Abstract
Background
Although numerous studies had revealed associations between autoimmune diseases (AIDs) and thyroid cancer (TC), the potential causal associations between the two remain poorly defined.
Methods
Using five approaches, two-sample Mendelian randomization (MR) analyses were carried out to determine the causal effects of 12 major AIDs on risk of TC. The sensitivity analyses were conducted to verify the reliability of the analysis. The reverse MR analysis was performed to evaluate the possibility of reverse causation.
Results
The results showed a significant causal association of systemic lupus erythematosus (SLE) and primary biliary cirrhosis (PBC) on the risk of TC. Genetically predicted PBC elevated the risk of TC (OR = 1.46, 95% CI = 1.06-2.02, p = 0.021). The risk of TC was also increased by genetically predicted SLE (OR = 6.52, 95% CI = 1.38-30.84, p = 0.018) with heterogeneity. After outlier-corrected analyses, the results still suggested that genetically predicted SLE increased the risk of TC (p = 0.019). No evidence of a causal relationship between the remaining 10 AIDs and TC was observed. No reverse causal effects of TC on AIDs were found in reverse MR analysis.
Conclusion
These findings support a significant causal association of SLE/PBC on the increased risk of TC, indicating that patients with SLE/PBC should be under a close monitoring of TC.
Keywords: autoimmune disease, thyroid cancer, Mendelian randomization, causality, systemic lupus erythematosus, primary biliary cirrhosis
1. Introduction
Thyroid cancer (TC) is a prevalent endocrine malignant tumor in China, with an estimated number of new cases exceeding 224,000 in 2022 (1). TC arises from endoderm-derived follicular cells or neural crest-derived C-cells and comprises papillary, follicular, medullary, and undifferentiated subtypes (2). With the increasing use of diagnostic imaging and surveillance, its incidence continues to rise steadily worldwide (3, 4). The precise causes of TC are multifactorial, complicated, and not well understood. Apart from the most-established risk factor for TC (childhood exposure to ionizing radiation), other possible risk factors such as estrogen, chromosomal and genetic alterations, lifestyle changes, autoimmune thyroid disease, and excess body weight have been reported (5). Converging lines of evidence indicate that the immune system exerts momentous roles during the multistep development of TC (6–8).
Autoimmune disease (AID) is an excessive host immune response with diverse clinical manifestations caused by the impairment of the host immune regulatory function (9). As we all know, both cancers and AIDs share the feature of immune system dysregulation. Accumulating evidence reveals that AIDs have been associated with multiple tumors. Zeev Elkoshi suggested that pre-existing AIDs promoted the initiation of cancer and its early growth, leading to an increased risk of cancer (10). A review indicates that the majority of autoimmune rheumatic diseases are linked to a slightly elevated risk of malignancy (11). A significant association to develop cancer in scleroderma patients has been observed when compared with the general population (12). Evidence supporting the intimate relationship between dermatomyositis and malignancies, such as non-Hodgkin lymphoma, lung, and colorectal cancers, has emerged (13). Hemminki et al. suggested that the autoimmune process in AIDs contributed to lung cancer susceptibility (14). Yang et al. indicated an association between Sjogren’s syndrome and increased risks of head and neck cancers, encompassing several sites including the oral cavity, oropharynx, nasopharynx, and thyroid (15). Chen et al. reported that patients with Graves’ disease (GD) exhibited a significantly higher risk of developing TC, with a 16-fold hazard within the first three years of diagnosis compared to non-GD patients (16). Due to the inherent limitations of observational studies, such as reporting biases and potential confounding factors, it cannot be proven whether there is a causal relationship between AIDs and TC.
By employing genetic variants as instrument variables (IVs), Mendelian randomization (MR) could evaluate the causality between an exposure and outcome (17). In this work, the two-sample MR analysis was employed to deduce the causative relationships between 12 major AIDs, including systemic lupus erythematosus (SLE), ankylosing spondylitis (AS), rheumatoid arthritis (RA), primary biliary cirrhosis (PBC), multiple sclerosis (MS), ulcerative colitis (UC), type 1 diabetes (T1D), celiac disease (CeD), Crohn’s disease (CD), asthma, psoriasis (PsO), and hypothyroidism, and the risk of TC using summary data from genome-wide association studies (GWAS). Moreover, a reverse MR analysis was conducted to investigate the potential for reverse causality.
2. Materials and methods
2.1. Data sources
To infer causal relationships between TC and AIDs, GWAS summary statistics on TC and 12 AIDs derived from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk) were downloaded. All individuals involved in this study were of European ancestry. Table 1 presented the specific data sources in detail.
Table 1.
Details of data sources.
| Disease | GWAS ID | Sample | Case | Control | SNP | Population | Author |
|---|---|---|---|---|---|---|---|
| Systemic lupus erythematosus | ebi-a-GCST003156 | 14267 | 5201 | 9066 | 7071163 | European | Bentham J |
| Rheumatoid arthritis | ebi-a-GCST90013534 | 58284 | 14361 | 43923 | 13108512 | European | Ha E |
| Multiple sclerosis | ebi-a-GCST005531 | 38582 | 14498 | 24091 | 132089 | European | Beecham AH |
| Crohn’s disease | ebi-a-GCST003044 | 20883 | 5956 | 14927 | 110583 | European | Liu JZ |
| Ulcerative colitis | ieu-a-970 | 47745 | 13768 | 33977 | 156116 | European | Liu |
| Ankylosing spondylitis | ebi-a-GCST005529 | 22647 | 9069 | 1550 | 99962 | European | Cortes A |
| Type 1 diabetes | ebi-a-GCST010681 | 24840 | 9266 | 15574 | 12783129 | European | Forgetta V |
| Primary biliary cirrhosis | ebi-a-GCST005581 | 11375 | 2861 | 8514 | 119756 | European | Liu JZ |
| Psoriasis | ebi-a-GCST005527 | 33394 | 10588 | 22806 | 138661 | European | Tsoi LC |
| Celiac disease | ebi-a-GCST000612 | 15283 | 4533 | 10750 | 518292 | European | Dubois PC |
| Asthma | ebi-a-GCST90014325 | 408442 | 56167 | 352255 | 34551291 | European | Valette K |
| Hypothyroidism | ebi-a-GCST90018862 | 410141 | 30155 | 379986 | 24138872 | European | Sakaue S |
| Thyroid cancer | ieu-a-1082 | 1080 | 649 | 431 | 572028 | European | Kohler A |
GWAS, genome-wide association study; SNP, single nucleotide polymorphism.
2.2. Single nucleotide polymorphism selection
SNPs were determined as the IVs with genome-wide significance (p <5×10-8). Linkage disequilibrium clumping was performed by setting r2 <0.001 and clump distance = 10,000 kb, ensuring the independence of SNPs associated with exposure. The F-statistic was calculated to determine instrument strength using the formula: F = (βexposure/SEexposure)2. The F-statistic larger than 10 indicates a slight possibility of a weak IV deviation.
2.3. Study design
In order to accurately infer potential causal relationships between TC and AIDs, 3 assumptions should be met: (1) IVs are significantly associated with the exposure; (2) IVs are independent of any confounding factor; and (3) IVs affect the outcomes exclusively via exposure instead of through any alternative pathway (18) ( Supplementary Figure S1 ). The process of this study was carried out in strict accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) Statement.
2.4. Statistical analysis
Five MR analysis methods based on different assumptions, including simple mode, inverse-variance-weighted (IVW), MR-Egger regression, weighted median (WM), and weighted mode, were applied to estimate the causal associations. IVW, as the main analysis method, combines the Wald ratio of each SNP to provide consistent causal estimates of the exposure on the outcome. The heterogeneity was assessed by Cochran’s Q test. The WM method offers unbiased causal estimates when the valid IVs account for more than half of the weight. The horizontal pleiotropy was evaluated with the MR-Egger method. We used the simple mode and weighted mode methods to supplement IVW estimates. The detection of outlier IVs and correction of horizontal pleiotropy were achieved by the MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) method. “TwoSampleMR” (version 0.5.8) and “MRPRESSO” (version 1.0) packages in R software (version 4.2.3) were employed to conduct MR analyses.
3. Results
3.1. IVs selection
The IVs that were significantly associated with 12 AIDs were obtained from the GWAS. Then, palindromic SNPs (ie., A/T or G/C) and SNPs that were not present in the outcome were eliminated. Finally, 8 IVs for SLE, 16 IVs for RA, 17 IVs for MS, 44 IVs for CD, 30 IVs for UC, 9 IVs for AS, 4 IVs for T1D, 5 IVs for PBC, 8 IVs for PsO, 7 IVs for CeD, 7 IVs for asthma, and 18 IVs for hypothyroidism (F-statistic > 10) were selected.
3.2. Causal effects of AIDs on TC
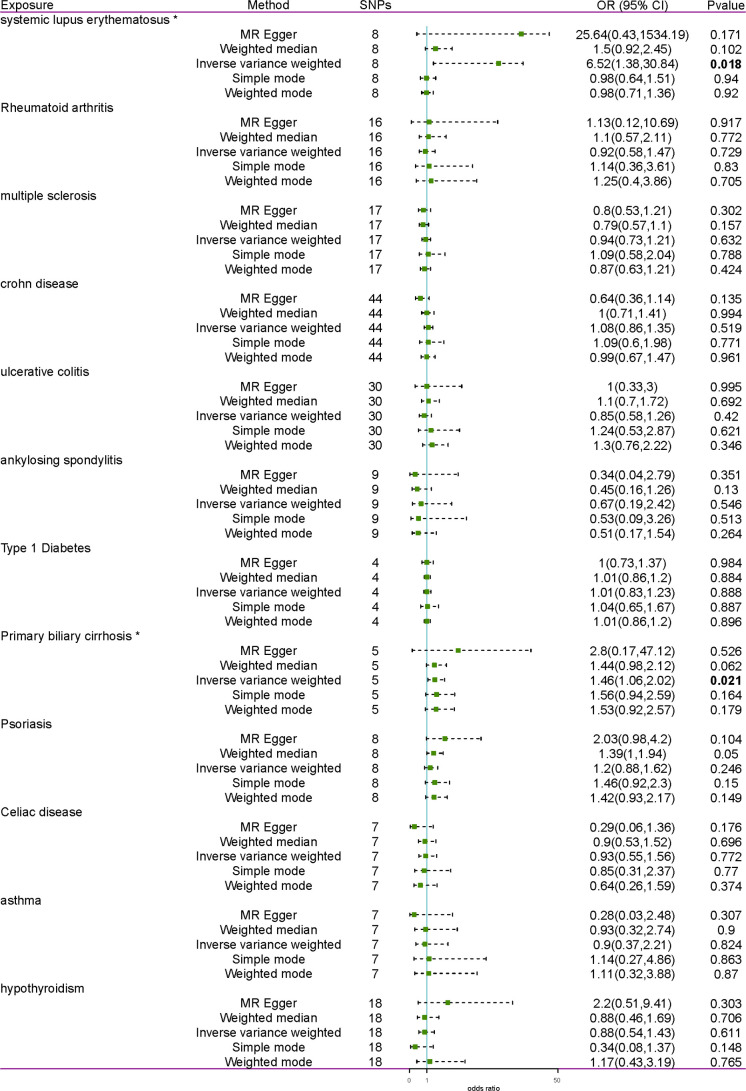
The MR analyses suggested a significant causal association of SLE and PBC on the risk of TC ( Figure 1 ). With the IVW method, genetically predicted PBC elevated the risk of TC (OR = 1.46, 95% CI = 1.06-2.02, p = 0.021). Cochran’s Q test revealed no heterogeneity (MR-Egger Cochran’s Q = 0.518, p = 0.915; IVW Cochran’s Q = 0.725, p = 0.948) ( Table 2 ). The risk of TC was likewise increased by genetically predicted SLE (OR = 6.52, 95% CI = 1.38-30.84, p = 0.018). Nonetheless, considerable heterogeneity was found in IVW (Cochran’s Q = 412.75, p = 4.393E-85) and MR-Egger (Cochran’s Q = 412.75, p = 4.393E-85). Then, the random effects IVW method was performed to assess the causal relationships. After removing 6 outliers (rs1143679, rs13019891, rs2431697, rs2459611, rs7097397, and rs7823055) detected by the MR-PRESSO test, only two SNPs remained. Hence, SNPs with genome-wide significance (p <5×10-6) were determined as the IVs. After eliminating linkage disequilibrium and deleting palindromic SNPs and SNPs not available in the outcome, a total of 12 SNPs were obtained. The IVW method was performed to estimate causality again, indicating a significant causal relationship between SLE and TC (p = 0.004). After MR-PRESSO detecting outliers, the results indicated that the risk of TC was elevated by genetically predicted SLE (p = 0.019).
Figure 1.
Forest plot of the effect of AIDs on TC. AID, autoimmune disease; TC, thyroid cancer; SNP, single- nucleotide polymorphism; OR, odds ratio. * indicates inverse variance weighted result with p <0.05. Results with a p <0.05 are bolded.
Table 2.
Pleiotropy and heterogeneity test of the MR analysis.
| Exposure | Heterogeneity test | Pleiotropy test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MR Egger | Inverse variance weighted | ||||||||
| Q | Q_df | Q_p-value | Q | Q_df | Q_p-value | Egger intercept | se | p-value | |
| Systemic lupus erythematosus | 380.437 | 6 | 4.478E-79 | 412.750 | 7 | 4.393E-85 | -0.526 | 0.740 | 0.502 |
| Rheumatoid arthritis | 13.222 | 14 | 0.509 | 13.255 | 15 | 0.583 | -0.02 | 0.111 | 0.858 |
| Multiple sclerosis | 16.821 | 15 | 0.330 | 17.907 | 16 | 0.329 | 0.038 | 0.039 | 0.341 |
| Crohn’s disease | 43.198 | 42 | 0.420 | 47.023 | 43 | 0.311 | 0.072 | 0.038 | 0.061 |
| Ulcerative colitis | 49.773 | 28 | 0.007 | 49.930 | 29 | 0.009 | -0.018 | 0.062 | 0.769 |
| Ankylosing spondylitis | 15.126 | 7 | 0.034 | 16.528 | 8 | 0.035 | 0.060 | 0.075 | 0.447 |
| Type 1 diabetes | 4.367 | 2 | 0.113 | 4.389 | 3 | 0.222 | 0.009 | 0.092 | 0.928 |
| Primary biliary cirrhosis | 0.518 | 3 | 0.915 | 0.725 | 4 | 0.948 | -0.162 | 0.357 | 0.680 |
| Psoriasis | 6.579 | 6 | 0.362 | 9.202 | 7 | 0.238 | -0.15 | 0.097 | 0.173 |
| Celiac disease | 8.906 | 5 | 0.113 | 13.184 | 6 | 0.040 | 0.270 | 0.174 | 0.182 |
| Asthma | 3.013 | 5 | 0.698 | 4.332 | 6 | 0.632 | 0.105 | 0.091 | 0.303 |
| Hypothyroidism | 19.520 | 16 | 0.243 | 21.604 | 17 | 0.200 | -0.093 | 0.071 | 0.210 |
MR, Mendelian randomization.
Except for SLE and PBC, the MR analyses showed no causal association between the remaining 10 AIDs and TC. Significant heterogeneity was detected by Cochran’s Q test in both the MR-Egger and IVW methods of UC and AS and the IVW method of CeD ( Table 2 ). Then, the random effects IVW method was performed again, indicating no causal association between UC, AS, CeD, and TC. The MR-Egger intercept test revealed no horizontal pleiotropy (p >0.05). Furthermore, leave-one-out analysis revealed that no single SNP can strongly affect the overall causal effect of AIDs on TC ( Supplementary Figure S2 ). In summary, the MR analyses showed that SLE and PBC were substantially linked to an elevated risk of TC after sensitivity and pleiotropy analyses at the Bonferroni-corrected significance level (p <0.05).
3.3. Causal effects of TC on AIDs
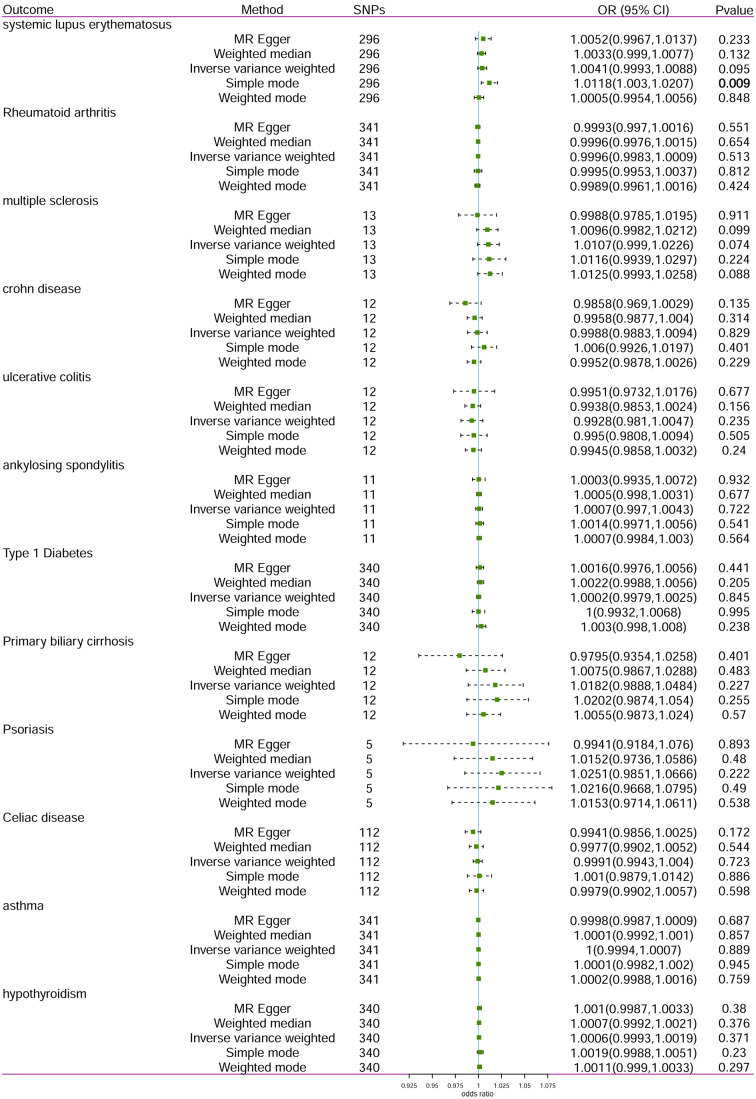
The reverse MR analysis was conducted to determine whether TC has any causal effect on AIDs using SNPs associated with TC as IVs. The reverse MR analysis used the identical IV selection criteria as the primary MR analysis. All MR approaches indicated that there was no reverse causal relationship between TC and AIDs with partial heterogeneity ( Figure 2 , Table 3 ). After MR-PRESSO detecting outliers, the results still support that there was no reverse causal relationships between TC and AIDs. In reverse MR analysis, the MR-Egger intercept test (p >0.05) showed no horizontal pleiotropy.
Figure 2.
Forest plot of the effect of TC on AIDs. TC, thyroid cancer; AID, autoimmune disease; SNP, single- nucleotide polymorphism; OR, odds ratio. Results with a p <0.05 are bolded.
Table 3.
Pleiotropy and heterogeneity test of the reverse MR analysis.
| Outcome | Heterogeneity test | Pleiotropy test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MR Egger | Inverse variance weighted | ||||||||
| Q | Q_df | Q_p-value | Q | Q_df | Q_p-value | Egger intercept | se | p-value | |
| Systemic lupus erythematosus | 921.760 | 294 | 6.62E-66 | 922.060 | 295 | 1.06E-65 | -0.002 | 0.005 | 0.757 |
| Rheumatoid arthritis | 366.406 | 339 | 0.147 | 366.486 | 340 | 0.155 | 0.000 | 0.001 | 0.786 |
| Multiple sclerosis | 20.790 | 11 | 0.036 | 24.260 | 12 | 0.019 | 0.018 | 0.013 | 0.203 |
| Crohn’s disease | 23.518 | 10 | 0.009 | 31.163 | 11 | 0.001 | 0.018 | 0.010 | 0.102 |
| Ulcerative colitis | 36.834 | 10 | 6.05E-05 | 37.063 | 11 | 0.000 | -0.003 | 0.013 | 0.808 |
| Ankylosing spondylitis | 37.056 | 9 | 2.57E-05 | 37.115 | 10 | 5.40E-05 | 0.001 | 0.005 | 0.907 |
| Type 1 diabetes | 366.824 | 338 | 0.135 | 367.525 | 339 | 0.138 | -0.002 | 0.003 | 0.422 |
| Primary biliary cirrhosis | 24.510 | 10 | 0.006 | 34.189 | 11 | 0.0003 | 0.052 | 0.026 | 0.075 |
| Psoriasis | 0.848 | 3 | 0.838 | 1.619 | 4 | 0.805 | 0.032 | 0.037 | 0.445 |
| Celiac disease | 112.013 | 110 | 0.429 | 114.072 | 111 | 0.402 | 0.007 | 0.005 | 0.158 |
| Asthma | 363.135 | 339 | 0.176 | 363.506 | 340 | 0.182 | 0.000 | 0.001 | 0.557 |
| Hypothyroidism | 695.921 | 338 | 5.51E-27 | 696.335 | 339 | 7.13E-27 | -0.001 | 0.001 | 0.654 |
MR, Mendelian randomization.
4. Discussion
AIDs are characterized by immune dysregulation and reactivity to self-antigens, resulting in immune-mediated destruction of own cells and tissues, which further leads to tissue damage and dysfunction (19). Due to the importance of immune system in recognition and elimination of tumors, immune system dysregulation is thought to increase the risk of cancer (20). Here, we performed the first two-sample MR analyses to determine the causal relationships between AIDs and TC. The MR analyses support a significant causal association of SLE and PBC on the risk of TC. However, no apparent genetic causal relationship between ten other AIDs (RA, MS, CD, UC, AS, T1D, PsO, CeD, asthma, and hypothyroidism) and TC was observed. The reverse MR analysis demonstrates that genetically predicted TC had no causal effect on AIDs (p >0.05).
Research on the association between SLE and cancer has been conducted for decades. The risk of prostate cancer was decreased in SLE patients, which may possibly be due to the low hypoadrenergic states that may occur in men with SLE (21). Bernatsky et al. reported a decreased risk of breast, ovarian, and endometrial cancers in SLE (22). The association between SLE and a small overall increased risk of certain cancers had been reported as well (23). A review based on the evidence from a meta-analysis revealed that SLE patients are at increased risk of developing lung, bladder, and liver cancers (24). In comparison to age-matched controls, SLE patients showed a higher prevalence of papillary TC in a case-control study (25). A review summarizes the scientific literature on the association between SLE and TC (26). A meta-analysis indicated a positive association between TC and SLE risk and concluded that patients with SLE had an increased risk of developing TC (27). Our results from MR analyses also support that genetically predicted SLE increases the risk of TC, indicating that patients with SLE should be under close surveillance for TC.
Increasing studies have indicated that PBC may be related to the risk of some cancers. Hepatocellular carcinoma (HCC) has been reported to be a potentially fatal complication for patients with PBC (28). HCC is not rare in Chinese PBC patients, with a significantly higher incidence in males than in females (29). A meta-analysis indicated that PBC patients had a higher risk of both HCC and overall cancer (30). Another study reported that genetically predisposed PBC reduced the risk of developing gastric cancer (31). Up to date, there are no studies linking PBC with TC. Our study is the first to support a significant causal association between PBC and TC, and patients with PBC are more vulnerable to developing TC, which may be a clinical issue worthy of contemplation.
The association between inflammatory bowel diseases (CD and UC) and TC risk has been reported but is controversial. The case-control study in the United States reported that patients with CD, not UC, were associated with a higher risk for TC than those with diverticulitis (32). Conversely, the meta-analysis performed by Lihong Cao suggested no association between CD and the increased risk of TC, and UC was related to the elevated risk of TC (33). The population-based cohort study from China showed that digestive cancers, TC, and hematological malignancies were the top three highest incidence cancers in UC patients, and no evidence supported the association between CD and cancer risk (34). In the present study, the MR analyses show no causal association between UC/CD and the risk of TC. This inconsistency may be caused by differences in genetic, environmental, or other confounding factors due to population differences in the study cohorts.
A large-scale cohort study in China indicated an increasing risk of developing TC in RA patients (35). A nationwide cohort study in Korea suggested a significantly elevated risk of TC in patients with RA compared with the control group without AIDs (36). A longitudinal analysis based on the Korean population exhibited higher odds of TC for male RA patients in the sex-stratified subgroup analyses (37). However, another early study in Korea reported that women patients with RA showed increased risks of TC (38). The nested case-control study in Korea reported that PsO was unrelated to the risk of TC in the overall adult population (39). In contrast, another cohort study, also in Korea, indicated that the TC risk was greater in patients with PsO than in those without PsO (40). In the nested case-control study in Finland, an elevated incidence of TC was observed in MS patients during the disease modifying treatment era (41). The nationwide cohort study in Taiwan indicated that patients with AS were at an increased risk of TC (42). The nationwide cohort of patients in the Swedish population showed that patients with CeD had no increased risk of TC, which was different from studies in Italy and the United States (43). A meta-analysis of cohort study reported that patients with diabetes mellitus were at increased risk of TC (44). Mäkimattila et al. indicated a marginally increased risk of TC in individuals with T1D compared to control individuals (45). The association between hypothyroidism and TC had been reported, with a higher prevalence of TC in patients with hypothyroidism (46). In addition, there is no study to describe the association between asthma and TC. Our findings showed no causal relationship between these AIDs and TC. Associations between these AIDs and TC found in previous observational research might be mediated by hitherto unknown confounding variables.
In conclusion, this is the first two-sample MR study to estimate the causality between AIDs and TC, which may offer more precise recommendations for TC monitoring in patients with AIDs. The results support a significant causal association of SLE and PBC on the risk of TC and do not support an association of the remaining ten AIDs with TC risk. It’s worth noting that the relationships between the remaining ten AIDs and TC risk may be weaker or require further exploration through extensive cohort studies. In addition, the reverse MR analyses demonstrate that genetically predicted TC has no causal effect on AIDs. However, the study population enrolled in the MR analyses was mainly from Europe, limiting the generalizability of our findings to individuals of other ethnicities. Importantly, these findings are based on predictions derived from genetic associations and should be considered preliminary. While our study provides statistical evidence for the causal associations between AIDs and TC, further research is needed to confirm these relationships and unravel the underlying mechanisms. Specifically, the connection between SLE, PBC, and TC risk requires additional confirmation. Furthermore, owing to limitations in the database, we were unable to delve into other confounding factors, notably non-genetic risk factors like exposure to ionizing radiation, endocrine disruptors, and medical radiation, which may cause potential residual confounding. Nevertheless, our results underscore the importance of closely monitoring patients with SLE or PBC for the development of TC, as this may inform early detection and intervention strategies.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
WP: Conceptualization, Writing – original draft. BX: Formal analysis, Writing – review & editing. HZ: Formal analysis, Writing – review & editing. JD: Data curation, Writing – review & editing. XG: Data curation, Writing – review & editing. SH: Conceptualization, Writing – original draft.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Talent Program of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (TRKYRC-yc202202) and Master and Doctor innovation talent base for endocrine and metabolic diseases (RCJD2021S03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1401458/full#supplementary-material
References
- 1. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fagin JA, Wells SA, Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. (2016) 375:1054–67. doi: 10.1056/NEJMra1501993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6 [DOI] [PubMed] [Google Scholar]
- 4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 5. Bogović Crnčić T, Ilić Tomaš M, Girotto N, Grbac Ivanković S. Risk factors for thyroid cancer: What do we know so far? Acta Clin Croat. (2020) 59:66–72. doi: 10.20471/acc.2020.59.s1.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menicali E, Guzzetti M, Morelli S, Moretti S, Puxeddu E. Immune landscape of thyroid cancers: New insights. Front Endocrinol (Lausanne). (2020) 11:637826. doi: 10.3389/fendo.2020.637826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mould RC, van Vloten JP, AuYeung AWK, Karimi K, Bridle BW. Immune responses in the thyroid cancer microenvironment: making immunotherapy a possible mission. Endocr Relat Cancer. (2017) 24:T311–t29. doi: 10.1530/ERC-17-0316 [DOI] [PubMed] [Google Scholar]
- 8. Ferrari SM, Fallahi P, Galdiero MR, Ruffilli I, Elia G, Ragusa F, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. (2019) 20:4413. doi: 10.3390/ijms20184413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yasunaga M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin Cancer Biol. (2020) 64:1–12. doi: 10.1016/j.semcancer.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 10. Elkoshi Z. Cancer and autoimmune diseases: A tale of two immunological opposites? Front Immunol. (2022) 13:821598. doi: 10.3389/fimmu.2022.821598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cappelli LC, Shah AA. The relationships between cancer and autoimmune rheumatic diseases. Best Pract Res Clin Rheumatol. (2020) 34:101472. doi: 10.1016/j.berh.2019.101472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonifazi M, Tramacere I, Pomponio G, Gabrielli B, Avvedimento EV, La Vecchia C, et al. Systemic sclerosis (scleroderma) and cancer risk: systematic review and meta-analysis of observational studies. Rheumatol (Oxford). (2013) 52:143–54. doi: 10.1093/rheumatology/kes303 [DOI] [PubMed] [Google Scholar]
- 13. Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. (2001) 357:96–100. doi: 10.1016/S0140-6736(00)03540-6 [DOI] [PubMed] [Google Scholar]
- 14. Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Effect of autoimmune diseases on risk and survival in histology-specific lung cancer. Eur Respir J. (2012) 40:1489–95. doi: 10.1183/09031936.00222911 [DOI] [PubMed] [Google Scholar]
- 15. Yang TH, Cheng YF, Chen CS, Lin HC. Increased prevalences of head and neck cancers in patients with Sjögren's syndrome. Head Neck. (2023) 45:2874–81. doi: 10.1002/hed.27518 [DOI] [PubMed] [Google Scholar]
- 16. Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC, et al. Cancer risk in patients with Graves' disease: a nationwide cohort study. Thyroid. (2013) 23:879–84. doi: 10.1089/thy.2012.0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219 [DOI] [PubMed] [Google Scholar]
- 19. Landgren AM, Landgren O, Gridley G, Dores GM, Linet MS, Morton LM. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4. 5 million US male veterans. Cancer. (2011) 117:1163–71. doi: 10.1002/cncr.25524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matza Porges S, Shamriz O. Genetics of immune dysregulation and cancer predisposition: Two sides of the same coin. Clin Exp Immunol. (2022) 210:114–27. doi: 10.1093/cei/uxac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernatsky S, Ramsey-Goldman R, Gordon C, Clarke AE. Prostate cancer in systemic lupus erythematosus. Int J Cancer. (2011) 129:2966–9. doi: 10.1002/ijc.25956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernatsky S, Ramsey-Goldman R, Foulkes WD, Gordon C, Clarke AE. Breast, ovarian, and endometrial Malignancies in systemic lupus erythematosus: a meta-analysis. Br J Cancer. (2011) 104:1478–81. doi: 10.1038/bjc.2011.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ladouceur A, Tessier-Cloutier B, Clarke AE, Ramsey-Goldman R, Gordon C, Hansen JE, et al. Cancer and systemic lupus erythematosus. Rheum Dis Clin North Am. (2020) 46:533–50. doi: 10.1016/j.rdc.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 24. Ni J, Qiu LJ, Hu LF, Cen H, Zhang M, Wen PF, et al. Lung, liver, prostate, bladder Malignancies risk in systemic lupus erythematosus: evidence from a meta-analysis. Lupus. (2014) 23:284–92. doi: 10.1177/0961203313520060 [DOI] [PubMed] [Google Scholar]
- 25. Antonelli A, Mosca M, Fallahi P, Neri R, Ferrari SM, D'Ascanio A, et al. Thyroid cancer in systemic lupus erythematosus: a case-control study. J Clin Endocrinol Metab. (2010) 95:314–8. doi: 10.1210/jc.2009-0677 [DOI] [PubMed] [Google Scholar]
- 26. Ferrari SM, Elia G, Virili C, Centanni M, Antonelli A, Fallahi P. Systemic lupus erythematosus and thyroid autoimmunity. Front Endocrinol (Lausanne). (2017) 8:138. doi: 10.3389/fendo.2017.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang M, Li XM, Wang GS, Qian L, Tao JH, Ma Y, et al. Thyroid cancer in systemic lupus erythematosus: a meta analysis. Int J Clin Exp Pathol. (2014) 7:6270–3. [PMC free article] [PubMed] [Google Scholar]
- 28. Sy AM, Ferreira RD, John BV. Hepatocellular carcinoma in primary biliary cholangitis. Clin Liver Dis. (2022) 26:691–704. doi: 10.1016/j.cld.2022.06.011 [DOI] [PubMed] [Google Scholar]
- 29. Zhang XX, Wang LF, Jin L, Li YY, Hao SL, Shi YC, et al. Primary biliary cirrhosis-associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol. (2015) 21:3554–63. doi: 10.3748/wjg.v21.i12.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang Y, Yang Z, Zhong R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. (2012) 56:1409–17. doi: 10.1002/hep.v56.4 [DOI] [PubMed] [Google Scholar]
- 31. Gu D, Zhang M, Wang Y, Bai Y, Wang X, Deng G. Causal effect of autoimmune liver diseases on cancer: Meta-analyses of cohort studies and Mendelian randomization study. Liver Int. (2022) 42:2216–26. doi: 10.1111/liv.15355 [DOI] [PubMed] [Google Scholar]
- 32. Wadhwa V, Lopez R, Shen B. Crohn's disease is associated with the risk for thyroid cancer. Inflammation Bowel Dis. (2016) 22:2902–6. doi: 10.1097/MIB.0000000000000963 [DOI] [PubMed] [Google Scholar]
- 33. Cao L. Assessment of thyroid cancer risk in more than 334,000 patients with inflammatory bowel disease: a case-control study and a meta-analysis. World J Surg Oncol. (2018) 16:182. doi: 10.1186/s12957-018-1485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Zhang M, Chen X, Guo M, Zhou R, Lv H, et al. Risk of Malignancy in patients with inflammatory bowel disease: A population-based cohort study from China. Int J Cancer. (2022) 150:1770–8. doi: 10.1002/ijc.33932 [DOI] [PubMed] [Google Scholar]
- 35. Zhou Z, Liu H, Yang Y, Zhou J, Zhao L, Chen H, et al. The five major autoimmune diseases increase the risk of cancer: epidemiological data from a large-scale cohort study in China. Cancer Commun (Lond). (2022) 42:435–46. doi: 10.1002/cac2.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park SK, Ryoo JH, Kim MH, Jung JY, Jung YS, Kim KN, et al. Association between eight autoimmune diseases and thyroid cancer: A nationwide cohort study. Thyroid. (2024) 34:206–14. doi: 10.1089/thy.2023.0353 [DOI] [PubMed] [Google Scholar]
- 37. Choi HG, Kang HS, Lim H, Kim JH, Kim JH, Cho SJ, et al. Potential cancer risk in patients with rheumatoid arthritis: A longitudinal Korean population-based analysis. J Pers Med. (2022) 12:965. doi: 10.3390/jpm12060965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee H. The risk of Malignancy in Korean patients with rheumatoid arthritis. Yonsei Med J. (2019) 60:223–9. doi: 10.3349/ymj.2019.60.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim SY, Yoo DM, Chung J, Choi HG. Thyroid cancer and psoriasis: A nested case-control study. Diagnostics (Basel). (2022) 12:2297. doi: 10.3390/diagnostics12102297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee JH, Kim HJ, Han KD, Kim HN, Park YM, Lee JY, et al. Cancer risk in 892 089 patients with psoriasis in Korea: A nationwide population-based cohort study. J Dermatol. (2019) 46:95–102. doi: 10.1111/1346-8138.14698 [DOI] [PubMed] [Google Scholar]
- 41. Hongell K, Kurki S, Sumelahti ML, Soilu-Hänninen M. Risk of cancer among Finnish multiple sclerosis patients. Mult Scler Relat Disord. (2019) 35:221–7. doi: 10.1016/j.msard.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 42. Chan TM, Luo SF, Yu KH, See LC, Huang LH, Kuo CF. Risk of cancer in patients with ankylosing spondylitis: a nationwide cohort study in Taiwan. Scand J Rheumatol. (2021) 50:132–8. doi: 10.1080/03009742.2020.1804612 [DOI] [PubMed] [Google Scholar]
- 43. Ludvigsson JF, Lebwohl B, Kämpe O, Murray JA, Green PH, Ekbom A. Risk of thyroid cancer in a nationwide cohort of patients with biopsy-verified celiac disease. Thyroid. (2013) 23:971–6. doi: 10.1089/thy.2012.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li H, Qian J. Association of diabetes mellitus with thyroid cancer risk: A meta-analysis of cohort studies. Med (Baltimore). (2017) 96:e8230. doi: 10.1097/MD.0000000000008230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mäkimattila S, Harjutsalo V, Feodoroff M, Groop P-H. Risk of thyroid cancer in people with type 1 diabetes by autoimmune thyroid diseases and tumor histology. J Endocrine Soc. (2024) 8:bvae054. doi: 10.1210/jendso/bvae054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zubčić Ž, Šestak A, Mihalj H, Kotromanović Ž, Včeva A, Prpić T, et al. The asSociation between type 2 diabetes mellitus, hypothyroidism, and thyroid cancer. Acta Clin Croat. (2020) 59:129–35. doi: 10.20471/acc.2020.59.s1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.