Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1) is a co-activator for AP-2α (activator protein 2α)-mediated transcriptional activation. In the present study, we find that the role of PARP-1 in AP-2α transcription is distinctly dualistic with opposing effects. Separate regions of PARP-1 interact with AP-2α and independently control its transcriptional activation. The C-terminus containing the catalytic domain strongly interacts with AP-2α, whereas low-affinity binding is seen in the middle region, which includes the breast-cancer susceptibility gene 1 C-terminal domain and automodification region. The middle region enhances AP-2α transcription. Even portions of this region independently interact and have partial effects on transcription. The catalytic domain strongly poly-(ADP-ribosyl)ates AP-2α. This modification, on the other hand, affects its DNA binding. 3-Aminobenzamide and 6(5H)-phenanthridinone that inhibit the enzymic activity significantly enhance the binding of AP-2α to its target sequence and increase its transcriptional activity. The enzymic activity of PARP-1 is known to be induced by stress conditions that damage cellular DNA, and the poly(ADP-ribosyl)ation of target proteins is transient in nature with a half-life of less than a minute. We hypothesize that PARP-1 enhances the transcriptional activity of AP-2α in normal circumstances, whereas its enzymic activity is used as a temporary shut-off mechanism during unfavourable conditions.
Keywords: activator protein 2α, co-activator, poly(ADP-ribose) polymerase-1, post-translational modification, transcriptional regulation
Abbreviations: Ab, antibody; 3-AB, 3-aminobenzamide; AP-2, activator protein 2; BRCT, breast-cancer susceptibility gene 1 C-terminal; CAT, chloramphenicol acetyltransferase; EMSA, electrophoretic mobility-shift assay; GST, glutathione S-transferase; HRP, horseradish peroxidase; IP, immunoprecipitation; MEF, mouse embryo fibroblast; NF-κB, nuclear factor κB; NLS, nuclear localization signal; PAR, poly(ADP-ribose); PARP-1, PAR polymerase-1; 6-Phen, 6(5H)-phenanthridinone
INTRODUCTION
Poly(ADP-ribose) polymerase-1 (PARP-1) is a ubiquitous nuclear protein widely known for its catalytic activity that uniquely modifies target proteins. It catalyses the transfer of ADP-ribose units from its substrate β-NAD+ covalently to several nuclear acceptor proteins including itself. Initial studies implicated this enzyme in many crucial biological functions including DNA repair, replication, recombination, apoptosis and cancer (reviewed in [1]). Evidence began to emerge from the later studies that it also had a profound role in transcription. PARP-1 was identified among the constituents of positive cofactor-1 complex [2]. A positive cofactor-1 complex is essential for the activity of transcription factors such as NF-κB (nuclear factor κB), Sp1 and Oct-1. Gene expression studies using microarray technology indicated that expression of more than 90 genes was affected by PARP-1 [3]. In recent years, the role of PARP-1 in transcription is well established with several independent studies revealing its potent effect on the activators AP-2 (activator protein 2), p53, NF-κB, HTLV-Tax, B-Myb and TEF-1/Max [4–10], but the mechanism is not clear (reviewed in [1,11,12]).
Transcription factor AP-2α regulates an array of genes involved in diverse cellular functions including development, differentiation, cancer and apoptosis. Of particular importance is the loss of its activity resulting in the induction of a number of carcinogenic events including the oncogenic signal-transduction pathway induced by the activated N-ras gene [13]. Multiple lines of studies indicate that the activity of AP-2α results in cell-cycle arrest and that it is a tumour suppressor gene [14,15]. PARP-1 also has been independently implicated in carcinogenic events and is considered to have a tumour suppressor role [1,11]. We have shown earlier that PARP-1 interacted with the C-terminus of AP-2α and enhanced its transcriptional activity [6]. To study the co-operation of PARP-1 with AP-2α in many of these events, we first investigated the PARP-1 molecule to understand its structural basis of transcriptional regulation. In the present study, we show the intriguing results that expose its dual-regulatory role.
EXPERIMENTAL
Cell culture
PARP-1−/− and PARP-1+/+ MEF (mouse embryo fibroblast) cell lines (gifts from Dr G. de Murcia, Centre National de la Recherche Scientifique, Paris, France) were grown in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA, U.S.A.) with 10% (v/v) fetal bovine serum (Hyclone, Logan, UT, U.S.A.) and antibiotics at 37 °C in 5% CO2/95% air. Mammary cell line MDA-MB-453 and PA-1 human teratocarcinoma cells were grown as described previously [16]. MDA-MB-453 stable cell line constitutively expressing Myc-tagged AP-2α was made by transfecting pCDNA6A-AP-2α and scoring for colonies resistant to the drug blasticidin.
Plasmid DNA constructions
Full-length PARP-1, 1–372, 1–656, 372–479, 372–656, 522–656, 656–910 and 522–1014 were PCR-amplified using the pSPARP-1 cDNA clone [6] as template and PfuUltra High-Fidelity DNA polymerase (Stratagene). The forward oligos contained a NotI restriction site, an ATG start codon and a Kozak box (5′-TGCGGCCGCAACATGGXn-3′) and reverse oligos an XhoI site (5′-TCTCGAGXn-3′), where Xn refers to 17–21 nt at the desired end-points of PARP-1 cDNA. The fragments were directionally cloned into the NotI and XhoI sites of the expression plasmid pCDNA6A (Invitrogen), which fused them in frame with His and Myc tags at their C-termini. The clones were sequenced using automated DNA sequencing to verify if they were error free, and in vitro synthesized using the TNT Quick Coupled Transcription/Translation System (Promega) and 35S-methionine (ICN Biomedicals, Irvine, CA, U.S.A.) to check whether they encoded the intended truncated proteins. To construct bacterial expression plasmids of PARP-1 and AP-2α, their full-length cDNA fragments from pCDNA6 plasmids along with the Myc and His tags were lifted out using NotI and PmeI and subcloned into pET-5a to generate pET-5a/PARP-1 and pET-5a/AP-2α.
Purification of recombinant protein
Plasmids pET-5a/PARP-1 and pET-5a/AP-2α expressing His-tagged proteins were transformed into M15 Escherichia coli strain and the proteins were induced to express using isopropyl-β-D-thiogalactoside. Nickel-affinity chromatography was employed to purify the proteins using the QIAexpress system (Qiagen, Chatsworth, CA, U.S.A.) in native form according to the manufacturer's instructions.
Poly(ADP-ribosyl)ation assay
Purified recombinant AP-2α and PARP-1 proteins were resolved by SDS/PAGE and transferred on to a PVDF membrane (Millipore). The proteins were reduced by treating with 0.7 M 2-mercaptoethanol in transfer buffer (25 mM Tris/HCl, pH 8.3, 192 mM glycine and 0.1% SDS) for 30 min at room temperature (25 °C), and renatured by incubating in renaturation buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 20 μM zinc acetate, 2 mM MgCl2, 1 mM dithiothreitol and 0.3% Tween 20) for 1 h at room temperature. Poly(ADP-ribosyl)ation assay was performed in 5 ml of the same buffer supplemented with 120 μM biotinylated β-NAD (Trevigen, Gaithersburg, MD, U.S.A.) or 25 nmol of [adenylate-32P]NAD (PerkinElmer Life and Analytical Sciences, Boston, MA, U.S.A.), 1 mM β-NAD, 10 μg of recombinant PARP-1 protein, 10 μg of activated DNA and incubating for 1 h at room temperature. PARP-1 protein was omitted from the assay while studying the automodification of membrane-bound PARP-1. The blot was washed twice in the renaturation buffer, and either probed with avidin–HRP (horseradish peroxidase) and the signals detected using enhanced chemiluminescence kit (ECL®; Amersham Biosciences) when biotinylated NAD+ was used or dried and directly autoradiographed when radiolabelled NAD+ was used. In vitro poly(ADP-ribosyl)ation of AP-2α in aqueous solution was performed in 25 μl of reaction buffer (25 mM Tris/HCl, pH 8.0, 10 mM MgCl2 and 50 μM ZnCl2) containing 5 μg of recombinant PARP-1 protein, 250 μM β-NAD and 20 ng of activated DNA and by incubating at room temperature for 30 min.
32P-labelled PAR polymer synthesis and blot overlay assay
Blot overlay assay was performed with synthesized 32P-labelled PAR polymers to study the non-covalent binding by adapting the procedures described by Pleschke et al. [17] and Griesenbeck et al. [18]. Briefly, 5 μg of PARP-1 was automodified in 25 μl of reaction buffer containing 20 ng of activated DNA and 6 nmol [adenylate-32P]NAD+ and incubated at room temperature for 30 min. This resulted in the synthesis of short oligomeric chains with less than 20 PAR units. To synthesize highly branched chains of PAR polymers, 1 mM of unlabelled β-NAD+ was added and the reaction was continued for an additional 30 min. Since both reactions contained the same amount of labelled NAD+, the radioactivity was similar in both preparations. Labelled PARP-1 protein was purified by passing through a Sephadex G50 column. The protein was destroyed by treating with 50 μg/ml of proteinase K for 1 h at 37 °C. PAR moieties were recovered by phenol/chloroform extraction and ethanol precipitation and verified by PAGE. AP-2α and histone H1 proteins (Trevigen) were separated on a 10% SDS/polyacrylamide gel and blotted on to PVDF membrane. The blot was overlaid with 5 ml of TBST (25 mM Tris/HCl, pH 7.4, 0.15 M NaCl and 0.05% Tween 20), containing 1×106 c.p.m. of synthesized PAR polymers and incubated for 1 h at room temperature. The blot was washed thrice with TBST containing 1 M NaCl to remove non-specific binding, dried and autoradiographed.
GST (glutathione S-transferase)–AP-2α binding assays
The GST–AP-2α binding assay was performed to identify the PARP-1 regions that specifically interacted with AP-2α. AP-2α and PARP-1 deletion proteins were in vitro synthesized using TNT Quick Coupled Transcription/Translation System and 35S-methionine. The template DNAs were removed by treating with 10 units of DNase I for 15 min at 37 °C. Recombinant GST–AP-2α protein was purified as described earlier [19]. GST–AP-2α (25 μg) was attached to glutathione–Sepharose beads and mixed with in vitro synthesized proteins. After rocking for 2 h at 4 °C, the mixture was washed four times in TBST. GST–AP-2α protein and its associated proteins were eluted from beads by adding 30 μl of 5 mM GSH (reduced glutathione) in 50 mM Tris/HCl (pH 8.0). The eluted proteins were resolved by SDS/PAGE (10% gel), amplified using Amplify reagent (Amersham Biosciences), dried and autoradiographed.
Western blotting
Transiently transfected cells were harvested and resuspended in PBS (pH 7.4), containing 1.5% (v/v) Triton X-100, 2 mM PMSF, 0.5 mM EDTA and a cocktail of protease inhibitors (Roche Diagnostics), lysed by three cycles of freezing and thawing and rocking for 30 min at 4 °C, and spun for 30 min at 14000 rev./min to clear the cell debris. Protein samples (or immunoprecipitated complexes, see below) were separated on a 14 or 18% SDS/polyacrylamide gel, transferred on to a PVDF membrane, probed with specific Ab (antibody) as needed and the signals were detected using anti-rabbit or anti-mouse IgG conjugated with HRP and ECL®.
IP (immunoprecipitation)
Cells were scraped off from culture dishes and washed with PBS and lysed in 1 ml of Nonidet P40 lysis buffer (50 mM Tris/HCl, pH 8.0, 150 mM NaCl, 1 mM PMSF and 10 μg/ml aprotinin) containing 1% Nonidet P40. Equal amount of protein samples (500 μg) was precleared using 2 μl of rabbit IgG and 30 μl of 50% (v/v) Protein A–Sepharose. The specific Ab (1–5 μl), i.e. rabbit polyclonal Ab raised against AP-2α (F87, laboratory made), PARP-1 or c-erbB-2/neu (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) was attached to the same amount of Protein A–Sepharose, mixed with the precleared protein sample and rocked overnight at 4 °C. The beads were then washed four times with Nonidet P40 lysis buffer, separated on a 14% SDS/polyacrylamide gel, transferred on to a PVDF membrane, probed with specific monoclonal Ab as needed and the signals were detected using anti-mouse IgG conjugated with HRP and ECL®.
For CoIP (co-immunoprecipitation) studies, equal amount of in vitro synthesized and 35S-labelled AP-2α and PARP-1-truncated proteins and 1 μl of specific Ab (F87 or Myc-Ab; Cell Signaling Technology, Beverley, MA, U.S.A.) were mixed in TBST containing 0.1% Triton X-100. Protein A–Sepharose (30 μl, 50%) slurry was added and rocked overnight at 4 °C. The beads with the co-immunoprecipitated complex were then washed three times in TBST containing 0.1% Triton X-100 and resolved on 14 or 18% SDS/polyacrylamide gel, amplified using Amplify reagent, dried and autoradiographed.
EMSA (electrophoretic mobility-shift assay)
A 30-bp double-stranded oligonucleotide 5′-GAACTGACCGCCCGCGGCCCGTGTGCAGAG-3′ corresponding to the AP-2-binding site of the human metallothionein gene IIa was endlabelled using T4 polynucleotide kinase and [γ-32P]ATP; 20000 c.p.m. of labelled oligonucleotide was mixed with 200 ng of His-tagged recombinant AP-2α, and the EMSA was performed as described previously [13]. For supershift analysis, 1 μl of F87 AP-2α-Ab was added and incubated on ice for 10 min. To test the effect of poly(ADP-ribosyl)ated AP-2α, in vitro poly(ADP-ribosyl)ation of the same amount of AP-2α was performed as described above and added to the reaction. Samples were separated on a 4% polyacrylamide gel, dried and autoradiographed.
Transient transfection and CAT (chloramphenicol acetyltransferase) assay
Transient transfection of PARP-1 expression plasmids and 3XAP-2hmtCAT using NovaFactor reagent (Venn Nova, Pompano Beach, FL, U.S.A.) into cells grown on 100 mm dishes, normalization of the transfections using β-galactosidase expression plasmid pCH110 and the CAT assays were all performed as described previously [16]. Acetylated forms of chloramphenicol were quantified by digitally analysing the radioactive spots using Storm Imager and ImageQuant software (Amersham Biosciences).
RESULTS
AP-2α is a substrate for the PARP-1 enzyme
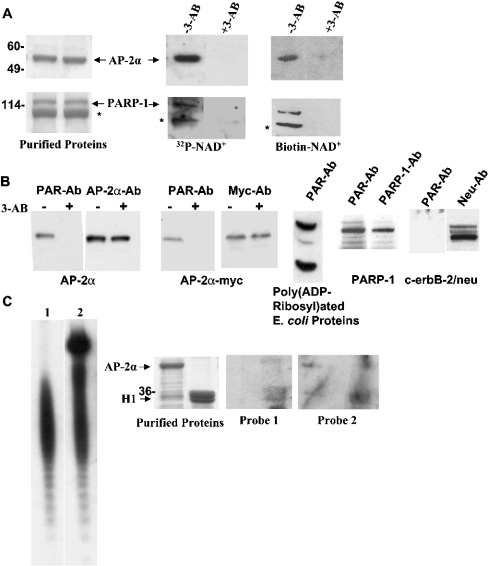
We have shown previously that PARP-1 was a co-activator for the human AP-2α-mediated transcriptional activation [6]. To gain further insight into the mechanism, we tested whether AP-2α was poly(ADP-ribosyl)ated by PAPR-1. Recombinant AP-2α was purified from bacteria and examined in in vitro poly(ADP ribosyl)ation assay using recombinant PARP-1 enzyme and [32P]NAD+ or biotinylated NAD+. Figure 1(A) shows that the positive control PARP-1 is autopoly(ADP-ribosyl)ated, which is reduced by 3-AB (3-aminobenzamide), an inhibitor of the enzyme [1]. AP-2α was also strongly poly(ADP-ribosyl)ated in these in vitro assays. We then examined whether cellular AP-2α undergoes this modification. AP-2α was immunoprecipitated from the breast cancer cell line MDA-MB-453 that has abundant AP-2α, Western-blotted and probed with a PAR moiety-specific Ab. It showed that cellular AP-2α was indeed poly(AD-ribosyl)ated (Figure 1B). In the same cell line, an exogenously expressed Myc-tagged AP-2α was also similarly modified. Treatment of these cells with 3-AB significantly reduced the poly(ADP-ribosyl)ation of both AP-2α and myc-tagged AP-2α, whereas the expression of these proteins was unaffected. Control IP experiments using rabbit and mouse IgG molecule did not show poly(ADP-ribosyl)ated AP-2α proteins (results not shown). Two positive controls, in vitro poly(ADP-ribosyl)ated E. coli proteins and PARP-1, were recognized by PAR-Ab but not the negative control membrane protein c-erbB-2, indicating the specificity of this Ab. These experiments reveal that AP-2α is a substrate for the PARP-1 enzyme and that AP-2α is covalently modified by PARP-1.
Figure 1. PARP-1 poly(ADP-ribosyl)ates AP-2α.
(A) In vitro poly(ADP-ribosyl)ation of AP-2α. Membrane-bound recombinant AP-2α protein was subjected to in vitro poly(ADP-ribosyl)ation assay using [32P]NAD+ or biotinylated NAD+ as substrate. 3-AB (8 mM) was used to inhibit the PARP-1 enzyme as indicated. The left panel shows two lanes of a Coomassie-stained gel with 2 μg of purified AP-2α protein in both lanes. Middle and right panels show the same amount of protein subjected to poly(ADP-ribosyl)ation assay. Automodification of PARP-1 protein (4 μg) was examined in parallel as positive control (bottom panels). Asterisk denotes a truncated PARP-1 that occurred during purification. (B) Poly(ADP-ribosyl)ation of AP-2α in MDA-MB-453 cells. AP-2α, Myc-tagged AP-2α, PARP-1 and c-erbB-2/neu proteins were immunoprecipitated using specific polyclonal antibodies (Santa Cruz Biotechnology) and examined for poly(ADP-ribosyl)ation using a monoclonal PAR-Ab (Trevigen). The membranes were stripped and reprobed with respective monoclonal antibodies [AP-2α-Ab and PARP-1-Ab from Serotec (Raleigh, NC, U.S.A.) and Neu-Ab from Santa Cruz Biotechnology] to visualize the proteins. E. coli proteins treated with PARP-1 (Trevigen) were used to verify the reactivity of PAR-Ab. As indicated on the AP-2α and AP-2α-myc panels, the cells were treated with 500 μM of 3-AB for 36 h to inhibit the PARP-1 enzyme activity before harvesting the cells. (C) Non-covalent binding of poly(ADP-ribose) polymers with AP-2α. 32P-labelled PAR polymers were synthesized as described in the Experimental section and resolved on a 20% PAGE (left panels). Each band in the ladder has one PAR unit more than the one below. Short oligomeric PAR chains were synthesized using 6 nmol of [32P]NAD+ as substrate (lane 1), and highly branched oligomeric chains were obtained by adding 1 mM of unlabelled β-NAD+ to the same reaction (lane 2). Coomassie-stained gel of 2 μg of purified AP-2α and H1 histone proteins is shown in the middle-left panel. Same amount of proteins were blotted on to a PVDF membrane and probed with the two PAR polymer probes as indicated (right two panels).
In addition to covalent modification, free PAR moieties have been shown to bind non-covalently with histones and a family of myristoylated alanine-rich C kinase substrate proteins and significantly affect their biological functions [17,20,21]. Transcription factor p53 is covalently modified by PARP-1 [22]. Chains of PAR units also non-covalently bind with p53 and the efficiency is comparable with that of their binding with histone H1 [23]. To examine whether oligomeric PAR moieties interact with AP-2α non-covalently, we prepared 32P-labelled oligomeric chains of PAR (Figure 1C). One preparation contained shorter oligomeric chains with fewer than 20 PAR units (probe 1) and another preparation contained highly branched chains of up to 200 units (probe 2). The probes were incubated with membrane-bound AP-2α and H1 histone proteins and washed with a high-salt buffer to remove non-specific binding. Probe 1 weakly bound to H1 but not to AP-2α; probe 2 significantly bound to H1. Weaker interaction with AP-2α could also be detected with this probe. These results indicate that in addition to covalent modification of PARP-1, highly branched chains of PAR units also non-covalently bind to AP-2α albeit at significantly reduced efficiency. Nonetheless, AP-2α is predominantly poly(ADP-ribosyl)ated by covalent linkage because when in vitro poly(ADP-ribosyl)ated AP-2α protein from the above studies was purified and then subjected to proteinase K proteolysis it yielded chains of PAR units similar to the studies in Figure 1(C) (results not shown).
Poly(ADP-ribosyl)ation of AP-2α affects its activity
To understand the role of this post-translational modification on AP-2α transcription, we examined the effect on AP-2α DNA binding. Recombinant AP-2α was purified and allowed to bind to the AP-2-target sequence (Figure 2). An AP-2α-Ab supershifted the complex indicating the specificity of the shift. Interestingly, when AP-2α protein was in vitro poly(ADP-ribosyl)ated, it no longer bound to the target sequence. When inhibitors such as 3-AB and 6-Phen [6(5H)-phenanthridinone] were added to block the enzymic activity, surprising results were seen; both significantly enhanced the DNA binding. The strong bindings were supershifted by AP-2α-Ab, confirming the presence of AP-2α in this complex. In contrast with the expectation, these results suggest that poly(ADP-ribosyl)ation has a negative effect on AP-2α transcription.
Figure 2. Poly(ADP-ribosyl)ation of AP-2α prevents DNA binding.
EMSAs were performed with a 32P-end-labelled double-stranded oligo containing an AP-2-binding site. Either 200 ng of recombinant AP-2α or the same amount of in vitro poly(ADP-ribosyl)ated protein was used in the binding assay. 3-AB or 6-Phen was added during the poly(ADP-ribosyl)ation as indicated. A single arrow indicates the AP-2α plus DNA complex and a double arrowhead the supershifted complex.
AP-2 activity is impaired in PARP-1−/− MEF cell lines
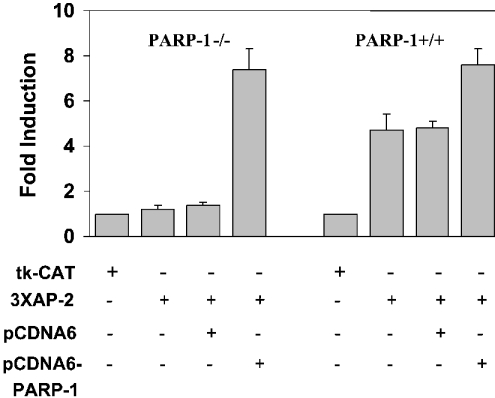
Human and murine AP-2α proteins are more than 98% similar [24]. To examine the status of AP-2 transcription in murine cells lacking PARP-1, MEF cells established from PARP-1-knockout mouse were transiently transfected with an AP-2-CAT reporter construct. As shown in Figure 3, these cells contain very little AP-2 activity, which is in contrast with the significant level found in similar cells established from PARP-1+/+ mouse. Co-transfecting an expression plasmid of PARP-1 into PARP-1−/− cells increased AP-2 activity by more than 7-fold. Raising the level of PARP-1 in PARP-1+/+ also significantly increased AP-2 activity. These results reiterate our previous observation that PARP-1 is a positive co-activator for AP-2 transcription.
Figure 3. PARP-1−/− MEF cells have reduced AP-2α transcription.
3XhmtAP2-CAT (4 μg) and pCDNA6/PARP-1 (4 μg) were transfected into MEF cells as indicated and CAT assays were performed. The activity of the tk-CAT plasmid (where tk stands for thymidine kinase) in each cell line was taken as 1 to calculate fold induction. Results from three independent experiments are shown.
PARP-1 makes multiple contacts with AP-2α
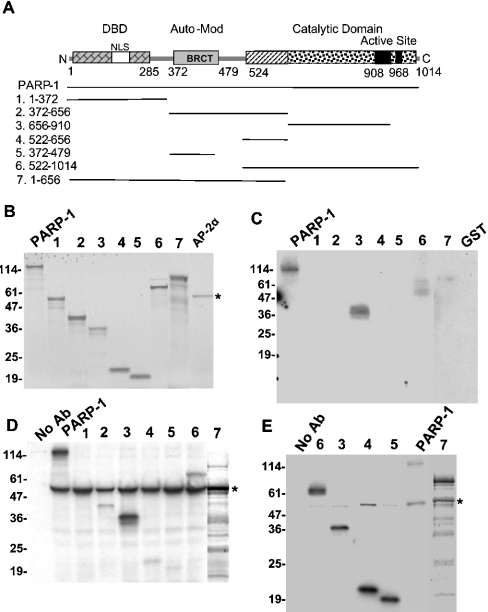
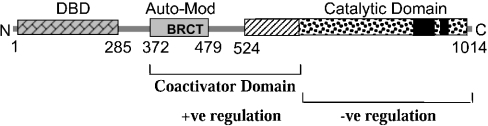
Our previous studies showed that full-length PARP-1 and AP-2α proteins obtained from various sources, mammalian cells, clonal bacteria and synthesized in vitro, physically interacted with each other in GST–AP-2α-binding assays and CoIP studies [6]. We made several deletions of the human PARP-1 cDNA to identify its region that interacts with AP-2α and thereby to obtain clues how it might regulate. The structure of PARP-1 has been studied in significant detail [11] (Figure 4A). The three known regions, DNA-binding domain, automodification/BRCT (breast-cancer susceptibility gene 1 C-terminal) region and the catalytic domain, were recovered separately on some of these truncated molecules, i.e. PARP-1/1–372, PARP-1/372–479 and PARP-1/522–1014 respectively. These deletion molecules were in vitro synthesized (Figure 4B) and their ability to bind to AP-2α was tested in a GST–AP-2α-binding assay (Figure 4C). Along with full-length PARP-1, the deletion molecule PARP-1/522–1014 also bound to AP-2α. Stronger binding was seen with the construct PARP-1/656–910, indicating that the sequence within this region makes the contact. Similar results were obtained in AP-2α-Ab CoIP studies using in vitro synthesized AP-2α and truncated PARP-1 proteins (Figure 4D). However, we repeatedly saw weaker interaction of the construct PARP-1/372–656 with AP-2α. Moreover, even when this truncated molecule was further fragmented into two, as 372–479 that contains the BRCT/automodification region and 522–656 with the adjacent region, they individually maintained the low-affinity interaction. The reason why the weaker interaction of these fragments was not clearly noticeable in the GST–AP-2α-binding assay may largely be due to the nature of the GST–AP-2α fusion protein. The fusion protein is not stable and degrades, which results in the loss of more than half of the AP-2α protein from the immobilized GST–AP-2α. The weaker interacting regions thus become difficult to detect because of their loss of the cleaved AP-2α, whereas this does not seriously affect stronger interacting regions. Indeed, increasing the amount of the input proteins did show the same pattern of results as the CoIP experiments including the low-affinity interaction with the co-activator domain of PARP-1 (results not shown). Reverse CoIP experiments using Myc-tag-specific Ab also revealed the interaction of the middle region of PARP-1 with AP-2α (Figure 4E), albeit with varied stoichiometric binding. It is noticeable from these results that the ratio of PARP-1 to AP-2α is higher, which may explain the variation in the binding and that not all immunoprecipitated PARP-1 molecules were bound to AP-2α.
Figure 4. Mapping the region of PARP-1 that interacts with AP-2α.
(A) The molecular structure of PARP-1. The deletion molecules used in the present study are shown. (B) In vitro synthesized PARP-1 deletion proteins. One-sixth of the truncated proteins and full-length AP-2α used in the binding assays are shown. The mobility of the molecular standards in kDa is marked on the left. (C) GST–AP-2α-binding assay. The binding of the PARP-1 deletion proteins with immobilized GST–AP-2α was examined. (D) CoIP of PARP-1 deletion molecules with full-length AP-2α. PARP-1 deletion protein and AP-2α were mixed together and immunoprecipitated with F87 AP-2α-Ab. (E) Reverse CoIP. Experiments were performed similar to (D) but using a Myc tag-Ab. The numbers in (B–E) correspond to the numbers of the PARP-1 deletion molecules in (A).
The middle region of PARP-1 enhances AP-2α transcriptional activity
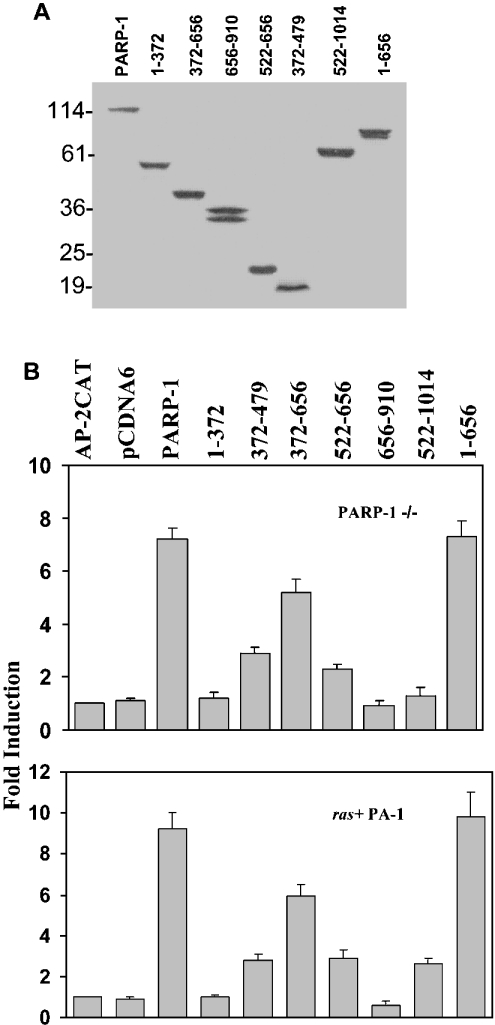
The effect of the PARP-1 deletion molecules on AP-2α transcriptional activity was tested in PARP-1−/− MEF cells, which lack PARP-1 and provide a clear background. The cytomegalovirus promoter-driven expression plasmids of the PARP-1 deletion constructs were transiently transfected into these cell lines along with an AP-2-CAT reporter plasmid and the CAT activity was determined. Figure 5(A) shows that all the transfected deletion molecules are comparably expressed in these cells. The construct PARP-1/372–656 significantly enhanced AP-2 activity (Figure 5B). Interestingly, two deletion constructs that contain portions of this region, 372–479 and 522–656, which weakly interacted with AP-2α also marginally enhanced the activity. The deletion construct PARP-1/522–1014 that contains the enzymic activity did not influence significantly. This truncated molecule had functional enzymic activity when subcloned into a bacterial expression vector pET-5a, expressed in E. coli, and examined in in vitro poly(ADP-ribosyl)ation assay (results not shown). The ras+ PA-1 human teratocarcinoma cells have a higher level of endogenous AP-2α [13]. Nevertheless, the AP-2 activity is low in these cells because of squelching [13,25]. Using reporter gene assays, we showed earlier that PARP-1 potentiated AP-2α transcriptional activity in these cells [6]. When we examined the effect of the various deletions of PARP-1 activity on AP-2α transactivation, we obtained the same pattern of results reiterating that the middle region of PARP-1 enhances transcription. Since all deletion constructs had a Myc tag at their C-termini, we stained the transfected cells immunofluorescently using Myc tag-Ab and studied where these proteins were located. On microscopical examination, full-length PARP-1 and PARP-1/1–372 proteins appear to be completely localized in the nucleus, whereas only a small amount of all the other deletion molecules were in the nucleus (results not shown). This observation was in agreement with an NLS (nuclear localization signal) present at the N-terminus of PARP-1 [11]. To investigate further the need for the NLS, we made a new construct PARP-1/1–656 containing the middle region and the NLS. This deletion molecule, which is transported completely into the nucleus, interacted with AP-2α (Figures 4D and 4E) and robustly enhanced its transcription (Figure 5B).
Figure 5. Mapping the regions of PARP-1 that affect AP-2 transcription.
(A) Expression of truncated PARP-1 molecules in transiently transfected PARP-1−/− MEF cells. Expression plasmids (4 μg) of PARP-1 deletion molecules were transfected into these cells. After 48 h, total protein was isolated and 100 μg of protein was resolved on an 18% SDS/polyacrylamide gel, Western-blotted and probed with Myc-Ab. (B) The middle region of PARP-1 enhances AP-2α transcription. Expression plasmids (4 μg) of PARP-1 deletion molecules and 4 μg of 3XAP-2hmtCAT were transiently transfected into PARP-1−/− and ras+ PA-1 cells and the CAT assays were performed. The activity of the reporter plasmid alone is taken as 1 to calculate fold induction. Results are from three independent experiments.
Inhibition of the enzymic activity of PARP-1 increases AP-2α transcription
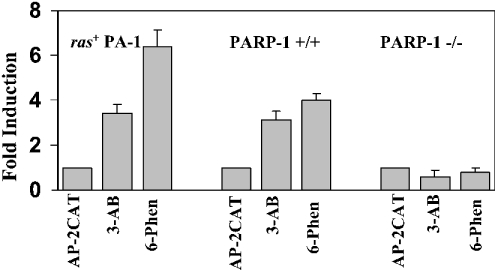
The results indicate that PARP-1 has a dual regulatory role; the middle region augments AP-2 transcription, whereas the enzymic activity represses it. If such is the case, then blocking the enzyme activity should increase AP-2α transcription. To verify this, we inhibited the enzymic activity of PARP-1 in PARP-1+/+ MEF and ras+ PA-1 cells by treating with the inhibitors 3-AB and 6-Phen. Indeed, inhibition of the catalytic activity of PARP-1 induced AP-2-activity 3–6-fold in both cells (Figure 6). Treatment of the PARP-1−/− cells with 3-AB and 6-Phen did not alter the level of AP-2α transactivation indicating that these inhibitors did not directly affect AP-2α and that the presence of PARP-1 is needed to enhance its transcription.
Figure 6. Blocking the enzymic activity of PARP-1 increases AP-2α transcription.
The ras+ PA-1, PARP-1+/+ and PARP-1−/− MEF cells were transiently transfected with 4 μg of 3XAP-2hmtCAT reporter plasmid and the cells were treated with 500 μM of 3-AB or 250 nM of 6-Phen for 36 h and the CAT activity was determined. The CAT activity in the absence of treatment was taken as 1 to calculate fold induction. Results are from three independent experiments.
DISCUSSION
Our studies reveal that PARP-1 has a dual regulatory role on AP-2α transcription with opposing effects (Figure 7). The C-terminus of PARP-1 strongly interacts with AP-2α. However, the low-affinity interaction of the middle region between amino acids 372–656 has positive effect on AP-2α transcription. The sequence between amino acids 372 and 479 harbours a BRCT domain. This motif is present in many proteins and is usually involved in associating with other proteins [26]. The sequence adjacent to this region, from amino acids 522–656, also independently maintains the ability to interact with AP-2α. It is possible that this sequence and the BRCT domain serve as independent bridges, which connect AP-2α to the general transcriptional machinery. The C-terminal enzymic domain of PARP-1, on the other hand, poly(ADP-ribosyl)ates AP-2α and affects its DNA binding and thereby its transcriptional activation. AP-2α is predominantly modified by covalent linkage of chains of PAR polymers because endogenous AP-2α molecules carry this modification, and proteolytic examination of the in vitro poly(ADP-ribosyl)ated AP-2α contain labelled PAR. Nevertheless, highly branched chains of free poly(ADP-ribose) units also non-covalently but weakly bind to AP-2α. This is similar to the transcription factor p53, which has been shown to be both covalently and non-covalently linked with chains of poly(ADP-ribose) [22,23]. In both events, poly(ADP-ribosyl)ation of these transcription factors is a consequence of the functioning of PARP-1 enzyme because it is the strongest poly(ADP-ribosyl)ating enzyme known so far [1,11].
Figure 7. Schematic representation of the dual-regulatory domains of PARP-1.
The role of PARP-1 in transcription has been confirmed recently by profuse reports that demonstrated its effect on various transcriptional events [11]. Nevertheless, the mechanism by which PARP-1 affects transcription lacks clarity. The ambiguity is evident for NF-κB-mediated transcription. One study demonstrates that poly(ADP-ribosyl)ation prevents the DNA binding of NF-κB and reduces its transcriptional activation [27], another study suggests that the catalytic activity of PARP-1 is needed for NF-κB-mediated transcription because reduced enzyme activity correlates with reduced transcription of an iNOS (inducible nitric oxide synthase) gene promoter, a target of NF-κB [8]; and yet another study shows that the middle region is the one that enhances NF-κB transcription and argues that the enzymic activity plays no role since a mutant of PARP-1 defective in the catalytic activity still promotes NF-κB transcription [28]. Another study attributes a general repressive effect for this enzyme for all RNA polymerase II-dependent transcriptions [29], whereas others show evidence that PARP-1 is needed for the transcriptions mediated by p53 and NF-κB since these transcriptions are affected in PARP-1−/− cells [9,10]. Analysing these reports, one can perceive that these studies have characterized and interpreted only one of the two effects.
A recent report shows that poly(ADP-ribosyl)ation activity is increased in Drosophila puff regions and that PARP-1 is needed to produce normal sized puffs [30], an indication that the enzymic activity promotes transcription. Histones are poly(ADP-ribosyl)ated, which causes loosening of chromatin due to the high negative charge and produce puffs. Along with histones, if AP-2α and other activators are simultaneously modified and repressed, then it would be counterproductive. Experiments by Griesenbeck et al. [18] reveal that automodification of PARP-1 plays a crucial role in choosing partners to interact with. It is possible that the state of automodification determines the time of interaction with AP-2α and histones. The structural overlap of the automodification region with the co-activator domain thus may represent an important built-in regulatory mechanism. This may also explain why we see weaker interaction of the AP-2α with this region.
Poly(ADP-ribosyl)ation is a temporary post-translational modification, which is promptly removed by another enzyme PAR glycohydrolase [31]. The half-life of the modification is less than a minute [32]. Since the catalytic activity of PARP-1 is stimulated during stress conditions by the presence of breaks in the DNA, this modification may be used to shut-off transiently AP-2α and possibly other transcriptions. PARP-1 has been well studied from the perspective of its enzymic activity. However, the role of catalytic activity on many target functions remains controversial. Our results indicate that PARP-1 has more roles other than its enzymic activity and warrants a new look at this molecule as a multi-faceted protein rather than as one with a single catalytic function with multiple effects.
Acknowledgments
This work was supported by the grant no. CA84278 to P.K. from the National Cancer Institute, National Institutes of Health. We thank Dr G. de Murcia for permitting us to use the MEF cells established from PARP-1 knockout and wild-type mice and Dr V. Kickhoefer (UCLA School of Medicine, Los Angeles, CA, U.S.A.) for making these cells available to us.
References
- 1.Virag L., Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 2.Guermah M., Malik S., Roeder R. G. Involvement of TFIID and USA components in transcriptional activation of the human immunodeficiency virus promoter by NF-κB and Sp1. Mol. Cell. Biol. 1998;18:3234–3244. doi: 10.1128/mcb.18.6.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simbulan-Rosenthal C. M., Ly D. H., Rosenthal D. S., Konopka G., Luo R., Wang Z. Q., Schultz P. G., Smulson M. E. Misregulation of gene expression in primary fibroblasts lacking poly(ADP-ribose) polymerase. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11274–11279. doi: 10.1073/pnas.200285797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M. G., Scoggin K. E., Simbulan-Rosenthal C. M., Steadman J. A. Identification of poly(ADP-ribose) polymerase as a transcriptional coactivator of the human T-cell leukemia virus type 1 Tax protein. J. Virol. 2000;74:2169–2177. doi: 10.1128/jvi.74.5.2169-2177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler A. J., Ordahl C. P. Poly(ADP-ribose) polymerase binds with transcription enhancer factor 1 to MCAT1 elements to regulate muscle-specific transcription. Mol. Cell. Biol. 1999;19:296–306. doi: 10.1128/mcb.19.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannan P., Yu Y., Wankhade S., Tainsky M. A. PolyADP-ribose polymerase is a coactivator for AP-2-mediated transcriptional activation. Nucleic Acids Res. 1999;27:866–874. doi: 10.1093/nar/27.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervellera M. N., Sala A. Poly(ADP-ribose) polymerase is a B-MYB coactivator. J. Biol. Chem. 2000;275:10692–10696. doi: 10.1074/jbc.275.14.10692. [DOI] [PubMed] [Google Scholar]
- 8.Le Page C., Sanceau J., Drapier J. C., Wietzerbin J. Inhibitors of ADP-ribosylation impair inducible nitric oxide synthase gene transcription through inhibition of NF-κB activation. Biochem. Biophys. Res. Commun. 1998;243:451–457. doi: 10.1006/bbrc.1998.8113. [DOI] [PubMed] [Google Scholar]
- 9.Whitacre C. M., Hashimoto H., Tsai M. L., Chatterjee S., Berger S. J., Berger N. A. Involvement of NAD-poly(ADP-ribose) metabolism in p53 regulation and its consequences. Cancer Res. 1995;55:3697–3701. [PubMed] [Google Scholar]
- 10.Oliver F. J., Menissier-De Murcia J., Nacci C., Decker P., Andriantsitohaina R., Muller S., de La R. G., Stoclet J. C., de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassa P. O., Hottiger M. O. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-κB in inflammatory disorders. Cell. Mol. Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraus W. L., Lis J. T. PARP goes transcription. Cell (Cambridge, Mass.) 2003;113:677–683. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 13.Kannan P., Buettner R., Chiao P. J., Yim S. O., Sarkiss M., Tainsky M. A. N-ras oncogene causes AP-2 transcriptional self-interference, which leads to transformation. Genes Dev. 1994;8:1258–1269. doi: 10.1101/gad.8.11.1258. [DOI] [PubMed] [Google Scholar]
- 14.McPherson L. A., Loktev A. V., Weigel R. J. Tumor suppressor activity of AP2α mediated through a direct interaction with p53. J. Biol. Chem. 2002;277:45028–45033. doi: 10.1074/jbc.M208924200. [DOI] [PubMed] [Google Scholar]
- 15.Wajapeyee N., Somasundaram K. Cell cycle arrest and apoptosis induction by activator protein 2α (AP-2α) and the role of p53 and p21WAF1/CIP1 in AP-2α mediated growth inhibition. J. Biol. Chem. 2003;278:52093–52101. doi: 10.1074/jbc.M305624200. [DOI] [PubMed] [Google Scholar]
- 16.Li M., Wang Y., Yu Y., Nishizawa M., Nakajima T., Ito S., Kannan P. The human transcription factor activation protein-2γ (AP-2γ): gene structure, promoter, and expression in mammary carcinoma cell lines. Gene. 2002;301:43–51. doi: 10.1016/s0378-1119(02)01057-0. [DOI] [PubMed] [Google Scholar]
- 17.Pleschke J. M., Kleczkowska H. E., Strohm M., Althaus F. R. Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 2000;275:40974–40980. doi: 10.1074/jbc.M006520200. [DOI] [PubMed] [Google Scholar]
- 18.Griesenbeck J., Oei S. L., Mayer-Kuckuk P., Ziegler M., Buchlow G., Schweiger M. Protein–protein interaction of the human poly(ADP-ribosyl)transferase depends on the functional state of the enzyme. Biochemistry. 1997;36:7297–7304. doi: 10.1021/bi962710g. [DOI] [PubMed] [Google Scholar]
- 19.Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 20.Panzeter P. L., Realini C. A., Althaus F. R. Noncovalent interactions of poly(adenosine diphosphate ribose) with histones. Biochemistry. 1992;31:1379–1385. doi: 10.1021/bi00120a014. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz A. A., Pleschke J. M., Kleczkowska H. E., Althaus F. R., Vergeres G. Poly(ADP-ribose) modulates the properties of MARCKS proteins. Biochemistry. 1998;37:9520–9527. doi: 10.1021/bi973063b. [DOI] [PubMed] [Google Scholar]
- 22.Kumari S. R., Mendoza-Alvarez H., Alvarez-Gonzalez R. Functional interactions of p53 with poly(ADP-ribose) polymerase (PARP) during apoptosis following DNA damage: covalent poly(ADP-ribosyl)ation of p53 by exogenous PARP and noncovalent binding of p53 to the M(r) 85000 proteolytic fragment. Cancer Res. 1998;58:5075–5078. [PubMed] [Google Scholar]
- 23.Malanga M., Pleschke J. M., Kleczkowska H. E., Althaus F. R. Poly(ADP-ribose) binds to specific domains of p53 and alters its DNA binding functions. J. Biol. Chem. 1998;273:11839–11843. doi: 10.1074/jbc.273.19.11839. [DOI] [PubMed] [Google Scholar]
- 24.Wankhade S., Yu Y., Weinberg J., Tainsky M. A., Kannan P. Characterization of the activation domains of AP-2 family transcription factors. J. Biol. Chem. 2000;275:29701–29708. doi: 10.1074/jbc.M000931200. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y., Wang Y., Li M., Kannan P. Tumorigenic effect of transcription factor hAP-2α and the intricate link between hAP-2α activation and squelching. Mol. Carcinog. 2002;34:172–179. doi: 10.1002/mc.10062. [DOI] [PubMed] [Google Scholar]
- 26.Callebaut I., Mornon J. P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 27.Kameoka M., Ota K., Tetsuka T., Tanaka Y., Itaya A., Okamoto T., Yoshihara K. Evidence for regulation of NF-κB by poly(ADP-ribose) polymerase. Biochem. J. 2000;346:641–649. [PMC free article] [PubMed] [Google Scholar]
- 28.Hassa P. O., Covic M., Hasan S., Imhof R., Hottiger M. O. The enzymatic and DNA binding activity of PARP-1 are not required for NF-κB coactivator function. J. Biol. Chem. 2001;276:45588–45597. doi: 10.1074/jbc.M106528200. [DOI] [PubMed] [Google Scholar]
- 29.Oei S. L., Griesenbeck J., Ziegler M., Schweiger M. A novel function of poly(ADP-ribosyl)ation: silencing of RNA polymerase II-dependent transcription. Biochemistry. 1998;37:1465–1469. doi: 10.1021/bi9727390. [DOI] [PubMed] [Google Scholar]
- 30.Tulin A., Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–562. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- 31.Davidovic L., Vodenicharov M., Affar E. B., Poirier G. G. Importance of poly(ADP-ribose) glycohydrolase in the control of poly(ADP-ribose) metabolism. Exp. Cell Res. 2001;268:7–13. doi: 10.1006/excr.2001.5263. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Gonzalez R., Althaus F. R. Poly(ADP-ribose) catabolism in mammalian cells exposed to DNA-damaging agents. Mutat. Res. 1989;218:67–74. doi: 10.1016/0921-8777(89)90012-8. [DOI] [PubMed] [Google Scholar]