Summary
The term “recurrent constellations of embryonic malformations” (RCEM) is used to describe a number of multiple malformation associations that affect three or more body structures. The causes of these disorders are currently unknown, and no diagnostic marker has been identified. Consequently, providing a definitive diagnosis in suspected individuals is challenging. In this study, genome-wide DNA methylation analysis was conducted on DNA samples obtained from the peripheral blood of 53 individuals with RCEM characterized by clinical features recognized as VACTERL and/or oculoauriculovertebral spectrum association. We identified a common DNA methylation episignature in 40 out of the 53 individuals. Subsequently, a sensitive and specific binary classifier was developed based on the DNA methylation episignature. This classifier can facilitate the use of RCEM episignature as a diagnostic biomarker in a clinical setting. The study also investigated the functional correlation of RCEM DNA methylation relative to other genetic disorders with known episignatures, highlighting the common genomic regulatory pathways involved in the pathophysiology of RCEM.
Keywords: epigenetics, DNA methylation, recurrent constellations of embryonic malformations, episignature, VACTERL, OAV
This study identified a common DNA methylation episignature in 40/53 individuals with recurrent constellations of embryonic malformations (RCEM). This episignature can serve as a diagnostic biomarker, enhancing diagnosis accuracy. Additionally, the study revealed shared genomic pathways between RCEM and other genetic disorders, advancing our understanding of their underlying mechanisms.
Introduction
Historically, a wide spectrum of structural congenital anomalies has been recognized to occur together more frequently than one would expect by chance and were referred to as associations. Many of these,—vertebral-anal-cardiac-tracheoesophageal fistula-renal-limb (VACTERL), pentalogy of Cantrell (POC), oculoauriculovertebral spectrum (OAV), limb-body wall defect (L-BWD), omphalocele-exstrophy-imperforate anus-spinal defects (OEIS), Mullerian duct aplasia-renal anomalies-cervicothoracic somite dysplasia (MURCS), urorectal septum malformation (URSM), and sirenomelia—are now referred to as recurrent constellations of embryonic malformations (RCEM),1,2,3,4 among which VACTERL and OAV are the foremost frequent. Vertebral anomalies are shared by all of these RCEM; cardiac, abdominal wall, renal and limb defects occur in several; epibulbar dermoids and anotia/microtia rarely occur except in OAV. Recently, it has been proposed that these associations may in fact represent a single spectrum of malformations rather than distinct associations.2,3,4 Few recurrences have been reported. The search for genetic or other etiologies of the associations have been largely unsuccessful, although maternal diabetes mellitus and twinning have been associated with an increased frequency.1 Individual subjects have been reported with genetic findings, but this alone was not sufficient to establish causality for specific gene defects in these associations.5 The majority of these case reports were molecularly confirmed diagnoses of known overlapping syndromes.6 The discovery of congenital anomalies affecting multiple organ systems in infants with biallelic alterations in genes associated with the tryptophan-kynurenine pathway (KYNU, HAAO, and NADSYN1), along with observations in mice subjected to tryptophan and vitamin B3 deficiency during pregnancy, have given rise to the hypothesis that a deficiency in nicotinamide adenine dinucleotide (NAD+) may underlie some of these conditions.3,7,8,9,10
Individuals clinically diagnosed with multiple malformation associations were included in this study. The two associations (VACTERL and OAV) were described more than 50 years ago with evolution in their nomenclature and phenotypic spectrum. Goldenhar syndrome, described in 1952 and renamed OAV, involves vertebral defects in addition to craniofacial anomalies.11,12 VATER association, described in 1973 and later expanded to VACTERL association, includes vertebral anomalies in combination with malformations below the neck such as thoracic, limb, and pelvic malformations.4,13,14,15,16
DNA methylation is one of the most extensively studied epigenetic mechanisms. To date, more than 60 disorders have been associated with distinct and precise DNA methylation patterns referred to as episignatures.17 Episignatures have proven to be stable and reliable biomarkers for diagnosing rare genetic disorders in clinical settings.18,19 In this study, a common genomic DNA methylation profile was identified in 40 out of 53 individuals diagnosed with RCEM, with clinical features spanning OAV and VACTERL, and used to develop a binary machine learning classifier enabling sensitive and specific molecular diagnosis of affected individuals. Identification of a shared DNA methylation profile across OAV and VACTERL provides further support for the emerging theory that some of the multiple malformation associations represent a single malformation spectrum. Functional correlation and gene network enrichment analysis were performed to correlate the epigenetically affected molecular pathways between RCEM and other rare disorders with established episignatures.
Material and methods
Subjects and cohorts
Datasets used in this study that are available publicly are previously described.20 The discovery cohort included DNA derived from peripheral blood of 53 individuals with a clinical diagnosis of VACTERL or OAV or overlapping features of VACTERL and OAV who were obtained from Imagine Institute in France (n = 25), Greenwood Genetic Center in the United States (n = 15), and Children’s Hospital of Eastern Ontario in Canada (n = 13). The study also included 5 monozygotic twin pairs, discordant for VACTERL presentation, recruited from across Europe by Radboud University Medical Center in the Netherlands. We considered a diagnosis of VACTERL if three of the six core features (vertebral, anal, cardiac anomalies, tracheoesophageal fistula/esophageal atresia, renal, and limb anomalies) were present. A diagnosis of OAV was given if the three features (ocular, auricular, and vertebral anomalies) were present or in the context of hemifacial microsomia plus two of the core features (Table S1). If the diagnostic criteria for VACTERL or OAV were not met, the individuals were classified into two other groups named (VACTERL) or (OAV). The diagnosis was considered to be “overlapping” if some of the features of either condition were present without meeting diagnostic criteria for one or both. The group of 53 individuals were classified as follows: VACTERL (n = 27), (VACTERL) (n = 2), OAV (n = 2), (OAV) (n = 5), and overlapping (n = 17). Within this cohort, there were 21 females and 32 males, with ages ranging from infancy to 17.9 years Table S1 describes the clinical features of the subgroups. Among the twins, there were three pairs of males and two pairs of females, all aged between 8 and 20 years old.
All samples and records were de-identified. Physicians obtained written consent from the families for use of their clinical information in this research. The research was conducted in accordance with all relevant ethical regulations. The study protocol was approved by the Western University Research Ethics Board (REB 106302 and 116108), Self Regional Healthcare (IRB Pro000085001), and the Children’s Hospital of Eastern Ontario Research Ethics Board (CTO 1577, CTO 3306). The Regional Committee on Research Involving Human Subjects Arnhem-Nijmegen approved the AGORA study protocol.
DNA methylation analysis
DNA methylation analysis was carried out using the Illumina Infinium Methylation EPIC Bead Chip microarrays following the manufacturer’s protocol (Illumina, San Diego, CA, USA). The DNA methylation analysis and discovery of episignatures were performed according to the previously described procedures.20,21 The data files, which consisted of methylated and unmethylated signal intensities, were analyzed using R version 4.2.0. The methylation data were normalized using the Illumina normalization method with background correction as implemented in the minfi package.22 The following probes were excluded: probes with a detection p value greater than 0.01, probes located on the X and Y chromosomes, probes containing SNPs near the CpG interrogation or single nucleotide extension sites, and probes showing cross-reactivity with chromosomal locations outside their target regions. In addition, arrays with a high probe failure rate (>5%) and those exhibiting a batch effect were removed from the dataset. The density of methylation data was evaluated for all samples to exclude those exhibiting a non-bimodal signal distribution. Principal component analysis (PCA) was performed to assess the batch structure and identify outliers.
Selection of matched controls
Controls were randomly selected from EpiSign Knowledge Database (EKD; https://episign.lhsc.on.ca/index.html) housed at the London Health Sciences Centre.20 Age, sex, and array type were considered to match controls and cases using the MatchIt package (version 4.5.1).23 Multiple matching trials were performed to obtain an optimized sample size. In each trial, the PCA was employed to examine the structure of matched controls, ensuring no outliers remained within the selected set. Samples displaying a standard deviation greater than 3 in the first two components of the PCA were excluded from the analysis. Selection of matched controls, methylation profiling, and model construction were first performed based on all RCEM samples. Subsequently, this process was repeated using subsets of the RCEM samples, considering the observed data substructure. The number of control samples used for probe selection and model construction in all analyses is listed in Table 1.
Table 1.
Summary of the probe selection criteria for the robust, intermediate, and combined cohorts
| Robust episignature | Intermediate episignature | Combined episignature | |
|---|---|---|---|
| Number of cases | 23 | 23 | 40 |
| Number of controls | 46 | 69 | 80 |
| 900 | 800 | 800 | |
| 450 | 400 | 400 | |
| 0.7 | 0.6 | 0.95 | |
| Number of selected probes | 271 | 255 | 377 |
| Model’s specificity | 99.16% | 98.76% | 98.32% |
: the number of probes with the highest products of mean methylation difference and negative of the logarithm of p values selected in the first step of probe selection; : the number of probes with the highest AUC selected in the second step of probe selection; : the cut-off for removing the highly correlated probes in the third step of probe selection.
DNA methylation profiling of the RCEM cohort
The methylation level (β value) for each probe was computed as the ratio of the methylated signal intensity to the sum of the methylated and unmethylated signal intensities. Β values, ranging between 0 (no methylation) and 1 (full methylation) were logit transformed into M values using the formula log2(β/(1-β)), ensuring the homoscedasticity required for subsequent linear modeling. A multivariate linear regression model was then employed to identify differentially methylated CpG probes using the limma package (version 3.52.4).24 The blood cell composition was estimated based on the procedure described by Houseman et al.25 and was incorporated into the linear regression model as a confounding variable. The eBayes function from the limma package was utilized to moderate the p values obtained from the linear modeling. In addition, the p values were adjusted for multiple testing using the Benjamini and Hochberg (BH) method.26
Three measurements were considered to select the most significant probe sets. Firstly, the absolute mean methylation difference between cases and controls were multiplied by the negative value of the log-transformed adjusted p value, and probes with the highest scores were selected for further analysis. Secondly, a receiver’s operating curve (ROC) characteristics analysis was employed to select probes with the highest area under the ROC curve (AUC). Lastly, to enhance genome-wide representation, Pearson’s correlation coefficient of probes was calculated between cases and controls, and highly correlated probes (correlation > ) were excluded from the analysis. The remaining probes were then used to perform a hierarchical clustering using Ward’s method on Euclidean distance. Moreover, the degree of differentiation between cases and controls was evaluated using a multidimensional scaling (MDS) model. In this model, pairwise Euclidean distances were scaled to assess the extent of distinction between the two groups. Three distinct analyses were conducted based on the observed methylation patterns within the case samples. Table 1 contains values pertaining to the probe selection criteria (designated as values ) as well as the quantity of selected probes in each analysis.
Construction of a classification model
A binary support vector machine (SVM) classification model was constructed using the e1071 R package (version 1.7.9) according to the procedure previously described.20,21 The classifier calculates a methylation variant pathogenicity (MVP) score, which predicts the probability of a sample’s methylation pattern associated with a specific episignature. MVP scores range from 0 to 1, with scores close to 1 indicating a high probability of the methylation pattern resembling the related syndrome. The model was constructed by training RCEM case samples against the matched controls, 75% of other controls, and 75% of subjects from other rare genetic disorders sourced from the previously published EpiSign v3 clinical classifier within the EKD.17 The remaining 25% of other controls and 25% of other disorders were then used as the testing set. In each of the three analyses, the model’s specificity was determined by calculating it using the “unresolved” column, which includes the subjects in our database that did not match any of the existing episignatures in the EpiSign v3 classifier, with a threshold of 0.25 for the MVP score. These results were then integrated into Table 1.
Cross-validation
To evaluate the reproducibility and robustness of the episignatures, multiple rounds of leave-one-out cross-validation (LOOCV) were performed. In each round, one of the training cases was left out as testing set while the other cases were utilized to select probes and construct the model. The heatmap, MDS, and MVP plots were then employed to visualize the distinction between testing case and other samples.
Analyses description
The probe selection, validation, and model construction procedures were performed using distinct subsets of the RCEM cohort through separate analyses. Initially, the entire RCEM sample set was employed for probe selection, and various combinations of , , and were tested in line with the earlier-described process. This involved ensuring that the remaining probes amounted to at least 200. Subsequently, hierarchical clustering and MDS plots were generated. As a result of this analysis, seven RCEM samples were excluded from classifier reference cohort due to their clustering with control samples in over 50% of these combinations.
The process of probe selection, validation, and model construction was repeated for the remaining 46 samples. Given the presence of two visually distinct groups (referred henceforth as the robust and intermediate groups) among the case samples, each consisting of 23 samples, two separate analyses were carried out. In each of these analyses, one of the two groups was employed for methylation profiling and model construction while the other group was used as the testing set.
The model generated using the intermediate group for probe selection and model construction successfully clustered 18 of the samples from the robust group. Consequently, an additional analysis was conducted. In this analysis, a sample from the previous iteration that had clustered with controls when subjected to LOOCV testing was excluded. Simultaneously, the 18 previously identified samples were incorporated into the probe selection cohort, constituting the Combined group. For a more detailed account of which samples were employed for probe selection and model construction in each analysis, refer to Table S1.
Functional correlation analysis
Comparison of cohorts and functional annotation were carried out following the procedure described by Levy et al..27 Separate functional correlation analyses were performed on the robust group, intermediate group, and the combined group. In each analysis, the RCEM subjects were compared to a set of controls consisting of individuals without any diagnosed disorder or a known episignature. These controls were also matched for age, sex, and array type. Probes with a mean methylation difference >5% between RCEM cases and matched controls and a BH adjusted p value <0.01 were then defined as differentially methylated probes (DMPs). The percentages of DMPs shared between RCEM cohort and other EpiSign v3 clinical classifier disorders were visualized using heatmap as implemented in pheatmap R package (version 1.0.12). The DMPs were ranked by p values, and the top 500 DMPs from each cohort were selected to evaluate the relationship between RCEM and 56 other disorders from the EKD. The Euclidean distances were measured using an agglomerative clustering method as described by Levy et al.27 and then visualized on a tree and leaf plot.
The genomic location of DMPs were then annotated in relation to CpG islands (CGIs) and genes using annotatr R package (version 1.22.0).28 The AnnotationHub (version 3.2.2) including the annotations hg19_cpgs, hg19_basicgenes, hg19_genes_intergenic, and hg19_genes_intronexonboundaries was employed as an annotation resource. The CGI annotations included CGI shores (0–2 kb on either side of CGIs), CGI shelves (2–4 kb on either side of CGIs), and inter-CGI regions (all remaining genomic regions). The gene annotations included promoters, promoter+, and gene body. The promoter was defined to cover the genomic region within 1 kb upstream of the transcription start site (TSS) while promoter+ encompassed the regions within 1–5 kb upstream of the TSS. The “gene body” category included untranslated regions (5ʹ UTR and 3ʹ UTR), exons, introns, and exon/intron boundaries.
Identification of differentially methylated regions
Differentially methylated regions (DMRs) were detected using the DMRcate package (version 2.10.0) in R. The DMR was defined as a region encompassing at least 5 different CpGs within 1 kb with a minimum absolute methylation difference of 10% between the case and control samples and a Fisher’s multiple comparison p value of <0.01.
Gene ontology enrichment analysis
The missMethyl (version 1.28.0) package of R29 was utilized to conduct gene ontology (GO) analysis on the identified DMRs. The DMRs were tested for GO terms enrichment, accounting for variations in the number of probes per gene on the array and the CpGs annotated to multiple genes. The analysis assumed that each gene has an equal likelihood of a significant CpG site associated with it. False discovery rates were calculated using the method of BH.21
Results
The study cohort consisted of 63 individuals, including a discovery cohort of 53 with RCEM (32 males and 21 females). Among the 53 individuals with RCEM, 29 met diagnostic criteria for VACTERL/(VACTERL), 7 met diagnostic criteria for OAV/(OAV), and 17 were classified as overlapping. The 5 sets of monozygotic twin pairs were each discordant for the clinical presentation of RCEM. All affected twins had VACTERL/(VACTERL). Table S1 summarizes the clinical characteristics of these individuals.
DNA methylation episignature discovery and model construction
All 53 individuals with RCEM presentations underwent methylation profiling. Consequently, 53 control samples were selected from the EKD and matched based on age, sex, and array type to the case samples. Using the technique outlined in the material and methodssection, various combinations of probe selection criteria were tested. For each combination, the selected probes were employed to generate hierarchical clustering and MDS plots (data not presented). Subsequently, seven samples that consistently clustered with the control samples in over 50% of probe selection criteria combinations (the no episignature group) were excluded from the discovery cohort.
Hence, 92 control samples were selected to match the 46 discovery cases. The probe selection process was repeated, yielding a set of 243 significant probes. This chosen probe set was then used to generate hierarchical clustering and MDS plots (Figure S1). This analysis yielded two distinct groups, each comprising 23 samples. One subgroup showed an intermediate methylation profile and clustered more proximate to the control samples in the MDS plot. Hierarchical clustering showed similar findings where the robust group exhibited a more pronounced difference in methylation patterns from the controls. Figure S2 highlights the seven samples that were excluded from the discovery cohort as the no episignature group relative to the case and control samples used for probe selection.
The samples in the robust group were paired with the 69 corresponding control samples for probe selection, resulting in selection of 271 probes (Table S2) that exhibited the most significant differentiation between the case samples and the controls. Hierarchical clustering and MDS visualization were performed using this selected probe set (Figures S3A and S3B). A clear separation between the case and control groups was observed in both plots.
Utilizing the selected probes, an SVM classifier was constructed. In this classifier, the 23 case samples were trained against the matched control samples, 75% of the other control samples, and 75% of samples from other rare genetic disorders with previously published episignatures from the EpiSign v3 clinical classifier within the EKD. The remaining 25% of the database samples were reserved for testing the model’s specificity. The constructed model was also applied to the remaining study samples (Figure S3D). The majority of the samples in the intermediate group received scores below 0.25. The classifier’s specificity was assessed using the unresolved samples with a cut-off MVP score of 0.25. The specificities of all the constructed models were recorded in Table 1.
Furthermore, 23 iterations of LOOCV were conducted. During each iteration, one case sample was excluded, probe selection was performed using the remaining 23 case samples and the 69 control samples, and MDS and MVP plots were generated. These plots visualized the excluded sample alongside the case and control samples used for probe selection. The high scores observed for the majority of case testing samples (Figure S3C) and the clustering of the majority of the case testing samples with the case samples used for probe selection (Figure S4) confirmed the reproducibility of the episignature.
The previously mentioned process was replicated using the samples in the intermediate group for methylation profiling, and the outcomes are shown in Figures S5A–S5D and S6. The hierarchical clustering (Figure S5A) and MDS (Figure S5B) plots demonstrated a clear separation between the case and control groups. In the summary of LOOCV MVP score plot (Figure S5C), the majority of case testing samples received MVP scores higher than the majority of the other database samples, indicating the relatively high sensitivity of the episignature. This result was further confirmed by observing the clustering of the majority of case testing samples with the case samples used for probe selection on the MDS plots (Figure S6). Individual 29 clustered entirely with control samples when omitted from the probe selection process (Figure S6). The model succeeded in clustering 18 samples from the robust group, employing an MVP score threshold of 0.25 (Figure S5D). The model’s specificity was also calculated using the unresolved column and the 0.25 MVP score cut-off value. The 255 selected probes are listed in Table S2.
Lastly, the combined cohort included the samples from the intermediate (excluding individual 29) and 18 samples from the robust cohort that achieved MVP scores >0.25 in the model based on the intermediate classifier. Model construction followed the aforementioned procedure, and results are presented in Figures 1A–1D and S7. The 377 selected probes are listed in Table S2. The heatmap (Figure 1A) displayed a distinct separation between the case and control samples. Furthermore, it was observed that the case samples were intermixed and not separated based on sub-grouping (Table S1). The distinct clusters of case and control groups can also be observed in the MDS plot (Figure 1B). In the summary of the LOOCV MVP score plot (Figure 1C), the majority of case testing samples received high MVP scores, indicating the high sensitivity and reproducibility of the episignature. The clustering of the majority of the case testing samples with the case samples used for probe selection in the MDS plots (Figure S7) further confirmed the episignature’s reproducibility. In the final MVP plot (Figure 1D), three of the samples from the robust group (individuals 5, 6, and 23) received scores above 0.25, indicating the similarity of their methylation patterns to the identified episignature. By utilizing the unresolved samples and applying a cut-off MVP score of 0.25, the model’s specificity was determined to be 98.32%.
Figure 1.
Verification of the probes selected for the combined episignature
(A) Hierarchical clustering was performed, with red representing individuals with a VACTERL phenotype, orange representing individuals with OAV, purple representing individuals with the overlapping phenotype, and blue representing control individuals.
(B) MDS analysis was conducted using the same color scheme as (A).
(C) The summary of MVP scores generated during LOOCV was visualized. Case testing samples are represented by red circles and other database samples by black circles.
(D) MVP scores calculated by the SVM classifier are plotted, with blue circles representing training samples and gray circles representing testing samples. Abbreviations of all cohorts ae listed in Table S3.
The methylation patterns of the five twin pairs were examined using hierarchical clustering and MDS plots (Figures S8 and S9). In 3 out of 5 pairs (pairs 1, 2, and 5), the twin with VACTERL phenotype and the unaffected twin clustered with cases and controls, respectively. The two other twin pairs did not show evidence of differential clustering based on their DNA methylation profiles.
Assessment of the VACTERL cohort episignature demonstrated significant overlap with the OAV cohort (Figure S10), providing evidence for a common DNA methylation disruption in a large proportion of samples across these clinical cohorts. It was observed that many OAV spectrum samples used for testing clustered with the VACTERL samples.
Clinical overlap between VACTERL and OAV individuals
Among the 23 subjects with the robust episignature, 15 (65%) individuals met clinical diagnostic criteria for VACTERL/(VACTERL), whereas 7 (30%) individuals were classified as overlapping (having features of both conditions), and only one (4%) individual met criteria for OAV/(OAV). Among the 23 subjects with the intermediate episignature, nine (39%) individuals met clinical diagnostic criteria for VACTERL/(VACTERL), nine (39%) were classified as overlapping, and five (22%) met criteria for OAV/(OAV).
Among the seven subjects in the no episignature group, five (71%) individuals met clinical diagnostic criteria for VACTERL/(VACTERL), one (14%) met criteria for OAV/(OAV), and one (14%) was classified as overlapping.
Functional correlation analysis
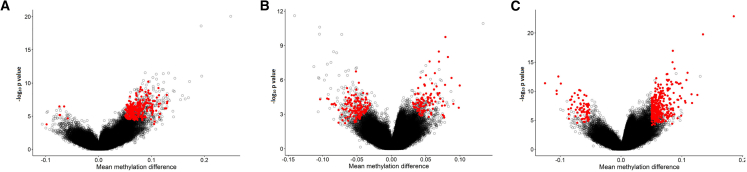
In Figure 2, the mean methylation differences between the case and control groups are graphed against the negative logarithm of the p values for the three episignatures. Notably, the robust cohort displayed the most pronounced hypermethylation, the intermediate cohort exhibited the most balanced methylation pattern, and the combined cohort displayed an intermediate pattern between these two cohorts.
Figure 2.
Visualization of mean methylation differences and the negative logarithm of p values for the three episignatures
(A) The robust episignature, (B) the intermediate episignature, and (C) the combined episignature. The selected probes are represented by red circles while other probes are marked with black circles.
We assessed the overlap of DMPs in the RCEM and 56 other neurodevelopmental cohorts included in the EpiSign v3 classifier,17 described in detail in Table S3. In total, 2,361 DMPs were identified for the robust cohort while the number of DMPs ranged from 279 to 151,848 in other episignature disorders. The robust cohort shared the highest percentages of DMPs (15%) with the SETD5-related intellectual developmental disorder (MRD23; MIM: 615761) cohort (Figure 3). The FAM50A-related X-linked intellectual developmental disorder, Armfield type (MRXSA; MIM: 300261) (11%) and CHARGE (MIM: 214800) (10%) were two other disorders that exhibited a higher proportion of DMPs shared with the robust cohort. Additionally, we identified 384 DMPs for the intermediate cohort and a total of 675 DMPs were identified for the combined cohort (Figures S11A and S12A, respectively). CHARGE syndrome had the highest percentage of overlapping DMPs with both the intermediate cohort (6%) and the combined cohort (7%).
Figure 3.
Overlap of DMPs among episignature disorders
Heatmap presenting the percentage of probes shared between each pair of cohorts. Colors range from white (indicating zero overlap) to red (indicated full overlap), representing the percentage of probes from the x axis cohort that are also present in the y axis cohort’s probes. Abbreviations for all cohorts are listed in Table S3.
The mean of DNA methylation levels (β values) was compared between the RCEM cohort and the other EKD disorders. This analysis revealed a predominant hypermethylation trend for RCEM subjects compared to the majority of other studied disorders (Figures 4A, S11B and S12B). The robust cohort showed highest mean methylation differences compared to the intermediate and combined cohorts.
Figure 4.
Functional correlation analysis of the robust group
(A) Methylation differences of all DMPs for each cohort. Red lines indicate mean methylation difference. Each circle represents one probe.
(B) Tree and leaf plot of hierarchical clustering of all 56 cohorts using the top DMPs for each cohort. A leaf node represents a cohort, with node sizes illustrating relative scales of the number of selected DMPs for the corresponding cohort, and node colors are indicative of the overall mean methylation difference.
(C) DMPs annotated in relation to CGIs (upper side) and genes (lower side). Abbreviations of all cohorts are listed in Table S3.
Up to 500 of the most significant DMPs from each cohort were examined for relatedness to other episignature disorders. The tree and leaf plot showed highest similarity of the robust (Figure 4B) and combined (Figure S12C) cohorts with Wolf-Hirschhorn syndrome (WHS; MIM: 194190) and the intermediate cohort with velocardiofacial syndrome (VCFS; MIM: 192430) and Cornelia de Lange syndrome (CdLS; MIM: 122470) (Figure S11C).
The DMPs were annotated in relation to CGIs and genes (Figure 4C). The CGI annotation revealed that a higher percentage of probes (∼38%) were located inside the CGIs for the robust and combined cohorts, whereas a slightly lower proportion (30.7%) was observed for the intermediate cohort. Similarly, a higher percentage of probes was annotated within promoter regions of the robust (28.7%) and the combined (27%) relative to the intermediate cohort (21.6%). On the other hand, a higher percentage of probes was located inside the coding sequences for the intermediate (40.3%) relative to the robust (31.9%) and the combined cohort (33%) (Figures S11D and S12D).
DMRs and gene ontology assessment
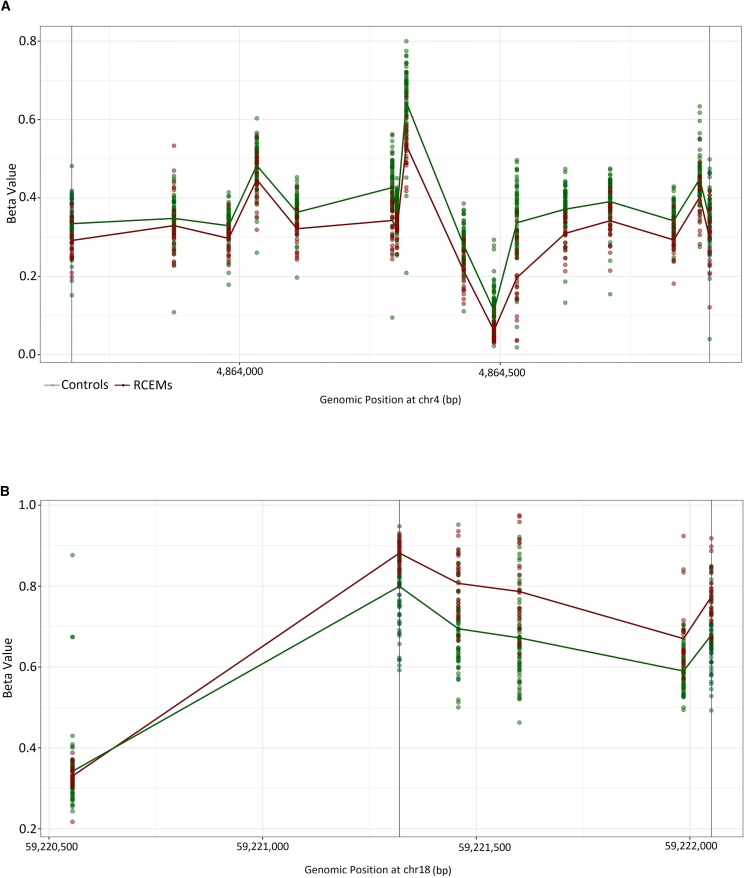
The robust cohort showed a total of 66 significant DMRs (Fisher’s multiple comparison p value <0.01) with a minimum mean methylation difference of 5%, 65 of which were hypermethylation events (Figure 5; Table S4). The combined cohort had 12 significant DMRs all overlapping with ones identified in the robust cohort. Only one significant hypomethylated DMR was identified in the intermediate group. Seven significant GO terms (false discovery rate [FDR] <0.01) were enriched in the robust cohort and included cell-cell adhesion, calcium ion binding, and being a component of plasma membrane (Table S5). IL-6, CELSR3, CDH20, and several members of protocadherin gamma gene cluster were overrepresented across these GO terms. No significant GO term was found for the DMRs associated with the intermediate and combined cohorts.
Figure 5.
DMRs in the intermediate and robust groups
(A) A general hypomethylation pattern was observed in the single DMR identified for the intermediate cluster located on chromosome 4.
(B) A predominant hypermethylation was observed in 65 out of 66 DMRs detected in the robust cluster. The DMR located on chromosome 18 was presented as an example. The details of other DMRs are provided in Table S4.
Discussion
The utility of DNA methylation episignatures for clinical diagnosis of rare genetic disorders and interpretation of ambiguous genetic findings has been established.18 The specific genetic etiology of a significant proportion of rare diseases, including RCEM, remains elusive. A clinical diagnosis of RCEM is often one of exclusion, involving extensive genetic testing to rule out other similar disorders, often associated with a long diagnostic odyssey. However, we have now identified a DNA methylation episignature that is a robust diagnostic biomarker for an RCEM characterized as VACTERL or OAV association that has the potential to dramatically change the way in which these conditions are diagnosed and will help to better differentiate them from other genetic conditions and understand their primary cause(s). The shared episignature between OAV and VACTERL also suggests a common underlying etiology for these two multiple malformation associations.
Stratification of the RCEM episignature to the robust and intermediate profile demonstrates evidence of a methylation profile gradient rather than a binary state. The model constructed using the robust cohort failed to detect samples in the intermediate cohort, whereas the intermediate classifier correctly assigned the majority of the robust cohort (Figures S3D and S5D). Of the individuals with the VACTERL/(VACTERL) (n = 29) diagnoses, 52% showed the robust signature, 31% had the intermediate profile, and 17% show no evidence of an episignature. Among the overlapping (n = 17) individuals, 41% had robust, 53% had intermediate, and 6% had no episignature. Finally, in the OAV/(OAV) (n = 7), 14.3% had robust, 71.4% had intermediate, and 14.3% had no episignature.
The functional correlation analysis revealed that the robust group showed a higher degree of similarity with WHS (Figure 4B). WHS is a multiple congenital anomaly syndrome due to a contiguous gene deletion at chromosome 4p16.3 characterized by prenatal onset growth deficiency, skeletal anomalies, a variable degree of developmental disability, characteristic craniofacial features, heart lesions, and closure defects,30 which overlap with some of the features observed in VACTERL and OAV (Table S6). While a similar pattern was also observed in the combined group, the tree and leaf plot clustered the intermediate group alongside the VCFS and CdLS (Figures S11C and S12C). However, it is important to acknowledge that this clustering analysis might be influenced by the limited probe selection with only 384 DMPs meeting significance criteria in the intermediate group rather than the top 500 significant DMRs selected for other cohorts.
MRD23 (15%), MRXSA (11%), and CHARGE syndrome (10%) presented the highest overlap of DMPs with the robust group, disorders that also share some common clinical features with RCEM (Table S6). SETD5-related neurodevelopmental disorder (MRD23) is primarily characterized by intellectual disability and delayed speech development. Variable skeletal abnormalities, including scoliosis, kyphosis, lordosis, and leg-length discrepancies, as well as congenital heart defects have been reported in individuals affected by this condition.31 MRXSA is another neurodevelopmental disorder characterized by intellectual disability, which also presents with other variable features including ocular anomalies, mild congenital heart defects, and renal anomalies.32 Lastly, CHARGE syndrome exhibits a wide spectrum of congenital anomalies including choanal atresia, cardiac malformations, ear abnormalities, and coloboma, which significantly overlap with those observed in RCEM.33 CHARGE syndrome also shared the highest percentage of DMPs with the intermediate (6%) and the combined group (7%), providing molecular pathophysiological association for these clinically overlapping syndromes. Interestingly, CHARGE syndrome was initially described as an association (i.e., a combination of co-occurring anomalies, observed together more frequently than expected for a random co-occurrence) similar to VACTERL and OAV and was designated an acronym until its underlying genetic defect (pathogenic variants in CHD7) was discovered.34
The DMR analyses of robust cluster revealed several genes potentially relevant to observed phenotypes or mechanisms suggested in RCEM (Table S4). The DMR identified on chromosome 3 involves CELSR3, which was associated with congenital anomalies of the kidneys and urinary tract as well as central nervous system anomalies.35
Several members of the protocadherin gamma gene cluster were found in the DMR located on chromosome 5. Although these genes play a crucial role in the establishment and function of specific cell-cell connections in brain and synaptic development in the spinal cord,36 recent studies have revealed their function in human congenital heart defects.37 In addition, the DMR located on chromosome 7 involves IL-6. While IL-6 has been widely known for its pro-inflammatory actions,38 there is some evidence revealing its role in kidney,39 congenital heart,40 and cardio-cerebrovascular diseases.41 This gene also contributes to the development of insulin resistance and type 2 diabetes mellitus,42 which is suggested as one of the maternal risks associated with RCEM. In addition, some studies have reported an association between NAD+ coenzymes and the expression level of IL-6.43,44 This link is intriguing, as there have been suggestions that NAD+ deficiency might serve as an underlying cause for various multiple malformation associations.3,4 Furthermore, the DMR located on chromosome 11 overlaps with FIBIN, which was associated with cardiomyocyte hypertrophy and cardiomyopathy in murine hearts.45
Considering the potential disruption of vertebral embryology in RCEM, we have also identified several genes associated with embryonic development. For example, the DMR identified on chromosome 6 overlapped with POU5F1, which is a transcription factor containing a POU homeodomain, and plays a critical role in human early embryonic development, stem cell pluripotency, and promoting cardiomyocyte development.46 It seems that IL-6 is also involved in human embryonic development.47 It has been reported that FIBIN plays a critical role during mouse embryonic development.48 Additionally, the DMR located on chromosome 18 overlaps CDH20, which has a prominent effect on neural tube compartmentalization during mouse embryogenesis.49
The DMR analysis of the intermediate group identified MSX1 located on chromosome 4 (Table S4). This gene is involved in several biological processes including embryonic limb morphogenesis, heart morphogenesis, face development, and skeletal system morphogenesis. Additionally, MSX1 encodes a transcription factor that plays a crucial role in multiple epithelial-mesenchymal interactions during vertebrate embryogenesis.50
In conclusion, etiological insight into any of the associations now recognized as part of the RECM group using omic technologies has previously been largely unpromising. The demonstration of a unified DNA methylation pattern for individuals with OAV and VACTERL association described herein provides a robust diagnostic biomarker and supports the concept that these two of the multiple malformation associations may represent a single malformation spectrum. Expanding the episignature identification to include other categories of RCEM beyond VACTERL and OAV is pinpointed as a future direction. These findings support the notion that the RCEM group of conditions should be considered a spectrum that could be studied as a group. Future studies that examine methylation signatures for additional associations (e.g., POC, L-BWD, OEIS, MURCS, URSM, and sirenomelia) will be helpful in further understanding these findings in the context of early embryonic development.
Data and code availability
The deposition of individual genomic, epigenomic, or any other personally identifiable data that has not previously been made publicly available for samples in the EpiSign Knowledge Database (EKD) is prohibited from deposition in publicly accessible databases due to institutional and ethical restrictions. Specifically, these include data and samples submitted from external institutions to London Health Sciences EKD that are subject to institutional material and data transfer agreements, data submitted to London Health Sciences for episignature assessment under Research Services Agreements, and research study cohorts under Institutional Research Ethics Approval (Western University REB 106302 and REB 116108). EpiSign is a commercial software and is not publicly available.
Acknowledgments
This work was funded in part by the government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-188) awarded to B.S., the Care4Rare Canada Consortium funded by Genome Canada and the Ontario Genomics Institute (OGI-147), the Canadian Institutes of Health Research (CIHR GP1-155867), Ontario Research Fund, Genome Alberta, Genome British Columbia, Génome Québec, and Children's Hospital of Eastern Ontario Foundation to K.M.B., and Greenwood Genetic Center Foundation to R.E.S. A.H. and H.B. were supported by the Solve-RD project. The Solve-RD project received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 779257. J.A.’s work is funded by MSDAVENIR (Devo-Decode project) and Mutuelles AXA (Chaire Tête et Cœur). The authors would like to thank Prof. Dr. Christoph Bock, CeMM and BSF, Vienna for his technical assistance.
Author contributions
Conceptualization: S.H., K.K., K.M.B., and B.S.; data curation: S.H., K.K., J.K., and J.R.; formal analysis: S.H., K.K., M.A.L., and R.R.; investigation: S.H., K.K., M.A.L., R.R., J.K., and J.R.; sample collection: R.E.S., A.M.W.-B, M.T.C., J.R., C.M.A., S.L.S., P.T.B., M.L.T., C.D.S., I.A.L.M.v.R., R.v.d.P., I.d.B., R.M.K., A.H., H.B., M.Z.E., A.P., J.A., S.L., T.B.B., and K.M.B.; methodology: S.H., K.K., M.A.L., and R.R.; project administration: B.S., K.M.B., H.M., and C.L.-Y.; software: S.H., K.K., M.A.L., and R.R.; writing – original draft: S.H., K.K., R.E.S., K.M.B., and B.S.; writing – review and editing: all authors.
Declaration of interests
B.S. is a shareholder in EpiSign Inc. involved in commercial uses of EpiSign technology.
Published: July 31, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2024.07.005.
Contributor Information
Kym M. Boycott, Email: kboycott@cheo.on.ca.
Bekim Sadikovic, Email: bekim.sadikovic@lhsc.on.ca.
Supplemental information
References
- 1.Adam A.P., Curry C.J., Hall J.G., Keppler-Noreuil K.M., Adam M.P., Dobyns W.B. Recurrent constellations of embryonic malformations re-conceptualized as an overlapping group of disorders with shared pathogenesis. Am. J. Med. Genet. 2020;182:2646–2661. doi: 10.1002/ajmg.a.61847. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson R.E. Common pathogenesis for sirenomelia, OEIS complex, limb-body wall defect, and other malformations of caudal structures. Am. J. Med. Genet. 2021;185:1379–1387. doi: 10.1002/ajmg.a.62103. [DOI] [PubMed] [Google Scholar]
- 3.Mark P.R. NAD+ deficiency in human congenital malformations and miscarriage: A new model of pleiotropy. Am. J. Med. Genet. 2022;188:2834–2849. doi: 10.1002/ajmg.a.62764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M., Bedard T., Crawford S., Grevers X., Lowry R. Craniofacial Microsomia, Associated Congenital Anomalies, and Risk Factors in 63 Cases from the Alberta Congenital Anomalies Surveillance System. J. Pediatr. 2023;261 doi: 10.1016/j.jpeds.2023.113528. [DOI] [PubMed] [Google Scholar]
- 5.Stevens S.J.C., Stumpel C.T.R.M., Diderich K.E.M., van Slegtenhorst M.A., Abbott M.A., Manning C., Balciuniene J., Pyle L.C., Leonard J., Murrell J.R., et al. The broader phenotypic spectrum of congenital caudal abnormalities associated with mutations in the caudal type homeobox 2 gene. Clin. Genet. 2022;101:183–189. doi: 10.1111/cge.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Putte R., Dworschak G.C., Brosens E., Reutter H.M., Marcelis C.L.M., Acuna-Hidalgo R., Kurtas N.E., Steehouwer M., Dunwoodie S.L., Schmiedeke E., et al. A Genetics-First Approach Revealed Monogenic Disorders in Patients With ARM and VACTERL Anomalies. Front. Pediatr. 2020;8:310. doi: 10.3389/fped.2020.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H., Enriquez A., Rapadas M., Martin E.M.M.A., Wang R., Moreau J., Lim C.K., Szot J.O., Ip E., Hughes J.N., et al. NAD Deficiency, Congenital Malformations, and Niacin Supplementation. N. Engl. J. Med. 2017;377:544–552. doi: 10.1056/nejmoa1616361. [DOI] [PubMed] [Google Scholar]
- 8.Szot J.O., Campagnolo C., Cao Y., Iyer K.R., Cuny H., Drysdale T., Flores-Daboub J.A., Bi W., Westerfield L., Liu P., et al. Bi-allelic Mutations in NADSYN1 Cause Multiple Organ Defects and Expand the Genotypic Spectrum of Congenital NAD Deficiency Disorders. Am. J. Hum. Genet. 2020;106:129–136. doi: 10.1016/j.ajhg.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuny H., Rapadas M., Gereis J., Martin E.M.M.A., Kirk R.B., Shi H., Dunwoodie S.L. NAD deficiency due to environmental factors or gene–environment interactions causes congenital malformations and miscarriage in mice. USA. 2020;117:3738–3747. doi: 10.1073/pnas.1916588117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mark P.R., Dunwoodie S.L. Viewing teratogens through the lens of nicotinamide adenine dinucleotide (NAD+) Birth Defects Res. 2022;114:1313–1323. doi: 10.1002/bdr2.2089. [DOI] [PubMed] [Google Scholar]
- 11.Goldenhar M. Associations malformatives de l’ oeil et l’ oreille, en particulierle syndrome dermoide epibu1baire- appendices auriculaires-fistula auris congenita et ses relations avec la dysostose mandibulo -faciale. J. Genet. Hum. 1952;1:243–283. [Google Scholar]
- 12.Gorlin R.J., Jue K.L., Jacobsen U., Goldschmidt E. Oculoauriculovertebral dysplasia. J. Pediatr. 1963;63:991–999. doi: 10.1016/S0022-3476(63)80233-4. [DOI] [PubMed] [Google Scholar]
- 13.Quan L., Smith D.W. The VATER association. Vertebral defects, anal atresia, T-E fistula with esophageal atresia, radial and renal dysplasia: A spectrum of associated defects. J. Pediatr. 1973;82:104–107. doi: 10.1016/S0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- 14.Temtamy S.A., Miller J.D. Extending the scope of the VATER association: Definition of the VATER syndrome. J. Pediatr. 1974;85:345–349. doi: 10.1016/S0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- 15.Nora A.H., Nora J.J. A Syndrome of Multiple Congenital Anomalies Associated With Teratogenic Exposure. Arch. Environ. Health. 1975;30:17–21. doi: 10.1080/00039896.1975.10666626. [DOI] [PubMed] [Google Scholar]
- 16.van de Putte R., van Rooij I.A.L.M., Marcelis C.L.M., Guo M., Brunner H.G., Addor M.C., Cavero-Carbonell C., Dias C.M., Draper E.S., Etxebarriarteun L., et al. Spectrum of congenital anomalies among VACTERL cases: a EUROCAT population-based study. Pediatr. Res. 2020;87:541–549. doi: 10.1038/s41390-019-0561-y. [DOI] [PubMed] [Google Scholar]
- 17.Levy M.A., McConkey H., Kerkhof J., Barat-Houari M., Bargiacchi S., Biamino E., Bralo M.P., Cappuccio G., Ciolfi A., Clarke A., et al. Novel diagnostic DNA methylation episignatures expand and refine the epigenetic landscapes of Mendelian disorders. HGG Adv. 2022;3 doi: 10.1016/j.xhgg.2021.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadikovic B., Levy M.A., Kerkhof J., Aref-Eshghi E., Schenkel L., Stuart A., McConkey H., Henneman P., Venema A., Schwartz C.E., et al. Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet. Med. 2021;23:1065–1074. doi: 10.1038/s41436-020-01096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerkhof J., Rastin C., Levy M.A., Relator R., McConkey H., Demain L., Dominguez-Garrido E., Donker Kaat L., Douzgou Houge S., DuPont B.R., et al. Diagnostic utility and reporting recommendations for clinical DNA methylation episignature testing in genetically undiagnosed rare diseases. Genet. Med. 2024;26 doi: 10.1016/j.gim.2024.101075. [DOI] [PubMed] [Google Scholar]
- 20.Aref-Eshghi E., Kerkhof J., Pedro V.P., Barat-Houari M., Ruiz-Pallares N., Andrau J.C., Lacombe D., Van-Gils J., Fergelot P., Dubourg C., et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am. J. Hum. Genet. 2020;106:356–370. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aref-Eshghi E., Rodenhiser D.I., Schenkel L.C., Lin H., Skinner C., Ainsworth P., Paré G., Hood R.L., Bulman D.E., Kernohan K.D., et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am. J. Hum. Genet. 2018;102:156–174. doi: 10.1016/j.ajhg.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho D.E., Imai K., King G., Stuart E.A. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit. Anal. 2007;15:199–236. doi: 10.1093/pan/mpl013. [DOI] [Google Scholar]
- 24.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43 doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houseman E.A., Accomando W.P., Koestler D.C., Christensen B.C., Marsit C.J., Nelson H.H., Wiencke J.K., Kelsey K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinf. 2012;13 doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y., Yosef H. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- 27.Levy M.A., Relator R., McConkey H., Pranckeviciene E., Kerkhof J., Barat-Houari M., Bargiacchi S., Biamino E., Palomares Bralo M., Cappuccio G., et al. Functional correlation of genome-wide DNA methylation profiles in genetic neurodevelopmental disorders. Hum. Mutat. 2022;43:1609–1628. doi: 10.1002/humu.24446. [DOI] [PubMed] [Google Scholar]
- 28.Cavalcante R.G., Sartor M.A. Annotatr: Genomic regions in context. Bioinformatics. 2017;33:2381–2383. doi: 10.1093/bioinformatics/btx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phipson B., Maksimovic J., Oshlack A. MissMethyl: An R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics. 2016;32:286–288. doi: 10.1093/bioinformatics/btv560. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia A., Filippi T., Carey J.C. Update on the clinical features and natural history of Wolf-Hirschhorn (4p-) syndrome: Experience with 87 patients and recommendations for routine health supervision. Am. J. Med. Genet. C Semin. Med. Genet. 2008;148C:246–251. doi: 10.1002/ajmg.c.30187. [DOI] [PubMed] [Google Scholar]
- 31.Grozeva D., Carss K., Spasic-Boskovic O., Parker M.J., Archer H., Firth H.V., Park S.M., Canham N., Holder S.E., Wilson M., et al. De novo loss-of-function mutations in SETD5, encoding a methyltransferase in a 3p25 microdeletion syndrome critical region, cause intellectual disability. Am. J. Hum. Genet. 2014;94:618–624. doi: 10.1016/j.ajhg.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y.R., Khan K., Armfield-Uhas K., Srikanth S., Thompson N.A., Pardo M., Yu L., Norris J.W., Peng Y., Gripp K.W., et al. Mutations in FAM50A suggest that Armfield XLID syndrome is a spliceosomopathy. Nat. Commun. 2020;11:3698. doi: 10.1038/s41467-020-17452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Källén K., Robert E., Mastroiacovo P., Castilla E.E., Källén B. CHARGE association in newborns: A registry-based study. Teratology. 1999;60:334–343. doi: 10.1002/(SICI)1096-9926(199912)60:6<334::AID-TERA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Pagon R.A., Graham J.M., Zonana J., Yong S.L. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J. Pediatr. 1981;99:223–227. doi: 10.1016/S0022-3476(81)80454-4. [DOI] [PubMed] [Google Scholar]
- 35.Stegmann J.D., Kalanithy J.C., Dworschak G.C., Ishorst N., Mingardo E., Lopes F.M., Ho Y.M., Grote P., Lindenberg T.T., Yilmaz Ö., et al. Bi-allelic variants in CELSR3 are implicated in central nervous system and urinary tract anomalies. npj Genomic Med. 2024;9:18. doi: 10.1038/s41525-024-00398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner J.A., Wang X., Tapia J.C., Sanes J.R. Gamma protocadherins are required for synaptic development in the spinal cord. USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu W., Chhibbar P., Lo C. The transcriptional landscape of the clustered protocadherins in the cardiovascular system. Eur. Heart J. 2021;42 doi: 10.1093/eurheartj/ehab724.3202. [DOI] [Google Scholar]
- 38.Spencer S., Bal S.K., Egner W., Allen H.L., Raza S.I., Ma C.A., Gürel M., Zhang Y., Sun G., Sabroe R.A., et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J. Exp. Med. 2019;216:1986–1998. doi: 10.1084/jem.20190344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H., Lei C.-T., Zhang C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q., Wang H., Xue J., Wu D. Associations between IL-6 Variations and Congenital Heart Disease Incidence among Chinese Han People. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26:e921032. doi: 10.12659/MSM.921032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su J.-H., Luo M.-Y., Liang N.-, Gong S.-X., Chen W., Huang W.-Q., Tian Y., Wang A.-P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.745061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehman K., Akash M.S.H., Liaqat A., Kamal S., Qadir M.I., Rasul A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017;27:229–236. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712. [DOI] [PubMed] [Google Scholar]
- 43.Chini C.C.S., Peclat T.R., Gomez L.S., Zeidler J.D., Warner G.M., Kashyap S., Mazdeh D.Z., Hayat F., Migaud M.E., Paulus A., et al. Dihydronicotinamide Riboside Is a Potent NAD+ Precursor Promoting a Pro-Inflammatory Phenotype in Macrophages. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.840246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elhassan Y.S., Kluckova K., Fletcher R.S., Schmidt M.S., Garten A., Doig C.L., Cartwright D.M., Oakey L., Burley C.V., Jenkinson N., et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019;28:1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen M., Schmiedel N., Dierck F., Hille S., Remes A., Senger F., Schmidt I., Lüllmann-Rauch R., Müller O.J., Frank D., et al. Fibin regulates cardiomyocyte hypertrophy and causes protein-aggregate-associated cardiomyopathy in vivo. Front. Mol. Biosci. 2023;10 doi: 10.3389/fmolb.2023.1169658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeVeale B., Brokhman I., Mohseni P., Babak T., Yoon C., Lin A., Onishi K., Tomilin A., Pevny L., Zandstra P.W., et al. Oct4 is required ∼E7.5 for proliferation in the primitive streak. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai N., Scarrow M., Lawson J., Kinzer D., Goldfarb J. Evaluation of the effect of interleukin-6 and human extracellullar matrix on embryonic development. Hum. Reprod. 1999;14:1588–1592. doi: 10.1093/humrep/14.6.1588. [DOI] [PubMed] [Google Scholar]
- 48.Lakner J., Seyer C., Hermsdorf T., Schöneberg T. Characterization of the expression, promoter activity and molecular architecture of fibin. BMC Biochem. 2011;12 doi: 10.1186/1471-2091-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore R., Champeval D., Denat L., Tan S.-S., Faure F., Julien-Grille S., Larue L. Involvement of cadherins 7 and 20 in mouse embryogenesis and melanocyte transformation. Oncogene. 2004;23:6726–6735. doi: 10.1038/sj.onc.1207675. [DOI] [PubMed] [Google Scholar]
- 50.Liang J., Von Den Hoff J., Lange J., Ren Y., Bian Z., Carels C.E.L. MSX1 mutations and associated disease phenotypes: Genotype-phenotype relations. Eur. J. Hum. Genet. 2016;24:1663–1670. doi: 10.1038/ejhg.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deposition of individual genomic, epigenomic, or any other personally identifiable data that has not previously been made publicly available for samples in the EpiSign Knowledge Database (EKD) is prohibited from deposition in publicly accessible databases due to institutional and ethical restrictions. Specifically, these include data and samples submitted from external institutions to London Health Sciences EKD that are subject to institutional material and data transfer agreements, data submitted to London Health Sciences for episignature assessment under Research Services Agreements, and research study cohorts under Institutional Research Ethics Approval (Western University REB 106302 and REB 116108). EpiSign is a commercial software and is not publicly available.