Abstract
A 15-month-old spayed female greater Swiss mountain dog was brought to our clinic because of relapsing episodes of urinary tract infection, present since her adoption at 2 mo of age. A diagnosis of chronic bacterial cystitis associated with an invasive, biofilm-forming uropathogenic Escherichia coli was made with bladder-wall histology and fluorescent in situ hybridization analysis. Local treatment with EDTA-tromethamine (EDTA-Tris) infusions along with parenteral cefquinome and prophylactic measures (Type-A proanthocyanidins and probiotics) coincided with clinical and bacterial remission. The dog has been free of clinical signs of urinary tract infection for >4 y. Biofilm-forming uropathogenic E. coli can cause chronic, recurrent cystitis due to low antibiotic efficacy and should be considered in cases of recurrent cystitis in dogs, especially in the absence of identified predisposing factors. This case report describes the diagnostic and therapeutic options that were used to manage a case of this type.
Key clinical message:
Fluorescent in situ hybridization analysis may be considered in the diagnosis of chronic bacterial cystitis in dogs, and intravesical instillations of EDTA-Tris may be helpful in managing such cases.
RÉSUMÉ
Traitement adjuvant intravésical avec de l’EDTA-trométhamine chez un chien présentant une cystite récurrente à Escherichia coli formant des biofilms
Une chienne grand bouvier suisse stérilisée de 15 mois nous a été présentée pour des épisodes d’infection du tractus urinaire récidivants depuis son adoption à l’âge de 2 mois. Une cystite bactérienne chronique associée à un Escherichia coli uropathogène formant des biofilms a été identifiée par l’examen histologique de la paroi vésicale et par hybridation in situ fluorescente. Des instillations intravésicales d’EDTA et trométhamine (EDTA-Tris) en complément d’une antibiothérapie parentérale de courte durée (cefquinome) et de mesures prophylactiques (proanthocyanidines de type A et probiotiques) ont permis une guérison clinique et bactériologique de la cystite pendant plus de 4 ans. Les infections par Escherichia coli formant des biofilms peuvent causer des cystites chroniques récurrentes dues à une faible efficacité des antibiotiques et doivent être incluses dans le diagnostic différentiel des cystites récurrentes chez le chien, particulièrement en l’absence d’autre facteur prédisposant. Ce rapport propose des stratégies diagnostiques et thérapeutiques ayant permis la prise en charge d’un de ces cas.
Message clinique clé :
L’analyse par hybridation in situ fluorescente peut être envisagé dans le diagnostic de cystite bactérienne chronique chez les chiens, et l’instillation intravésicale d’EDTA-Tris peut être utile dans la gestion de tels cas.
(Traduit par les auteurs)
Urinary tract infections (UTI) are frequent in both human and veterinary medicine and Escherichia coli is the most commonly isolated bacteria (1,2). Moreover, recurrent UTI are becoming more widely understood and a recent study noted an estimated prevalence of 0.25% in dogs (3). In human medicine, there is growing evidence that some recurrent UTI result from bacterial biofilm production or persistent intracellular bacterial community (IBC) formation (4–6). Such formations make UTI more challenging to treat, as they protect bacteria chemically and mechanically from host-immune response and antibacterial treatments. Indeed, growth in a biofilm provides an exopolysaccharide barrier to diffusion that decreases the effective concentration reaching the protected bacterial cells (5–9). Moreover, the existence of a heterogeneous community of both active and dormant cells is a hallmark of biofilm phenotype. Although antibiotics usually kill most of the active cells, some tolerant, persistent bacteria may remain and repopulate the biofilm, becoming responsible for the recalcitrance of those chronic infections (5–8). Similarly, IBC are structures that possess biofilm-like properties and develop within the cytoplasm of superficial urothelial cells after invasion and intracellular replication by uropathogen Escherichia coli (5–7). Hence, the current standard of care for therapeutic management of bacterial cystitis may not lead to clinical and bacterial cure.
Eradicating those pathogens requires antimicrobials that are effective intracellularly (4). Moreover, any treatment that disrupts biofilm structures, such as EDTA, may help in the dispersion of embedded bacteria and increase the exposure to antimicrobials (9). As a strategy to optimize drug concentrations at the site of infection, intravesical therapy is increasingly used to manage chronic bacterial cystitis in human medicine (4). Bladder infusion of EDTA-tromethamine (EDTA-Tris) in association with systemic antibacterial therapy was described in the veterinary literature > 25 y ago (10). However, the causes of recurrent cystitis with possible implication of biofilm-forming infections were not evaluated in that study.
This case report describes the involvement of a biofilmforming E. coli in a chronic, recurrent UTI in a young female dog and its successful treatment using intravesical instillations of EDTA-Tris as an adjuvant to systemic antibacterial therapy.
CASE DESCRIPTION
A 15-month-old spayed female greater Swiss mountain dog weighing 39.8 kg (86.0 lb) was referred to the National Veterinary School of Toulouse for the management of episodes of bacterial cystitis caused by E. coli infections that were recurrent since her adoption at 2 mo of age.
Initially, clinical signs attributed to the cystitis were pollakiuria, malodorous urine, perivulvar licking, and bouts of urinary incontinence. The primary veterinarian was consulted a few days after the adoption because of these signs. Bacterial culture on urine obtained by cystocentesis was positive for E. coli, which was resistant to 1st-line antibiotics (beta-lactams, 1st-generation cephalosporins and sulfonamides) but susceptible to fluoroquinolones. Marbofloxacin (dosage not reported) was prescribed for 10 d, with a complete clinical response. However, clinical signs reoccurred a few weeks after the end of treatment. This same scenario repeated 5 times until the dog was referred. Exact doses and durations of treatments were not available but multiple courses of marbofloxacin and nitrofurantoin were used for multiple weeks. Urine bacterial cultures were always positive for E. coli when clinical signs recurred, and negative at the end of each treatment course, with intervening subclinical periods ~3 wk in duration. Anatomic causes of recurrent UTI were thoroughly investigated and excluded. Abdominal ultrasound and computed tomography imaging with IV urography were both unremarkable. A cystoscopy was completed before referral, revealing only small bladder mucosal lesions.
The physical examination at the time of referral was unremarkable. The dog was still being treated with antibiotics (nitrofurantoin) and was described by the owners as free from clinical signs. Findings from a complete blood (cell) count, biochemistry panel, and urinalysis (urine-specific gravity, dipstick, and sediment examination) were unremarkable. Abdominal ultrasound and cystoscopy failed to reveal any possible cause for the recurrent UTI, though the urothelium appeared discretely hyperemic, with elevated, pinpoint lesions. Based on the age of the dog and clinicopathological results, other causes of recurrent UTI, such as hyperadrenocorticism, were considered highly unlikely and were excluded without further testing. The owners were instructed to stop antimicrobial treatment and return during the dog’s next relapse.
Three weeks later, the dog had a recurrence of malodorous urine and vulvar licking. At that time, abdominal ultrasound evaluation remained unremarkable, and urine was collected by cystocentesis for bacterial culture. Bladder-wall biopsies were obtained surgically and submitted for both histological examination and bacterial culture. Both cultures (urine and tissue) were positive for E. coli, though the susceptibility profiles were slightly different despite using similar break points (Table 1). Pulsed-field gel electrophoresis analysis was done on colonies from both locations and confirmed that the 2 isolated strains were indistinguishable.
TABLE 1.
Results from antimicrobial susceptibility testing of the Escherichia coli strain, depending on the specimen submitted to the laboratory.
| Tested antimicrobial | Urine specimen | Bladder wall biopsy |
|---|---|---|
| Penicillin G | R | R |
| Amoxicillin | R | R |
| Amoxicillin + clavulanic acid | I | I |
| Cefoxitin | R | S |
| Ceftiofur | S | S |
| Cefalexin | S | I |
| Cefquinome | S | S |
| Cefovecin | S | S |
| Gentamicin | R | S |
| Enrofloxacin | S | I |
| Marbofloxacin | S | S |
| Clindamycin | R | R |
| Sulfonamides + trimethoprim | S | R |
| Doxycycline | R | R |
| Fusidic acid | R | R |
| Chloramphenicol | S | S |
I — Intermediate; R — Resistant; S — Susceptible.
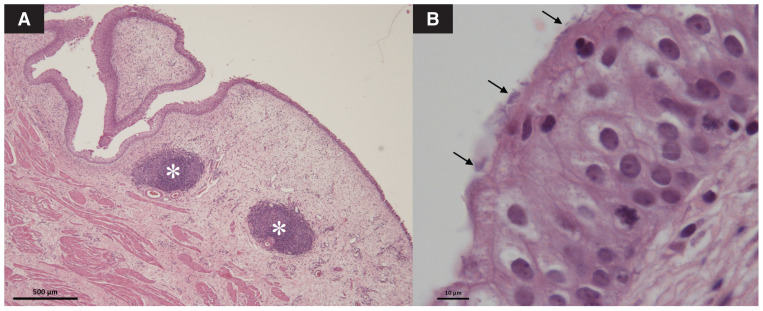
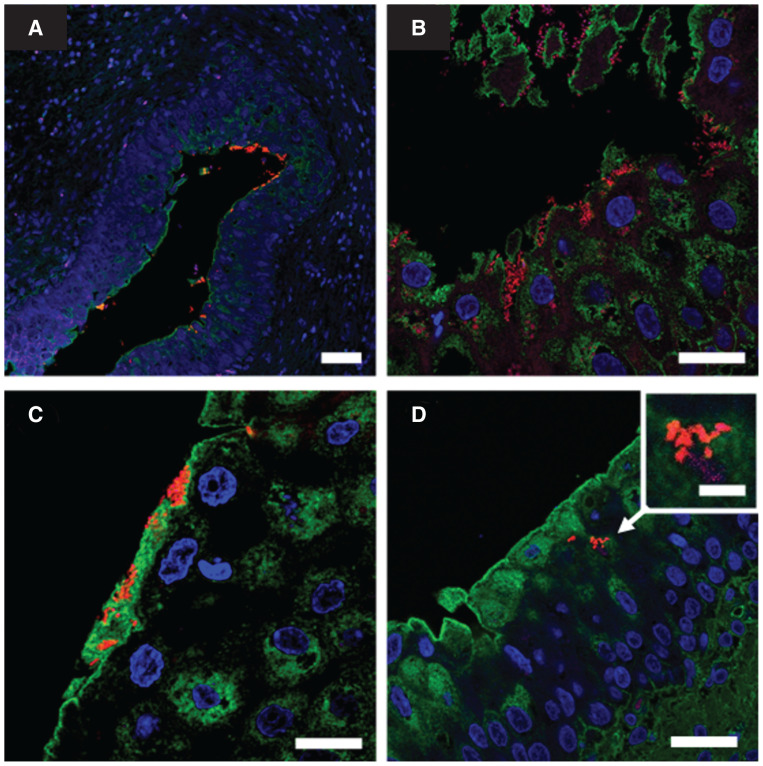
Conventional histological examination of the bladder wall revealed marked chronic hyperplastic and polypoid cystitis, characterized by an irregular thickening of the mucosa with several fibrovascular polyps covered by a hyperplastic, but intact and well-differentiated, urothelium. The lamina propria was diffusely edematous and hyperemic, with diffuse lymphocytic infiltrate and some follicular aggregates. In addition, small bacterial rods adherent to the apical region of urothelial cells were observed (Figure 1 A, B). A fluorescent in situ hybridization (FISH) analysis using a universal bacteria probe (EUB338) was conducted on saline-washed biopsies fixed in formaldehyde, revealing several rod-shaped bacteria adhering to the surface (Figure 2 A, B) and presence of IBC within superficial umbrella cells (Figure 2 C). Bacteria were also identified embedded and aggregated within a polysaccharide-rich material lying on the membrane surface, a characteristic reminiscent of a biofilm structure (Figure 2 D). The biofilm-forming capacity of the isolated E. coli was further assessed using a standard crystal violet assay, based on a technique described elsewhere (11). Both urine and bladder-wall isolates were biofilm-positive.
FIGURE 1.
Light microscopic evaluation of the urinary bladder wall in a female dog. A — The low-magnification image shows an irregular thickening of the mucosa with fibrovascular polyps and several follicular aggregates (*) (hematoxylin and eosin stain; scale bar = 500 μm). B — At high magnification, bacterial rods are observed adherent to the apical surface of urothelial cells (arrows) (hematoxylin and eosin stain; scale bar = 10 μm).
FIGURE 2.
Fluorescent in situ hybridization (FISH) analysis of bladder wall biopsies from a female dog. A — Patchy bacterial aggregates adhering to urothelium are visible at low magnification (scale bar = 50 μm). B — Invasive bacterial aggregates are observed in an area of urothelium erosion (scale bar = 25 μm). C — Bacterial aggregates embedded in a polysaccharide-rich matrix close to umbrella cells (scale bar = 25 μm). D — Image suggestive of bacterial aggregates within umbrella cells cytoplasm (scale bar = 25 μm); inset: intracellular bacterial community (scale bar = 5 μm). The FISH analysis stains are as follows: blue, 4′,6-diamidino-2-phenylindole (nuclear stain); green, wheat germ agglutinin (polysaccharide-rich material stain); red, Eub338 universal probe (bacterial stain).
Taken together, these results were suggestive of a chronic, recurrent UTI caused by a biofilm-forming E. coli strain, which would explain the relapsing clinical signs after antimicrobial withdrawal.
Treatment consisted of daily intravesical instillations of EDTA-Tris and IM injections of cefquinome (Cobactan 2.5%; MSD Animal Health, Beaucouze, France), 2.2 mg/kg, IM, q12h, based on susceptibility testing, for a total of 7 d. The EDTA-Tris infusions were made based on a previous description of a similar protocol (10). Briefly, the dog was sedated using 0.5 mg/kg of butorphanol (Butador; Boehringer Ingelheim Animal Health, Lyon, France) and 5 μg/kg of medetomidine (Domitor; Vetoquinol SA, Lure, France), IV. Urinary catheterization was achieved with a sterile 12-French Foley catheter. On Day 1, the bladder was completely emptied before being gently filled with sterile saline delivered through the catheter under ultrasonographic evaluation. This allowed us to evaluate the volume needed to subjectively obtain a sufficient distention of the bladder with the EDTA-Tris solution (220 mL in this case, corresponding to 5.5 mL/kg). The same volume of purposely manufactured EDTA-Tris (250 mM EDTA and 50 mM tromethamine) was then instilled, and the catheter was kept in place with the dog in sternal recumbency, recovering from the sedation, allowing the EDTA-Tris to remain within the bladder for 30 min before the catheter was removed. The bladder was not emptied before removal of the catheter, but the dog was allowed to urinate as soon as she was able to walk on her own.
Meloxicam (Metacam; Boehringer Ingelheim Animal Health), 0.2 mg/kg, IV, once; then 0.1 mg/kg, IV, q24h, was administered during the hospitalization. The dog exhibited no side effects except for a mild, self-limiting bloody vulvar discharge, attributed to repeated catheterization. The urine remained macroscopically clear during the treatment period. A urinalysis (cystocentesis) was completed on the last day of treatment and revealed only a microscopic hematuria (4+ for blood on dipstick analysis and < 250 red blood cells/high power field on sediment evaluation). The bacterial culture on this sample was negative. The dog was discharged and was prescribed oral meloxicam for 2 additional days; and cranberry extracts (Uripac; TVM Laboratory, Lempdes, France), 720 mg of type A proanthocyanidins, PO, q24h and probiotics (Vivomixx; Arkopharma, Carros, France), 225 billion lyophilized bacteria per 10 kg, PO, q24h, for 2 mo.
The dog remained free from clinical signs for the following 51 mo and was still doing clinically well at the time of writing. All urine bacterial cultures obtained after the end of the treatment were negative (after 22, 56, 190, 299, 421, 576, and 1071 d).
DISCUSSION
According to recent guidelines (12), recurrent bacterial cystitis is considered in individuals experiencing 3 or more episodes of clinical bacterial cystitis in the preceding 12 mo, or 2 or more episodes in the preceding 6 mo. The dog in this case clearly met this definition, as she had 6 episodes of relapsing cystitis secondary to an E. coli infection after her adoption, 13 mo before referral. Recurrent bacterial cystitis may result from relapsing or persistent infection, or reinfection, and efforts should be made to thoroughly explore the underlying cause in order to treat this condition accordingly (3,12). In some instances, as in this case, urinary bladder wall biopsies for histopathologic and bacterial culture may be necessary. The FISH analysis led us to suspect bacterial IBC and biofilm, which possibly explained the recurring clinical signs in this dog.
Applying a FISH analysis has been seldom described in veterinary uronephrology. Recently, it was studied in dogs with proliferative urethritis and follicular cystitis as a means to identify deep-seated bacterial infections (13,14) and in a dog suffering from malakoplakia of the urinary bladder (15). Most described applications of FISH in dogs concern granulomatous colitis (16,17) and, more anecdotally, hepatic diseases (18,19).
In human medicine, despite the lack of clear criteria to confirm its presence on tissues, FISH has been used to confirm biofilm formation based on the location of the bacteria (on the surface of tissues), their organization (aggregates), or both (20,21). The finding of a polysaccharide-rich material surrounding bacteria is also consistent with the chemical structure of a biofilm (8). To the authors’ knowledge, this is the first case where bacterial biofilm and IBC could be suspected, using FISH analysis, as a cause of recurrent cystitis in a dog. However, despite the descriptive criteria consistent with a biofilm, those conclusions should be taken with caution, as there is no established gold standard method to confirm our hypothesis. Additional studies about FISH analysis of bladder wall from dogs with and without lower urinary tract clinical signs would be necessary. Still, our confirmation of the in vitro biofilm-forming capacity of the causative E. coli in this case may be an additional argument for considering the diagnosis of biofilm-related bacterial cystitis.
Despite different antimicrobial susceptibility patterns, pulse-field gel electrophoresis analysis also helped to confirm that both E. coli isolated from the urine and the bladder wall were undistinguishable. A possible explanation for this discrepancy could be that bacteria implicated in a biofilm behave differently than during a planktonic phase. For instance, despite having no effect on planktonic bacteria, amino acid (especially leucine) depletion can increase the fluoroquinolones tolerance of E. coli in biofilms (22). Expression of some genes is also affected by biofilm conditions and has been shown to correlate with antimicrobial resistance (23,24). However, the different susceptibility patterns could also be explained by technical or dilution errors, though both analyses were done in the same laboratory with similar processes. This highlights the challenge to interpret antibiotic resistance profiles from different specimens with apparently undistinguishable bacterial strains.
Bladder infusion of EDTA-Tris was described in a study from Farca et al in which 4 dogs were treated for chronic cystitis (2 with E. coli infection) and were all reported to be free from clinical signs for 180 d (10). Unfortunately, histological evaluation of the bladder wall was not available from this study to determine whether these infections could have been associated with biofilm-formation or IBC. To the authors’ knowledge, there have been no other reports of the use of this treatment in dogs with chronic UTI. In the Farca et al study, it was speculated that EDTA-Tris potentiated the antimicrobial activity of selected antibiotics possibly by chelating divalent cations such as Mg2+, Ca2+, and Mn2+ (of which some are part of the bacterial plasma outer membrane), thus altering the bacterial cell wall permeability and ribosome stability. Other possible effects are interference with bacterial peptidoglycan biosynthesis and direct alteration of the bacterial surface, increasing the penetration of antimicrobials (10,25). Previous in vitro studies also showed that EDTA-Tris significantly decreased the minimal inhibitory concentrations of selected antibiotics against Gram-negative bacteria (26,27). Consequently, EDTA-Tris might also be an interesting therapeutic option in recurrent cystitis without biofilm or in cases where bladder-wall biopsy cannot be obtained.
Another known effect of EDTA-Tris is its anti-biofilm capacity, which may have been useful in the case reported here. The anti-biofilm effect of EDTA has been reported against various bacterial agents, including E. coli, Pseudomonas aeruginosa, and Staphylococcus aureus (8,9,28,29). Banin et al studied the effects of EDTA on biofilm-forming P. aeruginosa and showed that, by chelating iron and calcium ions (which are important to stabilize the biofilm matrix), EDTA provoked a loosening of the biofilm and massive detachments of bacteria (9). They also suggested a direct killing effect, though the mechanism remains unclear (9). Destabilizing the biofilm matrix, at least in part, through cation sequestration using EDTA-Tris, seems to be an interesting approach for in vivo biofilm eradication in chronic bacterial infections.
In the cases reported by Farca et al, the same, previously unsuccessful, antibiotic was used in each case (10). In the present report, we elected to choose a different antibiotic (cefquinome) based on bladder-wall bacterial culture and considering the in vitro susceptibility of the strain and previous inefficacy of fluoroquinolones. One advantage of cefquinome is that it has good distribution and tissue diffusion, which can be of interest for IBC (30). However, the use of a 4th-generation cephalosporin in that case can be questioned, as development of antimicrobial resistance is a major public health issue. Still, our treatment with a short course of antibiotics was associated with a clinical cure, which is the main therapeutic goal according to recent guidelines (12). Alternatively, local antimicrobial instillation (e.g., amikacin) could also have been considered to maximize efficacy by reaching higher concentration (31).
One of the disadvantages of a multimodal therapeutic approach is that it remains unclear which treatment was responsible for the clinical remission. The term “remission” is preferred here because of the absence of follow-up biopsies (declined by the owners) and the capacity of IBC to remain latent for months before relapsing (2). Moreover, although IBC seem to share similarities with biofilms, it is unclear whether EDTA can disrupt these intracellular communities.
The utility of Type-A proanthocyanidins and probiotics for preventing reinfection is not clear. Type-A proanthocyanidins have been demonstrated to inhibit the adherence of E. coli to the uroepithelium in vitro and ex vivo (32). However, in the veterinary literature, the only in vivo study available had low statistical power and was conducted in dogs with thoracolumbar disc herniation for which voluntary voiding was impaired, making reinfection more likely (33). Results from meta-analyses of human cases are contradictory, but one study by Jepson et al suggested that cranberry extracts could be effective in reducing the risk of recurrent infections in young, healthy women (34). Probiotics were useful in women with UTI who tended to have very different vaginal microbiomes compared to healthy women. However, such a difference was not confirmed in dogs (35), making the efficacy of probiotics uncertain for UTI prevention in this species. The effect of probiotic treatment on the gastrointestinal microbiome or the urobiome has not been studied in dogs.
The case reported here had some limitations and this report is not meant as a guideline to manage such cases. Indeed, although suspected in human medicine (4), the true contribution of the suspected biofilm to the recurrent cystitis could not be ascertained in this dog. Histological and FISH evaluations of biopsies taken during our initial cystoscopic evaluation, while the dog was still on antimicrobials, would have been interesting. Second, the choice to change the antibiotic was questionable based on previous results for EDTA-Tris in chronic cystitis (10). However, given current knowledge on biofilm and IBC roles in recurrent cystitis and the need for only a short course of antibiotic, we consider that local EDTA-Tris can be a therapeutic option in cases of recurrent cystitis, especially if biofilm or IBC are suspected. This case report may encourage further studies, ideally randomized controlled trials, to evaluate whether this treatment should be more routinely considered.
In summary, this case report describes the successful treatment of a chronic recurrent UTI likely caused by a biofilm-forming E. coli with IBC. The biofilm hypothesis should be considered in any dog with recurrent UTI, especially when no predisposing factor can be identified. Intravesical instillations of EDTA-Tris might be useful as an adjunctive therapy in such cases. Indeed, although the exact effects of EDTA-Tris are not well-understood, local infusions were associated with achieving the observed clinical remission in this dog. However, despite the cost-effectiveness of EDTA-Tris, the onerous nature of the present protocol (daily sedation and catheterization for 1 wk) may counterbalance this advantage. Further studies are warranted to determine if the diagnostic procedures exposed here should be applied to all dogs with similar presentation and to better assess the efficacy and tolerability of intravesical EDTA-Tris treatment.
ACKNOWLEDGMENTS
J-PM was funded by the EU’s Seventh Framework Programme N° FP7-609398 (AgreenSkills+ contract). We thank the staff at the histopathology core facility (F. Capilla and A. Alloy) and the cellular imaging facility (U1043, D. Daviaud). We also thank Dr. A. Franc, the primary veterinarian. CVJ
Funding Statement
J-PM was funded by the EU’s Seventh Framework Programme N° FP7-609398 (AgreenSkills+ contract).
Footnotes
Copyright is held by the Canadian Veterinary Medical Association. Individuals interested in obtaining reproductions of this article or permission to use this material elsewhere should contact Permissions.
REFERENCES
- 1.Byron JK. Urinary tract infection. Vet Clin North Am Small Anim Pract. 2019;49:211–221. doi: 10.1016/j.cvsm.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llido M, Vachon C, Dickinson M, et al. Transurethral cystoscopy in dogs with recurrent urinary tract infections: Retrospective study (2011–2018) J Vet Intern Med. 2020;34:790–796. doi: 10.1111/jvim.15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris CJ, Rohn JL, Glickman S, et al. Effective treatments of UTI: Is intravesical therapy the future? Pathogens. 2023;12:417. doi: 10.3390/pathogens12030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis AJ, Richards AC, Mulvey MA. Invasion of host cells and tissues by uropathogenic bacteria. Microbiol Spectrum. 2016;4 doi: 10.1128/microbiolspec.uti-0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terlizzi ME, Gribaudo G, Maffei ME. Uropathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010;54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RA, McCue PM, Borlee GI, et al. In vitro efficacy of non-antibiotic treatments on biofilm disruption of gram-negative pathogens and an in vivo model of infectious endometritis utilizing isolates from the equine uterus. J Clin Microbiol. 2016;54:631–639. doi: 10.1128/JCM.02861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banin E, Brady KM, Greenberg EP. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72:2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farca AM, Piromalli G, Maffei F, et al. Potentiating effect of EDTA-Tris on the activity of antibiotics against resistant bacteria associated with otitis, dermatitis and cystitis. J Small Anim Pract. 1997;38:243–245. doi: 10.1111/j.1748-5827.1997.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 11.Gilberte JM, Levent G, Norman KN, et al. Comprehensive phenotypic and genotypic characterization and comparison of virulence, biofilm, and antimicrobial resistance in urinary Escherichia coli isolated from canines. Vet Microbiol. 2020;249:108822. doi: 10.1016/j.vetmic.2020.108822. [DOI] [PubMed] [Google Scholar]
- 12.Weese JS, Blondeau J, Boothe D, et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J. 2019;247:8–25. doi: 10.1016/j.tvjl.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Borys A, Hulsebosch SE, Mohr FC, et al. Clinical, histopathologic, cystoscopic, and fluorescence in situ hydridization analysis of proliferative urethritis in 22 dogs. J Vet Intern Med. 2019;33:184–191. doi: 10.1111/jvim.15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viitanen SJ, Tuomisto L, Salonen N, et al. Escherichia coli-associated follicular cystitis in dogs: Clinical and pathologic characterization. J Vet Intern Med. 2023;37:1059–1066. doi: 10.1111/jvim.16719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brückner M. Malakoplakia of the urinary bladder in a young French bulldog. J Am Vet Med Assoc. 2021;260:543–548. doi: 10.2460/javma.20.12.0676. [DOI] [PubMed] [Google Scholar]
- 16.Simpson KW, Dogan B, Rishniw M, et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778–4792. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfield CS, James FE, Craven M, et al. Remission of histiocytic ulcerative colitis in boxer dogs correlated with eradication of invasive intramucosal Escherichia coli. J Vet Intern Med. 2009;23:964–969. doi: 10.1111/j.1939-1676.2009.0363.x. [DOI] [PubMed] [Google Scholar]
- 18.Im J, Burney DP, McDonough, et al. Canine hepatitis associated with intrahepatic bacteria in three dogs. J Am Anim Hosp Assoc. 2018;54:65–70. doi: 10.5326/JAAHA-MS-6492. [DOI] [PubMed] [Google Scholar]
- 19.McCallum KE, Constantino-Casas F, Cullen JM, et al. Hepatic leptospiral infections in dogs without obvious renal involvement. J Vet Intern Med. 2019;33:141–150. doi: 10.1111/jvim.15340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swidsinski A, Schlien P, Pernthaler A, et al. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 2005;54:388–395. doi: 10.1136/gut.2004.043059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadler N, Kvich L, Bjarnsholt T, et al. The discovery of bacterial biofilm in patients with muscle invasive bladder cancer. APMIS. 2021;129:265–270. doi: 10.1111/apm.13097. [DOI] [PubMed] [Google Scholar]
- 22.Bernier SP, Lebeaux D, DeFrancesco AS, et al. Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet. 2013;9:e1003144. doi: 10.1371/journal.pgen.1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison JJ, Wade WD, Akierman S, et al. The chromosomal toxin gene yafQ is a determinant of multidrug tolerance for Escherichia coli growing in a biofilm. Antimicrob Agents Chemother. 2009;53:2253–2258. doi: 10.1128/AAC.00043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch SV, Dixon L, Benoit MR, et al. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob Agents Chemother. 2007;51:3650–3658. doi: 10.1128/AAC.00601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wooley RE, Jones MS. Action of EDTA-Tris and antimicrobial agent combinations on selected pathogenic bacteria. Vet Microbiol. 1983;8:271–280. doi: 10.1016/0378-1135(83)90079-2. [DOI] [PubMed] [Google Scholar]
- 26.Farca AM, Nebbia P, Re G. Potentiation of the in vitro activity of some antimicrobial agents against selected gram-negative bacteria by EDTA-tromethamine. Vet Res Commun. 1993;17:77–84. doi: 10.1007/BF01839235. [DOI] [PubMed] [Google Scholar]
- 27.Aboelenin AM, Hassan R, Abdelmegeed ES. The effet of EDTA in combination with some antibiotics against clinical isolates of gram-negative bacteria in Mansoura, Egypt. Microb Pathog. 2021;154:104840. doi: 10.1016/j.micpath.2021.104840. [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre E, Vighetto C, Di Martino P, et al. Synergistic antibiofilm efficacy of various commercial antiseptics, enzymes and EDTA: A study of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Int J Antimicrob Agents. 2016;48:181–188. doi: 10.1016/j.ijantimicag.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Lin Y, Lu Q, et al. In vitro and in vivo activity of EDTA and antibacterial agents against the biofilm of mucoid Pseudomonas aeruginosa. Infection. 2017;45:23–31. doi: 10.1007/s15010-016-0905-z. [DOI] [PubMed] [Google Scholar]
- 30.Limbert M, Isert D, Klesel N, et al. Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1991;35:14–19. doi: 10.1128/aac.35.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres AR, Cooke K. Intravesical instillation of amikacin for treatment of lower urinary tract infection caused by Pseudomonas aeruginosa in a dog. J Am Vet Med Assoc. 2014;245:809–811. doi: 10.2460/javma.245.7.809. [DOI] [PubMed] [Google Scholar]
- 32.Raditic DM. Complementary and integrative therapies for lower urinary tract diseases. Vet Clin North Am Small Anim Pract. 2015;45:857–878. doi: 10.1016/j.cvsm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Olby NJ, Vaden SL, Williams VK, et al. Effect of cranberry extract on the frequency of bacteriuria in dogs with acute thoracolumbar disk herniation: A randomized controlled clinical trial. J Vet Intern Me. 2017;31:60–68. doi: 10.1111/jvim.14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2012; 10:CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchins RG, Vaden SL, Jacob ME, et al. Vaginal microbiota of spayed dogs with or without recurrent urinary tract infections. J Vet Intern Med. 2014;28:300–304. doi: 10.1111/jvim.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]