Abstract
Hallmarks of the inflammatory process in Type I diabetes are macrophage activation, local release of β-cell-toxic cytokines and infiltration of cytotoxic T lymphocytes. We have observed recently that mice overexpressing active FRK (fyn-related kinase)/RAK (previously named GTK/Bsk/IYK, where GTK stands for gut tyrosine kinase, Bsk for β-cell Src-homology kinase and IYK for intestinal tyrosine kinase) in β-cells exhibit increased susceptibility to β-cell-toxic events, and therefore, we now attempt to find a more precise role for FRK/RAK in these processes. Phosphopeptide mapping of baculovirus-produced mouse FRK/RAK revealed an autophosphorylation pattern compatible with Tyr-394 being the main site. No evidence for in vitro phosphorylation of the C-terminal regulatory sites Tyr-497 and Tyr-504 was obtained, nor was there any indication of in vitro regulation of FRK/RAK kinase activity. Screening a panel of known tyrosine kinase inhibitors for their ability to inhibit FRK/RAK revealed several compounds that inhibited FRK/RAK, with a potency similar to that reported for their ability to inhibit other tyrosine kinases. Cytokine-induced islet toxicity was reduced in islets isolated from FRK/RAK knockout mice and this occurred without effects on the production of nitric oxide. Addition of the nitric oxide inhibitor nitroarginine to FRK/RAK knockout islets exposed to cytokines decreased cell death to a basal level. In normal islets, cytokine-induced cell death was inhibited by the addition of two FRK/RAK inhibitors, SU4984 and D-65495, or by transfection with short interfering RNA against FRK/RAK. It is concluded that FRK/RAK contributes to cytokine-induced β-cell death, and inhibition of this kinase could provide means to suppress β-cell destruction in Type I diabetes.
Keywords: β-cell, cytokine, cytotoxicity, fyn-related kinase (FRK)/RAK, kinase inhibitor, knockout
Abbreviations: Bsk, β-cell Src-homology kinase; FRK, fyn-related kinase; GTK, gut tyrosine kinase; IFN-γ, interferon γ; IL-1β, interleukin-1β; IYK, intestinal tyrosine kinase; PDGFR, platelet-derived growth factor receptor; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; RNAi, RNA interference; SHB, SH2 protein in β-cells; siRNA, short interfering RNA
INTRODUCTION
The β-cell-destructive processes in Type I diabetes are not completely understood, but are supposed to involve multiple pathways [1]. At an early stage, tissue macrophages become activated in the vicinity of the islets, producing β-cell-cytotoxic cytokines such as IL-1β (interleukin-1β), tumour necrosis factor α and IFN-γ [2]. This process is supposed to induce partial β-cell damage [3,4]. Subsequent to this, cytotoxic T-cells are recruited to the site of inflammation and these eventually kill most of the remaining β-cells [5].
FRK (fyn-related kinase)/RAK (also named GTK/Bsk/IYK, where GTK stands for gut tyrosine kinase, Bsk for β-cell Src-homology kinase and IYK for intestinal tyrosine kinase) is a cytoplasmic tyrosine kinase belonging to the SRC family [6,7]. FRK/RAK is expressed in liver, lung, kidney, mammary-gland epithelium, intestine and the pancreas including islets [8,9]. Previous studies have suggested Tyr-497 and Tyr-504 in the C-terminus as potential regulators of kinase activity [10,11]. A transgenic mouse with active FRK/RAK expressed in β-cells under the control of the insulin promoter exhibits not only an increased β-cell mass, but also demonstrates enhanced susceptibility to the β-cell toxin streptozotocin and to the cytokines IL-1β and IFN-γ [12]. When such mice were subjected to partial pancreatectomy, they displayed a compensatory increase in β-cell replication [13]. The combined results suggest the dual role of FRK/RAK for β-cell function: increased cell replication and increased β-cell death under conditions of imposed toxicity.
To address further the role of FRK/RAK for the destruction of β-cells in response to cytotoxic cytokines, we first decided to characterize the kinase properties of mouse FRK/RAK. Furthermore, we addressed the role of the kinase in the β-cell-cytotoxic process after cytokine exposure by studies on islets isolated from FRK/RAK knockout mice. Finally, we investigated islet death in the presence of FRK/RAK inhibitors. The results suggest a role for FRK/RAK in the β-cell-destructive process in response to cytotoxic cytokines.
MATERIALS AND METHODS
Materials
Sf9 insect cells, pVL1393 and the Baculovirus Expression Vector System kit were obtained from PharMingen (San Diego, CA, U.S.A.); fetal calf serum and penicillin/streptomycin were from HyClone Europe (Cramlington, Northumberland, U.K.); Grace's insect medium, sodium orthovanadate, PMSF, dithiothreitol, Nonidet P40 and leupeptin were from Sigma (St. Louis, MO, U.S.A.), trypsin (modified sequencing grade) was from Promega (Madison, WI, U.S.A.), Protein A–Sepharose CL-4B and [γ-32P]ATP (3000 Ci/mmol) were from Amersham Biosciences (Uppsala, Sweden) and TC lactalbumin hydrolysate and TC yeastolate were from Difco Laboratories (Detroit, MI, U.S.A.). All kinase inhibitors except D-65495, D-68129 and AG1295 were obtained from Calbiochem (La Jolla, CA, U.S.A.). D-65495 and D-68129 [14] (compounds 53 and 67) and AG1295 were generously provided by Dr Frank Böhmer (Jena University, Jena, Germany). Specific siRNA (short interfering RNA) for FRK/RAK, scrambled oligonucleotide and Fluorescein-Luciferase GL2 duplex were obtained from Dharmacon Research (LaFayette, CO, U.S.A.). All other reagents were from Merck (Darmstadt, Germany).
Cell culture and transfection
Sf9 insect cells were kept in culture in Grace's insect medium supplemented with lactalbumin hydrolysate (1.67 g/500 ml), yeastoleate (1.67 g/500 ml), benzylpenicillin (100 units/ml), streptomycin (0.1 mg/ml) and 10% fetal calf serum at 27 °C in culture flasks. Full-length wild-type FRK/RAK cDNA or FRK/RAK cDNAs containing a Y497F (Tyr497→Phe) or Y504F single mutation or a Y497/504F double mutation [10] were subcloned into a pVL1393 baculovirus transfer vector. All mutations were verified by DNA sequencing. The construct was transfected into Sf9 cells using a Baculovirus Expression System Vector System kit according to the manufacturer's instructions. The conditioned medium containing baculovirus particles expressing FRK/RAK cDNA was used for amplification to obtain high-virus titres. The expression of FRK/RAK in Sf9 cells after infection with high-virus titre was confirmed by SDS/PAGE, followed by Western-blot analysis using FRK/RAK antiserum [10] as a probe. Sf9 cells (107) were infected with high-virus titre-conditioned medium for 3 days before phosphorylation experiments.
In vitro phosphorylation
Sf9 cells expressing wild-type or mutated FRK/RAK cDNA were harvested and lysed in RIPA buffer (150 mM NaCl, 30 mM Tris, pH 7.5, 10 mM EDTA, 1% Nonidet P40, 0.5% sodium deoxycholate and 0.1% SDS), supplemented with protease inhibitors (2 mM PMSF, 0.05 mM leupeptin and 1% Trasylol) and 0.1 mM sodium orthovanadate. Nuclei were removed by centrifugation and the cell extract was immunoprecipitated with FRK/RAK antiserum [10] and immobilized on Protein A–Sepharose CL-4B. The phosphorylation reaction was performed in kinase buffer (40 mM Hepes, pH 7.5, 10 mM MgCl2, 3 mM MnCl2 and 10% glycerol), supplemented with 0.1 μM [γ-32P]ATP, 0.1 mM sodium orthovanadate and 1 mM dithiothreitol for 15 min at room temperature, and the samples were subsequently subjected to phosphopeptide mapping. In some experiments, an FRK/RAK substrate peptide was included during the phosphorylation reaction, and substrate phosphorylation was determined at different concentrations of the peptide as described in [11].
Phosphopeptide mapping
Phosphopeptide mapping was performed as described in [15]. Briefly, the phosphorylated proteins were subjected to SDS/PAGE (7.5% gel), blotted on to Immobilon filters and exposed to Hyperfilm for 45 min at room temperature. Radioactive proteins of 58 kDa were excised from the filter and subjected to tryptic degradation [16]. The tryptic fragments were dissolved in a pH 1.9 buffer (formic acid/acetic acid/double-distilled water, 23:78:899) and applied on 0.1 mm cellulose TLC plates (Merck). First-dimension thin-layer electrophoresis was performed in the pH 1.9 buffer at 2000 V for 40 min using a Hunter thin-layer electrophoresis apparatus (HTLE-7000; CBS Scientific, Del Mar, CA, U.S.A.). Second-dimension ascending chromatography was run in isobutyric acid buffer (isobutyric acid/n-butyl alcohol/pyridine/acetic acid/double-distilled water, 625:19:48:29:279) for 12 h at room temperature. After exposure for 1 week to Hyperfilm at −70 °C or on a Bio-Imaging Analyzer screen (Fuji, Tokyo, Japan), spots corresponding to radioactive phosphopeptides were scraped from the plates, eluted in the pH 1.9 buffer and freeze-dried, and subsequently subjected to Edman degradation and two-dimensional phosphoamino acid analysis.
Kinase inhibitor experiments
Wild-type FRK/RAK produced in Sf9 cells was immunoprecipitated and subjected to in vitro kinase reactions as above, including the addition of different concentrations of the inhibitor. FRK/RAK autophosphorylation was determined and normalized for the amount of FRK/RAK present in the immunoprecipitates by Western-blot analysis. Approximate IC50 values were determined based on the profile of inhibition for each inhibitor. For inhibitors that displayed inhibitory effects, experiments were repeated 2–3 times.
FRK/RAK knockout mice
FRK/RAK knockout mice [17] were bred on the C57BL/KS strain of mice. After 3–4 generations of breeding, FRK/RAK −/− or +/+ littermates were used for experimentation.
Islet cell viability test, insulin secretion and NO (nitric oxide) production
Islets were isolated from either NMRI (Naval Marine Research Institute) mice or FRK/RAK +/+ or −/− mice on a mainly C57BLKS background by collagenase isolation. The islets were then cultured in RPMI 1640 containing 11 mM glucose, 10% fetal bovine serum and antibiotics for 3–7 days. The islets were then subjected (or not) to cytokine exposure for 18 h before insulin secretion experiments, NO determination or islet viability. In some experiments, tyrosine kinase inhibitors were added 10 min before the cytokines. Islet cell viability was determined by propidium iodide and Hoechst 33342 staining [18]. Insulin secretion, insulin content and NO were measured as described in [12].
FRK/RAK RNAi (RNA interference)
Freshly isolated islets or RIN-Y504F cells [11] were transfected by the LIPOFECTAMINE™ method with siRNA against FRK/RAK using either a double-stranded DNA/RNA oligonucleotide corresponding to the sequence AAGCGACTGGGATCTGGTCAGTT (nt 1217–1239 of the mouse FRK/RAK mRNA; the sense oligonucleotide GCGACUGGGAUCUGGUCAGdTdT and the antisense oligonucleotide CUGACCAGAUCCCAGUCGCdTdT) or a scrambled siRNA oligonucleotide (CAGUCGCGUUUGCGACUGG), which in some experiments was fluorescently labelled (Fluorescein-Luciferase GL2 duplex). The oligonucleotides had been converted into their 2′-hydroxyl form, annealed, purified and desalted. The transfection mixture contained 5 μl of LIPOFECTAMINE™ and 0.84 μg of oligonucleotide in 0.2 ml of Opti-MEM, which had been preincubated for 20 min at room temperature. This mixture was then added to serum-free islets or RINm5F cells. After 3 h in Opti-MEM, RPMI 1640 medium containing serum was added, which was changed after 24 h when cytokines were added as above. Alternatively, islet transfection efficiency was evaluated at that point after trypsinization and FACS analysis (Becton–Dickinson, San Diego, CA, U.S.A.). Cell viability was determined by staining with propidium iodide after an additional 18 h, and FRK/RAK protein expression in the RIN-Y504F cells was determined by Western-blot analysis.
RESULTS
Phosphopeptide analysis of FRK/RAK autokinase activity
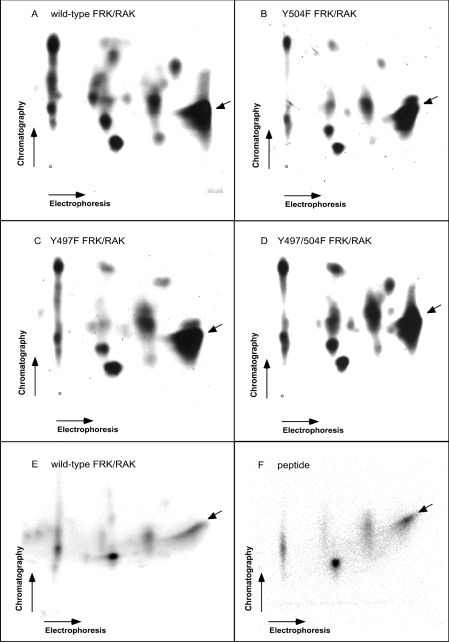
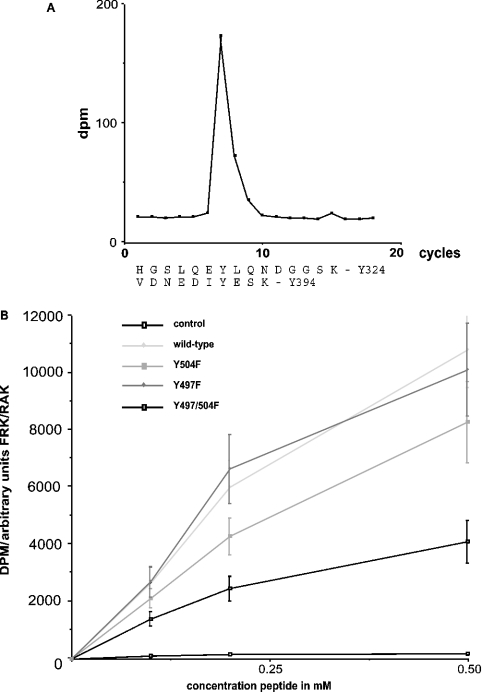
To investigate the properties of the FRK/RAK kinase, the protein and its Y497F, Y504F and Y497/504F mutants were expressed in Sf9 insect cells after baculovirus infection. Figure 1 shows the phosphopeptide map of FRK/RAK autokinase activity. The main spot was a charged peptide, which was seen in both the wild-type and the FRK/RAK mutants (Figures 1A–1D). Edman degradation of radioactive material in this spot after elution (Figure 2A) revealed radioactivity at position 7, which corresponds to two potential trypsin fragments containing tyrosine residue at position 7, Tyr-324 and Tyr-394. Tyr-416 in SRC has been established as the main autophosphorylation site [19]. On the basis of the structural resemblance between SRC and FRK/RAK, Tyr-394 is the FRK/RAK homologue to Tyr-416 in SRC. Thus our results are compatible with the view that the main autophosphorylation site in FRK/RAK is Tyr-394, sharing this feature with SRC. In addition, mutation of Tyr-394 to phenylalanine resulted in decreased FRK/RAK kinase activity when expressed in RINm5F cells [20].
Figure 1. Tryptic peptide fragments of baculovirus-produced FRK/RAK autophosphorylation sites.
Wild-type (A, E), Y504F (B), Y497F (C) and Y497/504F (D) murine FRK/RAK is shown. In (F), the phosphopeptide map of in vitro phosphorylated VDNEDIYESKHEIKKK peptide is shown, of which one position is identical with that of the wild-type FRK/RAK pattern. (E) is identical with (A), except that the experiment was performed at a different time.
Figure 2. In vitro characterization of FRK/RAK kinase.
(A) Edman degradation of the main autophosphorylation site (indicated with an arrow in Figure 1). The radioactive counts in each cycle are shown. Two tryptic fragments of murine FRK/RAK with tyrosine residue at position 7 are anticipated. (B) Kinase activity of baculovirus-produced wild-type and mutant FRK/RAK. A synthetic peptide was used as a substrate. Peptide phosphorylation values are given in dpm (disintegrations/min) after normalization for the amount of FRK/RAK kinase present in each reaction. Results are the means±S.E.M. for four independent measurements.
To verify that the main autophosphorylation site is one derived from the Tyr-394 trypsin-cleavage fragment, in vitro phosphorylation of the VDNEDIYESKHEIKKK peptide (corresponding to the sequence encompassing Tyr-394 in FRK/RAK) using baculovirus-produced FRK/RAK was performed. Phosphopeptide mapping of this in vitro phosphorylated peptide (Figures 1E and 1F) revealed several spots, which is compatible with the view that partial cleavage of the numerous lysine residues present in the peptide occurred. One of these spots was identical with the spot identified as the main FRK/RAK autophosphorylation site. This lends further support to the notion that the main autophosphorylation site in FRK/RAK is Tyr-394.
The FRK/RAK mutants displayed an identical phosphopeptide map (Figure 1) as the wild-type FRK/RAK, indicating that, in vitro, the kinase does not autophosphorylate the putative regulatory tyrosine residues in its C-terminus.
In vitro kinase activity of baculovirus-produced mutant or wild-type FRK/RAK was determined using a peptide substrate corresponding to the Tyr-394 site. Similar rates of activity were observed in the wild-type, Y497F and Y504F mutants, whereas the Y497/504F double mutant displayed somewhat lower activity (Figure 2B). This is in contrast with what was observed with FRK/RAK expressed in NIH3T3 cells [10] and RINm5F cells [11,20] and probably reflects the absence of regulatory phosphorylation of the C-terminal residues from baculovirus-produced FRK/RAK.
Cytokine-induced islet cell toxicity in FRK/RAK knockout islets
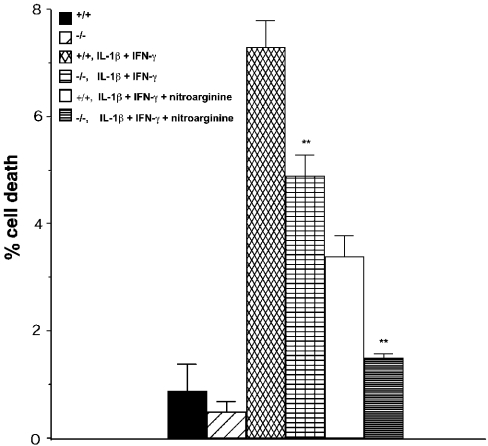
To assess directly the participation of FRK/RAK in cytokine-induced β-cell toxicity, islets were isolated from FRK/RAK −/− knockout mice or +/+ littermates. Islet cell viability after exposure to 75 units/ml IL-1β plus 1500 units/ml IFN-γ for 18 h is shown in Figure 3. Islet cell death was significantly reduced after cytokine exposure in the exposed −/− islets compared with the +/+ littermate controls. The subsequent addition of an inhibitor of NO formation, 2 mM nitroarginine, significantly reduced islet cell death. However, the difference between FRK/RAK +/+ and −/− islets remained, i.e. the latter exhibited a level of islet cell death in the combined presence of nitroarginine and cytokines that was similar to that of the islets not exposed to cytokines (Figure 3). Thus the results suggest that FRK/RAK is partly responsible for islet cell death in response to cytokines, an effect that appears not to involve NO production.
Figure 3. Islet cell death in FRK/RAK control (+/+) or knockout (−/−) islets after exposure to IL-1β (75 units/ml) + IFN-γ (1500 units/ml) for 18 h.
In two groups, the NO inhibitor nitroarginine (2 mM) was added. Results are the means±S.E.M. for six independent measurements.
Insulin secretion in response to 16.7 mM glucose was severely decreased after cytokine exposure, and no significant difference between the FRK/RAK +/+ or −/− islets was observed (Table 1). Similarly, cytokine-induced NO production was equal in the FRK/RAK +/+ and −/− islets. Thus the results suggest that FRK/RAK participates in an NO-independent manner in cytokine-induced islet cell death without influencing the suppression of insulin release.
Table 1. Insulin secretion, insulin content, NO production and DNA content of FRK/RAK +/+ and −/− islets in response to cytokine exposure for 18 h.
Values are means±S.E.M. for the number of observations given in parentheses.
| Insulin secretion | |||||
|---|---|---|---|---|---|
| 1.7 mM glucose (ng/10 islets) | 16.7 mM glucose (ng/10 islets) | Insulin content (ng/30 islets) | NO production [μmol·(25 islets)−1·(18 h)−1] | Islet DNA (ng/30 islets) | |
| FRK/RAK +/+ | 4.2±0.8 (6) | 39±8.0 (6) | 448±62 (6) | 0.4±0.1 (7) | 672±67 (4) |
| FRK/RAK +/+ 75 units/ml IL-1β+1500 units/ml IFN-γ | 8.3±2.2 (6) | 17±5.9 (6) | 360±60 (6) | 1.4±0.9 (7) | 552±96 (4) |
| FRK/RAK −/− | 3.8±0.9 (6) | 46±4.6 (6) | 663±155 (6) | 0.4±0.1 (7) | 610±148 (4) |
| FRK/RAK −/− 75 units/ml IL-1β+1500 units/ml IFN-γ | 4.8±1.0 (6) | 6.6±1.2 (6) | 419±94 (6) | 1.3±0.2 (7) | 568±132 (4) |
FRK/RAK RNAi inhibits cytokine-induced islet cell death
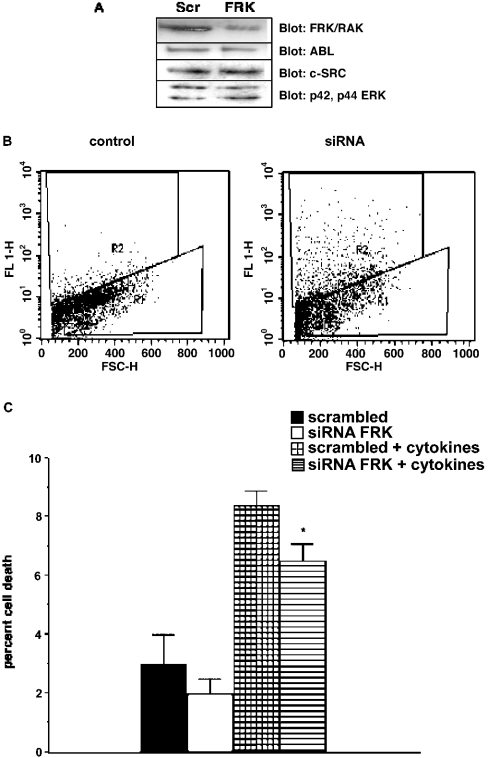
The RNAi procedure has been used to silence gene expression in insulin-producing cell lines [21,22]. When MIN6 (mouse insulinoma 6) or INS1 (insulinoma 1) insulin receptor, insulin-like growth factor-1 receptor or the plasma membrane-related Ca2+-ATPase-1 gene expression were assessed, the decrease was 91, 84 and 65–86% respectively. To assess the efficacy of RNAi silencing with respect to the decrease in FRK/RAK protein levels in our system, FRK/RAK-overexpressing RINm5F cells were transfected with siRNA against FRK/RAK (Figure 4A). This experiment was performed in RINm5F cells overexpressing FRK/RAK since FRK/RAK could not be detected by Western-blot analysis in isolated islets. FRK/RAK levels were reduced by 62±4.4% (n=3) using this procedure. The simultaneous transfection efficiency measured by uptake of a fluorescently labelled oligonucleotide was 57±4% (n=3). Probing the blot for c-Abl, c-Src and p42-p44 ERK revealed no effects of the FRK/RAK siRNA on the expression of these kinases. Next, the siRNA transfection efficiency in isolated mouse islets was assessed, since transfection of intact islets could be less efficient than that of RINm5F cells. Freshly isolated NMRI mouse islets were immediately transfected with a scrambled oligonucleotide containing a fluorescent tag, and on the next day, fluorescent cells were analysed by FACS after cell trypsinization (Figure 4B). The islets transfected with the fluorescent oligonucleotide displayed an R2 population that suggests, after subtracting the corresponding R2 value of the control cells, a transfection efficiency of 26%. This value is significantly higher than that anticipated for the corresponding DNA transfection, but agrees well with the general notion that siRNA is more efficiently transfected compared with DNA.
Figure 4. FRK/RAK knockdown by siRNA.
(A) FRK/RAK protein expression after transfection of FRK/RAK-overexpressing RINm5F cells with siRNA against murine FRK/RAK. Scrambled (Scr) oligonucleotide control transfection is shown. The blot was also probed for c-Abl, c-SRC and p42-p44 ERK to assess loading. (B) Islet transfection efficiency. Freshly isolated islets were transfected with a fluorescently scrambled siRNA oligonucleotide. On the next day, the islets were dispersed and analysed by FACS. A population of cells with increased fluorescence is indicated by R2. R1 indicates the viable untransfected cell population. (C) Islet cell viability with or without cytokine addition (50 units/ml IL-1β +1000 units/ml IFN-γ) after transfection with murine FRK/RAK RNAi. Results are the means±S.E.M. for three independent experiments. *P<0.05 with a paired Student's t test.
Finally, freshly isolated islets were transfected with siRNA against FRK/RAK, and cytokine-induced cell death was determined 2 days later (Figure 4C). Cytokine-induced islet cell death was significantly reduced after transfection with siRNA for FRK/RAK.
Inhibition of FRK/RAK kinase activity by tyrosine kinase inhibitors
To determine the characteristics of FRK/RAK autokinase activity, in vitro kinase reactions were performed on baculovirus-produced FRK/RAK. Different known tyrosine kinase inhibitors were added at three concentrations each, after which FRK/RAK autophosphorylation was determined. Approximate IC50 values for the inhibition of kinase activity were calculated and are shown in Table 2. Most inhibitors were not effective in inhibiting FRK/RAK. However, a few inhibitors show a profile of inhibition of FRK/RAK that is comparable with the known target tyrosine kinases (also listed in Table 1) of the corresponding inhibitors. Those that inhibited FRK/RAK kinase activity with a potency comparable with the reported inhibitory property were AG30 [IC50 FRK/RAK=0.1 mM, IC50 EGFR (epidermal growth factor receptor)=0.1 mM], AG1024 (IC50 FRK/RAK=15 μM, IC50 insulin R=10 μM), AG1872 (IC50 FRK/RAK=200 nM, IC50 SRC=100 nM), PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) (IC50 FRK/RAK=20 nM, IC50 SRC=5 nM), SU4984 (IC50 FRK/RAK=15 μM, IC50 fibroblast growth factor receptor 1=10 μM), D-65495 [IC50 FRK/RAK=100 nM, IC50 PDGFR (platelet-derived growth factor receptor)=100 nM] and D-68129 (IC50 FRK/RAK=100 nM, IC50 PDGFR=100 nM). Thus a number of tyrosine kinase inhibitors have been identified that will inhibit FRK/RAK.
Table 2. Characteristics of FRK/RAK autokinase properties in response to different inhibitors of tyrosine kinases.
FGFR, fibroblast growth factor receptor; JAK2, Janus kinase 2; VEGFR, vascular endothelial growth factor receptor.
| Mouse FRK/RAK IC50 (autokinase activity) | Reported inhibitory property (IC50) | Reference | |
|---|---|---|---|
| AG30 | 0.1 mM | EGFR=0.1 mM | [31,32] |
| AG370 | >50 nM | PDGFR=50 nM | [33] |
| AG490 | 0.1 mM | JAK2=100 nM | [34,35] |
| AG879 | >30 μM | TrkA=10 μM | [36] |
| AG957 | >3 μM | Bcr-Abl=1 μM | [37,38] |
| AG1024 | 15 μM | Insulin R=10 μM | [39] |
| AG1295 | >2 μM | PDGFR=1 μM | [40,41] |
| AG1296 | 0.1 mM | PDGFR=0.5 μM | [42] |
| AG1478 | >10 nM | EGFR=10 nM | [43,44] |
| AG1872 (PP1) | 0.2 μM | SRC=0.1 μM | [45] |
| Genistein | 10 mM | EGFR=20 μM | [46] |
| PP2 | 20 nM | SRC=5 nM | [45,47] |
| SU1498 | >3 mM | VEGFR-2=0.7 μM | [48,49] |
| SU4984 | 15 μM | FGFR-1=10 μM | [50] |
| D-65495 | 0.1 μM | PDGFR=0.1 μM | [14] |
| D-68129 | 0.1 μM | PDGFR=0.1 μM | [14] |
Effects of FRK/RAK kinase inhibitors on cytokine-induced islet cell cytotoxicity
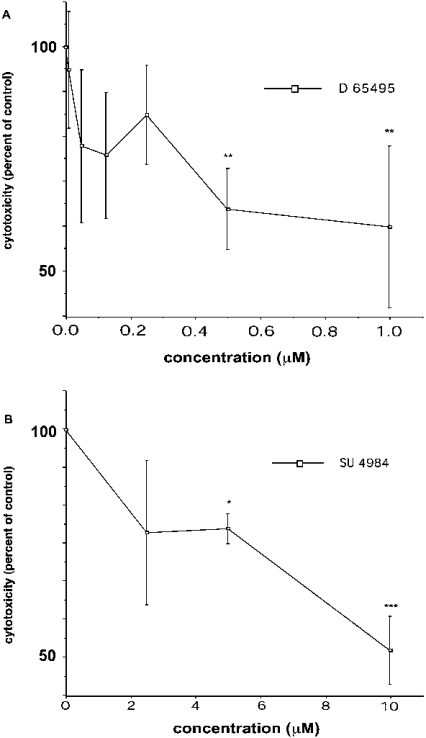
Among the identified tyrosine kinase inhibitors that would relatively potently inhibit FRK/RAK, AG1024 was considered unsuitable for further studies, since the insulin receptor has been shown to be essential for β-cell function [23]. When testing the others, the known SRC family inhibitors AG1872 and PP2, as well as the EGFR inhibitor AG30, were found to aggravate islet cell death under basal conditions and in response to cytokines (results not shown). However, D-65495 and SU4984 consistently inhibited cytokine-induced islet cell death in a dose-dependent manner (Figures 5A and 5B). This inhibitory effect occurred without concomitant effects on insulin secretion or NO production (results not shown). Thus certain inhibitors of FRK/RAK activity also counteract cytokine-induced islet cell death.
Figure 5. Dose–response of (A) D-65495 and (B) SU 4984 with respect to islet cell viability.
(A) Control islets were incubated with IL-1β (50 units/ml) +IFN-γ (1000 units/ml) for 18 h. D-65495 was added 30 min before cytokines in some groups at different concentrations. Islet cell viability was determined, and results are expressed as percentage death in relation to cytokine addition alone. (B) The same conditions as in (A) were used. Results are the means±S.E.M. for 4–8 independent measurements. *P<0.05, **P<0.01 and ***P<0.001 respectively for a paired Student's t test.
DISCUSSION
The aim of the present study was to assess to what extent the cytoplasmic tyrosine kinase FRK/RAK participates in cytokine-induced β-cell destruction. In a previous study, in which kinase-active FRK/RAK (also named GTK/Bsk/IYK) was expressed in β-cells in a transgenic mouse, increased susceptibility to cytokines with respect to β-cell death was observed [12]. For this reason, we proposed that FRK/RAK was a signalling component that participates in β-cell destruction in Type I diabetes. This disease is characterized by an early infiltration of tissue macrophages in the vicinity of islets [2]. The cause of macrophage infiltration has not been established, but it could be the consequence of an early insult to the β-cell that initiates an inflammatory reaction. The macrophages synthesize and release inflammatory cytokines, such as IL-1β, IFN-γ and tumour necrosis factor α [3]. These cytokines are known to cause β-cell necrosis and apoptosis [4] and this effect is partly mediated by NO production [24,25]. However, an NO-independent pathway for cytokine-induced β-cell death has also been suggested [26]. Further destruction of β-cells at this stage will probably aggravate and perpetuate the progression of disease, and eventually, the inflammation reaches a level of intensity that causes massive β-cell destruction after the activation of infiltrating cytotoxic T lymphocytes [5]. The duration of this process in humans may extend over several years. Any intervention that disrupts this vicious cycle of β-cell destruction and activation of the immune response may prevent the progression of disease.
Our current results with the FRK/RAK knockout mouse islets confirm our notion that FRK/RAK is a tyrosine kinase involved in β-cell destruction in response to cytotoxic cytokines. In addition, this effect appears not to involve NO production and, consequently, does not cause suppression of insulin secretion in response to the cytokines. Thus cytokines appear to signal through two pathways, one involving nuclear factor κB and NO production, and a second occurring via FRK/RAK. Whereas the former causes both suppression of insulin secretion and β-cell death, the latter only exerts cytotoxic effects.
Despite numerous attempts, we have not been able to characterize the conditions that regulate FRK/RAK kinase activity. Analogy with other SRC family members would suggest that FRK/RAK is activated by its binding to other proteins via its SH2 (Src homology 2) or SH3 domains [27]. So far, we have only identified one protein that associates with FRK/RAK and this is the SHB (SH2 protein in β-cells) adapter protein [20]. Curiously, SHB overexpression also causes enhanced cytokine-induced islet cell death [28] and, thus, the FRK/RAK–SHB signalling complex may be a key sensor of cytotoxic signals in β-cells. Events downstream of this complex in the cytotoxic pathway are so far unknown.
In addition to regulation of FRK/RAK kinase activity by association with signalling partners, Tyr-497 and Tyr-504 play a role in regulating the activity and properties of FRK/RAK [10,11]. In the present study, we show that these tyrosine residues are not autophosphorylation sites of FRK/RAK by two-dimensional phosphopeptide mapping. Instead, the results are compatible with the view that the major autophosphorylation site is Tyr-394, which by analogy with SRC is a positive regulator of kinase activity. FRK/RAK produced in Sf9 insect cells displays similar kinase activity irrespective of whether Tyr-497 and Tyr-504 are mutated or not. In contrast, when FRK/RAK-Y504F is expressed in RINm5F cells, its kinase activity is increased. Thus this residue does not become phosphorylated in the Sf9 cells and, consequently, fails to exert negative regulation of kinase activity unlike in the RINm5F cells. The kinase(s) responsible for phosphorylation of Tyr-497 and Tyr-504 in FRK/RAK remain(s) elusive. The Y497/504F double mutant exhibited decreased kinase activity in the Sf9 cells, suggesting a structural perturbation of FRK/RAK in this case.
Our screening of the known tyrosine kinase inhibitors revealed several chemical compounds that cause inhibition of FRK/RAK activity with relatively high specificity. Two were known inhibitors of SRC family of kinases and the others were inhibitors of receptor tyrosine kinases. When FRK/RAK was originally cloned, it was noted that in the kinase domain VI, FRK/RAK has the sequence DLAARN, which is conserved among tyrosine kinase receptors. Such a structural relationship may explain the finding that receptor tyrosine kinase inhibitors also inhibit FRK/RAK activity. Of the inhibitors tested, only SU4984 and D-65495 consistently inhibited cytokine-induced islet cell death. The others either aggravated the toxic response or had no effect. Particularly, the SRC family inhibitors were noxious to the islet cells. Thus it appears that inhibition of other kinases in islet cells, particularly SRC family members other than FRK/RAK, will exacerbate the toxic response. The failure of D-68129 to prevent the toxic response to cytokines at first appeared intriguing, since its inhibitory profile is very similar to that of D-65495. However, D-68129 is less soluble compared with D-65495 and this difference may explain the lower potency in cell culture.
The results obtained with the inhibitors do not exclude the possibility that a part of their effect relates to inhibition of tyrosine kinases other than FRK/RAK. This may be particularly true for SU4984, which decreased islet cell death to a larger extent than what was seen in the FRK/RAK knockout islets. However, contribution of FRK/RAK to this inhibitory effect is quite probable. The relatively non-specific tyrosine kinase inhibitors genistein, herbimycin A and tyrphostin have all been shown previously to reduce cytokine-induced nitrite formation and iNOS (inducible nitric oxide synthase) gene expression, but their effects on islet cell viability have not been investigated [29,30].
Taken as a whole, our study suggests that FRK/RAK plays a role in cytokine-induced islet cell death and specific inhibition of this kinase may alleviate the toxic response to cytokines. Finding a specific inhibitor of FRK/RAK that could be administered in vivo would thus provide the pharmacological means to intervene in the progression of Type I diabetes. The FRK/RAK knockout indicates that this gene is not essential for mouse viability. Thus pharmacological intervention under early phases of the development/progression of disease, which reduces β-cell death, could have an impact on the ensuing immune response in such a manner that disease progression is halted.
Acknowledgments
We are grateful to Dr Johan Sundelin and Dr Frank Böhmer for numerous comments and suggestions and for providing tyrosine kinase inhibitors and Dr Åke Engström for Edman degradation. The technical support from Ing-Britt Hallgren and Ing-Marie Mörsare is gratefully acknowledged. This work was supported by grants from Juvenile Diabetes Research Foundation, the Swedish Medical Research Council (31X-10822), the Swedish Diabetes Association, the Family Ernfors Fond and Innoventus Project AB.
References
- 1.Freiesleben De Blasio B., Bak P., Pociot F., Karlsen A. E., Nerup J. Onset of type 1 diabetes: a dynamical instability. Diabetes. 1999;48:1677–1685. doi: 10.2337/diabetes.48.9.1677. [DOI] [PubMed] [Google Scholar]
- 2.Rothe H., Kolb H. The APC1 concept of type I diabetes. Autoimmunity. 1998;27:179–184. doi: 10.3109/08916939809003865. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 1998;14:129–151. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Mandrup-Poulsen T. β-Cell apoptosis: stimuli and signaling. Diabetes. 2001;50(Suppl. 1):S58–S63. doi: 10.2337/diabetes.50.2007.s58. [DOI] [PubMed] [Google Scholar]
- 5.Petrovsky N., Schatz D. A. The immunology of human type 1 diabetes. In: Pickup J. C., Williams G., editors. Textbook of Diabetes, vol. 1. Malden, MA: Blackwell; 2003. pp. 18.1–18.14. [Google Scholar]
- 6.Cance W. G., Craven R. J., Bergman M., Xu L., Alitalo K., Liu E. T. Rak, a novel nuclear tyrosine kinase expressed in epithelial cells. Cell Growth Differ. 1994;5:1347–1355. [PubMed] [Google Scholar]
- 7.Lee J., Wang Z., Luoh S. M., Wood W. I., Scadden D. T. Cloning of FRK, a novel human intracellular SRC-like tyrosine kinase-encoding gene. Gene. 1994;138:247–251. doi: 10.1016/0378-1119(94)90817-6. [DOI] [PubMed] [Google Scholar]
- 8.Oberg-Welsh C., Welsh M. Cloning of BSK, a murine FRK homologue with a specific pattern of tissue distribution. Gene. 1995;152:239–242. doi: 10.1016/0378-1119(94)00718-8. [DOI] [PubMed] [Google Scholar]
- 9.Thuveson M., Albrecht D., Zurcher G., Andres A. C., Ziemiecki A. Iyk, a novel intracellular protein tyrosine kinase differentially expressed in the mouse mammary gland and intestine. Biochem. Biophys. Res. Commun. 1995;209:582–589. doi: 10.1006/bbrc.1995.1540. [DOI] [PubMed] [Google Scholar]
- 10.Oberg-Welsh C., Anneren C., Welsh M. Mutation of C-terminal tyrosine residues Y497/Y504 of the Src-family member Bsk/Iyk decreases NIH3T3 cell proliferation. Growth Factors. 1998;16:111–124. doi: 10.3109/08977199809002122. [DOI] [PubMed] [Google Scholar]
- 11.Anneren C., Welsh M. Role of the Bsk/Iyk non-receptor tyrosine kinase for the control of growth and hormone production in RINm5F cells. Growth Factors. 2000;17:233–247. doi: 10.3109/08977190009028969. [DOI] [PubMed] [Google Scholar]
- 12.Anneren C., Welsh M. Increased cytokine-induced cytotoxicity of pancreatic islet cells from transgenic mice expressing the Src-like tyrosine kinase GTK. Mol. Med. 2001;7:301–310. [PMC free article] [PubMed] [Google Scholar]
- 13.Anneren C. Dual role of the tyrosine kinase GTK and the adaptor protein SHB in β-cell growth: enhanced β-cell replication after 60% pancreatectomy and increased sensitivity to streptozotocin. J. Endocrinol. 2002;172:145–153. doi: 10.1677/joe.0.1720145. [DOI] [PubMed] [Google Scholar]
- 14.Mahboobi S., Teller S., Pongratz H., Hufsky H., Sellmer A., Botzki A., Uecker A., Beckers T., Baasner S., Schachtele C., et al. Bis(1H-2-indolyl)methanones as a novel class of inhibitors of the platelet-derived growth factor receptor kinase. J. Med. Chem. 2002;45:1002–1018. doi: 10.1021/jm010988n. [DOI] [PubMed] [Google Scholar]
- 15.Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 16.Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc. Natl. Acad. Sci. U.S.A. 1987;84:6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrasekharan S., Qiu T. H., Alkharouf N., Brantley K., Mitchell J. B., Liu E. T. Characterization of mice deficient in the Src family nonreceptor tyrosine kinase Frk/Rak. Mol. Cell. Biol. 2002;22:5235–5247. doi: 10.1128/MCB.22.14.5235-5247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saldeen J. Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology. 2000;141:2003–2010. doi: 10.1210/endo.141.6.7523. [DOI] [PubMed] [Google Scholar]
- 19.Kmiecik T. E., Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell (Cambridge, Mass.) 1987;49:65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- 20.Anneren C., Welsh M. GTK tyrosine kinase-induced alteration of IRS-protein signalling in insulin producing cells. Mol. Med. 2002;8:705–713. [PMC free article] [PubMed] [Google Scholar]
- 21.Da Silva Xavier G., Qian Q., Cullen P. J., Rutter G. A. Distinct roles for insulin and insulin-like growth factor-1 receptors in pancreatic β-cell glucose sensing revealed by RNA silencing. Biochem. J. 2004;377:149–158. doi: 10.1042/BJ20031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell K. J., Tsuboi T., Rutter G. A. Role for plasma membrane-related Ca2+-ATPase-1 (ATP2C1) in pancreatic β-cell Ca2+ homeostasis revealed by RNA silencing. Diabetes. 2004;53:393–400. doi: 10.2337/diabetes.53.2.393. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni R. N., Bruning J. C., Winnay J. N., Postic C., Magnuson M. A., Kahn C. R. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell (Cambridge, Mass.) 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 24.Corbett J. A., McDaniel M. L. Does nitric oxide mediate autoimmune destruction of β-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992;41:897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- 25.Eizirik D. L., Flodstrom M., Karlsen A. E., Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic β cells. Diabetologia. 1996;39:875–890. doi: 10.1007/BF00403906. [DOI] [PubMed] [Google Scholar]
- 26.Liu D., Pavlovic D., Chen M. C., Flodstrom M., Sandler S., Eizirik D. L. Cytokines induce apoptosis in β-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−) Diabetes. 2000;49:1116–1122. doi: 10.2337/diabetes.49.7.1116. [DOI] [PubMed] [Google Scholar]
- 27.Superti-Furga G., Gonfloni S. A crystal milestone: the structure of regulated Src. Bioessays. 1997;19:447–450. doi: 10.1002/bies.950190602. [DOI] [PubMed] [Google Scholar]
- 28.Welsh M., Christmansson L., Karlsson T., Sandler S., Welsh N. Transgenic mice expressing Shb adaptor protein under the control of rat insulin promoter exhibit altered viability of pancreatic islet cells. Mol. Med. 1999;5:169–180. [PMC free article] [PubMed] [Google Scholar]
- 29.Welsh N. A role for tyrosine kinase activation in interleukin-1 β induced nitric oxide production in the insulin producing cell line RINm-5F. Biosci. Rep. 1994;14:43–50. doi: 10.1007/BF01901637. [DOI] [PubMed] [Google Scholar]
- 30.Corbett J. A., Kwon G., Marino M. H., Rodi C. P., Sullivan P. M., Turk J., McDaniel M. L. Tyrosine kinase inhibitors prevent cytokine-induced expression of iNOS and COX-2 by human islets. Am. J. Physiol. 1996;270:C1581–C1587. doi: 10.1152/ajpcell.1996.270.6.C1581. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz B., Lamprecht S. A., Polak-Charcon S., Niv Y., Kim Y. S. Induction of the differentiated phenotype in human colon cancer cell is associated with the attenuation of subcellular tyrosine phosphorylation. Oncol. Res. 1995;7:277–287. [PubMed] [Google Scholar]
- 32.Wessely O., Mellitzer G., von Lindern M., Levitzki A., Gazit A., Ischenko I., Hayman M. J., Beug H. Distinct roles of the receptor tyrosine kinases c-ErbB and c-Kit in regulating the balance between erythroid cell proliferation and differentiation. Cell Growth Differ. 1997;8:481–493. [PubMed] [Google Scholar]
- 33.Bryckaert M. C., Eldor A., Fontenay M., Gazit A., Osherov N., Gilon C., Levitzki A., Tobelem G. Inhibition of platelet-derived growth factor-induced mitogenesis and tyrosine kinase activity in cultured bone marrow fibroblasts by tyrphostins. Exp. Cell Res. 1992;199:255–261. doi: 10.1016/0014-4827(92)90432-8. [DOI] [PubMed] [Google Scholar]
- 34.Meydan N., Grunberger T., Dadi H., Shahar M., Arpaia E., Lapidot Z., Leeder J. S., Freedman M., Cohen A., Gazit A., et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature (London) 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen M., Kaltoft K., Nordahl M., Ropke C., Geisler C., Mustelin T., Dobson P., Svejgaard A., Odum N. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohmichi M., Pang L., Ribon V., Gazit A., Levitzki A., Saltiel A. R. The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry. 1993;32:4650–4658. doi: 10.1021/bi00068a024. [DOI] [PubMed] [Google Scholar]
- 37.Anafi M., Gazit A., Gilon C., Ben-Neriah Y., Levitzki A. Selective interactions of transforming and normal abl proteins with ATP, tyrosine-copolymer substrates, and tyrphostins. J. Biol. Chem. 1992;267:4518–4523. [PubMed] [Google Scholar]
- 38.Kaur G., Sausville E. A. Altered physical state of p210bcr-abl in tyrphostin AG957-treated K562 cells. Anticancer Drugs. 1996;7:815–824. doi: 10.1097/00001813-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Parrizas M., Gazit A., Levitzki A., Wertheimer E., LeRoith D. Specific inhibition of insulin-like growth factor-1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins. Endocrinology. 1997;138:1427–1433. doi: 10.1210/endo.138.4.5092. [DOI] [PubMed] [Google Scholar]
- 40.Kovalenko M., Gazit A., Bohmer A., Rorsman C., Ronnstrand L., Heldin C. H., Waltenberger J., Bohmer F. D., Levitzki A. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- 41.Banai S., Wolf Y., Golomb G., Pearle A., Waltenberger J., Fishbein I., Schneider A., Gazit A., Perez L., Huber R., et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97:1960–1969. doi: 10.1161/01.cir.97.19.1960. [DOI] [PubMed] [Google Scholar]
- 42.Kovalenko M., Ronnstrand L., Heldin C. H., Loubtchenkov M., Gazit A., Levitzki A., Bohmer F. D. Phosphorylation site-specific inhibition of platelet-derived growth factor β-receptor autophosphorylation by the receptor blocking tyrphostin AG1296. Biochemistry. 1997;36:6260–6269. doi: 10.1021/bi962553l. [DOI] [PubMed] [Google Scholar]
- 43.Eguchi S., Numaguchi K., Iwasaki H., Matsumoto T., Yamakawa T., Utsunomiya H., Motley E. D., Kawakatsu H., Owada K. M., Hirata Y., et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 44.Liu W., Akhand A. A., Kato M., Yokoyama I., Miyata T., Kurokawa K., Uchida K., Nakashima I. 4-Hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J. Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 45.Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 46.Constantinou A., Huberman E. Genistein as an inducer of tumor cell differentiation: possible mechanisms of action. Proc. Soc. Exp. Biol. Med. 1995;208:109–115. doi: 10.3181/00379727-208-43841. [DOI] [PubMed] [Google Scholar]
- 47.Salazar E. P., Rozengurt E. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J. Biol. Chem. 1999;274:28371–28378. doi: 10.1074/jbc.274.40.28371. [DOI] [PubMed] [Google Scholar]
- 48.Strawn L. M., McMahon G., App H., Schreck R., Kuchler W. R., Longhi M. P., Hui T. H., Tang C., Levitzki A., Gazit A., et al. Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996;56:3540–3545. [PubMed] [Google Scholar]
- 49.Arbiser J. L., Larsson H., Claesson-Welsh L., Bai X., LaMontagne K., Weiss S. W., Soker S., Flynn E., Brown L. F. Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo. Am. J. Pathol. 2000;156:1469–1476. doi: 10.1016/S0002-9440(10)65015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]