Abstract
Chondroitin sulphate (CS) is a glycosaminoglycan widely distributed in animal tissues, which has anti-inflammatory and chondroprotective properties. We reported previously that chondroitin 4-sulphate (CS-A) up-regulates the antigen-specific Th1 immune response of murine splenocytes sensitized with ovalbumin in vitro, and that CS suppresses the antigen-specific IgE responses. We now demonstrate that a specific sulphation pattern of the CS polysaccharide is required for the Th1-promoted activity, as other polysaccharides such as dextran and dextran sulphate do not significantly induce this activity. While the presence of some O-sulpho groups appear to be essential for activity, CS-A, and synthetically prepared, partially O-sulphonated CS, induce higher Th1-promoted activity than synthetically prepared, fully O-sulphonated CS. CS-A induces an activity greater than chondroitin sulphate B (CS-B) or chondroitin 6-sulphate (CS-C). In addition, chondroitin sulphate E (CS-E) induces greater activity than CS-A or CS-D. These results suggest that the GlcA(β1-3)GalNAc(4,6-O-disulpho) sequence in CS-E is important for Th1-promoted activity. Furthermore, rat anti-mouse CD62L antibody, an antibody to L-selectin, inhibits the Th1-promoting activity of CS. These results suggest that the Th1-promoted activity could be associated with L-selectin on lymphocytes. These findings describe a new mechanism for the anti-inflammatory and chondroprotective properties of CS that may be useful in designing new therapeutic applications for CS used in the treatment of immediate-type hypersensitivity.
Keywords: chondroitin sulphate (CS), immunological activity, L-selectin, splenocyte, Th1
Abbreviations: 1D, one-dimensional; 2D, two-dimensional; CS, chondroitin sulphate; DS, dermatan sulphate; DX, dextran; DXS, dextran sulphate; FBS, fetal bovine serum; FSC, forward scatter; GAG, glycosaminoglycan; IdoA, iduronic acid; IFN, interferon; IL, interleukin; OVA, ovalbumin; SAR, structure–activity relationship; SSC, side scatter; TQF, triple quantum filtered
INTRODUCTION
Chondroitin sulphate (CS) is a family of structurally complex, sulphated, linear polysaccharides called glycosaminoglycans (GAGs). CS is composed of a repeating disaccharide unit of the structure, [-4)GlcA(β1-3)GalNAc(β1-]n, where GlcA is glucuronic acid and GalNAc is N-acetylgalactosamine [1]. CS can be substituted with O-sulpho groups at a variety of positions [2–4], it is an integral component of the proteoglycans found localized on cell surfaces and in the extracellular matrix, and is important for cell–cell communications [2,5–6]. Recently, there have been a number of reports on the biochemical activities of orally administrated exogenous CS, including its anti-inflammatory and chondroprotective properties [5–12]. It has been proposed that these activities result from an increase in the biosynthesis of connective tissue components, such as hyaluronan, at disease sites [13,14]. Both in vitro and in vivo studies have shown that CS regulates the formation of new cartilage by stimulating the chondrocyte synthesis of collagen, proteoglycans and hyaluronan [15,16].
Polysaccharides such as CS are poorly absorbed through the digestive system [17,18]. Moreover, we have shown that the half-life of CS in the circulatory system is 3 to 15 min, based on the pharmacokinetic study of intravenously administrated CS [19]. Accordingly, it appears unlikely that orally administered CS is systemically distributed to connective tissues such as cartilage and skin, and that exogenously administered CS actually directly stimulates chondrocyte synthesis of extracellular matrix components. This suggests that the mechanism of action of exogenously administrated CS might be mediated by other systems, such as the immunological system [20]. Our laboratory has already shown that CS up-regulates the in vitro antigen-specific Th1 immune response on murine splenocytes sensitized with ovalbumin (OVA), and that CS suppresses the antigen-specific IgE responses [21]. These findings suggest a therapeutic use of CS to control the IgE-mediated allergic response.
The number and position of O-sulpho groups varies among CS samples obtained from different sources [22–24]. We hypothesize that the immunological activity of CS might also be different among the several types of CS. It is important to determine the structure–activity relationship (SAR) of CS, particularly with respect to the number and position of O-sulpho groups in CS. Other related polysaccharides and modified polysaccharides have been examined as heparin analogues in the development of new drugs [25,26]. Knowledge of the SAR of CS will be necessary to further explore its effective use as a therapeutic agent.
The present study extends our previous work and examines the structural requirements for the immunological activity of CS and suggests the possibility of its association with receptors on lymphocytes. We report here the structural characterization of a CS that exhibits potent immunological activity.
EXPERIMENTAL
Animals
Inbred specific pathogen-free BALB/c mice (female, 6-weeks-old) were purchased from Charles River Japan (Yokohama, Japan). The mice were maintained in a temperature-controlled and light-controlled environment with free access to a sterile diet and water. They were acclimatized for at least a week before the study.
Reagents
CS-A prepared from bovine tracheal cartilage and having an average molecular weight of 15 kDa, was a gift from Shin-Nippon Yakugyo Co. (Tokyo, Japan). CS-C was kindly provided by San-Ei Gen FFI Co. (Osaka, Japan). CS-B, CS-D and CS-E were purchased from Seikagaku Kogyo Co. (Tokyo, Japan). OVA (chicken egg) was purchased from Sigma. RPMI 1640 medium was purchased from ICN Biomedicals (Aurora, OH, U.S.A.). Fetal bovine serum (FBS) was purchased from Sanko Junyaku (Tokyo, Japan), and heat-inactivated at 56 °C for 30 min prior to use. FITC-labelled rat anti-mouse CD62L monoclonal antibody (MEL-14) and phycoerythrin-labelled rat anti-mouse CD62P monoclonal antibody (Y-18) were purchased from Pharmingen (San Diego, CA, U.S.A.). Rat anti-mouse CD62L monoclonal antibody (MEL-14) was purchased from Chemicon International (Temecula, CA, U.S.A.). Rat anti-mouse CD62P monoclonal antibody (Y-18) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
Sensitization protocols
The mice (n=5) were intraperitoneally sensitized with 20 μg of OVA and 2 mg of an aluminium hydrate adjuvant {ALUM [Al (OH)3]; LSL Co., Japan} in a total volume of 400 μl of saline. Two weeks after the first sensitization, the mice were given a booster using the same doses of the antigen. On the following day, the mice were humanely killed and their spleens were harvested.
Preparation and stimulation of murine splenocytes in vitro
Mice were killed by cervical dislocation, their spleens were aseptically removed and cell suspensions made by passing them through a sterile cell strainer (Becton Dickinson, CA, U.S.A.). The cell suspensions were washed twice in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 units/ml penicillin, 50 μg/ml streptomycin, and 25 nM 2-mercaptoethanol, counted by Trypan Blue exclusion staining, and adjusted to 1×107 cells/ml. The cells were plated at 2 ml/well in a 24-well cell culture cluster (Corning, NY, U.S.A.) and challenged with OVA at a final concentration of 100 μg/ml. Furthermore, the samples (each at a final concentration of 1 μg/ml), or saline in vitro were added to the 24-well culture cluster at 37 °C in triplicate in a CO2 incubator for 3 days. The supernatants were then harvested for cytokine production analysis after 3 days of incubation.
Production of cytokine in vitro
The cytokines examined in this study were interferon (IFN)-γ, interleukin (IL)-2, IL-5 and IL-10. The amount of several cytokines in the culture medium after incubation was measured using an ELISA kit (OptEIA™ set, Pharmingen, San Diego, CA, U.S.A.). The principle of all ELISA kits was to employ the quantitative sandwich enzyme immunoassay technique. Absorbance was measured at 450 nm (E max, precision microplate reader, Molecular Devices, Sunnyvale, CA, U.S.A.).
Chemical preparation of CS with different contents of O-sulpho groups
The fully O-sulphonated CS, partially O-sulphonated CS and desulphonated CS were synthesized as described previously [27,28,29]. Briefly, chemical O-sulphonation was carried out under mild conditions with adducts of sulphur trioxide (SO3) in aprotic solvents to obtain fully sulphated CS and partially sulphated CS. Fully sulphated CS was prepared from the tributylammonium salt, obtained from 100 mg of CS-A sodium salt by strong cationexchange chromatography, and concentration by freeze-drying. The resulting salt was dissolved in 0.8 ml of N,N-dimethylformamide (DMF) to which a required excess (15 mol/equivalent of available hydroxyl group in CS) of the pyridine-SO3 complex had been added. After 1 h at 40 °C (0 °C in the case of partially sulphated CS), the reaction was terminated through the addition of 1.6 ml of water, and the crude product was precipitated with 3 volumes of cold ethanol saturated with anhydrous sodium acetate, and then collected by centrifugation. The resulting fully sulphated CS and partially sulphated CS were dissolved in water, dialysed to remove the salts, and freeze-dried.
1H-NMR spectroscopy was performed using the conditions described previously by Toida et al. [3]. The sample (approx. 1–3 mg) was kept in a desiccator over phosphorus pentoxide in vacuo overnight at 22 °C. The thoroughly dried sample was then dissolved in 500 μl of 2H2O (100%; Aldrich Japan, Tokyo, Japan), and passed through a 0.45 μm syringe filter and transferred to an NMR tube. One-dimensional (1D) and two-dimensional (2D) NMR experiments were performed using a JEOL α500 MHz spectrometer equipped with a 5 mm field gradient tunable probe with standard JEOL software at 60 °C, for all the experiments with 500 μl samples. The 2H2O signal was suppressed by presaturation during 3 s or 1.5 s for the 1D or 2D spectra respectively. To obtain the 2D spectra, 512 experiments resulting in 1024 data points for a spectral width of 2000 Hz were measured, and the time domain data were multiplied after zero filling (data matrix size, 1K×1K) with a shifted sine-bell window functions for 2D double quantum filtered (DQF) and triple quantum filtered (TQF)-COSY, NOESY or TOCSY experiments. An MLEV-17 mixing sequence of 100 ms was used for the 2D TOCSY experiments, and the NOESY experiments, by using 150, 250 and 500 ms as the mixing time.
FACS analysis of cultured splenocytes
Splenocytes (5.0×106 cells/ml) were co-cultured with OVA (final concentration of 100 μg/ml) and/or the rat anti-mouse CD62L antibody (final concentration of 2 μg/ml) at 37 °C in a CO2 incubator for 3 days. The cultured splenocytes were washed twice in PBS containing 2% FBS and then cell-staining analysis was carried out in the following way. Briefly, 100 μl of cultured splenocytes at 2.0×106 cells/ml, in PBS containing 2% FBS and 1 μl of anti-CD16/CD32 monoclonal antibody, were reacted for 5 min at 4 °C. FITC-labelled anti-CD62L antibodies at 0.125 μg in 10 μl (this is the optimal concentration of labelled antibodies as determined by preliminary experiments) were added to the above tubes and mixed, then incubated for 30 min at 4 °C in the dark. After staining, the cells were washed three times with PBS. After washing, the cells were poured into FACS tubes in PBS containing 2% FBS and 0.1% NaN3 in a total volume of 500 μl.
Flow cytometric analysis was performed with FACSCalibur™ flow cytometer (Becton Dickinson, CA, U.S.A.) equipped with a 488 nm argon laser and detectors for forward scatter (FSC), 90° light scatter (side scatter, SSC), and FL1 (band pass filter wavelength 530 nm) and FL2 (585 nm) fluorescence emission in the green and red/orange parts of the spectrum respectively. Splenocytes were identified by their characteristic appearance on a dot plot of FSC versus SSC and electronically gated to exclude platelets, red cells or cell debris. The results were indicated as the percentage of positive cells within a gate.
Disaccharide and compositional analysis
The determination of unsaturated disaccharides, prepared from various CS and CS structural variants, was performed on the lyasedigested samples using HPLC [30,31].
Statistical analysis
Differences between the mean values were analysed by an unpaired Student's t test. Comparisons among groups were made with a factorial ANOVA. In all cases, significance was set as P<0.05.
RESULTS
Dose response of CS on in vitro cytokine production by splenocytes
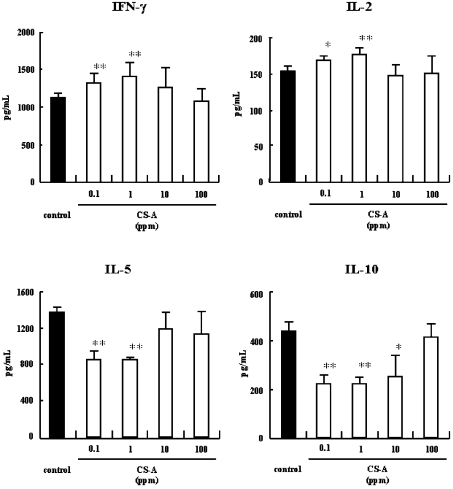
We had reported previously that CS-A promoted the secretion of Th1-type cytokines (IFN-γ, IL-2, and IL-12) but suppressed the secretion of Th2-type cytokines (IL-5 and IL-10) secretion by OVA-sensitized splenocytes [21]. To determine the concentration of CS-A giving maximal response, we examined the dose response of CS-A on in vitro cytokine production by splenocytes. As shown in Figure 1, of the four concentrations tested (0.1, 1, 10 and 100 μg/ml), the Th1-promoting activity of CS-A was the greatest at 1 μg/ml. The Th2-inhibitory activity of CS-A also appeared to be the greatest at 1 μg/ml. Higher concentrations of CS-A, such as 10 or 100 μg/ml, never showed any improvement in activity over that with 1 μg/ml. Therefore, we compared the activities of various CS preparations at 1 μg/ml.
Figure 1. Dose response of CS on the cytokine production of murine splenocyte in vitro.
BALB/c mice (n=5) were intraperitoneally injected on day 0 and day 13 with 20 μg of OVA and 2 mg of Al(OH)3 at a total volume of 400 μl. Splenocytes (5.0×106 cells/ml) were collected on day 14 and were co-cultured with OVA (final concentration 100 μg/ml). The amounts of cytokines in the supernatant were measured by ELISA. *P<0.05, **P<0.01 (significantly different from control value). Error bars represent means±S.D. for 6 wells.
Effects of polysaccharide sulphation on in vitro cytokine productions by splenocytes
The influence of the presence of polysaccharide sulphation on the cytokine production of the OVA-sensitized splenocytes, following in vitro re-stimulation, was examined using dextran (DX) as a neutral polysaccharide. CS-A, DX and dextran sulphate (DXS) (Figure 2) were used in this assay (Figure 3). Only CS-A significantly promoted the Th1-type cytokine (IFN-γ and IL-2) production. For Th2 type cytokine production, IL-5 production was significantly inhibited by CS-A or DXS when compared with control. IL-10 production was significantly inhibited only by CS-A. These results suggest that only certain acid polysaccharides induce Th1-promoted activity of the OVA-sensitized mice splenocytes on OVA re-stimulation.
Figure 2. Chemical structure of polysaccharides.
Figure 3. Effects of polysaccharide on the cytokine production of murine splenocyte in vitro.
BALB/c mice (n=5) were intraperitoneally injected on day 0 and day 13 with 20 μg of OVA and 2 mg of Al(OH)3 in a total volume of 400 μl. Splenocytes (5.0×106 cells/ml) were collected on day 14 and were co-cultured with OVA (final concentration 100 μg/ml). The samples (each at a final concentration of 1 μg/ml), or saline in vitro were added to the 24-well culture cluster in triplicate at 37 °C in a CO2 incubator for 3 days. The amounts of cytokines in the supernatant were measured by ELISA. *P<0.05, **P<0.01 (significantly different from control value). Error bars represent means±S.D. for 6 wells.
Effects of CS O-sulpho group content on the in vitro cytokine production of splenocytes
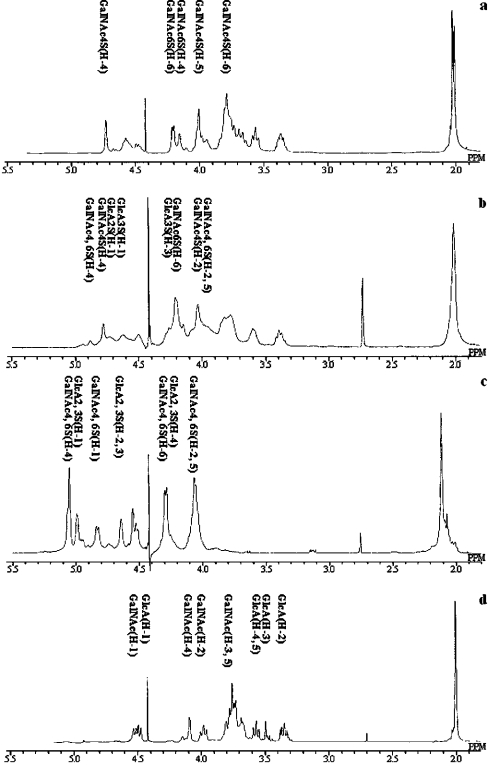
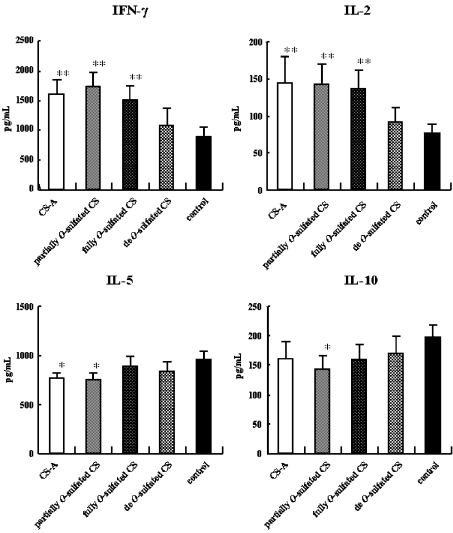
Various types of CS were chemically synthesized from CS-A. The 1D 1H-NMR spectra of these modified CS derivatives confirmed their structures (Figure 4). CS-A, partially O-sulphated CS, fully O-sulphated CS and de-O-sulphated CS differ only by their degree of sulphation. The spectra of the parent CS showed a substantial level of structural heterogeneity resulting from the presence and/or absence of O-sulpho groups at the 4- and/or 6-positions of the GalNAc residue with an average of one O-sulpho group per disaccharide repeating unit. Partial chemical O-sulphonation at 0 °C adds additional O-sulpho groups at the C-4 and C-6 of GalNAc and at the 2- and 3- positions of GlcA (with 2–3 O-sulpho groups per disaccharide repeating unit), and this derivative shows the expected [32,33] increase in the structural heterogeneity compared with the parent CS-A. In contrast, chemical O-sulphonation at 40 °C results in full substitution with O-sulpho groups (with four O-sulpho groups per disaccharide repeating unit) affording a considerably less complex 1D 1H NMR spectrum, consistent with the reduced structural heterogeneity of this product. Table 1 also shows the disaccharide composition of various CS chains used in this study. Fully O-sulphated CS-A could not be digested with chondroitinase ABC. Using these CS derivatives, we examined the effects of CS O-sulpho group content on in vitro cytokine production of OVA-sensitized splenocytes upon OVA re-stimulation. CS-A, partially sulphated CS, and fully sulphated CS all significantly promoted the Th1-type cytokine production compared with the control (Figure 5). There was no significant difference between the effect of desulphated CS and that of the control on the Th1-type cytokine production. In contrast, CS-A and partially sulphated CS significantly inhibited IL-5 production compared with the control. In addition, only partially sulphated CS significantly inhibited IL-10 production compared with the control. These results suggest that an appropriate level of CS sulphation could greatly induce Th1-promoted activity and Th2-inhibitory activity of the OVA-sensitized mice splenocytes upon OVA re-stimulation. There is some discrepancy between the significance of the data in Figure 5 and Figure 1 with regard to the ability of 1 μg/ml CS-A to inhibit IL-10. Nevertheless, a similar trend is observed in both experiments. Since it is difficult to obtain identical results in independent experiments, in terms of the Th2 response, we report the relative response of sample to control.
Figure 4. 1H-NMR spectra of CS-A and CS derivatives.
(a) Intact CS-A, (b) partially O-sulphated CS-A, (c) fully O-sulphated CS-A, (d) de-O-sulphated CS-A recorded in 2H2O at 333 K (or 60 °C). The letters in the spectra refer to the corresponding residues in the structures. Assignments were determined using 2D NMR [27].
Table 1. Disaccharide components of various CSs and chemically derived CS.
Disaccharide composition (%) of each CS was determined by HPLC on an amino-bound silica column after chondroitinase ABC digestion as described previously [31]. ΔDi-0S, Δ4,5HexA(α1-3)GalNAc; ΔDi-4S, Δ4,5HexA(α1-3)GalNAc(4-O-sulphate);ΔDi-6S, Δ4,5HexA(α1-3)GalNAc(6-O-sulphate); ΔDi-diSE, Δ4,5HexA(α1-3)GalNAc(4,6-O-disulphate); ΔDi-diSB, Δ4,5HexA(2-O-sulphate)(α1-3)GalNAc(4-O-sulphate); ΔDi-diSD, Δ4,5HexA(2-O-sulphate)(α1-3)GalNAc(4-O-sulphate); ΔDi-tirS, Δ4,5HexA(2-O-sulphate)(α1-3)GalNAc(4,6-O-disulphate); n.d., not determined.
| Unsaturated disaccharide (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| CS | Source (see main text) | ΔDi-0S | ΔDi-4S | ΔDi-6S | ΔDi-diSE | ΔDi-diSB | ΔDi-diSD | ΔDi-triS |
| CS-A | Shin-Nihon Yakugyo Co. | 10.1 | 49.2 | 40.0 | 0.2 | 0.0 | 0.4 | n.d. |
| CS-B | Seikagaku Kogyo Co. | 1.5 | 91.1 | 1.7 | n.d. | 5.5 | 0.2 | n.d. |
| CS-C | San-Ei Gen FFI Co. | 6.2 | 21.7 | 62.0 | 1.7 | n.d. | 8.4 | n.d. |
| CS-D | Seikagaku Kogyo Co. | 1.9 | 36.3 | 41.0 | 0.6 | 0.8 | 19.1 | 0.3 |
| CS-E | Seikagaku Kogyo Co. | 11.9 | 22.2 | 10.0 | 55.5 | n.d. | n.d. | 0.4 |
| Fully O-sulphated CS-A* | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Partially O-sulphated CS-A | 2.1 | 2.3 | 37.7 | 33.1 | n.d. | 7.8 | 17.1 | |
| de O-sulphated CS-A | 100.0 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
* Fully O-sulphated CS-A is highly inhibitory to the action of chondroitinase ABC.
Figure 5. Effects of CS-A and CS derivatives on the cytokine production of murine splenocyte in vitro.
BALB/c mice (n=5) were intraperitoneally injected on day 0 and day 13 with 20 μg of OVA and 2 mg of Al(OH)3 in a total volume of 400 μl. Splenocytes (5.0×106 cells/ml) were collected on day 14 and were co-cultured with OVA (final concentration 100 μg/ml). The samples (each at a final concentration of 1 μg/ml), or saline in vitro were added to the 24-well culture cluster in triplicate at 37 °C in a CO2 incubator for 3 days. The amounts of cytokines in the supernatant were measured by ELISA. *P<0.05, **P<0.01 (significantly different from control value). Error bars represent means±S.D. for 6 wells.
Effects of O-sulpho group position in monosulphated CS on in vitro cytokine production of splenocytes
CS-A {[-4)GlcA(β1-3)GalNAc4S(β1-]n, where S is sulpho and the average molecular mass is 15 kDa}, CS-C {[-4)GlcA(β1-3)GalNAc6S(β1-]n; average molecular mass 13 kDa} and CS-B {[-4)IdoA(β1-3)GalNAc4S(β1-]n; where IdoA is iduronic acid and CS-B has an average molecular mass 16 kDa}, each contain one O-sulpho group in their disaccharide repeating unit (Table 1). As shown in Figure 6, CS-A and CS-C significantly promoted the Th1-type cytokine production compared with the control. Surprisingly, there was no significant difference between the effect of CS-B and that of the control on the Th1-type cytokine production, although CS-B appeared to be slightly more promoting. Both CS-A and CS-C significantly inhibited the IL-5 production compared with the control. In terms of the IL-10 production, only CS-A significantly inhibited the IL-10 production compared with the control, but CS-C also appeared to be slightly inhibiting. These results demonstrate that CS-A induces the Th1-promoted activity and Th2-inhibitory activity to a greater extent than CS-C and CS-B.
Figure 6. Effects of various CS on the cytokine production of murine splenocyte in vitro.
BALB/c mice (n=5) were intraperitoneally injected on day 0 and day 13 with 20 μg of OVA and 2 mg of Al(OH)3 in a total volume of 400 μl. Splenocytes (5.0×106 cells/ml) were collected on day 14 and were co-cultured with OVA (final concentration 100 μg/ml). The samples (each at a final concentration of 1 μg/ml), or saline in vitro were added to the 24-well culture cluster in triplicate at 37 °C in a CO2 incubator for 3 days. The amounts of cytokines in the supernatant were measured by ELISA. *P<0.05, **P<0.01 (significantly different from control value). Error bars represent means±S.D. for 6 wells.
Effects of O-sulpho group position in disulphated CS on in vitro cytokine production of splenocytes
Since partially sulphated CS (containing 2–3 O-sulpho groups per disaccharide repeating unit) showed the greatest effect on cytokine production (Figure 6) we decided to investigate the effect of O-sulpho position in disulphated CS on the cytokine production of the splenocytes. CS-A, CS-E ([-4)GlcA(β1-3)GalNAc4S6S-(β1-)n; average molecular mass 30 kDa) and CS-D ([-4]GlcA2S-(β1-3)GalNAc6S(β1-]n; average molecular mass 26 kDa). CS-A, CS-E and CS-D all significantly promoted the Th1-type cytokine production compared with the control (Figure 7, Table 1). In addition, CS-A and CS-E significantly inhibited the IL-5 production compared with the control. Only CS-E significantly inhibited the IL-10 production compared with the control in this experiment, but CS-A and CS-D also appeared to inhibit them. These results demonstrate that CS-E induces the greatest Th1-promoted activity and Th2-inhibitory activity among these samples.
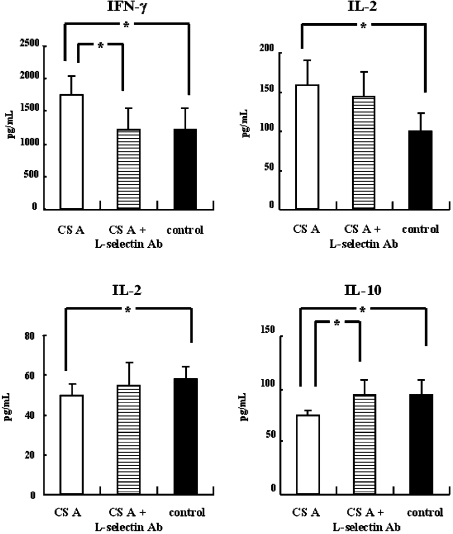
Figure 7. CS receptor associated with Th1-promoted activity on splenocytes in vitro.
BALB/c mice (n=5) were intraperitoneally injected on day 0 and day 13 with 20 μg of OVA and 2 mg of Al(OH)3 in a total volume of 400 μl. Splenocytes (5.0×106 cells/ml) were collected on day 14 and were co-cultured with OVA (final concentration 100 μg/ml). The samples (each at a final concentration of 1 μg/ml), or saline in vitro were added to the 24-well culture cluster in triplicate at 37 °C in a CO2 incubator for 3 days. The amounts of cytokines in the supernatant were measured by ELISA. *P<0.05, **P<0.01 (significantly different from control value). Error bars represent means±S.D. for 6 wells.
CS receptor associated with Th1-promoted activity on splenocytes in vitro
We next examined whether the Th1-promoted activity could be associated with L-selectin (CD62L) and P-selectin (CD62P) on the murine splenocytes. As shown in Figure 7, the Th1-promoted activity and Th2-inhibitory activity of CS-A was not observed in the presence of the rat anti-mouse CD62L antibody. Spiking of the culture with rat anti-mouse P-selectin antibody resulted in no difference in the Th1-promoted activity or the Th2-inhibitory activity of CS-A (results not shown). These results suggest that the activity of CS-A is associated with only L-selectin binding.
In FACS analyses using FITC-labelled anti-mouse CD62L antibody, after we carried out the splenocyte culture with the anti-mouse CD62L antibody and CS-A, cell staining was inhibited compared with the control (Figure 8). However, when cells were incubated with only CS-A in advance, and then stained with the anti-mouse CD62L antibody after washing, cell staining was not inhibited compared with the control (Figure 8). These results suggest that the Th1-promoted and Th2-inhibitory activity of CS-A could be ascribed to a reversible interaction with L-selectin (CD62L).
Figure 8. Effect of spiked CS-A on binding to L-selectin.
Splenocytes (5.0×106 cells/ml) were cocultured with OVA (final concentration 100 μg/ml) and/or the rat anti-mouse CD62L antibody (final concentration 2 μg/ml) in triplicate at 37 °C in a CO2 incubator for 3 days. The colleted cells were then staining with FITC-labelled anti anti-mouse CD62L antibody (MEL-14) and analysed on FACSCalibur™ flow cytometer (Becton Dickinson). (A) control, (B) after culture with CS, and (C) after culture with CS and the rat anti-mouse CD62L antibody.
DISCUSSION
It is generally accepted that CD4+ T cells are subpopulations containing 2 cell types (Th1 and Th2), based on their different patterns of cytokine secretion [34,35]. Th1 cells secrete IFN-γ, IL-2 and IL-12. Th2 cells produce IL-4, IL-5 and IL-10. IFN-γ and IL-12 induce the differentiation of Th0 cells to Th1 cells, whereas IL-4 induces the differentiation to Th2 cells. Therefore, it is believed that an increase in IFN-γ and IL-12 shifts the Th1/Th2 cell balance to predominantly Th1, while an increase in IL-5 and IL-10 shifts the balance to predominantly Th2 [36,37]. We reported previously that CS induced Th1-type cytokine (IFN-γ, IL-2, and IL-12) secretion but suppressed Th2-type cytokine (IL-5 and IL-10) secretion by the OVA-sensitized splenocytes. In the present study, we show that both O-sulpho group content and position in CS is important for the Th1-promoted activity of murine splenocytes, in terms of the cytokine production and Th1/Th2 balance. We first examined whether the activity was associated with the O-sulpho groups in CS, and confirmed that the sulphation of a polysaccharide plays an important role in the activity. We showed that CS induced the Th1-promoted activity, while DX, a neutral polysaccharide, did not (Figure 3). These results suggest that the polysaccharide sulphation is critical for the Th1-promoted activity.
We subsequently showed the effect of the level of CS sulphation on the Th1-promoted activity. While fully sulphated CS exhibits Th1-promoted activity, CS-A and the partially O-sulphated CS, demonstrate higher activity CS (Figure 5). These results suggest that excess sulpho groups in CS could decrease the Th1-promoted and Th2-inhibitory activities of CS.
Among the monosulphated CS, CS-A, CS-C and CS-B, the CS-A sample showed highest activity (Figure 6). This result suggests that the [-4)GlcA(β1-3)GalNAc4S(β1-]n sequence is more important for activity than the [-4)GlcA(β1-3)GalNAc6S(β1-]n or [-4)IdoA(β1-3)GalNAc4S(β1-]n sequences characteriztic of CS-C and CS-B respectively. CS-B (dermatan sulphate), while nearly structurally identical to CS-A (it contains IdoA in place of GlcA), shows lower activity. This is surprising as the greater flexibility of the IdoA residue in CS-B (compared with the GlcA residue in CS-A) is commonly used to explain the propensity of IdoA-containing GAGs to interact with proteins and display a large number of different biological activities [48]. Examination of the disulphated CS samples shows that the effects of CS-E on the Th2-inhibitory activity were higher than those of CS-D or CS-A (Figure 7). These results suggest that the [-4)GlcA(β1-3)GalNAc4S6S(β1-]n sequence in CS-E is more important for high activity than the [-4)GlcA2S(β1-3)GalNAc6S(β1-]n sequence characteristically found in CS-D. Furthermore, these experiments demonstrate that the [-4)GlcA(β1-3)GalNAc4S(β1-]n and [-4)GlcA(β1-3)GalNAc4S6S(β1-]n sequences in CS are more critical for high activity.
Researchers have reported many biological activities for sulphated polysaccharides [24,25,38–40]. In most cases the number of sulpho groups in the polysaccharide directly correlates with the level of bioactivity [38–40]. Koyanagi et al. [40], have shown that by increasing the number of sulpho groups in the fucoidan molecule, its anti-angiogenic and antitumour activities can be potentiated. Our laboratory has also reported the many biological activities of the chemically fully sulphated poly- and oligosaccharides [38–40].
CS has been found in many tissues [41] and cells [42–44], and has been reported to interact with various biologically important molecules and regulate their functions. The present study demonstrates the importance of the content, position and number of O-sulpho groups in CS for immunological activity of the OVA-stimulated murine splenocytes in vitro.
We have also shown that Th1-promoted and Th2-inhibitory activity of CS on murine splenocytes could be associated with binding to L-selectin. It has been reported that a large CS/DS (dermatan sulphate) proteoglycan interacts through its CS/DS chains with the adhesion molecules L- and P-selectin, CD44, and chemokines. Kawashima and co-workers [45–48] and others [49] reported that oversulphated CS/DS, containing [-4)GlcA(β1-3)GalNAc4S6S(β1-]n sequences, interacts with L-selectin, P-selectin and chemokines. Our findings indicate that these same [-4)GlcA(β1-3)GalNAc4S6S(β1-]n sequences in CS would be associated with the strongest effects on the promotion of the Th1-type cytokine production and the inhibition of the Th2-type cytokine production. The present structural characterization of CS to Th1-promoted and Th2-inhibitory activity is consistent with the high affinity binding of CS, containing the [-4)GlcA(β1-3)GalNAc4S(β1-]n and [-4)GlcA(β1-3)GalNAc4S6S(β1-]n sequences, to L-selectin [48]. These findings support our hypothesis that this activity could be associated with the binding of CS-A to L-selectin on T cells. This study also demonstrates that differences in the content and position of O-sulpho groups in CS could markedly influence Th1-promoted and Th2-inhibitory activities, as do differences between GlcA and IdoA residues in CS. In this study, however, the inhibition of CS-binding to L-selectin could not be shown by FACS analysis using labelled anti-L-selectin monoclonal antibody. This result suggests that the epitope region on L-selectin to anti-L-selectin monoclonal antibody might not be located in the region of the lectin that binds to GAGs. We have not yet established the relationship between the various CS samples and the immunological activities. Thus, further studies are required on the relative L-selectin binding affinity of different types of CS to fully elucidate the importance of L-selectin–CS binding.
The effect of heparin was examined on Th1/Th2 balance and found to demonstrate the same level of activity as CS at identical doses (results not shown). We are considering future studies to assess the effects of heparin and its derivatives on these activities to fully elucidate the SAR of GAGs.
These findings provide a new mechanism for the anti-inflammatory and chemoprotective properties of CS, and may be useful for designing new therapeutic applications for CS in the treatment of immediate-type hypersensitivity.
Acknowledgments
The study was supported by a grant from the Japan Health Sciences Foundation and the San-Ei Gen Foundation for Food Chemical Research. We thank Dr. R. Teshima and Dr. Y. Kikuchi in National Institute of Health Sciences for giving useful suggestions in these studies and Ms. H. Akiyama-Okunuki in National Institute of Health Sciences for teaching us the technique.
References
- 1.Roden L. New York: Plenum Press; 1980. The biochemistry of glycoproteins and proteoglycans; pp. 267–371. [Google Scholar]
- 2.Kimata K., Okayama M., Oohira A., Suzuki S. Cytodifferentiation and proteoglycan biosynthesis. Mol. Cell. Biochem. 1973;1:211–228. doi: 10.1007/BF01659331. [DOI] [PubMed] [Google Scholar]
- 3.Toida T., Toyoda H., Imanari T. High-resolution proton nuclear magnetic resonance studies on chondroitin sulphates. Anal. Sci. 1993;9:53–58. [Google Scholar]
- 4.Scott J. E. Structure and function in extracellular matrices depend on interactions between anionic glycosaminoglycans. Pathol. Biol. (Paris) 2001;49:284–289. doi: 10.1016/s0369-8114(01)00152-3. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem. J. 1986;236:313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourin M. C., Ohlin A. K., Lane D., Stenflo J., Lindahl U. Relationship between anticoagulant activities and polyanionic properties of rabbit thrombomodulin. J. Biol. Chem. 1988;263:8044–8052. [PubMed] [Google Scholar]
- 7.Kelly G. S. The role of glucosamine sulphate and chondroitin sulphates in the treatment of degenerative joint disease. Alt. Med. Rev. 1998;3:27–39. [PubMed] [Google Scholar]
- 8.Omata T., Segawa Y., Itokazu Y., Inoue N., Tanaka Y. Effects of chondroitin sulphate-C on bradykinin-induced proteoglycan depletion in rats. Arzneim.-Forsch./Drug Res. 1999;49:577–581. doi: 10.1055/s-0031-1300465. [DOI] [PubMed] [Google Scholar]
- 9.Beren J., Hill S. L., D-West M., Rose N. R. Effect of pre-loading oral glucosamine HCl/chondroitin sulphate/manganese ascorbate combination on experimental arthritis in rats. Exp. Biol. Med. 2001;226:144–151. doi: 10.1177/153537020122600213. [DOI] [PubMed] [Google Scholar]
- 10.Conrozier T. Chondroitin sulphates (CS 4&6): practical applications and economic impact. Presse Med. 1998;27:1862–1865. [PubMed] [Google Scholar]
- 11.Rovetta G. Galactosaminoglycuronoglycan sulphate (matrix) in therapy of tibiofibular osteoarthritis of the knee. Drugs Explt. Clin. Res. 1991;17:53–57. [PubMed] [Google Scholar]
- 12.Bourgeois P., Chales G., Dehais J., Delcambre B., Kuntz J. L., Rozenberg S. Efficacy and tolerability of chondroitin sulphate 1200 mg/day vs chondroitin sulphate 3×400 mg/day vs placebo. Osteoarthritis Cartilage. 1998;6:25–30. doi: 10.1016/s1063-4584(98)80008-3. [DOI] [PubMed] [Google Scholar]
- 13.Theoharides T. C., Patra P., Boucher W., Letourneau R., Kempuraj D., Chiang G., Jeudy S., Hasse L., Athanasiou A. Chondroitin sulphate inhibits connective tissue mast cells. Br. J. Pharmacol. 2000;131:1039–1049. doi: 10.1038/sj.bjp.0703672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bali J. P., Cousse H., Neuzil E. Biochemical basis of the pharmacologic action of chondroitin sulphates on the osteoarticular system. Semin. Arthritis Rheum. 2001;31:58–68. doi: 10.1053/sarh.2000.24874. [DOI] [PubMed] [Google Scholar]
- 15.McCarty M. F., Russell A. L., Seed M. P. Sulphated glycosaminoglycans and glucosamine may synergize in promoting synovial hyaluronic acid synthesis. Med. Hypotheses. 2000;54:798–802. doi: 10.1054/mehy.1999.0954. [DOI] [PubMed] [Google Scholar]
- 16.Ishizeki K., Hiraki Y., Kubo M., Nawa T. Sequential synthesis of cartilage and bone marker proteins during transdifferentiation of mouse Meckel's cartilage chondrocytes in vitro. Int. J. Dev. Biol. 1997;41:83–89. [PubMed] [Google Scholar]
- 17.Conte A., Volpi N., Palmieri L., Bahous I., Ronca G. Biochemical and pharmacokinetic aspects of oral treatment with chondroitin sulphate. Arzneim.-Forsch./Drug Res. 1995;45:918–925. [PubMed] [Google Scholar]
- 18.Baici A., Horler D., Moser B., Hofer H., Fehr K., Wagenhauser F. Analysis of glycosaminoglycans in human serum after oral administration of chondroitin sulphate. Rheumatol. Int. 1992;12:81–88. doi: 10.1007/BF00290259. [DOI] [PubMed] [Google Scholar]
- 19.Sakai S., Onose J., Nakamura N., Toyoda H., Toida T., Imanari I., Linhardt R. J. Pretreatment procedure for the microdetermination of chondroitin sulphate in plasma and urine. Anal. Biochem. 2002;302:169–174. doi: 10.1006/abio.2001.5545. [DOI] [PubMed] [Google Scholar]
- 20.Wrenshall L. E., Stevens R. B., Cerra F. B., Platt J. L. Modulation of macrophage and B cell function by glycosaminoglycans. J. Leuk. Biol. 1999;66:391–400. doi: 10.1002/jlb.66.3.391. [DOI] [PubMed] [Google Scholar]
- 21.Sakai S., Akiyama H., Harikai N., Toyoda H., Toida T., Maitani T., Imanari I. Effect of chondroitin sulphate on murine splenocytes sensitized with ovalbumin. Immunol. Lett. 2002;84:211–216. doi: 10.1016/s0165-2478(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 22.Santos J. A., Mulloy B., Mourao P. A. S. Structural diversity among sulphated α-L-galactans from ascidians (tunicates). Studies on the species Ciona intestinalis and Herdmania monus. Eur. J. Biochem. 1992;204:669–677. doi: 10.1111/j.1432-1033.1992.tb16680.x. [DOI] [PubMed] [Google Scholar]
- 23.Alves A. P., Mulloy B., Diniz J. A., Mourao P. A. S. Sulphated polysaccharides from the egg jelly layer are species-specific inducers of acrosomal reaction in sperms of sea urchins. J. Biol. Chem. 1997;272:6965–6971. doi: 10.1074/jbc.272.11.6965. [DOI] [PubMed] [Google Scholar]
- 24.Farias W. R., Valente A., Pereira M. S., Mourao P. A. S. Structure and anticoagulant activity of sulphated galactans. Isolation of a unique sulphated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulphated galactans from invertebrates. J. Biol. Chem. 2000;275:29299–29307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- 25.Linhardt R. J., Toida T. Heparin oligosaccharides: new analogs development and applications. In: Witczak Z. B., Nieforth K. A., editors. Carbohydrates as drugs. New York: Marcel Dekker; 1997. pp. 277–341. [Google Scholar]
- 26.Razi N., Feyzi E., Bjork I., Naggi A., Casu B., Lindahl U. Structural and functional properties of heparin analogues obtained by chemical sulphation of Escherichia coli K5 capsular polysaccharide. Biochem. J. 1995;309:465–472. doi: 10.1042/bj3090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama T., Toida T., Imanari T., Yu G., Linhardt R. J. Conformational changes and anticoagulant activity of chondroitin sulphate following its O-sulphonation. Carbohydr. Res. 1998;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa K., Uchiyama H., Wajima N. Chemical sulphation of preparations of chondroitin 4- and 6-sulphate, and dermatan sulphate: preparation of chondroitin sulphate E-like materials from chondroitin 4-sulphate. Carbohydr. Res. 1986;158:183–190. [Google Scholar]
- 29.Toida T., Suzuki A., Nakajima K., Chaidedgumjorn A., Imanari T. Effect of 6-O-sulphonate hexosamine residue on anticoagulant activity of fully O-sulphonated glycosaminoglycans. Glycoconj. J. 2000;17:393–399. doi: 10.1023/a:1007108131223. [DOI] [PubMed] [Google Scholar]
- 30.Toyoda H., Shinomiya K., Yamanashi S., Koshiishi I., Imanari T. Microdetermination of unsaturated disaccharides produced from chondroitin sulphates in rabbit plasma by high performance liquid chromatography with fluorometric detection. Anal. Sci. 1988;4:381–384. [Google Scholar]
- 31.Toyoda H., Motoki H., Tanikawa M., Shinomiya K., Akiyama H., Imanari T. Determination of human urinary hyaluronic acid, chondroitin sulphate and dermatan sulphate as their unsaturated disaccharides by high-performance liquid chromatography. J. Chromatogr. 1991;565:141–148. doi: 10.1016/0378-4347(91)80378-p. [DOI] [PubMed] [Google Scholar]
- 32.Maaroufi R. M., Tapon-Bretandiere J., Mardiguian J., Sternberg C., Dauztenberg M. D., Fischer A. M. Influence of the oversulphation method and the degree of sulphation on the anticoagulant properties of dermatan sulphate derivatives. Thromb. Res. 1990;59:749–758. doi: 10.1016/0049-3848(90)90056-i. [DOI] [PubMed] [Google Scholar]
- 33.Bartoulucci C., Cellai L., Lannelli M. A., Lamba D., Liverani L., Mascellani G., Perola E. Inhibition of human leukocyte elastase by chemically and naturally oversulphated galactosaminoglycans. Carbohydr. Res. 1995;276:401–408. doi: 10.1016/0008-6215(95)00179-w. [DOI] [PubMed] [Google Scholar]
- 34.Mossmann R. T., Coffman L. R. TH1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 35.Mossmann R. T., Coffman L. R. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama H., Hoshino K., Tokuzumi M., Teshima R., Mori H., Inakuma T., Ishiguro Y., Goda Y., Sawada J., Toyoda M. The effect of feeding carrots on immunoglobulin E production and anaphylactic response in mice. Biol. Pharm. Bull. 1999;22:551–555. doi: 10.1248/bpb.22.551. [DOI] [PubMed] [Google Scholar]
- 37.Nagafuchi S., Hachimura S., Totsuka M., Takahashi T., Goto M., Yajima T., Kuwata T., Habu S., Kaminogawa S. Dietary nucleotides can up-regulate antigen-specific Th1 immune responses and suppress antigen-specific IgE responses in mice. Int. Arch. Allergy Immunol. 2000;122:33–41. doi: 10.1159/000024356. [DOI] [PubMed] [Google Scholar]
- 38.Toida T., Maruyama T., Ogita Y., Suzuki A., Toyoda H., Imanari T., Linhardt R. J. Preparation and anticoagulant activity of fully O-sulphonated glycosaminoglycans. Int. J. Biol. Macromol. 1999;26:233–241. doi: 10.1016/s0141-8130(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 39.Chaidedgumjorn A., Toyoda H., Woo E. R., Lee K. B., Kim Y. S., Toida T., Imanari T. Effect of (1-3)- and (1-4)-linkages of fully sulphated polysaccharides on their anticoagulant activity. Carbohydrate Res. 2002;337:925–933. doi: 10.1016/s0008-6215(02)00078-2. [DOI] [PubMed] [Google Scholar]
- 40.Koyanagi S., Tanigawa N., Nakagawa H., Soeda S., Shimeno H. Oversulphation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003;65:173–179. doi: 10.1016/s0006-2952(02)01478-8. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S., Satio H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulphated disaccharides from chondroitin sulphates by chondroitinase digestion. J. Biol. Chem. 1968;243:1543–1550. [PubMed] [Google Scholar]
- 42.Ohhashi Y., Hasumi F., Mori Y. Comparative study on glycosaminoglycans synthesized in peripheral and peritoneal polymorphonuclear leucocytes from guinea pigs. Biochem. J. 1984;217:199–207. doi: 10.1042/bj2170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens R. L., Fox C. C., Lichtenstein L. M., Austen K. F. Identification of chondroitin sulphate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:2284–2287. doi: 10.1073/pnas.85.7.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen R. L., Brandt E., Lindahl U., Spillmann D. Characterization of a neutrophil cell surface glycosaminoglycan that mediates binding of platelet factor 4. J. Biol. Chem. 1999;274:12376–12382. doi: 10.1074/jbc.274.18.12376. [DOI] [PubMed] [Google Scholar]
- 45.Kawashima H., Li Y.-F., Watanabe N., Hirose J., Hirose M., Miyasaka M. Identification and characterization of ligands for L-selectin in the kidney. I. Versican, a large chondroitin sulphate proteoglycan, is a ligand for L-selectin. Int. Immunol. 1999;11:393–405. doi: 10.1093/intimm/11.3.393. [DOI] [PubMed] [Google Scholar]
- 46.Kawashima H., Hirose M., Hirose J., Nagakubo D., Plaas A. H. K., Miyasaka M. Binding of a large chondroitin sulphate/dermatan sulphate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J. Biol. Chem. 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 47.Hirose J., Kawashima H., Yoshie O., Tashiro K., Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J. Biol. Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- 48.Kawashima H., Atarashi K., Hirose M., Hirose J., Yamada S., Sugahara K., Miyasaka M. Oversulphated chondroitin/dermatan sulphates containing GlcAβ1/IdoAα1–3GalNAc (4,6-O-disulphate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- 49.Capila I., Linhardt R. J. Heparin–protein interactions. Angew. Chem. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]