Abstract
NAD+ and its metabolites serve important functions in intracellular signalling. NAD+-mediated regulatory processes also take place on the cell surface, particularly of immune cells. In this issue of the Biochemical Journal, Gerth et al. have demonstrated a new mechanism of Ca2+ uptake into monocytes which is triggered by NAD+ or its degradation product, ADP-ribose. These observations point to a hitherto unknown Ca2+-influx mechanism and underscore the potential significance of NAD+ and ADP-ribose as signalling molecules on the extracellular side of the plasma membrane.
Keywords: ADP-ribose, calcium, ion transport, immune cell, NAD+, pyridine nucleotide
From most textbooks of biochemistry one may still get the impression that the pyridine nucleotides have been invented just to make our lives a bit easier – they mediate the majority of redox reactions as cofactors (and there are few alternatives). Moreover, pyridine nucleotides contain an AMP moiety, which is also part of ATP. Therefore efforts to memorize the structures of NAD(P) can be limited to nicotinamide mononucleotide, the other half of these molecules. However, as a result of research over the past 15–20 years a far more complicated picture has emerged also placing NAD(P)+ at key positions in cellular signal transduction [1]. Most remarkably, NAD(P)+ undergoes structural conversions in these regulatory pathways, including the generation of new molecules [among them, cADPR (cyclic ADP-ribose), O-acetyl ADP-ribose and NAADP (nicotinic acid–adenine dinucleotide phosphate)]; compounds that are not known to participate in any other biochemical pathway.
So far, most of the NAD+-dependent signalling processes have been shown to contribute to the control of intracellular events. For example, poly-ADP ribosylation takes place primarily in the nucleus and regulates DNA repair, transcription, apoptosis and other fundamental cellular processes. The NAD+-derivative cADPR activates Ca2+ release from the endoplasmic reticulum and thereby Ca2+-dependent pathways. On the other hand, although also present within cells, mono-ADP-ribosylation appears to be largely an extracellular mechanism of NAD+-mediated regulation. All identified ARTs (mono-ADP-ribosyl transferases) of higher eukaryotes are either GPI (glycosylphosphatidylinositol)-anchored or secreted [2,3]. These enzymes transfer the ADPR (ADP-ribose) moiety of NAD+ on to acceptor proteins, thereby modulating their biological activities. ARTs are apparently primarily expressed in blood cells and may contribute to immune functions by modifying proteins, for example, of the extracellular matrix [2,3]. Surprisingly, the known mammalian enzymes catalysing the synthesis of cADPR, designated ADP-ribosyl cyclases, are also located on the cell surface (again, primarily of blood cells). This is unexpected inasmuch as the generated messenger, cADPR, has an intracellular function. Despite appreciable efforts, so far no mammalian intracellular ADP-ribosyl cyclase has been characterized at the molecular level. Several possibilities have been invoked to resolve this apparent contradiction. For example, the best-studied mammalian ADP-ribosyl cyclase, CD38, has been proposed to be internalized upon stimulation [4,5]. Alternatively, transport systems may exist which permit entry of the synthesized cADPR into cells [6]. To add further complication, CD38 (as well as other known ADP-ribosyl cyclases), besides generating the cyclic molecule, cADPR, also produces ADPR by simple hydrolytic cleavage of the nicotinamide moiety from NAD+. In fact, at least in the test tube, this is by far the predominant reaction. Some evidence has been presented indicating that ADPR may also have a regulatory role.
In this issue of the Biochemical Journal, Gerth et al. [7] have reported observations suggesting the existence of yet another mechanism which involves extracellular NAD+ and ADPR as signal transducers. They show that these nucleotides, when added to human monocytes, mediate a transient rise of the intracellular Ca2+ concentration. Moreover, they demonstrated that the increase of the cytosolic Ca2+ concentration is brought about by Ca2+ influx from the medium and is independent of release from intracellular stores. Given the complexity of NAD+-converting processes on the surface of immune cells (Figure 1; besides being a substrate for ARTs and CD38, NAD+ can also be cleaved by phosphodiesterase to AMP and NMN), it is not an easy task to verify that the effect is direct and not mediated by any of these enzyme activities. The authors [7] excluded the involvement of CD38 using a potent inhibitor (2′-deoxy-2′-fluoroarabinoside), which did not alter the Ca2+ uptake initiated by NAD+ or ADPR. Therefore it is most likely that NAD+ acted directly, without first being cleaved to ADPR. Based on previous work, they also eliminated the possibility of significant degradation of NAD+ or ADPR (e.g. into ADP or AMP), at least for the time frame of the experiments. As discussed in their paper [7], the authors cannot rule out the involvement of ADP-ribosylation in the Ca2+-uptake mechanism; however, this possibility appears rather unlikely. So, what is it then?
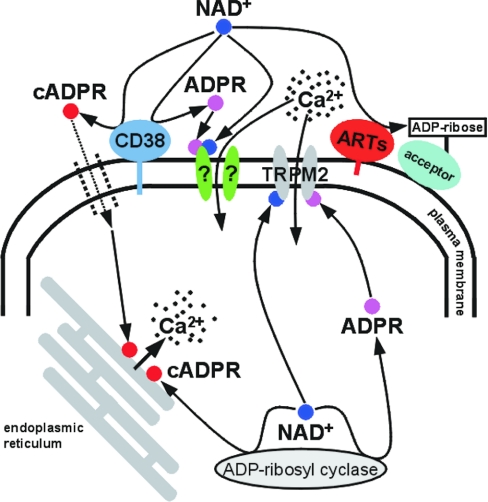
Figure 1. NAD+ and its derivatives as intra- and extra-cellular Ca2+-mobilizing molecules.
As well as serving as a substrate for ARTs, extracellular NAD+ converted into cADPR by membrane-associated CD38 might elicit Ca2+ release from intracellular stores following uptake into the cell. The same mechanism is supposed to be activated by cADPR generated by yet uncharacterized ADP-ribosyl cyclase(s) within the cell. Ca2+ entry from the extracellular fluid may be triggered by intracellular NAD+ and its derivative, ADPR, by acting on TRPM2. In this issue of the Biochemical Journal Gerth et al. [7] propose a similar mechanism, however, triggered by extracellular NAD+ or ADPR operating on a different, still to be identified, ligand-gated Ca2+ channel.
Interestingly, Ca2+ influx into immunocytes via the transient receptor potential channel TRPM2 (formerly termed LTRP2) has been demonstrated previously, and was found to be initiated by intracellular NAD+ or ADPR [8,9]. That is, a very similar transient Ca2+-influx phenomenon is triggered by the same compounds, but on the opposite (internal) side of the plasma membrane. This raises the question of whether NAD+ and ADPR could have been taken up by the cells (although it is not clear how this occurs) and then promoted Ca2+ influx by activating TRPM2. Indeed, following addition of NAD+ or ADPR to the monocytes, Ca2+ influx starts only after a lag phase of 20–30 s. It is argued by Gerth et al. [7] that, in contrast with TRPM2, the observed Ca2+ uptake can also be initiated by NADP+. However, the potential presence of phosphatases on the surface of monocytes would weaken this notion. Another Ca2+ conductance, recently described for rat peritoneal macrophages, shares several characteristics with TRPM2, particularly, the activation by intracellular NAD+ and ADPR [10]. In this case, the effective concentration of ADPR (10–100 μM) appeared to be at least ten times lower than that of NAD+. Gerth et al. [7] reported that the Ca2+ influx in monocytes was similar when triggered by either NAD+ or ADPR at 200 μM. While the physiological concentrations of these nucleotides in the extracellular fluids are unlikely to reach these levels, it may be possible that ADPR could be active at a far lower concentration. The fura 2-based measurement of intracellular Ca2+ used in their study [7] precluded the application of reduced pyridine nucleotides (NADH and NADPH), which also fluoresce when excited at 340 nm. Consequently, there is a possibility that, perhaps, NADH might also initiate the described Ca2+ uptake.
The potential function of ADPR in regulatory events has long escaped detection. The molecule had been well known before cADPR or ADP-ribosylation processes and was regarded as an ordinary degradation product of NAD+ with no particular biological function. Only recent reports [8–10] indicate that ADPR may serve as activator of Ca2+ channels (see above). The detection of a Ca2+-influx mechanism triggered by extracellular ADPR clearly underscores the potential role of this molecule for signalling events.
The study by Gerth et al. [7] has provided convincing evidence to support the existence of a plasma membrane Ca2+-uptake mechanism which is triggered by extracellular NAD+ or ADPR. It shares some intriguing similarities with TRPM2-mediated Ca2+ influx, except the binding sites for the ligands are most likely located on opposite sides of the plasma membrane. Consequently, the newly reported Ca2+-influx system would possess a distinct property: the ligand (NAD+ or ADPR) would bind to the outer surface of the plasma membrane, similar to Ca2+, which is then transported across the membrane (Figure 1). In any case, Ca2+ uptake by this mechanism is the result of an extracellular event, as opposed to the TPRM2-mediated cytosolic Ca2+ increase. No doubt, this novel pathway for Ca2+ to enter cells will attract attention, but we will also keep watching how NAD+-mediated regulatory events gain entry into the textbooks.
References
- 1.Berger F., Ramírez-Hernández M. H., Ziegler M. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem. Sci. 2004;29:111–118. doi: 10.1016/j.tibs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki I. J., Moss J. Glycosylphosphatidylinositol-anchored and secretory isoforms of mono-ADP-ribosyltransferases. J. Biol. Chem. 1998;273:23617–23620. doi: 10.1074/jbc.273.37.23617. [DOI] [PubMed] [Google Scholar]
- 3.Glowacki G., Braren R., Firner K., Nissen M., Kuhl M., Reche P., Bazan F., Cetkovic-Cvrlje M., Leiter E., Haag F., Koch-Nolte F. The family of toxin-related ecto-ADP-ribosyltransferases in humans and the mouse. Protein Sci. 2002;11:1657–1670. doi: 10.1110/ps.0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funaro A., Reinis M., Trubiani O., Santi S., Di Primio R., Malavasi F. CD38 functions are regulated through an internalization step. J. Immunol. 1998;160:2238–2247. [PubMed] [Google Scholar]
- 5.Zocchi E., Usai C., Guida L., Franco L., Bruzzone S., Passalacqua M., De Flora A. Ligand-induced internalization of CD38 results in intracellular Ca2+ mobilization: role of NAD+ transport across cell membranes. FASEB J. 1999;13:273–283. doi: 10.1096/fasebj.13.2.273. [DOI] [PubMed] [Google Scholar]
- 6.Guida L., Franco L., Bruzzone S., Sturla L., Zocchi E., Basile G., Usai C., De Flora A. Concentrative influx of functionally active cyclic ADP-ribose in dimethyl sulfoxide-differentiated HL-60 cells. J. Biol. Chem. 2004;279:22066–22075. doi: 10.1074/jbc.M314137200. [DOI] [PubMed] [Google Scholar]
- 7.Gerth A., Nieber K., Oppenheimer N. J., Hauschildt S. Extracellular NAD+ regulates intracellular free calcium concentration in human monocytes. Biochem. J. 2004;382:849–856. doi: 10.1042/BJ20040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sano Y., Inamura K., Miyake A., Mochizuki S., Yokoi H., Matsushime H., Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 9.Perraud A. L., Fleig A., Dunn C. A., Bagley L. A., Launay P., Schmitz C., Stokes A. J, Zhu Q., Bessman M. J., Penner R., et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature (London) 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 10.Campo B., Surprenant A., North R. A. Sustained depolarization and ADP-ribose activate a common ionic current in rat peritoneal macrophages. J. Immunol. 2003;170:1167–1173. doi: 10.4049/jimmunol.170.3.1167. [DOI] [PubMed] [Google Scholar]