Abstract
Ca2+ ions play a critical role in the biochemical cascade of signal transduction pathways, leading to the activation of immune cells. In the present study, we show that the exposure of freshly isolated human monocytes to NAD+ results in a rapid concentration-dependent elevation of [Ca2+]i (intracellular free Ca2+ concentration) caused by the influx of extracellular Ca2+. NAD+ derivatives containing a modified adenine or nicotinamide ring failed to trigger a Ca2+ increase. Treating monocytes with ADPR (ADP-ribose), a major degradation product of NAD+, also resulted in a rise in [Ca2+]i. Selective inhibition of CD38, an NAD-glycohydrolase that generates free ADPR from NAD+, does not abolish the effect of NAD+, excluding the possibility that NAD+ might act via ADPR. The NAD+-induced Ca2+ response was prevented by the prior addition of ADPR and vice versa, indicating that both compounds share some mechanisms mediating the rise in [Ca2+]i. NAD+, as well as ADPR, were ineffective when applied following ATP, suggesting that ATP controls events that intersect with NAD+ and ADPR signalling.
Keywords: ADP-ribose, ATP, calcium, CD38, monocyte, NAD+
Abbreviations: ADPR, ADP-ribose; cADPR, cyclic ADP-ribose; β-araF-NAD, 2′-deoxy-2′-fluoroarabinoside NAD; ART, mono-ADP-ribosyltransferase; [Ca2+]i, intracellular free Ca2+ concentration; fMLP, N-formylmethionyl-leucylphenylalanine; fura 2/AM, fura 2 acetoxymethyl ester; NADase, NAD+-glycohydrolase; β-riboF-NAD, 2′-deoxy-2′-F-ribose-NAD; TRP, transient receptor potential
INTRODUCTION
NAD+ and its metabolites are mainly known as important regulators of numerous intracellular processes. Only lately, has NAD+ gained much attention as an extracellular molecule that is involved in cellular signalling [1–4]. The ability to carry out such dual functions is not restricted to NAD+. It is shared by ATP, which has been established as an important molecule in signalling pathways [5–10], besides its well-known function in energy transduction. The relatively recent identification of extracellular membrane-bound NAD+-metabolizing enzymes, including CD38 [11], a 45 kDa type II integral transmembrane glycoprotein, and ARTs (mono-ADP-ribosyltransferases) [12] led to the suggestion that NAD+ as a substrate of these enzymes has regulatory activities.
ARTs catalyse the transfer of the ADP-ribose moiety from NAD+ to specific amino acids; e.g. arginine or cysteine of target proteins [13,14], thereby usually modifying their function. In T-cells, ADP-ribosylation of cell-surface molecules has been reported to inhibit association of receptors into a signal-transmitting cluster [15] and to induce apoptosis [2,3]. NAD+ is also used for the synthesis of cADPR (cyclic ADP-ribose) generated by an ADP-ribosyl cyclase and degraded by a cADPR hydrolase [16]. In mammals, both activities are expressed at the outer surface of the cell by CD38. CD38 also functions as an NAD+-glycohydrolase (NADase), which cleaves, similarly to ARTs, NAD+ at the adenosine diphosphoribosyl–nicotinamide linkage and thus generates free ADPR (ADP-ribose), which has been shown to attach covalently to proteins [17]. cADPR is a potent Ca2+-mobilizing agent [18] that activates a family of Ca2+-release channels known as ryanodine receptors that are expressed in eukaryotic cell microsomal membranes [19]. The observed increase in [Ca2+]i (intracellular free Ca2+ concentration) in response to extracellular NAD+ has been ascribed to a CD38-dependent release of intracellular Ca2+ [20–22].
A number of models have been proposed to explain how extracellular NAD+ exerts such an effect. Franco et al. [20] have suggested that extracellularly generated cADPR could be translocated through CD38 itself. Alternatively, Zocchi et al. [21,22] proposed that NAD+ binding to CD38 may cause its internalization, so that cADPR can be generated cytosolically. Finally, Sun et al. [23] have suggested that the response depends on the entry of NAD+ into cells and on the presence of functional intracellular CD38.
In view of the importance of Ca2+ in monocyte functions such as chemotaxis [24], phagocytosis [25] and cytokine production [26], and the increasing evidence that NAD+ is capable of regulating immune functions, we studied the effect of NAD+ on the intracellular Ca2+ concentration in human monocytes. As the main effector cells at sites of inflammation and tissue injury, they are likely to be exposed to extracellular NAD+ released from damaged or lysed cells. In the present paper, we give evidence that NAD+ is a potent inducer of [Ca2+]i in monocytes, and that it shares this ability with ADPR, a compound so far mainly recognized as an NAD+-degradation product.
The mechanism whereby NAD+ and ADPR induce a Ca2+ response is unknown; however, according to our data, it may interfere with ATP signalling. Our findings suggest that NAD+ and ADPR induce the activation of a Ca2+ channel through a pathway that involves Ca2+ influx, but not depletion of Ca2+ stores. This mode of Ca2+ entry clearly differs from the capacitative Ca2+ entry, which, triggered by depletion of Ins(1,4,5)P3-sensitive Ca2+ stores, is a major component of agonist-induced Ca2+ signalling in non-excitable cells [27].
EXPERIMENTAL
Reagents
LPS (lipopolysaccharide; from Escherichia coli 055:B5), ADPR, ATP, 1,N6-etheno-NAD+, α-NAD+ and nicotinamide were purchased from Sigma–Aldrich (Taufkirchen, Germany). AMP, ADP and MnCl2 were from AppliChem GmbH (Darmstadt, Germany). NAD+ and NADP+ were from Roche Diagnostics GmbH (Mannheim, Germany), and β-araF-NAD (2′-deoxy-2′-fluoroarabinoside NAD) and β-riboF-NAD (2′-deoxy-2′-fluoro-ribose-NAD) have been described previously [28,29].
Cell separation and cell culture
Human PBMCs (peripheral blood mononuclear cells) from healthy donors were obtained by centrifugation at 700 g for 40 min at 20 °C over a Ficoll-Isopaque (Amersham Biosciences, Freiburg, Germany) density gradient. After repeated washing (500 g for 10 min at 4 °C) in PBS containing 0.3 mM EDTA, the monocytes were isolated by counterflow elutriation using the JE-6B elutriation system (Beckman Instruments, Palo Alto, CA, U.S.A.), as described previously [30]. The purity of the monocyte preparation was >90%, as assessed by morphological screening and immunofluorescence staining with a monoclonal antibody against CD14 (BL-M/G14; DiaMak, Leipzig, Germany).
Monocytes (2×106/ml) were suspended in RPMI 1640 medium (Sigma–Aldrich) supplemented with 10% (v/v) foetal calf serum (Sigma–Aldrich), 1% glutamine (Seromed® Biochrom KG, Berlin, Germany) and 1% penicillin/streptomycin (Seromed® Biochrom KG).
Measurement of [Ca2+]i by Ca2+ imaging
A sample (300 μl) of cell suspension (2×106/ml) was seeded on to 30-mm-diameter sterile glass coverslips (Marienfeld Laboratory Glassware, Bad Mergentheim, Germany) incubated for 30 min at 37 °C and in 5% CO2. Monocytes that adhered to coverslips were incubated with 10 μM fura 2/AM (fura 2 acetoxymethyl ester) (TEF Labs, Austin, TX, U.S.A.) and 0.0125% Pluronic® F-127 (TEF Labs) in 1 ml of standard Ca2+ solution [125 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM Hepes and 7.5 mM glucose (adjusted to pH 7.4 with NaOH)] at room temperature (21 °C) for 30 min in the dark.
The coverslips were placed in a recording chamber and were continuously perfused at room temperature at a rate of 2 ml/min. Solutions were removed by a vacuum pump. Experiments were performed on a Zeiss Axiovert 135 microscope (Carl Zeiss Jena GmbH, Jena, Germany) equipped with Axiovert 135 UV transparent optics (Carl Zeiss Jena GmbH). Dye-excitation illumination was provided by a dual-wavelength illuminator system (T.I.L.L. Photonics GmbH, Gräfelfing, Germany) consisting of a xenon arc lamp, a variable-speed reflective optic chopper and two monochromators both under computer control. The excitation wavelengths used were 340 and 380 nm. Emitted fluorescence filtered at 510 nm was collected by a photomultiplier tube and photon-counting photometer. Changes in [Ca2+]i were expressed as the ratio (R) of dye fluorescence at 340 and 380 nm. Fluorescence intensities for both excitation wavelengths were acquired in intervals of 2 s. Ca2+ measurements were performed on fields containing 45–70 cell bodies.
Mn2+-uptake assay
Cells were loaded with fura 2/AM in Ca2+-free solution [125 mM NaCl, 5 mM KCl, 2 mM MgCl2·6H2O, 1 mM EGTA, 10 mM Hepes and 7.5 mM glucose (adjusted to pH 7.4 with NaOH)], in the presence and absence of thapsigargin (0.3 μM). Before the addition of NAD+ (200 μM) or NAD+ and thapsigargin, the cells were incubated with 0.6 mM MnCl2 for 20 s. Fluorescence was monitored as described above at a wavelength of 360 nm. Data were normalized using 360 nm values obtained immediately before the addition of NAD+.
Determination of NADase activity
The assay was carried out according to Stoeckler et al. [31] with minor modifications. Monocytes (4×106/ml) were pre-incubated in the absence and presence of β-araF-NAD (500 nM) in PBS supplemented with 20 mM Hepes (pH 7.2) for 30 min before the addition of 0.6 mM NAD+. Controls were run in the absence of cells. The samples were incubated under constant shaking at 37 °C. After 60 min, the reaction mixture was stored on ice for 5 min and then centrifuged at 3000 g for 2 min at 4 °C. The supernatant (0.2 ml) was mixed with 0.6 ml of 1.33 M KCN and incubated for 5 min at room temperature. The cyanide complex of NAD+ was measured spectrophotometrically at 325 nm (Ultroscope 2000; Amersham Biosciences, Uppsala, Sweden). The mean difference in absorbance between reactions stopped at 0 and 60 min was used as an arbitrary unit of NADase activity.
Analysis of P2X7-dependent pore formation by flow cytometry
A sample (300 μl) of human monocytes (2×106/ml) was resuspended in medium containing 25 mM Hepes, 130 mM potassium glutamate, 5 mM KCl, 0.5 mM CaCl2, 10 mM glucose, 0.5% BSA (adjusted to pH 7.4 with KOH) and 2.5 mM ethidium bromide. The cells were incubated with ATP (2 mM), NAD+ (2 mM) or ADPR (2 mM) for 15 min at 37 °C, washed twice and measured by FACS analysis (Becton Dickinson, Heidelberg, Germany) using 488 nm excitation and 585 nm emission filters.
RESULTS
NAD+ induces a rise in [Ca2+]i
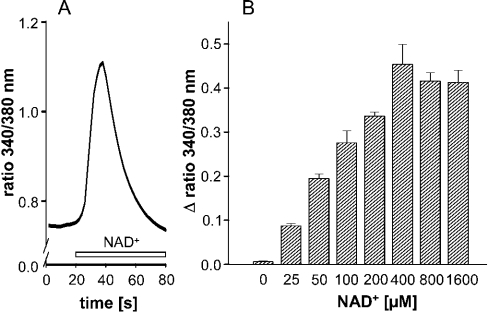
Incubating fura 2-loaded human monocytes with 200 μM NAD+ results in a rapid increase in [Ca2+]i, reaching peak values within 10–20 s and declining thereafter to baseline levels (Figure 1A). The increase was concentration-dependent in the range 25–400 μM, and reached a maximum at 400 μM (Figure 1B). All further experiments were performed at 200 μM NAD+.
Figure 1. NAD+ induces a rise in cytosolic Ca2+ in human monocytes.
(A) Representative trace of an increase in intracellular Ca2+ following application of NAD+ (200 μM, open bar) (n=31). Shown is the 340 nm/380 nm emission ratio from one out of 20 experiments. (B) Dose-dependent effect of NAD+ on [Ca2+]i measured as the change in the 340 nm/380 nm emission ratio. Results are means±S.E.M. (n=15–39 cells) of one out of three experiments.
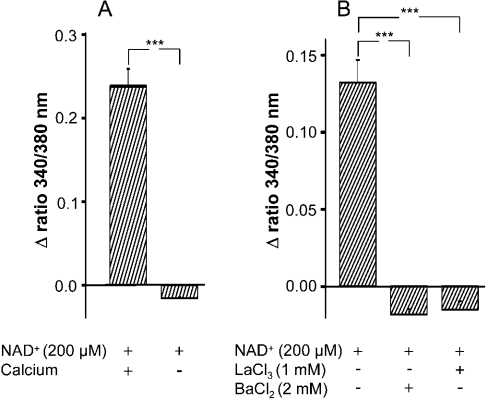
To determine if extracellular Ca2+ was required for the observed intracellular Ca2+ increase, NAD+ was added to monocytes in Ca2+-free medium (Ca2+-free solution plus 0.5 mM EGTA). As seen in Figure 2(A), in the absence of extracellular Ca2+, NAD+ failed to induce an increase in [Ca2+]i. To test the involvement of Ca2+-channels in the NAD+-induced elevation of [Ca2+]i, the cells were pre-treated with the Ca2+-channel-blockers LaCl3 or BaCl2, before NAD+ was added. Both inhibitors prevented Ca2+ mobilization (Figure 2B), indicating that the elevation of [Ca2+]i following addition of NAD+ requires extracellular Ca2+, the influx being mediated by Ca2+ channels.
Figure 2. NAD+-induced rise in [Ca2+]i depends on extracellular Ca2+.
(A) Monocytes were incubated with NAD+ (200 μM) in a Ca2+-containing or Ca2+-free solution. Results are means±S.E.M. (n=35–49 cells) of one out of three experiments. ***P<0.001 compared with control (Ca2+-containing solution); Student's t test. (B) Monocytes were pre-incubated with LaCl3 (1 mM) or BaCl2 (2 mM) for 30 min before NAD+ (200 μM) was added. Results are means±S.E.M. (n=20–55 cells) of one out of three experiments. ***P<0.001 compared with control (in the absence of LaCl3 or BaCl2); Student's t test.
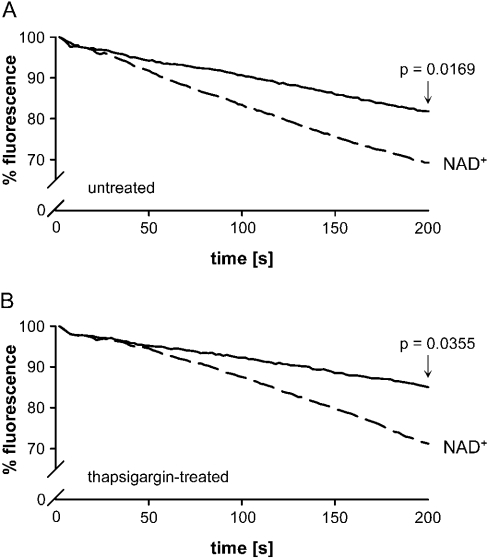
To verify that the NAD+-induced increase in [Ca2+]i depends on Ca2+ influx, extracellular Ca2+ was replaced by Mn2+. Mn2+, which can serve as a Ca2+ surrogate [32,33], has the advantage that it cannot be modulated by a number of processes such as buffering, extrusion and sequestering that may interfere with the interpretation of studies on Ca2+ uptake. Activation of a Ca2+-channel should allow MnCl2 to permeate cells and quench fura 2. Mn2+ has a higher affinity to fura 2 than Ca2+ does [34]. In the presence of 0.6 mM MnCl2, we observed a decrease in fluorescence after application of 200 μM NAD+ caused by the influx of MnCl2 and quenching of fura 2 fluorescence (Figure 3A).
Figure 3. NAD+-induced uptake of Mn2+.
Monocytes were loaded with fura 2/AM in the absence (A) and presence (B) of thapsigargin (0.3 μM). Fluorescence monitoring at an emission wavelength of 360 nm was performed for 200 s starting with the addition of MnCl2 (0.6 mM) for 20 s before the addition of NAD+ (200 μM). A fluorescence value of 100% refers to the 360 nm values obtained immediately before the addition of NAD+. (A) Values measured after 200 s in the absence of NAD+ were 83% and in the presence of NAD+ 72% (P=0.0169; n=5; Student's t test). (B) Values measured after 200 s in the presence of thapsigargin were 85%, and in the presence of thapsigargin and NAD+ were 71% (P=0.0355; n=5; Student's t test).
To test whether Ca2+ released from endoplasmic reticulum stores may contribute to the entry of cations, cells were treated with thapsigargin to deplete intracellular stores [35] before NAD+ was applied. As seen in Figure 3(B), thapsigargin-treated monocytes react in the same way as untreated cells. A MnCl2-induced quenching of the fluorescence could be observed in the presence and absence of thapsigargin, indicating that the [Ca2+]i increase in response to NAD+ is due to Ca2+ influx and is independent of internal Ca2+ mobilization.
ADPR induces a rise in [Ca2+]i
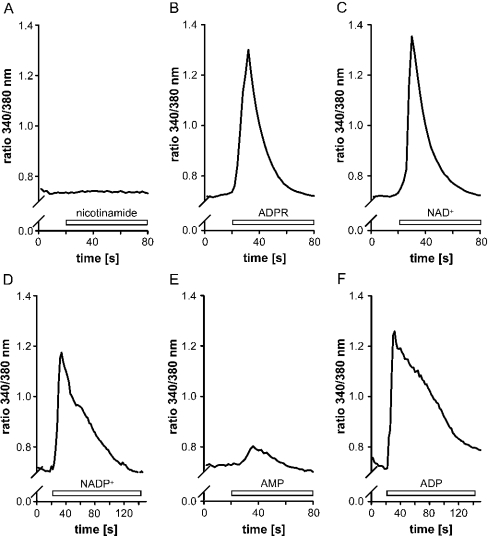
We described above that extracellularly added NAD+ is rapidly degraded by intact monocytes to nicotinamide and ADPR and to minor amounts of AMP, ADP and cADPR [36]. To test whether the main NAD+ metabolites had similar effects as NAD+, monocytes were incubated in the presence of ADPR or nicotinamide and the [Ca2+]i was determined. Whereas monocytes did not respond to nicotinamide (Figure 4A), the addition of ADPR elicited a rise in [Ca2+]i (Figure 4B), which resembled that induced by NAD+ (Figure 4C). Similarly to NAD+, ADPR had no effect in the absence of extracellular Ca2+ (results not shown).
Figure 4. Effects of nicotinamide, ADPR, NAD+, NADP+, AMP and ADP on the [Ca2+]i in monocytes.
Cells were incubated with 200 μM of the following compounds (open bar): (A) nicotinamide, (B) ADPR, (C) NAD+, (D) NADP+, (E) AMP or (F) ADP, and the intracellular Ca2+ levels were measured as the 340 nm/380 nm emission ratio. Representative traces (n=30–60 cells) from one out of three experiments are shown.
Other NAD+-degradation products such as ADP, AMP or adenosine can be excluded as putative inducers of [Ca2+]i, because the NAD+-induced rise is too short (seconds) for these metabolites to be formed in substantial amounts. As shown in previous experiments, albeit carried out at lower NAD+ concentrations, even after 60 min of incubation, minor amounts of AMP and ADP are formed, and adenosine was not detectable at all [36]. Furthermore, AMP had hardly any effect on the [Ca2+]i (Figure 4E), and, although ADP induced a rapid rise in [Ca2+]i (Figure 4F), it only declined gradually over the next 2 min to baseline levels and thus differs from the NAD+-induced increase. An increase in [Ca2+]i was also observed when NADP+ was used instead of NAD+ (Figure 4D). The effect of the reduced forms of the nucleotides could not be measured because the high fluorescence of the compounds disturbed the interpretation of the fura 2 fluorescence data.
NAD+ does not act via its degradation product ADPR
Because ADPR was equally effective as NAD+, we raised the question of whether NAD+ itself and/or its degradation product ADPR was responsible for the observed Ca2+ mobilization.
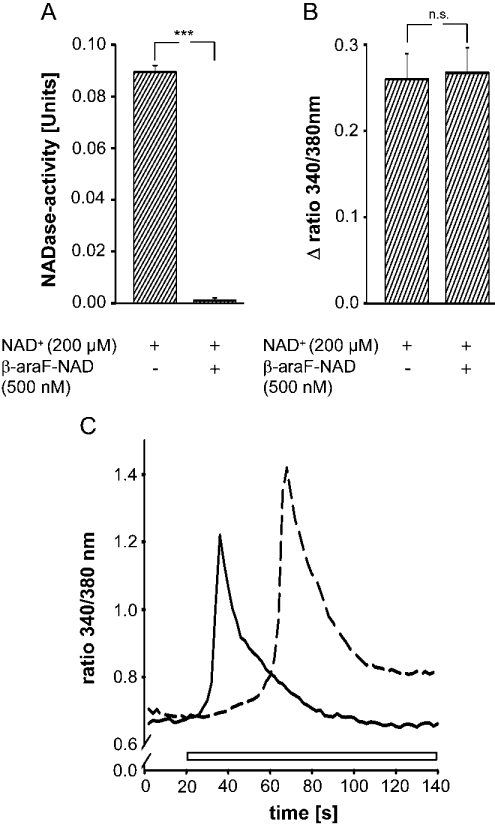
Monocytes were pre-incubated with the NAD+ analogue, β-araF-NAD for 30 min before NAD+ was administered. β-araF-NAD is one of a series of potent, slow-binding and selective inhibitors of CD38 [37] and has a Ki for human CD38 of 1.7 nM [38]. NAD+-glycohydrolases, like CD38, generate free ADPR by cleaving NAD+ at the adenosine diphosphoribosyl–nicotinamide linkage. The data presented in Figure 5(A) demonstrate that β-araF-NAD inhibited NADase activity completely, but had no effect on the NAD+-induced increase in [Ca2+]i (Figure 5B). These results establish that degradation of NAD+ to ADPR is very likely to be mediated by CD38, and that this reaction is not a prerequisite for NAD+ to induce changes in [Ca2+]i. Adding β-riboF-NAD (200 μM), which is resistant to hydrolysis by CD38 [29], to monocytes also results in an increase in [Ca2+]i (Figure 5C). The response is slightly delayed compared with that induced by NAD+.
Figure 5. Effect of β-araF-NAD and β-riboF-NAD on the [Ca2+]i in monocytes. β-araF-NAD inhibits NAD+ glycohydrolase activity.
(A) Cells were incubated with NAD+ (200 μM) in the presence and absence of β-araF-NAD (500 nM) for 30 min, and the cyanide complex of NAD+ in the supernatant was measured at 325 nm. Units are the decrease in absorbance per h per 106 cells. Results are the means±S.E.M. of three separate experiments. ***P<0.001 compared with control (in the absence of β-araF-NAD). (B) Cells were incubated in the presence and absence of β-araF-NAD (500 nM) for 30 min before the addition of NAD+ (200 μM). Intracellular Ca2+ levels were measured as the change in the 340 nm/380 nm emission ratio. Results are means±S.E.M. of three experiments (n=25–35 cells). n.s., not significant. (C) Representative trace of an increase in intracellular Ca2+ following application of NAD+ (200 μM, solid line, open bar) or β-riboF-NAD (200 μM, broken line, open bar) (n=25). The 340 nm/380 nm emission ratio from one out of three experiments is shown.
In further experiments, we studied the ability of NAD+ derivatives to mobilize intracellular Ca2+. Instead of NAD+, we treated the cells with 1,N6-etheno-NAD+ or α-NAD+ that contain a modified adenine or nicotinamide ring respectively. Both derivatives failed to trigger a Ca2+ release (results not shown), suggesting that, for NAD+ to induce a Ca2+ response, its structural properties seem to play an important role.
NAD+ prevents the ADPR-induced rise in [Ca2+]i and vice versa
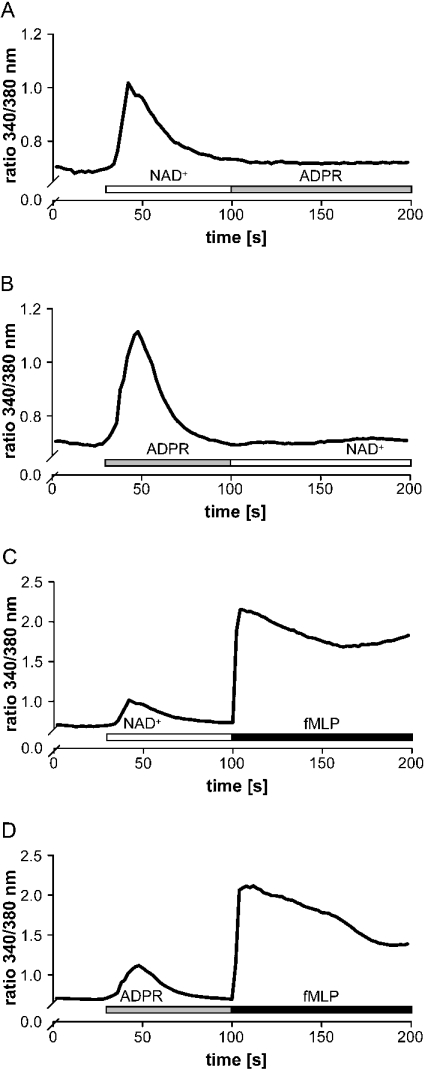
Having shown that NAD+ and ADPR both induce an influx in Ca2+, we tested whether the compounds interfered with each other. We therefore treated monocytes with ADPR before the addition of NAD+. Figure 6(A) shows that application of NAD+ prevented monocytes from responding to the subsequent application of ADPR. This desensitization was also observed when the stimuli were applied in reversed order (Figure 6B). In contrast, when cells were treated with NAD+ or ADPR first and then re-stimulated with fMLP (N-formylmethionyl-leucylphenylalanine), they responded with a large increase in [Ca2+]i (Figures 6C and 6D). These data indicate that NAD+ and ADPR may share common components or pathways to induce a Ca2+ response, while fMLP exerts its effects via different mechanisms.
Figure 6. NAD+ (followed by ADPR) treatment prevents stimulation with ADPR (NAD+), but not with fMLP.
Monocytes were treated with NAD+ (200 μM, open bar) (A and C) or ADPR (200 μM, grey bar) (B and D) before the addition of ADPR (200 μM, grey bar) (A) or NAD+ (200 μM, open bar) (B) or fMLP (1 μM, black bar) (C and D). Intracellular Ca2+ levels were measured as the 340 nm/380 nm emission ratio. Representative traces (n=40–45 cells) from one out of three experiments are shown.
ATP prevents the NAD+- and ADPR-induced rise in [Ca2+]i and, in contrast with NAD+ and ADPR, triggers pore formation
We next tested in how far extracellular ATP has any influence on NAD+- and ADPR-mediated Ca2+-influx, and whether it shares functional events with ADPR and NAD+.
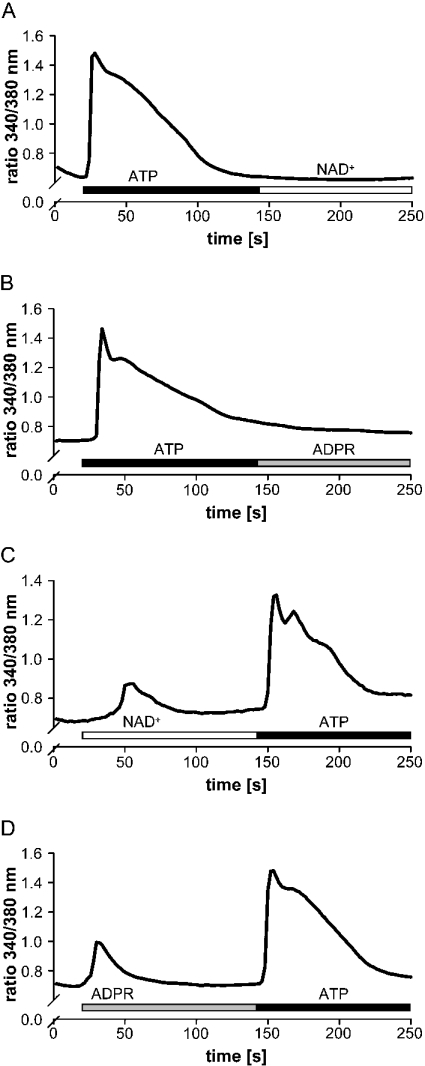
As seen in Figure 7, when monocytes were treated with ATP before the addition of NAD+ (Figure 7A) or ADPR (Figure 7B), ATP rendered the cells insensitive to both compounds. The inhibitory action of ATP might be consistent with several mechanisms. ATP may occupy binding sites also used by NAD+ and ADPR. It may interfere with NAD+-/ADPR-mediated downstream effects or modulate signalling events that may prevent ADPR and NAD+ from causing an influx in Ca2+.
Figure 7. ATP prevents stimulation with NAD+ (followed by ADPR).
Monocytes were treated with 100 μM ATP (A and B, black bar) before the addition of 200 μM NAD+ (A, open bar) and 200 μM ADPR (B, grey bar) or with 200 μM NAD+ (C, open bar) and 200 μM ADPR (D, grey bar) before the addition of 100 μM ATP (C and D, black bar). Intracellular Ca2+ levels were measured as the 340 nm/380 nm emission ratio. Representative traces (n=45–60 cells) from one out of three experiments are shown.
On the other hand, if NAD+ (Figure 7C) and ADPR (Figure 7D) were applied before ATP, the cells were fully responsive. This would imply that putative binding sites occupied by NAD+ or ADPR only represent a minor part of receptors that have been described to mediate ATP-induced Ca2+ signalling [39]. Among the ATP receptors the P2X7 receptor has been examined extensively [40].
Upon brief exposure to ATP, it triggers depolarization, Ca2+ influx and rapid equilibration of Na+ and K+ gradients, whereas sustained activation of P2X7 results in the formation of a non-selective pore that permits the passage of solutes of molecular mass as large as 800 Da [41]. The molecular mechanism underlying pore formation remains to be established. It is not clear whether P2X7 receptors themselves form pores or induce pore formation by binding to other proteins. In contrast with human macrophages, human monocytes, which are P2X7-receptor-positive [42], are resistant to pore formation by ATP when stimulated in standard NaCl-based salines [43,44]. However, when changing the ionic composition of the extracellular medium to a high K+ and low Na+ solution wherein organic anions substitute for Cl− [45], the cells become responsive to ATP, confirming previous studies that showed that the activation of the P2X7 receptor by ATP is extremely sensitive to the surrounding ionic composition [41,46,47].
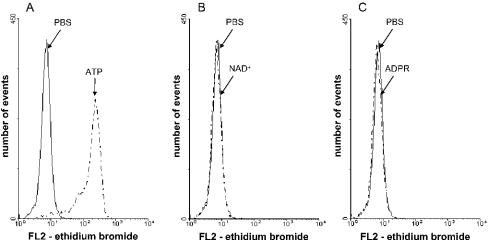
To test whether NAD+ and ADPR share the ability of ATP to induce non-selective pore formation, monocytes were suspended in potassium glutamate basic salt solution in the presence of ethidium bromide before they were incubated in the presence and absence of 2 mM ATP, NAD+ or ADPR at 37 °C for 15 min. Under these experimental conditions, which have been described as optimal for pore formation [45], ATP triggers the uptake of ethidium bromide, whereas neither NAD+ nor ADPR had any effect on the uptake during the test incubation (Figure 8). Thus NAD+ and ADPR, unlike ATP, lack the ability to induce pore formation in human monocytes.
Figure 8. Effects of ATP, NAD+ and ADPR on ethidium bromide uptake in human monocytes.
Monocytes were suspended in potassium glutamate basic salt solution in the presence of 2.5 μM ethidium bromide. The cells were incubated with 2 mM ATP (A), 2 mM NAD+ (B) or 2 mM ADPR (C) for 15 min at 37 °C, and washed twice with PBS. Ethidium bromide uptake was measured by FACS analysis. Traces from one out of two independent experiments are shown.
DISCUSSION
The potential of extracellular NAD+ to participate in the regulation of immune cells has gained much interest in recent years [2–4,15]. In the present paper, we show that extracellular NAD+ induces a rapid (seconds) transient rise in cytosolic Ca2+ levels in human monocytes. A similar immediate response to NAD+ has been detected in various cells, including astrocytes [48], MC3T3-E1 [23], CD38-transfected HeLa− [20] and NIH3T3 cells [23]. In those cells, cADPR generated from CD38 catalysis has been implicated to play a critical role in mediating the Ca2+ increase via the release of Ca2+ from internal stores. Our data indicate that the action of NAD+ in monocytes appears to be mediated via influx of extracellular Ca2+, since removal of extracellular Ca2+ from the external medium completely blocked the response.
The ability of NAD+ to induce an increase in [Ca2+]i, which is shared by ADPR, is not due to the CD38-catalysed formation of ADPR, as established by the use of the CD38 inhibitor β-araF-NAD.
Based on these results, the central question is how NAD+ triggers the rapid rise in [Ca2+]i if the CD38/cADPR system is not responsible for it. There is some evidence that cell membranes are permeable to pyridine nucleotides [23], including NAD+, NADH, NADP+ and NADPH [22]. According to Zocchi et al. [22], the NAD+ flux depends on a concentration gradient and does not require any energy stores. The transport system is characterized by low dinucleotide specificity, and transportation is supposed to occur across a channel rather than involving a transporter. Under physiological conditions, the extracellular NAD+ concentration is too low to allow the entry of NAD+ into cells; however, high enough concentrations may be reached under specific conditions in which the intracellular content of cells is released, such as cell death or by the regulated release from specific cells such as fibroblasts and epithelial cells [21]. How far this may also apply to ADPR is not yet known. Intracellular ADPR and NAD+ may directly enable Ca2+ influx as suggested by Sano et al. [49] who observed that both compounds activate a member of the TRP (transient receptor potential) channel family (LTRPC2), which functions as a Ca2+-permeant non-selective cation channel in immunocytes. Only recently, Heiner et al. [50] provided evidence for a prominent role of the LTRPC2 as a Na+ and Ca2+ entry pathway in neutrophil granulocytes regulated by intracellular ADPR and NAD+, and Campo et al. [51] reported on a non-selective ionic current, which is activated by sustained depolarization, intracellular ADPR and NAD+ in rat peritoneal macrophages.
For LTRPC2 to operate as described [49–51], one would have to postulate the uptake of NAD+ and ADPR across the cell membrane, a process which is difficult to envisage. Furthermore the fact that extracellular NADP+ induces a rise in [Ca2+]i, whereas it fails to activate ion currents when applied into the cell [51], suggests that, if LTRPC2 were engaged, they might have different properties.
How far NAD+ and ADPR act via similar mechanisms is not known. However, they do share some properties which may point to some similar mode of action. Both compounds induce an extracellular Ca2+-dependent rise in [Ca2+]i and NAD+ when applied to the cells before ADPR renders the cells insensitive to the second stimulus and vice versa. However, a challenge with fMLP following the application of NAD+ and ADPR always results in a Ca2+ response. It is conceivable that NAD+ and ADPR use similar binding components and/or share signal transduction pathways that are distinct from those described for the chemotactic peptide fMLP. fMLP mediates its effect by binding to a G-protein-coupled receptor. Signalling through the receptor results in mobilization of Ins(1,4,5)P3-sensitive Ca2+ stores, activation of plasma membrane influx pathways and stimulation of diacylglycerol-dependent protein kinase C enzymes [52].
Desensitization was also observed when ATP was applied before NAD+ or ADPR. Speculations might include that purinergic receptors are involved in NAD+ and ADPR-induced Ca2+ signalling or that ATP controls a signalling event that intersects with NAD+ and ADPR signalling. Rapid desensitization of ATP receptors, compared with other types of receptors, has been reported previously [53]. ATP can activate cells via P2 receptors of which there are two classes [54]. P2Y receptors are coupled to a G-protein which activates phospholipase C, leading to the production of Ins(1,4,5)P3 and Ins(1,4,5)P3-induced Ca2+ release [55]. Among the P2X receptors, which are ligand-gated ion channels, the P2X7 receptor is of special interest, since, in the presence of ATP, it converts into a pore that allows passage of molecules as large as 800 Da [56]. In the present paper, we show that in monocytes, which express various purinergic receptors [57,58], including P2X7 receptors, ATP triggers pore formation, whereas NAD+ and ADPR are unable to activate non-selective pores. This implies that ATP-induced pore formation involves mechanisms that are distinct from the ones that interfere with NAD+/ADPR-mediated Ca2+ signalling.
When considering the mechanism whereby NAD+ induces a rise in [Ca2+]i, it cannot be excluded that, as a substrate for ARTs, NAD+ modifies signalling events by stimulating ADP-ribosylation of proteins. Thus exposure of mouse T-cells to NAD+ has been reported to result in cell death by activating the P2X7 receptor via ART-2-catalysed ADP-ribosylation [59]. Similarly to NAD+, the highly reactive molecule ADPR has the potential to modify proteins by being non-enzymically transferred to specific amino acids of a target protein [60]. The fact that extracellular NAD+ induces Ca2+ mobilization in human monocytes raises the question of how effective concentrations (μM) of extracellular NAD+ can be reached in vivo.
Under physiological conditions, the concentration of NAD+ in extracellular body fluids is in the submicromolar range, whereas cytosolic NAD+ can reach 1 mM [22,61]. An increase could occur under specific conditions and in selective tissues. One possibility is that NAD+ is released into the extracellular compartment from cells as a consequence of lysis during tissue injury and inflammatory immune reactions. ADPR could then be formed by degradation of NAD+. As cell damage would also cause the efflux of other nucleotides such as ATP, a well-described immune modulator [62], it is likely that these purine nucleotides and their degradation products at sites of inflammation influence the outcome of an immune response in a concerted manner.
Acknowledgments
This study was supported in part by Deutsche Forschungsgemeinschaft contract grant HA 2484/1-4.
References
- 1.Wang J., Nemoto E., Kots A. Y., Kaslow H. R., Dennert G. Regulation of cytotoxic T cells by ecto-nicotinamide adenine dinucleotide (NAD) correlates with cell surface GPI-anchored/arginine ADP-ribosyltransferase. J. Immunol. 1994;153:4048–4058. [PubMed] [Google Scholar]
- 2.Liu Z. X., Azhipa O., Okamoto S., Govindarajan S., Dennert G. Extracellular nicotinamide adenine dinucleotide induces T cell apoptosis in vivo and in vitro. J. Immunol. 2001;167:4942–4947. doi: 10.4049/jimmunol.167.9.4942. [DOI] [PubMed] [Google Scholar]
- 3.Adriouch S., Ohlrogge W., Haag F., Koch-Nolte F., Seman M. Rapid induction of naive T cell apoptosis by ecto-nicotinamide adenine dinucleotide: requirement for mono(ADP-ribosyl)transferase 2 and a downstream effector. J. Immunol. 2001;167:196–203. doi: 10.4049/jimmunol.167.1.196. [DOI] [PubMed] [Google Scholar]
- 4.Bortell R., Moss J., McKenna R. C., Rigby M. R., Niedzwiecki D., Stevens L. A., Patton W. A., Mordes J. P., Greiner D. L., Rossini A. A. Nicotinamide adenine dinucleotide (NAD) and its metabolites inhibit T lymphocyte proliferation: role of cell surface NAD glycohydrolase and pyrophosphatase activities. J. Immunol. 2001;167:2049–2059. doi: 10.4049/jimmunol.167.4.2049. [DOI] [PubMed] [Google Scholar]
- 5.Dubyak G. R. Role of P2 receptors in the immune system. In: Abbracchio M. P., Williams M., editors. Handbook of Experimental Pharmacology: Purinergic and Pyrimidinergic Signalling, vol. 151/II. Berlin: Springer-Verlag; 2001. p. 323. [Google Scholar]
- 6.Di Virgilio F., Chiozzi P., Ferrari D., Falzoni S., Sanz J. M., Morelli A., Torboli M., Bolognesi G., Baricordi O. R. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 7.Hogquist K. A., Nett M. A., Unanue E. R., Chaplin D. D. Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths R. J., Stam E. J., Downs J. T., Otterness I. G. ATP induces the release of IL-1 from LPS-primed cells in vivo. J. Immunol. 1995;154:2821–2828. [PubMed] [Google Scholar]
- 9.Falzoni S., Chiozzi P., Ferrari D., Buell G., Di Virgilio F. P2X7 receptor and polykarion formation. Mol. Biol. Cell. 2000;11:3169–3176. doi: 10.1091/mbc.11.9.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lammas D. A., Stober C., Harvey C. J., Kendrick N., Panchalingam S., Kumararatne D. S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 11.Mehta K., Shahid U., Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J. 1996;10:1408–1417. doi: 10.1096/fasebj.10.12.8903511. [DOI] [PubMed] [Google Scholar]
- 12.Zolkiewska A., Nightingale M. S., Moss J. Molecular characterization of NAD:arginine ADP-ribosyltransferase from rabbit skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11352–11356. doi: 10.1073/pnas.89.23.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludden P. W. Reversible ADP-ribosylation as a mechanism of enzyme regulation in procaryotes. Mol. Cell. Biochem. 1994;138:123–129. doi: 10.1007/BF00928453. [DOI] [PubMed] [Google Scholar]
- 14.Koch-Nolte F., Haag F. Mono(ADP-ribosyl)transferases and related enzymes in animal tissues: emerging gene families. Adv. Exp. Med. Biol. 1997;419:1–13. doi: 10.1007/978-1-4419-8632-0_1. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z. X., Yu Y., Dennert G. A cell surface ADP-ribosyltransferase modulates T cell receptor association and signaling. J. Biol. Chem. 1999;274:17399–17401. doi: 10.1074/jbc.274.25.17399. [DOI] [PubMed] [Google Scholar]
- 16.Zocchi E., Franco L., Guida L., Benfatti U., Bargellesi A., Malavasi F., Lee H. C., de Flora A. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem. Biophys. Res. Commun. 1993;196:1459–1465. doi: 10.1006/bbrc.1993.2416. [DOI] [PubMed] [Google Scholar]
- 17.McDonald L. J., Moss J. Enzymatic and nonenzymatic ADP-ribosylation of cysteine. Mol. Cell. Biochem. 1994;138:221–226. doi: 10.1007/BF00928465. [DOI] [PubMed] [Google Scholar]
- 18.Lee H. C. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol. Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 19.Lee H. C., Galione A., Walseth T. F. Cyclic ADP-ribose: metabolism and calcium mobilizing function. Vitam. Horm. 1994;48:199–257. doi: 10.1016/s0083-6729(08)60499-9. [DOI] [PubMed] [Google Scholar]
- 20.Franco L., Guida L., Buzzone S., Zocchi E., Usai C., de Flora A. The transmembrane glycoprotein CD38 is a catalytically active transporter responsible for generation and influx of the second messenger cyclic ADP-ribose across membranes. FASEB J. 1998;12:1507–1520. doi: 10.1096/fasebj.12.14.1507. [DOI] [PubMed] [Google Scholar]
- 21.Zocchi E., Franco L., Guida L., Piccini D., Tacchetti C., de Flora A. NAD+-dependent internalization of the transmembrane glycoprotein CD38 in human Namalwa B cells. FEBS Lett. 1996;396:327–332. doi: 10.1016/0014-5793(96)01125-8. [DOI] [PubMed] [Google Scholar]
- 22.Zocchi E., Usai C., Guida L., Franco L., Buzzone S., Passalacqua M., de Flora A. Ligand-induced internalization of CD38 results in intracellular Ca2+ mobilization: role of NAD+ transport across cell membranes. FASEB J. 1999;13:273–283. doi: 10.1096/fasebj.13.2.273. [DOI] [PubMed] [Google Scholar]
- 23.Sun L., Adebanjo O. A., Koval A., Anandatheerthavarada H. K., Iqbal J., Wu X. Y., Moonga B. S., Wu X. B., Biswas G., Bevis P. J., et al. A novel mechanism for coupling cellular intermediary metabolism to cytosolic Ca2+ signaling via CD38/ADP-ribosyl cyclase, a putative intracellular NAD+ sensor. FASEB J. 2002;16:302–314. doi: 10.1096/fj.01-0705com. [DOI] [PubMed] [Google Scholar]
- 24.Badolato R., Johnston J. A., Wang J. M., McVicar D., Xu L. L., Oppenheim J. J., Kelvin D. J. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J. Immunol. 1995;155:4004–4010. [PubMed] [Google Scholar]
- 25.Hishikawa T., Cheung J. Y., Yelamarty R. V., Knutson D. W. Calcium transients during Fc receptor-mediated and nonspecific phagocytosis by murine peritoneal macrophages. J. Cell Biol. 1991;115:59–66. doi: 10.1083/jcb.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacIntyre J. P., Pope B. L. The involvement of protein kinase C, calcium, and 5-lipoxygenase in the production of tumor necrosis factor by a cloned interleukin-3 dependent cell line with natural cytotoxic activity. Int. J. Immunopharmacol. 1991;13:175–184. doi: 10.1016/0192-0561(91)90096-p. [DOI] [PubMed] [Google Scholar]
- 27.Sargeant P., Sage S. O. Calcium signalling in platelets and other nonexcitable cells. Pharmacol. Ther. 1994;64:395–443. doi: 10.1016/0163-7258(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Sleath P. R., Handlon A. L., Oppenheimer N. J. Pyridine coenzyme analogues. 3. Synthesis of three NAD+-analogues containing a 2′-deoxy-2′-substituted nicotinamide arabinofuranosyl moiety. J. Org. Chem. 1991;56:3608–3613. [Google Scholar]
- 29.Handlon A. L., Xu C., Muller-Steffner H. M., Schuber F., Oppenheimer N. J. 2′-Ribose substituent effects on the chemical and enzymatic hydrolysis of NAD+ J. Am. Chem. Soc. 1994;116:12087–12088. [Google Scholar]
- 30.Grage-Griebenow E., Lorenzen D., Fetting R., Flad H. D., Ernst M. Phenotypical and functional characterization of Fcγ receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur. J. Immunol. 1993;23:3126–3135. doi: 10.1002/eji.1830231213. [DOI] [PubMed] [Google Scholar]
- 31.Stoeckler J. D., Stoeckler H. A., Kouttab N., Maizel A. L. 1α,25-Dihydroxyvitamin D3 modulates CD38 expression on human lymphocytes. J. Immunol. 1996;157:4908–4917. [PubMed] [Google Scholar]
- 32.Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J. Biol. Chem. 1992;267:2318–2324. [PubMed] [Google Scholar]
- 33.Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985;186:175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- 34.Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes: identification and characterization using manganese. J. Biol. Chem. 1990;265:17486–17492. [PubMed] [Google Scholar]
- 35.Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells: evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J. Biol. Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 36.Pfister M., Ogilvie A., da Silva C. P., Grahnert A., Guse A. H., Hauschildt S. NAD degradation and regulation of CD38 expression by human monocytes/macrophages. Eur. J. Biochem. 2001;268:5601–5608. doi: 10.1046/j.1432-1033.2001.02495.x. [DOI] [PubMed] [Google Scholar]
- 37.Müller-Steffner H. M., Malver O., Hosie L., Oppenheimer N. J., Schuber F. Slow-binding inhibition of NAD+ glycohydrolase by arabino analogues of β-NAD. J. Biol. Chem. 1992;267:9606–9611. [PubMed] [Google Scholar]
- 38.Berthelier V., Tixier J. M., Muller-Steffner H., Schuber F., Deterre P. Human CD38 is an authentic NAD(P)+ glycohydrolase. Biochem. J. 1998;330:1383–1390. doi: 10.1042/bj3301383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy C. The discovery and development of P2 receptor subtypes. J. Auton. Nerv. Syst. 2000;81:158–163. doi: 10.1016/s0165-1838(00)00133-8. [DOI] [PubMed] [Google Scholar]
- 40.Di Virgilio F., Falzoni S., Chiozzi P., Sanz J. M., Ferrari D., Buell G. N. ATP receptors and giant cell formation. J. Leukocyte Biol. 1999;66:723–726. doi: 10.1002/jlb.66.5.723. [DOI] [PubMed] [Google Scholar]
- 41.Michel A. D., Chessell I. P., Humphrey P. P. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedeberg's Arch. Pharmacol. 1999;359:102–109. doi: 10.1007/pl00005328. [DOI] [PubMed] [Google Scholar]
- 42.Buell G., Chessell I. P., Michel A. D., Collo G., Salazzo M., Herren S., Gretener D., Grahames C., Kaur R., Kosco-Vilbois M. H., Humphrey P. P. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. [PubMed] [Google Scholar]
- 43.Falzoni S., Munerati M., Ferrari D., Spisani S., Moretti S., Di Virgilio F. The purinergic P2Z receptor of human macrophage cells: characterization and possible physiological role. J. Clin. Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickman S. E., Khoury J. E., Greenberg S., Schieren I., Silverstein S. C. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–2456. [PubMed] [Google Scholar]
- 45.Gudipaty L., Humphreys B. D., Buell G., Dubyak G. R. Regulation of P2X7 nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am. J. Physiol. Cell. Physiol. 2001;280:C943–C953. doi: 10.1152/ajpcell.2001.280.4.C943. [DOI] [PubMed] [Google Scholar]
- 46.Wiley J. S., Chen R., Wiley M. J., Jamieson G. P. The ATP4-receptor-operated ion channel of human lymphocytes: inhibition by ion fluxes by amiloride analogs and by extracellular sodium ions. Arch. Biochem. Biophys. 1992;292:411–418. doi: 10.1016/0003-9861(92)90010-t. [DOI] [PubMed] [Google Scholar]
- 47.Gu B., Zhang W. Y., Bendall L. J., Chessell I. P., Buell G. N., Wiley J. S. Expression of P2X7 purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X7 receptors. Am. J. Physiol. Cell. Physiol. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- 48.Verderio C., Buzzone S., Zocchi E., Fedele E., Schenk U., de Flora A., Matteoli M. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J. Neurochem. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 49.Sano Y., Inamura K., Miyake A., Mochizuki S., Yokoi H., Matsushime H., Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 50.Heiner I., Eisfeld J., Halaszovich C., Wehage E., Jüngling E., Zitt C., Lückhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem. J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campo B., Surprenant A., North R. A. Sustained depolarization and ADP-ribose activate a common ionic current in rat peritoneal macrophages. J. Immunol. 2003;170:1167–1173. doi: 10.4049/jimmunol.170.3.1167. [DOI] [PubMed] [Google Scholar]
- 52.Panaro M. A., Mitolo V. Cellular responses to FMLP challenging: a mini-review. Immunopharmacol. Immunotoxicol. 1999;21:397–419. doi: 10.3109/08923979909007117. [DOI] [PubMed] [Google Scholar]
- 53.Bischof G., Serwold T. F., Machen T. E. Does nitric oxide regulate capacitative Ca influx in HEK 293 cells? Cell Calcium. 1997;21:135–142. doi: 10.1016/s0143-4160(97)90037-3. [DOI] [PubMed] [Google Scholar]
- 54.Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 55.Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 56.Surprenant A., Rassendren F., Kawashima E., North R. A., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 57.Berchtold S., Ogilvie A. L., Bogdan C., Muhl-Zurbes P., Ogilvie A., Schuler G., Steinkasserer A. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 58.Freter R., Eschke D., Hauschildt S., Nieber K. Intracellular [Ca2+]i changes induced by ATP in human monocytes. Naunyn Schmiedeberg's Arch. Pharmacol. 2001;363:R14. [Google Scholar]
- 59.Seman M., Adriouch S., Scheuplein F., Krebs C., Freese D., Glowacki G., Deterre P., Haag F., Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/s1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 60.McDonald L. J., Wainschel L. A., Oppenheimer N. J., Moss J. Amino acid-specific ADP-ribosylation: structural characterization and chemical differentiation of ADP-ribose-cysteine adducts formed nonenzymatically and in a pertussis toxin-catalyzed reaction. Biochemistry. 1992;31:11881–11887. doi: 10.1021/bi00162a029. [DOI] [PubMed] [Google Scholar]
- 61.Jacobson E. L., Jacobson M. K. Tissue NAD as a biochemical measure of niacin status in humans. Methods Enzymol. 1997;280:221–230. doi: 10.1016/s0076-6879(97)80113-9. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari D., di Virgilio F. Purinergic P2X7-mediated responses in immune cells. Mod. Asp. Immunobiol. 2000;1:156–159. [Google Scholar]