Abstract
We have previously shown that the LG4 (laminin G-like) domain of the laminin α4 chain is responsible for the significantly higher affinity of the α4 chain to heparin than found for other α chains [Yamaguchi, Yamashita, Mori, Okazaki, Nomizu, Beck and Kitagawa (2000) J. Biol. Chem. 275, 29458–29465]; four basic residues were identified to be essential for this activity [Yamashita, Beck and Kitagawa (2004) J. Mol. Biol. 335, 1145–1149]. By creating GST (glutathione S-transferase)-fused LG1, LG2, LG4 and LG5 proteins, we found that only LG4 is active for the adhesion of human HT1080 cells, human umbilical vein endothelial cells and Drosophila haemocytes Kc167 with a half-saturating concentration of 20 μg/ml. Adhesion was counteracted by treatment of the cells with heparin, heparan sulphate and heparitinase I. Upon mutating the four basic residues essential for heparin binding within LG4, the adhesion activity was abolished. Pull-down experiments using glutathione beads/GST-fusion proteins indicate a direct interaction of LG4 with syndecan-4, which might be the major receptor for cell adhesion. Neither the release of glypican-1 by treating human cells with phosphatidylinositol-specific phospholipase C nor targeted knockdown of dally or dally-like protein impaired the cell-adhesion activity. As the LG4–LG5 domain of the α4 chain is cleaved in vivo from the main body of laminin-8 (α4β1γ1), we suggest that the heparan sulphate proteoglycan-binding activity of LG4 is significant in modulating the signalling of Wnt, Decapentaplegic and fibroblast growth factors.
Keywords: heparin, laminin, RNA interference, syndecans
Abbreviations: Dpp, Decapentaplegic; DMEM, Dulbecco's modified Eagle's medium; ds, double-stranded; FGF, fibroblast growth factor; GST, glutathione S-transferase; HSPG, heparan sulphate proteoglycan; HUVEC, human umbilical vein endothelial cell; LG, laminin G-like; PI-PLC, phosphatidylinositol-specific phospholipase C; RT, reverse transcription
INTRODUCTION
Laminins are major glycoproteins of basement membranes, and are composed of α, β and γ chains assembled into αβγ heterotrimers via a three-stranded α-helical coiled-coil domain. Currently, 15 heterotrimers of different chain composition have been described containing one of five α (α1–α5), three β (β1–β3) and three γ (γ1–γ3) chains [1,2]. Although certain laminin isoforms such as laminin-10/11 (α5β1γ1/α5β2γ1) show widespread tissue distribution, the expression of most laminin isoforms is tissue-specific [3–5]. This indicates that each laminin isoform has specific functions in controlling adhesion, migration, proliferation, survival and differentiation of tissue cells at specified stages and sites during animal development. For example, laminin-2 (α2β1γ1) is expressed in the basement membrane of skeletal muscle, and plays an important role in clustering of the acetylcholine receptor at the neuromuscular junction [6,7]. Laminin-5 (α3β3γ2) is a constituent of the basement membrane of epithelial tissue, where it creates stable adhesion of epithelium to the connective tissue [8,9]. Laminin-8 (α4β1γ1) was first found in endothelial cell lines and 3T3-L1 fibroblasts having a high potential for differentiating into adipocytes [10–12]. Expression of the laminin-8-specific α4 chain is restricted to vascular endothelial basement membranes of brain, muscle and bone marrow, and perineurium of peripheral nerves, heart, developing skeletal muscle and developing kidney [13–16].
In contrast with the α1, α2 and α5 chains, the α4 chain has a truncated N-terminus [12–14,17] similar to the α3 chain contained in laminin-5. Like all other α chains, the α4 chain possesses a large C-terminal laminin G-like (LG) domain consisting of five structurally similar, but functionally distinct, modules: LG1–LG5 [17–20]. Fujiwara et al. [21] showed that the integrin heterodimers α3β1 and α6β1 may function as cell-surface-adhesion receptors for the α4 chain-containing laminin-8. Recombinant laminin-8 produced in a mammalian expression system promoted adhesion of human fibrosarcoma HT1080 cells by binding to α6β1 integrin [22]. The same activity of laminin-8 from RN22 cells was inhibited by antibodies against α4 LG1–LG3, but not against α4 LG4–LG5, suggesting that α4 LG1–LG3 contributes to the adhesion of HT1080 cells [18]. Gonzales et al. [22] suggested that αvβ3 integrin in endothelial cells adhered to α4-chain-containing laminins when it co-distributed in focal contact structures. Moreover, antibodies against αvβ3 integrin inhibited endothelial cell adhesion to a G-domain fragment of the laminin α4 chain [23]. The binding site of the α4 G domain to αvβ3 integrin has been attributed to the LG1–LG2 module pair [24].
We have shown previously that the G domain of the α4 chain has a substantially higher affinity for heparin than that of other α chains, and specified LG4 to be responsible for this activity [25]. By preparing site-directed mutants covering all basic residues, we have recently identified four basic residues crucial for the binding of LG4 to heparin [26]. Although these data suggested that besides the LG1–LG2 pair, the LG4 domain contains a second site within the laminin α4 chain for interactions with cells probably through cell-surface HSPGs (heparan sulphate proteoglycans), the LG4–LG5 part of the α4 chain has been shown to be proteolytically cleaved in vivo from the main body of laminin-8, and it is not retained within the basement membrane [18,27]. It is therefore important to investigate whether such heparin-binding activity may be of biological significance. In the present study, we have focused on the heparin-binding activity of α4 LG4, and found that it can strongly adhere to HT1080 cells and HUVECs (human umbilical vein endothelial cells). We show that syndecans, but not glypicans, are important for this binding not only to mammalian cells, but also to Drosophila haemocyte Kc167 cells.
MATERIALS AND METHODS
Expression vectors for GST (glutathione S-transferase)-fusion proteins
cDNA fragments corresponding to the mouse laminin α4 chain LG domains (LG1: nt 2503–3099; LG2: nt 3100–3675; LG3: nt 3676–4353; LG4: nt 4357–4908; LG5: nt 4909–5445 [13]) were amplified by PCR using pEFneoα4myc [28] as a template. PCR was carried out with Taq DNA polymerase (KODplus polymerase; Toyobo Biochemicals, Osaka, Japan). The primers used were: LG1 forward primer, 5′-CCGGAATTCCTCAGCTGTCGAAGTGCACCCCAAAGTC-3′; reverse primer, 5′-CCGCTCGAGTTTATCCCTGGCACAGGGCACTGAC-3′; LG2 forward primer, 5′-CCGGAATTCCCTGGCTTTCACTCAGAGTAGGGCTGCC-3′; reverse primer, 5′-CCGCTCGAGAGAGTCCTCTGGGCATCCATAACC-3′; LG3 forward primer, 5′-CGCGTCGACCTGATATCTCGCAGAGCATATTTCAATGGG-3′; reverse primer, 5′-ATAAGAATGCGGCCGCGAGGTGGCAGTGGGAATCTCTTGGAGC-3′; LG4 forward primer, 5′-CGCGGATCCAGCAGCCCCAGGGCAATAGAACATGCC-3′; reverse primer, 5′-CCGGAATTCCTGGCCCTTCAAAGCAAGGGGTCACGC-3′; LG5 forward primer, 5′-CGCGGATCCATGGAAACAGGAACTTATTTTTCCACAG-3′; reverse primer, 5′-CCGGAATTCGGCTGTGGGACAGGAGTTGATGCTCAC-3′. Amplified LG1 and LG2 fragments were digested with EcoRI and XhoI, the LG3 fragment with SalI and NotI, the LG5 fragment with EcoRI and BamHI, and inserted into corresponding sites of the pGEX6p-3 expression vector (Amersham Biosciences). The LG4 fragment was digested with EcoRI and BamHI and inserted into EcoRI/BamHI sites of the pGEX 2T expression vector (Amersham Biosciences). The vector expressing GST–LG4 was subjected to sitedirected mutagenesis to exchange residues Arg1520, Lys1531, Lys1533 and Lys1539 [26] with alanine residues using the QuikChange® Site-Directed Mutagenesis kit (Stratagene).
Purification of GST-fusion proteins
Small-scale production of GST-fused mouse laminin α4 chain modules LG1–LG5 and GST–LG4 mutants for analysis by SDS/PAGE and immunoblotting (see Figure 1) was carried out with 30 ml cultures. After overnight induction by 1 mM isopropyl-β-D-thiogalactoside, bacteria were collected by centrifugation at 4000 g for 5 min, suspended in 1.0 ml buffer containing 50 mM Tris/HCl (pH 7.4), 2 mM EDTA and a protease inhibitor cocktail (Roche Diagnostics GmbH; 1 tablet/50 ml), and sonicated on ice. Glutathione–Sepharose 4 beads (10 μl; Amersham Biosciences) were added to the extract, incubated for 5 min, washed twice with 1.0 ml PBS containing 0.1% (v/v) Triton X-100, and the bound proteins were extracted by 20 μl sample buffer for SDS/PAGE. The extraction procedures were completed within 10 min. Large-scale production for cell-binding assays was carried out with 3 litre cultures. Recombinant LG modules were purified as described above at a 100-fold scale, and the washed glutathione beads were packed into open columns. The columns were eluted with buffer containing 50 mM Tris/HCl (pH 8.0) and 10 mM reduced glutathione. Eluted fractions were combined and dialysed overnight against 2 litres of buffer containing 50 mM Tris/HCl (pH 7.4). Mouse α4 chain G domain (LG1–LG5) and LG4–LG5 modules were prepared as described [25].
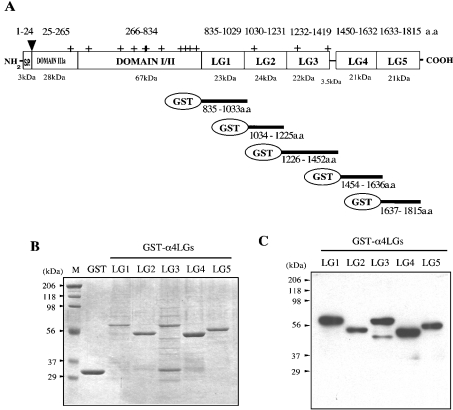
Figure 1. GST-fusion proteins with laminin α4 LG modules.
(A) Domain arrangement of the mouse laminin α4 chain. Sizes of each domain are calculated from the amino acid sequence. Sequences of the five recombinant laminin α4 LG GST fusion proteins are summarized. S.P., signal sequence; ▾, acceptor site of O-linked chondroitin sulphate glycan chain; +, putative acceptor sites for N-linked glycosylation. (B) SDS/PAGE (12% gel) of GST–LG fusion proteins run under reducing conditions and stained with Coomassie Brilliant Blue. Marker proteins (lane M) and their masses (in kDa) are shown on the left. (C) Western blot of GST–LG proteins detected with a polyclonal antibody against the laminin α4 G domain.
Antibodies
Rabbit antiserum against mouse laminin α4 chain G domain was generated as described in [25]. Rabbit antisera were generated against GST and mouse laminin α4 LG4–LG5 modules by two injections of the recombinant antigens in complete Freund's adjuvant. Monoclonal antibody 3G10 recognizing heparan sulphate stub epitopes after treatment with heparitinase I [29] was obtained from Seikagaku Kogyo (Tokyo, Japan). Antibody 8G3 recognizing the cytoplasmic domain of syndecan-4 [30,31] was a gift from Dr Guido David (University of Leuven, Leuven, Belgium).
Cell culture
Human fibrosarcoma HT1080 cells and HUVECs were maintained under a humidified atmosphere containing 5% CO2 at 37 °C in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) foetal bovine serum, 50 units/ml penicillin and 50 mg/ml streptomycin. The Drosophila haemocyte-derived Kc167 cells were maintained under air at 25 °C in HyQ CCM3 serum-free medium (Hyclone).
Cell-binding assays
Cell binding to mouse laminin α4 chain fragments was assayed in 96-well plates (Nunc). For HT1080 cells and HUVECs, plates were coated with various fragments diluted in Dulbecco's PBS overnight at 4 °C, rinsed with Dulbecco's PBS and blocked with 1% BSA in DMEM (300 μl) for 1 h at 37 °C and washed twice with 0.1% BSA in DMEM. Cells were trypsinized and returned to DMEM containing 10% (v/v) foetal bovine serum for 10 min at 37 °C in a humidified atmosphere containing 5% CO2, followed by rinsing twice with 20 μl of serum-free DMEM. A 100 μl volume of the cell suspension (2×106 cells/ml) were plated on the laminin-fragment-coated wells for 1 h at 37 °C in a humidified atmosphere containing 5% CO2. After washing off unattached cells three times with Dulbecco's PBS, attached cells were stained for 30 min with 0.4% (w/v) Crystal Violet in 50% (v/v) methanol. After washing three times with water, cells were dissolved in 50 μl of 0.1 M citrate in 50% (v/v) ethanol, and the absorbance at 595 nm was measured. For Drosophila Kc167 cells, the laminin-fragment-coated plates were blocked with 1% BSA and washed with HyQ CCM3 serum-free medium containing BSA. Cells in suspension culture were collected by centrifugation at 70 g for 3 min, and suspended in the same medium at 2×107 cells/ml, and 100 μl aliquots were incubated in laminin-fragment-coated wells for 2 h at 25 °C under air. Other details were as described for the HT1080 cells and HUVECs. To determine the effects of heparin and EDTA on cell binding, cells were pre-incubated with 10 μg/ml heparin (Sigma) or 5 mM EDTA for 10 min and then subjected to the binding assay. The effects of heparan sulphate (Sigma), and chondroitin sulphate A and C (Sigma) were studied as described above by pre-incubating with these glycan chains at 10 μg/ml. For heparitinase I (Seikagaku Kogyo) experiments, cells were pre-cultured for 2 h with 2 μg/ml cycloheximide, collected as described above, suspended in the same medium, incubated with 0.060 unit/ml of heparitinase I for 90 min, and then subjected to the binding assay.
RT (reverse transcription)-PCR of syndecan mRNAs
To detect the expression of members of the syndecan gene family in HT1080 cells and HUVECs, total RNA was isolated from both cells by the guanidine thiocyanate/phenol/chloroform method [32]. Total RNA digested with RQ1 RNase-free DNaseI (Promega) was incubated for 10 min at 70 °C, chilled on ice, and added to a 25 μl aliquot of a reaction mixture containing 5 μl of M-MLV RT 5× buffer (Promega), 1.25 μl of 10 mM mixture of four dNTPs, 25 units of RNase inhibitor (Promega) and 50 ng of random primers. M-MLV Reverse Transcriptase (200 units), RNaseH Minus (Point Mutant; Promega) were added and the incubation continued for 1 h at 42 °C. Single-stranded cDNAs were amplified by 30 cycles of first PCR using Taq DNA polymerase (Promega). Denaturation was carried out at 94 °C for 1 min, annealing at 60 °C for 30 s, and extension at 74 °C for 2 min. Second PCR was carried out using 2 μl of first PCR products as a template. The amplified first and second PCR products were electrophoresed on 1% (w/v) agarose gels and sized using HindIII/EcoRI digest of λDNA as the marker. RT-PCR was performed using the following primer sets: syndecan-1 forward primer, 5′-ATGAGGCGCGCGGCGCTCTGGCTCTGGCTG-3′; reverse primer, 5′-TCAGGCATAGAATTCCTCCTGTTTGGTGGG-3′; syndecan-2 forward primer, 5′-CCGGAATTCATGCGGCGCGCGTGGATCCTGCTCACCTTG-3′; reverse primer, 5′-CCCATCGATCGCATAAAACTCCTTAGTAGGTGCCTTCTG-3′; syndecan-3 forward primer, 5′-ATGAAGCCGGGGCCGCCGCACCGTGCCGGG-3′; reverse primer, 5′-CTAGGCATAGAACTCCTCCTGCTTGTCAGG-3′; syndecan-4 forward primer, 5′-CCGGAATTCATGGCCCCCGCCCGTCTGTTCGCGCTGCTG-3′; reverse primer, 5′-CCCATCGATCGCGTAGAACTCATTGGTGGGGGCTTTCTT-3′.
Immunoblot analysis of syndecan and glypican proteins
HT1080 cells and HUVECs were trypsinized and recovered in DMEM containing 10% (v/v) foetal bovine serum. After washing with serum-free DMEM, cells (106 total) were suspended in 50 μl of serum-free DMEM. For the identification of glypican, cells were first digested with PI-PLC (phosphatidylinositol-specific phospholipase C) [33,34] (Molecular Probes). After separation of the supernatant by centrifugation at 70 g for 3 min, cells were suspended in 50 μl of serum-free DMEM and treated at 37 °C for 40 min with 0.060 unit/ml heparitinase I. Cells were lysed by adding 30 μl of lysis buffer (PBS containing 2% Triton X-100 and 1 tablet/50 ml of protease inhibitor cocktail) and centrifuged for 30 min at 70 g. Together with the supernatant from PI-PLC treatment, the supernatants were boiled in reducing sample buffer for SDS/PAGE. After separation by SDS/PAGE on an 8% polyacrylamide gel, immunoblotting was performed. In brief, separated proteins were transferred on to a nitrocellulose membrane, which was then blocked for 1 h with PBS containing 5% (w/v) dried skimmed milk and 0.1% (v/v) Tween-20, reacted with 3G10 (1/1000 dilution) and 8G3 (1/1000 dilution) as a first antibody for 16 h at 4 °C, washed with PBS containing 0.1% (v/v) Tween-20 and reacted with horseradish-peroxidase-conjugated anti-mouse IgG (1/1000 dilution) for 1 h. The ECL® (enhanced chemiluminescence) Western-blotting system (Amersham Biosciences) was used for detection of the signal.
Pull down of syndecan-4 bound to GST–LG4
HUVECs cultured in ten 150-mm-diameter dishes were scraped off, suspended, washed with PBS and lysed with 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% (v/v) Nonidet P40, 0.5% (w/v) deoxycholic acid, 0.1% (w/v) SDS and a protease inhibitor cocktail on ice for 30 min. To reduce non-specifically bound proteins, the lysate was pre-incubated on ice with 50 μl of glutathione–Sepharose 4B beads for 10 min. To the supernatant, the same amount of beads preliminarily incubated with GST proteins or GST–LG4 were added respectively and incubated for 1 h on ice. Beads were washed three times with 50 mM Tris/HCl (pH 7.4) and transferred to a digestion buffer containing 20 mM sodium acetate, 5 mM CaCl2 (pH 7.0) and incubated for 40 min with 0.060 unit/ml heparitinase I at 42 °C. Digests were mixed with reducing sample buffer for SDS/PAGE and boiled. For immunoblot analysis, syndecan-4 bound to GST–LG4 was detected with anti-syndecan-4 antibody 8G3.
RNA-interference of syndecan, dally and dally-like protein in Drosophila Kc167 cells
RNA interference was carried out essentially as described in [35,36]. Templates for the preparation of ds (double-stranded) RNA were PCR-derived fragments sandwiched by two T7 promoter sequences. The amplified cDNA fragments for each gene were: Drosophila syndecan (nt 329–868, GenBank® accession no. U03282), dally (nt 689–1528, GenBank® accession no. U31985), dally-like protein (nt 1–1380, AF317090). Primers for dsRNA were used as follows. Drosophila syndecan forward primer, 5′-TAATACGACTCACTATAGGGAGACCACATGAAGCCAAAGCAGAAGATTTCCGTA-3′; Drosophila syndecan reverse primer, 5′-TAATACGACTCACTATAGGGAGACCACTGGCGGGAACAGCGGTTGCTGCGGTTC-3′; dally forward primer, 5′-TAATACGACTCACTATAGGGAGACCACATGGCGGCCAGGAGCGTGCGGCTAGCG-3′; dally reverse primer, 5′-TAATACGACTCACTATAGGGAGACCACCAGGGCATTCATGAAGACATTGGACAT-3′; dally-like protein forward primer, 5′-TAATACGACTCACTATAGGGAGACCACATGCTACATCAGGAGCAACAACAACAA-3′; dally-like protein reverse primer, 5′-TAATACGACTCACTATAGGGAGACCACAACTCCTTGCTCTTGAGACTGTTGCGA-3′.
Kc167 cells were plated into six-well multidish plates (Nunc) and transfected with 6 μg of dsRNAs using Effectene Transfection Reagent (Qiagen). After 3 days of incubation, the transfected Kc167 cells were used for the cell-binding assay. To confirm the suppression of mRNA levels, RT-PCR was performed on mRNA preparations from the same Kc167 cells for the cellbinding assay using sets of primers amplifying the sequences of Drosophila syndecan (nt 929–1513), dally (nt 1649–2566), dally-like protein (nt 1441–2295) and Hsc70-3 (nt 1506–1906, GenBank® accession no. NM_167308) as an internal control. Primers for RT-PCR were used as follows: Drosophila syndecan forward primer, 5′-GACGATGAGGACGATGGCGACGATAAGGAC-3′; Drosophila syndecan reverse primer, 5′-GGCGTAGAACTCGCGGTTATTCGCATTTTT-3′; dally forward primer, 5′-AGTTCTAGGAGCGAAACGAAATTGTGCTAT-3′; dally reverse primer, 5′-ACTACAGCTACTAAAGAGCATTGTGACTAG-3′; dally-like protein forward primer, 5′-CGAAAGCAGCAGCAACAGCGACGCAAACAG-3′; dally-like protein reverse primer, 5′-CAGCAAATCGGCGCAGACGCCACCGAACCA-3′; Hsc70-3 forward primer, 5′-CAGGCTGGCGTGCTCTCTGGCGAACAAGA-3′; Hsc70-3 reverse primer, 5′-CGATCGATATCCTCGGGCGTCAGGCGGTTCT-3′
RESULTS
The laminin α4 chain LG4 domain has cell-binding activity
To screen the LG modules of the laminin α4 G domain for cell-binding activity, we prepared a series of GST-fusion proteins with LG1–LG5. For this purpose, plasmids with cDNA fragments encoding the amino acid sequences of corresponding LG modules (Figure 1A) at the 3′ end of the GST sequence were constructed. When the Escherichia coli products were analysed by SDS/PAGE after small-scale purification (Figure 1B), major bands with expected sizes were detected for each LG module, except for LG3. Since these major bands gave strong signals in immunoblots when tested with antiserum against mouse α4 LG1–LG5 (Figure 1C), we concluded that the plasmids were constructed correctly. For GST–LG3, the purified preparation showed minor and faster migrating bands (Figures 1B and 1C). This might indicate that the configuration of GST–LG3 is sensitive to endogenous proteases. Since this degradation proceeded during large-scale purification to prepare samples for the binding assay, no reliable data about its cell binding activity could be obtained.
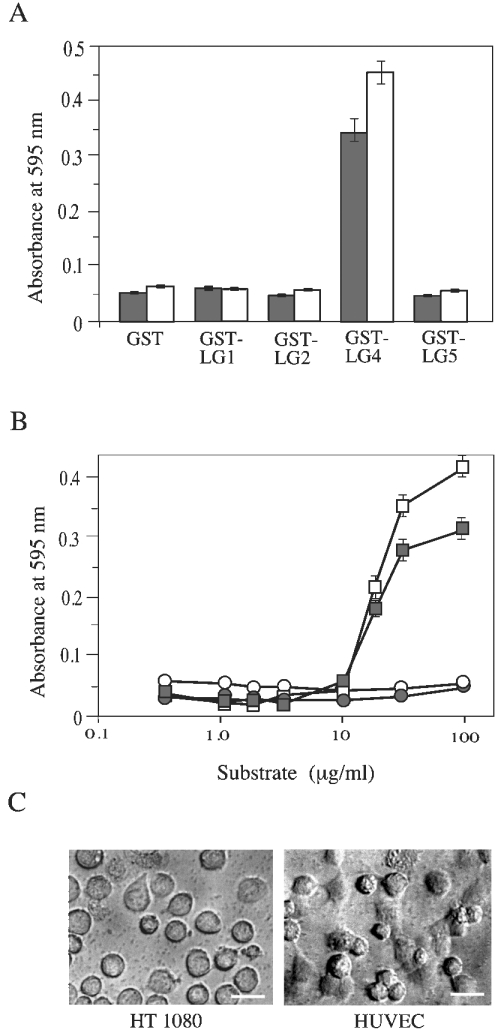
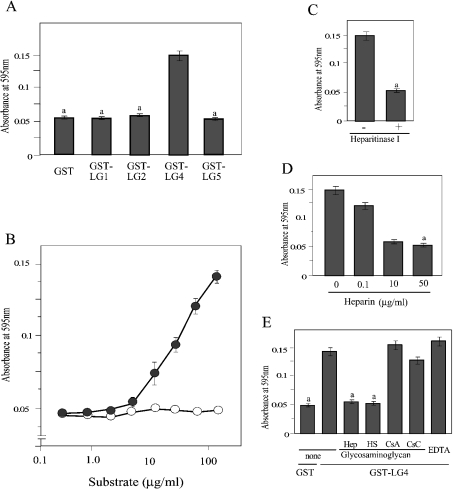
When the surface of culture dishes was coated with these GST-fusion proteins, only GST–LG4 showed binding activity for both HT1080 cells and HUVECs (Figure 2A). Since other fusion proteins gave only background absorbance comparable with that of GST alone, we concluded that LG1, LG2 and LG5 have no cell-binding activity. By coating dishes with different concentrations of GST–LG4, we estimated the half-saturating concentration for both HT1080 cells and HUVECs to be approx. 20 μg/ml (Figure 2B). Microscopy of bound cells (Figure 2C) indicated that both cell lines spread on the surface coated with GST–LG4, and the binding density was higher for HUVECs.
Figure 2. Adhesion of HT1080 cells and HUVECs to GST–LG proteins.
(A) Microtitre plates (96-well) were coated with 100 μg/ml of GST–LG proteins. HT1080 cells (grey bars) and HUVECs (white bars) were allowed to adhere at 37 °C for 1 h. After washing off non-adhering cells, the remaining cells were stained with Crystal Violet. After solubilization, absorbance was measured at 595 nm. (B) Wells were coated with the indicated concentrations of GST (circles) or GST-LG4 (squares). Adhesion was assayed for HT1080 cells (white symbols) and HUVECs (grey symbols). Results in (A) and (B) are means±S.E.M. (n=3). (C) Micrographs of HT1080 cells and HUVECs in wells coated with 100 μg/ml GST–LG4 are shown. Scale bars, 100 μm.
α4-chain-containing recombinant laminin-8 binds to integrins α3β1 and α6β1 dimers as cell-surface-adhesion receptors [21,22]. Binding of the same recombinant laminin-8 to HT1080 cells was inhibited by an antibody against α4 LG1–LG3, but not against α4 LG4–LG5 [18]. Gonzales et al. [22] suggested that αvβ3 integrin in endothelial cells adhered to α4-chain-containing laminins [23]. The binding site of the α4 chain to αvβ3 integrin was localized to the LG1–LG2 pair, since an antibody against residues 919–1207 of the α4 chain inhibited angiogenesis in a mouse–human chimaeric model [24]. Interestingly, the recombinant 919–1207 residue fragment showed half-maximal binding to endothelial cells at 1.4 nM, whereas shorter fragments covering residues 919–1018 (within LG1) and 1016–1207 (mainly within LG2) showed only weak cell-binding activity. This might explain that neither GST–LG1 nor GST–LG2 showed binding activity (Figure 2A), since the active 919–1207 residues were separated into two GST-fusion proteins.
When the cell-binding activity of the laminin α4 LG1–LG5 fragment was compared with that of GST–LG4 at a molar concentration level (Figure 3A), it showed a half-saturating concentration of approx. 1/50. This suggests that the binding activity of the LG1–LG2 pair to cells through integrins is much greater than that of LG4. The data also show that the affinity of α4 LG4–LG5 was 3-fold higher than that of GST–LG4. This could be indicative of another cell-binding sequence in LG5 or at the boundary between LG4 and LG5, but it is more likely that configurational hindrance of the GST domain might reduce the affinity of GST–LG4. Also the affinity of GST–LG4 for the heparin column was slightly lower than that of the α4 LG4–LG5 fragment (results not shown).
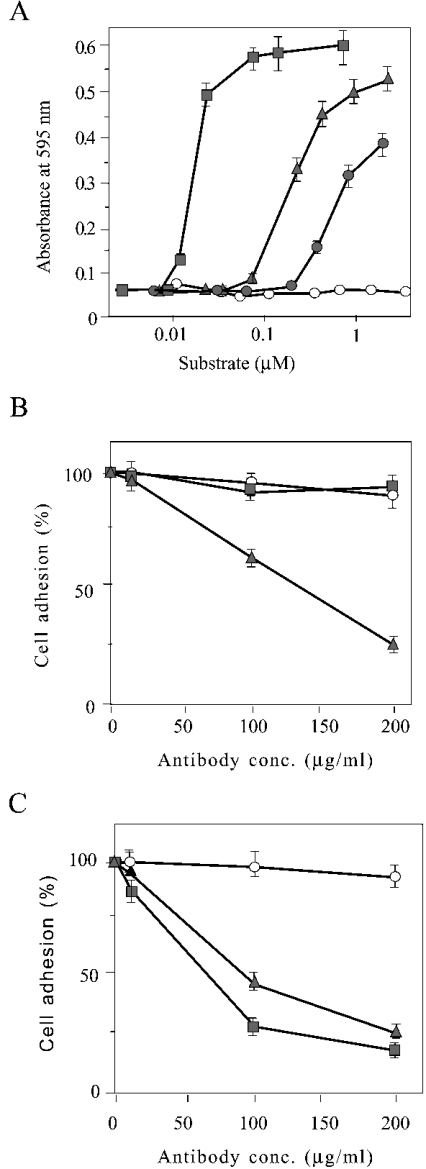
Figure 3. The LG4 domain is a second cell-binding site within the laminin α4 G domain.
(A) Adhesion of HUVECs to wells coated with different concentrations of GST (white circles), GST–LG4 (grey circles), recombinant α4 LG4–LG5 (triangles) and recombinant α4 LG1–LG5 (squares) is shown. Adhesion of HUVECs to wells coated with 100 μg/ml of recombinant α4 LG1–LG5 (B) or 100 μg/ml of GST–LG4 (C) was determined in the presence of various concentrations of pre-immune serum (circles), antiserum against α4 LG1–LG5 (triangles) and antiserum against α4 LG4–LG5 (squares). Results are means±S.E.M. (n=3).
For further confirmation of two distinct cell-binding sites within the laminin α4 G domain, the inhibitory effect of polyclonal antibodies recognizing α4 LG1–LG5 and α4 LG4–LG5 to the binding of HUVECs to the surface coated with α4 LG1–LG5 (Figure 3B) or α4 LG4–LG5 (Figure 3C) was compared. Cell binding to α4 LG1–LG5 was inhibited by the antibody recognizing α4 LG1–LG5, but not by the antibody recognizing α4 LG4–LG5. In contrast, binding to the α4 LG4–LG5 fragment was strongly inhibited by the antibody recognizing α4 LG4–LG5. Weaker inhibition by the antibody against α4 LG1–LG5 (Figure 3C) was probably due to a population of immunoglobulin recognizing LG4. We thus conclude that LG4 is a second and weaker cell-binding site within the laminin α4 G domain. We obtained essentially the same results as in Figure 3 by replacing HUVECs with HT1080 cells (results not shown).
Cell-binding activity of laminin α4 LG4 depends on its heparin-binding activity
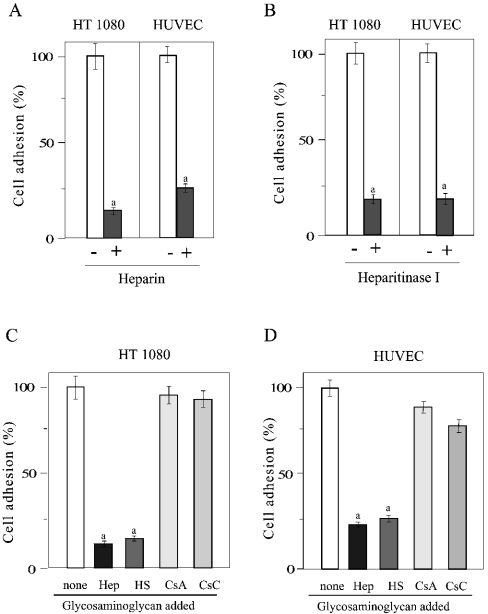
The binding of HT1080 cells and HUVECs to the surface coated with GST–LG4 was counteracted by heparin (Figure 4A). The activity was lost by treatment of both HT1080 cells and HUVECs with heparitinase I (Figure 4B). As well as for heparin, the binding activity was counteracted by heparan sulphate, but not by chondroitin sulphate A and C (Figures 4C and 4D). This indicates that the cell-binding activity of the laminin α4 LG4 domain is due to its heparin- and heparan-sulphate-binding activity.
Figure 4. Inhibition of cell adhesion to GST–LG4 by heparin, heparan sulphate and heparitinase I.
(A) HT1080 cells and HUVECs were pre-incubated without (open bars) or with 10 μg/ml of heparin (closed bars) and plated on wells coated with 100 μg/ml of GST–LG4. a, significantly different (P<0.01) from samples without heparin. (B) Cells were pre-incubated without (open bars) or with (closed bars) heparitinase I (0.060 unit/ml) and analysed as in (A). a, significantly different (P<0.01) from samples without heparitinase. HT1080 cells (C) and HUVECs (D) were pre-incubated without or with 10 μg/ml of heparin (Hep), heparan sulphate (HS), chondroitin sulphate A (CsA) or chondroitin sulphate C (CsC) and analysed as in (A). a, significantly different (P<0.01) from samples without glycosaminoglycan. Results are means±S.E.M. (n=3).
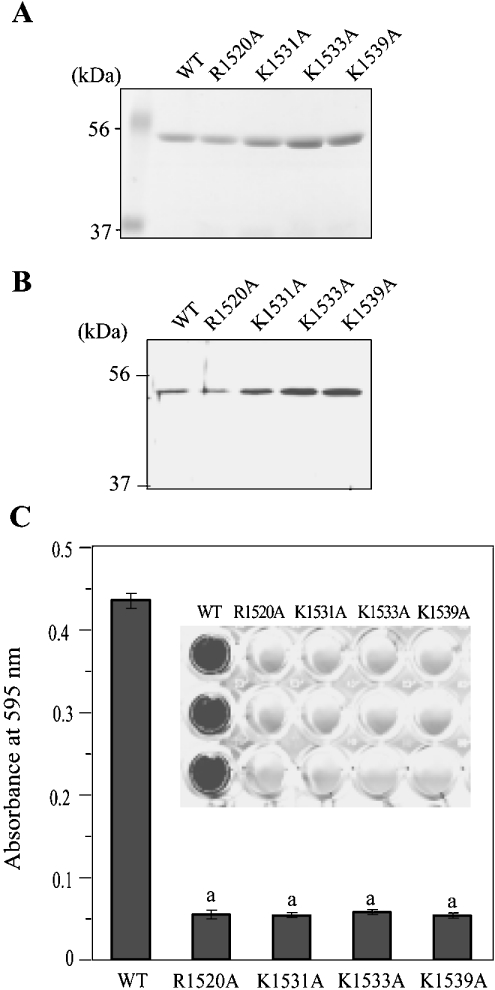
By preparing site-directed mutants of the laminin α4 LG4 module in which codons for all basic residues (arginine, lysine and histidine) were mutated to residues coding for alanine, we have recently identified four residues that are crucial for heparin binding [26]. To confirm further the dependency of the cell-binding activity of α4 LG4 to its heparin-binding activity, we took advantage of these mutants [R1520A (Arg1520→Ala), K1531A (Lys1531→Ala), K1533A (Lys1533→Ala) and K1539A (Lys1539→Ala); Figures 5A and 5B]. In contrast with the surface coated with wild-type GST–LG4, HUVECs did not bind to the surface coated with these mutants (Figure 5C). These data together suggest that the binding of HT1080 cells and HUVECs to GST–LG4 depends on its activity of binding to HSPGs at the cell surface.
Figure 5. Adhesion of HUVECs to GST–LG4 mutants.
(A) Purity of GST–LG4 mutants [26] was checked by SDS/PAGE and staining with Coomassie Brilliant Blue and (B) by Western blotting with an antibody against α4 LG1-LG5. (C) HUVECs were plated in wells coated with 100 μg/ml of mutants. Results are means±S.E.M. (n=3). a, significantly different (P<0.01) from wild-type.
Syndecan(s), but not glypican, is responsible for the binding of mammalian cells to laminin α4 LG4
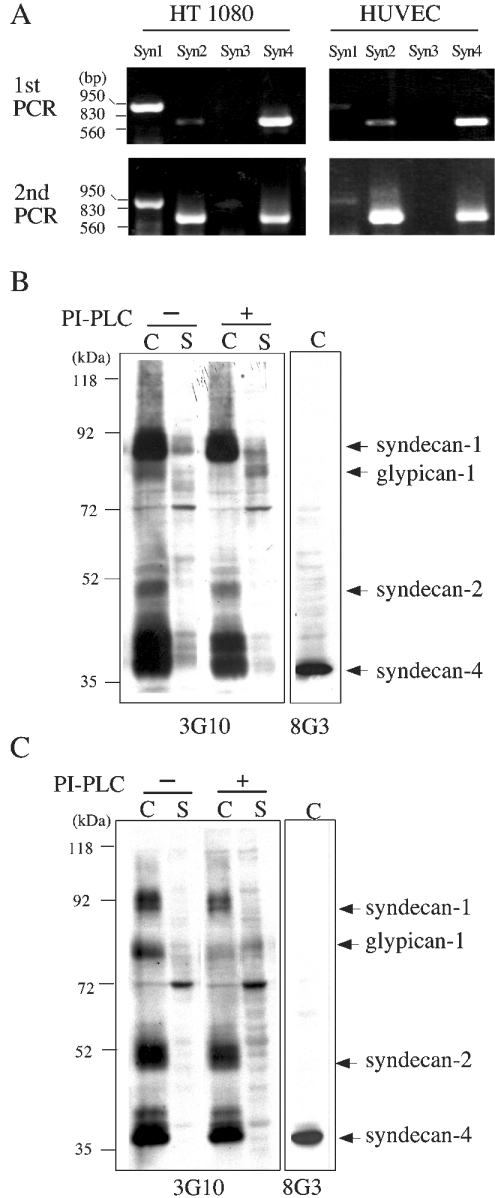
To explore membrane-anchored HSPGs responsible for cell binding, we first screened for the expression of members of the syndecan gene family by RT-PCR on RNA extracted from HT1080 cells and HUVECs (Figure 6A). Amplification of cDNA encoding syndecan-1, -2 and -4 could be confirmed for both cell lines, but expression of syndecan-3 could not be detected even after a second round of RT-PCR. For the detection of HSPGs in the membrane fraction of HT1080 cells (Figure 6B) and HUVECs (Figure 6C), we used monoclonal antibody 3G10 recognizing heparan sulphate stub epitopes which remain after treatment with heparitinase [29]. Based on the size of the core proteins detected in immunoblots, we confirmed the synthesis and localization to membrane fractions of HT1080 cells and HUVECs of syndecan-1, -2 and -4. For syndecan-4, in addition, antibody 8G3 recognizing its cytoplasmic domain was used (Figures 6B and 6C). For the identification of glypican-1, which is anchored to the outer leaflet of the plasma membrane through a phosphatidylinositol linkage [33,34], we first treated the cells with PI-PLC and detected it as it specifically appeared in the soluble fraction (Figures 6B and 6C).
Figure 6. Expression of members of the syndecan gene family and glypican-1.
(A) mRNAs encoding syndecans were detected by RT-PCR on RNA extracted from HT1080 cells and HUVECs. Electrophoresis patterns of a first and a second round of RT-PCR are shown; positions of markers (in bp) are indicated on the left. HT1080 cells (B) and HUVECs (C) were first treated with (+) or without (−) PI-PLC; cell (C) and supernatant (S) fractions were incubated with heparitinase I and analysed by SDS/PAGE (8% gels) under reducing conditions. Immunoblots were performed using monoclonal antibody 3G10 recognizing stubs of heparan sulphate glycan or monoclonal antibody 8G3 recognizing the cytoplasmic domain of syndecan-4. Migration positions of size markers (in kDa) are indicated on the left.
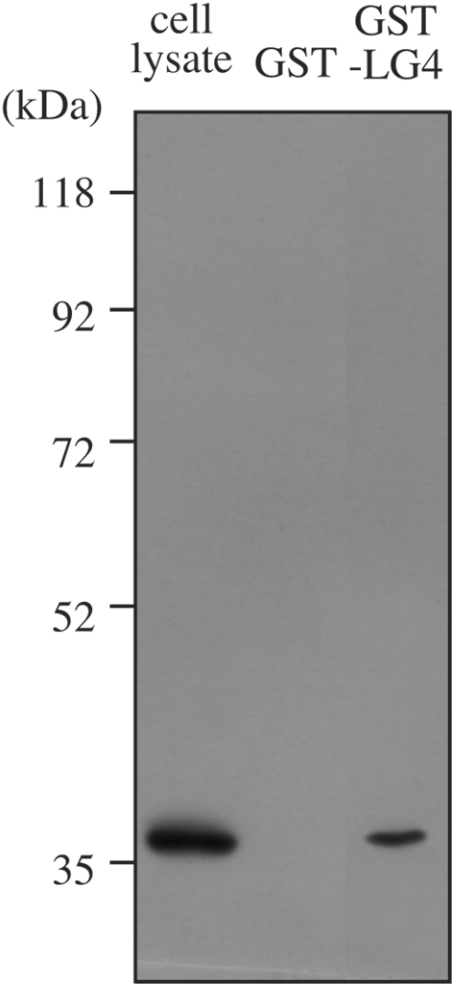
To obtain direct evidence for any interaction of laminin α4 LG4 with membrane-anchored HSPG, we designed a pull-down assay based on the interaction of GST–LG4 with glutathione beads. Immunoblots with monoclonal antibody 3G10 of membrane proteins bound to glutathione beads in the presence of GST–LG4 suggested the interaction with syndecans; however, the possibility of non-specific interactions with the beads could not be excluded. A complex pattern and bands of similar sizes were observed even in a control experiment adding GST alone (results not shown). Antibodies against syndecan-1 and -2 available to us also gave complex patterns that were difficult to interpret, probably due to non-specific interaction (results not shown). Immunoblots with monoclonal antibody 8G3 (Figure 7), however, gave clear evidence for a specific interaction of syndecan-4 with GST–LG4, but not with GST alone. Thus we suggest that laminin α4 LG4 can interact at least with syndecan-4 at the surface of HUVECs.
Figure 7. GST–LG4 pull-down of syndecan-4.
Glutathione-conjugated beads incubated with GST or GST–LG4 were added to proteins solubilized from HUVECs. Beads were washed and treated with heparitinase I. Digests were separated by SDS/PAGE. The cell lysate lane shows a corresponding amount of HUVEC proteins loaded after heparitinase I digestion. Immunoblotting was carried out using monoclonal antibody 8G3. Migration positions of size markers (in kDa) are indicated on the left.
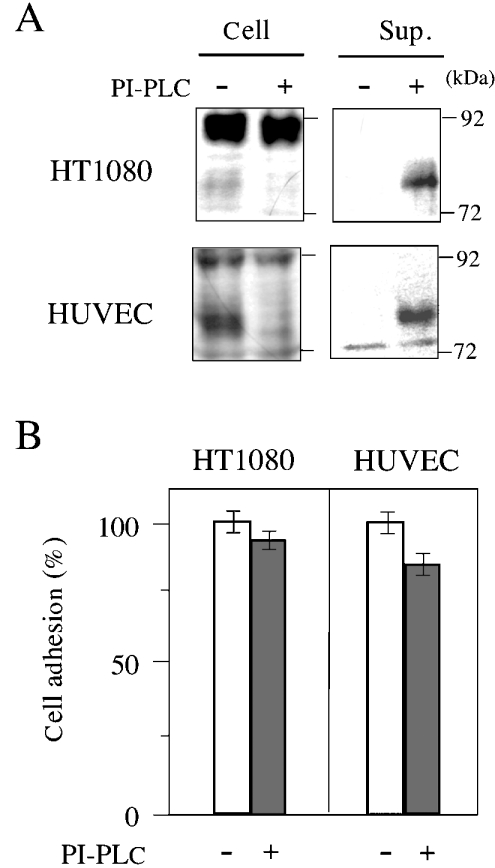
As the specific release of glypican-1 from the cell surface (Figure 6) might allow an estimate of its contribution to cell binding to GST–LG4, we prepared HT1080 cells and HUVECs almost completely free from glypican-1 by treatment with PI-PLC (Figure 8A). When these cells were plated on dishes coated with GST–LG4, they showed almost full binding activity (Figure 8B). We thus found that glypican-1 is not needed for binding of HT1080 cells and HUVECs to laminin α4 LG4.
Figure 8. Glypican-1 is dispensable for adhesion of HT1080 cells and HUVECs to laminin α4 LG4.
After confirming the release of glypican-1 depending on PI-PLC treatment of HT1080 cells and HUVECs (A), their adhesion to the well coated with 100 μg/ml of GST–LG4 protein was tested (B). Results are means±S.E.M. (n=3).
Drosophila haemocyte Kc167 cells bind to mouse laminin α4 LG4 through syndecan, but not glypicans (dally and dally-like protein)
Although the interaction of Drosophila cells with mouse laminin is not of direct physiological relevance, the data summarized in Figure 9 show that the basic mechanism of binding of laminin α4 LG4 to mammalian cells can be shared with corresponding molecules in Drosophila haemocyte Kc167. Among the GST-fusion proteins with α4 LG1, LG2, LG4 and LG5, only GST–LG4 could bind the cells (Figure 9A). Although binding was not saturated even at the highest possible concentration, binding was observed at GST–LG4 concentrations greater than 10 μg/ml (Figure 9B) as was found for mammalian cells (Figure 2B). Binding was diminished by preliminary treatment of the cells with heparitinase I (Figure 9C). It was counteracted by pre-incubation of the cells with heparin and heparan sulphate, but not with chondroitin sulphate A and C (Figures 9D and 9E). No effect of EDTA could be found (Figure 9E), suggesting that bivalent metal ions are not involved in the binding of Kc167 cells to α4 LG4.
Figure 9. Adhesion of Drosophila Kc167 cells to GST–LG proteins.
Adhesion of Kc167 cells was measured as described in Figures 2 and 4. In (A), (C), (D) and (E), wells were coated with 140 μg/ml of GST–LG protein. In (B), wells were coated with the indicated concentrations of GST–LG4 protein (closed circle) or GST protein (open circle). Results are means±S.E.M. (n=3). a, significantly different (P<0.01) from the binding of GST–LG4 (A), from without heparitinase (B), from without heparin (D) and from the binding of GST–LG4 to the untreated surface.
An advantage of the Drosophila system is that expression of the gene of interest can be knocked down just by the addition of dsRNA encoding a partial sequence of mRNA [35,36] to the culture medium. In the Drosophila genome, only three core proteins of HSPGs, namely syndecan, dally and dally-like protein, have been identified. The latter two are similar to mammalian glypicans. Therefore it is easy to design experiments addressing the question of which HSPG is important for the binding of Kc167 cells to laminin α4 LG4. As shown in Figure 10(A), incubation of the cells with dsRNA encoding the mRNA sequence of syndecan, dally or dally-like protein caused specific reduction of corresponding mRNA detected by RT-PCR. When the binding activity of such cells to the dishes coated with GST–LG4 was tested, only the knockdown of syndecan resulted in a statistically significant reduction of the binding activity (Figure 10B). Knock-down of dally and dally-like protein caused only a slight decrease in the binding activity.
Figure 10. Adhesion of Drosophila Kc167 cells and knockdown variants to GST–LG4.
(A) Selective knockdown of syndecan (Syn), dally (Dal) and dally-like protein (Dlp) by transfection of Kc167 cells with corresponding dsRNA was confirmed by detecting mRNAs by RT-PCR. (B) Adhesion of knockdown variants to wells coated with 140 μg/ml of GST–LG4 was tested. Results are means±S.E.M. (n=3). a, significantly different (P<0.01) from the binding of the cells transfected with none.
DISCUSSION
In the present study, the binding activity of laminin α4 LG4 domain was analysed mainly by using GST-fused mouse α4 LG4. It is important to give evidence that LG4 expressed in bacteria is functionally equal to the eukaryotic protein. We addressed this point in experiments not shown and cleaved GST-fused α4 LG4 to obtain a ∼23 kDa fragment having the N-terminal sequence of GSSSPR. Separately, we cleaved mouse laminin α4 LG1–LG5 produced in CHO (Chinese-hamster ovary) cells [25] by trypsin to obtain a ∼23 kDa fragment having the N-terminal sequence of QYGGTANSR. Except for minor difference in the N- and C-terminal sequences, both fragments covered the main body of α4 LG4 domain. They showed essentially the same elution profile from the heparin column by stepwise increase of NaCl concentration. Furthermore, the elution pattern was the same as that for GST-fused mouse laminin α4 LG4 wild-type shown previously [26]. We thus concluded that GST-fused α4 LG4 is a functional representative for the eukaryotic protein.
Talts et al. [18] concluded that both α4 LG1–LG3 and LG4–LG5 fragments were poor substrates for cell adhesion and had only a low affinity for the α-dystroglycan receptor when compared with the G domains of α1 and α2 chains. However, our evaluation showed that substantially higher affinity of α4 chain LG4 for heparin than other LG domains [25,26] can create the scaffold for adhesion of mammalian and Drosophila cells. Since cell-binding tests using a series of GST-fusion proteins with α4 LG1–LG5 and differential inhibition of the binding by antibodies recognizing α4 LG1–LG5 and α4 LG4–LG5 showed that this cell-binding site is distinct from that demonstrated for α4 LG1–LG2 [18,22,24], we suggest that the LG4 contains a second cell-binding site in the laminin α4 G domain. When compared on a molar basis, however, the affinity of LG4 for cells was only approx. 1/50 of that of the LG1–LG2 pair. Considering the report on laminin-1 (α1β1γ1), which shares β1 and γ1 chains with laminin-8 (α4β1γ1), that cell-attachment activity was greatly increased by the presence of the C-terminal sequences of β1 and γ1 chains [37], the contribution of this syndecan-mediated cell attachment to LG4 is even less strong. Therefore the localization of such a weak cell-binding site adjacent to the stronger sites opens the question about its biological significance. Interestingly, it was found that the LG4–LG5 domains of the α4 chain are proteolytically cleaved in vivo from the main body of laminin-8 [18,27]. This suggests the possibility that the cell-binding activity of α4 LG4 becomes significant only after its release from laminin-8.
In a model experiment of preparing mouse L cells expressing myc-tagged Wnt1, the product of Wnt1-myc was tightly bound to the cells. It could be released to the culture medium only after addition of sodium suramin. This is due to the interaction of Wnt with cell-surface HSPG [38], and functional studies of HSPG in Drosophila have shown that dally is required for Wnt signalling [39]. They are also implicated in affecting signalling of Dpp (Decapentaplegic) [40]. Our interesting observation was that addition of a peptide fragment covering the α4 LG4 module at less than 10 nM released Wnt1-myc to the medium. This implies that the LG4–LG5 domains cleaved off from laminin-8 can modify the signalling of morphogenic factors such as Wnt and Dpp. A similar modification is possible for the signalling of FGFs (fibroblast growth factors) and PDGFs (platelet-derived growth factors) which bind to cell-surface HSPGs before the final signalling through the specific receptors. In an ongoing study in our laboratory, we found that de novo adipogenesis in mice at the site of injection of basement membrane (Matrigel) and basic FGF [41] was inhibited by injecting α4 LG4–LG5 together at a concentration of less than 20 nM. These observations together suggest an important function for α4 LG4–LG5: by competing for the binding to HSPGs, α4 LG4–LG5 might modify the signalling of many factors interacting with cell-surface HSGPs before the final signalling step.
The pull-down experiment using glutathione-conjugated beads showed direct interaction of GST–LG4 with at least syndecan-4. The core protein of syndecans is characterized by divergent extracellular domains and highly conserved cytoplasmic tails that contain two constant regions separated by a variable region unique to each family member [42]. Specifically, the variable region of syndecan-4 contains a unique seven-residue binding site for PtdIns(4,5)P2 involved in protein kinase C activation [42–45] and multimerization [43,46], which is critical for cell spreading and cytoskeletal reorganization [47]. We thus suggest that syndecan-4 is the major HSPG responsible for the binding of cells to dishes coated with GST–LG4.
By specific release of glypican-1 from the surface of HT1080 cells and HUVECs, we have shown that glypican-1 is dispensable for cell binding. We obtained another line of evidence for this conclusion in Drosophila Kc167 cells by targeted knockdown of syndecan, dally or dally-like protein. Whereas knockdown of Drosophila syndecan impaired the biding of Kc167 cells to GST–LG4, knockdown of dally or dally-like protein did not show a marked effect. Considering that the Hep II domain of fibronectin can bind heparan sulphate chains from glypican and syndecan with similar affinity, despite distinct molecular masses of the glycans and differences in the disaccharide and sulphation patterns [47], it is difficult to understand that α4 LG4 could distinguish between syndecan and glypican to result in the difference in cell-binding activity. Our pull-down experiment suggested binding of α4 LG4 also to glypican-1, but the possibility of its non-specific binding to the beads was difficult to exclude. An important difference of glypicans in contrast with syndecans is that they are anchored to the outer leaflet of the plasma membrane through phosphatidylinositol linkage [33,34] and do not have a cytoplasmic domain. A plausible explanation for the dispensability of glypican for cell binding might be that even in the case that glypican binds to α4 LG4 in a similar manner as syndecan-4 does, this binding could not create the reorganization of the cytoskeleton as it is essential for cell spreading and adhesion.
Acknowledgments
We thank Dr Motoyoshi Nomizu (Graduate School of Environmental Earth Sciences, Hokkaido University, Hokkaido, Japan) for providing us with human fibrosarcoma HT1080 cells, Professor Shinji Iijima (Graduate School of Engineering, Nagoya University) for HUVECs, Professor Guido David (Center for Human Genetics, University of Leuven, Leuven, Belgium) for antibodies against syndecans, Dr Kayoko Oguri (Clinical Research Institute, National Nagoya Hospital, Nagoya, Japan) for instructions on proteoglycan analysis, Professor Hitoshi Mori (Graduate School of Bioagricultural Sciences, Nagoya University) for microsequencing of peptide fragments and Dr Konrad Beck (Department of Dental Health and Biological Sciences, University of Wales College of Medicine, Cardiff, U.K.) for proofreading of the manuscript pre-submission and comments. This work was supported by a Grant-in-aid for Scientific Research (13460039) from the Ministry of Education, Science, Culture and Sports of Japan awarded to Y. K. and by a grant from Kyowa Hakko Kogyo Co. Ltd.
References
- 1.Colognato H., Yurchenco P. D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Libby R. T., Champliaud M. F., Claudepierre T., Xu Y., Gibbons E. P., Koch M., Burgeson R. E., Hunter D. D., Brunken W. J. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J. Neurosci. 2000;20:6517–6528. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aumailley M., Smyth N. The role of laminins in basement membrane function. J. Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timpl R., Brown J. C. The laminins. Matrix Biol. 1994;14:275–281. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 5.Tunggal P., Smyth N., Paulsson M., Ott M.-C. Laminins: structure and genetic regulation. Microsc. Res. Tech. 2000;51:214–227. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Patton B. L. Laminins of the neuromuscular system. Microsc. Res. Tech. 2000;51:247–261. doi: 10.1002/1097-0029(20001101)51:3<247::AID-JEMT5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Smirnov S., McDearmon E., Shaohua L., Ervasti J., Tryggvason K., Yurchenco P. D. Contributions of the LG modules and furin processing to laminin-2 functions. J. Biol. Chem. 2002;277:18928–18937. doi: 10.1074/jbc.M201880200. [DOI] [PubMed] [Google Scholar]
- 8.Baker S. E., Hopkinson S. B., Fitchmun M., Andreason G. L., Frasier F., Plopper G., Quaranta V., Jones J. C. R. Laminin-5 and hemidesmosomes: role of the α3 chain subunit in hemidesmosome stability and assembly. J. Cell Sci. 1996;109:2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- 9.McGowan K., Marinkovich M. P. Laminins and human disease. Microsc. Res. Tech. 2000;51:262–279. doi: 10.1002/1097-0029(20001101)51:3<262::AID-JEMT6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Aratani Y., Kitagawa Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J. Biol. Chem. 1988;263:16163–16169. [PubMed] [Google Scholar]
- 11.Tokida Y., Aratani Y., Morita A., Kitagawa Y. Production of two variant laminin forms by endothelial cells and shift of their relative levels by angiostatic steroids. J. Biol. Chem. 1990;265:18123–18129. [PubMed] [Google Scholar]
- 12.Niimi T., Kumagai C., Okano M., Kitagawa Y. Differentiation-dependent expression of laminin-8 (α4β1γ1) mRNAs in mouse 3T3-L1 adipocytes. Matrix Biol. 1997;16:223–230. doi: 10.1016/s0945-053x(97)90011-1. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Mayne R. The complete cDNA coding sequence and tissue-specific expression of the mouse laminin α4 chain. Matrix Biol. 1996;15:433–437. doi: 10.1016/s0945-053x(96)90162-6. [DOI] [PubMed] [Google Scholar]
- 14.Richards A., Al-Imara L., Pope F. M. The structural organisation of LAMA4, the gene encoding laminin α4. Eur. J. Biochem. 1996;238:813–821. doi: 10.1111/j.1432-1033.1997.t01-1-00015.x. [DOI] [PubMed] [Google Scholar]
- 15.Miner J. H., Patton B. L., Lentz S. I., Gilbert D. J., Snider W. D., Jenkins N. A., Copeland N. G., Sanes J. R. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J. Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iivanainen A., Kortesmaa J., Sahlberg C., Morita T., Bergmann U., Thesleff I., Tryggvason K. Primary structure, developmental expression, and immunolocalization of the murine laminin α4 chain. J. Biol. Chem. 1997;272:27862–27868. doi: 10.1074/jbc.272.44.27862. [DOI] [PubMed] [Google Scholar]
- 17.Frieser M., Nockel H., Pausch F., Roder C., Hahn A., Deutzmann R., Sorokin L. M. Cloning of the mouse laminin α4 cDNA: expression in a subset of endothelium. Eur. J. Biochem. 1997;246:727–735. doi: 10.1111/j.1432-1033.1997.t01-1-00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Talts J. F., Sasaki T., Miosge N., Gohring W., Mann K., Mayne R., Timpl R. Structural and functional analysis of the recombinant G domain of the laminin α4 chain and its proteolytic processing in tissues. J. Biol. Chem. 2000;275:35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- 19.Iivanainen A., Sainio H., Tryggvason K. Primary structure and expression of a novel human laminin α4 chain. FEBS Lett. 1995;365:183–188. doi: 10.1016/0014-5793(95)00462-i. [DOI] [PubMed] [Google Scholar]
- 20.Miner J. H., Patton B. L., Lentz S. I., Gilbert D. J., Snider W. D., Jenkins N. A., Copeland N. G., Sanes J. R. The laminin α chains: expression, developmental transitions, and chromosomal locations of α5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J. Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara H., Kikkawa Y., Sanzen N., Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through α3β1 and α6β1 integrins. J. Biol. Chem. 2001;276:17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 22.Gonzales M., Weksler B., Tsuruta D., Golman R. D., Yoon K. J., Hopkinson S. B., Flitney F. W., Jones J. C. R. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kortesmaa J., Yurchenco P., Tryggvason K. Recombinant laminin-8 (α4β1γ1): production, purification, and interactions with integrins. J. Biol. Chem. 2000;275:14853–14859. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez A. M., Gonzales M., Herron G. S., Nagavarapu U., Hopkinson S. B., Tsuruta D., Jones J. C. R. Complex interactions between the laminin α4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16075–16080. doi: 10.1073/pnas.252649399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi H., Yamashita H., Mori H., Okazaki I., Nomizu M., Beck K., Kitagawa Y. High and low affinity heparin-binding sites in the G domain of the mouse laminin α4 chain. J. Biol. Chem. 2000;275:29458–29465. doi: 10.1074/jbc.M003103200. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita H., Beck K., Kitagawa Y. Heparin binds to the laminin α4 chain LG4 domain at a site different from that found for other laminins. J. Mol. Biol. 2004;335:1145–1149. doi: 10.1016/j.jmb.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 27.Timpl R., Tisi D., Talts J. F., Andac Z., Sasaki T., Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–317l. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 28.Niimi T., Miki K., Kitagawa Y. Expression of the long arm sequence of mouse laminin α1, β1, or γ1 chain in COS1 cells and assembly of monkey-mouse hybrid laminin. J. Biochem. (Tokyo) 1997;121:854–861. doi: 10.1093/oxfordjournals.jbchem.a021665. [DOI] [PubMed] [Google Scholar]
- 29.Lories V., Cassiman J. J., Van den Berghe H., David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J. Biol. Chem. 1989;264:7009–7016. [PubMed] [Google Scholar]
- 30.Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J. Biol. Chem. 1992;267:1116–1122. [PubMed] [Google Scholar]
- 31.David G., Van der Schueren B., Marynen P., Cassiman J. J., Van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J. Cell Biol. 1992;118:961–969. doi: 10.1083/jcb.118.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.Carey D. J., Stahl R. C. Identification of a lipid-anchored heparan sulfate proteoglycan in Schwann cells. J. Cell Biol. 1990;111:2053–2062. doi: 10.1083/jcb.111.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner G., Gabrilove J., Rifkin D. B., Wilson E. L. Phospholipase C release of basic fibroblast growth factor from human bone marrow cultures as a biologically active complex with a phosphatidylinositol-anchored heparan sulfate proteoglycan. J. Cell Biol. 1991;114:1275–1283. doi: 10.1083/jcb.114.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto A., Matsushima Y., Kadowaki T., Kitagawa Y. Drosophila mitochondrial transcription factor A (d-TFAM) is dispensable for the transcription of mitochondrial DNA in Kc167 cells. Biochem. J. 2001;354:43–48. doi: 10.1042/0264-6021:3540243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto A., Aoki M., Ichihara S., Kitagawa Y. α-, β- or γ-chain-specific RNA interference of laminin assembly in Drosophila Kc167 cells. Biochem. J. 2001;360:167–172. doi: 10.1042/0264-6021:3600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung U., O'Rear J. J., Yurchenco P. D. Cell and heparin binding in the distal long arm of laminin: identification of active and cryptic sites with recombinant and hybrid glycoprotein. J. Cell Biol. 1993;123:1255–1268. doi: 10.1083/jcb.123.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baeg G. H., Lin X., Khare N., Baumgartner S., Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Reichsman F., Smith L., Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J. Cell Biol. 1996;135:819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson S. M., Nakato H., Sugiura M., Jannuzi A., Oakes R., Kaluza V., Golden C., Selleck S. B. dally, a Drosophila glypican, controls cellular responses to the TGF-β-related morphogen, Dpp. Development. 1997;124:4113–4120. doi: 10.1242/dev.124.20.4113. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi N., Toriyama K., Nicodemou-Lena E., Inou K., Torii S., Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Couchman J. R., Woods A. Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J. Cell Sci. 1999;112:3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- 43.Oh E. S., Woods A., Couchman J. R. Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 1997;272:8133–8136. doi: 10.1074/jbc.272.13.8133. [DOI] [PubMed] [Google Scholar]
- 44.Oh E. S., Woods A., Lim S. T., Theibert A. W., Couchman J. R. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4,5-bisphosphate coordinately regulate protein kinase C activity. J. Biol. Chem. 1998;273:10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- 45.Oh E. S., Woods A., Couchman J. R. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J. Biol. Chem. 1997;272:11805–11811. doi: 10.1074/jbc.272.18.11805. [DOI] [PubMed] [Google Scholar]
- 46.Longley R. L., Woods A., Fleetwood A., Cowling G. J., Gallagher J. T., Couchman J. R. Control of morphology, cytoskeleton and migration by syndecan-4. J. Cell Sci. 1999;112:3421–3431. doi: 10.1242/jcs.112.20.3421. [DOI] [PubMed] [Google Scholar]
- 47.Couchman J. R., Woods A. Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J. Cell Sci. 1999;112:3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]