Abstract
Kidney cancer is a common malignancy that constitutes around 5% of all cancer cases. Males are twice as likely to acquire renal cell carcinoma (RCC) compared to females and experience a higher rate of mortality. These disparities indicate that sex hormone (SH)-dependent pathways may have an impact on the aetiology and pathophysiology of RCC. Examination of SH involvement in conventional signalling pathways, as well as genetics and genomics, especially the involvement of ribonucleic acid, reveal further insights into sex-related differences. An understanding of SHs and their influence on kidney cancer is essential to offer patients individualized medicine that would better meet their needs in terms of prevention, diagnosis and treatment. This review presents the understanding of sex-related differences in the clinical manifestation of kidney cancer patients and the underlying biological processes.
Keywords: androgen, hormone signalling axis, immune checkpoint inhibitors, oestrogen, renal cell carcinoma, RNA, sex, sex hormones, tumour microenvironment
Introduction
Renal cell carcinoma (RCC) represents around 5% of newly diagnosed cancer cases in men and 3% in women, ranking the sixth most common malignancy in males and tenth in females with a more than doubled incidence over the past half-century in the developed world. Three main histological subtypes can be distinguished, with the most common being clear cell RCC (ccRCC), followed by papillary RCC (pRCC) and chromophobe RCC (chRCC). 1 Most cases are discovered incidentally during imaging, leading to a survival rate being dependent on the stage at diagnosis. General risk and modifiable risk factors include obesity, smoking, poorly controlled hypertension and renal failure.
Underlying genetic predispositions significantly contribute to tumour development, further course of the disease and even therapy response. An example that has fundamentally changed the therapy approach in RCC is the finding of mutations in the gene. The VHL gene mutations are associated with the Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signalling pathway, which results in the overexpression of VEGF and platelet-derived growth factor (PDGF) receptors.2,3 This finding leads to the notably angiogenic characteristic of RCC and to the development of targeted therapies for metastasized disease, such as tyrosine kinase inhibitors (TKIs), mammalian target of rapamycin (mTOR) inhibitors and recombinant humanized monoclonal IgG1 antibodies that oppose VEGF.4–11
The highly immunogenic nature is a further characteristic of RCC, 12 being the basis for already outdated therapeutic approaches with interleukin (IL)-2 and interferon-α (IFN-α) as well as the to date cutting-edge RCC treatment with immune checkpoint inhibitors (ICIs).10,13–20
In addition to characteristics such as vascularization and immunogenicity, emerging evidence highlights the importance of sex hormone (SH) signalling in various solid tumours. Previous pan-cancer analyses have identified sex-specific characteristics in the tumour microenvironment (TME), impacting tumour mutation burden (TMB), immune cell counts, immune checkpoint genes and related functional pathways in the TME. 21 SH and their corresponding receptors, including the androgen receptor (AR), oestrogen receptor (ER) and progesterone receptor (PR), act as ligand-dependent transcription factors and play critical roles in cellular growth and differentiation, both in neoplastic and non-neoplastic states. 22 Moreover, there is increasing evidence that these receptors can also stimulate gene expression through pathways that are independent of ligand interactions. 23 This role is well documented in hormone-dependent organs such as breast, prostate and gynaecologic cancers24–26 and the significance of these pathways is evident in the efficacy of hormone withdrawal treatments using specific inhibitors.26–28 Furthermore, exposure to diethylstilbestrol (DES), a synthetic oestrogen or environmental chemicals mimicking androgens, increases the risk of clear cell adenocarcinoma of the cervix or vagina in women and promotes the proliferation of prostate cancer in men, respectively.29–31
However, there is increasing attention to intriguing sex-based disparities in cancer incidence, particularly the higher rates observed in men compared to women, affecting nonreproductive organs.32–36 AR activation mediated by testosterone and its derivatives has been described as a stimulator of tumour cell proliferation and migration, which may contribute to the higher incidence of many cancers in men.37–42 Conversely, female SHs oestrogen (E2) and PR may inhibit tumour cell proliferation and migration, primarily by inducing apoptosis, thereby reducing the risk of tumour development in certain contexts. Notably, the effects of E2 may vary or even oppose each other depending on the forms of ER (ER-α vs ER-β), its genetic variations (ERα36) and, finally, the target organ.43–47
As with many neoplasms, RCC is more common in men, suggesting that underlying sex-specific pathophysiology and pathways may underlie the epidemiological differences in tumour development.48,49 Initial findings indicate that hormones also play a role in the development and progression of RCC. 50 The implication of the role of steroid receptor signalling pathways has been described similarly, as regression of metastatic RCC during the administration of progestin or androgen was reported. 51 Other sex-related hormonal factors, like age at first childbirth, parity, oral contraceptive use and the condition after hysterectomy, have even more interestingly shown to affect the risk of RCC.52–54 However, these epidemiological studies present a challenge in differentiating between sex, which pertains to biological and physiological attributes, and gender, which refers to socially constructed attributes.55–57
Nevertheless, it has shown to be crucial to elucidate and gain a more detailed understanding of the hormonal-molecular pathway mechanisms involved in RCC development and progression to further promote individualized tumour prevention and treatment strategies. Furthermore, although the role of ICI in the primary therapy setting will not as quickly be displaced, potential as well as supportive or alternative treatments will be discussed. As sex therefore entails both individual genetic and pathophysiological attributes, we aim to describe, understand and analyse the role of sex itself and SH in kidney cancer formation.
Methods
Literature research was conducted between January 2023 and September 2023 to identify studies reporting on the association between SH and RCC development. The search was performed using commonly used databases, including PubMed, Medline and Google Scholar. The study language was limited to English. The following medical subject heading terms were used to identify relevant results: ‘kidney cancer’, ‘renal cell cancer’, ‘metastatic RCC’, ‘tumour microenvironment’, ‘sex hormones’, ‘steroid receptors’, ‘oestrogen’, ‘testosterone’, ‘progesterone’, ‘androgen’, ‘oestrogen receptor’, ‘androgen receptor’, ‘progesterone receptors’, ‘RNA’, ‘tyrosine kinase inhibitor’, ‘immunotherapy’ and ‘checkpoint inhibitor’. Thereafter, 38 on general topics and 151 studies that investigate specific aspects of the research area were included in this review.
SHs and signal transduction pathways in renal cell cancer
Signal transduction pathway-mediated processes refer to the series of molecular events that transmit signals from the cell surface to the nucleus, leading to specific cellular responses. These processes play a fundamental role in various biological functions, including cell growth, differentiation, proliferation and survival. Interaction with this mechanism by different signal molecules like AR, ER, PR and their ligands causes activation or inactivation of downstream signalling components.58,59 Here, we present evidence strongly suggesting that the interplay between the SH axis and signalling pathways implicated in oncogenesis, observed in other cancer types, may also have relevance in the development of RCC (Table 1).
Table 1.
Studies characterizing sex hormone interactions with signalling pathways during cross-talk in renal cell cancer.
| Sex hormone axis | Receptor | Regulation | Pathway | Effect | Reference |
|---|---|---|---|---|---|
| Androgen | AR | ↑ | HIF2α/VEGF/VHL | • Tumour progression • Cell migration and invasion |
(60) |
| AR | ↑ | PI3K/AKT/NF-κB/CXCL5 | • Tumour progression • Vascular endothelial cell proliferation and recruitment |
(61) | |
| AR | ↑ | Neutrophil/c-Myc | • Tumour cell proliferation | (62) | |
| AR | ↑ | STAT5 phosphorylation | • Tumour cell proliferation | (63) | |
| Oestrogen | ER-α | ↑ | VHL/HIF-1α/p53 | • Tumour cell proliferation | (64) |
| ER-α | ↑ | VEGFa/HIF-2α | • Tumour cell proliferation | (65) | |
| GPER | ↑ | PI3K/AKT/MMP-9 | • Tumour progression • Cell migration |
(66) | |
| ER-β | ↓ | AKT/ERK/JAK/Bid → Caspase-3, -8, -9 |
• Enhancement of tumour apoptosis | (67) | |
| ER-β | ↑ | TGF-β1/SMAD3 | • Tumour progression • Cell migration |
(68) | |
| ER-β | ↑ | Angiopoietin-2/Tie-2 | • Vascular endothelial cell proliferation and recruitment | (69) |
AR, androgen receptor; Bid, BH3 interacting-domain death agonist; ER, oestrogen receptor; ERK, extracellular signal-regulated kinase; GPER, G-protein coupled oestrogen receptor; HIF-2α, hypoxia-inducible factor-2α; JAK, janus kinase; MMP-9, matrix metalloproteinase-2; NF-κB, nuclear factor-κB; PI3K, phosphoinositide 3-kinases; pVHL, von Hippel-Lindau protein; RCC, renal cell carcinoma; TGF-β1, transforming growth factor beta 1; VEGF, vascular endothelial growth factor; ↑, promoting effect; ↓, inhibiting effect.
Androgens
The androgen signalling axis is described as a key player in the development and progression of prostate cancer, 26 which is recognizably already influenced by sex in its primary development. Yet, an increasing number of studies suggest a potential contribution in RCC as well, which is gender-independent at first glance. For instance, a varying AR expression (15%–55%) in RCC with mixed correlations to outcomes was revealed.70–74 A noteworthy correlation between AR expression and lower pathological stage and grading at diagnosis, as well as subsequent better outcomes, has been described.70,71,75,76 Controversially, studies have indicated worse oncological prognosis and overall outcome in correlation to AR expression72,73,77 as well.
In this context, the AR has been identified as a potential co-regulator of the HIF2a/VEGF signalling pathway. 78 Its activation induces HIF2α/VEGF expression in RCC tumour tissue, subsequently promoting RCC progression. In a preclinical mouse model, targeting AR with AR degradation enhancers showed an effectively suppressed RCC progression, indicating a promising therapeutic approach. 60 Guan et al. described a further pro-angiogenic effect of AR by demonstrating its function as a regulator of the PI3K/AKT → NF-κB → CXCL5 signalling pathway, which is known to influence RCC progression and endothelial cell recruitment. The group highlighted the involvement of NF-κB, a chemokine responsible for tumourigenesis and inflammation in cancer cells. NF-κB has been suggested to be an additional potential mediator in the PI3K/AKT signalling pathway, contributing to RCC progression. 61
RCC is widely recognized as a typical ‘hot tumour’, characterized by an abundant infiltration of CD8+ T cells in the TME.79–81 While a higher infiltration of CD8+ T cells predicts a better prognosis in many cancers due to their cytotoxic function,82–84 this correlation does not apply to RCC patients. 85 However, CD8+ T cells have distinct subtypes, with conventional cytotoxic CD8+ T cells having an anticancer role, whereas exhausted CD8+ T cells become dysfunctional. Studies investigating sex bias have identified the exhausted and dysfunctional state of CD8+ T-cells in various cancers, including bladder, prostate and liver cancers. In these contexts, the AR has been implicated both as a transcription factor promoting exhausted CD8+ T-cell formation and as an inhibitor of CD8+ T-cell function, activity and stemness.86–88 Investigations into the differences in the TME of RCC between male and female patients have revealed that male RCC TME exhibits higher infiltration and exhaustion of CD8+ T cells compared to females. Additionally, the crucial role of the androgen-AR axis in inducing CD8+ T-cell exhaustion in RCC could be shown. 89 The presence of intratumoural neutrophils in RCC has been associated with a negative prognosis in RCC as well. High-grade RCC patients exhibited a higher degree of neutrophil infiltration in tumour tissue compared to low grade, indicating the potential role of cancer-induced immunosuppression in promoting cancer progression through the modulation of neutrophils. 90 Focusing on N2 neutrophils, which are known to be involved in carcinogenesis, angiogenesis and immunosuppression, Song et al. 62 described their ability to promote RCC proliferation by upregulating AR expression via the AR-c-Myc signalling pathway.
Moreover, high levels of dihydrotestosterone (DHT) receptors were found more often in higher staged RCC tumours. 91 In this context, He et al. conducted an experimental model demonstrating that transfected normal human kidney cells with AR with a subsequent exposure to a carcinogen, resulted in a higher incidence of larger cell colonies and growth. Furthermore, inoculating functional AR into stable RCC cell lines resulted in increased tumour proliferation. 60 Pak et al. observed that treatment with DHT led to an increase in cell proliferation in both AR-positive and AR-negative RCC cells, depending on the concentration of DHT. The study also showed a dose-dependent rise of STAT5 in RCC cells following DHT treatment. Yet, as AR was knocked down with small interfering ribonucleic acid (siRNA), there was a reduction in cell proliferation specifically in AR-positive cells. Interestingly, the decrease in phosphorylated STAT5 levels after AR knockdown mirrored these findings, implicating STAT5 activation as a potential mechanism behind DHT-induced RCC cell growth. 63 These observations provide evidence that the androgen axis affects cell proliferation and migration and therefore must be relevant in the context of RCC development.92–95
Oestrogens
The E2 signalling axis has been shown to influence the development of RCC significantly as well. Two distinct forms of ER, ER-α and ER-β, are present in normal renal tissue. 96 ERs have furthermore already been detected in stromal tumours, cystic nephromas and angiomyolipoma.97–102 ERs in RCC seem to be highly variable in their expression with controversial data. Earlier studies indicate a high ER presence (30%) 100 followed by more recent observations, describing a low ER distribution (1.1%). E2 is suggested to affect RCC cell viability, DNA damage, oxidative stress, nuclear factor phosphorylation, clearly influencing tumour growth and additionally may affect the balance between autophagy and apoptosis in RCC.67,103
Oestrogen receptor-α
Genetic variations within the ER-α gene have a notable role in RCC development. An exploratory analysis of 113 RCC cases revealed differences in genotype distribution at codon 10 on exon-1 of the ER-α gene compared to healthy individuals.104,105 ERα36 is known to be another splice variant, found in the cytoplasm and plasma membrane. Elevated ERα36 levels in these locations are linked to adverse prognostic factors in RCC, including poor disease-free survival, larger tumour size and advanced clinical stages.46,106
A complex underlying interaction in the RCC formation involves the interplay between ER-α, VHL, HIF-1α and p53. ER-α is assumed to be a target for proteasomal degradation by the tumour suppressor von Hippel-Lindau protein (pVHL) E3 ligase. Overexpression of pVHL suppresses ER-α in RCC, while pVHL downregulation increases ER-α expression. 64 Elevated ER-α expression has been found to enhance the activity of the HIF-1α transcription factor. In VHL-deficient cells, the expression of both ER-α and HIF-1α persists, and blocking ER-α using its inhibitor can effectively inhibit the proliferation of VHL-deficient cells. Notably, the anti-proliferative effect of faslodex, an ER-α inhibitor, in VHL-deficient cells by inducing the expression of p53 could be demonstrated. 64 ER-α furthermore promotes the transcription of growth-related factors, driving gene expression, mitosis, proliferation, cancer development and tumour progression. 54
The G-protein-coupled oestrogen receptor (GPER), distinct from nuclear ER, also plays a role in oestrogen-dependent development and progression of cancers, including RCC.107–110 RCC cell lines express GPER abundantly and its activation promotes RCC cell migration and invasion by upregulating matrix metalloproteinase-2 (MMP-2) and MMP-9, as well as activating downstream signalling pathways, particularly MAPK and PI3K/AKT, promoting cell migration via the PI3K/AKT/MMP-9 pathway. 66
Oestrogen receptor-β
The role of ERβ remains controversial. While some studies show an inhibitory effect on cell proliferation, others suggest that ERβ expression may promote cancer development.111–113 However, initial investigations suggest that ER-β signalling appears to have a tumour-suppressive role in RCC 114 characterized by anti-proliferative functions, inhibition of migration, suppression of invasion and enhancement of apoptosis.54,67,103
The inhibitory effects of oestrogen via ER-β activation in RCC involve dampening downstream hormone signalling, including AKT, extracellular signal-regulated kinase (ERK) and janus kinase (JAK) activation, while increasing the expression of apoptotic genes such as BH3 interacting-domain death agonist (Bid), Caspase-3, Caspase-8 and Caspase-9.54,67 Conversely, subsequent investigations revealed the contrary role of ER-β in RCC. Clinical data showed increased ER-β expression in advanced-stage or high-grade tumours, correlating with unfavourable survival outcomes and reduced disease-free survival for RCC patients.68,69,115,116 ER-β has been identified as a promoter of RCC cell invasion through the augmentation of the TGF-β1/SMAD3 signalling axis. Targeting this pathway with anti-oestrogens or TGF-β receptor inhibitors effectively reduced RCC tumour growth and invasion. 68 ER-β was also found to regulate Angiopoietin-2 (ANGPT-2)in RCC cells through oestrogen response elements (EREs) on the ANGPT-2 promoter. The escalated ANGPT-2 levels in RCC cells exert a stimulatory influence on angiogenesis by engaging and phosphorylating the Tie-2 receptor, leading to the formation of HUVEC tubes. Targeting the ER-β/ANGPT-2/Tie-2 pathway with faslodex-enhanced RCC sensitivity to the TKI sunitinib treatment emerges as a promising therapy strategy. 69 ER-β signalling has been implicated in inducing the VEGFa/HIF2α pathway as well. 65 Infiltrating immune cells, particularly T cells, can modify ER-β expression and can promote RCC invasion. T-cell co-cultures with RCC cells resulted in elevated levels of T-cell-attracting factors, including IFN-γ, C-C motif chemokine ligand (CCL) 3 and CCL5, suggesting the establishment of a positive regulatory feedback mechanism. Simultaneously, infiltrating T cells appears to contribute to RCC cell invasion by influencing ER-β expression and concurrently suppressing the expression of DAB2IP. Intriguingly, the suppression of DAB2IP could subsequently reverse the T-cell-mediated promotion of RCC cell invasion. 117 The interaction in this course concerning immunotherapy treatment with the checkpoint inhibitor nivolumab in RCC patients has been recently described. This therapeutic approach, which elicits immunomodulatory effects on neutrophils, has been found to induce alterations in the expression of sex SH, particularly oestrogen, during the treatment in patients with mRCC. 118 Notably, an intriguing observation emerged with the administration of TKIs sunitinib and axitinib, leading to increased expression and stability of ER-β in RCC cell lines.119,120
Progesterone
Progesterone signalling axis has also been shown to potentially influence the development of RCC. PR expression is observed in normal and carcinomatous kidney tissue, with varying levels in different histological RCC subtypes.51,121,122 PR presence is reported in benign renal tumours and metaplastic nodules, 121 at a high rate than in malignant tumours of the kidney. Higher levels of expression were notably observed in cases of renal oncocytoma (RO). 60 Literature also suggests that PR demonstrates nuclear reactivity in a range of 10%–50% of tumour cells across all RO cases. Interestingly, up to 20% higher PR expression has been described in chRCC. In comparison, non-neoplastic renal tissue displays scattered stromal cells and tubular cells that show reactivity for both ER and PR, as in comparison fewer than 1% of RCC cases show reactivity for these receptors. 123
Progestin and adipoQ receptor 5 (PAQR5), a membrane-bound PR, can activate downstream signalling pathways affecting various cellular processes, including cell proliferation, migration and invasion. In RCC, decreased expression of PAQR5 is linked to tumour stage, cancer grade, lymph node invasion and distal metastasis, all in all suggesting a role in RCC progression. While the precise mechanisms underlying PAQR5s regulation of downstream signalling pathways remain unclear, its functions are thought to align with pathways related to ribosome function, focal adhesion and intracellular signal transduction pathways such as PI3K-AKT, MAPK and mTOR. 124 Progesterone Receptor Membrane Component 1 (PGRMC1) is another member of the membrane-associated PR protein family, which has been shown to distribute key functions in various cancer types, including RCC. 125 Elevated PGRMC1 levels in RCC tissues are correlated with higher tumour stage and are more common in poorly differentiated tumours. High expression of PGRMC1 in RCC tissue compared to adjacent non-cancerous tissues evinces its potential utility as a diagnostic and prognostic biomarker for RCC, as it has already demonstrated the ability to influence cancer cell susceptibility to chemotherapy, further emphasizing its correlation with tumour malignancy and progression. 125
SHs and RNA-mediated processes in RCC
Noncoding RNA (ncRNA) and their influence on regulatory processes open up a further field of interest concerning hormone-dependent modulation of RCC initiation and progression at a molecular level. NcRNA encompasses approximately 90% of the human transcriptome and does not encode proteins. Its involvement in tumour initiation and progression has gained significant attention in recent years.
MicroRNA (miRNA) consists of a small RNA molecule that is capable of binding to the 3′-untranslated region (3′-UTR) of target gene transcripts, leading to translational repression or mRNA destabilization. Long noncoding RNA (lncRNA) exceeding 200 nucleotides in length has also been shown to play a regulatory role in cancer biology. 126 These types of RNA can either act as oncogenes or tumour suppressor genes, exerting influence by modulating diverse signalling pathways. 127 Another category of ncRNA, referred to as pseudogenes which are non-functional duplicates of genes, has been identified as a significant contributor to cancer 128 and may act as competitive endogenous RNAs (ceRNA). 129 Finally, circular RNAs (circRNA) constitute a newly discovered group of ncRNAs, primarily formed as loop structures at the exons due to non-canonical splicing. Recent data suggest that aberrant circRNA expression is linked to the onset and progression of diseases, particularly various types of human malignancies.130,131 The following chapters will present current findings on the connection between ncRNA and the signalling pathways of SH, analysing their potential involvement in the progression of RCC (Table 2 and Figure 1).
Table 2.
Studies characterizing sex hormone interactions with ncRNA during cross-talk in renal cell cancer.
| Sex hormone axis | HR/ncRNA interaction | ncRNA effect | Pathway | Regulation | Effect on RCC | Reference |
|---|---|---|---|---|---|---|
| Androgen | AR/ASS1P3 | ↓ ASS1P3 | miRNA-34a-5p/ASS1 | ↑ | • Tumour proliferation • Tumour progression |
(132) |
| AR/HOTAIR | ↑ HOTAIR | GLI2/VEGFA + PDGFA/CSC (SOX2, OCT4, NANOG, SLUG) | ↑ | • Tumour angiogenesis • Cancer stemness |
(133) | |
| lncRNA-SARCC/AR/miRNA-143-3p | AR ↓ miRNA-143-3p lncRNA-SARCC ↓ AR |
AKT; MMP-13; K-RAS; P-ERK | ↑ | • Tumour cell migration • Tumour proliferation • Tumour progression |
(134) | |
| lncRNA-SARCC/AR | lncRNA-SARCC modulates the AR under different oxygen conditions | HIF-2α/C-MYC | ↑↓ | • Suppresses tumour progression under hypoxia • Induces tumour progression under normoxia |
(135) | |
| AR/miRNA-185-5p | AR ↑ miRNA-185-5p expression miRNA-185-5p ↓ VEGF-c and ↑ HIF-2α/VEGFA expression |
VEGF-c HIF-2α/VEGFA |
↑↓ | • Increases haematogenous metastasis • Decreases lymphatic metastasis |
(136) | |
| AR/circHIAT1 | AR ↓ circHIAT1 resulting in deregulating miR-195-5p/29a-3p/29c-3p expressions, which ↑ CDC42 expression | miRNA-195-5p, miRNA-29-3p, miRNA-29-3p/CDC42 |
↑ | • Tumour cell migration • Tumour cell invasion |
(137) | |
| AR /miRNA-145 | AR ↓ miRNA-145 | HIF-2α/VEGFA, MMP-9, CCND1 | ↑ | • Tumour cell invasion • Tumour cell proliferation |
(138) | |
| Oestrogen | ER-β/circATP2B1 | ER-β ↓ the expression of circATP2B1 CircATP2B1 ↓ miRNA-204-3p, which caused the ↑ expression of FN1 |
miRNA-204-3p/FN1 | ↑ | • Tumour cell migration • Tumour progression |
(116) |
| ER-β/HOTAIR | ER-β ↑ HOTAIR expression HOTAIR ↓ various miRNA and their suppressive effect on different oncogenes |
miRNA-138/ADAM9 miRNA-204/CCND2 miRNA-217/VEGFA, VIM, ZEB1, ZEB2 miRNA-200c/ZEB1, ZEB2 |
↑ | • Tumour cell migration • Tumour proliferation • Tumour cell invasion |
(115) | |
| lncRNA-ECVSR/ER-β | lncRNA-ECVSR ↑ ER-β miRNA stability ER-β → Hif2-α ↑ Hif2-α → CSC phenotype and VM formation ↑ |
HIF-2α/VM formation | ↑ | • Tumour angiogenesis | (119) | |
| ER-β/circDGKD | ER-β → circDGKD expression ↑ CircDGKD sponge miRNA-125-5p miRNA-125-5p → VE-cadherin ↑ |
miRNA-125-5p/VE-cadherin | ↑ | • Tumour angiogenesis | (120) |
AR, androgen receptor; ASS1, argininosuccinate synthase 1; CDC42, cell division cycle 42 protein; circATP2B1, circular RNA ATPase plasma membrane transporter 2B1; circDGKD, circular RNA DGKD; CSC, cancer stem cell; ER, oestrogen receptor; FN1, fibronectin 1; HOTAIR, HOX transcript antisense intergenic RNA; K-RAS, Kirsten rat sarcoma viral oncogene; lncRNA, long noncoding RNA; miRNA, micro RNA; ncRNA, noncoding RNA; PDGFA, Platelet derived growth factor subunit A; RCC, renal cell carcinoma; VE-cadherin, vascular endothelial cadherin; VEGF, vascular endothelial growth factor; VM, vasculogenic mimicry; ↑, promoting effect; ↓, inhibiting effect.
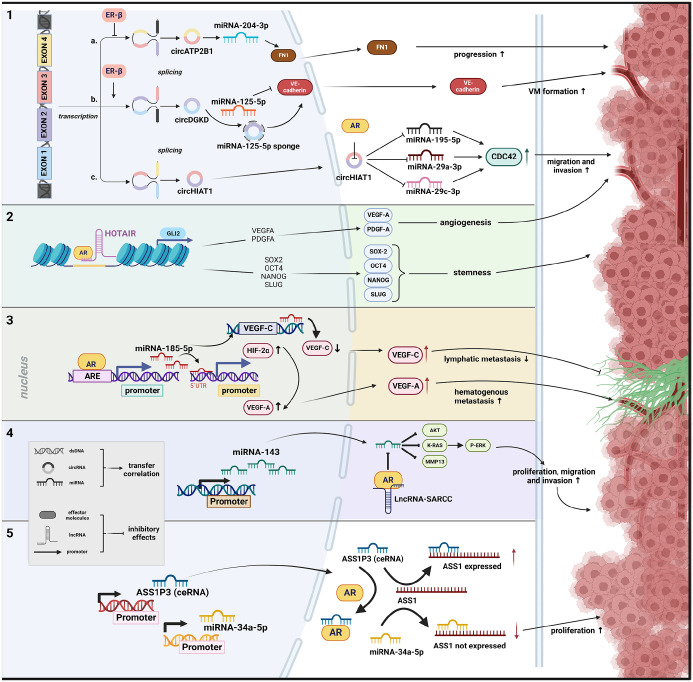
Figure 1.
Schematic representation of potential interaction mechanisms between sex hormones and ncRNA regarding RCC formation. (1) Function via altering circRNA with inhibiting effect of ER-β on transcription of circRNA, 116 promoting effect of ER-β on transcription of circRNA 120 and direct effect of AR on circRNA function. 137 (2) The interaction between AR and lncRNA with chromatin has a synergistic effect on the activities that affect the intrachromosomal genes located nearby. 133 (3) The effect of AR on miRNA expression occurs through the direct binding of its candidate AREs in the promoter region. 136 (4) The function and stability of a protein is influenced by its interaction with lncRNA, thereby impacting interactions between the protein and miRNA. 134 (5) Direct interaction with AR can regulate the ceRNA activity, resulting in an increased effect of the competing miRNA. 132
Source: Created with BioRender.com.
ARE, androgen response elements; ceRNA, competitive endogenous RNA; circRNA, circular RNAs; lncRNA, long noncoding RNA; miRNA, microRNA.
Androgens
Argininosuccinate synthase 1 (ASS1) expression showed a negative correlation to tumour staging, indicating a reduced ASS1 expression during tumour progression. Recent studies have additionally highlighted the significance of decreased ASS1 activity in promoting tumour growth. Immunohistochemical staining of 40 each primary RCC and adjacent normal renal tissue samples demonstrated a lower ASS1 expression in RCC tissue. Following this, one could implicate that decreased ASS1 expression is associated with a poorer prognosis in RCC, highlighting the possible role of ASS1. Increasing AR expression in vitro led to decreased ASS1 expression, promoting cell proliferation. Conversely, AR knockdown resulted in increased ASS1 expression. In this context, miRNA-34a-5p has been identified as a regulator of ASS1, capable of negatively modulating its expression. Notably, ASS1P3 has been indicated as a pseudogene, which could function as a ceRNA, to modulate the expression of its corresponding gene, ASS1, by competing with miRNA-34a-5p. Reduced ASS1P3 expression inhibited ASS1 by miRNA-34a-5p, resulting in increased cell proliferation. Although a direct correlation between AR and ASS1P3 expression was not detected by the authors, it was postulated that AR may physically interact with ASS1P3, thereby impeding the interaction between ASS1P3 and miRNA-34a-5p. 132 Bai et al. 133 investigated the relationship between the lncRNA known as HOX transcript antisense intergenic RNA (HOTAIR) and AR in human ccRCC. HOTAIR is a trans-acting lncRNA located on chromosome 12q13.13, with a regulatory boundary in the HOXC cluster. 139 HOTAIR, along with other lncRNAs associated with the HOX locus, such as HOTAIRM1 and HOTTIP, plays significant roles in the development of ccRCC,140–142 through various mechanisms.115,143–145 The Hedgehog-GLI (HH-GLI) signalling pathway has been implicated in promoting cellular proliferation, differentiation, vascularization and stem cell maintenance. In RCC, GLI1/2 is activated by PI3K/AKT signalling. 146 Here, it was shown that GLI2 serves as a target gene for both HOTAIR and AR synergistically. HOTAIR and AR cooperatively bind to the GLI2 promoter, leading to an increase in its transcriptional activity. Consequently, GLI2 and its downstream genes, including cancer stem cell (CSC) transcription factors, vascular endothelial growth factor A (VEGFA) and PDGFA, were upregulated. This upregulation promotes tumour angiogenesis and enhances cancer stemness in RCC cells both in vitro and in vivo. 133
MiRNA-143-3p, identified as a tumour suppressor, is frequently down-regulated in various cancers, including RCC.147–150 Its decreased expression has been associated with the promotion of RCC cell invasion, migration and proliferation through downstream signalling molecules, including AKT, MMP-13, K-RAS and P-ERK. In a study by Zhai et al. AR influence of miRNA-143-3p expression by direct binding to its potential androgen response elements (AREs) in its promoter, thereby transcriptionally suppressing miRNA-143-3p was discovered. Additionally, a long noncoding RNA called suppressing AR in RCC (lncRNA-SARCC) was identified to directly bind and suppress the AR function by post-transcriptionally modulating the AR protein, consequently increasing miRNA-143-3p expression and suppressing the RCC progression. Consequently, expression of lncRNA-SARCC was found to be reduced in ccRCC and metastatic ccRCC compared to surrounding non-tumour and non-metastatic tissues, and this reduction correlated with a poorer prognosis in ccRCC patients. Interestingly, the authors observed that Sunitinib induces the expression of lncRNA-SARCC, thereby reducing the resistance of RCC cells to this drug. 134 An additional suppressive effect of LncRNA-SARCC on RCC development could be revealed through its regulation of the AR/HIF-2α/C-MYC axis signalling pathway. Interestingly, the expression of lncRNA-SARCC was found to be mediated differently in response to hypoxia. Under hypoxic conditions, lncRNA-SARCC suppressed AR expression, leading to a decrease in the HIF-2α/C-MYC axis and its cell proliferating and tumourigenic effect. In return, lncRNA-SARCC expression can be transcriptionally regulated by HIF-2α through its binding to hypoxia-responsive elements on the lncRNA-SARCC promoter, suggesting the presence of a negative feedback loop. 135 These findings provide valuable insights into the role of lncRNA-SARCC as a suppressor of RCC progression and highlight new therapeutic strategies for the treatment of RCC, specifically focusing on the regulation of AR and miRNA interactions.
Huang et al. investigated a novel interaction mechanism of AR with HIF-2α through miRNA regulation in ccRCC. Elevated AR expression was associated with increased haematogenous metastasis to the lung but reduced lymphatic metastases, through enhanced miRNA-185-5p expression by binding to its promoter region, leading to the suppression of vascular endothelial growth factor C (VEGF-C). Conversely, AR-mediated upregulation of miRNA-185-5p promoted HIF-2α/VEGF-A expression. This unique interplay between AR, miRNA-185-5p, VEGF-C and HIF-2α/VEGF-A highlighted AR’s dual role in facilitating or inhibiting ccRCC metastasis. 136 In addition, AR has been found to exert differential regulation on VEGF-A and VEGF-C in VHL wild-type ccRCC, depending on the oxygen conditions (normoxia vs hypoxia), thereby impacting the metastasis processes in distinct ways. Under normoxic conditions, the down-regulation of miRNA-185-5p results in the up-regulation of both VEGF-A and VEGF-C. Conversely, in a hypoxic environment, the upregulation of miRNA-185-5p leads to a decrease in both VEGF-A and VEGF-C expression. However, the activation of HIF-2α in hypoxia leads to the transcriptional upregulation of VEGF-A, outweighing miRNA-185-5p’s down-regulation, resulting in elevated VEGF-A expression. 151
Literature also reveals another study describing showing the link between AR and miRNAs in RCC, indicating that AR affects ccRCC cell migration and invasion by changing circHIAT1/miRNA-195-5p/29a-3p/29c-3p/cell division cycle 42 protein (CDC42) signalling. Suppression of circulating RNA circHIAT1 by AR resulted in altered miRNA-195-5p/29a-3p/29c-3p expression, which increased CDC42 expression, leading to intensified cell migration and invasion. 137
It has been shown that AR can bind to the ARE on the promoter region of miRNA-145, leading to the reduced ability of p53 to induce miRNA-145 expression. MiRNA-145 normally acts to suppress the expression of HIF-2α, VEGF, MMP9 and CCND1, which are key factors involved in RCC progression. Suppressing AR or introducing miRNA-145 mimics reduced RCC progression, regardless of VHL status. In a preclinical RCC mouse model, miRNA-145 mimic administration effectively suppressed RCC progression. 138
Oestrogens
As a transcription factor, ER-β can bind to specific DNA sequences in the promoter regions of target genes, either activating or repressing their transcription. ER-β was identified as a suppressor of circRNA ATPase plasma membrane transporter 2B1 (circATP2B1) expression by directly binding to the 5′ promoter region of its host gene ATPase plasma membrane Ca2+ transporting 1 (ATP2B1), which encodes circATP2B1. CircATP2B1 is implicated in regulating miRNA-204-3p in ccRCC cells, significantly increasing miRNA-204-3p by circATP2B1 addition. Moreover, circATP2B1 may function as a so-called ‘reservoir’ to stabilize miRNA-204-3p expression, as it interacts directly with miRNA-204-3p. This interplay results in elevated fibronectin 1 (FN1) expression in ccRCC cells, as miRNA-204-3p directly targets FN1 mRNA’s 3′ UTR, suppressing FN1 protein expression. Inhibition of miRNA-204-3p increases FN1 expression, while miRNA-204-3p overexpression decreases FN1 levels in ccRCC cells. Analysis of the ccRCC patient survival data from the Cancer Genome Atlas indicates worse overall survival (OS) for patients with elevated ER-β and FN1 expression, while higher miRNA-204-3p expression correlates with significantly better OS, emphasizing the clinical relevance of the ER-β/circATP2B1/miRNA-204-3p/FN1 axis in ccRCC progression. 116 Furthermore, ER-β facilitates an increase in HOTAIR expression by binding to its promoter in RCC. Consequently, HOTAIR assumes a role in counteracting the effects of various miRNAs. Subsequently, HOTAIR counteracts the effects of several miRNAs. By antagonizing miRNA-138, (targeting ADAM9), miRNA-204 (targeting CCND2), miRNA-217 (targeting genes like VEGFA, VIM, ZEB1 and ZEB2) and miRNA-200c (targeting ZEB1 and ZEB2), it results in collective interaction with RCC cell, proliferation, migration and invasion. 115
Higher ER-β expression correlates with elevated VE-cadherin, a pivotal adhesion molecule in vasculogenic mimicry (VM) formation. VM is a process where tumour cells mimic blood vessel-like structures to secure nutrients and oxygen, potentially contributing to ccRCC progression and metastasis. 119 One supposed mechanism by which sunitinib promotes VM formation in RCC cells involves the modulation of lncRNA called lncRNA-ECVSR. Sunitinib treatment can increase the expression of lncRNA-ECVSR, which enhances the stability of ER-β mRNA. This increased ER-β expression can then function via transcriptional up-regulation of Hif2-α by binding to ERE, specifically to ERE1, in the promoter region of Hif2-α. Hif2-α, in turn, promotes the CSC phenotype, which is associated with increased VM formation. The sunitinib/lncRNA-ECVSR-increased ERβ expression can transcriptionally regulate lncRNA-ECVSR expression via a positive-feedback loop, further enhancing the effects of sunitinib on VM formation. 119
TKI-induced ER-β transcriptionally up-regulates the circular RNA DGKD (circDGKD), which functions as a miRNA-125-5p sponge. MiRNA-125-5p interacts with the 3′ UTR of VE-cadherin mRNA, leading to its degradation or translational inhibition. In RCC, miRNA-125-5p down-regulation results in increased VE-cadherin expression, promoting VM formation. Targeting circDGKD tempers sunitinib-induced RCC VM formation, reduces metastasis and enhances survival in experimental animal models. The authors propose that intervening in ERβ/circDGKD signalling may enhance TKI effectiveness and offer novel combination therapies for the management of metastatic RCC. 120
SHs and treatment of RCC
Androgen treatment in RCC
The potential significance of the androgen-signalling axis in the progression of RCC has led to the thought that interfering with the hormonal axis may be a potential strategy to enhance patient survival. Clinical trials are already investigating the efficacy of therapeutic agents targeting AR in RCC. The BARE trial (Blockade of Androgens in RCC using Enzalutamide, NCT02885649, www.clinicaltrials.gov, accessed on 9 September 2021) was designed to elucidate the impact of the AR inhibitor enzalutamide on tumour growth prior to surgical resection. Regrettably, the trial was prematurely terminated due to unavailability of funding.
Flutamide, a nonsteroidal anti-androgen, was also investigated in patients with RCC. Among 25 cases treated, one patient exhibited partial remission and two patients experienced a state of stabilization of disease. Nevertheless, flutamide did not demonstrate any anti-tumour activity in individuals with metastatic RCC. 122 Enzalutamide and abiraterone acetate, a CYP17A1 inhibitor that inhibits androgen production, showed more promising results in in vivo studies, demonstrating a substantial reduction in tumour size. 152
Knockdown of the epigenetic co-regulator lysine-specific histone demethylase 1, along with enzalutamide, slowed RCC growth and migration in a mouse model. 153 In a patient-derived xenograft model with sunitinib-resistant RCC, AR upregulation was observed. Enzalutamide treatment led to AR degradation and decreased AR activity, resulting in effective tumour regression when combined with sunitinib. 154 These findings highlight the importance of the androgen axis in RCC and suggest AR as a potential target for a therapeutic approach.
Oestrogen treatment in RCC
Investigation on hormonal carcinogenesis, specifically highlighting the involvement of the SH in RCC pathogenesis, has yielded encouraging findings. Particularly outcomes in the domain of RCC therapy targeting ER remain promising, providing notable advantages for managing metastatic disease.
In a hamster model, RCC was successfully induced through the chronic administration of DES and polydiethylstilbestrol phosphate, underlining the possibly important involvement of oestrogens in RCC aetiology.155–157 Conversely, the inhibitory impact on tumour formation was demonstrated with the antioestrogen agent nafoxidine. 158 In vitro investigations proposed that potentially reactive oestrogen intermediates might act as instigators of experimental nephron-carcinogenesis, inducing substantial oxidative stress within renal cells upon prolonged oestrogen exposure.159,160
The potential utility of tamoxifen, a selective ER modulator commonly used in breast cancer treatment, was explored as a therapeutic approach for small cohorts of patients with RCC, yielding varied outcomes. In one study, 34 patients with progressive RCC were treated with high-dose tamoxifen (100 mg/ml 2 daily) until disease progression. An overall partial response of 10%, including one complete remission, was observed. Favourable survival outcomes were noted in patients with pulmonary metastases, good performance status and prior nephrectomy. 161
Another investigation involved 10 patients with advanced RCC treated using combined chemo-endocrine therapy comprising tegafur, a prodrug of fluorouracil, and tamoxifen. Positive responses were observed, particularly in patients with ER-positive and ER-negative tumours. 162 A comparison study evaluated tamoxifen alone against IL-2/IFN-α therapy combined with tamoxifen. Although tamoxifen was included due to its non-toxic behaviour and potential enhancement of IL-2’s anti-tumour activity, no significant survival differences were found between treatment arms. 163 While high-dose tamoxifen demonstrated some anti-tumour effects in specific cases, combined hormonal therapy did not confer a significant therapeutic advantage for advanced RCC. Despite this, subsequent years have seen limited research exploring the potential of hormone modulators in RCC. This is noteworthy considering the emergence of new therapeutic strategies such as TKIs and immune-based therapies, which have shown promise in metastatic RCC management.
Progesterone treatment in RCC
Hormonal agents, like medroxyprogesterone, were found to have some effectiveness in treating metastatic RCC in early studies. However, limited data show response rates in different studies, ranging from 7% to 25%, and still need further evaluation in larger studies to confirm oncological ongoing response.164–166
Sex and immunotherapy in RCC
Previous research has shown differences in immune responses, particularly anti-tumour responses, between sexes.167,168 Meta-analyses propose that ICIs may provide greater benefits for male cancer patients in comparison to females.169–171 The controversially discussed data do not necessarily apply to RCC.172,173 A recent comprehensive review focused on the efficacy of ICI in urological cancers, including RCC, indicating an improved OS, regardless of sex. 174 Meanwhile, adjuvant ICI monotherapies reduce the risk of disease recurrence in women with locally advanced RCC, yet not in men. Furthermore, ranking analyses revealed distinct outcomes for RCC treatment between the sexes, suggesting that sex may influence clinical decision-making. 174 However, there is evidence of a divergent response to ICI treatment in patients with advanced RCC, with a less marked effect observed in females compared to male patients, indicating that sex is a crucial factor in clinical decision-making. 175
Another intriguing aspect of sex differences lies in the occurrence of adverse events associated with ICI. Immunotherapy disrupts immune balance, potentially leading to immune-related adverse events (irAEs) affecting various organ systems. 176 Women exhibit higher innate and adaptive immune responses than men, along with an increased susceptibility to autoimmune diseases, leading to a higher risk of irAEs. 177 Consequently, female sex has been identified as a predictive biomarker for irAE occurrence in patients treated with ICIs. 178 Notably, a study by Unger et al. 179 examining gender disparities in therapy responses, particularly to immunotherapy, revealed that women receiving immunotherapy exhibited a 49% greater risk of irAEs than men, with the severity of irAEs being higher among women.
Some further nuanced relationships between sex and molecular predictors of ICI response could be revealed. TMB has been associated with a positive ICI response in men with certain cancers such as melanoma, bladder, head and neck, and RCC. 180 Conversely, other molecular markers, such as activated T-cell frequency and expression of immune checkpoint proteins, including both inhibitory (programmed cell death protein1 1 (PD-1), cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4), Lag3) and stimulatory (OX40, ICOS, CD27) markers, are associated with a positive ICI response in female patients. 181
In this context, SHs are suspected to influence the TME and underlie the differences in immune responses between men and women. 182 E2 increases immunoglobulin production, while androgens such as dihydrotestosterone (DHT) and testosterone have been shown to reduce immune activity. 167 Regulatory T cells (Treg) rise when E2 levels increase. 183 Reduced levels of E2 encourage differentiation of T helper (Th) cells towards Th1, whereas higher levels of E2 promote the Th2 phenotype. 184 Additionally, E2 is linked with heightened expression of PD-1. 183
The persistence of androgen-induced exhaustion of CD8+ T cells may limit the efficacy of ICIs in male RCC, necessitating additional therapeutic strategies. 89 Androgen deprivation therapy (ADT) can prevent CD8+ T-cell exhaustion in the TME and improve the efficacy of ICIs, particularly anti-PD-1 treatment.86,87 Combination therapy with androgen receptor inhibitors (ARi) and ICIs has shown synergistic effects in RCC in vivo, possibly because ARi reverses androgen-induced immunosuppression in the TME of male RCC. 89 Other investigations suggest that ADT can enhance the effectiveness of immunotherapy by modulating immune cell function. 185 Moreover, studies have shown that dendritic cells are better able to stimulate T-cell responses when ADT is administered after immunotherapy.
Nevertheless, the regulatory impact of SH on immunotherapy in cancer, particularly the association between SH and ICI in metastatic RCC, has not been thoroughly evaluated. There is a notable lack of data and the interpretation is complicated, also due to the insufficient exploration of the reciprocal effects of checkpoint inhibition on SH and vice versa. However, anti-PD-L1 therapy has been shown to significantly downregulate SH levels in male mice, but not in female mice, thereby enhancing the anti-tumour efficacy of anti-PD-L1. 186 In contrast, an increase in estradiol and luteinising hormone (LH)/follicle-stimulating hormone (FSH) ratio in male patients receiving nivolumab monotherapy for metastatic RCC has been recently reported and an association between progression free survival (PFS) and objective response rate (ORR) with increased LH/FSH ratio during nivolumab therapy has been demonstrated. 118
Additionally, survival outcomes among RCC patients may be influenced by gender-related factors such as behaviour, as well as genetics and hormones.57,167,187 Therefore, there is still an ongoing debate about sex-related differences in oncological outcomes for patients with metastatic RCC. Pooled meta-analyses may not fully capture the nuanced interactions of sex and ICI response. Further investigation into possible sex-based differences in the immune response to ICIs is essential for identifying patients who are most likely to benefit from particular ICI-based combination therapies.
Summary and conclusion
Investigations of the pathobiology of sex steroid hormones and their receptors in RCC significantly expand our understanding of crucial aspects of RCC development and progression. Currently, the molecular role of SH in RCC remains to be elucidated to provide a precise model of hormonal interactions with oncogenesis. Unfortunately, the limited number of publications in this area suggests that there is a lack of research priority.
As a physiological fact, variable expression of steroid receptors has been noted when comparing normal kidney tissue and RCC tissue, which is to be seen as the potential basis for the discovered differences in the development of RCC. SH signalling in RCC suggests a not yet fully understood multifaceted dual role, influencing processes such as proliferation, invasion, apoptosis and angiogenesis through distinct molecular mechanisms. In this review, we presented comprehensive examples of both the oncogenic and tumour-suppressive effects of SH in RCC. For instance, E2 has been observed to interact with various signalling pathways, including VEGF/HIF2α, PI3K/AKT/MMP-9, TGF-β1/SMAD3 and ER-β/ANGPT-2/Tie-2, primarily through ER, whereas the activation of the AR has been linked to pathways involving PI3K/AKT → NF-κB → CXCL5, AR-c-Myc and STAT5 regulation.
Recent research has emphasized the critical role of ncRNAs in various biological functions and their profound impact on cancer, including RCC. Notably, the substantial influence of SH on the expression and functionality of numerous ncRNAs involved in the complex process of RCC development has been highlighted. Although our understanding of ncRNA function in RCC is evolving, this review underscores their potential role in initiating, promoting and progressing RCC through different miRNA/target gene axes via activation of AR and ER. Their contribution to RCC development shown in this review highlights their importance as potential therapeutic targets and biomarkers for more effective RCC therapies. However, our understanding of ncRNA function in RCC, particularly in relation to steroid hormones, remains limited, and further research is needed to explore their full functional spectrum.188,189
The approval of a wide range of immunotherapies is considered to be the most significant breakthrough in the treatment of advanced RCC in recent years. However, it is to be noted that many exploratory studies investigating hormone manipulation as a potential strategy for the treatment of metastatic RCC have also shown promising clinical results. This highlights the need to investigate whether ncRNAs can modulate the immune pathways involved in RCC, particularly in the context of SH involvement.
Notably, there is a lack of specific data or evidence on the interaction between SH and the efficacy of immunotherapeutic interventions in non-ccRCC subtypes due to the lower prevalence of pRCC and chRCC. As a result, the existing body of evidence does not adequately address the potential impact of SH on carcinogenesis or TME in this particular subset of RCC. Further investigation is warranted to fully elucidate this relationship in patients with non-ccRCC.
In summary, the interactions between SH and the intricate pathways within RCC are complex and not fully elucidated. Nevertheless, these findings suggest that SH plays a role in the differential response rates observed in male and female RCC patients. This review aims to rekindle interest in studying steroid hormones and their receptors in RCC, as they hold promise for therapy and biomarker development. Conducting sex-specific research, especially in the context of clinical treatments, is crucial, highlighting the importance of in-depth scientific exploration in this field.
Acknowledgments
None.
Footnotes
ORCID iD: Gennadi Tulchiner  https://orcid.org/0000-0002-2964-1544
https://orcid.org/0000-0002-2964-1544
Contributor Information
Michael Ladurner, Department of Urology, Medical University of Innsbruck, Innsbruck, Austria.
Andrea Katharina Lindner, Department of Urology, General Hospital Hall in Tirol, Hall in Tirol, Austria.
Peter Rehder, Department of Urology, Medical University of Innsbruck, Innsbruck, Austria.
Gennadi Tulchiner, Department of Urology, Medical University of Innsbruck, Anichstrasse 35, Innsbruck 6020, Austria.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Michael Ladurner: Conceptualization; Data curation; Formal analysis; Validation; Writing – original draft.
Andrea Katharina Lindner: Data curation; Investigation; Visualization; Writing – review & editing.
Peter Rehder: Methodology; Supervision; Validation; Writing – review & editing.
Gennadi Tulchiner: Conceptualization; Data curation; Formal analysis; Supervision; Validation; Visualization; Writing – original draft.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Low G, Huang G, Fu W, et al. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 2016; 8(5): 484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buczek M, Escudier B, Bartnik E, et al. Resistance to tyrosine kinase inhibitors in clear cell renal cell carcinoma: from the patient’s bed to molecular mechanisms. Biochim Biophys Acta 2014; 1845(1): 31–41. [DOI] [PubMed] [Google Scholar]
- 3. Kornakiewicz A, Solarek W, Bielecka ZF, et al. Mammalian target of rapamycin inhibitors resistance mechanisms in clear cell renal cell carcinoma. Curr Signal Transduct Ther 2014; 8(3): 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lalani AA, McGregor BA, Albiges L, et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: current paradigms, use of immunotherapy, and future directions. Eur Urol 2019; 75(1): 100–110. [DOI] [PubMed] [Google Scholar]
- 5. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27(22): 3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369(8): 722–731. [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372(9637): 449–456. [DOI] [PubMed] [Google Scholar]
- 8. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015; 16(15): 1473–1482. [DOI] [PubMed] [Google Scholar]
- 9. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016; 17(7): 917–927. [DOI] [PubMed] [Google Scholar]
- 10. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378(9807): 1931–1939. [DOI] [PubMed] [Google Scholar]
- 11. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010; 28(6): 1061–1068. [DOI] [PubMed] [Google Scholar]
- 12. Van Poppel H, Joniau S, Van Gool SW. Vaccine therapy in patients with renal cell carcinoma. Eur Urol 2009; 55(6): 1333–1342. [DOI] [PubMed] [Google Scholar]
- 13. Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002; 20(1): 289–296. [DOI] [PubMed] [Google Scholar]
- 14. Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 2007; 110(11): 2468–2477. [DOI] [PubMed] [Google Scholar]
- 15. Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003; 21(16): 3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021; 384(9): 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373(19): 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378(14): 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380(12): 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384(14): 1289–1300. [DOI] [PubMed] [Google Scholar]
- 21. Han J, Yang Y, Li X, et al. Pan-cancer analysis reveals sex-specific signatures in the tumor microenvironment. Mol Oncol 2022; 16(11): 2153–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol 2016; 17(12): 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol 2011; 31(23): 4663–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol 2010; 28(26): 4038–4044. [DOI] [PubMed] [Google Scholar]
- 25. Liao RS, Ma S, Miao L, et al. Androgen receptor-mediated non-genomic regulation of prostate cancer cell proliferation. Transl Androl Urol 2013; 2(3): 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, Zhou Y, Xing Z, et al. Androgen metabolism and response in prostate cancer anti-androgen therapy resistance. Int J Mol Sci 2022; 23(21): 13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gershanovich M, Chaudri HA, Campos D, et al. Letrozole, a new oral aromatase inhibitor: randomised trial comparing 2.5 mg daily, 0.5 mg daily and aminoglutethimide in postmenopausal women with advanced breast cancer. Letrozole International Trial Group (AR/BC3). Ann Oncol 1998; 9(6): 639–645. [DOI] [PubMed] [Google Scholar]
- 28. MacNeill FA, Jones AL, Jacobs S, et al. The influence of aminoglutethimide and its analogue rogletimide on peripheral aromatisation in breast cancer. Br J Cancer 1992; 66(4): 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hatch EE, Palmer JR, Titus-Ernstoff L, et al. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA 1998; 280(7): 630–634. [DOI] [PubMed] [Google Scholar]
- 30. Tournaire M, Devouche E, Espie M, et al. Cancer risk in women exposed to diethylstilbestrol in utero. Therapie 2015; 70(5): 433–441. [DOI] [PubMed] [Google Scholar]
- 31. Singh VK, Pal R, Srivastava P, et al. Exposure of androgen mimicking environmental chemicals enhances proliferation of prostate cancer (LNCaP) cells by inducing AR expression and epigenetic modifications. Environ Pollut 2021; 272: 116397. [DOI] [PubMed] [Google Scholar]
- 32. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74(3): 229–263. [DOI] [PubMed] [Google Scholar]
- 33. Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol 2017; 115(5): 564–579. [DOI] [PubMed] [Google Scholar]
- 34. Bilski K, Zapala L, Skrzypczyk MA, et al. Review on gender differences in non-muscle invasive bladder cancer. Transl Androl Urol 2019; 8(1): 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKinley BP, Michalek AM, Fenstermaker RA, et al. The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. J Neurosurg 2000; 93(6): 932–939. [DOI] [PubMed] [Google Scholar]
- 36. Lin JH, Zhang SM, Rexrode KM, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol 2013; 11(4): 419–424.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boorjian S, Ugras S, Mongan NP, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology 2004; 64(2): 383–388. [DOI] [PubMed] [Google Scholar]
- 38. Palethorpe HM, Drew PA, Smith E. Androgen signaling in esophageal adenocarcinoma cell lines in vitro. Dig Dis Sci 2017; 62(12): 3402–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu X, Jiang Y, Wei W, et al. Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumour Biol 2015; 36(2): 967–972. [DOI] [PubMed] [Google Scholar]
- 40. Mizushima T, Tirador KA, Miyamoto H. Androgen receptor activation: a prospective therapeutic target for bladder cancer? Expert Opin Ther Targets 2017; 21(3): 249–257. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Lozano DC, Pina-Medina AG, Hansberg-Pastor V, et al. Testosterone promotes glioblastoma cell proliferation, migration, and invasion through androgen receptor activation. Front Endocrinol (Lausanne) 2019; 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gil D, Zarzycka M, Dulinska-Litewka J, et al. Dihydrotestosterone increases the risk of bladder cancer in men. Hum Cell 2019; 32(3): 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu HH, Kuo WW, Ju DT, et al. Estradiol agonists inhibit human LoVo colorectal-cancer cell proliferation and migration through p53. World J Gastroenterol 2014; 20(44): 16665–16673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilkins HR, Doucet K, Duke V, et al. Estrogen prevents sustained COLO-205 human colon cancer cell growth by inducing apoptosis, decreasing c-myb protein, and decreasing transcription of the anti-apoptotic protein bcl-2. Tumour Biol 2010; 31(1): 16–22. [DOI] [PubMed] [Google Scholar]
- 45. Chen C, Gong X, Yang X, et al. The roles of estrogen and estrogen receptors in gastrointestinal disease. Oncol Lett 2019; 18(6): 5673–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Q, Zhang W, Yang J, et al. High ERα36 expression level and membrane location predict poor prognosis in renal cell carcinoma. Medicine (Baltimore) 2015; 94(26): e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tyulmenkov VV, Jernigan SC, Klinge CM. Comparison of transcriptional synergy of estrogen receptors alpha and beta from multiple tandem estrogen response elements. Mol Cell Endocrinol 2000; 165(1–2): 151–161. [DOI] [PubMed] [Google Scholar]
- 48. Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010; 7(5): 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127(12): 2893–2917. [DOI] [PubMed] [Google Scholar]
- 50. Concolino G, Marocchi A, Conti C, et al. Human renal cell carcinoma as a hormone-dependent tumor. Cancer Res 1978; 38(11 Pt 2): 4340–4344. [PubMed] [Google Scholar]
- 51. Concolino G, Di Silverio F, Marocchi A, et al. Renal cancer steroid receptors: biochemical basis for endocrine therapy. Eur Urol 1979; 5(5): 319–322. [DOI] [PubMed] [Google Scholar]
- 52. Zucchetto A, Talamini R, Dal Maso L, et al. Reproductive, menstrual, and other hormone-related factors and risk of renal cell cancer. Int J Cancer 2008; 123(9): 2213–2216. [DOI] [PubMed] [Google Scholar]
- 53. Kabat GC, Silvera SA, Miller AB, et al. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer 2007; 96(5): 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu CP, Ho JY, Huang YT, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PLoS One 2013; 8(2): e56667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kautzky-Willer A. [Gender medicine. Sex- and gender-specific aspects of clinical medicine]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014; 57(9): 1022–1030. [DOI] [PubMed] [Google Scholar]
- 56. Becher E, Oertelt-Prigione S. The impact of sex and gender in medicine and pharmacology. In: Tsirka SE, Acosta-Martinez M. (eds) Sex and Gender Effects in Pharmacology. Handbook of Experimental Pharmacology, Vol. 282, Springer, Cham. 10.1007/164_2023_688 [DOI] [PubMed] [Google Scholar]
- 57. Wagner AD, Oertelt-Prigione S, Adjei A, et al. Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol 2019; 30(12): 1914–1924. [DOI] [PubMed] [Google Scholar]
- 58. Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells 2020; 9(10): 2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu WJ, Zhao G, Zhang CY, et al. Comparison of the roles of estrogens and androgens in breast cancer and prostate cancer. J Cell Biochem 2020; 121(4): 2756–2769. [DOI] [PubMed] [Google Scholar]
- 60. He D, Li L, Zhu G, et al. ASC-J9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2alpha/VEGF signaling pathway. Cancer Res 2014; 74(16): 4420–4430. [DOI] [PubMed] [Google Scholar]
- 61. Guan Z, Li C, Fan J, et al. Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT → NF-kappaB → CXCL5 signaling. Sci Rep 2016; 6: 37085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Song W, Li L, He D, et al. Infiltrating neutrophils promote renal cell carcinoma (RCC) proliferation via modulating androgen receptor (AR) → c-Myc signals. Cancer Lett 2015; 368(1): 71–78. [DOI] [PubMed] [Google Scholar]
- 63. Pak S, Kim W, Kim Y, et al. Dihydrotestosterone promotes kidney cancer cell proliferation by activating the STAT5 pathway via androgen and glucocorticoid receptors. J Cancer Res Clin Oncol 2019; 145(9): 2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jung YS, Lee SJ, Yoon MH, et al. Estrogen receptor alpha is a novel target of the Von Hippel-Lindau protein and is responsible for the proliferation of VHL-deficient cells under hypoxic conditions. Cell Cycle 2012; 11(23): 4462–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Song W, Yeh CR, He D, et al. Infiltrating neutrophils promote renal cell carcinoma progression via VEGFa/HIF2alpha and estrogen receptor beta signals. Oncotarget 2015; 6(22): 19290–19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guan BZ, Yan RL, Huang JW, et al. Activation of G protein coupled estrogen receptor (GPER) promotes the migration of renal cell carcinoma via the PI3K/AKT/MMP-9 signals. Cell Adh Migr 2018; 12(2): 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen KC, Lin CM, Huang CJ, et al. Dual roles of 17-beta estradiol in estrogen receptor-dependent growth inhibition in renal cell carcinoma. Cancer Genomics Proteomics 2016; 13(3): 219–230. [PubMed] [Google Scholar]
- 68. Song W, He D, Chen Y, et al. Targeting newly identified ERbeta/TGF-beta1/SMAD3 signals with the FDA-approved anti-estrogen Faslodex or an ERbeta selective antagonist in renal cell carcinoma. Mol Oncol 2018; 12(12): 2055–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gu J, Zhang Y, Han Z, et al. Targeting the ERbeta/angiopoietin-2/Tie-2 signaling-mediated angiogenesis with the FDA-approved anti-estrogen Faslodex to increase the Sunitinib sensitivity in RCC. Cell Death Dis 2020; 11(5): 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Langner C, Ratschek M, Rehak P, et al. Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J Urol 2004; 171(2 Pt 1): 611–614. [DOI] [PubMed] [Google Scholar]
- 71. Zhu G, Liang L, Li L, et al. The expression and evaluation of androgen receptor in human renal cell carcinoma. Urology 2014; 83(2): 510.e19-24. [DOI] [PubMed] [Google Scholar]
- 72. Ha YS, Lee GT, Modi P, et al. Increased expression of androgen receptor mRNA in human renal cell carcinoma cells is associated with poor prognosis in patients with localized renal cell carcinoma. J Urol 2015; 194(5): 1441–1448. [DOI] [PubMed] [Google Scholar]
- 73. Yuan P, Ge Y, Liu X, et al. The association of androgen receptor expression with renal cell carcinoma risk: a systematic review and meta-analysis. Pathol Oncol Res 2020; 26(2): 605–614. [DOI] [PubMed] [Google Scholar]
- 74. Kimura N, Mizokami A, Oonuma T, et al. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 1993; 41(5): 671–678. [DOI] [PubMed] [Google Scholar]
- 75. Brown DF, Dababo MA, Hladik CL, et al. Hormone receptor immunoreactivity in hemangioblastomas and clear cell renal cell carcinomas. Mod Pathol 1998; 11(1): 55–59. [PubMed] [Google Scholar]
- 76. Foersch S, Schindeldecker M, Keith M, et al. Prognostic relevance of androgen receptor expression in renal cell carcinomas. Oncotarget 2017; 8(45): 78545–78555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Noh SJ, Kang MJ, Kim KM, et al. Acetylation status of P53 and the expression of DBC1, SIRT1, and androgen receptor are associated with survival in clear cell renal cell carcinoma patients. Pathology 2013; 45(6): 574–580. [DOI] [PubMed] [Google Scholar]
- 78. Baldewijns MM, van Vlodrop IJ, Vermeulen PB, et al. VHL and HIF signalling in renal cell carcinogenesis. J Pathol 2010; 221(2): 125–138. [DOI] [PubMed] [Google Scholar]
- 79. Zhang Y, Narayanan SP, Mannan R, et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc Natl Acad Sci U S A 2021; 118(24): e2103240118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Motzer RJ, Robbins PB, Powles T, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med 2020; 26(11): 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet 2014; 46(3): 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018; 24(5): 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sasson SC, Slevin SM, Cheung VTF, et al. Interferon-gamma-producing CD8(+) tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. Gastroenterology 2021; 161(4): 1229–1244.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang T, Shen Y, Luyten S, et al. Tissue-resident memory CD8(+) T cells in cancer immunology and immunotherapy. Pharmacol Res 2020; 159: 104876. [DOI] [PubMed] [Google Scholar]
- 85. Braun DA, Hou Y, Bakouny Z, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med 2020; 26(6): 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kwon H, Schafer JM, Song NJ, et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci Immunol 2022; 7(73): eabq2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guan X, Polesso F, Wang C, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022; 606(7915): 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang C, Jin J, Yang Y, et al. Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity 2022; 55(9): 1747. [DOI] [PubMed] [Google Scholar]
- 89. Ning K, Peng Y, Jiang Y, et al. Sex differences in renal cell carcinoma: a single-cell analysis reveals exhausted CD8(+) T-cells highly infiltrated in males. Biol Sex Differ 2023; 14(1): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jensen HK, Donskov F, Marcussen N, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009; 27(28): 4709–4717. [DOI] [PubMed] [Google Scholar]
- 91. Noronha RF, Rao BR. Increased dihydrotestosterone receptor levels in high-stage renal adenocarcinoma. Cancer 1985; 56(1): 134–137. [DOI] [PubMed] [Google Scholar]
- 92. Matsumoto T, Sakari M, Okada M, et al. The androgen receptor in health and disease. Annu Rev Physiol 2013; 75: 201–224. [DOI] [PubMed] [Google Scholar]
- 93. Bennett NC, Gardiner RA, Hooper JD, et al. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol 2010; 42(6): 813–827. [DOI] [PubMed] [Google Scholar]
- 94. Yonekura S, Terauchi F, Hoshi K, et al. Androgen receptor predicts first and multiple recurrences in non-muscle invasive urothelial carcinoma of the bladder. Pathol Oncol Res 2019; 25(3): 987–994. [DOI] [PubMed] [Google Scholar]
- 95. Olsen JR, Azeem W, Hellem MR, et al. Context dependent regulatory patterns of the androgen receptor and androgen receptor target genes. BMC Cancer 2016; 16: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bennett NC, Rajandram R, Ng KL, et al. Evaluation of steroid hormones and their receptors in development and progression of renal cell carcinoma. J Kidney Cancer VHL 2014; 1(2): 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Adsay NV, Eble JN, Srigley JR, et al. Mixed epithelial and stromal tumor of the kidney. Am J Surg Pathol 2000; 24(7): 958–970. [DOI] [PubMed] [Google Scholar]
- 98. Orovan WL, Ryan ED. Estrogen and progesterone binding sites in renal cell carcinoma. Urology 1989; 34(1): 65–67. [DOI] [PubMed] [Google Scholar]
- 99. Ronchi E, Pizzocaro G, Miodini P, et al. Steroid hormone receptors in normal and malignant human renal tissue: relationship with progestin therapy. J Steroid Biochem 1984; 21(3): 329–335. [DOI] [PubMed] [Google Scholar]
- 100. Hemstreet GP, 3rd, Wittliff JL, Sarrif AM, et al. Comparison of steroid receptor levels in renal-cell carcinoma and autologous normal kidney. Int J Cancer 1980; 26(6): 769–775. [DOI] [PubMed] [Google Scholar]
- 101. Karr JP, Pontes JE, Schneider S, et al. Clinical aspects of steroid hormone receptors in human renal cell carcinoma. J Surg Oncol 1983; 23(2): 117–124. [DOI] [PubMed] [Google Scholar]
- 102. Pearson J, Friedman MA, Hoffman PG., Jr. Hormone receptors in renal cell carcinoma. Their utility as predictors of response to endocrine therapy. Cancer Chemother Pharmacol 1981; 6(2): 151–154. [DOI] [PubMed] [Google Scholar]
- 103. Wu ST, Ku WC, Huang CJ, et al. Cellular effects induced by 17-beta-estradiol to reduce the survival of renal cell carcinoma cells. J Biomed Sci 2016; 23(1): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tanaka Y, Sasaki M, Kaneuchi M, et al. Estrogen receptor alpha polymorphisms and renal cell carcinoma – a possible risk. Mol Cell Endocrinol 2003; 202(1–2): 109–116. [DOI] [PubMed] [Google Scholar]
- 105. Tanaka Y, Sasaki M, Kaneuchi M, et al. Single nucleotide polymorphisms of estrogen receptor alpha in human renal cell carcinoma. Biochem Biophys Res Commun 2002; 296(5): 1200–1206. [DOI] [PubMed] [Google Scholar]
- 106. El-Deek HEM, Ahmed AM, Hassan TS, et al. Expression and localization of estrogen receptors in human renal cell carcinoma and their clinical significance. Int J Clin Exp Pathol 2018; 11(6): 3176–3185. [PMC free article] [PubMed] [Google Scholar]
- 107. Luo LJ, Liu F, Lin ZK, et al. Genistein regulates the IL-1 beta induced activation of MAPKs in human periodontal ligament cells through G protein-coupled receptor 30. Arch Biochem Biophys 2012; 522(1): 9–16. [DOI] [PubMed] [Google Scholar]
- 108. Ge C, Yu M, Zhang C. G protein-coupled receptor 30 mediates estrogen-induced proliferation of primordial germ cells via EGFR/Akt/beta-catenin signaling pathway. Endocrinology 2012; 153(7): 3504–3516. [DOI] [PubMed] [Google Scholar]
- 109. Du GQ, Zhou L, Chen XY, et al. The G protein-coupled receptor GPR30 mediates the proliferative and invasive effects induced by hydroxytamoxifen in endometrial cancer cells. Biochem Biophys Res Commun 2012; 420(2): 343–349. [DOI] [PubMed] [Google Scholar]
- 110. He YY, Cai B, Yang YX, et al. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci 2009; 100(6): 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schleipen B, Hertrampf T, Fritzemeier KH, et al. ERbeta-specific agonists and genistein inhibit proliferation and induce apoptosis in the large and small intestine. Carcinogenesis 2011; 32(11): 1675–1683. [DOI] [PubMed] [Google Scholar]
- 112. Godoy G, Gakis G, Smith CL, et al. Effects of androgen and estrogen receptor signaling pathways on bladder cancer initiation and progression. Bladder Cancer 2016; 2(2): 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pinton G, Thomas W, Bellini P, et al. Estrogen receptor beta exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. PLoS One 2010; 5(11): e14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011; 469(7331): 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ding J, Yeh CR, Sun Y, et al. Estrogen receptor beta promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene 2018; 37(37): 5037–5053. [DOI] [PubMed] [Google Scholar]
- 116. Han Z, Zhang Y, Sun Y, et al. ERbeta-mediated alteration of circATP2B1 and miR-204-3p signaling promotes invasion of clear cell renal cell carcinoma. Cancer Res 2018; 78(10): 2550–2563. [DOI] [PubMed] [Google Scholar]
- 117. Yeh CR, Ou ZY, Xiao GQ, et al. Infiltrating T cells promote renal cell carcinoma (RCC) progression via altering the estrogen receptor beta-DAB2IP signals. Oncotarget 2015; 6(42): 44346–44359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tulchiner G, Pichler R, Ulmer H, et al. Sex-specific hormone changes during immunotherapy and its influence on survival in metastatic renal cell carcinoma. Cancer Immunol Immunother 2021; 70(10): 2805–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. He M, Yang H, Shi H, et al. Sunitinib increases the cancer stem cells and vasculogenic mimicry formation via modulating the lncRNA-ECVSR/ERbeta/Hif2-alpha signaling. Cancer Lett 2022; 524: 15–28. [DOI] [PubMed] [Google Scholar]
- 120. Ding J, Cui XG, Chen HJ, et al. Targeting circDGKD intercepts TKI’s effects on up-regulation of estrogen receptor beta and vasculogenic mimicry in renal cell carcinoma. Cancers (Basel) 2022; 14(7): 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tickoo SK, Gopalan A, Tu JJ, et al. Estrogen and progesterone-receptor-positive stroma as a non-tumorous proliferation in kidneys: a possible metaplastic response to obstruction. Mod Pathol 2008; 21(1): 60–65. [DOI] [PubMed] [Google Scholar]
- 122. Ahmed T, Benedetto P, Yagoda A, et al. Estrogen, progesterone, and androgen-binding sites in renal cell carcinoma. Observations obtained in phase II trial of flutamide. Cancer 1984; 54(3): 477–481. [DOI] [PubMed] [Google Scholar]
- 123. Mai KT, Kohler DM, Robertson SJ, et al. Oncocytic papillary renal cell carcinoma with solid architecture: mimic of renal oncocytoma. Pathol Int 2008; 58(3): 164–168. [DOI] [PubMed] [Google Scholar]
- 124. Tao C, Liu W, Yan X, et al. PAQR5 expression is suppressed by TGFbeta1 and associated with a poor survival outcome in renal clear cell carcinoma. Front Oncol 2021; 11: 827344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang D, Xia X, Wang X, et al. PGRMC1 is a novel potential tumor biomarker of human renal cell carcinoma based on quantitative proteomic and integrative biological assessments. PLoS One 2017; 12(1): e0170453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018; 172(3): 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017; 36(41): 5661–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stasiak M, Kolenda T, Kozlowska-Maslon J, et al. The world of pseudogenes: new diagnostic and therapeutic targets in cancers or still mystery molecules? Life (Basel) 2021; 11(12): 1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol 2020; 21(8): 475–490. [DOI] [PubMed] [Google Scholar]
- 130. Guarnerio J, Zhang Y, Cheloni G, et al. Intragenic antagonistic roles of protein and circRNA in tumorigenesis. Cell Res 2019; 29(8): 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rajappa A, Banerjee S, Sharma V, et al. Circular RNAs: emerging role in cancer diagnostics and therapeutics. Front Mol Biosci 2020; 7: 577938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang K, Sun Y, Guo C, et al. Androgen receptor regulates ASS1P3/miR-34a-5p/ASS1 signaling to promote renal cell carcinoma cell growth. Cell Death Dis 2019; 10(5): 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bai JY, Jin B, Ma JB, et al. HOTAIR and androgen receptor synergistically increase GLI2 transcription to promote tumor angiogenesis and cancer stemness in renal cell carcinoma. Cancer Lett 2021; 498: 70–79. [DOI] [PubMed] [Google Scholar]
- 134. Zhai W, Sun Y, Guo C, et al. LncRNA-SARCC suppresses renal cell carcinoma (RCC) progression via altering the androgen receptor(AR)/miRNA-143-3p signals. Cell Death Differ 2017; 24(9): 1502–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhai W, Sun Y, Jiang M, et al. Differential regulation of LncRNA-SARCC suppresses VHL-mutant RCC cell proliferation yet promotes VHL-normal RCC cell proliferation via modulating androgen receptor/HIF-2alpha/C-MYC axis under hypoxia. Oncogene 2017; 36(31): 4525. [DOI] [PubMed] [Google Scholar]
- 136. Huang Q, Sun Y, Ma X, et al. Androgen receptor increases hematogenous metastasis yet decreases lymphatic metastasis of renal cell carcinoma. Nat Commun 2017; 8(1): 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wang K, Sun Y, Tao W, et al. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett 2017; 394: 1–12. [DOI] [PubMed] [Google Scholar]
- 138. Chen Y, Sun Y, Rao Q, et al. Androgen receptor (AR) suppresses miRNA-145 to promote renal cell carcinoma (RCC) progression independent of VHL status. Oncotarget 2015; 6(31): 31203–31215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007; 129(7): 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yuan C, Ning Y, Pan Y. Emerging roles of HOTAIR in human cancer. J Cell Biochem 2020; 121(5–6): 3235–3247. [DOI] [PubMed] [Google Scholar]
- 141. Hamilton MJ, Young M, Jang K, et al. HOTAIRM1 lncRNA is downregulated in clear cell renal cell carcinoma and inhibits the hypoxia pathway. Cancer Lett 2020; 472: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu Z, Wang Z, Wang X, et al. Long noncoding RNA HOTTIP serves as an independent predictive biomarker for the prognosis of patients with clear cell renal cell carcinoma. Int J Genomics 2020; 2020: 4301634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hu G, Dong B, Zhang J, et al. The long noncoding RNA HOTAIR activates the Hippo pathway by directly binding to SAV1 in renal cell carcinoma. Oncotarget 2017; 8(35): 58654–58667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hong Q, Li O, Zheng W, et al. LncRNA HOTAIR regulates HIF-1alpha/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis 2017; 8(5): e2772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145. Pan Y, Wu Y, Hu J, et al. Long noncoding RNA HOTAIR promotes renal cell carcinoma malignancy through alpha-2, 8-sialyltransferase 4 by sponging microRNA-124. Cell Prolif 2018; 51(6): e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhou J, Zhu G, Huang J, et al. Non-canonical GLI1/2 activation by PI3K/AKT signaling in renal cell carcinoma: a novel potential therapeutic target. Cancer Lett 2016; 370(2): 313–323. [DOI] [PubMed] [Google Scholar]
- 147. Kent OA, Chivukula RR, Mullendore M, et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev 2010; 24(24): 2754–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chen X, Guo X, Zhang H, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene 2009; 28(10): 1385–1392. [DOI] [PubMed] [Google Scholar]
- 149. Yoshino H, Enokida H, Itesako T, et al. Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in renal cell carcinoma. Cancer Sci 2013; 104(12): 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang H, Pu J, Qi T, et al. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene 2014; 33(3): 387–397. [DOI] [PubMed] [Google Scholar]
- 151. Huang Q, Sun Y, Zhai W, et al. Androgen receptor modulates metastatic routes of VHL wild-type clear cell renal cell carcinoma in an oxygen-dependent manner. Oncogene 2020; 39(43): 6677–6691. [DOI] [PubMed] [Google Scholar]
- 152. Lee GT, Han CS, Kwon YS, et al. Intracrine androgen biosynthesis in renal cell carcinoma. Br J Cancer 2017; 116(7): 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lee KH, Kim BC, Jeong SH, et al. Histone demethylase LSD1 regulates kidney cancer progression by modulating androgen receptor activity. Int J Mol Sci 2020; 21(17): 6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Adelaiye-Ogala R, Damayanti NP, Orillion AR, et al. Therapeutic targeting of sunitinib-induced AR phosphorylation in renal cell carcinoma. Cancer Res 2018; 78(11): 2886–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li JJ, Weroha SJ, Davis MF, et al. ER and PR in renomedullary interstitial cells during Syrian hamster estrogen-induced tumorigenesis: evidence for receptor-mediated oncogenesis. Endocrinology 2001; 142(9): 4006–4014. [DOI] [PubMed] [Google Scholar]
- 156. Kirkman H, Bacon RL. Malignant renal tumors in male hamsters (Cricetus auratus) treated with estrogen. Cancer Res 1950; 10(2): 122–124. [PubMed] [Google Scholar]
- 157. Reznik-Schuller H. Carcinogenic effects of diethylstilbestrol in male Syrian golden hamsters and European hamsters. J Natl Cancer Inst 1979; 62(4): 1083–1088. [PubMed] [Google Scholar]
- 158. Antonio P, Gabaldon M, Lacomba T, et al. Effect of the antiestrogen nafoxidine on the occurrence of estrogen-dependent renal tumors in hamster. Horm Metab Res 1974; 6(6): 522–524. [DOI] [PubMed] [Google Scholar]
- 159. Bhat HK, Calaf G, Hei TK, et al. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci U S A 2003; 100(7): 3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Stefaniak T, Krajewski J, Kobiela J, et al. Protein oxidation in male Syrian hamster kidney during estrogen-induced carcinogenesis. Pathophysiology 2002; 8(4): 269–273. [DOI] [PubMed] [Google Scholar]
- 161. Papac RJ, Keohane MF. Hormonal therapy for metastatic renal cell carcinoma combined androgen and provera followed by high dose tamoxifen. Eur J Cancer 1993; 29A(7): 997–999. [DOI] [PubMed] [Google Scholar]
- 162. Wada T, Nishiyama K, Maeda M, et al. Combined chemoendocrine treatment with tegafur and tamoxifen for advanced renal cell carcinoma. Anticancer Res 1995; 15(4): 1581–1584. [PubMed] [Google Scholar]
- 163. Henriksson R, Nilsson S, Colleen S, et al. Survival in renal cell carcinoma – a randomized evaluation of tamoxifen vs interleukin 2, alpha-interferon (leucocyte) and tamoxifen. Br J Cancer 1998; 77(8): 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Harris DT. Hormonal therapy and chemotherapy of renal-cell carcinoma. Semin Oncol 1983; 10(4): 422–430. [PubMed] [Google Scholar]
- 165. Bloom HJ. Medroxyprogesterone acetate (Provera) in the treatment of metastatic renal cancer. Br J Cancer 1971; 25(2): 250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bloom HJ, Wallace DM. Hormones and the kidney: possible therapeutic role of testosterone in a patient with regression of metastases from renal adenocarcinoma. Br Med J 1964; 2(5407): 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16(10): 626–638. [DOI] [PubMed] [Google Scholar]
- 168. Wang S, Cowley LA, Liu XS. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules 2019; 24(18): 3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Botticelli A, Onesti CE, Zizzari I, et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017; 8(59): 99336–99346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 2018; 19(6): 737–746. [DOI] [PubMed] [Google Scholar]
- 171. Pinto JA, Vallejos CS, Raez LE, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018; 3(3): e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Graham J, Abdel-Rahman O, Choueiri TK, et al.; International mRCC Database Consortium. Re: Conforti Fabio, Pala Laura, Bagnardi Vincenzo, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 2018; 19: 737–746: outcomes of metastatic renal cell carcinoma by gender: contrasting results from the international mRCC database consortium. Eur Urol 2018; 74(6): e139–e140. [DOI] [PubMed] [Google Scholar]
- 173. Hassler MR, Abufaraj M, Kimura S, et al. Impact of patients’ gender on efficacy of immunotherapy in patients with metastatic kidney cancer: a systematic review and meta-analysis. Clin Genitourin Cancer 2020; 18(2): 88–94.e2. [DOI] [PubMed] [Google Scholar]
- 174. Yanagisawa T, Kawada T, Quhal F, et al. Impact of sex on the efficacy of immune checkpoint inhibitors in kidney and urothelial cancers: a systematic review and meta-analysis. World J Urol 2023; 41(7): 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Nemoto Y, Ishihara H, Nakamura K, et al. Impact of sex on prognosis in patients with advanced renal cell carcinoma treated with immune checkpoint inhibitors. Jpn J Clin Oncol 2023; 53(7): 611–618. [DOI] [PubMed] [Google Scholar]
- 176. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin 2020; 70(2): 86–104. [DOI] [PubMed] [Google Scholar]
- 177. Pala L, De Pas T, Catania C, et al. Sex and cancer immunotherapy: current understanding and challenges. Cancer Cell 2022; 40(7): 695–700. [DOI] [PubMed] [Google Scholar]
- 178. Wang J, Ma Y, Lin H, et al. Predictive biomarkers for immune-related adverse events in cancer patients treated with immune-checkpoint inhibitors. BMC Immunol 2024; 25(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Unger JM, Vaidya R, Albain KS, et al. Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J Clin Oncol 2022; 40(13): 1474–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wang S, Zhang J, He Z, et al. The predictive power of tumor mutational burden in lung cancer immunotherapy response is influenced by patients’ sex. Int J Cancer 2019; 145(10): 2840–2849. [DOI] [PubMed] [Google Scholar]
- 181. Ye Y, Jing Y, Li L, et al. Sex-associated molecular differences for cancer immunotherapy. Nat Commun 2020; 11(1): 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ozdemir BC, Dotto GP. Sex hormones and anticancer immunity. Clin Cancer Res 2019; 25(15): 4603–4610. [DOI] [PubMed] [Google Scholar]
- 183. Polanczyk MJ, Hopke C, Vandenbark AA, et al. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 2006; 84(2): 370–378. [DOI] [PubMed] [Google Scholar]
- 184. Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy 2004; 3(1): 97–104. [DOI] [PubMed] [Google Scholar]
- 185. Koh YT, Gray A, Higgins SA, et al. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 2009; 69(6): 571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wang L, Jiang G, Jing N, et al. Downregulating testosterone levels enhance immunotherapy efficiency. Oncoimmunology 2021; 10(1): 1981570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. De Martinis M, Sirufo MM, Suppa M, et al. Sex and gender aspects for patient stratification in allergy prevention and treatment. Int J Mol Sci 2020; 21(4): 1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ma Z, Shuai Y, Gao X, et al. Circular RNAs in the tumour microenvironment. Mol Cancer 2020; 19(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Del Vecchio F, Lee GH, Hawezi J, et al. Long non-coding RNAs within the tumour microenvironment and their role in tumour-stroma cross-talk. Cancer Lett 2018; 421: 94–102. [DOI] [PubMed] [Google Scholar]