin gene. FEBS Lett. 1990;260:305–308. doi: 10.1016/0014-5793(90)80130-b. [DOI] [PubMed] [Google Scholar]
- 27.Pardy K., Adan R. A., Carter D. A., Seah V., Burbach J. P., Murphy D. The identification of a cis-acting element involved in cyclic 3′,5′-adenosine monophosphate regulation of bovine vasopressin gene expression. J. Biol. Chem. 1992;267:21746–21752. [PubMed] [Google Scholar]
- 28.Verbeeck M. A., Sutanto W., Burbach J. P. Regulation of vasopressin messenger RNA levels in the small cell lung carcinoma cell line GLC-8: interactions between glucocorticoids and second messengers. Mol. Endocrinol. 1991;5:795–801. doi: 10.1210/mend-5-6-795. [DOI] [PubMed] [Google Scholar]
- 29.Adan R. A., Walther N., Cox J. J., Ivell R., Burbach J. P. Comparison of the estrogen responsiveness of the rat and bovine oxytocin gene promoters. Biochem. Biophys. Res. Commun. 1991;175:117–122. doi: 10.1016/s0006-291x(05)81208-2. [DOI] [PubMed] [Google Scholar]
- 30.Adan R. A., Cox J. J., van Kats J. P., Burbach J. P. Thyroid hormone regulates the oxytocin gene. J. Biol. Chem. 1992;267:3771–3777. [PubMed] [Google Scholar]
- 31.Adan R. A., Cox J. J., Beischlag T. V., Burbach J. P. A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Mol. Endocrinol. 1993;7:47–57. doi: 10.1210/mend.7.1.8383287. [DOI] [PubMed] [Google Scholar]
- 32.Burbach J. P., Lopes da Silva S., Cox J. J., Adan R. A., Cooney A. J., Tsai M. J., Tsai S. Y. Repression of estrogen-dependent stimulation of the oxytocin gene by chicken ovalbumin upstream promoter transcription factor I. J. Biol. Chem. 1994;269:15046–15053. [PubMed] [Google Scholar]
- 33.Mohr E., Schmitz E. Functional characterization of estrogen and glucocorticoid responsive elements in the rat oxytocin gene. Brain Res. Mol. Brain Res. 1991;9:293–298. doi: 10.1016/0169-328x(91)90075-9. [DOI] [PubMed] [Google Scholar]
- 34.Richard S., Zingg H. H. The human oxytocin gene promoter is regulated by estrogens. J. Biol. Chem. 1990;265:6098–6103. [PubMed] [Google Scholar]
- 35.Wehrenberg U., Ivell R., Walther N. The COUP transcription factor (COUP-TF) is directly involved in the regulation of oxytocin gene expression in luteinizing bovine granulosa cells. Biochem. Biophys. Res. Commun. 1992;189:496–503. doi: 10.1016/0006-291x(92)91585-e. [DOI] [PubMed] [Google Scholar]
- 36.Takahama H., Haibara K., Oumi T., Ukena K., Morishita F., Furukawa Y., Matsushima O., Minakata H., Nomoto K. Immunohistochemical localization of annetocin, an earthworm oxytocin-related peptide, and identification and ultrastructural characteristics of the annetocin-secretory cells in the oligochaete earthworm Eisenia foetida. Zool. Sci. 1998;15:381–388. doi: 10.2108/zsj.15.381. [DOI] [PubMed] [Google Scholar]
- 37.Barnes R. D. Invertebrate Zoology. 5th edn. New York: Saunders College Publishing; 1987. The annelids; pp. 263–341. [Google Scholar]