Abstract
Extracts of normal mature articular cartilage contain aggrecan molecules which bear the G1 domain (the N-terminal globular domain of aggrecan) and are C-terminally truncated by proteolysis at a number of sites. A proportion of these molecules are generated by an aggrecanase and/or matrix-metalloproteinase-mediated cleavage in the IGD (interglobular domain between the G1 and G2 domains of aggrecan). However, the proteinase(s) responsible for formation of the majority of the larger G1-G2 and glycosaminoglycan-bearing truncated species is (are) unknown. N-terminal sequencing of aggrecan core fragments generated by m-calpain digestion of bovine aggrecan has identified four novel cleavage sites: one within the CS (chondroitin sulphate)-1 domain (at one or more of the bonds Ser1229–Val1230, Ser1249–Val1250, Ser1287–Val1288, Gly1307–Val1308 and Ser1346–Val1347), two within the IGD (at bonds Ala474–Ala475 and Gly365–Gly366) and one within the KS (keratan sulphate) domain (at Ala719–Ala720). A new monoclonal antibody (SK-28) to the C-terminal neoepitope at M710VTQVGPGVA719 showed that aggrecan products generated by this cleavage are present in high abundance in mature bovine articular cartilage extracts. We conclude that m-calpain, or an unidentified proteinase with the capacity to cleave at the same site, is active during aggrecan biosynthesis/secretion by mature chondrocytes or in the matrix of mature bovine articular cartilage in vivo.
Keywords: aggrecanase, calcium-dependent proteolysis, calpain, chondroitin sulphate, interglobular domain
Abbreviations: A1A1D1–A1A1D6, CsCl gradient fractions used in aggrecan purification; ADAMTS, adisintegrin and metalloproteinase with a thrombospondin motif; CS, chondroitin sulphate; G1, the N-terminal globular domain of aggrecan; IGD, interglobular domain between the G1 and G2 domains of aggrecan; KLH, keyhole-limpet (Megathura crenulata) haemocyanin; KS, keratan sulphate; mAb, monoclonal antibody; MMP, matrix metalloproteinase
INTRODUCTION
Aggrecan is the major space-filling proteoglycan present in articular cartilage, and it provides the tissue with mechanical properties of reversible compressibility. In joint pathology, such as that seen in osteoarthritis and rheumatoid arthritis, aggrecan loss leads to progressive cartilage degradation. It is now well established that aggrecan core protein in mature articular cartilages is present as multiple species generated by C-terminal truncation of the full-length protein [1–4]. Indeed, the major aggrecan core species present in bovine [5,6] and rat aggrecan [7] have similar electrophoretic properties to the species characterized as 1, a, b, c, d, 6 and e in human articular cartilage extracts [3]. Despite intensive study of these C-terminally truncated species [4,8–11] and attempts to identify the proteinases responsible for their generation in vivo, the precise structure has been established only for species 1, 6 and e, which represent the full-length core protein, the aggrecanase [one or more of ADAMTS (a disintegrin and metalloproteinase with a thrombospondin motif)-1, -4, -5, -8, -9 or -15]-generated G1-NITEGE and the matrix metalloproteinase (MMP)/ADAMTS4/cathepsin-B-generated G1-VDIPEN repectively [3,10,12–22] (G1 is the N-terminal globular domain of aggrecan).
By comparison of the electrophoretic behaviour of the unidentified G1-bearing species (a, b, c and d [3]) with that of all the aggrecan fragments of known structure, we predicted [3] that the C-termini of these species would be in the region of residue 1300 (species a), 653–755 (species b) and 374–480 (species c and d) (these species are also identified in Figure 1A, right lane, and Figure 4, below).
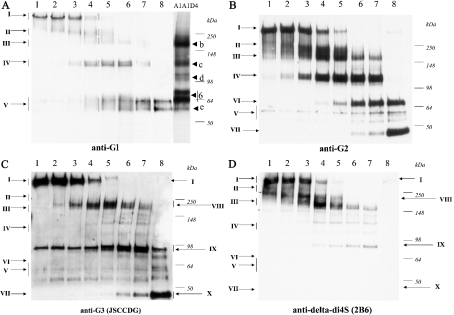
Figure 1. Western-blot analyses of products generated by digestion of bovine aggrecan with m-calpain.
Mature bovine aggrecan (100 pmol of A1A1D1) was digested with increasing amounts of m-calpain (lanes 1–8). The products were deglycosylated and analysed by Western blot with antiserum to aggrecan G1 domain (A), G2 domain (B), G3 domain (C), and Δdi4S [‘delta-di4S’; 2-acetamido-2-deoxy-3-O-(β-D-gluco-4-enepyranosyluronic acid)-4-O-sulpho-D-galactose] stubs (D). Also shown is mature bovine aggrecan A1A1D4 (A, right lane). For the conditions of incubation, see the text. See Figure 2 for a description of the species I–V. Also note that the extent of migration of all the species in (D) is about 15% less than on the other gels, for unknown reasons.
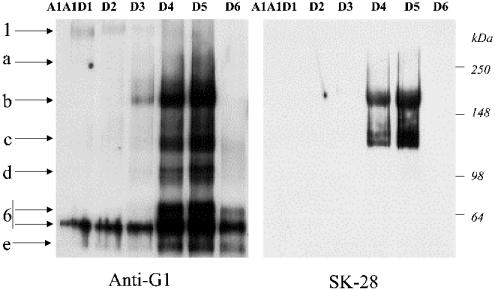
Figure 4. Western-blot analysis of mature bovine articular aggrecan fractions with anti-G1 and SK-28.
CsCl fractions (A1A1D1–A1A1D6) were deglycosylated and analysed by Western blotting with anti-G1 (left panel) and SK-28 (right panel). The abundant G1-bearing species (labelled as b and c) in D4 and D5 also react very strongly with SK-28. The proposed structure for band b (described as species III) and band c (described as species IV) are shown on Figure 2 and discussed in the text.
For species a, which appears as a broad heterogeneous band on gels, there is no known proteolytic cleavage site near residue 1300; the electrophoretic properties of species a indicate that it is slightly smaller than the aggrecanase-generated product formed by cleavage at Glu1564 [23] (human) or Glu1499 ([24], but see erratum [24a]) (bovine). In addition, it has been suggested [3,25] that species b, c and d may be products of MMP activity, since MMP-1 cleaves at Gly675–Ileu676 [26] and a range of MMPs cleave at Asp460–Leu461 [27], both of which are near the predicted sites [3].
The demonstration [28,29] that m-calpain cleaves efficiently at the Ala719–Ala720 and Ala474-Ala475 bonds of bovine aggrecan (residue numbers from [24,24a]) raised the possibility that the naturally occurring species, b, c and d, might instead result from proteolysis at these sites. Here we report the generation and use of a mAb (monoclonal antibody), SK-28, which reveals that the naturally occurring bovine aggrecan species b (also called band b in [7], species c in [6] and species b in [3]) and species c (also called species D in [6] and species c in [3]) are the products of m-calpain, or an unidentified proteinase with the same substrate specificity for bovine aggrecan cleavage. The calpains are currently defined as a family of calcium-dependent neutral cysteine proteinases which are located inside cells in either cytoplasmic or membrane-associated forms [30]. Defined structural similarities in the catalytic domain determine the members of the calpain superfamily. The two major calpains, which are very widely distributed, are termed calpain 1 (μ-calpain) and calpain 2 (m-calpain), owing to the fact that they are activated at micro- and milli-molar concentrations of Ca2+ respectively. Within the diarthrodial joint, m-calpain has been identified in articular chondrocytes [31–33], synoviocytes [34] and growth-cartilage chondrocytes [35].
The findings reported here provide new insight into the proteinases responsible for C-terminal truncation of aggrecan in vivo and establish the presence of a novel proteolytic pathway for aggrecanolysis in the cells and/or matrix of mature articular cartilages.
EXPERIMENTAL
Materials
Porcine kidney m-calpain was purchased from Calbiochem. Chondroitinase ABC, endo-β-galactosidase and keratanase II were obtained from Seikagaku America (East Falmouth, MA, U.S.A.). Goat anti-mouse secondary antibody and mouse mAb isotyping kit were from Amersham Biosciences (Little Chalfont, Amersham, Bucks., U.K.). The affinity column HiTrap™ Protein A HP and Sepharose CL-2B were from Amersham Biosciences (Uppsala, Sweden).
Preparation of mAb SK-28
The antigen used for immunization was the ovalbumin-linked peptide CGGMVTQVGPGVA (the italicized portion is the linker and spacer sequence). Procedures for immunization, cell fusion and hybridoma selection were as described in [36], with modification. Hybridomas showing reactivity with the immunizing antigen were expanded in cell culture, cloned by limiting dilution and used for ascites production. The SK-28 antibodies were purified on HiTrap Protein A and the subclass of SK-28 was IgG1κ. Specificity was characterized with synthetic peptides (MVTQVGPGVA and MVTQVGPGVAAVP) by competitive-inhibition ELISA using a p-nitrophenyl phosphate substrate and monitoring absorbance of the product, p-nitrophenol, at 405 nm.
Bovine aggrecan isolation and calpain digestion
For core analysis and calpain-digestion studies of fetal and mature bovine aggrecan, the A1A1D1–A1A1D6 fractions (densities 1.65, 1.56, 1.52, 1.47, 1.41 and 1.37 g/cm3 respectively) of fetal epiphyseal and mature articular cartilages were prepared in the presence of proteinase-inhibitor mixture as described in [1]. Aggrecan aggregate preparations for N-terminal-analysis studies were prepared with mature bovine aggrecan (D1) and 5% (w/w) hyaluronan. Degradation of aggrecan by m-calpain was generally performed under standard conditions for 30 min at 30 °C [28]. Briefly, the reaction mixture (200 μl) contained 100 μg of aggrecan (≈100 pmol) and m-calpain (≈0.01–10.0 pmol) in 5 mM 2-mercaptoethanol/1 mM EGTA/110 mM imidazol buffer, pH 7.5. Digestion was started by addition of CaCl2 to a final concentration of 7 mM (resulting in 6 mM free Ca2+ over EGTA) and terminated by addition of EDTA to a final concentration of 10 mM or monoiodoacetic acid to a final concentration of 20 mM.
SDS/PAGE and Western blotting
After calpain digestion, aggrecan was deglycosylated with chondroitinase ABC, endo-β-galactosidase and keratanase II and subjected to 4–12%-PAGE as previously described [3]. Anti-G1 [3], JSCATE, previously described as anti-ATEGQV [37], and SK-28 were used at 1:3000 dilution. Anti-aggrecan G3 domain (JSCCDG) was prepared by immunization of rabbits with the peptide CDGHPMQFENWRPNQPDN conjugated to KLH [keyhole-limpet (Megathura crenulata) haemocyanin]. This antiserum was previously described as LEC-7 [7]. An anti-G2 serum (raised against a 27-mer peptide immunogen KCYAGWLADGSLRYPIVTPRPACGGDK from the B′ loop of the human G2 domain (conjugated to KLH) [4] was very kindly provided by Dr Amanda Fosang, University of Melbourne, Department of Paediatrics, Cell and Matrix Biology Research Unit and Murdoch Childrens Research Institute, Royal Children's Hospital, Parkville, Vic., Australia, and used at 1:5000 dilution. Monoclonal antibodies 2-B-6 and 3-B-3, which are highly reactive with unsaturated 4-sulphated and 6-sulphated disaccharide stubs on chondroitinase-digested aggrecan core protein respectively [38], were kindly provided by Dr Bruce Caterson, Connective Tissue Laboratories, Cardiff School of Biosciences, University of Cardiff, Cardiff, Wales, U.K.
Isolation of aggrecan fragments and N-terminal amino-acid-sequence analysis
Calpain digests were fractionated on Superose 12, Sepharose CL-2B (10 mm×100 cm), TSK4000 (TOSHO, Tokyo, Japan) and Biosilect SEC400 (Bio-Rad Inc.), the products were desalted by dialysis and applied to an Applied Biosystem 477A sequencer.
RESULTS
Western analysis of m-calpain digestion products of bovine aggrecan and comparison with naturally occurring core species
For this purpose we set up 30 min digestions containing 100 pmol of mature bovine aggrecan A1A1D1 and increasing amounts (0, 0.01, 0.02, 0.1, 0.2, 1.0 and 2.0 pmol) of m-calpain. Also included was an extensive digestion of 100 pmol of aggrecan with 10 pmol of m-calpain for 2 h. The products of all digests were deglycosylated and analysed by Western blot with anti-(G1 domain) serum (Figure 1A, lanes 1–8). Shown for comparison is an analysis of an A1A1D4 sample of mature bovine articular cartilage which contains the major naturally occurring G1-bearing species labelled here as b, c, d, 6 (doublet) and e. Five major G1-immunoreactive bands (I–V) were detected in the m-calpain digests with apparent molecular masses of ≈450 (I), ≈250 (II), ≈160 (III), ≈120 (doublet, IV) and a doublet at ≈60 kDa (V). Band I is the full-length core protein, since it reacts with anti-G1, anti-G2 and anti-G3 antibodies (see Figures 1A, 1B and 1C). Band II, a diffuse region at ≈250 kDa, was generated at low calpain concentrations (lanes 2–5) and was destroyed by concentrations of 1.0 pmol of calpain and above (lanes 6–8); this species migrates to a position similar to that of the previously described band a [3]. Species III, at ≈160 kDa, was detected at very low abundance and only at intermediate calpain concentrations (lanes 3–7); it migrates to a position similar to that of the natural band b shown in the right lane. Species IV, at ≈120 kDa, was detected in quite high abundance at intermediate calpain concentrations; it was destroyed by high calpain concentrations, and it migrated to a position identical with that of the natural band c shown in the A1A1D4 sample. Species V, a diffuse product becoming a clear doublet at the highest calpain concentration (lane 8), migrates to a position very similar to that of band 6 (doublet of G1-NITEGE392; numbering from [24,24a]) and band e (G1-VDIPES360; sequence and numbering from [24,24a]) shown in the A1A1D4 sample. The presence of the G1 domain on these species (I–V) was confirmed by analysis of these samples with antibody JSCATE, which recognizes a peptide in the A-loop of the G1 domain (results not shown). A schematic illustration of the structures consistent with the anti-G1 Western-blot analysis is given in Figure 2. Western-blot analysis of the same samples with anti-G2 (Figure 1B) fully supported these proposed structures for species I–IV (G2-positive) and species V (G2-negative) and also revealed the presence of products VI and VII, characterized by reactivity with anti-G2, but not with anti-G1.
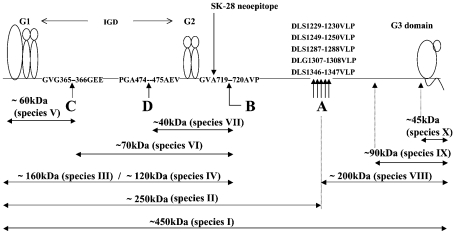
Figure 2. Schematic illustration of calpain-generated core species.
The Figure summarizes the cleavage-site data and immunoreactivity profiles obtained with products of calpain digestion of mature bovine aggrecan. The location of cleavages and the order of cleavage (A, B, C and D in that order) were established by N-terminal analysis, product size and immunoreactivity profiles of species I–X. The N-termini of species IX and X have not been determined, and the structure of species III has not been definitively established (see the text for an explanation).
Western-blot analysis of the same calpain digests with anti-G3 (Figure 1C) showed, as expected, a very different pattern from that shown in Figures 1(A) and 1(B). There were three major G3-bearing products generated from the full-length band I, with apparent sizes of ≈200 kDa (species VIII), 90 kDa (species IX) and 45 kDa (species X). The sharp 90 kDa band present in all samples appears to be a non-specific reactivity of the antiserum. Analysis of these same samples with mAb 2B6 (Figure 1D) and 3B3 (results not shown) gave similar patterns to each other and showed that, as expected, only the high-molecular-mass species (above 160 kDa) are highly antigenic, owing to substitution with chondroitin sulphate at multiple sites (see Figure 2 for a schematic summary of cleavage sites and proposed species identification).
N-terminal sequence analysis identifies four major calpain cleavage sites in aggrecan
The Western-blot analyses (Figures 1A–1D) suggested that m-calpain cleaves aggrecan at four or more sites and that the product sizes decrease with increasing enzyme concentration. To establish the sequences at the cleavage sites, we generated products for N-terminal analysis by digestion of bovine A1D1 aggrecan in aggregate form [up to 10 nmol of substrate in aggregates with hyaluronan (5%, w/w)] for 30 min under conditions which corresponded to a ratio of 0.03, 0.25, 2.5 and 12.5 pmol of calpain per 100 pmol of aggrecan. In each case the products were separated on an associative Sepharose CL-2B column and the non-aggregating fragments were purified on TSK4000 and/or Biosilect SEC400 columns for N-terminal analysis. Under mild digestion conditions, a single major new N-terminal sequence, namely VLPXG, was detected, which could be generated by cleavage at one or more of the following bonds: Ser1229–Val1230, Ser1249–Val1250, Ser1287–Val1288, Gly1307–Val1308 and Ser1346–Val1347 (see cleavage A, Figure 2). Any of these cleavages generate a G1-bearing peptide of about 1300 residues which corresponds in size to species II (Figures 1A and 1B). This is consistent with the finding that the major hyaluronan-bound product of this digestion migrated at the position of species II on SDS/PAGE (results not shown).
Under more extensive digestion, a new major N-terminus, AVP, was detected, confirming the previous finding [28,29] of a cleavage site at Ala719–Ala720 (see cleavage B, Figure 2). Indeed, the same analysis done with human and rat aggrecan indicated the N-termini AVPVEE and AVPL respectively, consistent with cleavage at the equivalent Ala–Ala bond in these core proteins. Under more exhaustive digestion a new N-terminus, GEEDI, was obtained, corresponding to a cleavage at Gly365–Gly366 (cleavage C, Figure 2). Finally, the digest generated with 12.5 pmol of calpain per 100 pmol of aggrecan was deglycosylated, separated by SDS/PAGE and blotted on to PVDF membrane. A major ≈40 kDa product was obtained and was found to have the N-terminal sequence AEVPGQPXLGP, confirming cleavage at the Ala474–Ala475 bond (cleavage D, Figure 2) [29] at very high calpain concentrations. This final cleavage generates the intact G2-domain with IGD and KS domain extensions of about 30 residues each (see species VII, Figures 1B and 2). This analysis confirmed the previously described cleavage sites B and D [28,29] and established the presence of two more sites (see cleavages A and C in Figure 2). The same general product patterns seen in Figure 1(A) (anti-G1) and Figure 3 (SK-28) were obtained in a separate set of incubations done at 37 °C instead of 30 °C, supporting the physiological relevance of these cleavage sites (results not shown).
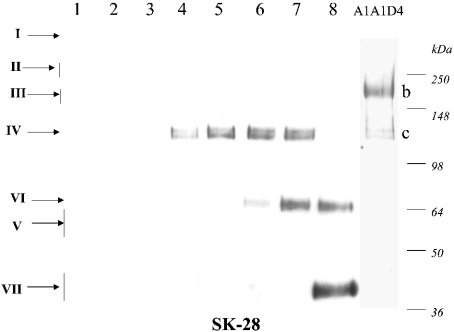
Figure 3. Core products generated by digestion of bovine aggrecan with m-calpain which react with mAb SK-28.
Mature bovine aggrecan (100 pmol of A1A1D1) was digested with increasing amounts of m-calpain (lanes 1–8), the products were deglycosylated and analysed by Western blotting with MAb SK-28 raised to the calpain-generated C-terminal sequence MVTQVGPVGA719. For a further description of MAb SK-28 and the conditions of incubation, see the text. See Figure 2 for a description of the species I–X. Also shown is mature bovine aggrecan A1A1D4 (right lane).
Summary of in vitro aggrecan cleavages by m-calpain
The Western-blot data (Figures 1A, 1B, 1C and 1D), along with the known cleavage locations from N-terminal analysis, were used to generate a schematic map of species I–X (Figure 2). The minimum m-calpain concentration required to generate (and eliminate) the individual products shown on Western-blot analysis proved that cleavage (from the most sensitive to the least sensitive bond) was in the order A–D shown in Figure 2. This priority is based on the order of product appearance with increasing enzyme concentration (that is, II followed by III/IV followed by V followed by VI/IX and finally VII/X). It should be noted that this structural summary includes an assumption that species III and species IV are the same aggrecan core species, despite their obvious difference in migration behaviour (Figures 1A and 1B). Possible explanations for this apparent anomaly are provided in the Discussion. It also shows that the disulphide-bonded globular domains of aggrecan (G1, G2 and G3) resist calpain digestion, since the G1 domain in species V, the G2 domain in species VII, and the G3 domain in species X, were all preserved, and found as major terminal products.
The specific sites of cleavage within the CS-2 domain, which are responsible for the generation of species IX and species X, have not been identified. Analysis of digestion products by Western blot with antibody 2-B-6 (Figure 1D) and by Sepharose CL-2B chromatography (results not shown) suggested that, at low enzyme concentrations, cleavage occurs at only two or three sites. The apparent sizes suggest that they would be at about residue 1950 for species IX and residue 2100 for species X. An inspection of the bovine sequence in these regions suggest suitable sites for these cleavages at Ala1948–Ala1950 and Gly2102–Gly2103 respectively. However, at intermediate to high enzyme concentration (lanes 5–8, Figure 1D), further cleavages must occur, since the CS-bearing species VIII and IX are eliminated and the isolated G3 domain (species X) is formed (Figure 1C).
Cleavage-specific neoepitope mAb SK-28
To further examine the cleavage of bovine aggrecan with m-calpain, we next prepared a neoepitope mAb (SK-28) to the ovalbumin-conjugated peptide CGGMVTQVGPGVA719, the expected C-terminal sequence generated by cleavage at site B (Figure 2). To test the reactivity and the specificity of SK-28, we did inhibition ELISA with MVTQVGPGVA and MVTQVGPGVAAVP, which showed that the antibodies are directed exclusively to the neoepitope generated by cleavage at Ala719–Ala720 (Results not shown).
We also did Western analysis (Figure 3) of the same samples shown in Figures 1(A)–1(D). The SK-28 mAb recognized three products of ≈120 (close doublet), ≈70 and 40 kDa, generated with increasing enzyme concentration. This confirmed the generation of species IV, VI and VII respectively, and the order of cleavages, B–D, as shown in Figure 2. The apparent size summations for species IV, V and VI are consistent with the conclusion that IV (≈120 kDa) is cleaved directly to V (≈60 kDa) and VI (≈70 kDa), before VI is cleaved to VII (≈40 kDa). An overlay comparison of these Western blots showed that the ≈120 kDa (close doublet) species IV was the only species in the digests which reacted strongly with the anti-G1, anti-G2 and SK-28 antibodies, and, most interestingly, it also appears to be present in the A1A1D4 sample, where it is labelled as ‘band c’ (Figures 1A, 3 and 4).
Two major components of C-terminally truncated aggrecan in mature bovine cartilage react with the SK-28 antibody
The natural band c exhibited all of the properties of species IV, including migration behaviour, doublet appearance and reactivity with anti-G1, anti-G2 and SK-28. On the other hand, the natural band b could not be definitively identified, since there was apparently no equivalent SK-28-reactive species formed in the calpain digests (Figure 3). The analysis of mature bovine aggrecan A1A1D4 with anti-G1 (Figure 1A) and anti-G2 (results not shown) confirmed that the natural band b has an immunoreactivity profile (G1-positive and G2-positive) and migration behaviour consistent with its identification as species III.
To examine this matter further, we analysed CsCl gradient fractions of fetal epiphyseal and mature bovine aggrecan (A1A1D1–A1A1D6) by Western-blot analysis with anti-G1 and SK-28 (Figure 4). The fetal samples were composed primarily of full-length aggrecan (species I), and there was no evidence for the presence of species b or c with either the anti-G1 antibody or SK-28. These species were also undetectable in aggrecan purified from all zones of fetal bovine rib growth-plate cartilages (fetal results not shown).
However, with the mature bovine aggrecan, the high-buoyant-density aggrecan (A1A1D1–A1A1D3) was essentially pure full-length core protein (species I), whereas the low-buoyant-density fractions (A1A1D4–A1A1D6) contained abundant C-terminally truncated species, labelled here as b, c, d, 6 and e (Figure 4, left panel). The apparent low abundance of species I and a on this Western-blot analysis is due to the relatively poor transfer rate of high-molecular-mass aggrecan core proteins from SDS/PAGE gel to nitrocellulose membrane.
Most interestingly, the naturally occurring proteins in the A1A1D4 and A1A1D5 fractions, labelled as b and c, both reacted strongly with both anti-G1 (left panel), anti-G2 (results not shown) and SK-28 (right panel). When taken together, all the data obtained support the identification of the natural band c (Figures 1A, 3 and 4) as the calpain-generated species IV (Figures 1A, 1B and 2). The identification of the more abundant natural band b appears more problematic. While it appears to be equivalent to band III (Figure 1A), the small amount of band III which appeared to be generated in calpain digests of A1A1D1 was not detected by SK-28 (Figure 2). Exhaustive digestion of A1A1D4 samples with both O-glycanase and N-glycanase II did not markedly alter the electrophoretic behaviour of band b or band c, making it unlikely that they represent glycovariants of the same peptide.
The SK-28 antibody detected two other prominent species in the calpain digests, namely VI at ≈70 kDa and VII at 40 kDa. These were readily identified, since they reacted with anti-G2 (Figure 1B) but not with anti-G1 or anti-G3 (Figures 1A and 1C). They were, however, apparently not present in native bovine aggrecan preparations.
DISCUSSION
The work presented here provides the first direct evidence that m-calpain, or a proteinase that shares the same substrate specificity, is responsible for the generation of a major proportion (perhaps 40%) of the C-terminally truncated species of aggrecan present in the extracellular matrix of mature articular cartilages in vivo. It has been clearly shown that m-calpain can be readily detected by enzymic activity and immunological characterization in chondrocyte cultures, cartilage extracts and synovial fluids [33,35], and its abundance has been shown to correlate with the severity of arthritis in both animal models [31,32] and human disease [34]. The mAb SK-28, which is described for the first time in the present paper, has been used here to investigate the specific role and natural substrate of m-calpain in chondrocytes or normal cartilage matrix in situ. The SK-28 antibody, which reacts with a neoepitope generated by m-calpain-mediated cleavage of bovine aggrecan at Ala719–Ala720, detects two species in bovine articular-cartilage aggrecan preparations (b and c, Figures 1A, 2 and 4) which together appear to represent a major naturally occurring pool of C-terminally truncated aggrecan in this tissue. Since the site of truncation is within the KS-rich domain, it appears that these calpain-generated species represent KS-bearing, but CS-deficient, aggrecan, and they may therefore contribute organizational rather than water-retaining properties to the tissue. It should be noted that the demonstration in the present paper that the 120 kDa natural band c (species IV) includes the G2 domain and has a C-terminus at Ala719 (bovine) shows that the earlier prediction [3] about the size of the human band c was in error. The results presented here show clearly that only species which migrate at about 100 kDa and below have C-termini within the IGD.
The possibility that these SK-28-reactive products were generated by uncontrolled proteolysis during extraction of the aggrecan from the tissue can be almost certainly excluded for two reasons. First, the preparations analysed here were prepared under established conditions (with the inclusion of proteinase inhibitors) designed to prevent autolytic activity by endogenous proteinases such as calpains [1]. Secondly, aggrecan prepared from fetal bovine cartilage under identical conditions did not contain the SK-28-reactive species, showing that the products appear to be formed in the tissue during growth and/or maturation in vivo.
Since band c of mature bovine cartilage and species IV generated by calpain digestion of A1A1D1 aggrecan have identical size, immunoreactivity profiles and similar relative immunoreactivities (G1 to SK-28), it seems probable that the majority of the natural band c is indeed generated in vivo by m-calpain or a related proteinase. On the other hand, we are unable at present to definitively identify the natural band b, which, like band c, also reacts strongly with anti-G1, anti-G2 and monoclonal antibody SK-28. The electrophoretic properties of band b (which is about 40 kDa larger and more diffuse than band c) are consistent with it representing a modified form of band c. In this regard we are investigating the possibility that band b might represent a cross-linked advanced glycation end product of band c [39,40] or a cartilage-matrix-protein-substituted band c generated by transglutaminase activity [41]. Such an explanation might explain why we are unable to generate detectable amounts of SK-28-reactive band b in digests of bovine aggrecan A1A1D1. Thus the derivatized form may only be generated in vivo following cross-linking reactions which occur within truncated species such as band c over extended periods of dwell time in the matrix.
If, as seems very likely, calpain is responsible in vivo for the generation of both bands b and c, it might follow that calpain is also responsible for the generation of band a, the other major C-terminally truncated glycosaminoglycan-bearing species present in articular cartilages. This population [3] appears as a broad band at ≈250 kDa on most Western-blot analyses of bovine and human aggrecan preparations. Such a population is indeed generated very readily on calpain digestion (species II, Figures 1A and 1B) and the multiple possible C-termini (from Ser1229 to Ser1346) might explain the apparent heterogeneity of the naturally occurring species. Preparation of neoepitope antisera to these C-termini should be capable of resolving this question.
In this regard it will also be important to determine whether SK-28 can be used to determine the extent to which the same C-terminal processing occurs in human aggrecan in situ. On this point we have already shown that calpain digests of human aggrecan contain SK-28- reactive products at 95 kDa and 50 kDa (H. Oshita, Y. Bai, J. D. Sandy and K. Suzuki, unpublished work). However, these products have not been fully characterized. The present results also suggest that m-calpain might be an important new tool for isolation of natural molecular domains of aggrecan for analysis. Thus the composition of the exhaustive digest (lane 8, Figures 1A–1D and 3) shows that it appears to be a good source for purification of all three of the globular domains (G1, G2 and G3).
Since it is clear that a proportion of the aggrecan extracted from mature articular cartilage has been C-terminally truncated by m-calpain or a closely related proteinase, it now becomes important to definitively identify the proteinase(s) responsible and the precise location (intracellular, cell membrane, intercellular matrix) where the cleavage occurs. While both cathepsin S and cathepsin L have been shown to cleave at the Ala719–Ala720 bond (SK-28 epitope) in bovine aggrecan digests [42], it seems very unlikely that they are responsible for generation of bands b and c in cartilage, since the cathepsins have a pH optimum which is appropriate only for lysosomal activity in vivo [43]. In addition, we have been unable to generate SK-28-reactive species by digestion of bovine aggrecan with MMP-3 and ADAMTS4 (Y. Bai and J. D. Sandy, unpublished work), suggesting that metalloproteinases are not likely to be involved.
Whereas m-calpain is primarily considered to be a cytosolic protein, it can translocate to both focal complexes/adhesions or the plasma membrane [32,44,45], and there is also evidence that it is active at the endoplasmic reticulum/Golgi apparatus interface and in membrane lipid rafts [46]. These findings raise the possibility that calpain-mediated aggrecanolysis might occur during aggrecan biosynthesis and/or secretion, a suggestion which is consistent with biosynthetic studies showing the generation of large and small populations of aggregating species over short labelling periods [47,48]. Indeed, further work is necessary not only to confirm that m-calpain is responsible, and the location of the proteolysis, but also to determine whether this type of aggrecan processing achieves cartilage matrix organizational and biomechanical properties which promote or compromise the function of the tissue. Immunohistochemical localization of m-calpain and the SK-28-positive products in mature bovine cartilages should provide insights into these questions.
Acknowledgments
We gratefully acknowledge the collaboration with, and provision of the very important anti-G2 antibody by, Dr Amanda Fosang. This work was supported by the Uehara Memorial Foundation and Grants-in Aid for Scientific Research (Category B, No. 14370360) from The Japanese Society for the Promotion of Science (JSPS), a Grant from Hip Joint Foundation of Japan and a grant from the Florida Chapter of the Arthritis Foundation to J.D.S. We thank Dr Bruce Caterson for teaching us antibody production techniques. Dr Larry Rosenberg (Montefiore Hospital, New York, NY, U.S.A.) kindly provided the purified bovine aggrecan samples.
References
- 1.Rosenberg L., Wolfenstein-Todel C., Margolis R., Pal S., Strider W. Proteoglycans from bovine proximal humeral articular cartilage. Structural basis for the polydispersity of proteoglycan subunit. J. Biol. Chem. 1976;251:6439–6444. [PubMed] [Google Scholar]
- 2.Dudhia J., Davidson C. M., Wells T. M., Vynios D. H., Hardingham T. E., Bayliss M. T. Age-related changes in the content of the C-terminal region of aggrecan in human articular cartilage. Biochem. J. 1996;313:933–940. doi: 10.1042/bj3130933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandy J. D., Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem. J. 2001;358:615–626. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilim V., Fosang A. J. Proteoglycans isolated from dissociative extracts of differently aged human articular cartilage: characterization of naturally occurring hyaluronan-binding fragments of aggrecan. Biochem J. 1994;304:887–894. doi: 10.1042/bj3040887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandy J. D., Plaas A. H., Koob T. J. Pathways of aggrecan processing in joint tissues. Implications for disease mechanism and monitoring. Acta Orthop. Scand. 1995;266(Suppl.):26–32. [PubMed] [Google Scholar]
- 6.Patwari P., Kurz B., Sandy J. D., Grodzinsky A. J. Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch. Biochem. Biophys. 2000;374:79–85. doi: 10.1006/abbi.1999.1538. [DOI] [PubMed] [Google Scholar]
- 7.Sandy J. D., Thompson V., Doege K., Verscharen C. The intermediates of aggrecanase-dependent cleavage of aggrecan in rat chondrosarcoma cells treated with interleukin-1. Biochem. J. 2000;351:161–166. doi: 10.1042/0264-6021:3510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem. J. 1976;157:127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinegard D., Wieslander J., Sheehan J., Paulsson M., Sommarin Y. Separation and characterization of two populations of aggregating proteoglycans from cartilage. Biochem. J. 1985;225:95–106. doi: 10.1042/bj2250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes C. E., Caterson B., Fosang A. J., Roughley P. J., Mort J. S. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem. J. 1995;305:799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilic M. Z., Robinson H. C., Handley C. J. Characterization of aggrecan retained and lost from the extracellular matrix of articular cartilage. Involvement of carboxyl-terminal processing in the catabolism of aggrecan. J. Biol. Chem. 1998;273:17451–17458. doi: 10.1074/jbc.273.28.17451. [DOI] [PubMed] [Google Scholar]
- 12.Westling J., Fosang A. J., Last K., Thompson V. P., Tomkinson K. N., Hebert T., McDonagh T., Collins-Racie L. A., LaVallie E. R., Morris E. A., Sandy J. D. ADAMTS4 cleaves at the aggrecanase site (Glu373–Ala374) and secondarily at the matrix metalloproteinase site (Asn341–Phe342) in the aggrecan interglobular domain. J. Biol. Chem. 2002;277:16059–16066. doi: 10.1074/jbc.M108607200. [DOI] [PubMed] [Google Scholar]
- 13.Caterson B., Flannery C. R., Hughes C. E., Little C. B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Cole A. A., Kuettner K. E. MMP-8 (neutrophil collagenase) mRNA and aggrecanase cleavage products are present in normal and osteoarthritic human articular cartilage. Acta Orthop. Scand. Suppl. 1995;266:98–102. [PubMed] [Google Scholar]
- 15.Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J. Biol. Chem. 1992;267:1008–1014. [PubMed] [Google Scholar]
- 16.Fosang A. J., Last K., Neame P. J., Murphy G., Knauper V., Tschesche H., Hughes C. E., Caterson B., Hardingham T. E. Neutrophil collagenase (MMP-8) cleaves at the aggrecanase site E373–A374 in the interglobular domain of cartilage aggrecan. Biochem. J. 1994;304:347–351. doi: 10.1042/bj3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lark M. W., Gordy J. T., Weidner J. R., Ayala J., Kimura J. H., Williams H. R., Mumford R. A., Flannery C. R., Carlson S. S., Iwata M., et al. Cell-mediated catabolism of aggrecan. Evidence that cleavage at the “aggrecanase” site (Glu373–Ala374) is a primary event in proteolysis of the interglobular domain. J. Biol. Chem. 1995;270:2550–2556. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- 18.Mort J. S., Magny M. C., Lee E. R. Cathepsin B: an alternative protease for the generation of an aggrecan ‘metalloproteinase’ cleavage neoepitope. Biochem. J. 1998;335:491–494. doi: 10.1042/bj3350491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratta M. A., Yao W., Decicco C., Tortorella M. D., Liu R. Q., Copeland R. A., Magolda R., Newton R. C., Trzaskos J. M., Arner E. C. Aggrecan protects cartilage collagen from proteolytic cleavage. J. Biol. Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 20.Ragan P. M., Chin V. I., Hung H. H., Masuda K., Thonar E. J., Arner E. C., Grodzinsky A. J., Sandy J. D. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch. Biochem. Biophys. 2000;383:256–264. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 21.Sztrolovics R., White R. J., Roughley P. J., Mort J. S. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem. J. 2002;362:465–472. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tortorella M. D., Pratta M., Liu R. Q., Austin J., Ross O. H., Abbaszade I., Burn T., Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4) J. Biol. Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- 23.Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J. Biol. Chem. 1991;266:894–902. [PubMed] [Google Scholar]
- 24.Hering T. M., Kollar J., Huynh T. D. Complete coding sequence of bovine aggrecan: comparative structural analysis. Arch. Biochem. Biophys. 1997;345:259–270. doi: 10.1006/abbi.1997.0261. [DOI] [PubMed] [Google Scholar]
- 24a.Erratum. Arch. Biochem. Biophys. 1999;367:151. [Google Scholar]
- 25.Fosang A. J., Last K., Maciewicz R. A. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J. Clin. Invest. 1996;98:2292–2299. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flannery C., Sandy J. Aggrecan catabolism in cartilage:studies on the nature of a novel proteinase (“aggrecanase”) which cleaves the Glu373–Ala374 bond of the interglobular domain. Trans. Orthop. Res. Soc. 1993;17:677. [Google Scholar]
- 27.Fosang A. J., Last K., Knauper V., Neame P. J., Murphy G., Hardingham T. E., Tschesche H., Hamilton J. A. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem. J. 1993;295:273–276. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K., Shimizu K., Sandy J. Degradation of bovine aggrecan by m-calpain. Trans. Annu. Meet. Orthop. Res. Soc. 1994;40:319. [Google Scholar]
- 29.Suzuki K., Neame P., Sandy J. Isolation of aggrecan G2 domain. Trans. Annu. Meet. Orthop. Res. Soc. 1995;41:425. [Google Scholar]
- 30.Gafni J., Hermel E., Young J. E., Wellington C. L., Hayden M. R., Ellerby L. M. Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J. Biol. Chem. 2004;279:20211–20220. doi: 10.1074/jbc.M401267200. [DOI] [PubMed] [Google Scholar]
- 31.Fujimori Y., Shimizu K., Suzuki K., Nakagawa Y., Yamamoto S., Yamamuro T. Immunohistochemical demonstration of calcium-dependent cysteine proteinase (calpain) in collagen-induced arthritis in mice. Z. Rheumatol. 1994;53:72–75. [PubMed] [Google Scholar]
- 32.Szomor Z., Shimizu K., Fujimori Y., Yamamoto S., Yamamuro T. Appearance of calpain correlates with arthritis and cartilage destruction in collagen induced arthritic knee joints of mice. Ann. Rheum. Dis. 1995;54:477–483. doi: 10.1136/ard.54.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szomor Z., Shimizu K., Yamamoto S., Yasuda T., Ishikawa H., Nakamura T. Externalization of calpain (calcium-dependent neutral cysteine proteinase) in human arthritic cartilage. Clin. Exp. Rheumatol. 1999;17:569–574. [PubMed] [Google Scholar]
- 34.Yamamoto S., Shimizu K., Suzuki K., Nakagawa Y., Yamamuro T. Calcium-dependent cysteine proteinase (calpain) in human arthritic synovial joints. Arth. Rheum. 1992;35:1309–1317. doi: 10.1002/art.1780351111. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda T., Shimizu K., Nakagawa Y., Yamamoto S., Niibayashi H., Yamamuro T. m-Calpain in rat growth plate chondrocyte cultures: its involvement in the matrix mineralization process. Dev. Biol. 1995;170:159–168. doi: 10.1006/dbio.1995.1204. [DOI] [PubMed] [Google Scholar]
- 36.Caterson B., Christner J. E., Baker J. R. Identification of a mAb that specifically recognizes corneal and skeletal keratan sulphate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 1983;258:8848–8854. [PubMed] [Google Scholar]
- 37.Lemons M. L., Sandy J. D., Anderson D. K., Howland D. R. Intact aggrecan and fragments generated by both aggrecanse and metalloproteinase-like activities are present in the developing and adult rat spinal cord and their relative abundance is altered by injury. J. Neurosci. 2001;21:4772–4781. doi: 10.1523/JNEUROSCI.21-13-04772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed. Proc. Fed. Am. Soc. Exp. Biol. 1985;44:386–393. [PubMed] [Google Scholar]
- 39.Verzijl N., DeGroot J., Bank R. A., Bayliss M. T., Bijlsma J. W., Lafeber F. P., Maroudas A., TeKoppele J. M. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 40.Pokharna H. K., Pottenger L. A. Glycation induced crosslinking of link proteins, in vivo and in vitro. J. Surg. Res. 2000;94:35–42. doi: 10.1006/jsre.2000.6000. [DOI] [PubMed] [Google Scholar]
- 41.Hauser N., Paulsson M., Heinegard D., Morgelin M. Interaction of cartilage matrix protein with aggrecan. Increased covalent cross-linking with tissue maturation. J. Biol. Chem. 1996;271:32247–32252. doi: 10.1074/jbc.271.50.32247. [DOI] [PubMed] [Google Scholar]
- 42.Hascall V., Sandy J., Handley C. Regulation of proteoglycan metabolism in articular cartilage. In: Caterson B. A. C., Benjamin M., Ralphs J., editors. Biology of The Synovial Joint. Reading: Harwood Academic Publishers; 1999. pp. 101–120. [Google Scholar]
- 43.Wilkins R. J., Hall A. C. Control of matrix synthesis in isolated bovine chondrocytes by extracellular and intracellular pH. J. Cell Physiol. 1995;164:474–481. doi: 10.1002/jcp.1041640305. [DOI] [PubMed] [Google Scholar]
- 44.Cuevas B. D., Abell A. N., Witowsky J. A., Yujiri T., Johnson N. L., Kesavan K., Ware M., Jones P. L., Weed S. A., DeBiasi R. L., et al. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontremoli S., Melloni E., Salamino F., Patrone M., Michetti M., Horecker B. L. Activation of neutrophil calpain following its translocation to the plasma membrane induced by phorbol ester or fMet-Leu-Phe. Biochem. Biophys. Res. Commun. 1989;160:737–743. doi: 10.1016/0006-291x(89)92495-9. [DOI] [PubMed] [Google Scholar]
- 46.Hood J. L., Logan B. B., Sinai A. P., Brooks W. H., Roszman T. L. Association of the calpain/calpastatin network with subcellular organelles. Biochem. Biophys. Res. Commun. 2003;310:1200–1212. doi: 10.1016/j.bbrc.2003.09.142. [DOI] [PubMed] [Google Scholar]
- 47.Carney S. L., Bayliss M. T., Collier J. M., Muir H. Electrophoresis of 35S-labeled proteoglycans on polyacrylamide-agarose composite gels and their visualization by fluorography. Anal. Biochem. 1986;156:38–44. doi: 10.1016/0003-2697(86)90150-8. [DOI] [PubMed] [Google Scholar]
- 48.Sandy J. D., Plaas A. H. Studies on the hyaluronate binding properties of newly synthesized proteoglycans purified from articular chondrocyte cultures. Arch. Biochem. Biophys. 1989;271:300–314. doi: 10.1016/0003-9861(89)90280-4. [DOI] [PubMed] [Google Scholar]