Abstract
Background:
Central nervous system (CNS) relapse in non-Hodgkin lymphoma (NHL) is a rare but serious complication that carries a poor prognosis. The use of infusional etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R) for frontline treatment of diffuse large B cell lymphoma (DLBCL) is increasing, though little is known about incidence of and risk factors for CNS relapse with this regimen
Patients and methods:
We completed a chart review of patients with NHL who received EPOCH-R as front line therapy. Data obtained included baseline and treatment characteristics including if patients received CNS directed therapy. We measured overall survival (OS), progression free survival (PFS), and progression to CNS involvement.
Results:
We identified 223 patients who met the inclusion criteria, 72% had DLBCL. Of all the patients, 5.8% experienced CNS relapse, and 38.6% were treated with CNS prophylaxis. There was no difference in rate of CNS relapse, OS, or PFS between patients who had and had not received CNS prophylaxis. Patients whose serum lactate dehydrogenase was greater than twice the upper limit of normal at diagnosis and those with extranodal disease were significantly more likely to have CNS relapse (P = .0247 and 0.022, respectively) than their counterparts.
Conclusions:
The rate of CNS relapse in this patient population approaches 6%, not significantly different from reports on those receiving R-CHOP. The results of this study suggest that CNS prophylaxis might be more selectively used among patients treated with EPOCH-R with certain high-risk features.
1 |. INTRODUCTION
Although the incidence of central nervous system (CNS) relapse in non-Hodgkin lymphoma (NHL) patients is low at 2%−9%, prognosis is poor and the outcome is almost universally fatal with a median overall survival (OS) of <6 months.1–6 Several risk factors have been associated with a higher risk of CNS relapse in NHL,7 including Burkitt and lymphoblastic histologies,8 elevated serum lactate dehydrogenase (LDH) levels, presence of extranodal disease, and increased international prognostic index (IPI) scores.6,9,10 Treatment options for CNS relapse are limited, and include intrathecal (IT) chemotherapy with methotrexate or cytarabine, high-dose intravenous (IV) methotrexate, or palliative radiotherapy.5,11,12 New therapeutic options have been suggested with some improvement in outcomes,13 but prevention of CNS disease remains the ultimate objective. Some studies suggest the addition of high dose IV methotrexate or cytarabine is superior to IT chemotherapy at preventing CNS relapse, but data is not conclusive.14 Given the above, effective CNS-directed prophylactic measures in NHL patients are needed, but the optimal approach has yet to be defined.15
Notably, almost all recent data regarding CNS treatment and prophylaxis have been generated from studies in which diffuse large B cell lymphoma (DLBCL) patients received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as frontline therapy. With increased appreciation of features associated with failure of R-CHOP, more patients are now being treated with etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R). In fact, EPOCH-R is arguably the treatment of choice for patients with primary mediastinal lymphoma and double hit lymphomas.16–20 A phase III randomized trial comparing R-CHOP to EPOCH-R in DLBCL has completed accrual, and early data demonstrated no difference in event free survival or OS, but effect on CNS relapse as well as subset analyses are still pending (NCT00118209). Importantly, data on the frequency and predictors of CNS events in patients treated with EPOCH-R are very limited; moreover, no data are available on the efficacy of CNS prophylaxis in these patients. Data for patients with AIDS related lymphomas showed no improvement in CNS outcomes with use of EPOCH-R,21 though these results are not widely applicable to all patients with NHL. As this regimen is increasingly used in DLBCL, a better understanding of the utility of CNS prophylaxis in this population is needed.
Accordingly, we analyzed NHL patients who were treated with frontline EPOCH-R with the objectives of (1) determining the rates and predictors of CNS events in these patients, and (2) to study outcomes of patients receiving CNS prophylaxis as they compare to those not receiving prophylaxis.
2 |. METHODS
2.1 |. Chart review
We completed a chart review of 223 patients who received EPOCH-R as front line therapy for NHL between 2004 and 2014. Key inclusion criteria included: diagnosis of NHL confirmed by biopsy, age >18 years, no other first line therapy prior to EPOCH-R, at least 2 cycles of EPOCH-R received, no consolidation with hematopoietic stem cell transplantation, and no known evidence of CNS involvement at the time of diagnosis. All patients included with Richter transformation or transformed follicular lymphoma underwent transformation prior to therapy. Because of the retrospective nature of this study, employment of CNS prophylaxis was strictly at the discretion of the treating physician, and therefore potentially subject to personal preference or institutional guidelines. Data obtained were: age, histology, Eastern Cooperative Oncology Group (ECOG) performance status, presence of B symptoms, white blood cell count, serum LDH, HIV status, extranodal (EN) sites of involvement, bone marrow status, and stage. Initial and subsequent staging was based on the treating physician’s assessment. Data on treatment included number of cycles received and CNS directed therapy if applicable. We measured OS, progression free survival (PFS), and progression to CNS involvement starting from the date of diagnosis. CNS relapse was defined as any disease progression involving the CNS, parenchymal or leptomeningeal, in the presence or absence of systemic progression. The study was approved by the IRB of each participating institution (Northwestern University, the University of Iowa, the University of Chicago, and the Mayo Clinic).
2.2 |. Statistical analysis
Two-tailed Fisher’s exact test was used to compare the subgroups of patients who received CNS prophylaxis to those who did not based on baseline features. Two-tailed Fisher’s exact test was also used to determine whether any of the following baseline features was associated with risk of CNS relapse: age, histology, ECOG performance status, presence of B symptoms, white blood cell count, serum LDH, HIV status, EN sites of involvement, bone marrow status, and stage. A P-value of <.05 was considered statistically significant. We compared, using Kaplan-Meier survival curves with log-rank analyses, the patterns of OS and PFS between patients receiving CNS prophylaxis and those who did not receive it, as well as OS for patients who experienced CNS relapse.22 All analyses were performed with Prism (Graphpad Software, San Diego, CA) and SAS (Cary, NC).
3 |. RESULTS
3.1 |. Patients characteristics
We identified 223 patients with a median age at diagnosis of 54 (range 18–83), of whom 61.9% were males. Other patients’ characteristics are noted in Table 1. Notably, 72% had DLBCL; 68% had ECOG PS 0–1; 74.9% had LDH above upper limit of normal (ULN); 35.0% had 2 or more EN sites; and 71.3% had Stage III-IV disease at time of diagnosis.
TABLE 1.
Baseline characteristics of all patients
| Total patients | 223 |
|---|---|
| Males | 138 (61.9%) |
| Median age at diagnosis | 54 (range 18–83) |
| Patients ≥ 60-years old at diagnosis | 79 (35.4%) |
| ECOG PS 0 or 1 | 151 (67.7%) |
| ECOG PS ≥ 2 | 72 (32.3%) |
| Elevated LDH | 167 (74.9%) |
| > 1 site of EN disease | 78 (35.0%) |
| Stage I or II disease | 64 (28.7%) |
| Stage III or IV disease | 159 (71.3%) |
| Histologic diagnosis | |
| DLBCL | 161 (72.2%) |
| FL | 5 (2.2%) |
| Richter transformation | 12 (5.4%) |
| PMBCL | 24 (10.8%) |
| Transformed from FL | 5 (2.2%) |
| Gray zone | 7 (3.1%) |
| Received CNS prophylaxis | 86 (38.6%) |
| IT methotrexate | 83 |
| IT cytarabine | 2 |
| IT methotrexate plus cytarabine | 1 |
3.2 |. Use of CNS prophylaxis
Of all patients, 38.6% received CNS prophylaxis, most using IT methotrexate but 2 were treated with IT cytarabine and 1 patient was given both methotrexate and cytarabine. Patients who had a baseline IPI score of 0 or 1 or with stage I or II disease were significantly less likely to receive CNS prophylaxis (P < .001 for both). There was no significant difference in use of CNS prophylaxis in patients with other baseline characteristics including presence of B symptoms, bone marrow involvement, baseline ECOG performance status, or LDH 2 times above the ULN (Table 2).
TABLE 2.
Comparison of patients who received CNS prophylaxis and those who did not
| Received CNS ppx | No CNS ppx | P value | |
|---|---|---|---|
| Total | 86 (38.6%) | 137 (61.4%) | |
| CNS relapses, n (% [95% CI]) | 5 (5.8%, [3.0–13.0]) | 8 (5.8%, [3.0–11]) | 1.00 |
| Male | 55 (39.8%) | 83 (60.1%) | .6718 |
| Female | 31 (36.4%) | 54 (63.5%) | |
| Median age at diagnosis | 54 | 54 | |
| Patients ≥ 60-years old | 35 (40.1%) | 54 (39.4%) | .8887 |
| ECOG PS 0 or 1 | 55 (64.0%) | 90 (65.7%) | .8854 |
| ECOG PS ≥ 2 | 31 (36.0%) | 47 (34.3%) | |
| Histologic diagnosis | |||
| DLBCL | 72 (83.7%) | 89 (64.9%) | |
| FL | 2 (2.3%) | 3 (2.2%) | |
| Richter | 1 (1.0%) | 11 (8.0% | |
| PMBCL | 2 (2.3%) | 22 (16.0%) | |
| Transformed from FL | 4 (4.7%) | 1 (0.7%) | |
| Gray zone | 2 (2.3%) | 5 (3.6%) | |
| Elevated LDH | 68 (79.1%) | 103 (75.2%) | .5209 |
| LDH/ULN >2 | 27 (31.4%) | 41 (29.9%) | .8815 |
| Stage I or II disease | 7 (8.1%) | 57 (41.6%) | <.001 |
| Stage III or IV disease | 79 (91.9%) | 80 (58.4%) | |
| IPI score 0–1 | 10 (11.6%) | 52 (37.9%) | <.001 |
| IPI score ≥ 2 | 76 (88.4%) | 85 (62.0%) | |
| B symptoms | 33 (38.4%) | 63 (46.0%) | .2707 |
| Bone marrow involvement | 18 (32.1%) | 18 (13.1%) | .1374 |
| 24-month OS | 78.7% | 81.1% | .7323 |
| 48-month OS | 69.7% | 74.1% | .4455 |
| 24-month PFS | 75.8% | 73.0% | .7544 |
| 48month PFS | 61.2% | 67.6% | .386 |
3.3 |. Outcomes
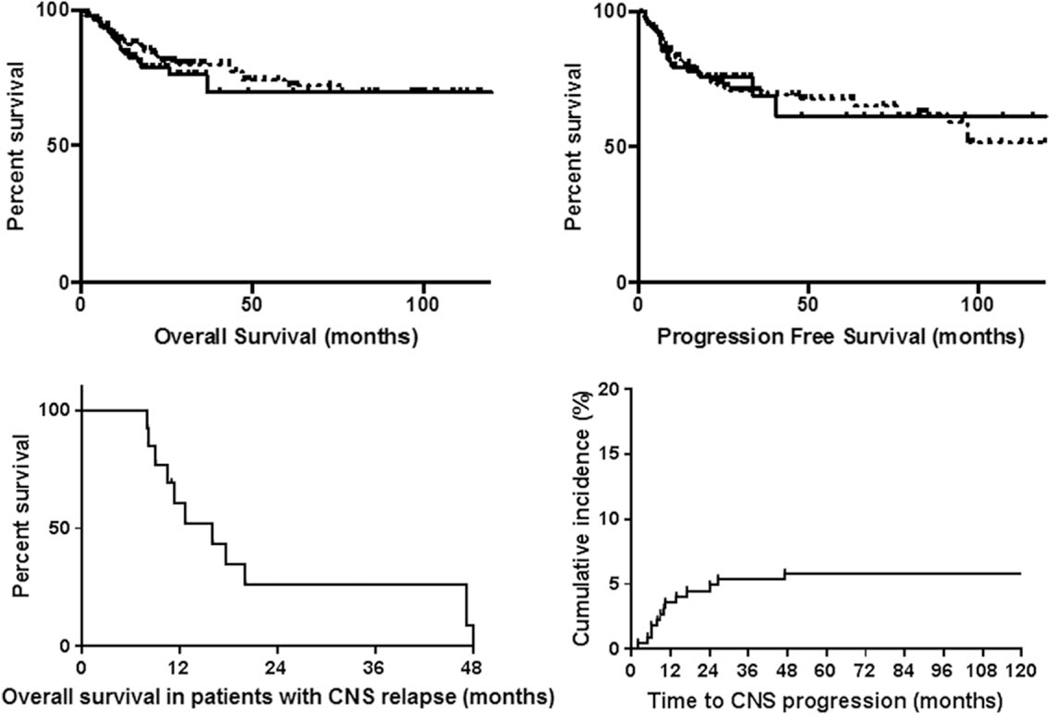
The 24- and 48-month OS for all patients were 80.0% and 72.6%, respectively. The 24-month OS for patients who received CNS prophylaxis was 78.7% compared with 81.1% (P = .7323); the 48-month OS were 69.7% and 74.1% (P = .4455), respectively (Figure 1). The 24 and 48 month PFS for all patients were 73.7% and 66.9%, respectively. The 24-month PFS for patients receiving CNS prophylaxis was 75.8% compared with 73.0% for those who did not (P = .7544); the 48-month PFS were 61.2% and 67.6% (P = .386), respectively (Figure 1).
FIGURE 1.
Top left: OS in NHL patients treated with first line dose adjusted EPOCH plus rituximab who received CNS prophylaxis compared with those who did not. Top right: PFS in NHL patients treated with first line dose adjusted EPOCH plus rituximab who received CNS prophylaxis compared with those who did not. In the top 2 figures, the solid line represents patient who received CNS prophylaxis and the dotted line represents those who did not. Bottom left: OS in NHL patients treated with first line dose adjusted EPOCH plus rituximab who experienced CNS relapse. Bottom right: Time to CNS progression in NHL patients treated with first line dose adjusted EPOCH plus rituximab
Of the patients who received CNS prophylaxis with IT methotrexate, 5 experienced CNS relapse (5.8%). Of the patients who did not receive CNS prophylaxis, 8 experienced CNS relapse (5.8%). There was no difference in the rate of CNS relapse between patients who did or did not receive CNS prophylaxis (P = 1.00). Of the 13 CNS relapses we observed, four had parenchymal involvement, 5 had leptomeningeal involvement, and 1 had both parenchymal and leptomeningeal involvement. We were unable to obtain where in the CNS the relapse had occurred for the other 3 patients with CNS relapse. Among patients in whom CNS relapse was observed, 7 had a complete response to front line therapy and 2 had a partial response. We do not have data for the other 4 patients who experienced CNS relapse. Patients whose LDH was greater than twice the ULN at diagnosis and those with EN disease were significantly more likely to have CNS relapse (P = .0247 and .022, respectively) than their counterparts. No other clinical or disease-related factors were associated with CNS relapse in our cohort. There was no difference identified in rates of CNS relapse among patients with more than 1 site of EN disease at time of diagnosis and patients who had 1 or no sites of EN disease, though there was a trend toward significance (P = .0675). We did not identify any particular sites of EN involvement that had a significant difference in CNS relapse (Table 3). The median OS and PFS for patients who had CNS relapse were 12.5 and 8.9 months, respectively (Figure 1). The median time to CNS progression was 10 months (range 2.1–27.0 months) (Figure 1). A cox regression analysis was undertaken to identify any risk factors for CNS relapse in multivariate fashion. This failed to identify any statistically significant factors (P < .05).
TABLE 3.
Comparison of patients who experienced CNS relapse to patients who did not
| All patients (n = 223) | CNS relapse (n = 13) | No CNS relapse (n = 210) | P value (CNS relapse vs. No CNS relapse | |
|---|---|---|---|---|
| Male | 138 (61.8%) | 7 (53.8%) | 131 (62.3%) | 0.5659 |
| Female | 85 (38.1%) | 6 (46.2%) | 79 (37.6%) | |
| Median age | 54 | 52 | 55 | |
| Patient ≥ 60 years old | 79 (35.4%) | 4 (30.8%) | 75 (33.3%) | 1.0000 |
| ECOG PS 0 or 1 | 151 (67.7%) | 7 (53.8%) | 144 (68.6%) | 0.3589 |
| ECOG PS ≥ 2 | 72 (32.3%) | 6 (46.2%) | 66 (31.4%) | |
| HIV positive | 24 (10.8%) | 3 (23.1%) | 21 (10.0%) | 0.1521 |
| Histologic diagnosis | ||||
| DLBCL | 161 (72.2%) | 10 (76.9%) | 151 (71.9%) | |
| FL | 5 (0.9%) | 2 (15.4%) | 3 (1.4%) | |
| Richter | 12 (5.4%) | 0 | 12 (5.7%) | |
| PMBCL | 24 (10.8%) | 0 | 24 (11.4%) | |
| Transformed from FL | 5 (0.9%) | 0 | 5 (2.4%) | |
| Gray zone | 7 (3.1%) | 1 (7.7%) | 6 (2.9%) | |
| Clinical Feature | ||||
| Elevated LDH | 167 (74.9%) | 11 (84.6%) | 156 (74.3%) | 0.5252 |
| LDH/ULN >2 | 68 (30.5%) | 8 (61.5%) | 60 (28.6%) | 0.0247 |
| Stage I or II disease | 64 (28.7%) | 3 (23.1%) | 61 (29.0%) | 0.7618 |
| Stage III or IV disease | 159 (71.3%) | 10 (76.9%) | 149 (71.0%) | |
| IPI score 0–1 | 62 (27.8%) | 3 (23.1%) | 59 (28.1%) | 1.00 |
| IPI score ≥ 2 | 161 (72.2%) | 10 (76.9%) | 151 (71.9%) | |
| B symptoms | 96 (43.0%) | 9 (69.2%) | 87 (41.4%) | 0.0800 |
| Bone marrow involvement | 35 (15.7%) | 4 (30.8%) | 31 (14.8%) | 0.1278 |
| B symptoms and bone marrow involvement | 18 (8.1%) | 3 (23.1%) | 15 (7.1%) | 0.1058 |
| Extranodal Involvement Features | ||||
| >1 site of EN disease | 78 (35.0%) | 8 (61.5%) | 70 (33.3%) | 0.0675 |
| 1 site of EN disease | 81 (36.3%) | 5 (12.3%) | 76 (36.2%) | 1.00 |
| No EN disease | 64 (28.7%) | 0 | 64 (30.4%) | 0.022 |
| GI/hepatic | 53 (23.8%) | 3 (23.1%) | 50 (23.8%) | 1.00 |
| Skeletal | 58 (26.0%) | 5 (38.5%) | 53 (25.2%) | 0.3305 |
| Cutaneous | 9 (4.0%) | 0 | 9 (4.3%) | 1.00 |
| Pulmonary/thorax | 43 (19.3%) | 4 (30.8%) | 39 (18.6%) | 0.2827 |
| Genitourinary | 24 (10.8%) | 3 (23.1%) | 21 (10.0%) | 0.1521 |
| Received CNS prophylaxis | 85 (38.1%) | 5 (38.5%) | 80 (38.1%) | 1.00 |
| Median OS (months) | NR | 12.5 | NR | |
| Median PFS (months) | NR | 8.9 | NR |
NR, not reached.
3.4 |. DLBCL subgroup analysis
A separate subgroup analysis was performed on patients diagnosed with DLBCL, excluding those diagnosed with HIV. Of the 139 patients included, 7 (5%) experienced CNS relapse, 58 (42%) received CNS prophylaxis. There was no statistically significant difference in CNS relapse rates between patients who did and did not receive CNS prophylaxis (P = .6991). There was no difference found in use of CNS prophylaxis in this population based on ECOG performance status, LDH, IPI score, presence of B symptoms, or bone marrow involvement at time of diagnosis. There was no significant difference in OS or PFS (P = .8822 and .7122, respectively) (see Supporting Information Table S4).
4 |. DISCUSSION
In this large series of patients treated with EPOCH-R, we show that CNS prophylaxis did not affect rates of CNS relapse, though it is probable that those who received CNS prophylaxis had a higher baseline risk of CNS relapse. We confirmed that patients who relapse in the CNS have universally poor outcomes. Further, we establish that EN disease and elevated LDH predict CNS relapse analogous to what was previously published in patients treated with R-CHOP.
Our series specifically studied patients treated with EPOCH-R as, to our knowledge, no data on rates of CNS relapse and outcomes have been published in patients treated with this regimen. EPOCH-R is generally used in DLBCL patients with more aggressive disease features than those receiving R-CHOP, though no formal comparisons were made between rates of aggressive disease features in our cohort as compared with those in reported cohorts of patients treated with R-CHOP. In our analysis, 44% of patients had IPI scores of 3 or 4, which suggests a 5-year OS of 46% and 32%, respectively,23 and implies that EPOCH-R are generally offered for high-risk DLBCL patients. Our population also had an 11% incidence of HIV infection. It is important to acknowledge the high risk characteristics of this study population when considering the findings reported here. We were unable to obtain sufficient data to make any conclusions with respect to the impact of cell of origin on outcomes for patients in our cohort.
The overall rate of CNS relapse in patients receiving dose adjusted EPOCH-R was 5.8%, which is similar to what has been previously reported for patients who received R-CHOP.1,5–7 Importantly, we also did not find a significant difference in CNS relapse rates amongst patients who did and did not receive IT CNS prophylaxis, though only 86 patients (38.6%) in this series received CNS prophylaxis, limiting the power of any conclusions. Patients who were selected for prophylaxis were more likely to have a higher IPI score, but we were not able to identify any other factors associated with physician choice to use IT CNS prophylaxis. While all participating institutions used methotrexate as the agent of choice, 1 institution implemented prophylaxis in 55% of patients while the other 2 utilized this strategy in 21% and 24%, respectively. Although this may reflect differences in referral patterns, it also raises a question as to whether more broadly implemented guidelines regarding prophylaxis are warranted.
We identified LDH levels above 2 times the institutional ULN, and presence of extranodal disease, as the 2 baseline factors most associated with risk of CNS relapse. Interestingly, we did not find a significant difference in rates of CNS relapse in patients who had B symptoms, bone marrow involvement, or renal or adrenal extranodal disease as prior studies have suggested for patients receiving R-CHOP and other regimens not containing rituximab.2,10 This could represent a real difference in risk factors for relapse in patients treated with EPOCH-R compared with R-CHOP. It is possible it could be due to an insufficient number of CNS events to detect a difference.
We performed a separate subgroup analysis on the population of patients diagnosed with DLBCL, excluding those diagnosed with HIV. We identified a significantly lower use of CNS prophylaxis in patients with an IPI score of 0–1 (P = .0243), as well as a trend toward significance in higher use of CNS prophylaxis in patients with bone marrow involvement (P = .0892). We found no significant difference in CNS relapse rates, OS, or PFS in patients who did or did not receive CNS prophylaxis in this population.
The CNS IPI score as presented by Schmitz et al.10 has been accepted as a model to predict risk of CNS relapse in patients with DLBCL treated with R-CHOP. It includes the IPI score as well as presence of genitourinary or adrenal diseases to risk stratify patients. We calculated the CNS-IPI score on the subpopulation of DLBCL patients without HIV, and found 6 patients with a CNS-IPI score of 0, 20 patients with a score of 1, 31 patients with a score of 2, 38 patients with a score of 3, 29 patients with a score of 4, 13 patients with a score of 5, and 2 patients with a score of 6. Of the 7 patients in this subgroup who experienced CNS relapse, 2 had a score of 5, 3 had a score of 4, 1 had a score of 3, and 1 had a score of 2. Based on the risk of CNS relapse provided by Schmitz et al., the weighted risk of CNS relapse in this cohort is 5.05%, which correlated with our findings in this group. Supporting Information Table S5 demonstrates the use of CNS prophylaxis stratified by CNS-IPI score.
Our study is not without limitations. The retrospective nature of this analysis led to missing data. The lack of adequate cell of origin data limited our ability to assess whether rates of CNS relapse correlate with cell of origin or any molecular drivers, such as MYC/BCL-2 expression. Furthermore, while the sample size of 223 is relatively large, patients were treated at 4 large academic centers which represents its own selection bias. Another limitation is the heterogenous nature of the diseases included as well as small size of subgroups with involvement of each extranodal organ. Nonetheless, the results of this study suggest that CNS prophylaxis might be more selectively deployed among patients treated with EPOCH-R, particularly those with certain high-risk features. The rate of CNS relapse in this patient population approaches 6%, not significantly different from reports on those receiving R-CHOP. It should also be noted that our effort provides data regarding the use or omission of IT chemotherapy, but this should be not be conflated with any conclusions regarding either the usage of high-dose systemic CNS prophylaxis, or of IT treatment for known CNS disease (as opposed to prophylaxis). Given the poor prognosis of patients who suffer from CNS relapse, novel strategies and clinical trials addressing this issue are urgently needed.
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- [1].Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113: 3896–3902. [DOI] [PubMed] [Google Scholar]
- [2].Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma–a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann. Oncol 2007;18:149–157. [DOI] [PubMed] [Google Scholar]
- [3].Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol. 2004;15:129–133. [DOI] [PubMed] [Google Scholar]
- [4].Levitt LJ, Dawson DM, Rosenthal DS, et al. CNS involvement in the non-Hodgkin’s lymphomas. Cancer. 1980;45:545–552. [DOI] [PubMed] [Google Scholar]
- [5].Arkenau HT, Chong G, Cunningham D, et al. The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Ann. Oncol 2007;18:541–545. [DOI] [PubMed] [Google Scholar]
- [6].Schmitz N, Zeynalova S, Glass B, et al. CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Ann. Oncol 2012;23:1267–1273. [DOI] [PubMed] [Google Scholar]
- [7].Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann. Oncol 2002;13:1099–1107. [DOI] [PubMed] [Google Scholar]
- [8].Cheah CY, Seymour JF. Central nervous system prophylaxis in non-Hodgkin lymphoma: who, what, and when?. Curr. Oncol. Rep 2015; 17:25. [DOI] [PubMed] [Google Scholar]
- [9].van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91:1178–1184. [PubMed] [Google Scholar]
- [10].Schmitz N, Zeynalova S, Nickelsen M, et al. CNS international prognostic index: a risk model for cns relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J. Clin. Oncol 2016;34:3150–3156. [DOI] [PubMed] [Google Scholar]
- [11].Chua SL, Seymour JF, Streater J, et al. Intrathecal chemotherapy alone is inadequate central nervous system prophylaxis in patients with intermediate-grade non-Hodgkin’s lymphoma. Leuk. Lymphoma 2002;43:1783–1788. [DOI] [PubMed] [Google Scholar]
- [12].Ferreri AJ, Bruno-Ventre M, Donadoni G, et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br. J. Haematol 2015;168:654–662. [DOI] [PubMed] [Google Scholar]
- [13].Ferreri AJ, Donadoni G, Cabras MG, et al. High doses of antimetabolites followed by high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation in patients with systemic B-cell lymphoma and secondary CNS involvement: final results of a multicenter Phase II trial. J. Clin. Oncol 2015;33:3903–3910. [DOI] [PubMed] [Google Scholar]
- [14].Cheah CY, Herbert KE, O’rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br. J. Cancer 2014;111:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 – the Southwest Oncology Group. J. Clin. Oncol 2009;27:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilson WH, Jung SH, Porcu P, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Purroy N, Bergua J, Gallur L, et al. Long-term follow-up of dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor prognosis large B-cell lymphoma. A phase II study conducted by the Spanish PETHEMA Group. Br. J. Haematol 2015;169:188–198. [DOI] [PubMed] [Google Scholar]
- [18].Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124: 2354–2361. [DOI] [PubMed] [Google Scholar]
- [19].Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br. J. Haematol 2014; 166:891–901. [DOI] [PubMed] [Google Scholar]
- [20].Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med 2013;368:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barta SK, Joshi J, Mounier N, et al. Central nervous system involvement in AIDS-related lymphomas. Br. J. Haematol 2016;173:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc 1958;53:457–481. [Google Scholar]
- [23].A predictive model for aggressive non-Hodgkin’s lymphoma. The international non-Hodgkin’s lymphoma prognostic factors project. N. Engl. J. Med 1993;329:987–994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.