Abstract
PPM (polyprenol monophosphomannose) has been shown to act as a glycosyl donor in the biosynthesis of the Man (mannose)-rich mycobacterial lipoglycans LM (lipomannan) and LAM (lipoarabinomannan). The Mycobacterium tuberculosis PPM synthase (Mt-Ppm1) catalyses the transfer of Man from GDP-Man to polyprenyl phosphates. The resulting PPM then serves as a donor of Man residues leading to the formation of an α(1→6)LM intermediate through a PPM-dependent α(1→6)mannosyltransferase. In the present study, we prepared a series of ten novel prenyl-related photoactivatable probes based on benzophenone with lipophilic spacers replacing several internal isoprene units. These probes were excellent substrates for the recombinant PPM synthase Mt-Ppm1/D2 and, on photoactivation, several inhibited its activity in vitro. The protection of the PPM synthase activity by a ‘natural’ C75 polyprenyl acceptor during phototreatment is consistent with probe-mediated photoinhibition occurring via specific covalent modification of the enzyme active site. In addition, the unique mannosylated derivatives of the photoreactive probes were all donors of Man residues, through a PPM-dependent mycobacterial α(1→6)mannosyltransferase, to a synthetic Manp(1→6)-Manp-O-C10:1 disaccharide acceptor (where Manp stands for mannopyranose). Photoactivation of probe 7 led to striking-specific inhibition of the M. smegmatis α(1→6)mannosyltransferase. The present study represents the first application of photoreactive probes to the study of mycobacterial glycosyltransferases involved in LM and LAM biosynthesis. These preliminary findings suggest that the probes will prove useful in investigating the polyprenyl-dependent steps of the complex biosynthetic pathways to the mycobacterial lipoglycans, aiding in the identification of novel glycosyltransferases.
Keywords: benzophenone, inhibition, lipoarabinomannan, mannosyltransferase, mycobacterial, photoprobe
Abbreviations: ESI–MS, electrospray ionization mass spectrometry; LAM, lipoarabinomannan; LB, Luria–Bertani; LM, lipomannan; mAGP, mycolyl–arabinogalactan–peptidoglycan; Man, mannose; ManLAM, LAM with Man caps; Manp, mannopyranose; PILAM, LAM with phosphoinositide caps; PIM, phosphatidyl-myo-inositol mannoside; PPM, polyprenol monophosphomannose
INTRODUCTION
The biosynthetically related lipoglycans, LAM (lipoarabinomannan), LM (lipomannan) and the PIMs (phosphatidyl-myo-inositol mannosides) are important constituents of the cell envelope of mycobacteria, including pathogens such as Mycobacterium tuberculosis [1]. The basic structural features of LAMs are well described; they possess an amphipathic tripartite structure [2,3] in which common domains include a mannosyl-phosphatidyl-myo-inositol anchor and a polysaccharide backbone consisting of mannose (Manp, mannopyranose) and arabinose (Araf, arabino-furanose) residues. These molecules, termed AraLAM [4], are subject to further species-specific elaborations, either mannooligosaccharide or phosphoinositol caps, resulting in ManLAM (LAM with Man caps) [5,6] or PILAM (LAM with phosphoinositide caps) [7,8] respectively.
Both ManLAM and PILAM exhibit a broad range of immunomodulatory activities. For example, ManLAM predominantly found in the slow-growing pathogenic mycobacteria, inhibits a number of immune system effector functions, including interferon-γ-mediated activation of macrophages [9–11]. ManLAM also inhibits the production of the proinflammatory cytokines interleukin-12 [12] and tumour necrosis factor α [13]. Thus it appears that slow-growing mycobacteria possess a virulence factor enabling their persistence within the host. Conversely, PILAM, which is characteristic of the fast-growing saprophytic mycobacteria, can induce a proinflammatory response in a Toll-like 2 receptor-dependent manner [14], and as a consequence PILAM probably favours the killing of these mycobacteria. The biological importance of the capping motifs found in ManLAM is now clear and reinforces the emerging paradigm that ‘capped’ LAM molecules possess immunomodulatory properties [12,15,16].
Although the structure of mycobacterial LAM has been well documented [2,3], the genetics of its biosynthesis remains largely ill defined. Intuitively, it should proceed via the sequential addition of Manp residues to PI to produce PIMs (2–6 Manp residues) and LM (up to 20 Manp residues), which is then extensively arabinosylated to form AraLAM. The biosynthetic relationship of PI→PIMs→LM→AraLAM has recently been supported by biochemical [17] and genetic studies [18–21]. Besra et al. [17] demonstrated that Ac1PIM2 (according to the nomenclature of Kordulakova et al. [21]) is specifically extended by an addition of Manp residues from the alkali-stable sugar donor C50/C40/C35-PPM (where PPM stands for polyprenol monophosphomannose) to form higher PIMs and further to a ‘linear’ α(1→6)-LM via a PPM-dependent α(1→6)mannosyltransferase. The generation of PPM in M. tuberculosis H37Rv results from the transfer of Manp from GDP-Manp to polyprenyl phosphates catalysed by the C-terminal domain(s) of a PPM synthase (Mt-Ppm1), now termed as Mt-Ppm1/D2 [22]. However, the identity of the mycobacterial PPM-dependent α(1→6)mannosyltransferase is unknown.
In the present study, we have chemically synthesized a structurally novel series of compounds based on a modified photoreactive benzophenone-linked prenyl phosphate as analogues of the mycobacterial C50/C40/C35 polyprenyl phosphates. The compounds were designed specifically to investigate their utilization as substrates by the PPM synthase Mt-Ppm1/D2 and its subsequent inhibition after photoactivation. Similarly, through the enzymic synthesis of the corresponding mannosylated derivatives of the photoreactive probes by recombinant Mt-Ppm1/D2 and using a Manpα(1→6)Manp-O-C10:1 neoglycolipid acceptor assay [23], we demonstrate their utilization as Man donors and their inhibitory properties after photoactivation in relation to the mycobacterial PPM:(α1→6)mannosyltransferase. The present study describes the first use of photoreactive probes in the study of glycosyltransferases involved in mycobacterial LM and LAM biosynthesis. More generally, in combination with modern proteomic and genomic methodologies, these probes represent novel tools to aid in the identification of glycosyltransferases dependent on polyprenylphosphate sugar donors and the definition of their ligand interaction sites.
EXPERIMENTAL
Chemistry
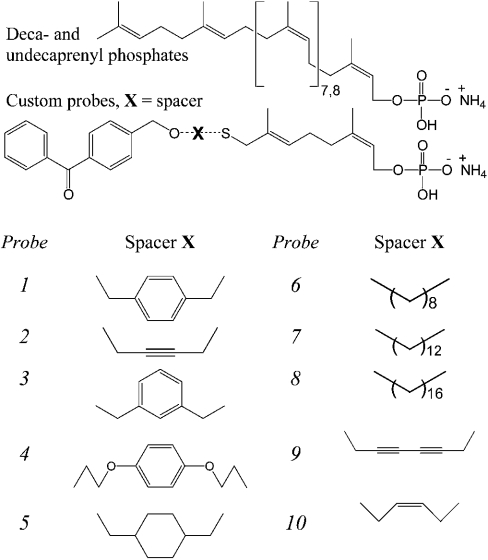
A general description of the route to the synthesis of the benzophenone-linked prenyl phosphates leading to probes 1–10 (Figure 1) will be documented separately (G. S. Besra, unpublished work).
Figure 1. Chemical structures of decaprenyl (C50)/undecaprenyl (C55) polyprenol phosphates and synthetic benzophenone-containing polyprenol phosphate-based compounds (probes 1–10).
Bacterial strains and growth conditions
All cloning steps were performed in Epicurian Coli™ XL1-Blue (Stratagene, La Jolla, CA, U.S.A.). M. smegmatis mc2155 was a gift from W. R. Jacobs (Albert Einstein College of Medicine, Bronx, NY, U.S.A.). Liquid cultures of M. smegmatis mc2155 were grown at 37 °C in LB (Luria–Bertani) broth (Difco, Detroit, MI, U.S.A.) supplemented with 0.05% Tween 80. Sequences corresponding to the C-terminal domain of the Rv2051c gene product (Mt-ppm/D2) required for cloning into pUC8 were obtained by PCR using Vent polymerase (New England Biolabs, Beverly, MA, U.S.A.) as described previously [22]. Liquid cultures of Escherichia coli (pUC8-Mt-ppm1/D2) were grown in LB broth at 37 °C with 100 μg/ml ampicillin to an A600 0.4 and induced for 4 h with 1 mM isopropyl β-D-thiogalactoside [22]. Bacterial strains were grown in LB broth, supplemented with antibiotics, grown to mid-exponential phase, harvested by centrifugation, washed with PBS (50 mM, pH 7.5) and stored at −20 °C until further use [22].
Preparation of enzyme extracts
M. smegmatis mc2155 and E. coli (pUC8-Mt-ppm1/D2) were grown as described above. Mycobacterial cells (10 g wet weight) were washed and re-suspended in 30 ml of buffer A, containing 50 mM Mops (adjusted to pH 8.0 with KOH), 5 mM 2-mercaptoethanol and 10 mM MgCl2 at 4 °C and subjected to sonication (Soniprep 150; MSE Sanyo Gallenkamp, Crawley, Sussex, U.K.; 1 cm probe) for a total time of 10 min in 60 s pulses and 90 s cooling intervals. E. coli cells were disrupted in a similar fashion using 30 s pulses and 30 s cooling intervals. The sonicated materials were centrifuged at 27000 g for 20 min at 4 °C. The supernatant fractions from E. coli (pUC8-Mt-ppm1/D2) were used in all subsequent PPM experiments. Membrane fractions from M. smegmatis mc2155 were obtained by further centrifugation of the 27000 g supernatant at 100000 g for 1 h at 4 °C and used in the α(1→6)mannosyltransferase neoglycolipid acceptor assay [23]. The supernatant was carefully removed and the membranes were gently re-suspended in buffer A at a protein concentration of 20 mg/ml. Protein concentrations were determined using the BCA Protein Assay Reagent kit (Pierce Europe, Oud-Beijerland, The Netherlands).
PPM synthase assay
Reaction mixtures for assessing [14C]Man incorporation consisted of 2.4 μM GDP-[U-14C]Man (DuPont–New England Nuclear, Stevenage, Herts., U.K.; 321 mCi/mmol and 0.25 μCi), 62.5 μM ATP, 10 μM MgCl2 and the supernatant fraction from Epicurian Coli™ XL1-Blue (pUC8-Mt-ppm1/D2) corresponding to 100–250 μg of protein in a final volume of 50 μl. Exogenous lipid monophosphate substrates were added to the reaction mixtures at a final concentration of 0.25 mM in 0.25% CHAPS. The reaction mixtures were then incubated at 37 °C for 30 min. In addition, photolysis experiments were conducted at 4 °C in the absence of GDP-[14C]Man, for a 45–90 min exposure time, using a UV lamp (365 nm) placed over the open microcentrifuge tubes at a distance of 3 cm, both control and photolysed samples were then incubated at 37 °C for 30 min with GDP-[14C]Man and further processed as described below using extraction method 2. In competition/protection experiments, probe 7 was used at 0.2 mM (a value which consistently resulted in photoinactivation of approx. 50%) and indicated amounts of C75 polyprenyl phosphate were added as appropriate before photolysis.
Extraction and characterization of 14C-Man-labelled products from reaction mixtures
Method 1
The enzymic reactions were terminated by the addition of chloroform/methanol/0.8 M sodium hydroxide (10:10:3, by vol.; 700 μl per 50 μl of reaction mixture) followed by further incubation at 50 °C for 20 min. After base treatment, the reaction mixtures were partitioned between the phases arising from n-butanol and water. The water-saturated n-butanol layer was recovered, backwashed twice with n-butanol-saturated water and dried. The resulting C55-P[14C]Man and the probe-P[14C]Man were resuspended in 200 μl of chloroform/methanol (2:1, v/v) and an aliquot (10 μl) dried under a stream of argon in a scintillation vial before scintillation counting using 10 ml of EcoScintA (National Diagnostics, Atlanta, GA, U.S.A.). TLC of the reaction products, usually aliquots representing 10% of the reaction mixtures, was performed on aluminium-backed plates of silica gel 60 F254 (E. Merck, Darmstadt, Germany) using chloroform/methanol/1.8 M ammonium hydroxide (65:25:4.1, by vol.). Autoradiograms were obtained by exposing chromatograms to Kodak X-Omat AR films for 2–3 days at −70 °C.
Method 2
Alternatively, 5 μl of the crude reaction mixture was loaded directly on to the concentrating zone of a glass-backed HPTLC (high-performance TLC) plate (E. Merck) and developed using chloroform/methanol/1.8 M ammonium hydroxide (65:25:4.1, by vol.). Autoradiograms were obtained by exposing chromatograms to Kodak X-Omat AR films for 2–3 days at −80 °C.
Analysis of reaction products
Large-scale reaction mixtures containing unlabelled GDP-Man (80 mM) and the other components were prepared and processed as described above using extraction method 1. The reaction products were dried, applied to preparative TLC plates (plastic-backed plates of silica gel 60 F254; E. Merck) along with radiolabelled material (50000 c.p.m.) to trace the unlabelled enzymically synthesized products and developed in chloroform/methanol/1.8 M ammonium hydroxide (65:25:4.1, by vol.) Autoradiography was performed as described previously [22]. The band corresponding to the synthesized products were recovered from plates using water-saturated n-butanol. The water-saturated n-butanol extracts were backwashed twice with n-butanol-saturated water, dried under a stream of nitrogen and analysed using ESI–MS (electrospray ionization mass spectrometry) as described previously [22].
α(1→6)Mannosyltransferase neoglycolipid-based acceptor assay
The synthetic Manp(1→6)Manp-O-C10:1 disaccharide acceptor (0.5 mM) [23] and either C50-P[14C]Man or probe-P[14C]Man (100000 c.p.m., stored in chloroform/methanol, 2:1, v/v) were dried under a stream of argon in a microcentrifuge tube (1.5 ml) and placed in a vacuum desiccator for 15 min to remove any residual solvent. The dried constituents of the assay were then resuspended in 8 μl of a 1% aqueous solution of Igepal CA-630. The remaining constituents of the mannosyltransferase assay containing 50 mM Mops (adjusted to pH 8.0 with KOH), 5 mM 2-mercaptoethanol, 10 mM MgCl2, 1 mM ATP and M. smegmatis mc2155 (250 μg) were added to a final reaction volume of 80 μl. The reaction mixtures were then incubated at 37 °C for 1 h. A chloroform/methanol (533 μl, 1:1, v/v) solution was then added to the incubation tubes and the entire contents centrifuged at 18000 g. The supernatant was recovered and dried under a stream of argon and re-suspended in C2H5OH/water (1 ml, 1:1, v/v) and loaded on to a preequilibrated (C2H5OH/water, 1:1, v/v) 1 ml Whatman strong anion-exchange (SAX) cartridge, which was washed with 3 ml of ethanol. The eluate was dried and the resulting products were partitioned between the two phases arising from a mixture of n-butanol (3 ml) and water (3 ml). The resulting organic phase was recovered after centrifugation at 3500 g and the aqueous phase was extracted again twice with 3 ml of water-saturated n-butanol; the pooled extracts were back-washed twice with n-butanol-saturated water (3 ml). The water-saturated n-butanol fraction was dried and re-suspended in 200 μl of n-butanol. The extracted radiolabelled material was quantified by liquid-scintillation counting of 20 μl of the extract in 10 ml of EcoScintA (National Diagnostics). The incorporation of [14C]Manp was determined by subtracting counts present in control assays (incubation of the reaction components in the absence of the synthetic disaccharide acceptor). Another 20 μl of the labelled material was subjected to TLC in chloroform/methanol/1.8 M ammonium hydroxide (65:25:4.1, by vol.) on aluminium-backed Silica Gel 60 F254 plates (E. Merck). Autoradiograms were obtained by exposing TLCs to X-ray film (Kodak X-Omat) for 3 days. Photolysis experiments were conducted at 4 °C in the absence of the synthetic Manp(1→6)Manp-O-C10:1 disaccharide acceptor using a UV lamp (365 nm) for a 90 min exposure time placed over the open microcentrifuge tube at a distance of 3 cm. Control and photolysed samples were then incubated at 37 °C for 1 h along with the synthetic Manp(1→6)Manp-O-C10:1 disaccharide acceptor and further processed as described above to determine the extent of product formation. In competition/protection experiments, C75 polyprenyl phosphomannose was added before photolysis.
RESULTS
Chemistry – synthetic strategy
A simple convergent approach was utilized for the synthesis of candidate photoaffinity target molecules based on polyprenol moieties. The basic strategy conceived was to prepare a series of modified polyprenyl phosphates with a pendant benzophenone group attached to the end of the polyprenol chain via a diethyl ether linkage. In a related approach, photoreactive diethyl ether-linked benzophenone farnesyl diphosphate analogues have been utilized previously in the study of protein prenyltransferases [24]. It was assumed at the outset that at least one prenyl unit would probably be required for molecular recognition. However, to facilitate photoprobe synthesis and enable investigation of possible effects due to different phosphate–photoprobe distances, it was decided to replace the remaining prenyl units with a range of lipophilic spacers. The final targets in the present study retain two isoprene units attached to the spacers via a thioether. The replacement of the ‘middle’ prenyl section of the molecule by an accessible unit attached through a diethyl ether/thioether linkage provides modified probes that retain the essential physicochemical characteristics of the natural substrates. A series of ten spacer units were selected from commercially available symmetrical diols spanning a range of different chain lengths with varying degrees of flexibility and the corresponding prenyl phosphate-based probes synthesized (G. S. Besra, unpublished work) (Figure 1). The photoreactive probes 1–10 formed the basis of a preliminary study to interrogate the binding of Mt-Ppm1 substrate analogues and to test the structural constraints of molecular recognition and substrate binding for future photoinactivation experiments.
Enzymic mannosylation of benzophenone-linked prenyl phosphate substrate analogues by recombinant Mt-Ppm1/D2
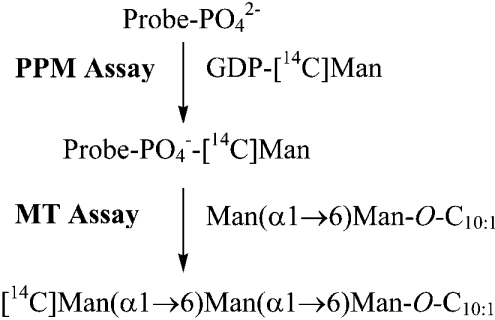
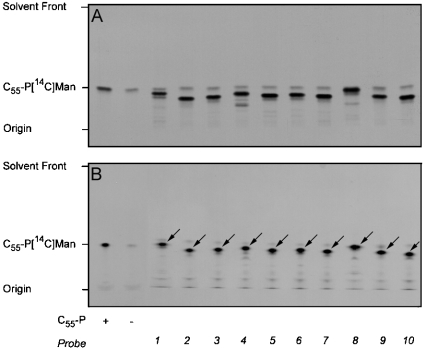
To establish whether the synthetic benzophenone-linked prenyl phosphate analogues were substrates for the M. tuberculosis PPM synthase component Mt-Ppm1/D2, we incubated extracts prepared from Epicurian Coli™ XL1-Blue expressing recombinant Mt-ppm1/D2 with the analogues and GDP-[14C]Man (Scheme 1). The E. coli (pUC8-Mt-ppm1/D2) extract possessed a moderate level of activity in the absence of added acceptor (Table 1), which after analysis by TLC/autoradiography (Figures 2A and 2B), was attributed to the utilization of endogenous C55-polyprenyl monophosphate. Supplementing the extracts with exogenous C55 polyprenyl phosphate led to increased formation of C55-P[14C]Man (Table 1 and Figures 2A and 2B). Introduction of the benzophenone-linked prenyl phosphates and GDP-[14C]Man led to the synthesis of the [14C]mannosylated derivatives of the benzophenone-linked prenyl phosphates (Table 1 and Figures 2A and 2B). Interestingly, with all ten novel synthetic probes, Mt-Ppm1/D2 displayed greater activity when compared with the C55-polyprenyl phosphate standard (Table 1 and Figures 2A and 2B).
Scheme 1. Illustration of substrates and donors for the PPM and α(1→6)mannosyltransferase (MT) assays.
Table 1. Performance of probes as [14C]Man acceptors and inhibitors in PPM synthase assays.
Mannosyltransferase activities (pmol/min) determined for recombinant Mt-Ppm1/D2 with each probe. NA, not applicable.
| PPM activity*† (pmol/min) | Photoinactivation‡ (%) | |
|---|---|---|
| No exogenous substrate | 1.20 | NA |
| C55-P | 3.48 | 0 |
| Probe 1 | 6.33 | 0 |
| Probe 2 | 10.56 | 28.2 |
| Probe 3 | 10.91 | 34.1 |
| Probe 4 | 11.32 | 11.6 |
| Probe 5 | 12.73 | 0 |
| Probe 6 | 11.29 | 2.9 |
| Probe 7 | 14.17 | 83.9 |
| Probe 8 | 9.21 | 9.5 |
| Probe 9 | 9.96 | 34.9 |
| Probe 10 | 13.55 | 32.0 |
* The [14C]mannosylated products were isolated as described in [22] and represent an average of triplicates from three independent experiments.
† Specific activities are reported after subtracting counts observed from assays performed with no exogenous substrate (see Figure 2A).
‡ The values given for percentage photoinactivation reflect the reduction in absorbance of autoradiogram relative to non-UV-treated reactions above the chromatographic origin after HPTLC/autoradiography. The values presented are typical and are those given for the experiment depicted in Figure 4.
Figure 2. Transfer of [14C]Man from GDP-[14C]Man to exogenous prenylated monophosphates by Mt-Ppm1/D2.
The standard reaction mixture contained the indicated lipid monophosphate substrate at a final concentration of 0.25 mM in 0.25% CHAPS. Incubations were performed for 30 min at 37 °C with extracts from Epicurian Coli™ XL1-Blue (pUC8-Mt-ppm1/D2) and the resulting [14C]Man products isolated following either base treatment and butanol/water extraction (A) or examined by directly loading 10% of the reaction material on to a concentrating zone of a HPTLC plate (B). An aliquot of the n-butanol-extracted products (10%) was subjected to scintillation counting (see Table 1). A second aliquot (10%) (A) along with the material applied to the HPTLC plate (B) was then subjected to TLC/autoradiography using chloroform/methanol/1.8 M ammonium hydroxide (65/25/4.1). The C55-P[14C]Man product is shown for reference and PPM products in relation to probes 1–10 indicated by the arrows on (B) for clarity. Assays were performed in triplicate using freshly prepared membranes; the TLC was representative of a number of samples from independent experiments.
In addition, a useful method for the direct analysis of reaction products by HPTLC was developed, eliminating the need for mild base/n-butanol extraction [22]. As samples of reactions (10%, by vol.) analysed by HPTLC/autoradiography yielded similar results to our traditional analyses (Figures 2A and 2B), the HPTLC-based method was adopted for subsequent experiments.
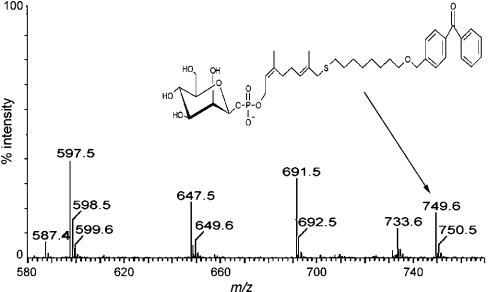
Similar extracts containing recombinant Mt-Ppm1/D2 were used to produce non-radioactive reaction products for each benzophenone-linked prenyl phosphate acceptor using unlabelled GDP-Man as the sugar donor. After purification by preparative TLC, these were characterized using ESI–MS. The reaction products yielded (M-H)− ions for the precursor lipid phosphates and, more importantly, the addition of m/z 162 was observed confirming mannosylation of the lipid phosphates as shown for probe 6 (Figure 3). Thus Mt-Ppm1/D2 appears to tolerate substantial changes in the lipid moiety, such as the incorporation of aromatic, cyclohexyl rings, oxygen and sulphur substitutions.
Figure 3. Negative ion ESI–MS of in vitro synthesized mannosylated probe 6.
Following extraction of polyprenyl species after in vitro mannosylation using a lysate of Epicurian Coli™ XL1-Blue (pUC8-Mt-ppm1/D2), the resulting products of probe 6 were assigned to the molecular ion of the mannosylated probe 6 product at m/z 749.6. In addition, m/z 587.4 corresponds to the molecular ion of probe 6.
Photoactivatable inhibition of Mt-ppm1/D2 with benzophenone-linked prenyl phosphate substrate analogues
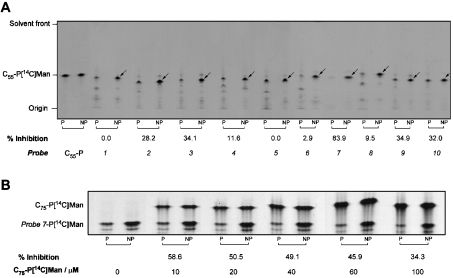
Having established that probes 1–10 were excellent novel substrates for the M. tuberculosis PPM synthase, we conducted photoinactivation experiments using cell-free lysates of E. coli containing recombinant Mt-Ppm1/D2. The PPM synthase activities of identical samples were assayed both with and without prior UV irradiation (for 45 min at 4 °C). When C55-polyprenyl phosphate was used as the [14C]Man acceptor, we noted no significant decay in enzyme activity due to non-specific effects of this UV treatment. However, when probes 1–10 were added as acceptors, significant reductions in mannosylated acceptor product were seen in most of the cases (Figure 4A). Several unidentified labelled species were clearly visible on autoradiograms, migrating with decreased mobility relative to the relevant probe on HPTLC. We suggest that these species may be generated by reaction of the probe with small molecule components of the assay mixture or may represent probe oligomers formed during the irradiation period, i.e. before exposure to GDP-[14C]Man. Alternatively, some degradation of the probe resulting in the loss of the benzophenone group may occur. Irrespective of their route of generation, their incorporation of radioactivity from the nucleotide sugar donor clearly demonstrates that these remain competent as sugar acceptors. Consequently, radiolabelling of these species has been incorporated into our measurements for quantification of Mt-Ppm1/D2 activity and the extent of any photoinhibition (Figure 4A). As probe 7 appeared less prone to these effects, it was used in subsequent protection assays to simplify analyses (Figure 4A).
Figure 4. HPTLC analysis of photoprobe-dependent photoactivable inhibition of Mt-Ppm1/D2 and its protection using C75 polyprenyl monophosphate.
(A) The standard reaction mixture contained 250 μg of protein from a cell-free extract of E. coli pUC8-ppm1/D2 and the indicated lipid monophosphate substrates at a final concentration of 0.25 mM in 0.25% CHAPS. Photoinactivation was conducted at 4 °C in the absence of GDP-[14C]Man using a UV lamp (365 nm) for a 45 min exposure time and then both non-photolysed (NP) and photolysed (P) samples were incubated at 37 °C for 30 min with GDP-[14C]Man. The resulting probe-P[14C]Man products were examined by direct loading of 10% of the reaction material on to the concentrating zone of a HPTLC plate and subjected to TLC/autoradiography using chloroform/methanol/1.8 M ammonium hydroxide (65:25:4.1). The C55-P[14C]Man product is shown for reference, and PPM products in relation to probes 1–10 are indicated by arrows on NP lanes for clarity. Assays were performed in triplicate using freshly prepared membranes, with the TLC representative of a number of samples from independent experiments. (B) As in (A) but using 100 μg of protein with 0.2 mM probe 7 and increasing concentrations (indicated) of C75 polyprenyl monophosphate added to the mixtures before photolysis.
Quantification of all migrating radiolabelled species, in identical samples with or without UV-treatment before assay, demonstrated that several of the probes appear to cause bona fide photoinhibition of Mt-Ppm1/D2. Probe 7 was particularly potent with UV treatment leading to a reduction in mannosyltransferase activity of 83.9% (Figure 4A, Table 1). Photoactivation of several probes caused reductions of approx. 30% in activity with others having very small (approx. 10%) or immeasurable effects on overall mannosyltransferase activity. Interestingly, the effects of UV treatment of probes 6–8, i.e. those containing the simple alkyl spacer units, on activity were particularly diverse.
Natural polyprenyl phosphates protect mannosyltransferase activity of Mt-Ppm1/D2
We elaborated on the above experiments by introducing increasing amounts of a ‘natural’ polyprenyl phosphate acceptor to determine whether this might facilitate protection of the enzyme by competing with probe 7 for occupancy of its active site. To visualize the individual mannosylated products, we used C75 polyprenyl phosphate, which on mannosylation, migrates at a significantly higher Rf value than the corresponding probe 7 product. Our previous studies have shown that C75 polyprenyl phosphate acts as a good Man acceptor from Mt-Ppm1/D2 producing a similar reaction rate to our probes [22].
We used probe 7 at 0.2 mM, as this concentration consistently produced approx. 50% photoactivatable inhibition in our experiments. Our results clearly show that the extent of the photoinhibition of the mannosyltransferase activity is diminished with increasing C75 polyprenyl phosphate concentration (Figure 4B). This relationship suggests that the degree of protection from the inhibition by photoactivated probe 7 increases with more frequent occupancy of the active site by the competing ‘natural’ ligand and supports the hypothesis that photoactivated probe 7 specifically inhibits this mannosyltransferase by covalent modification of its active site.
Probe-P[14C]Man act as sugar donors for a mycobacterial α(1→6)mannosyltransferase and photoactivated probe 7-P-Man inhibits activity
Previously, we established that synthetic glycosides could be utilized as acceptor substrate analogues in assays for a variety of mycobacterial glycosyltransferases [23,25,26]. In particular, we demonstrated that the transfer of Man from the mycobacterial C50/C40/C35-P[14C]Man donor to the synthetic Manp(1→6)-Manp-O-C10:1 neoglycolipid acceptor was facilitated by a α(1→6)mannosyltransferase in M. smegmatis membrane preparations forming a [14C]Manp(1→6)Manp(1→6)Manp-O-C10:1 trisaccharide product [23]. In the present study, we reproduced these assays using mannosylated probes, generated via recombinant Mt-Ppm1/D2, as the radiolabelled Man donor. The mannosylated derivatives of probes 1–10 all behaved as donors of [14C]Man for the M. smegmatis α(1→6)mannosyltransferase (Table 2), suggesting that this enzyme tolerates a degree of alteration in the structure of its sugar donor. Furthermore, the mannosylated probes represented better substrates than the natural C50-P-Man donor.
Table 2. Performance of [14C]Man-P-probes as glycosyl donors in α(1→6)mannosyltransferase assays.
Mannosyltransferase activities (pmol/min) determined for M. smegmatis α(1→6)mannosyltransferase with each mannosylated probe.
| Sugar donor | Mannosyltransferase activity* (pmol/min) |
|---|---|
| C50-P-Man | 0.017 |
| Probe 1-Man | 0.080 |
| Probe 2-Man | 0.043 |
| Probe 3-Man | 0.118 |
| Probe 4-Man | 0.081 |
| Probe 5-Man | 0.081 |
| Probe 6-Man | 0.065 |
| Probe 7-Man | 0.082 |
| Probe 8-Man | 0.050 |
| Probe 9-Man | 0.048 |
| Probe 10-Man | 0.032 |
* Specific activities are reported after subtracting counts observed from assays performed with no exogenous substrate. Each reaction contained 250 μg of protein from a membrane fraction of M. smegmatis mc2155 and 56 pmol (40000 c.p.m.) [14C]Man-P-probe/lipid. The reaction was performed at 37 °C for 1 h.
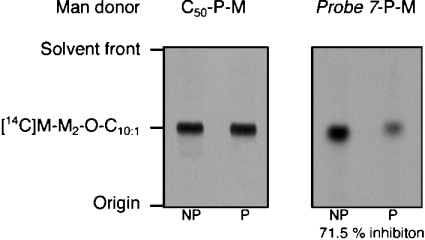
The processing of samples using our neoglycolipid disaccharide acceptor assays is designed to exclude donor species from the final sample. Thus the degradation or non-specific reactivity seen with some probes in our experiments with Mt-Ppm1/D2 would make the results of photoactivation experiments uncertain or, at best, difficult to analyse. Since in these experiments, probe 7 had appeared free of these effects, we selected probe 7-P[14C]Man to conduct a photoinactivation experiment with the M. smegmatis α(1→6)mannosyltransferase. The [14C]Man donors were mixed with a M. smegmatis membrane preparation and UV irradiated over a 90 min period at 4 °C. The synthetic Manp(1→6)Manp-O-C10:1 disaccharide acceptor was added, and the transfer of [14C]Man to the neoglycolipid acceptor was initiated by warming the reaction mixture to 37 °C and holding for 1 h. As before, identical reactions were performed without photoactivation to determine the mannosyltransferase activity of the extract. When using C50-P[14C]Man as the Man donor, the effect of UV treatment on α(1→6)mannosyltransferase activity was found to be negligible (Figure 5). However, it was clear that in addition to behaving as a donor of [14C]Man in the assay, probe 7-P[14C]-Man produced a strong photoinactivation of the α(1→6)mannosyltransferase (Figure 5), reducing its activity by 71.5%.
Figure 5. Probe 7-P[14C]Man is a glycosyl donor photoinhibitor of M. smegmatis α(1→6)mannosyltransferase.
The indicated mannosylated lipid monophosphate substrates (100000 c.p.m., 140 pmol) in 0.25% CHAPS were mixed with a M. smegmatis membrane preparation and some were photolysed at 4 °C using a UV lamp (365 nm) for 90 min. The non-photolysed (NP) and photolysed (P) samples were then incubated at 37 °C for 1 h along with the synthetic Manp(1→6)Manp-O-C10:1 disaccharide acceptor and processed and subjected to TLC/autoradiography as described previously [23] to determine the extent of product formation {[14C]M-M2-O-C10:1, [14C]Manp-(1→6)Manp(1→6)Manp-O-C10:1}.
As with Mt-Ppm1/D2, we analysed the ability of a ‘natural’ donor, C75-P-Man, to protect this M. smegmatis α(1→6)mannosyltransferase activity from photoinactivation by probe 7-P[14C]Man using a protection experiment similar to that described for Mt-Ppm1/D2. Although (often complete) protection from photoinactivation was always evident (results not shown), the reproducibility of these results was not as robust as seen with the recombinant Mt-Ppm1/D2, which in part may be due to the relatively low donor concentrations to which we are currently limited in this assay.
DISCUSSION
Although photoaffinity labelling has evolved considerably, the basic principle remains unchanged [27,28]. Benzophenone-based photophores have been exploited in biological systems as tethered photoactivatable agents to functionalize specific remote C–H bonds in steroids and to map conformations of flexible chains in solution, micelles and membranes [29]. They are more chemically stable than diazo esters, aryl azides and diazirines, enabling them to be manipulated under normal physiological conditions. They are activated at wavelengths of approx. 350–360 nm, which is not detrimental to the biochemical systems in which they are being utilized. These properties lead to highly efficient covalent modifications of macromolecules, often with considerable site specificity. Typically, the benzophenone unit in biochemical probes is coupled with active ligands via linkers bearing either moderately flexible (1–5 CH2 units, O, NH) or moderately rigid (>C=O, −COOH) functionalities.
Photoaffinity probes based on prenylated structures have been developed mainly to study protein prenylation in Ras proteins with farnesyltransferase inhibitors in particular [28], and a number of groups have incorporated benzophenone into the design of inhibitors and probes to target farnesyl protein transferase(s) [30–34]. In these studies, it has been shown that photoaffinity probes, based on naturally occurring prenols, are recognized by transferase enzymes and are taken up in much the same way as the natural substrates. Recently, fluorescent-based dolichyl phosphates modified at either internal β-, γ- or ω-isoprene units have been used as acceptors to examine the dolichyl phosphate Man synthase from Saccharomyces cerevisiae [35–37].
Glycosyl-monophosphoryl polyprenols have been implicated in the biosynthesis of the arabinan domains of the mAGP (mycolyl–arabinogalactan–peptidoglycan) complex, and arabinan and mannan domains of LAM and LM respectively [1,38]. In addition, decaprenyl diphosphate N-acetylglucosamine is involved in the biosynthesis of peptidoglycan and the initial stages of the ‘linker unit’ synthesis for mAGP in M. tuberculosis [39]. Clearly, enzymes that utilize polyprenyl phosphate carriers, whether for mycolylation or polysaccharide synthesis, are attractive targets for drug development due to the essentiality of PIM/LM and the mAGP complex and their subsequent identification key to future chemotherapeutic strategies [1,38].
In this preliminary investigation, we have focused on the synthesis of novel photoactivatable probes to study key stages involved in LM and LAM biosynthesis. Mycobacterial LAM is now well accepted as an important immunomodulatory molecule, and plays a significant role in the pathogenesis of tuberculosis. Indeed, a recent study has defined the precise role played by ManLAM in the molecular mechanism of the mycobacterial-induced arrest in phagosome maturation [40]. A recent review on LAM suggests that much remains to be discovered about the role of this important virulence factor for tuberculosis [3]. On the basis of the literature precedent for the use of photoprobes incorporating benzophenone, a unique series of compounds were designed specifically to probe the biosynthesis of the mannan portion of LM and LAM, through the utilization of PPM synthase and a PPM-dependent α(1→6)mannosyltransferase. The probes are chemically novel in that they incorporate a flexible linker unit attached to the photophore, reducing the isoprene-related structure from 7 to 10 units in mycobacteria [17] to just 2 units thereby removing the need for elaborate syntheses of modified isoprenoid units. The results were very gratifying in relation to substrate specificity and photoinhibition. All ten probes were excellent substrates for the PPM synthase, illustrating that changes within the lipid portion [22], such as overall chain length, saturation, unsaturation, benzyl and cyclohexyl rings, are tolerated without a substantial decrease in activity [22,35–37] (Table 1). Probe 7 yielded the best activity for the PPM synthase assay (Table 1), presumably representing an optimum in terms of hydrophobicity and solubility of the substrate. Interestingly, the activities of Mt-Ppm1/D2 with probes 1 and 5 as substrates differed by 2-fold in the PPM synthase assay (Table 1) and may reflect the change from a planar to a chair conformation of the utilized spacer unit (Figure 1). After photoactivation, probe 7 yielded the most significant inhibition (83.9%) in the PPM synthase assay, whereas probes 1, 5 and 6 produced little or no enzyme inhibition (Table 1). Given the similarities in reaction rate gained using probes 6 and 7 as Man acceptors and their structural similarity, the 12C spacer must define a particularly important parameter in terms of the protein–acceptor interactions culminating in effective photoinhibition. The specificity of this probe-mediated photoinhibition was supported by our observation that a ‘natural’ C75 polyprenyl phosphate acceptor protected the mannosyltransferase activity of Mt-Ppm1/D2 from photoinhibition by probe 7.
The mannosylated versions of the probes were all donors of [14C]Man for the M. smegmatis α(1→6)mannosyltransferase involved in LM/LAM biosynthesis. This was surprising and to our knowledge represents the first description of a synthetic prenylated non-natural donor for a glycosyltransferase. Probe 7 was the best donor in the synthetic disaccharide acceptor assay (Table 2) and its photoactivation led to the specific inhibition of the M. smegmatis α(1→6)mannosyltransferase activity by 71.5%.
In the absence of structural information, the generation of a panel of probes that incorporate a variety of spacer groups and display a range of potencies as enzyme inhibitors represents a useful ‘toolbox’ facilitating further study of lipoglycan-related glycosyltransferases in mycobacteria. It is envisaged that radiolabelling of the mannosylated and non-mannosylated forms of these probes should facilitate the study of the polyprenyl-ligand binding/recognition sites of these enzymes, as well as identifying the mycobacterial PPM-dependent α(1→6)mannosyltransferase, which is pivotal to the formation of LM and LAMs. Using a combined proteomic and genomic approach, it may now also be possible to examine several other complex pathways, such as the biosynthesis of the arabinose donor decaprenyl monophosphoarabinofuranose, polyprenyl-related arabinosyltransferases and mannosyltransferases involved in mAGP and LAM biosynthesis through the use of these modified prenylated probes, which will ultimately aid target identification and the drug discovery process towards new and effective treatments for M. tuberculosis infection.
Acknowledgments
G.S.B. acknowledges the support as a Lister Institute-Jenner Research Fellow. This work was supported by the Medical Research Council (G9901077 and G9901078), The Wellcome Trust (058972 and 060750) and EPSRC CASE Award (M.R.G.) with GlaxoSmithKline ActionTB.
References
- 1.Brennan P. J., Nikaido H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee D., Khoo K. H. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 3.Nigou J., Gilleron M., Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochemie. 2003;85:153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 4.Guerardel Y., Maes E., Elass E., Leroy Y., Timmermann P., Besra G. S., Locht C., Strecker G., Kremer L. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with α1,3-mannopyranose side chains. J. Biol. Chem. 2002;277:30635–30648. doi: 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- 5.Venisse A., Berjeaud J. M., Chaurand P., Gilleron M., Puzo G. Structural features of lipoarabinomannan from Mycobacterium bovis BCG. Determination of molecular mass by laser desorption mass spectrometry. J. Biol. Chem. 1993;268:12401–12411. [PubMed] [Google Scholar]
- 6.Chatterjee D., Lowell K., Rivoire B., McNeil M. R., Brennan P. J. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 1992;267:6234–6239. [PubMed] [Google Scholar]
- 7.Khoo K. H., Dell A., Morris H. R., Brennan P. J., Chatterjee D. Inositol phosphate capping of the non-reducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J. Biol. Chem. 1995;270:12380–12389. doi: 10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- 8.Gilleron M., Himoudi N., Adam O., Constant P., Venisse A., Riviere M., Puzo G. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans. Structure and localisation of alkali-labile and alkali-stable phosphoinositides. J. Biol. Chem. 1997;272:117–124. doi: 10.1074/jbc.272.1.117. [DOI] [PubMed] [Google Scholar]
- 9.Chan J., Fan X. D., Hunter S. W., Brennan P. J., Bloom B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibley L. D., Adams L. B., Krahenbuhl J. L. Inhibition of interferon-γ-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin. Exp. Immunol. 1990;80:141–148. doi: 10.1111/j.1365-2249.1990.tb06454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits γ interferon-mediated activation of macrophages. Infect. Immun. 1988;56:1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigou J., Zelle-Rieser C., Gilleron M., Thurnher M., Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 13.Knutson K. L., Hmama Z., Herrera-Velit P., Rochford R., Reiner N. E. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. Role of the Src homology 2 containing tyrosine phosphatase 1. J. Biol. Chem. 1998;273:645–652. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- 14.Means T. K., Wang S., Lien E., Yoshimura A., Golenbock D. T., Fenton M. J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 15.Maeda N., Nigou J., Herrmann J. L., Jackson M., Amara A., Lagrange P. H., Puzo G., Gicquel B., Neyrolles O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- 16.Sidobre S., Puzo G., Riviere M. Lipid-restricted recognition of mycobacterial lipoglycans by human pulmonary surfactant protein A: a surface-plasmon-resonance study. Biochem. J. 2002;365:89–97. doi: 10.1042/BJ20011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besra G. S., Morehouse C. B., Rittner C. M., Waechter C. J., Brennan P. J. Biosynthesis of mycobacterial lipoarabinomannan. J. Biol. Chem. 1997;272:18460–18466. doi: 10.1074/jbc.272.29.18460. [DOI] [PubMed] [Google Scholar]
- 18.Kremer L., Gurcha S. S., Bifani P., Hitchen P. G., Baulard A., Morris H. R., Dell A., Brennan P. J., Besra G. S. Characterisation of a putative α-mannosyltransferase involved in phosphatidylinositol trimannoside biosynthesis in Mycobacterium tuberculosis. Biochem. J. 2002;363:437–447. doi: 10.1042/0264-6021:3630437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kordulakova J., Gilleron M., Mikusova K., Puzo G., Brennan P. J., Gicquel B., Jackson M. Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis. PimA is essential for growth of mycobacteria. J. Biol. Chem. 2002;277:31335–31344. doi: 10.1074/jbc.M204060200. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer M. L., Khoo K. H., Besra G. S., Chatterjee D., Brennan P. J., Belisle J. T., Inamine J. M. The pimB gene of Mycobacterium tuberculosis encodes a mannosyltransferase involved in lipoarabinomannan biosynthesis. J. Biol. Chem. 1999;274:31625–31631. doi: 10.1074/jbc.274.44.31625. [DOI] [PubMed] [Google Scholar]
- 21.Kordulakova J., Gilleron M., Puzo G., Brennan P. J., Gicquel B., Mikusova K., Jackson M. Identification of the required acyltransferase step in the biosynthesis of the phosphatidylinositol mannosides of mycobacterium species. J. Biol. Chem. 2003;278:36285–36295. doi: 10.1074/jbc.M303639200. [DOI] [PubMed] [Google Scholar]
- 22.Gurcha S. S., Baulard A. R., Kremer L., Locht C., Moody D. B., Muhlecker W., Costello C. E., Crick D. C., Brennan P. J., Besra G. S. Ppm1, a novel polyprenol monophosphomannose synthase from Mycobacterium tuberculosis. Biochem. J. 2002;365:441–450. doi: 10.1042/BJ20020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown J. R., Field R. A., Barker A., Guy M., Grewal R., Khoo K. H., Brennan P. J., Besra G. S., Chatterjee D. Synthetic mannosides acts as acceptors for mycobacterial α1,6-mannosyltransferase. Bioorg. Med. Chem. Lett. 2001;9:815–824. doi: 10.1016/s0968-0896(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 24.Turek T. C., Gaon I., Distefano M. D., Strickland C. L. Synthesis of farnesyl diphosphate analogues containing ether-linked photoactive benzophenones and their application in studies of protein prenyltransferases. J. Org. Chem. 2001;66:3253–3264. doi: 10.1021/jo991130x. [DOI] [PubMed] [Google Scholar]
- 25.Lee R. E., Brennan P. J., Besra G. S. Mycobacterial arabinan biosynthesis: the use of synthetic arabinoside acceptors in the development of an arabinosyl transfer assay. Glycobiology. 1997;7:1121–1128. doi: 10.1093/glycob/7.8.1121. [DOI] [PubMed] [Google Scholar]
- 26.Kremer L., Dover L. G., Morehouse C., Hitchin P., Everett M., Morris H. R., Dell A., Brennan P. J., McNeil M. R., Flaherty C., et al. Galactan biosynthesis in Mycobacterium tuberculosis. Identification of a bifunctional UDP-galactofuranosyltransferase. J. Biol. Chem. 2001;276:26430–26440. doi: 10.1074/jbc.M102022200. [DOI] [PubMed] [Google Scholar]
- 27.Singh A., Thornton E. R., Westheimer F. H. The photolysis of diazoacetylchymotrypsin. J. Biol. Chem. 1962;237:3006–3008. [PubMed] [Google Scholar]
- 28.Kale T. A., Hsieh S. A., Rose M. W., Distefano M. D. Use of synthetic isoprenoid analogues for understanding protein prenyltransferase mechanism and structure. Curr. Top. Med. Chem. 2003;3:1043–1074. doi: 10.2174/1568026033452087. [DOI] [PubMed] [Google Scholar]
- 29.Dorman G., Prestwich G. D. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 30.Williams N., Ackerman S. H., Coleman P. S. Benzophenone-ATP – a photoaffinity label for the active-site of ATPases. Methods Enzymol. 1986;126:667–682. doi: 10.1016/s0076-6879(86)26070-x. [DOI] [PubMed] [Google Scholar]
- 31.Turek T. C., Gaon I., Distefano M. D. Analogs of farnesyl pyrophosphate incorporating internal benzoylbenzoate esters: synthesis, inhibition kinetics and photoinactivation of yeast protein farnesyltransferase. Tetrahedron Lett. 1996;37:4845–4848. doi: 10.1021/jo9602736. [DOI] [PubMed] [Google Scholar]
- 32.Bohm M., Mitsch A., Wissner P., Sattler I., Schlitzer M. Exploration of novel aryl binding sites of farnesyltransferase using molecular modeling and benzophenone-based farnesyltransferase inhibitors. J. Med. Chem. 2001;44:3117–3124. doi: 10.1021/jm010873j. [DOI] [PubMed] [Google Scholar]
- 33.Cox A. D., Der C. J. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim. Biophys. Acta. 1997;1333:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 34.Marecak D. M., Horiuchi Y., Arai H., Shimonaga M., Maki Y., Koyama T., Ogura K., Prestwich G. D. Benzoylphenoxy analogs of isoprenoid diphosphates as photoactivatable substrates for bacterial prenyltransferases. Bioorg. Med. Chem. Lett. 1997;7:1973–1978. [Google Scholar]
- 35.Xing J., Forsee W. T., Lamani E., Maltsev S. D., Danilov L. L., Shibaev V. N., Schutzbach J. S., Cheung H. C., Jedrzejas M. J. Investigations of the active site of Saccharomyces cerevisiae dolichyl-phosphate-mannose synthase using fluorescent labeled dolichyl-phosphate derivatives. Biochemistry. 2000;39:7886–7894. doi: 10.1021/bi0003240. [DOI] [PubMed] [Google Scholar]
- 36.Shibaev V. N., Veselovsky V. V., Lozanova A. V., Maltsev S. D., Danilov L. L., Forsee W. T., Xing J., Cheung H. C., Jedrzejas M. J. Synthesis of dolichyl phosphate derivatives with fluorescent label at the ω-end of the chain, new tools to study protein glycosylation. Bioorg. Med. Chem. Lett. 2000;10:189–192. doi: 10.1016/s0960-894x(99)00662-9. [DOI] [PubMed] [Google Scholar]
- 37.Grigorieva N. Y., Pinsker O. A., Maltsev S. D., Danilov L. L., Shibaev V. N., Jedrzejas M. J. Dolichyl phosphate derivatives with a fluorescent label at an internal isoprene unit. Mend. Commun. 2000;3:92–93. [Google Scholar]
- 38.Kremer L., Besra G. S. Current status and future development of antitubercular chemotherapy. Exp. Opin. Invest. Drugs. 2002;11:1033–1049. doi: 10.1517/13543784.11.8.1033. [DOI] [PubMed] [Google Scholar]
- 39.Mikusová K., Mikus M., Besra G. S., Hancock I., Brennan P. J. Biosynthesis of the linkage region of the mycobacterial cell wall. J. Biol. Chem. 1996;271:7820–7828. doi: 10.1074/jbc.271.13.7820. [DOI] [PubMed] [Google Scholar]
- 40.Fratti R. A., Chua J., Vergne I., Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]