Abstract

Closely spaced promoters are ubiquitous in prokaryotic and eukaryotic genomes. How their structure and dynamics relate remains unclear, particularly for tandem formations. To study their transcriptional interference, we engineered two pairs and one trio of synthetic promoters in nonoverlapping, tandem formation, in single-copy plasmids transformed into Escherichia coli cells. From in vivo measurements, we found that these promoters in tandem formation can have attenuated transcription rates. The attenuation strength can be widely fine-tuned by the promoters’ positioning, natural regulatory mechanisms, and other factors, including the antibiotic rifampicin, which is known to hamper RNAP promoter escape. From this, and supported by in silico models, we concluded that the attenuation in these constructs emerges from premature terminations generated by collisions between RNAPs elongating from upstream promoters and RNAPs occupying downstream promoters. Moreover, we found that these collisions can cause one or both RNAPs to falloff. Finally, the broad spectrum of possible, externally regulated, attenuation strengths observed in our synthetic tandem promoters suggests that they could become useful as externally controllable regulators of future synthetic circuits.
Introduction
Closely spaced promoters in convergent, divergent, and tandem geometries are widely present in living organisms, including Escherichia coli.1−7 They are known to have high conservation levels,2,8 but it remains unclear what are their selective advantages.
Provided that the elongation regions of two genes overlap, RNAPs starting from different promoters can be expected to interact. Studies have reported that promoters in convergent formation have weakened RNA production due to collisions between the RNAPs elongating from one transcription start site (TSS) and the RNAPs bound to the other TSS9 (likely followed, in some cases, by premature terminations). Meanwhile, in promoters in tandem formation, RNAPs bound to one promoter can block (at least transiently) RNAPs elongating from the other promoter.10 Moreover, natural genes controlled by tandem promoters whose TSSs are closer than ∼35 bp have notably weaker expression rates (on average) than genes controlled by tandem promoters separated by longer distances.11 This could be explained by the phenomenon of occlusion of one of the TSSs following RNAP occupation of the other TSS, since RNAPs are known to occupy that DNA length during transcription initiation.12
It should be possible to use the interactivity between RNAPs of promoters closely spaced, in tandem formation, as a means to engineer synthetic genes with fine-tuned dynamics.11 Models have explored the potential dynamics of promoters in tandem formation as a function of properties such as the component promoters’ strength.11,13 However, empirical validation is largely lacking, and the potential influence of other parameters (e.g., transcription factor regulation) remains largely unexplored. Also, empirical data are lacking on the outcome of collisions between elongating RNAPs and RNAPs bound to promoters and how it could be regulated (e.g., by tuning promoter escape rates).
Here, we studied interference between promoters in tandem formation. For this, we engineered synthetic constructs of promoters in tandem formation using three genetically modified natural promoters: PLacO3O1, PtetA, and PBAD, as building blocks.14−16 The internal composition of our synthetic constructs is illustrated in Figure 1. In all constructs, the promoter(s) control the expression of an mCherry protein to track their transcription dynamics. We used the constructs to study the interference as a function of the regulation state of the component promoters and when subject to an antibiotic that directly interferes with transcription initiation.17
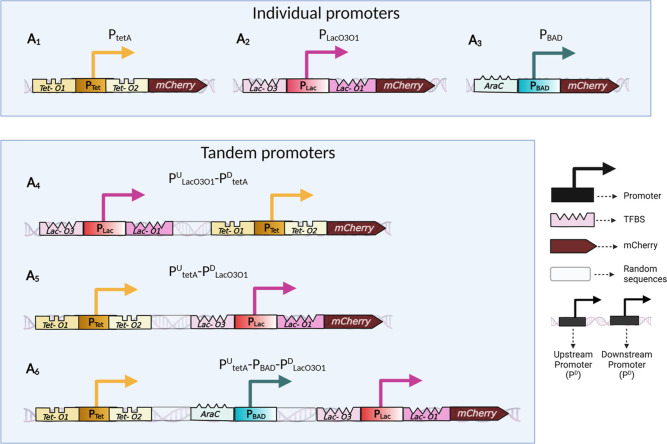
Figure 1.
Schematic representation of the synthetic promoters in tandem formation along with their transcription factor binding sites. Bioparts (A1–A6) were each inserted into single-copy pBAC plasmids. Each biopart is followed by an mCherry coding region. Bioparts (A1,A2,A3) are the individual promoter constructs. Bioparts (A4,A5,A6) are the constructs with (pairs or trios of) tandem promoters. The component promoters in individual formation are PtetA, PLacO3O1, and PBAD. The two dual synthetic promoters in tandem formation are PLacO3O1U-PtetAD and PtetAU-PLacO3O1D, (U and D stand for upstream and downstream, respectively). Finally, the trio of promoters in tandem formation is PtetAU-PBAD-PLacO3O1D.
From Figure 1, the fluorescence probes of the tandem constructs allow tracking the overall dynamics of the component promoters (Figure 1A4 to 1A6) but not the dynamics of each component promoter independently. For that, we used individual promoter constructs (Figure 1A1–A3). From here onward, we referred to the constructs carrying promoters in tandem formation as “tandem promoters” (e.g., tandem promoters “PtetAU-PLacO3O1D”). Meanwhile, we referred to the constructs carrying a single promoter as “individual promoters” (e.g., “individual promoter PtetA”).
Results
The promoters used to build the tandem promoters have been profusely studied. PLacO3O1 was engineered from the natural Lac promoter of the lactose operon in E. coli,18 by removing the operator O2.19,20 This decreases by 2- to 3-fold the repression strength of the wild-type tetrameric Lac repressor (LacI).19 The homotetrameric Lac repressor protein represses by binding the DNA operator sequences.21 The binding forces DNA loops22,23 that make the promoter less accessible.24 Contrarily, IPTG, a structural analogue of the natural inducer Lactose,25 indirectly induces PLacO3O1 by binding to LacI, which hampers LacI’s ability to bind to the promoter.21
The second promoter, PtetA, used was extracted in its natural form from the Tet operon in E. coli, where it controls the expression of the tetA gene.26 The Tet operon is involved in tetracycline resistance and can self-repress due to carrying a second gene, tetR, coding for TetR.27 This protein binds to the operator sites of the promoters of tetA and tetR and prevents their transcription.28 Meanwhile, tetracycline binds to TetR, hampering its ability to bind to the DNA, thus enabling the expression of tetA and tetR.29 aTc, an analogue of tetracycline, can induce PtetA by the same process.30
The final promoter used, PBAD, was also extracted in its natural form. Originally, it is a component of the l-arabinose operon of E. coli.31 It can be repressed by dimers of AraC, which can form a DNA loop that blocks transcription.32 Arabinose can induce PBAD by binding to the dimeric AraC. This binding changes the conformation of AraC, which breaks the DNA loop, allowing RNAP to bind to PBAD.33
A recent work used similar promoters to study the dynamics of genes in convergent, divergent, and tandem formations.34 However, unlike in our constructs, the elongation regions were separated by Rho independent hairpin loops. Thus, the RNAPs transcribing from one promoter should not collide with RNAPs transcribing from the other promoter. Among others, they used the constructs to study the influence of supercoiling buildup on closely spaced genes.
Next, we describe the assembly of the genetic constructs. Afterward, we study their dynamics and show that, in tandem formation, their strength is reduced due to collisions between RNAPs leading to premature transcription terminations. We also show that this phenomenon can be fine-tuned by inducers of the regulatory mechanisms of the component promoters and by a transcription-targeting antibiotic. Moreover, we use a stochastic model to show that the phenomena identified suffices to explain the observed dynamics of the promoters in tandem formation. In the end, we discuss how our constructs may become valuable components of future synthetic genetic circuits.
Assembly of the Synthetic, Nonoverlapping Tandem Promoters in Single-Copy Plasmids
We designed the tandem constructs using Snapgene (GSL Biotech) and assembled them at Integrated DNA Technology, Iowa, U.S.A. Next, we introduced them into single-copy plasmids (pBAC).35 The original sequences of PLacO3O1, PtetA, and PBAD (Methods Section “Bacterial strains, growth conditions, induction, and antibiotic”) were not altered. We distanced the TSSs of the pairs of promoters in tandem formation by 150 bp (Figure 1A4,A5), while the trio of promoters were separated by 200 bp, each (Figure 1A6). For that, we introduced random sequences between the promoters generated using Random.org. Snapgene confirmed the absence of sequences known to lead to the formation of secondary RNA structures (e.g., hairpin loops) as well as sequences coding for translational products.
In addition to the tandem promoters (“biopart”), each plasmid has a sequence coding for a fluorescent protein, mCherry36 (plasmid accession number KX264176.135,36), immediately downstream of the biopart. The DNA code includes a strong RBS to ensure strong translation rates to robustly track promoter activity. Moreover, the maturation time of mCherry (FPbase ID: ZERB6) is 15 min37 to closely report RNA production rates. We also produced three control strains, each carrying one individual promoter (PLacO3O1, PtetA, and PBAD) (Figure 1A1–A3).
Since all constructs have the same RNA degradation rate and mCherry translation and degradation rates, differences in fluorescence intensities between strains should be caused mostly by differences in overall transcription rates alone. To best ensure this, small differences in growth rates between strains were accounted for (next section). Finally, since some tetracycline derivatives exhibit fluorescence,38 we tested if the inducer aTc could interfere with the measurements. However, within the range of concentrations used, aTc did not affect cell fluorescence substantially (Figure S1).
Cell Morphology and Physiology; Variability in Single Cell Expression Levels
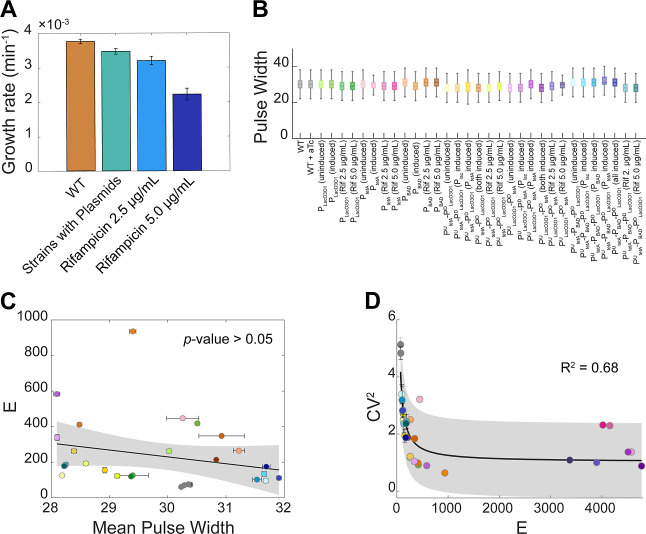
We searched for potential physiological and morphological differences between strains that could affect the constructs’ expression. First, we monitored cell growth rates of all strains in all measurement conditions studied throughout the manuscript (Figure 2A). Strains carrying the plasmids have a slightly slower growth rate than the wilt type, WT, strain (8% weaker). For comparison, rifampicin (5 μg/mL) reduced growth by much more (41%). Given the existence of differences, from here onward, we accounted for them by normalizing mean population expression levels obtained by spectrophotometry using data on cell population sizes from O.D.600 measurements (Methods Section “Spectrophotometry”).
Figure 2.
(A) Average growth rates (O.D.600 measurements by spectrophotometry). (B) Boxplots of the distributions of single-cell pulse width, used as a proxy for cell size. The outliers (values higher or lower than 1.5·IQR, where IQR is the interquartile range) are not shown. (C) Scatter plot of the mean single-cell fluorescence, E, and the mean pulse width. Also shown are the best-fitting line and corresponding p-value, after excluding outliers (not shown) (Methods Section” Fitting and statistical analysis”). The correlation is not significant at the 5% significance level, even when including outliers (Figure S2). (D) Scatter plot between the CV2 and the mean single-cell fluorescence intensities (E). Also shown are the best-fitting curve and the corresponding R2 (Methods Section “Fitting and statistical analysis”). The shaded area represents the 95% confidence interval. Also shown is a best (black line) fitting curve, whose equation and coefficient values are shown in Table S4p. Finally, the error bars in (A,C,D) correspond to the standard error of the mean of 3 biological replicates.
Next, we observed cell sizes using pulse width from flow cytometry as a proxy.39 The pulse width differs little between strains and conditions (Figure 2B). In agreement, we also could not find a significant correlation between mean single-cell protein expression levels and mean single-cell pulse widths (Figure 2C). Thus, gene expression intensities obtained by flow cytometry were not normalized by cell sizes.
Finally, we searched for correlations (in flow cytometry data) between mean single-cell expression levels (E) and cell-to-cell variability (as measured by the squared coefficient of variation, CV2) in our tandem constructs. Past studies40,41 suggest that in E. coli, at the genome wide level, these two quantities should correlate in accordance with eq 1
| 1 |
where ‘c’ is the ratio between noise and mean, and “n” is the “noise floor”42 (i.e., is the lower limit of noise). From Figure 2D, given the high R2 value, we conclude that our constructs also exhibit the relationship in (1).
Interpromoter Spacing between Synthetic, Nonoverlapping, Tandem Promoters Does Not Necessarily Influence Their Dynamics
A previous study reported the single-cell dynamics of several natural tandem promoters of E. coli under standard growth conditions and in poor media.11 When plotting the mean expression levels of the genes controlled by these promoters as a function of the nucleotide distance between their TSSs, it was observed that the most influential variable was whether the promoters were close enough so that two RNAPs bounded to the TSSs would, or not, overlap. Aside this, the distance between the promoters was not influential in that set of tandem promoters. Using our synthetic constructs, here we also studied whether the interpromoter spacing influences the expression dynamics of the constructs. For this, we produced two new constructs whose interpromoter spacing is larger than the original constructs. Specifically, we added 100 bp to the original PULacO3O1-PDtetA and 200 bp to the original PUtetA-PDLacO3O1 (also designed by Snapgene, assembled at Integrated DNA Technology, Iowa, U.S.A., and introduced into single-copy plasmids pBAC).
These distances were selected to account for most of the known interpromoter distances of natural tandem promoters of E. coli. To determine this, we obtained information from RegulonDB43 (v. 12.5.0) regarding the approximately 330 genes of E. coli’s controlled by two and only two promoters in tandem formation. Interspacing distances were limited to 1000 bp to remove outliers, likely produced by present limited knowledge on which sequences cause elongation terminations (e.g., from RegulonDB, we found that approximately 800 operons have been identified in E. coli, but only approximately 200 of them have a known TTS). The resulting distribution of interpromoter spacings is shown in Figure S8. Visibly, the bulk of the distribution (approximately 85%) is below 350 bp.
We compared the gene expression levels of the new strains with the original strains, when subject to the same (maximum) induction schemes (Materials and Methods Section “Bacterial strains, growth conditions, induction, and antibiotics”). From Figure S9, the mean response strengths are similar, i.e., the expression differs by less than 4% in both cases. Moreover, we found relatively small standard deviations and standard errors of the mean between the three repeats in both cases. Overall, this suggests that, in the case of these synthetic constructs of nonoverlapping tandem promoters, the interpromoter spacing does not exert a strong influence in the expression levels, within the range of spacing tested. Thus, for simplicity, from here onward, we use only the strains carrying the plasmids whose TSSs are distanced by 150 base pairs.
Noteworthy, these conclusions do not necessarily apply to other synthetic tandem constructs. Moreover, for several reasons, they may not apply to many natural promoters in tandem formation. For example, in highly expressing regions of the chromosome, one can expect high rates of positive supercoiling buildup.44 Likely, in such regions, the distance between tandem promoters could be an influential parameter. Similarly, in DNA regions with high rates of RNAP premature transcription terminations, we expect the distance between tandem promoters to be influential as well.
Additive Model of Transcription of Promoters in Nonoverlapping Tandem Formation
Our promoters, in individual formation, produce standard nonlinear saturation dose–response curves of protein expression levels (‘E’) (Figure S6). These saturations of the maximum expression rates are caused by finite, non-negligible RNAP occupancy times of the TSSs prior to promoter escape, finite number of RNAPs in the cells, and finite rates for RNAP-promoter binding rates, among others. Similarly, at the translation level, there are finite number of ribosomes in the cells and finite rates of ribosome-RNA binding. Meanwhile, the differences in these limits between genes are made possible by differences in promoter sequences and the specific regulatory mechanisms. In general, the expression protein levels of all constructs are expected to follow eq 2
| 2 |
here, ai represents the maximum expression level of a gene and bi represents the steepness at which the expression increases with the increase in the concentration of transcription limiting factors, x. Finally, ci is the level of limiting factors “x” at which the expression is half of its maximum value.
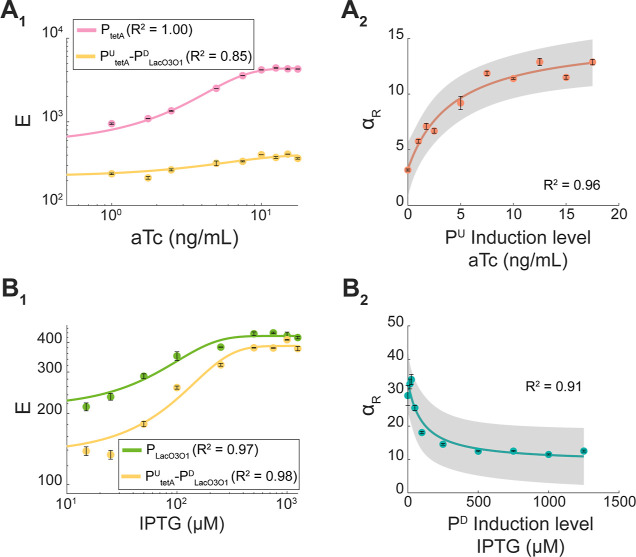
Importantly, below, we show that the tandem constructs studied here also exhibit expression levels that can be well-fit by eq 2 (Figure 5A1,B1).
Figure 5.
(A1) Mean single-cell expression level (E) of PtetA and of PtetAU-PLacO3O1D, when inducing PtetA. In all conditions, PLacO3O1D is fully induced. (A2) Interactivity leading to attenuation (i.e., αR > 1) of the tandem promoters as a function of the induction strength of the upstream promoter. (B1) Mean single-cell expression level (E) of PLacO3O1 and of PtetAU-PLacO3O1D, when inducing PLacO3O1. In all conditions, PtetAU is fully induced. (B2) Interactivity leading to attenuation (i.e., αR > 1) of the tandem promoters as a function of the induction strength of the downstream promoter. In all plots, the error bars correspond to the standard error mean of three biological replicates. Also shown are best fitting curves and the corresponding R2 (Methods Section” Fitting and statistical analysis”). The shaded areas represent the 95% confidence intervals. The equation and coefficient values of the fitted curve are shown in Table S4. Note that the axes of (B1) and (A1) are in logarithmic scale. All fitting curves and best fitting parameter values are shown in Table S4. Data points of the induction curves in (A1) and (B1) were, as expected, well fitted by logistic functions. Meanwhile, the data points in (A2,B2) are well-fitted by rational curves.
To study the dynamics of nonoverlapping, tandem promoters, let α be the difference between their mean expression level and the summed mean expression levels of the component promoters in individual formation. Also, let T stand for the tandem promoters’ formation, while U and D stand for the upstream and downstream promoters of that formation, respectively. Contrarily, the abbreviation “ind” informs that a specific mean expression level corresponds (instead) to a promoter in individual formation. Given this, α in tandem promoters is quantified by
| 3 |
According to this formula, α will be positive if the promoters in tandem formation have a lower expression level than the sum of their expression levels in individual formation, when under the same induction scheme (i.e., have attenuated RNA production). Contrarily, α will be negative if the tandem construct has higher expression than the summed individual expression levels (suggesting enhanced RNA production). Finally, α should equal 0 only when the tandem promoters do not interact with each other.
Noteworthy, this model assumes that no factor influences the dynamics of nonoverlapping tandem promoters, other than the interference resulting from the interactions between the RNAPs starting from the upstream promoter and the RNAP or TFs bound to the downstream promoter. For example, based on the results of the previous section, the model assumes that placing the upstream promoter more distanced from the region codding for mCherry, in order to obtain a nonoverlapping tandem formation, does not influence its transcription initiation dynamics tangibly (when compared to when in the individual formation).
Promoters in Nonoverlapping Tandem Formation can Have Attenuated Overall Expression Rates
Next, we studied the dynamics of the dual synthetic tandem promoters PLacO3O1U-PtetAD and PtetAU-PLacO3O1D from single-cell fluorescence data under three induction schemes: (i) upstream promoter induced; (ii) downstream promoter induced; and (iii) both promoters induced. For comparison, we also studied the individual promoters PLacO3O1 and PtetA, when and when not induced. The mean (“E”), standard deviation, CV2, and skewness and kurtosis of the distributions of single-cell expression levels are shown in Supporting Information.
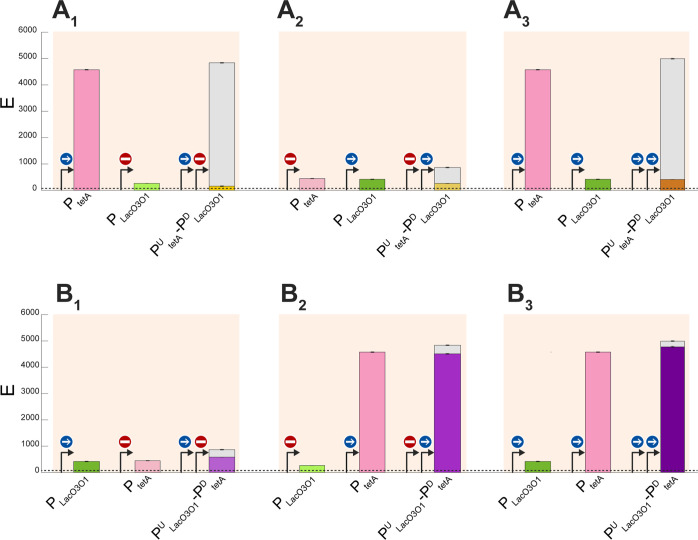
From Figure 3, in both tandem constructs under all induction schemes, α > 0. Thus, in general, placing these promoters in tandem formation attenuates overall protein expression rates.
Figure 3.
Mean expression intensity (E) of the tandem and the individual promoters, under various induction schemes. The blue sign with a straight white arrow stands for “fully induced”, while the red “Do Not Enter” sign stands for “fully repressed” in (A1–A3) PtetA, PLacO3O1, and PtetAU-PLacO3O1D and (B1–B3) PtetA, PLacO3O1, and PLacO3O1D-PtetAU. In all plots, the height of the gray bars is a measure of the interactivity, as quantified by α defined in eq 3. The error bars correspond to the standard error of the mean of three biological replicates. The dashed horizontal line near 0 marks the autofluorescence intensity of WT cells. The expression levels of the individual promoters, PtetA and PLacO3O1, are shown more than once to facilitate comparing the tandem constructs with the component promoters in individual formation.
Meanwhile, for PtetAU-PLacO3O1D alone: ET < EUind and ET < EDind (Figure 3A1–A3), i.e., placing the weaker promoter downstream heavily reduced the RNA production rate of the upstream promoter, causing PtetAU-PLacO3O1D expression to be weaker than the downstream promoter alone.
Conversely, for PLacO3O1U-PtetAD, in one condition, we find that ET > EUind and ET < EDind (Figure 3B2). Interestingly, we also observe that when PLacO3O1 is induced: ET > EUind and ET > EDind (Figure 3B1,B3), i.e., the tandem promoters PLacO3O1U-PtetAD express more strongly than the individual downstream promoter.
Finally, inducing the upstream promoter when the downstream promoter is already active (i.e., comparing Figure 3A3 with 3A2) can result in increased overall expression of the tandem constructs, along with increased attenuation. Dynamically, as the number of RNAPs elongating from the upstream promoter increases, so will increase RNA production. However, expectedly, since the downstream promoter is transiently occupied, this will also cause more of the RNAPs elongating from the upstream promoter to be blocked due to collisions, enhancing attenuation when compared to the individual promoter activity.
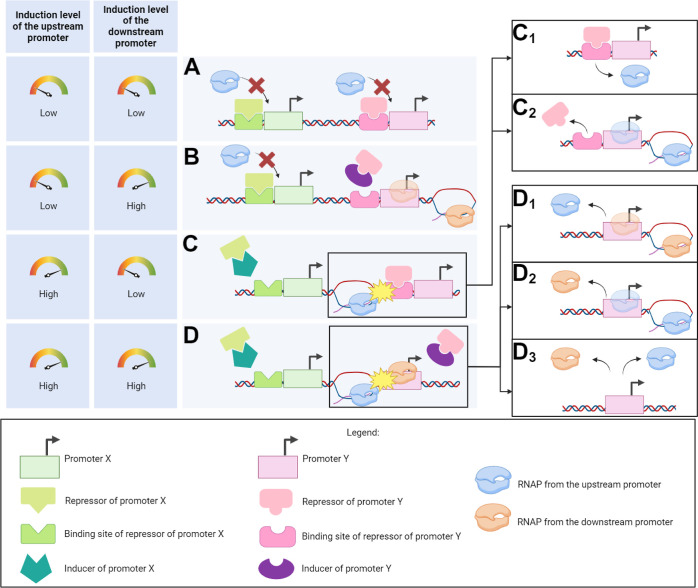
From the above, we interpret α > 0 in both constructs, in all conditions (Figure 3A1–A3,B1–3B3), as evidence that a significant number of RNAPs elongating from upstream promoters are prematurely terminated (falloff), explaining the observed attenuation. When the downstream promoter is repressed, the fall-offs are likely due to the repressors’ binding (Figure 4C). Conversely, when the downstream promoter is active, the fall-offs are likely due to collisions between the elongating RNAP from the upstream promoter with an RNAP bound to the downstream promoter (Figure 4D).
Figure 4.
Illustration of transcription interference events generating attenuation in tandem promoters under different induction schemes. (A) Weak-to-no induction of both the upstream and the downstream promoters. (B) Weak-to-no induction of the upstream promoter along with high induction pf the downstream promoter. (C) High induction of the upstream promoter and weak-to-no induction of the downstream promoter. (D) High induction of both the upstream and downstream promoters. In (C), collisions between RNAPs elongating from the upstream promoter and the repressor at the downstream promoter are likely to occur. Those collisions can cause (C1) falloff of the elongating RNAP or (C2) falloff of the repressor bound to the operator site. Similarly, in (D), the RNAP elongating from the upstream promoter can collide with the RNAP occupying the downstream promoter. This causes the elongating RNAP to falloff in (D1), the RNAP at the downstream promoter to falloff in (D2), or both RNAPs to falloff in (D3).
Moreover, we interpret the existence of a tandem construct with weaker activity than either of its component promoters (Figure 3A1–A3) as evidence that several collisions cause fall-offs of both the RNAP bound to the downstream promoter as well as the elongating RNAP (Figure 4D3). If this never occurred, the tandem promoters should be at least as strong as the downstream promoter alone.
Finally, we interpret the existence of a tandem construct with stronger activity than the individual downstream promoter (Figure 3B1,B3) as evidence that, in some tandem promoters, neither the repressors (Figure 3B1) nor RNAPs initiating transcription (Figure 3B3) at the downstream promoter can block all RNAPs elongating from the upstream promoter (Figure 4C2,D2).
Interactivity in Synthetic Tandem Constructs can be Fine-Tuned by Induction of the Component Promoters
We investigated if the interactivity can be fine-tuned, i.e., sensitive to external regulation of the activity of the component promoters. We tested in PtetAU-PLacO3OD, since it exhibited stronger attenuation. For this, let αR be the ratio between the summed expression levels of the promoters in individual formation and the expression level of the corresponding promoters in tandem formation
| 4 |
We started with a fully induced PLacO3O1D and then gradually induced PtetAU. Visibly, PtetAU-PLacO3O1D is less induced than PtetA alone (Figure 5A1), and αR increases with induction of the upstream promoter (Figure 5A2). This can be explained by an increase in the fraction of RNAPs from the upstream promoter that fail to complete elongation. Such fall-offs could be explained by the transient occupation of the (active) downstream promoter by RNAPs initiating transcription.
Next, we started with a fully induced PUtetA and gradually induced the downstream promoter, PDLacO3O1. Again, the individual promoter (PtetA) is more strongly induced than the tandem construct, implying the existence of attenuation (Figure 5B1). Nevertheless, contrary to before, αR decreased with induction (Figure 5B2). This is expected since repressors bound to the DNA are expected to be more efficient in blocking RNAPs elongating from the upstream promoter than RNAPs occupying the downstream promoter. Consequently, as the repression mechanism of the downstream promoter is inactivated, more RNAPs from the upstream promoter can complete elongation.
Given all of the above, we conclude that αR can be fine-tuned by the regulatory mechanisms of the component promoters in diverse ways. Moreover, we expect it to be possible to achieve more complex behaviors than solely increasing or decreasing with induction levels (as assumed in eqs 2 and 3), depending on how we combine individual promoters (e.g., it could be possible for αR to first increase and then decrease with induction strength, etc.). Nevertheless, since the expression levels of the component promoters saturate, so will αR.
Finally, as a note, in the case of natural promoters in tandem formation, rather than using chemicals for the external regulation αR, it should be possible to make use of their natural transcription factor regulators. This may be less disturbing for the basic biology of the cells.
Adding Another Promoter in Tandem Formation can Enhance Attenuation
We next introduced an additional promoter, PBAD, in between two tandem promoters (PtetAU-PLacO3O1D). From this resulted a trio of promoters in tandem formation: PtetAU-PBAD-PLacO3O1D. To study their interactivity, we modified α defined in eq 3, so as to account for the additional promoter in the middle. Let M stands for “Middle”. We define αtrio as
| 5 |
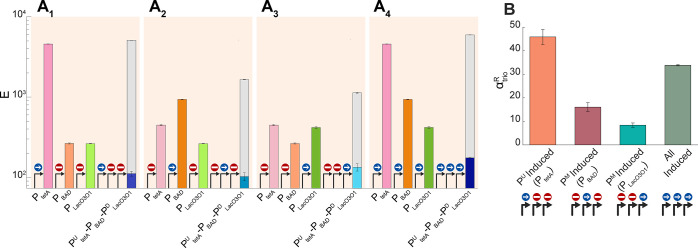
As expected, PtetAU-PBAD-PLacO3O1D activity is also weaker than the sum of individual promoters’ activity, i.e., in all induction schemes (Figure 6A1–A4), αtrio > 0.
Figure 6.
(A1–A4) Mean expression intensities (E, in logarithmic scale) of the trio tandem promoters (PtetAU–PBAD-PLacO1O3D) and of each individual promoter. We tested inducing only the most upstream promoter (A1), inducing only the middle promoter (A2), inducing only the most downstream promoter (A3), and inducing all promoters at the same time (A4). The gray bars measure the interactivity as quantified by, αtrio, defined in eq 5. (B) Interactivity, as quantified by αtrioR (eq 5) of the trio of tandem promoters under various induction schemes. In all plots, error bars correspond to the standard error of the mean of 3 biological replicates.
Conversely, unlike the dual tandem promoters, the ET of the trio of tandem promoters is smaller than EUind, EMind, and EDind, alone, in all induction schemes (Figure 6A): ET < EUind, EMind, EDind. This is evidence for the occurrence of significant collisions between RNAPs elongating from the upstream promoters with repressors (causing the falloff of the RNAP) and with RNAPs occupying the downstream promoters (causing the fall-offs of both colliding RNAPs).
Meanwhile, the strongest relative αtrio (eq 6) still occurs when the most upstream promoter is the only one induced (Figure 6B), suggesting that, as expected, collisions between elongating RNAPs and repressors bound to the DNA usually cause the collision of the RNAP. Interestingly, αtrioR is also high when all promoters are induced. The simplest explanation is that numerous RNAPs elongating from the upstream promoters are prematurely terminated by collisions with RNAPs occupying the downstream promoters and that several collisions cause both colliding RNAPs to falloff.
| 6 |
Attenuation is Enhanced When Hampering Promoters Escape by Adding Rifampicin
Above, we argued that several RNAPs are prematurely terminated by collisions of RNAPs elongating from upstream promoters with RNAPs sitting at downstream promoters. If this holds true, increasing the fraction of time spent by RNAPs occupying a downstream promoter should increase αR.
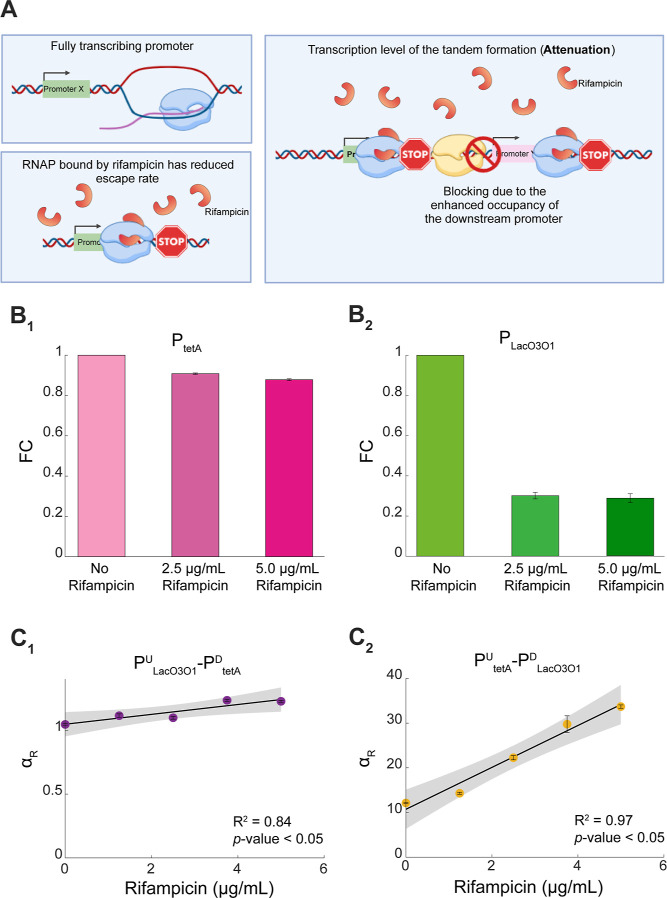
To test this, we subjected cells to rifampicin (Methods Section “Fitting and statistical analysis”). This antibiotic binds to the β subunit of RNAP.45 When bound, the RNAP cannot escape beyond 2–3 nucleotides away from the TSS.46,47 Thus, rifampicin should not only reduce the activity of each component promoter but also increase αR due to increasing the occupancy time of RNAPs at the downstream promoter (illustrated in Figure 7A). Other direct effects on gene expression are not expected since, e.g., rifampicin does not affect stable transcription elongation complexes.48,49
Figure 7.
Effects of rifampicin on the interactivity in tandem promoters. (A) Illustration of how rifampicin reduces the activity of an individual promoter, as well as of promoters in tandem formation. The latter should be further affected by enhanced attenuation. (B1–B2) Fold-change in mean expression levels (FC) of PtetA and PLacO3O1 due to rifampicin. (C1–C2) αR of PtetAU-PLacO3O1D and PLacO3O1U-PtetAD, when subject to rifampicin. The error bars correspond to the standard error of the mean of three biological replicates. Shown are the best-fitting lines and their p-value and R2 (Methods Section “Fitting and Statistical Analysis”). The shaded areas represent the 95% confidence interval. The equation and coefficient values of the fitted lines are shown in Table S4.
As expected, rifampicin reduced the activity of both PtetA as well as PLacO3O1 in individual formations (Figure 7B1,B2, respectively). PLacO3O1 was (relatively) more affected than PtetA, for unknown reasons (also visible in spectrophotometry data in Figure S3). Also, for unknown reasons, PtetA activity reduction was approximately linear with rifampicin concentration, while PLacO3O1 had a sharp initial decrease in expression, but further increasing rifampicin concentration did have notable additional effects.
Most importantly, we also observed that rifampicin gradually increased αR in both tandem constructs (Figure 7C1,C2), as hypothesized. Noteworthy, the higher sensitivity of PLacO3O1 to rifampicin explains why αR of PtetAU-PLacO3O1D changed the most (Figure 7C1,C2).
These results exemplify how the interactivity in tandem promoters can be controlled by external regulation and, with that, produce different (yet predictable) gene expression dynamics.
Notably, it is likely that many natural tandem promoters will not respond to rifampicin identically to what we observed. First, rifampicin has broad effects on the genome-wide expression levels of E. coli cells and can harm cell health. Thus, antibiotic response mechanisms may have evolved that, at least partially, will cause natural tandem promoters to respond to rifampicin differently from our constructs. In line with this, our synthetic promoters are transformed into single copy plasmids and are not subject to natural TF’s regulation, among other, which should influence how their kinetics changes with rifampicin. Nevertheless, on average, at a genome-wide level, we expect that increasing rifampicin should increase the propensity for collisions between RNAPs from natural closely spaced promoters in tandem formation.
Model of Transcription Interactivity of Promoters in Tandem Formation due to RNAP Collisions and Consequent Fall-Offs can Explain the Empirical Data
Above, we observed that the tandem promoters always exhibit attenuation. In PtetAU-PLacO3O1D, the attenuation was strong, in general, making expression levels lower than in the individual downstream promoter. Instead, in PLacO3O1U-PtetAD, the attenuation was weak, allowing, in some conditions, almost as strong expression as the sum of expressions of the individual component promoters. In both tandem promoters, attenuation was present when the downstream promoter was activated, as well as when it was repressed, in which case it was stronger.
In this section, we propose a general stochastic model for tandem promoters. Shortly, the model includes a two-step transcription initiation process at each promoter, to account for promoter occupancy by RNAPs. Repressors can also occlude promoters, while inducers inactivate repressors. Also, RNAs degrade. Finally, we model collisions between RNAPs, as well as between RNAPs and repressors occluding promoters. Critically, we assume that the collisions between RNAPs can cause fall-offs of one or both RNAPs. Meanwhile, collisions between RNAPs and repressors always cause the RNAPs to falloff. A complete model description is provided in Section S1. Using this model, we show that various degrees of attenuation observed above can be explained by fall-offs emerging from RNAP–RNAP and RNAP-repressor collisions.
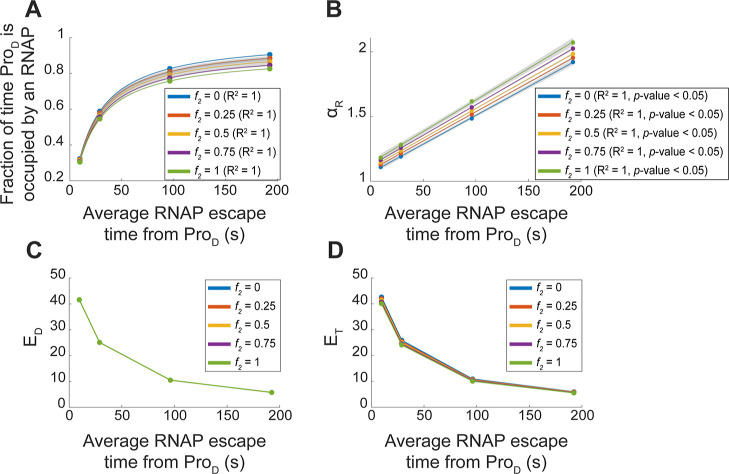
First, we tested whether increasing the time that the downstream promoter is occupied by initiating RNAPs increases attenuation (due to increased rate of collisions and subsequent fall-offs). For this, we decreased the rate of RNAP escape from the downstream promoter (kescD) (similar to the effects of increasing rifampicin, Figure 7C1–C2). This increased the mean amount of time that the downstream promoter is occupied by RNAPs (Figure 8A). This, in turn, increased linearly αR (Figure 8B), as in Figure 7C1–C2. Also, in agreement with the empirical data (Supporting Information), it decreased EDind (Figure 8C) and ET (Figure 8D).
Figure 8.
Estimations using the stochastic models of the effects of increasing the average time for RNAPs to escape the downstream promoter (ProD), by decreasing kescD, as well as of changing the frequency with which RNAP collisions cause both RNAPs to falloff, instead of only one falling-off. (A) Fraction of time that the downstream promoter is occupied by an RNAP as a function of the inverse of the RNAP escape rate. Note that the latter is affected also by collisions between RNAPs. (B) Interactivity as measured by αR in tandem promoters as a function of the average RNAP escape times from downstream promoters. (C,D) Expression levels of the individual downstream promoter (EDind) and of the tandem promoters (ET) as a function of the average RNAP escape times from the downstream promoter. In all plots, each colored line represents a different relative frequency (f2) of both RNAPs falling-off upon colliding (as opposed to only one RNAP falling-off). (A,B) also show the best fitting functions along with their R2 values (and p-values in case of linear fits) (Methods Section” Fitting and statistical analysis”). The shaded areas represent the 95% confidence intervals. The equation and coefficient values of the fitted lines are shown in Table S4.
Next, we tested increasing the frequency f2 with which RNAP–RNAP collisions cause both RNAPs to falloff (instead of only one RNAP falling-off). We observed, first, a small decrease in the fraction of time that the downstream promoter is occupied by RNAPs (Figure 8A), as expected from the increased fall-offs of the initiating RNAPs. In agreement, we observe increased αR with increased f2 (Figure 8B). Meanwhile, the quantitative relationships between EDind and ET with the average RNAP escape rates (Figure 8C,D) are not influenced (since both change accordingly).
We also observed that, for higher frequencies of double RNAP fall-offs, ET can become slightly weaker than EDind (Figure S4A). We explored this by tuning the binding and escape rates of RNAPs to ProU and ProD. From Figure S4B, there is a wide range of parameter values (Table S3) for which ET can be considerably weaker than EDind (in agreement with the empirical data in Figure 3A1–A3).
Finally, we tested with the model if the induction-repression mechanisms can increase and decrease αR with increasing induction, depending on whether it is the upstream or the downstream promoter that are initially repressed. Figure S5 supports that this is possible.
Overall, we conclude that the model can explain all behaviors observed in the two tandem constructs.
Discussion
Above, we found that premature transcription terminations of RNAPs elongating from upstream promoters and of RNAPs occupying downstream promoters can cause substantial attenuation of tandem constructs. The attenuation can differ widely with the active transcription initiation dynamics of the component promoters, allowing for the expression of the tandem constructs to range from weaker than either component promoter up to similar (but never higher) than the sum of the expression dynamics of the two promoters. Moreover, the attenuation can be further externally tuned using the natural regulatory mechanisms of each promoter and/or using antibiotics targeting, e.g., transcription. This tunability could allow tandem formations to execute fine-tuned expression dynamics.
The dynamics of our tandem constructs were largely predictable by a simple model that only requires knowledge of the dynamics of the component promoters in individual formation, along with the relative rate of collisions that lead to two (instead of one) RNAP fall-offs. This rate should differ with the binding affinity of RNAPs or repressors to the downstream promoter and can be empirically estimated. This model-based predictability is of importance since it remains an important and difficult challenge in synthetic biology. For example, we are yet to efficiently predict how random DNA sequences express, even in bacteria, albeit notable recent successes.50−57
On the contrary, knowledge of the dynamics of individual, natural promoters (e.g., in E. coli) is relatively easy to acquire, and the data are rapidly increasing.41,43,58−60 Using these increasing libraries, along with the model proposed, it should be feasible to engineer novel tandem promoters with desired, diverse dynamics. These novel constructs could then be used, e.g., as building blocks for future, more complex circuits.
As an example, genetic switches are circuits composed of two genes repressing one another. Because of this, they are expected to have two possible states: either one gene is “ON” and the other one is “OFF”, or vice versa. One of the most promising applications of these circuits is as components of information processing circuits (e.g., for storing information). The main difficulty in achieving this is in tuning a switch to be both sufficiently sensitive to changes supposed to control their state, while also being robust in maintaining the state, if no relevant changes occur in the cell. This requires specific expression levels of both genes of the switch61 and depends on promoter occupancy times by RNAP and repressors, repressor-activator binding and unbinding rates, and other parameters.62 Finding natural promoters with the desired features or changing their sequence to achieve them is presently complex. Meanwhile, our results suggest that starting with a large library (such as41,60) and then using the model to find combinations of two promoters in specific states that achieve a desired dynamics is a more promising approach.
Notably, it is possible that many natural tandem promoters will not necessarily follow accurately the behavior of the constructs studied here, for several reasons. First, our constructs are on plasmids. Thus, the levels of positive supercoiling buildup should differ from the ones that natural promoters are subject to, which influences transcription initiation dynamics.44,63 Second, natural genes are subject to several regulatory mechanisms (e.g., TF regulation) that can affect, among other, how RNAP and promoters interact. These two reasons alone suffice to predict that there will be a significant diversity in the outcome of RNAP collisions between natural promoters in tandem formation.
In this regard, while not observed here, we do not exclude the possibility that a few promoters in tandem formation can be stronger than the sum of the two component promoters independently. For example, this could potentially be possible if elongating RNAPs from the upstream promoter would dislodge repressors at the downstream promoter, while not falling-off themselves. Weak repressor-DNA binding affinities could make this possible. However, we do not expect it to be common in natural genomes. Nevertheless, removing repressor binding sites (or altering their sequences) could facilitate it in synthetic circuits.
In the future, we plan to explore broadly how to tune the interactivity in synthetic, as well as in natural promoters in tandem formation. In addition to repression mechanisms and antibiotics hampering RNAP promoter escape, it may be possible to use supercoiling regulation for this, as it affects RNAP-promoter binding.44,64 In that case, the location of the tandem promoters in the DNA (e.g., if in highly or weakly expressing topological domains65,66) could already suffice to influence the interactivity. Other influential variables are the durations of open complex formation and promoter escape, as they influence RNAP occupancy times of the promoter.67 Changing both could even allow changing the interactivity without changing transcription rates.11
Overall, our findings, including the model proposed to predict interactivity levels, should allow establishing a novel pipeline for engineering promoters (in tandem formation) whose overall kinetics can be fined-tuned within a relative wide dynamic state space. This pipeline should contribute to the development of novel components for complex synthetic genetic circuits with predictable behaviors, making their assembly faster and cheaper. Subsequently, the novel complex circuits could contribute to the engineering of bacterial strains whose metabolic tasks have minimal resource consumption, so as to improve the efficiency of bioindustrial processes.
Materials and Methods
Bacterial Strains, Growth Conditions, Induction, and Antibiotics
We used the E. coli strain DH5α-PRO (identical to DH5αZ1, here named “wild type”, WT).14,15,68 This strain produces the necessary regulatory proteins (LacI, AraC, and TetR) that tightly regulate each of our promoter constructs.14
First, chemically competent (CC) E. coli DH5α-PRO cells were prepared for plasmid transformations. For each strain, 10 ng of the plasmid DNA was mixed with 50 μL of DH5α-PRO CC (1:10 ratio), and the mixture was incubated on ice for 30 min. Next, the mixture was kept at 42 °C in a water bath for 1 min. Finally, 800 μL of LB medium was added to the mixture, which was kept at 37 °C under aeration at 250 rpm for 1 h.
From each mixture, 200 μL was plated using the spread plate method on fresh LB agar plates (2%), prepared by supplementing with antibiotics (34 μg/mL chloramphenicol). Finally, the plates were kept overnight at 37 °C. The next day, three colonies were picked from each plate and inoculated in fresh LB medium, supplemented with antibiotics (34 μg/mL chloramphenicol). Afterward, the cells were incubated at 30 °C overnight with shaking at 250 rpm. The resulting cells were diluted into fresh M9 media (0.03 O.D.600) and were supplemented with 0.4% glycerol, amino acids, and vitamin solutions.11,15
The control condition was M9 medium at 30 °C. Cells were induced and given antibiotic treatment at the midexponential phase (O.D.600 ≈ 0.25–0.3). Flow cytometry data were collected 180 min after induction. The spectrophotometry time series started immediately after induction and continued for 700 min.
The LacO3O1 promoter was induced with 1 μM IPTG, the TetA promoter was induced with 15 ng/mL aTc, and the BAD promoter was induced with 0.1% arabinose (induction curves in Figure S6).15,68,69 The promoters are referred to as PLacO3O1, PtetA, and PBAD, respectively, for simplicity. In some experiments, cells were subjected to rifampicin (2.5, 1.25, 3.75, and 5.0 μg/mL).70 Rifampicin (on its own) does not influence cell fluorescence (Figure S7).
Spectrophotometry
We performed spectrophotometry to measure optical density at 600 nm (O.D.600), which we used to monitor cell growth. We used a Biotek Synergy HTX Multi-Mode Reader. We also measured mean cell fluorescence. We used the excitation and emission wavelengths for mCherry (575/15 nm and 620/15 nm, respectively) with a gain of 50. Time series data were captured every 20 min. We normalized the fluorescence by the O.D.600 to estimate the average single-cell fluorescence. For this, O.D.600 curves were time-shifted so that they reached 5% of their maximal O.D.600 at the same time point., as in.60
Flow Cytometry
We performed flow cytometry using an ACEA NovoCyte Flow Cytometer controlled by the software Novo Express V1.50. Cells were diluted 1:10000 into 1 mL of PBS, vortexed for 10 s. For each condition, we performed 3 biological replicates, acquiring 50,000 events for each replicate. We detected mCherry using the PE-Texas Red channel with an excitation of 561 nm and emission 615/20. We collected events at a flow rate of 14 μL/min (core diameter of 7.7 μM). We have set a minimum detection threshold in FSC-H at 5000 to remove small particle interference. We also discarded the 1% of the highest PE-Texas Red-H intensities detected, to remove outliers.
Fittings and Statistical Analysis
All best-fitting lines (e.g., Figure 2C) were obtained by linear regression (MATLAB). Fits of other functions (Figures 2D and 8A) were obtained by a nonlinear least-squares method based on the Trust-Region-Reflective algorithm (MATLAB). The goodness of fit of the best-fitting lines was estimated from R2 values.
Meanwhile, to determine if there is a significant correlation between any pair of variables, y and x, we used t-statistic with the null hypothesis that the slope of the best-fitting line (of a scatter plot between the variables) is not different from zero. For p-values, smaller than 0.05, we reject the null hypothesis.
To identify outlier data points (in Figure 2C), we used an iterative method to discard
outliers in linear correlations.40 Data
points (x,y) were classified as
outliers if their vertical distance from the best linear fit was larger
than the standard deviation of the distribution of y values (Figure S2). When applied, the
method identified all outliers in a single reading of the data, without
needing further iterations. Finally, the associated standard error
of the mean for calculations with fluorescence was calculated using
an error propagation method. In general, if z = f(x,y), then the error
of z is  , where
, where 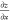 and
and 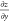 are the partial derivatives of z with respect to x and y, respectively.
are the partial derivatives of z with respect to x and y, respectively.
Stochastic Simulations
We used stochastic models to estimate how changes in the dynamics of the promoters (e.g., RNAP occupancy time, repressor occupancy time, and frequency of dual RNAP fall-offs compared to one RNAP falloff) influence the interactivity in tandem promoter formations. The model is described in Section S1. Simulations were performed using SGNS2,71 a stochastic gene networks simulator whose dynamics follow the Stochastic Simulation Algorithm.72 The time length of each simulation was set to 5 × 105 seconds (with a sampling interval of 100 s). This time length sufficed to reach quasi-equilibrium in RNA numbers. The average behavior of each condition was characterized from 100 independent runs, which always sufficed to obtain consistent results. Finally, for the various conditions, we assumed the parameter values in Table S1 and the initial amounts of reactants in Table S2.
Acknowledgments
The authors also acknowledge the Tampere Flow Cytometry Facility for their service. Figures 1, 4, 7A, and TOC were created with BioRender.com. The authors thank Cristina S. D. Palma for valuable discussions.
Data Availability Statement
A data package is deposited in Dryad with flow cytometry data, spectrophotometry data, and the constructs sequences and maps. The data are available at the link: https://doi.org/10.5061/dryad.jm63xsjgt. The constructs will be available for distribution in Addgene.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.4c00012.
Stochastic model of non-overlapping tandem promoters, single-cell fluorescence levels, probability density function of mean single-cell fluorescence levels, average single-cell fluorescence levels, in silico expression levels of individual downstream promoters, induction curves, fluorescence levels, distribution of inter-promoter spacing, and mean single-cell expression levels (PDF)
Tables with the mean, standard deviation, CV2, skewness, and Kurtosis of single-cell distributions of expression levels obtained by flow-cytometry (XLSX)
Author Contributions
A.S.R. and V.C. conceived the study. A.S.R. supervised the study. V.C. designed the constructs and planned and executed most measurements. I.S.C.B. executed most data analysis. SD assisted in the design of the constructs and measurements. A.M.A. conducted measurements. V.C. and R.J. contributed to the data analysis. A.S.R, R.J., and I.S.C.B. developed the model. I.S.C.B. implemented the model. A.S.R., V.C., and I.S.C.B. drafted all documents, which were revised by all coauthors.
We thank Jane and Aatos Erkko Foundation (10-10524-38 to A.S.R.); Sigrid Jusélius Foundation (to A.S.R.); Suomalainen Tiedeakatemia (to SD and to R.J.); EDUFI Fellowship (TM-21-11655 to R.J. and TM-23-11984 to A.M.A.); Finnish Cultural Foundation (00222452 and 00232457 to I.S.C.B.). Tampere University Graduate Program (to V.C.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Adachi N.; Lieber M. R. Bidirectional Gene Organization: A Common Architectural Feature of the Human Genome. Cell 2002, 109, 807–809. 10.1016/S0092-8674(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Trinklein N. D.; Aldred S. F.; Hartman S. J.; Schroeder D. I.; Otillar R. P.; Myers R. M. An abundance of bidirectional promoters in the human genome. Genome Res. 2004, 14, 62–66. 10.1101/gr.1982804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert M.; Kolb A.; Buc H. Overlapping promoters and their control in Escherichia coli: the gal case. Proc. Natl. Acad. Sci. U.S.A. 1986, 83, 2807–2811. 10.1073/pnas.83.9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen K. M.; Erdossy J.; Csiszovszki Z.; Svenningsen S. L.; Sneppen K.; Krishna S.; Semsey S. Direct and indirect effects in the regulation of overlapping promoters. Nucleic Acids Res. 2011, 39, 6879–6885. 10.1093/nar/gkr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F.; Warren R. A. Divergent promoters, a common form of gene organization. Microbiol. Rev. 1988, 52, 318–326. 10.1128/mr.52.3.318-326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott E. M.; Proudfoot N. J. Transcriptional collision between convergent genes in budding yeast. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 8796–8801. 10.1073/pnas.132270899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A.; Parsons G. D. Tandem promoters in the gene for ribosomal protein S20. J. Biol. Chem. 1983, 258, 7840–7846. 10.1016/S0021-9258(18)32255-5. [DOI] [PubMed] [Google Scholar]
- Korbel J. O.; Jensen L. J.; von Mering C.; Bork P. Analysis of genomic context: prediction of functional associations from conserved bidirectionally transcribed gene pairs. Nat. Biotechnol. 2004, 22, 911–917. 10.1038/nbt988. [DOI] [PubMed] [Google Scholar]
- Callen B. P.; Shearwin K. E.; Egan J. B. Transcriptional interference between convergent promoters caused by elongation over the promoter. Mol. Cell 2004, 14, 647–656. 10.1016/j.molcel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Horowitz H.; Platt T. Regulation of transcription from tandem and convergent promoters. Nucleic Acids Res. 1982, 10, 5447–5465. 10.1093/nar/10.18.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V.; Bahrudeen M. N. M.; Palma C. S. D.; Baptista I. S. C.; Almeida B. L. B.; Dash S.; Kandavalli V.; Ribeiro A. S. Analytical kinetic model of native tandem promoters in E. coli. PLoS Comput. Biol. 2022, 18, e1009824 10.1371/journal.pcbi.1009824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deHaseth P. L.; Zupancic M. L.; Record M. T. Jr RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 1998, 180, 3019–3025. 10.1128/JB.180.12.3019-3025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneppen K.; Dodd I. B.; Shearwin K. E.; Palmer A. C.; Schubert R. A.; Callen B. P.; Egan J. B. A mathematical model for transcriptional interference by RNA polymerase traffic in Escherichia coli. J. Mol. Biol. 2005, 346, 399–409. 10.1016/j.jmb.2004.11.075. [DOI] [PubMed] [Google Scholar]
- Lutz R.; Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997, 25, 1203–1210. 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S. M. D.; Häkkinen A.; Lloyd-Price J.; Tran H.; Kandavalli V.; Ribeiro A. S. Temperature-Dependent Model of Multi-step Transcription Initiation in Escherichia coli Based on Live Single-Cell Measurements. PLoS Comput. Biol. 2016, 12, e1005174 10.1371/journal.pcbi.1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan A. B.; Kandhavelu M.; Lloyd-Price J.; Kudasov F.; Chowdhury S.; Yli-Harja O.; Ribeiro A. S. Dynamics of transcription driven by the tetA promoter, one event at a time, in live Escherichia coli cells. Nucleic Acids Res. 2012, 40, 8472–8483. 10.1093/nar/gks583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. A.; Korzheva N.; Mustaev A.; Murakami K.; Nair S.; Goldfarb A.; Darst S. A. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 2001, 104, 901–912. 10.1016/S0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- Jacob F.; Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 1961, 3, 318–356. 10.1016/S0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Oehler S.; Eismann E. R.; Krämer H.; Müller-Hill B. The three operators of the lac operon cooperate in repression. EMBO J. 1990, 9, 973–979. 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler S.; Amouyal M.; Kolkhof P.; von Wilcken-Bergmann B.; Müller-Hill B. Quality and position of the three lac operators of E. coli define efficiency of repression. EMBO J. 1994, 13, 3348–3355. 10.1002/j.1460-2075.1994.tb06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W.; Müller-Hill B. Isolation of the lac repressor. Proc. Natl. Acad. Sci. U.S.A. 1966, 56, 1891–1898. 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigon D.; Coleman B. D.; Olson W. K. Modeling the Lac repressor-operator assembly: the influence of DNA looping on Lac repressor conformation. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 9879–9884. 10.1073/pnas.0603557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N. A.; Peters J. P.; Maher L. J. 3rd; Lionberger T. A. Mechanism of promoter repression by Lac repressor-DNA loops. Nucleic Acids Res. 2013, 41, 156–166. 10.1093/nar/gks1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H.; Niemöller M.; Amouyal M.; Revet B.; von Wilcken-Bergmann B.; Müller-Hill B. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 1987, 6, 1481–1491. 10.1002/j.1460-2075.1987.tb02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.; Chang G.; Horton N. C.; Kercher M. A.; Pace H. C.; Schumacher M. A.; Brennan R. G.; Lu P. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science 1996, 271, 1247–1254. 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- Hillen W.; Schollmeier K.; Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J. Mol. Biol. 1984, 172, 185–201. 10.1016/S0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Beck C. F.; Mutzel R.; Barbé J.; Müller W. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J. Bacteriol. 1982, 150, 633–642. 10.1128/jb.150.2.633-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W.; Berens C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 1994, 48, 345–369. 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- Orth P.; Schnappinger D.; Hillen W.; Saenger W.; Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat. Struct. Biol. 2000, 7, 215–219. 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- Murphy K. F.; Adams R. M.; Wang X.; Balázsi G.; Collins J. J. Tuning and controlling gene expression noise in synthetic gene networks. Nucleic Acids Res. 2010, 38, 2712–2726. 10.1093/nar/gkq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Regulation of the L-arabinose operon of Escherichia coli. Trends Genet. 2000, 16, 559–565. 10.1016/S0168-9525(00)02153-3. [DOI] [PubMed] [Google Scholar]
- Seabold R. R.; Schleif R. F. Apo-AraC actively seeks to loop. J. Mol. Biol. 1998, 278, 529–538. 10.1006/jmbi.1998.1713. [DOI] [PubMed] [Google Scholar]
- Smith B. R.; Schleif R. Nucleotide sequence of the L-arabinose regulatory region of Escherichia coli K12. J. Biol. Chem. 1978, 253, 6931–6933. 10.1016/S0021-9258(17)38009-2. [DOI] [PubMed] [Google Scholar]
- Yeung E.; Dy A. J.; Martin K. B.; Ng A. H.; Del Vecchio D.; Beck J. L.; Collins J. J.; Murray R. M. Biophysical Constraints Arising from Compositional Context in Synthetic Gene Networks. Cell Syst. 2017, 5, 11–24.e12. 10.1016/j.cels.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Lee J. W.; Gyorgy A.; Cameron D. E.; Pyenson N.; Choi K. R.; Way J. C.; Silver P. A.; Del Vecchio D.; Collins J. J. Creating Single-Copy Genetic Circuits. Mol. Cell 2016, 63, 329–336. 10.1016/j.molcel.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N. C.; Campbell R. E.; Steinbach P. A.; Giepmans B. N. G.; Palmer A. E.; Tsien R. Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Lambert T. J. FPbase: a community-editable fluorescent protein database. Nat. Methods 2019, 16, 277–278. 10.1038/s41592-019-0352-8. [DOI] [PubMed] [Google Scholar]
- Pautke C.; Vogt S.; Kreutzer K.; Haczek C.; Wexel G.; Kolk A.; Imhoff A. B.; Zitzelsberger H.; Milz S.; Tischer T. Characterization of eight different tetracyclines: advances in fluorescence bone labeling. J. Anat. 2010, 217, 76–82. 10.1111/j.1469-7580.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A.Analysis of Microalgae and Cyanobacteria by Flow Cytometry. In Flow Cytometry in Microbiology; Lloyd D., Ed.; Springer London: London, 1993; pp 131–142. [Google Scholar]
- Bar-Even A.; Paulsson J.; Maheshri N.; Carmi M.; O’Shea E.; Pilpel Y.; Barkai N. Noise in protein expression scales with natural protein abundance. Nat. Genet. 2006, 38, 636–643. 10.1038/ng1807. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y.; Choi P. J.; Li G.-W.; Chen H.; Babu M.; Hearn J.; Emili A.; Xie X. S. Quantifying E. coli Proteome and Transcriptome with Single-Molecule Sensitivity in Single Cells. Science 2010, 329, 533–538. 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser J.; Mayo A.; Keren L.; Alon U. Central dogma rates and the trade-off between precision and economy in gene expression. Nat. Commun. 2019, 10, 68. 10.1038/s41467-018-07391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H.; Gama-Castro S.; Lara P.; Mejia-Almonte C.; Alarcón-Carranza G.; López-Almazo A. G.; Betancourt-Figueroa F.; Peña-Loredo P.; Alquicira-Hernández S.; Ledezma-Tejeida D.; Arizmendi-Zagal L.; Mendez-Hernandez F.; Diaz-Gomez A. K.; Ochoa-Praxedis E.; Muñiz-Rascado L. J.; García-Sotelo J. S.; Flores-Gallegos F. A.; Gómez L.; Bonavides-Martínez C.; del Moral-Chávez V. M.; Hernández-Alvarez A. J.; Santos-Zavaleta A.; Capella-Gutierrez S.; Gelpi J. L.; Collado-Vides J. RegulonDB v12.0: a comprehensive resource of transcriptional regulation in E. coli K-12. Nucleic Acids Res. 2024, 52, D255–D264. 10.1093/nar/gkad1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S.; Chen C.; Ge H.; Xie X. S. Mechanism of transcriptional bursting in bacteria. Cell 2014, 158, 314–326. 10.1016/j.cell.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkoorn R. C.; Sala C.; Magnet S. J.; Chen J. M.; Pojer F.; Cole S. T. Sigma factor F does not prevent rifampin inhibition of RNA polymerase or cause rifampin tolerance in Mycobacterium tuberculosis. J. Bacteriol. 2010, 192, 5472–5479. 10.1128/JB.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. A.; Pavlova O.; Zenkin N.; Leon F.; Irschik H.; Jansen R.; Severinov K.; Darst S. A. Structural, functional, and genetic analysis of sorangicin inhibition of bacterial RNA polymerase. EMBO J. 2005, 24, 674–682. 10.1038/sj.emboj.7600499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R.; Cech C. L. On the mechanism of rifampicin inhibition of RNA synthesis. J. Biol. Chem. 1978, 253, 8949–8956. 10.1016/S0021-9258(17)34269-2. [DOI] [PubMed] [Google Scholar]
- Sippel A. E.; Hartmann G. R. Rifampicin resistance of RNA polymerase in the binary complex with DNA. Eur. J. Biochem. 1970, 16, 152–157. 10.1111/j.1432-1033.1970.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Sippel A.; Hartmann G. Mode of action of rifamycin on the RNA polymerase reaction. Biochim. Biophys. Acta, Nucleic Acids Protein Synth. 1968, 157, 218–219. 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Agarwal V.; Shendure J. Predicting mRNA Abundance Directly from Genomic Sequence Using Deep Convolutional Neural Networks. Cell Rep. 2020, 31, 107663. 10.1016/j.celrep.2020.107663. [DOI] [PubMed] [Google Scholar]
- Avsec Z.; Agarwal V.; Visentin D.; Ledsam J. R.; Grabska-Barwinska A.; Taylor K. R.; Assael Y.; Jumper J.; Kohli P.; Kelley D. R. Effective gene expression prediction from sequence by integrating long-range interactions. Nat. Methods 2021, 18, 1196–1203. 10.1038/s41592-021-01252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster R. C.; Jones D. L.; Phillips R. Tuning Promoter Strength through RNA Polymerase Binding Site Design in Escherichia coli. PLoS Comput. Biol. 2012, 8, e1002811 10.1371/journal.pcbi.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einav T.; Phillips R. How the avidity of polymerase binding to the – 35/–10 promoter sites affects gene expression. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 13340–13345. 10.1073/pnas.1905615116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. R. Cross-species regulatory sequence activity prediction. PLoS Comput. Biol. 2020, 16, e1008050 10.1371/journal.pcbi.1008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. R.; Reshef Y. A.; Bileschi M.; Belanger D.; McLean C. Y.; Snoek J. Sequential regulatory activity prediction across chromosomes with convolutional neural networks. Genome Res. 2018, 28, 739–750. 10.1101/gr.227819.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtecho G.; Tripp A. D.; Insigne K. D.; Kim H.; Kosuri S. Systematic Dissection of Sequence Elements Controlling σ70 Promoters Using a Genomically Encoded Multiplexed Reporter Assay in Escherichia coli. Biochemistry 2019, 58, 1539–1551. 10.1021/acs.biochem.7b01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Theesfeld C. L.; Yao K.; Chen K. M.; Wong A. K.; Troyanskaya O. G. Deep learning sequence-based ab initio prediction of variant effects on expression and disease risk. Nat. Genet. 2018, 50, 1171–1179. 10.1038/s41588-018-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keseler I. M.; Gama-Castro S.; Mackie A.; Billington R.; Bonavides-Martínez C.; Caspi R.; Kothari A.; Krummenacker M.; Midford P. E.; Muñiz-Rascado L.; Ong W. K.; Paley S.; Santos-Zavaleta A.; Subhraveti P.; Tierrafría V. H.; Wolfe A. J.; Collado-Vides J.; Paulsen I. T.; Karp P. D. The EcoCyc Database in 2021. Front. Microbiol. 2021, 12, 711077. 10.3389/fmicb.2021.711077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisutham V.; Chhabra S.; Ali M. Z.; Brewster R. C. Tunable transcription factor library for robust quantification of regulatory properties in Escherichia coli. Mol. Syst. Biol. 2022, 18, e10843 10.15252/msb.202110843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslaver A.; Bren A.; Ronen M.; Itzkovitz S.; Kikoin I.; Shavit S.; Liebermeister W.; Surette M. G.; Alon U. A comprehensive library of fluorescent transcriptional reporters for Escherichia coli. Nat. Methods 2006, 3, 623–628. 10.1038/nmeth895. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. S. Dynamics and evolution of stochastic bistable gene networks with sensing in fluctuating environments. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 2008, 78, 061902. 10.1103/PhysRevE.78.061902. [DOI] [PubMed] [Google Scholar]
- Potapov I.; Lloyd-Price J.; Yli-Harja O.; Ribeiro A. S. Dynamics of a genetic toggle switch at the nucleotide and codon levels. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 2011, 84, 031903. 10.1103/PhysRevE.84.031903. [DOI] [PubMed] [Google Scholar]
- Travers A.; Muskhelishvili G. DNA supercoiling — a global transcriptional regulator for enterobacterial growth?. Nat. Rev. Microbiol. 2005, 3, 157–169. 10.1038/nrmicro1088. [DOI] [PubMed] [Google Scholar]
- Ma J.; Wang M. D. DNA supercoiling during transcription. Biophys. Rev. 2016, 8, 75–87. 10.1007/s12551-016-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow L.; Hardy C. D.; Arsuaga J.; Cozzarelli N. R. Topological domain structure of the Escherichia coli chromosome. Genes Dev. 2004, 18, 1766–1779. 10.1101/gad.1207504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock H.; Ussery D. W. Chromatin architecture and gene expression in Escherichia coli. Genome Biol. 2004, 5, 252. 10.1186/gb-2004-5-12-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu. Rev. Biochem. 1985, 54, 171–204. 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Startceva S.; Kandavalli V. K.; Visa A.; Ribeiro A. S. Regulation of asymmetries in the kinetics and protein numbers of bacterial gene expression. Biochim. Biophys. Acta, Gene Regul. Mech. 2019, 1862, 119–128. 10.1016/j.bbagrm.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Kandavalli V. K.; Tran H.; Ribeiro A. S. Effects of σ factor competition are promoter initiation kinetics dependent. Biochim. Biophys. Acta 2016, 1859, 1281–1288. 10.1016/j.bbagrm.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Weinstein Z. B.; Zaman M. H. Evolution of Rifampin Resistance in Escherichia coli and Mycobacterium smegmatis Due to Substandard Drugs. Antimicrob. Agents Chemother. 2019, 63, 10. 10.1128/aac.01243-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J.; Gupta A.; Ribeiro A. S. SGNS2: a compartmentalized stochastic chemical kinetics simulator for dynamic cell populations. Bioinformatics 2012, 28, 3004–3005. 10.1093/bioinformatics/bts556. [DOI] [PubMed] [Google Scholar]
- Gillespie D. T. Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 1977, 81, 2340–2361. 10.1021/j100540a008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A data package is deposited in Dryad with flow cytometry data, spectrophotometry data, and the constructs sequences and maps. The data are available at the link: https://doi.org/10.5061/dryad.jm63xsjgt. The constructs will be available for distribution in Addgene.