Abstract
A 10-year-old spayed female shih tzu dog was brought to the hospital because of recurring syncope that occurred simultaneously with a cough. Physical examination did not reveal an abnormal heart rhythm or abnormal heart sounds. Electrocardiography revealed sinus arrest of 4.7 s with intermittent escape beats during coughing. Additional examinations, including thoracic radiography, clinical pathology, and echocardiography, revealed no abnormalities of concern. Forty-eight-hour Holter monitoring captured 1 syncopal episode following severe coughing, during which the longest sinus arrest lasted 16 s with intermittent escape beats. This observation confirmed our strong suspicion that coughing was the cause of varying degrees of sinus arrest in this dog. Theophylline, codeine, and short-term prednisolone were prescribed to treat the dog’s cough. The daily episodes of syncope ceased and coughing decreased. Subsequent 48-hour Holter monitoring revealed no abnormal pauses, and the owner did not report syncope. Theophylline and codeine were continued for 5 mo, during which time no syncope occurred. To our knowledge, this case provides the first clear evidence of a correlation between cough-induced sinus arrest and syncope in a veterinary patient, as confirmed by Holter monitoring and electrocardiography.
Key clinical message:
Cough-induced severe bradycardia and syncope were identified in a shih tzu dog. After the antitussive medication was adjusted, the signs resolved.
RÉSUMÉ
Bradycardie sévère et syncope provoquées par la toux chez un chien
Une chienne shih tzu stérilisée âgée de 10 ans a été amenée à l’hôpital en raison d’une syncope récurrente survenue simultanément avec une toux. L’examen physique n’a révélé aucun rythme cardiaque anormal ni bruits cardiaques anormaux. L’électrocardiographie a révélé un arrêt sinusal de 4,7 s avec des battements d’échappements intermittents lors de la toux. Des examens complémentaires, notamment une radiographie thoracique, des analyses en pathologie clinique et une échocardiographie, n’ont révélé aucune anomalie préoccupante. Une surveillance Holter de 48 heures a capturé 1 épisode syncopal à la suite d’une toux sévère, au cours duquel l’arrêt sinusal le plus long a duré 16 s avec des battements d’échappements intermittents. Cette observation a confirmé nos fortes suspicions selon lesquelles la toux était la cause de divers degrés d’arrêt sinusal chez ce chien. De la théophylline, de la codéine et de la prednisolone de courte durée ont été prescrites pour traiter la toux du chien. Les épisodes quotidiens de syncope ont cessé et la toux a diminué. Une surveillance Holter ultérieure de 48 heures n’a révélé aucune pause anormale et le propriétaire n’a pas signalé de syncope. La théophylline et la codéine ont été poursuivies pendant 5 mois, période pendant laquelle aucune syncope ne s’est produite. À notre connaissance, ce cas constitue la première preuve claire d’une corrélation entre l’arrêt sinusal induit par la toux et la syncope chez un patient vétérinaire, comme le confirme la surveillance Holter et l’électrocardiographie.
Message clinique clé :
Une bradycardie et une syncope sévères induites par la toux ont été identifiées chez un chien shih tzu. Après ajustement du traitement antitussif, les signes ont disparu.
(Traduit par Dr Serge Messier)
Cough-induced syncope, a fainting spell triggered by vigorous coughing, has garnered attention in human medical literature (1–3). The pathophysiological mechanisms underlying this phenomenon have been explored, revealing that cerebral anoxia and syncope may arise from increased intrathoracic pressure leading to decreased venous return and cardiac output (4). In addition, a hypersensitive bronchopulmonary reflex has been suggested as a cause of bradyarrhythmia, including high-degree atrioventricular block or sinus arrest, further contributing to cough-induced syncope (5,6). Although cough syncope has been documented in humans, its prevalence remains relatively low, accounting for only 2.6% of syncopal cases (1). Nevertheless, the risk of syncope due to cough is notably increased in individuals with obstructive airway disease (1,7). Interestingly, in contrast to its recognition in human medicine, reports of cough-induced syncope in veterinary medicine are scarce, resulting in a paucity of epidemiological studies in this area. Fox et al have suggested that syncope due to coughing may be more prevalent in small, brachycephalic dog breeds (8). However, limited evidence exists, with only a few cases highly suspicious for lung lobe torsion as the primary cause of cough-induced syncope in pugs (9). This disparity can likely be attributed to the inherent challenges associated with determining the correlation between syncope and coughing in animals compared to in humans. In this context, we present a compelling case of a dog experiencing repeated syncopal episodes clearly associated with coughing bouts, accompanied by severe bradycardia.
CASE DESCRIPTION
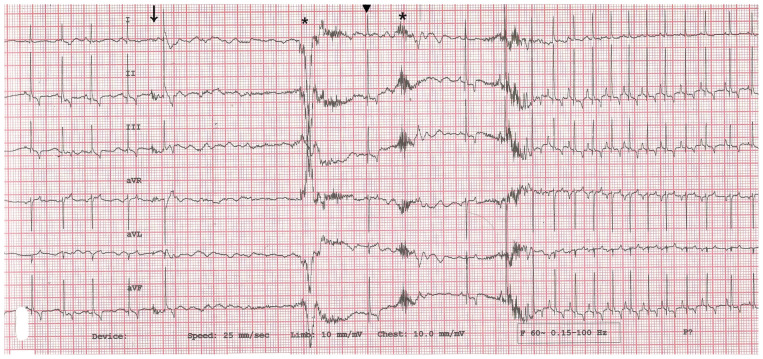
A 10-year-old spayed female shih tzu dog was brought to the Seoul Animal Heart Hospital after multiple events of squealing and fainting after coughing. The dog was bright, alert, and responsive, and physical examination did not reveal any abnormal heart sounds or arrhythmias of concern. Her heart rate was 132 bpm and her femoral pulses were normal. Results from blood testing, including the complete blood (cell) count, biochemistry analysis, total T4 concentration, and N-terminal pro-brain natriuretic peptide concentration, were unremarkable. Thoracic radiography and echocardiography revealed no abnormalities associated with serious cardiac disease. No airway or pulmonary abnormalities were detected. The lung parenchyma appeared normal, with no interstitial, alveolar, or bronchial pattern identified. Electrocardiography (ECG) in the frontal plane incidentally revealed that the dog experienced sudden sinus arrest after coughing. Subsequently, we induced coughing by massaging the dog’s trachea. The dog simultaneously experienced sinus arrest and coughing (Video 1, available online from: Supplementary Materials), during which sinus arrest lasted ~4.7 s (Figure 1). Therefore, we strongly suspected that the dog’s syncope was directly associated with cough-induced sinus arrest.
FIGURE 1.
Part of an electrocardiogram (ECG) recording obtained in the hospital from a female dog. A sinus arrest of 4.7 s with intermittent escape beat (arrowhead) and artifacts (asterisks) was recorded after coughing (arrow). Paper speed = 25 mm/s; 1 cm = 1 mV.
According to the owner, during Holter monitoring, 1 syncopal episode occurred and coincided with a long sinus arrest on the ECG. The sinus arrest lasted for 16 s following a bradycardic episode with a heart rate of 60 bpm (Figure 2). As syncope can be induced by the failure of vital organ perfusion when sinus arrest lasts for more than 5 to 6 s (2), the 16-second sinus arrest was very likely the primary cause of syncope. Therefore, we diagnosed the dog with cardiac syncope due to cough.
FIGURE 2.
A — Full disclosure of an electrocardiogram (ECG) recording obtained with a Holter monitor from a female dog during a syncope event. Bradycardia started at 06:41:00, followed by 16 s of sinus arrest, from 06:41:17 to 06:41:33. Paper speed = 25 mm/s; 1 cm = 1 mV.
B — An ECG recording obtained with a Holter monitor during a syncope event. Before the occurrence of sinus arrest, the PR interval remained constant, but the RR interval gradually started to irregularly lengthen. Subsequently, coughing occurred (arrow, as mentioned by the owner), accompanied by the onset of sinus arrest. Intermittent escape beats were observed (arrowheads) and the return to a clear sinus rhythm was noted (asterisk). The ECGs are not continuous. Paper speed = 25 mm/s; 1 cm = 1 mV.
To treat the dog’s syncope, we administered theophylline (10 mg/kg body weight, PO, q12h), codeine (0.5 mg/kg body weight, PO, q12h), and prednisolone (0.5 mg/kg body weight, PO, q12h for 3 d), which reduced her coughing. No further syncope occurred. Additional 48-hour Holter monitoring revealed normal sinus rhythms (Figure 3). Theophylline and codeine were discontinued 5 mo later, during which time the dog experienced occasional events of mild coughing but no syncope.
FIGURE 3.
Full disclosure of an electrocardiogram (ECG) recording obtained with a Holter monitor from a female dog after antitussive medication. No signs of arrhythmia were observed. Paper speed = 25 mm/s; 1 cm = 1 mV.
DISCUSSION
In this case, syncope was directly induced by coughing, as confirmed by Holter monitoring and ECG. In addition, we captured a video depicting coughing that coincided with the sinus arrest on the ECG. Coughing episodes were accompanied by varying degrees of sinus arrest.
Several mechanisms have been proposed to explain cough-induced syncope, including i) increased intrathoracic pressure leading to decreased venous return and cardiac output and diminished cerebral blood flow, and ii) an extreme vagal response that provokes bradyarrhythmia (1–3,10). Increased intrathoracic pressure during cough has traditionally been considered the major cause of cough syncope (4). In terms of the vagal response to cough, Saito et al suggested that hypersensitivity of bronchopulmonary receptors may be pivotal to cough syncope (6). In this regard, coughing may induce a bronchopulmonary reflex, which can cause hypervagotonia, provoking severe bradyarrhythmia due to 3rd-degree heart block and sinus arrest (5,11). Additional reports related to this neuronal reflex in humans have been published (12,13). In this case, we describe a dog experiencing syncopal episodes immediately after coughing. Further examinations, including a Holter monitor, confirmed that the coughing induced a sinus arrest severe enough to cause fainting.
The association between severe bradyarrhythmia and cough in humans can be determined via ECG, Holter monitoring, carotid sinus massage, orthostatic challenge testing, tilt-table testing, and the Valsalva maneuver, among other methods. (14). In addition to these tests, patients’ descriptions of their experiences during the events improve the accuracy and efficacy of diagnosis (15). In veterinary medicine, anamnesis from the owner, physical examination, video recording, ECG, Holter monitoring, and other tests can be used to diagnose syncope (16). Among these, ECG and Holter monitoring during coughing are the best measures to confirm the association between coughing and severe bradyarrhythmia (17). In this case, as cough-induced sinus arrest occurred during ECG in the hospital, we strongly suspected that severe bradyarrhythmia was triggered by coughing. Every cough we induced by means of massaging the dog’s trachea immediately caused her to experience sinus arrest, although syncope did not occur. The dog experienced a syncopal episode after discharge from the hospital. The Holter monitor ECG verified that cough-induced severe sinus arrest occurred long enough to cause the syncope. With treatment, Holter monitor findings revealed no abnormalities. When the cough resolved, no additional bradyarrhythmias occurred, which provided further evidence for a diagnosis of cough-induced bradyarrhythmia and syncope.
The most crucial element in the treatment of cough-related syncope is to reduce the coughing (1). Human patients with cough-related syncope are educated to avoid coughing triggers and take medication to reduce coughing (14,18). In cases where other treatments are not effective, a pacemaker can be implanted to prevent extreme bradycardia. (14,19). We focused on reducing the dog’s coughing via medication. To evaluate the dog’s persistent cough, we conducted a comprehensive assessment, including a review of the dog’s medical history, physical examination, chest radiography, and echocardiography. Our initial assessment strongly suggested bronchomalacia and bronchitis as the primary underlying conditions contributing to the cough (16). Consequently, we considered theophylline, codeine, and prednisolone as the treatment of choice (20,21). Theophylline, an adenosine receptor antagonist and cyclic phosphodiesterase inhibitor, is well-known for its efficacy in relaxing bronchial smooth muscles and reducing coughing (22). In addition, theophylline exhibits a weak chronotropic action (22), which we believed could be beneficial in improving the dog’s severe bradycardia induced by coughing episodes. Codeine, a selective agonist for the μ-opioid receptor, was chosen to suppress the cough reflex by acting on the cough center located in the pons and upper brain stem (20). Prednisolone, an anti-inflammatory agent, was included in the treatment regimen due to its effectiveness in managing bronchitis (23). Holter monitoring conducted after resolution of the cough revealed an almost-normal sinus rhythm, and the dog did not experience any syncopal episodes following administration of the medication. Although the dog’s cough responded favorably to this treatment approach, it is important to acknowledge that further investigations are warranted to definitively determine the underlying causes of the cough. Our diagnostic approach involved X-ray imaging, auscultation, echocardiography, blood tests, and physical examinations, which did not reveal significant pneumonia, preexisting lung inflammation, or pronounced tracheal collapse as potential causes of the cough. However, it is essential to note that additional, precise examinations may be required to further differentiate the underlying causes of cough. Unfortunately, due to circumstances with the dog and owner, we were unable to pursue further investigations.
In this case, clear evidence of an association between cough and syncope in a dog was observed through Holter monitoring and general ECG. Although reports of coughing and syncope in veterinary medicine are not new, this case is important because it demonstrates the relationship between these signs and the potential effectiveness of drug-based treatment. CVJ
Footnotes
Unpublished supplementary material (Video 1) is available online from: Supplementary Materials
Copyright is held by the Canadian Veterinary Medical Association. Individuals interested in obtaining reproductions of this article or permission to use this material elsewhere should contact Permissions.
REFERENCES
- 1.Dicpinigaitis PV, Lim L, Farmakidis C. Cough syncope. Respir Med. 2014;108:144–251. doi: 10.1016/j.rmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Grabczak EM, Stec S, Dabrowska M, Plevkova J, Krenke R. Cough as a cause and consequence of heart dysfunction: Current state of art. Physiol Res. 2020;69:S105–S121. doi: 10.33549/physiolres.934408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao AC, Lin RT, Liu CK, Wang PY, Hsu HY. Mechanisms of cough syncope as evaluated by valsalva maneuver. Kaohsiung J Med Sci. 2007;23:55–62. doi: 10.1016/S1607-551X(09)70375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpey-Shafer EP. Syncope. Br Med J. 1956;1:506–509. doi: 10.1136/bmj.1.4965.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D, Beldner S, Pollaro F, Jadonath R, Maccaro P, Goldner B. Cough-induced heart block. Pacing Clin Electrophysiol. 1999;22:1270–1271. doi: 10.1111/j.1540-8159.1999.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Saito D, Matsuno S, Matsushita K, et al. Cough syncope due to atrio-ventricular conduction block. Jpn Heart J. 1982;23:1015–1020. doi: 10.1536/ihj.23.1015. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor WN. Evaluation and outcome of patients with syncope. Medicine. 1990;69:160–175. doi: 10.1097/00005792-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fox PR, Sisson, David M, Sydney N. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice. Philadelphia, Pennsylvania: WB Saunders; 1999. pp. 446–454. [Google Scholar]
- 9.Davies JA, Snead EC, Pharr JW. Tussive syncope in a pug with lung-lobe torsion. Can Vet J. 2011;52:656–660. [PMC free article] [PubMed] [Google Scholar]
- 10.Davidow EB, Woodfield JA, Proulx J. Syncope: Pathophysiology and differential diagnosis. Compendium. 2001;26:608–620. [Google Scholar]
- 11.Hart G, Oldershaw PJ, Cull RE, Humphrey P, Ward D. Syncope caused by cough-induced complete atrioventricular block. Pacing Clin Electrophysiol. 1982;5:564–566. doi: 10.1111/j.1540-8159.1982.tb02279.x. [DOI] [PubMed] [Google Scholar]
- 12.Choi YS, Kim JJ, Oh BH, Park YB, Seo JD, Lee YW. Cough syncope caused by sinus arrest in a patient with sick sinus syndrome. Pacing Clin Electrophysiol. 1989;12:883–886. doi: 10.1111/j.1540-8159.1989.tb05024.x. [DOI] [PubMed] [Google Scholar]
- 13.Aliyev F, Kiliçkesmez KO, Çeliker C, Türkoğlu C. Cough-induced sinus arrest resulting in recurrent episodes of syncope is it really transient? J Cardiovasc Med. 2012;13:468–470. doi: 10.2459/JCM.0b013e32833892c4. [DOI] [PubMed] [Google Scholar]
- 14.Brignole M, Moya A, de Lange FJ, et al. [2018 ESC guidelines for the diagnosis and management of syncope [article in Polish]]. Kardiol Pol. 2018;76:1119–1198. doi: 10.5603/KP.2018.0161. [DOI] [PubMed] [Google Scholar]
- 15.Willis J. Syncope. Pediatr Rev. 2000;21:201–204. doi: 10.1542/pir.21-6-201. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger SJ, Feldman EC. Textbook of Veterinary Internal medicine: Diseases of the Dog and Cat. Philadelphia, Pennsylvania: WB Saunders; 2017. pp. 503–536. [Google Scholar]
- 17.Bright JM, Cali JV. Clinical usefulness of cardiac event recording in dogs and cats examined because of syncope, episodic collapse, or intermittent weakness: 60 cases (1997–1999) J Am Vet Med Assoc. 2000;216:1110–1114. doi: 10.2460/javma.2000.216.1110. [DOI] [PubMed] [Google Scholar]
- 18.White CM, Tsikouris JP. A review of pathophysiology and therapy of patients with vasovagal syncope. Pharmacotherapy. 2000;20:158–165. doi: 10.1592/phco.20.3.158.34786. [DOI] [PubMed] [Google Scholar]
- 19.Alboni P, Menozzi C, Brignole M, et al. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome — the THEOPACE study: A randomized controlled trial. Circulation. 1997;96:260–266. doi: 10.1161/01.cir.96.1.260. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh BM, Beets AK. Coughing in small animal patients. Front Vet Sci. 2020;6:513. doi: 10.3389/fvets.2019.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubuis E, Wortley MA, Grace MS, et al. Theophylline inhibits the cough reflex through a novel mechanism of action. J Allergy Clin Immunol. 2014;133:1588–1598. doi: 10.1016/j.jaci.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plumb DC. Veterinary Drug Handbook: Pocket Edition. Ames, Iowa: Iowa State University Press; 2018. pp. 1564–1567. [Google Scholar]
- 23.Garrison MM, Christakis DA, Harvey E, Cummings P, Davis RL. Systemic corticosteroids in infant bronchiolitis: A meta-analysis. Pediatrics. 2000;105:E44. doi: 10.1542/peds.105.4.e44. [DOI] [PubMed] [Google Scholar]