Abstract
Metformin, a drug widely used in the treatment of Type II diabetes, has recently received attention owing to new findings regarding its mitochondrial and cellular effects. In the present study, the effects of metformin on respiration, complex 1 activity, mitochondrial permeability transition, cytochrome c release and cell death were investigated in cultured cells from a human carcinoma-derived cell line (KB cells). Metformin significantly decreased respiration both in intact cells and after permeabilization. This was due to a mild and specific inhibition of the respiratory chain complex 1. In addition, metformin prevented to a significant extent mitochondrial permeability transition both in permeabilized cells, as induced by calcium, and in intact cells, as induced by the glutathione-oxidizing agent t-butyl hydroperoxide. This effect was equivalent to that of cyclosporin A, the reference inhibitor. Finally, metformin impaired the t-butyl hydroperoxide-induced cell death, as judged by Trypan Blue exclusion, propidium iodide staining and cytochrome c release. We propose that metformin prevents the permeability transition-related commitment to cell death in relation to its mild inhibitory effect on complex 1, which is responsible for a decreased probability of mitochondrial permeability transition.
Keywords: cell death, complex 1, metformin, mitochondria, oxidative stress, permeability transition pore (PTP)
Abbreviations: AMPK, AMP-activated protein kinase; CCCP, carbonyl cyanide m-chlorophenylhydrazone; CsA, cyclosporin A; PLSD, protected least-significant difference; PTP, permeability transition pore; ROS, reactive oxygen species; tBH, t-butyl hydroperoxide; TMPD, N,N,N′,N′-tetramethyl-1,4-phenylenediamine; VDAC, voltage-dependent anion channel
INTRODUCTION
A significant increase in the prevalence of Type II diabetes is a leading health problem worldwide which occurs in both developed and developing countries [1,2]. It is now believed that hyperglycaemia is not only a marker, but is also a key event responsible for most of the deleterious consequences of this disease. Hyperglycaemia, which is the major metabolic abnormality in Type II diabetes [3], has also been recently emphasized as an important factor in the prognosis of intensive care patients with inflammation-related insulin resistance [4]. Regarding the deleterious effect of hyperglycaemia, Brownlee [5] has proposed a unifying hypothesis on the basis of the superoxide overproduction from the mitochondrial electron-transport chain as a consequence of hyperglycaemia-related increased glycolysis [5]. Hence, if reduction of hyperglycaemia as early and as profoundly as possible remains the cornerstone for the treatment of diabetes [6], decreasing the reactive oxygen species-related glucose toxicity at the cellular level may represent an additional attractive proposition.
Among the different drugs used for the treatment of Type II diabetes, metformin is widely used [7–9] since it lowers glucose levels by increasing the glucose uptake of muscles [10] and by decreasing hepatic glucose production [11,12]. However, its cellular mechanism of action is still poorly understood. Recently, Zhou et al. [13] demonstrated that metformin activated the AMPK (AMP-activated protein kinase) both in hepatocytes and skeletal muscles. This finding is of importance since AMPK is involved in the regulation of both glucose production and fatty acid oxidation; however, the relationship between metformin and AMPK activation is not yet clear, since it does not seem to change the intracellular AMP/ATP ratio [14,15]. Recently, we reported that metformin also mildly inhibits the respiratory chain in liver cells [16]. This effect, which is exclusively located on the respiratory chain complex 1, occurs only in intact cells and not in isolated mitochondria or permeabilized cells, and it vanishes at low temperatures [16].
Studies during the last decade have emphasized the central role of mitochondria, besides their prominent function in cellular energy metabolism, in several other major processes such as control of cell death. Although the exact mechanism linking mitochondria and cell death still remains to be clarified, it is probable that the mitochondrial PTP (permeability transition pore) is involved via the release of cytochrome c [17]. Moreover, there is further evidence to suggest that a PTP-independent pathway involving Bcl-2 family proteins may also contribute to cytochrome c release from the mitochondrial intermembrane space to the cytosol. Both mechanisms, i.e. the PTP-dependent and -independent mechanisms, can potentially contribute to the commitment to cell death [18].
The molecular nature of PTP is still unknown, but its modulation by several physiological factors has been widely studied [17]. Among these, Ca2+ is certainly the most important inducer, whereas matrix pH, transmembrane electrical potential, Mg2+, Pi, cyclophilin D, oxidative stress and adenine nucleotides are also effective regulators [17,19]. In addition, CsA (cyclosporin A) is regarded as a specific reference inhibitor of PTP. We reported previously that PTP is also modulated by electron flux through the respiratory chain complex 1 [17,19]. This was initially proposed because a different amount of Ca2+ was necessary to induce the permeability transition according to the nature of the respiratory substrates, i.e. glutamate versus succinate. This observation, together with other considerations [20], allowed us to propose that the respiratory chain complex 1 may be part of the PTP [17,19,20]. By investigating the effects of the complex 1 inhibitor rotenone, we found that a significant inhibition of PTP was associated with the prevention of cell death [21].
In the light of the mitochondrial effect of metformin on the respiratory chain [16], we hypothesized that this drug, by its inhibition of complex 1, modulates the mitochondrial permeability transition and thereby prevents the cell death due to PTP-related cytochrome c release.
MATERIALS AND METHODS
Materials and products
Cells from an oral squamous carcinoma cell line, namely KB cells [22], were maintained in exponential growth phase using RPMI 1640 culture medium, supplemented with 10% (v/v) fetal calf serum, 2 mM glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin. These cells were purchased from A.T.C.C. (reference CCL-17). Calcein-acetomethoxyl ester and Calcium Green-5N were obtained from Molecular Probes; monoclonal antibodies were from BD Biosciences Pharmingen (San Diego, CA, U.S.A.). Metformin was a gift from Merck-Lipha. All other chemicals were purchased from Sigma.
Measurement of oxygen consumption rate in intact cells
KB cells (107 cells/ml) were incubated in closed vials in a shaking water bath in 2.5 ml of RPMI 1640 medium saturated with a mixture of O2/CO2 (19:1). Incubations were performed at 37 °C, unless otherwise indicated (15 °C), with or without 10 mM metformin. After 30 min, 2 ml of the suspension was removed from vials and placed in a stirred oxygraph vessel, which was thermostatically maintained at 37 °C and equipped with a Clark oxygen electrode. The oxygen consumption rate (VO2) was first measured in the absence of any addition; subsequently, 1.25 μM rotenone, 0.5 μM CCCP (carbonyl cyanide m-chlorophenylhydrazone), 3.8 μM myxothiazol and 1 mM TMPD (N,N,N′,N′-tetramethyl-1,4-phenylenediamine) +5 mM ascorbate were successively added, as indicated.
Measurement of VO2 in permeabilized cells
After 30 min preincubation as described above, intact KB cell suspensions were centrifuged and the cell pellets were carefully resuspended in KCl medium (125 mM KCl, 20 mM Tris/HCl, 1 mM EGTA and 5 mM Pi/Tris, pH 7.2) containing 200 μg/ml digitonin. Cells were first permeabilized for 2 min at 37 °C and then the suspension was removed from the vial and placed in the oxygraph as described above. As indicated, either 5 mM glutamate/Tris+2.5 mM malate/Tris or 5 mM succinate/Tris+0.5 mM malate/Tris+1.25 μM rotenone were added. VO2 was measured before and after the successive additions of 1 mM ADP/Tris, 0.75 μg/ml oligomycin, 0.5 μM CCCP, 3.8 μM myxothiazol and 1 mM TMPD+5 mM ascorbate.
Measurement of complex 1 and citrate synthase activities
After preincubation in RPMI 1640 medium with or without 10 mM (30 min) or 100 μM (24 h) metformin, KB cell suspensions were centrifuged and the cell pellets were resuspended in a cold buffer containing 40 mM KCl, 250 mM sucrose, 2 mM EGTA, 20 mM Tris (pH 7.2) and 200 μg/ml digitonin. After 5 min incubation on ice, cells were spun down (12000 g for 10 min) to eliminate possible cytosolic contaminating enzyme activities. The permeabilized KB cells were then carefully washed and resuspended either in the above buffer devoid of digitonin for assaying complex 1 or in a lysis buffer (100 mM KH2PO4, 2 mM EDTA and 1 mM dithiothreitol, pH 7.3) containing 0.1% Triton X-100 for assaying the citrate synthase activity. Protein concentrations were measured using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, U.S.A.).
Citrate synthase activity was measured by the method of Srere [23], whereas complex 1 activity was determined fluorimetrically in a Kontron SFM23 spectrofluorimeter by monitoring NADH oxidation with the excitation and emission wavelengths set at 340 and 460 nm respectively. In brief, permeabilized cells (8×106) were placed in 800 μl of water in a well-stirred glass cuvette for 2 min at 30 °C to break mitochondrial membranes by hypo-osmotic shock. Tris solution (200 μl, 50 mM, pH 8.0) containing 150 μM NADH was then added for 1 min and the reaction was started by adding 100 μM decylubiquinone as the final electron acceptor. Rotenone-sensitive complex 1 activity was obtained after subtraction of the remaining signal in the presence of 6 μM rotenone.
Determination of permeability transition in permeabilized cells
Intact KB cells (5×106) were incubated for 30 min with or without 10 mM metformin as described above. The cells were then centrifuged and resuspended in a medium containing 250 mM sucrose, 10 mM Mops, 1 mM Pi/Tris and 50 μg/ml digitonin (pH 7.35) and placed in a spectrofluorimeter glass cuvette, continuously stirred and thermostatically maintained at 25 °C. After 2 min, cells were permeabilized and 1 μM CsA or vehicle was also added to the medium as indicated. After signal stabilization, 10 μl of 1 mM Ca2+ pulses was successively added at 2 min intervals until the opening of PTP, as indicated by the release of Ca2+ in the medium. Measurements of Ca2+ were performed fluorimetrically with a PTI Quantamaster C61 spectrofluorimeter. Free Ca2+ was measured in the presence of 0.25 μM Calcium Green-5N with excitation and emission wavelengths set at 506 and 532 nm respectively.
Determination of permeability transition in intact cells
Calcein staining in KB cells was achieved after the cells (5×104) were grown for 48 h on 22-mm diameter round glass coverslips and exposed for 15 min at 37 °C to a PBS medium supplemented with 5 mM glucose, 0.35 mM pyruvate, 1 mM CoCl2 and 1 μM calcein-acetomethoxyl ester as described in [24]. After loading, cells were washed free of calcein and CoCl2 and further incubated for 20 min at 37 °C in PBS/glucose/pyruvate medium, supplemented with either 10 mM metformin, 1 μM CsA or vehicle. For low concentrations of metformin, KB cells were first preincubated for 24 h with or without metformin (100 μM) before the calcein and CoCl2 loading step. Coverslips were then mounted on the stage of an inverted microscope and PTP opening was achieved by adding 50 μM tBH (t-butyl hydroperoxide), a glutathione-oxidizing agent. Changes in cellular fluorescence were quantified using the NIH image software. The intensity of fluorescence of ten cells was followed in time after the addition of tBH.
Determination of cellular death
KB cells (2×107) were preincubated in Petri dishes with either 10 mM metformin and 1 μM CsA or vehicle for 30 min or preincubated for 24 h at 37 °C with or without 100 μM metformin. Cells were washed with PBS before subsequent exposure to 0.2 mM tBH for 45 min. Cells were again washed with PBS and incubated at 37 °C for 6 or 24 h in a complete RPMI 1640 medium. Cytotoxicity was evaluated either by staining necrotic cells with 20 μg/ml propidium iodide or by using a Trypan Blue (5%, v/v) exclusion assay.
Cellular images were obtained at 25 °C with a Nikon TE200 microscope (Nikon France, Champigny-sur-Marne, France), which was equipped for epifluorescent illumination and included a xenon light source (75 W) and a 12-bit digital-cooled charge-coupled-device camera (SPOT-RT; Diagnostic Instruments, Sterling Heights, MI, U.S.A.). For calcein fluorescence, 488±5/525±10 nm excitation/emission filter settings were used, and images were collected every minute with a constant exposure time using a 60×/1.40 Plan Apo oil immersion objective (Nikon). For detection of propidium iodide, five randomly selected fields were acquired from each Petri dish using an excitation/emission cube of 550±10/580 longpass and an ELWD 20×/0.45 Plan Fluor objective (Nikon). The corresponding bright field images were also obtained, and the two channels were overlaid using the appropriate function of the SPOT™ 3.0.6 software.
Cytochrome c was assessed in both mitochondrial and cytosolic spaces after KB cells were fractionated using the digitonin method [25]. Cytosolic (3 μg) and mitochondrial proteins (15 μg) were separated by SDS/PAGE (10% gel) in Mes buffer, followed by Western-blot analysis. Membranes were probed with a monoclonal antibody against cytochrome c (1 μg/ml) clone 7H8.2C12, and developed with a secondary goat anti-mouse horseradish peroxidase-labelled antibody, followed by chemiluminescent detection. Quantification was performed using the NIH image software.
Statistics
Results are expressed as means±S.E.M. and statistically significant differences were assessed by ANOVA, followed by Fisher's PLSD (protected least-significant difference) post hoc test or by paired or unpaired Student's t test (StatView®, Abacus Concepts, Berkeley, CA, U.S.A.) as indicated.
RESULTS
Specific inhibition of respiratory chain complex 1 by metformin in KB cells
As shown in Table 1, 10 mM metformin significantly inhibited respiration in intact KB cells by 53% (P<0.01), whereas rotenone led to a stronger inhibition (P<0.01) regardless of the presence of metformin (89 and 79% respectively with or without metformin). Metformin also inhibited respiration when cells were uncoupled by CCCP (58%, P<0.01), indicating that the inhibition was not related to mitochondrial adenine nucleotide phosphorylation. Finally, the lack of effect of metformin in the presence of TMPD+ascorbate, while assessing cytochrome oxidase activity, indicated that metformin did not affect this complex of the respiratory chain. The inhibitory effect on respiration was not present when cells were incubated with metformin at 15 °C before the determination of oxygen consumption, which was followed at 37 °C (results not shown).
Table 1. Inhibition of oxygen uptake by metformin in intact KB cells.
KB cells (107 cells/ml) were incubated in closed vials at 37 °C in RPMI 1640 medium saturated with a mixture of O2/CO2 (19:1) with or without 10 mM metformin. After 30 min, oxygen uptake (VO2) was measured before and after the successive additions of 1.25 μM rotenone, 0.5 μM CCCP, 3.8 μM myxothiazol and 1 mM TMPD+5 mM ascorbate (TMPD+a). Myxothiazol-sensitive VO2 was calculated and the results are expressed as means±S.E.M. (n=5 independent experiments, each performed in triplicate). *P<0.01 versus control.
| VO2 [nmol atoms O·min−1·(106 cells)−1] | ||
|---|---|---|
| Control | Metformin | |
| No addition | 1.9±0.1 | 0.9±0.1* |
| Rotenone | 0.4±0.1 | 0.1±0.0* |
| CCCP | 2.6±0.2 | 1.1±0.1* |
| TMPD+a | 10.0±0.3 | 10.5±0.5 |
The respiratory effect of metformin in intact cells was further investigated after digitonin permeabilization of the plasma membrane, allowing investigation of the oxidative mitochondrial phosphorylation pathway in situ. Mean values of myxothiazol-sensitive oxygen consumption obtained from repeated experiments are given in Table 2. With glutamate and malate as respiratory substrates, metformin inhibited oxygen consumption (33%, P<0.01), which persisted in the presence of ADP (state 3, 48%, P<0.01) or in an uncoupled state (CCCP addition, 36%, P<0.01). In state 4 (i.e. non-phosphorylating conditions obtained in the presence of oligomycin, an inhibitor of the Fo subunit of ATP synthase), the inhibition by metformin did not reach a significant level. As already found in intact cells (see Table 1), metformin did not affect the respiratory rate in the presence of TMPD+ascorbate. Furthermore, metformin affected none of these parameters with succinate and malate as respiratory substrates (Table 2).
Table 2. Inhibitory effect of metformin on VO2 in permeabilized KB cells.
Permeabilization of KB cells was achieved after intact cells had been previously incubated for 30 min with or without 10 mM metformin. Digitonin was added to intact cells (see the Materials and methods section) in a medium containing 125 mM KCl, 20 mM Tris/HCl, 1 mM EGTA and 5 mM Pi/Tris (pH 7.2) at 37 °C. Permeabilized cells were then incubated in the presence of either 5 mM glutamate+2.5 mM malate or 5 mM succinate+0.5 mM malate+1.25 μM rotenone. VO2 was measured before and after the successive additions of 1 mM ADP/Tris, 0.75 μg/ml oligomycin, 0.5 μM CCCP, 3.8 μM myxothiazol and 1 mM TMPD+5 mM ascorbate. Myxothiazol-sensitive respiration was calculated and the results are expressed as means±S.E.M. (n=5 independent experiments, each performed in triplicate). *P<0.01 versus control.
| VO2 [nmol atoms O·min−1·(106 cells)−1] | ||||
|---|---|---|---|---|
| Glutamate/malate | Succinate/malate | |||
| Control | Metformin | Control | Metformin | |
| No addition | 1.5±0.1 | 1.0±0.1* | 2.4±0.2 | 2.4±0.2 |
| ADP | 5.0±0.2 | 2.6±0.2* | 7.5±0.3 | 7.7±0.4 |
| Oligomycin | 1.0±0.1 | 0.8±0.1 | 1.5±0.3 | 1.6±0.3 |
| CCCP | 6.1±0.5 | 3.9±0.3* | 8.2±0.6 | 8.4±0.6 |
| TMPD+a | 11.2±0.3 | 11.5±0.3 | 10.4±0.3 | 10.7±0.3 |
These results allowed us to postulate that metformin affects mitochondrial respiration in KB cells by inhibiting respiratory chain complex 1, as already found in liver cells or in Xenopus laevis oocytes [16,26]. To confirm this hypothesis, rotenone-sensitive NADH decylubiquinone reductase activity was measured in metformin-treated and control KB cells after cell permeabilization and osmotic shock. Table 3 summarizes the results obtained after a short-time, high-concentration preincubation and a long-time, low-concentration preincubation of the drug; the results clearly show that, in both situations (10 mM or 100 μM), metformin inhibited rotenone-sensitive oxidation of NADH without altering the citrate synthase activity. The maximal effect of metformin was obtained at 10 mM (34%, P<0.01) and remained significant even at a concentration close to the therapeutic range (100 μM, 24%, P<0.05).
Table 3. Inhibitory effect of metformin on the isolated mitochondrial complex 1 in permeabilized KB cells.
Permeabilization of KB cells was achieved after intact cells had been previously preincubated in the absence or presence of 10 mM or 100 μM metformin. Digitonin was added to intact cells in a medium containing 250 mM sucrose, 40 mM KCl, 2 mM EGTA and 20 mM Tris, pH 7.2 (see the Materials and methods section). Complex 1 activity was measured after hypo-osmotic shock-induced mitochondrial membrane rupture, and NADH oxidation was then monitored in the simultaneous presence of 150 μM NADH and 100 μM decylubiquinone before and after the addition of 6 μM rotenone. Citrate synthase activity was measured after the lysis of permeabilized KB cells in a buffer (100 mM KH2PO4, 2 mM EDTA and 1 mM dithiothreitol, pH 7.3) containing 0.1% Triton X-100. Each enzyme activity and the corresponding ratio of both were calculated and are expressed as means±S.E.M. (n=4 independent experiments, each performed in duplicate). *P<0.05; **P<0.01 versus control.
| Metformin | |||
|---|---|---|---|
| Control | 10 mM for 30 min | 100 μM for 24 h | |
| Rotenone-sensitive activity of complex 1 [nmol of NADH·min−1·(mg of protein)−1] | 3.8±0.5 | 2.5±0.5** | 2.9±0.5* |
| Citrate synthase activity [pmol·min−1·(mg of protein)−1] | 42.6±8.5 | 41.7±8 | 40.4±11.3 |
| Enzyme activities ratio (nmol of NADH/unit of citrate synthase) | 90.3±7.4 | 59.9±4.1** | 73.2±7.5* |
Metformin prevents mitochondrial PTP opening in both permeabilized and intact KB cells
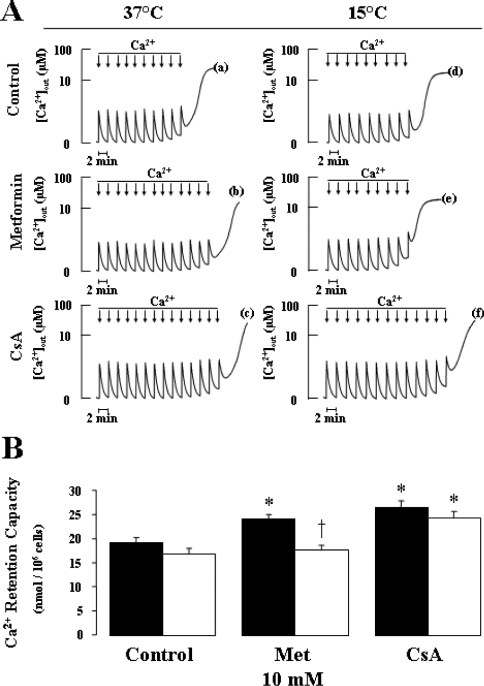
The regulation of PTP opening by calcium was studied by determining the amount of calcium required for inducing a permeability transition, as demonstrated by calcium release to the medium from digitonin-permeabilized cells. Figure 1(A) shows typical experiments and Figure 1(B) presents the results of repeated experiments. Compared with controls, metformin significantly increased the calcium requirement for achieving the permeability transition (27%, P<0.01), an effect only slightly lower (NS) than that of the reference inhibitor CsA (38%, P<0.01). Since the effect of metformin on respiration was temperature-dependent in KB cells (results not shown), as found in rat liver cells [16], we studied the effect of metformin on calcium retention in permeabilized cells after exposure to metformin at a low temperature (15 °C). Although the low temperature did not affect basal or CsA-inhibited calcium retention, the metformin effect was completely abolished, indicating that, in KB cells, it was also temperature-dependent.
Figure 1. Metformin delays permeability transition in permeabilized KB cells.
After 30 min preincubation in RPMI 1640 medium with or without 10 mM metformin at 37 or 15 °C, 5×106 KB cells were added to a medium containing 250 mM sucrose, 10 mM Mops and 1 mM Pi/Tris at 25 °C (pH 7.35). The medium was supplemented with 5 mM succinate/Tris and 0.25 μM Calcium Green-5N, followed by the addition of vehicle (A, traces a, b, d and e) or 1 μM CsA (A, traces c and f). Experiments were started 3 min after permeabilization with 50 μg/ml digitonin. Where indicated, 10 μl of 1 mM Ca2+ pulses was added every 2 min (arrows) until the opening of PTP, as indicated by the release of Ca2+ into the medium. Typical experiments performed on KB cells preincubated at 37 °C (A, left panels) and 15 °C (A, right panels) are shown. (B) Effects of preincubation with or without 10 mM metformin (Met) at different temperatures (37 °C, black bar; 15 °C, open bar) on the Ca2+-retention capacity of permeabilized KB cells. Results are expressed as the means±S.E.M. for five independent experiments. *P<0.05 versus control; †P<0.05 versus 37 °C, ANOVA, followed by Fisher's PLSD post hoc test and unpaired Student's t test.
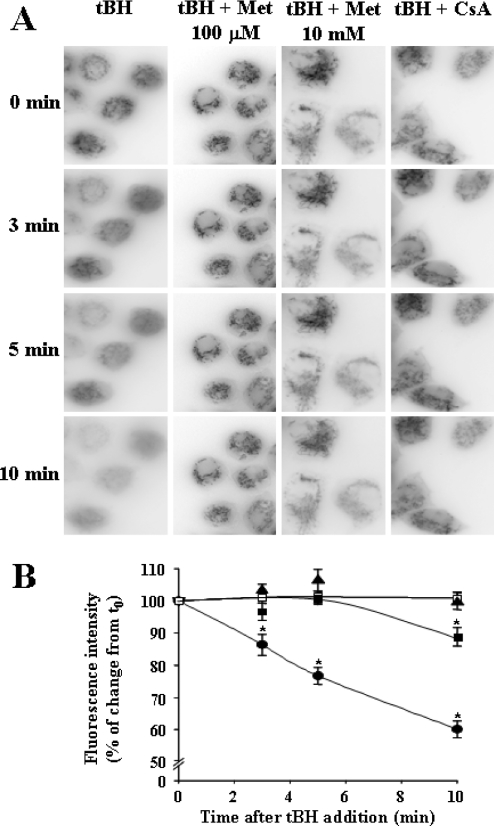
The effect of metformin on the regulation of the permeability transition was also investigated in intact cells, where PTP opening was achieved using the glutathione-oxidizing agent tBH. PTP opening was directly assessed by measuring mitochondrial permeability to calcein and cobalt [24]. After PTP opening, cobalt diffuses from the cytoplasm into the mitochondrial matrix, thus quenching the calcein-related fluorescence in this compartment. Hence, after a change in permeability, intracellular fluorescence is progressively decompartmentalized and quenched. As shown in Figure 2, this occurred in cells after the addition of tBH. Indeed, the cellular heterogeneity of fluorescence was less marked after exposure to tBH alone (Figure 2A, first column, from top to bottom), and this effect was associated with a quenching of fluorescence, which was already significant after 3 min and became more pronounced after 5 and 10 min (Figure 2B). Cellular heterogeneity and intensity of fluorescence persisted for up to 10 min with either metformin or CsA (Figures 2A). Furthermore, the effect of tBH on the fluorescence quenching was significantly decreased and delayed with 10 mM metformin, whereas it was completely abolished after 10 min with 100 μM metformin, an effect equivalent to that of CsA (Figures 2B). From these results, we conclude that metformin prevents mitochondrial permeability transition both in intact and permeabilized KB cells, and this effect is not different from that of CsA, the reference inhibitor of PTP.
Figure 2. Metformin prevents tBH-induced PTP opening in intact KB cells.
KB cells (5×104) were grown for 48 h on glass coverslips and loaded for 15 min at 37 °C with 1 μM calcein-acetomethoxyl ester in a PBS medium supplemented with 5 mM glucose, 0.35 mM pyruvate and 1 mM CoCl2. Vehicle, 1 μM CsA or 10 mM metformin (Met) were added after calcein loading and post-incubated for 20 min at 37 °C. When low concentrations of metformin were used, cells were first preincubated for 24 h with or without 100 μM metformin. Images were collected at 1 min intervals with an inverted microscope using a ×60 oil immersion objective before and after the addition of 50 μM tBH. (A) Cells at 0 min (immediately after tBH addition) and 3, 5 and 10 min after addition are shown; results are representative of a typical experiment. (B) Quantification of the fluorescence intensity using the NIH image software. Light intensities of ten different cells were monitored 3, 5 and 10 min after the addition of tBH to four different cell preparations for each condition: tBH (•), 100 μM metformin (□), 10 mM metformin (▪) and CsA (▴). Results are expressed as means±S.E.M. and represent the percentage of change from the initial value, i.e. before tBH addition (t0). *P<0.05, statistical comparisons between each value and its own reference at t0 were achieved by using paired Student's t test.
Prevention of tBH-induced cellular death by metformin
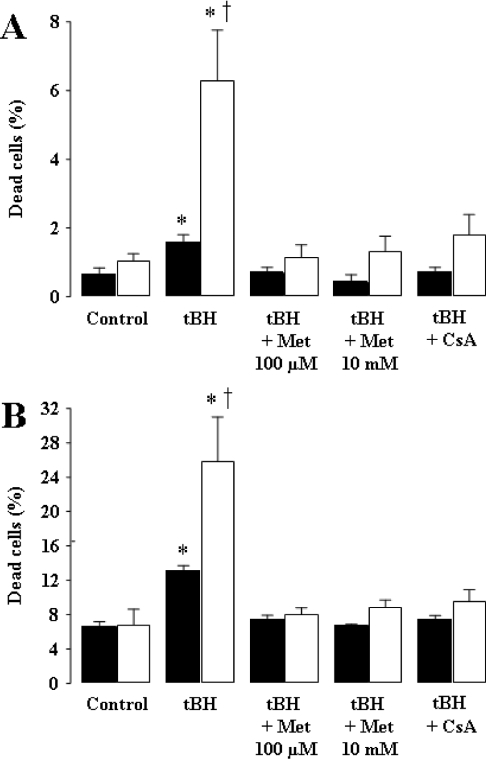
The effect of metformin on cellular death was first investigated by using propidium iodide staining, allowing us to evaluate the number of dead cells. As shown in Figure 3(A), exposure to tBH resulted in an increase in the percentage of cells stained by the dye. The magnitude of the effect was rather limited when observed just 6 h after tBH exposure (Figure 3A, black bars); however, a large and significant effect of this exogenous oxidizing agent was obtained when observed after a longer period. Interestingly, the detrimental effect of tBH was completely prevented by metformin, independent of the time and concentration used (100 μM or 10 mM), and also by CsA. The protective effect of metformin on cell death was also assessed by Trypan Blue exclusion. As shown in Figure 3(B), metformin (100 μM and 10 mM) or CsA significantly protected KB cells from cell death as induced by tBH.
Figure 3. Metformin inhibits tBH-induced cell death.
KB cells (2×107), either preincubated with vehicle, 1 μM CsA or 10 mM metformin for 30 min in RPMI 1640 medium or after 24 h preincubation with 100 μM metformin, were exposed to 0.2 mM tBH for 45 min, washed with PBS and incubated at 37 °C for 6 h (black bars) or 24 h (open bars) in a complete RPMI 1640 medium. Control, cells not exposed to tBH. (A) Quantification of the percentage of cell death by staining with propidium iodide. (B) Percentage of cell death determined using the Trypan Blue exclusion assay. Results are expressed as the means±S.E.M. for 5–8 independent experiments; more than 1000 cells were counted and analysed during each assay. *P<0.05 versus control; †P<0.05 versus 6 h, ANOVA, followed by Fisher's PLSD post hoc test.
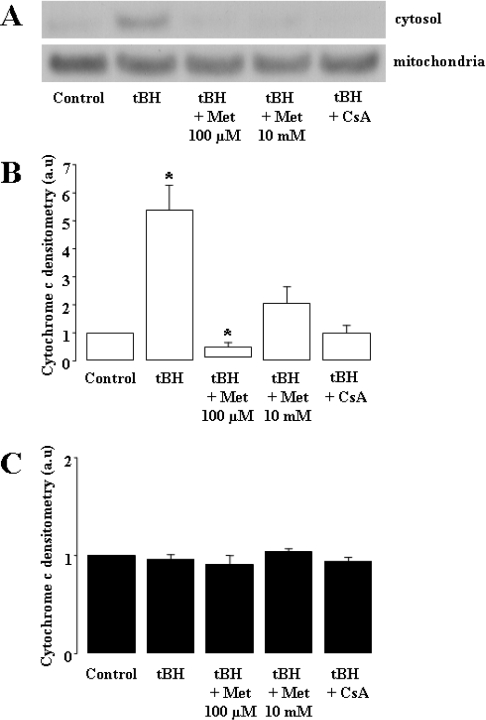
Finally, the effect on cell death was confirmed by determining the release of cytochrome c from the mitochondrial intermembrane space to the cytoplasm. This criterion is recognized, together with other factors, as a proapoptotic event in the commitment to cell death. The cytochrome c content of both mitochondrial and cytosolic spaces was assessed by Western-blot analysis (Figure 4A shows a typical experiment). We found that, although cytochrome c was hardly detected in the cytoplasm of control cells, tBH exposure resulted in a significant increase in cytosolic levels (Figure 4B). This increase was completely prevented by metformin (100 μM or 10 mM) or CsA. None of the different conditions affected the mitochondrial content of cytochrome c, indicating that only a very small fraction was released into the cytoplasm.
Figure 4. Metformin prevents tBH-induced release of cytochrome c into the cytoplasm of KB cells.
Mitochondrial and cytosolic spaces of KB cells were separated using the digitonin fractionation method. Cytosolic (3 μg) and mitochondrial (15 μg) proteins were separated by SDS/PAGE (10% gel) in Mes buffer, followed by Western-blot analysis. (A) A typical immunoblot each of cytosolic (upper panel) and mitochondrial (lower panel) cytochrome c. Means±S.E.M. for five independent experiments of cytosolic (B) and mitochondrial (C) cytochrome c levels are also shown. *P<0.05 versus control, ANOVA, followed by Fisher's PLSD post hoc test.
DISCUSSION
Results of the present study indicate that metformin inhibits complex 1 of the mitochondrial respiratory chain in a specific and temperature-dependent manner in KB cells, a finding that agrees with other results obtained in rat liver cells [16] and X. laevis oocytes [26]. However, the main finding presented here is that metformin decreases mitochondrial PTP opening and prevents the release of cytochrome c, this being associated with a decreased occurrence of cell death after the addition of the glutathione-oxidizing agent tBH. Such a result is of interest when considering the clinical use of metformin as antidiabetic agent, since this effect was observed not only at 10 mM, a high pharmacological concentration, but also at a value close to the therapeutic range (100 μM).
Use of KB cells as an experimental model for studying the effect of metformin on cell death
The KB cell line was used in the present study as an experimental model to investigate the effect of metformin on the relationship between respiratory chain complex 1, PTP regulation and cell death. Since KB cells are very flat, the pictures obtained from epifluorescence microscopy allow an accurate assessment of the changes in fluorescence distribution of calcein after the induction of oxidative stress. In a previous study, we have shown that mitochondria from KB cells exhibit a regulation by rotenone of the PTP subsequent to either a calcium challenge (after permeabilization) or oxidative stress (addition of tBH to intact cells) [21]. Hence, KB cells represent a suitable model to investigate the effects of metformin on mitochondria and cell death.
Cellular action of metformin
Despite the progress made in recent studies [13,14,16], the cellular action of metformin is still not fully understood. In the present study, we have used two concentrations: a high saturating one (10 mM) to investigate the maximal effect on respiratory chain complex 1 and a lower one corresponding to the therapeutic range (100 μM). From previous reports, it is clear that the mitochondrial effect of metformin occurs only when the drug is administered to intact cells, but not to isolated mitochondria or permeabilized cells [16,26]. Similar results were also found with KB cells (results not shown). However, when the cells were permeabilized after metformin exposure, the mitochondrial effect is still present, indicating that the putative mitochondrial change persisted. The lack of effect of metformin on isolated mitochondria has been challenged by results showing that metformin can directly inhibit complex 1 [27,28]. However, this effect was only observed at very high concentrations (K0.5=79 mM) and after very long incubation times at 8 °C (225–400 min), contrasting with the rapid effects (20 min) observed in intact cells [16]. (The K0.5 value was extracted from [27] and it represented the concentration of metformin necessary for an inhibition of 50% of the respiratory rate. The requirement of intact cells for achieving the mitochondrial inhibition together with its complete suppression at low temperature, and the failure to find any interference between metformin and several inhibitors of the main signalling pathways, allowed us to suggest that metformin could act via a plasma-membrane-related event. This was further supported by results obtained in X. laevis oocytes, where it was shown that a direct microinjection of metformin in the cytosol had no effect, whereas injection of liposome-encapsulated metformin inhibited complex 1 [26]. The mitochondrial effect was specifically located on complex 1 in KB cells, as in rat liver cells or X. laevis oocytes. The inhibitory effect was moderate, since the highest metformin concentration used (10 mM) resulted in a significantly lower inhibition compared with rotenone.
Recently, metformin was shown to activate AMPK, although its precise mechanism is not clear [13–15]. If AMPK represents a primary cellular target of the drug, metformin probably affects cell death via a first activation of this kinase, which might also be responsible for the inhibitory effect on the respiratory chain. Indeed, it cannot be excluded that AMPK might phosphorylate one of the components of complex 1 [29], leading to its inhibition and subsequent events affecting PTP regulation, cytochrome c release and, ultimately, cell death. However, such a putative mechanism cannot be the consequence of a direct interaction, since AMPK appears to be located in the cytoplasm and nucleus of cells [30] and, therefore, is probably inaccessible to the inner mitochondrial membrane complex 1. An indirect mechanism, involving a cascade of phosphorylation events of some proteins located in the outer mitochondrial membrane, could be proposed. Recent findings suggest that VDAC (voltage-dependent anion channel) may play a role in the regulation of mitochondria-mediated cell death by direct [31] or indirect modulation [32,33] of its conformation. Whereas a direct link between AMPK and VDAC has never been demonstrated, a modification of hexokinase binding to VDAC through phosphorylation of one of the protein-binding sites of the channel by AMPK (or a downstream kinase) or through an indirect pathway could also be envisaged. However, the previous finding that metformin inhibits complex 1 in isolated mitochondria or disrupted tissues only when exposed to a large concentration and/or a long incubation time [27,28] does not favour a direct involvement of AMPK.
Finally, the possibility that AMPK activation by metformin is not the result of a direct causal effect of the drug but rather the consequence of its mitochondrial effect through an unknown downstream mechanism that remains to be elucidated cannot be excluded. However, results of our recent work indicate that AMPK activation by AICAriboside (5-amino-4-imidazolecarboxamide riboside) does not prevent the tBH-related induction of cell death in KB cells (D. Detaille, B. Guigas and X. Leverve, unpublished work) as metformin does.
Metformin inhibits mitochondrial permeability transition and cell death
Under the conditions described here, metformin was found to modulate PTP similar to a previous finding concerning rotenone [21]. This was shown in permeabilized cells, where metformin modulated PTP opening after a calcium challenge. PTP is regulated by NADH, ADP and the mitochondrial membrane potential, among other factors [17]. These parameters are potentially affected by respiratory chain inhibition by metformin. However, in the experiments presented in Figure 1 investigating the effect of metformin on calcium retention, the permeabilized cells were incubated with succinate as substrate, a condition where metformin does not affect the respiratory chain (see Table 1). Hence, the significant increase in calcium retention presented in Figure 1 is related to the inhibition of complex 1 by metformin, but not as a consequence of oxidative phosphorylation. Interestingly, metformin also inhibited PTP opening in intact cells, although in this case, such a phenomenon was related to tBH-induced oxidative stress and not to calcium exposure. As described by Petronilli et al. [24], the quenching of the intracellular fluorescent probe calcein by cobalt could be proposed as direct evidence of PTP opening in intact cells. Of note, these effects of metformin, even at the lowest concentration, are equivalent to those obtained with CsA, and metformin appears to be as potent as the reference modulator of PTP, i.e. CsA. Prevention of tBH-related cytochrome c release in the cytoplasm and of cell death by metformin is also very significant and, here too, a low concentration of metformin appears to be as potent as CsA (see Figure 2). Involvement of PTP in the commitment to cell death was proposed from the finding that CsA was a protective agent in several models of cell death [34–37]. Other results further support this view, such as early mitochondrial depolarization [38] preceding cytochrome c release [39] or the inhibition of cellular death by rotenone [21]. Results of the present study also clearly account for such an effect of PTP modulation by complex 1 inhibition on the regulation of cell death, metformin being a novel agent of this regulation.
Considering that (i) most of the deleterious complications of diabetes seem to be due to the glucose-induced production of reactive oxygen species by the respiratory chain [40], (ii) exogenous oxidative stress-related cell death is mediated by PTP opening [21] and (iii) inhibition of complex 1 prevents PTP opening [21], we propose that metformin, which acts as a mild complex 1 inhibitor, prevents oxidative stress-related cytochrome c release and commitment to cell death. This finding may represent an important new direction in the treatment of hyperglycaemia-related detrimental effects. If hyperglycaemia-related deleterious consequences are linked to superoxide overproduction at the level of the mitochondrial respiratory chain [5], whereas mitochondrial superoxide generation, or exogenous oxidative stress, leads to apoptosis, then inhibition of PTP opening may efficiently prevent the effect of hyperglycaemia. Hence, if metformin prevents PTP opening on exogenous oxidative stress as well as its effects on apoptosis, this may suggest that metformin also prevents hyperglycaemia-induced cellular death. This finding may provide a new direction for the treatment of hyperglycaemia-related complications: besides the necessity for lowering the blood glucose level, it is possible to diminish the mitochondria-related toxicity of hyperglycaemia.
Acknowledgments
We are grateful to Dr Nicolas Wiernsperger (MERCK SANTE/INSERM U585, Villeurbanne, France) for stimulating discussions and to Professor Mark H. Rider (Hormone and Metabolic Research Unit, Institute of Cellular Pathology, Brussels, Belgium) and our colleague Dr Christiane Keriel for revision of this paper. This work was supported by INSERM, MERT (Ministère de l'Enseignement, de la Recherche et de la Technologie) and Merck (to B.G.).
References
- 1.Anonymous. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. The Diabetes Prevention Program Research Group. Diabetes Care. 2000;23:1619–1629. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., Nathan D. M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner R. C., Cull C. A., Frighi V., Holman R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA, J. Am. Med. Assoc. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 4.van den Berghe G., Wouters P., Weekers F., Verwaest C., Bruyninckx F., Schetz M., Vlasselaers D., Ferdinande P., Lauwers P., Bouillon R. Intensive insulin therapy in the critically ill patients. N. Engl. J. Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature (London) 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo R. A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bailey C. J. Biguanides and NIDDM. Diabetes Care. 1992;15:755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 8.Bailey C. J., Turner R. C. Metformin. N. Engl. J. Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 9.Stumvoll M., Nurjhan N., Perriello G., Dailey G., Gerich J. E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 10.Hundal H. S., Ramlal T., Reyes R., Leiter L. A., Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- 11.Argaud D., Roth H., Wiernsperger N., Leverve X. M. Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur. J. Biochem. 1993;213:1341–1348. doi: 10.1111/j.1432-1033.1993.tb17886.x. [DOI] [PubMed] [Google Scholar]
- 12.Hundal R. S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S. E., Schumann W. C., Petersen K. F., Landau B. R., et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryer L. G., Parbu-Patel A., Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 15.Hawley S. A., Gadalla A. E., Olsen G. S., Hardie D. G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 16.El-Mir M. Y., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 17.Bernardi P., Scorrano L., Colonna R., Petronilli V., Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 18.Scorrano L., Korsmeyer S. J. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 19.Fontaine E., Eriksson O., Ichas F., Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex I. J. Biol. Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 20.Fontaine E., Bernardi P. Progress on the mitochondrial permeability transition pore: regulation by complex I and ubiquinone analogs. J. Bioenerg. Biomembr. 1999;31:335–345. doi: 10.1023/a:1005475802350. [DOI] [PubMed] [Google Scholar]
- 21.Chauvin C., De Oliveira F., Ronot X., Mousseau M., Leverve X., Fontaine E. Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. J. Biol. Chem. 2001;276:41394–41398. doi: 10.1074/jbc.M106417200. [DOI] [PubMed] [Google Scholar]
- 22.Charalampous F. C., Gonatas N. K. The plasma membrane of the KB cells: isolation and properties. Methods Cell Biol. 1975;9:259–280. doi: 10.1016/s0091-679x(08)60078-3. [DOI] [PubMed] [Google Scholar]
- 23.Srere P. A. The citrate synthase. Methods Enzymol. 1969;13:3–26. [Google Scholar]
- 24.Petronilli V., Miotto G., Canton M., Brini M., Colonna R., Bernardi P., Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuurendonk P. F., Tischler M. E., Akerboom T. P., Van Der Meer R., Williamson J. R., Tager J. M. Rapid separation of particulate and soluble fractions from isolated cell preparations (digitonin and cell cavitation procedures) Methods Enzymol. 1979;56:207–223. doi: 10.1016/0076-6879(79)56023-6. [DOI] [PubMed] [Google Scholar]
- 26.Detaille D., Guigas B., Leverve X., Wiernsperger N., Devos P. Obligatory role of membrane events in the regulatory effect of metformin on the respiratory chain function. Biochem. Pharmacol. 2002;63:1259–1272. doi: 10.1016/s0006-2952(02)00858-4. [DOI] [PubMed] [Google Scholar]
- 27.Owen M. R., Doran E., Halestrap A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 28.Brunmair B., Staniek K., Gras F., Scharf N., Althaym A., Clara R., Roden M., Gnaiger E., Nohl H., Waldhausl W., et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 29.Chen R., Fearnley I. M., Peak-Chew S. Y., Walker J. E. The phosphorylation of subunits of complex I from bovine heart mitochondria. J. Biol. Chem. 2004;279:26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- 30.Salt I., Celler J. W., Hawley S. A., Prescott A., Woods A., Carling D., Hardie D. G. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem. J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bera A. K., Ghosh S. Dual mode of gating of voltage-dependent anion channel as revealed by phosphorylation. J. Struct. Biol. 2001;135:67–72. doi: 10.1006/jsbi.2001.4399. [DOI] [PubMed] [Google Scholar]
- 32.Azoulay-Zohar H., Israelson A., Abu-Hamad S., Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R. B., Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crompton M. Mitochondrial intermembrane junctional complexes and their role in cell death. J. Physiol. (Cambridge, U.K.) 2000;529:11–21. doi: 10.1111/j.1469-7793.2000.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichas F., Mazat J. P. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim. Biophys. Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 36.Kroemer G., Reed J. C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 37.Lemasters J. J., Nieminen A. L., Qian T., Trost L. C., Elmore S. P., Nishimura Y., Crowe R. A., Cascio W. E., Bradham C. A., Brenner D. A., et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 38.Vayssiere J. L., Petit P. X., Risler Y., Mignotte B. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11752–11756. doi: 10.1073/pnas.91.24.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Desagher S., Martinou J. C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 40.Du X. L., Edelstein D., Rossetti L., Fantus I. G., Goldberg H., Ziyadeh F., Wu J., Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]