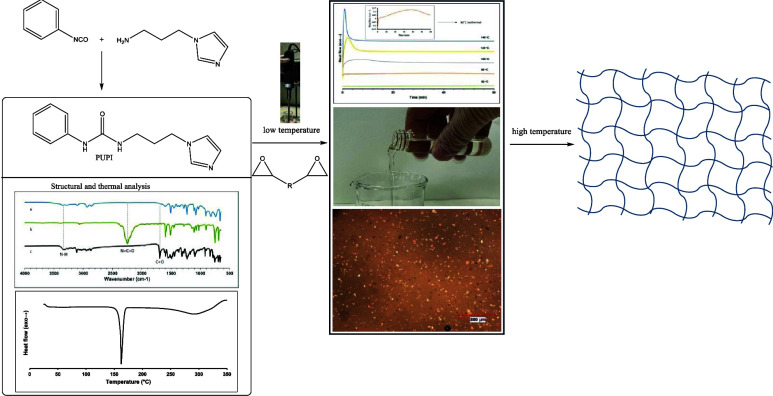
Abstract
This study investigates the formulation and curing behavior of a one-component epoxy resin (OCER). The OCER was formulated using phenylurea propyl imidazole (PUPI) as a thermal latent curing agent and diglycidylether bisphenol A (DGEBA). PUPI was synthesized by reacting phenyl isocyanate with 1-(3-aminopropyl)imidazole and characterized using 1H NMR, Fourier transform infrared (FTIR), thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC). PUPI and DGEBA were mixed with a homogenizer at ambient temperature. Cure kinetics studies were conducted using DSC and a rheometer. The OCER achieved conversions of 97.6 and 99.3% at 120 and 140 °C in 5.17 and 1.95 min, respectively, and exhibited stability of 3 days at ambient temperature. Furthermore, PUPI acted as an accelerator for the dicyandiamide curing agent in the curing of DGEBA. Upon the incorporation of PUPI at various amounts (1, 5, and 10% by weight), the left-limit and peak temperatures of curing were significantly lowered from 173 and 204 °C to 72–111 °C and 122–155 °C, respectively.
1. Introduction
Composites have become indispensable for various engineering applications in industries such as aerospace, automotive, and coatings due to their exceptional mechanical properties and tailored performance characteristics.1 They consist of a matrix material that encapsulates and binds a reinforcing phase, resulting in a material with properties beyond those of its individual components. One important subclass of composites is “prepreg composites”, where the reinforcing fibers are preimpregnated with matrix material (prepreg) prior to the fabrication process.2 This preimpregnation ensures uniform distribution of the matrix within the reinforcement, enabling precise control over the composite’s thermal and mechanical properties. The matrix material in prepreg is often in a tacky, partially cured state, facilitating ease of handling during setup and subsequent processing. In advanced composite manufacturing, epoxy prepregs have emerged as a crucial material, playing a pivotal role in industries ranging from aerospace to automotive due to their exceptional mechanical properties, dimensional stability, and ease of application.3,4
In the preparation of prepregs, both two- and one-component epoxy resins are utilized. In two-component epoxy resins, the resin and hardener components are kept separate and mixed just prior to application. Conversely, in one-component epoxy resins (OCERs), the resin and a latent curing agent are preformulated together in a single package, simplifying handling and application processes.5 OCERs have gained importance in commercial applications due to their increased manufacturing efficiency and improved storage conditions compared to conventional two-component resins.1−3,6 The inclusion of latent curing agents in OCERs prevents curing at room temperature and helps reach targeted cure temperatures. Latent curing agents can be classified according to their sources of stimuli such as thermal, ultrasonic, electron beam, and photo irradiation.7 Among these, thermal latent curing agents (TLCs) have been extensively investigated for their stability performance and handling properties.8,9 TLCs contribute to the room temperature stability of epoxy resins and can be activated upon application of heat, reacting with epoxy resins at the desired temperatures. Various TLCs including dicyandiamide (DICY), dihydrazides, amino acids, and imidazole derivatives have been employed in OCERs in the literature.10−14 These TLCs enhance the application and performance of epoxy prepreg systems, enabling controlled curing kinetics as well as tailorable prepreg properties such as drapeability and tackiness.1,6,15,16
TLCs can offer physical or chemical latency. In physical latency, either a curing agent is encapsulated within a polymer, preventing its reaction with epoxy resin, or an insoluble curing agent is employed, which can only be activated following its melting and dissolution in epoxy resin. In chemical latency, either the active site of the curing agent is sterically hindered or the curing agent is conjugated to another small molecule or polymer to reduce its reactivity with epoxy resin. Two commonly used curing agents with epoxy resins are DICY and imidazole (or its derivatives). DICY is an insoluble TLC in epoxy resin at room temperature, with a melting temperature typically ranging from 208 to 212 °C.17 The high melting point of DICY prevents its dissolution at room temperature, thereby providing thermal latency. However, DICY can be activated at very high temperatures during curing, typically exceeding 170 °C, (>170 °C), which necessitates high energy for curing and can lead to issues such as explosive evolution of ammonia and other gases, particularly in large part manufacturing processes such as composite parts. Consequently, DICY is typically suitable only for applications involving thin parts.17 Additionally, concerns arise regarding the variation in the dispersion quality of DICY in epoxy resin, which can result in challenges related to cure kinetics and heterogeneity in the final product properties.
While imidazole readily reacts with epoxy groups at room temperature, several less reactive imidazole derivatives have been developed as TLCs.18−21 Strategies such as encapsulation of imidazole, utilization of 1-substituted imidazole derivatives, and restraining of the tertiary amine via metal–organic coordination or hydrogen bond have been employed to reduce imidazole reactivity.18 Kohlan et al. conducted a study on imidazole-epoxy reactions and discovered that the curing onset temperature of an OCER formulated with 5 wt % of imidazole encapsulated in a copolymer and diglycidylether bisphenol A (DGEBA) resin is at 78 °C.22 The encapsulation method has been reported to be inefficient due to challenges such as difficulty in mixing encapsulation materials with epoxy and the potential for premature release of curing agent, leading to stability issues. Furthermore, as encapsulated agents remain within the cross-linked network, they can adversely affect the thermal and mechanical performance of cured epoxy matrices.18 Yin et al. demonstrated prolonged shelf life by encapsulating a thermal latent curing agent using urea-formaldehyde capsules.23 Li et al. introduced a novel method for encapsulating imidazole adducts through thio-click reaction in an oil–water emulsion, claiming stability for up to 30 days with the encapsulated imidazole.7
Our study involves the synthesis and characterization of PUPI, its incorporation into DGEBA to formulate an OCER, and the investigation of the curing behavior and stability of the OCER via differential scanning calorimetry (DSC) and rheology studies. The novelty of this work stems from the utilization of prepared PUPI as a thermal latent curing agent for one-component epoxy resins (OCERs). Although synthesized previously, PUPI has not been investigated in detail as a thermal latent curing agent for OCERs. In this study, in addition to providing a simple method for the synthesis and extensive characterization of PUPI, the curing behavior of PUPI as a thermal latent curing agent alone and as a catalyst for DICY in the curing of DGEBA resin and the stability of the OCERs were thoroughly investigated.
2. Experimental Section
2.1. Materials
DGEBA (EEW: 170.2 g/mol), phenyl isocyanate (≥99.8%), 1-(3-aminopropyl)imidazole (≥97%), and dichloromethane (DCM) (≥99.8%) were purchased from Merck Corporation Sigma-Aldrich. DICY was purchased from Ataman Chemicals.
2.2. Instruments
1H NMR spectra are recorded in CD3OD and CDCl3 on a 500 MHz Varian spectrometer. Fourier transform infrared (FTIR) spectra are obtained with a Thermo Scientific Nicolet iS50 FTIR spectrometer using a Smart iTR Accessory, employing transmission mode with a resolution of 4 cm–1. DSC measurements are conducted using a Mettler Toledo DSC 3+ instrument with sample sizes of 8–10 mg. For dynamic DSC measurements, the temperature range spans from 25 to 250 °C, with heating and nitrogen flow rates set at 10 °C/min and 50 mL/min, respectively. Isothermal DSC measurements are performed for 3 h at various temperatures, followed by dynamic DSC tests from 25 to 300 °C at a heating rate of 10 °C/min to measure residual enthalpy. Thermogravimetric analysis (TGA) measurements are executed on a Mettler Toledo TGA/DSC 3+ instrument within a temperature range of 25–800 °C. Samples weighing 10–100 mg are heated at 10 °C/min under a nitrogen atmosphere with a flow rate of 100 mL/min. Optical microscope images are captured using a Nikon Eclipse LV100ND optical microscope equipped with CFI60–2 lenses at magnifications of 25×, 50×, 100×, and 200×. Bright-field illumination is applied from beneath the material during imaging. Viscosity measurements are conducted using a Brookfield DV2T viscometer with an SC4–21-type spindle at 40 °C for 10 min and a rotation speed of 100 rpm. Data are collected at 30 s intervals, with a reported accuracy of ±5 mPa·s. Following each measurement, the sample is retrieved and stored at room temperature in a sealed vial. The vial is purged with nitrogen for 1 min before being sealed with parafilm.
2.3. Methods
2.3.1. Synthesis of Phenylurea Propyl Imidazole (PUPI)
PUPI is synthesized by reacting 1-(3-aminopropyl)imidazole (3.75 g, 0.029 mol, 1 equiv) with phenyl isocyanate (3.57 g, 0.029 mol, 1 equiv) in 60 mL of dichloromethane (DCM) (Figure 1). Initially, 1-(3-aminopropyl)imidazole is added to DCM in a 100 mL round-bottom flask equipped with a magnetic stir bar, and the solution is cooled to 0 °C using an ice bath. Subsequently, phenyl isocyanate is added dropwise into the flask. The solution is then allowed to gradually warm up to room temperature and stirred for 24 h. Upon completion of the reaction, DCM is evaporated with a rotary evaporator at room temperature to yield a white powdery product (6.83 g, yield: 93%).
Figure 1.

Synthesis of PUPI.
2.3.2. Preparation of the OCER
PUPI (0.35 g) and DGEBA (5.0 g, EEW: 170.2 g/mol) are mixed for 5 min at 1000 rpm using a homogenizer in a glass vial to achieve a 5 mol % concentration of PUPI in the mixture. Following mixing, the vial is purged with nitrogen, sealed with a cap, and covered with parafilm to ensure safe storage at room temperature.
3. Results
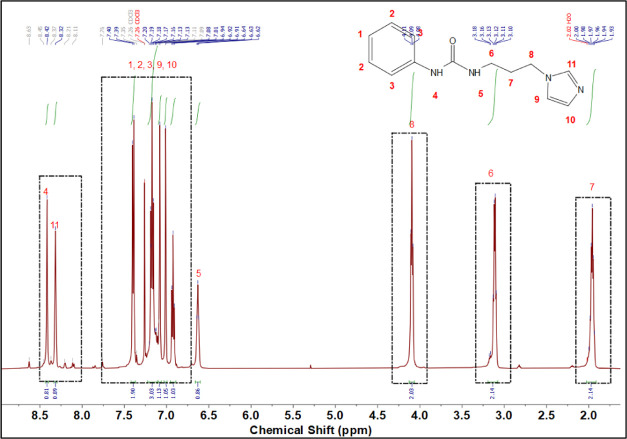
3.1. 1H NMR Analysis of PUPI
The 1H NMR spectrum of PUPI, along with the peak assignments, is presented in Figure 2. The presence of urea −NH peaks at 8.42 (peak 4) and 6.63 ppm (peak 5), along with the shift of CH2 peaks of 1-(3-aminopropyl) imidazole adjacent to −NH2 from 1.85 and 2.64 ppm to 1.97 (peak 7) and 3.11 ppm (peak 6) in PUPI, indicates the successful progression of the reaction. Additionally, the absence of the −NH2 peak of 1-(3-aminopropyl)imidazole at 7.69 ppm confirms its complete reaction with phenyl isocyanate. The imidazole −CH peak (peak 11) shifted from 7.47 to 8.32 ppm due to hydrogen bonding with the urea group. Furthermore, all other peaks of 1-(3-aminopropyl)imidazole (peaks 8, 9, 10) and aromatic peaks (peaks 1, 2, and 3) of phenyl isocyanate were observed in the spectrum. In Figure 2, peaks 4, 5, 6, 7, 8, and 11 were separately integrated, whereas peaks 1, 2, 3, 9, and 10 were integrated together and the corresponding integral peaks were provided. The integral values of the peaks align well with the number of protons assigned for them and show that the intended structure (PUPI) was obtained.
Figure 2.
1H NMR spectrum of PUPI (in CDCl3).
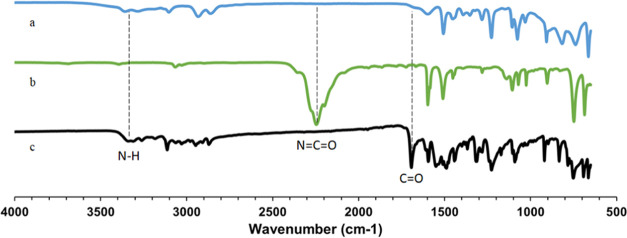
3.2. FTIR Analysis of PUPI
FTIR spectra of 1-(3-aminopropyl)imidazole, phenyl isocyanate, and PUPI are shown in Figure 3. The peak observed at 2246 cm–1 for phenyl isocyanate corresponds to the isocyanate (−N=C=O) group, which is absent in the spectrum of PUPI due to its reaction with 1-(3-aminopropyl)imidazole. In the FTIR spectra of 1-(3-aminopropyl)imidazole, peaks associated with N–H bending and stretching are observed at 1597 and 3358 cm–1, respectively. Additionally, the urea N–H peak of PUPI is observed around 3350 cm–1. The appearance of the urea carbonyl peak (−NH–CO–NH−) at 1693 cm–1 in the PUPI spectrum confirms the successful synthesis of the product.24,25
Figure 3.
FTIR spectra of (a) 1-(3-aminopropyl)imidazole, (b) phenyl isocyanate, and (c) PUPI.
3.3. TGA Analysis of PUPI
The TGA thermogram of PUPI and its derivative are shown in Figure 4. PUPI undergoes two main decomposition steps: the first occurs between 180 and 330 °C, resulting in a 45% weight loss, while the second takes place within the range of 330–500 °C, leading to a 50% weight loss. The initial decomposition is attributed to the breakage of the urea bond and the evaporation of the released phenyl isocyanate, while the subsequent decomposition is associated with the degradation of 1-(3-aminopropyl)imidazole. The weight loss percentages align with the molecular weight ratios of phenyl isocyanate (119.12/244.29 g/mol = 48.8%) and 1-(3-aminopropyl)imidazole (125.17/244.29 g/mol = 51.2%) in PUPI (molecular weight: 244.29 g/mol).
Figure 4.
TGA thermogram of PUPI (solid line) and its derivative (dashed line).
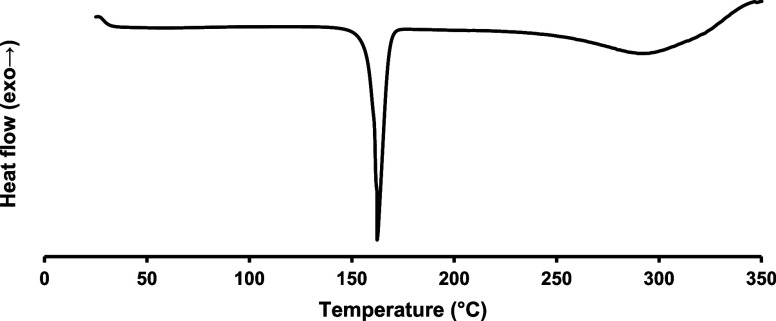
3.4. DSC Analysis of PUPI
In the DSC thermogram of PUPI (Figure 5), the melting temperature of PUPI is observed in the range of 145–175 °C, with a peak temperature of 162 °C. PUPI starts to decompose at around 200 °C, a finding consistent with the TGA results.
Figure 5.
DSC thermogram of PUPI.
3.4.1. Dynamic DSC Analysis of the OCER Formulated with PUPI
The dynamic DSC thermogram of OCER is shown in Figure 6. Upon mixing PUPI with DGEBA at 5% mole concentration, the resin solidifies after cooling down to room temperature. Consequently, the initial peak around 40 °C corresponds to the melting of DGEBA. The left-limit, onset, peak, and right-limit temperatures of curing are observed at 82, 104, 131, and 215 °C, respectively. The curing reaction enthalpy is 439.62 J/g.
Figure 6.
Dynamic DSC thermogram of the OCER formulated with PUPI.
3.4.2. Isothermal DSC Results of the OCER Formulated with PUPI
The isothermal DSC thermograms of the OCER (5% PUPI in DGEBA) are shown at varying temperatures in Figure 7. In an isothermal DSC test, the temperature is kept constant during the run, and curing behavior is observed at a particular temperature. At 140 and 120 °C, sharp and narrow curing peaks with curing completion times of 1.97 and 5.17 min, respectively, are observed, indicating that the epoxy curing reaction takes place rapidly at these temperatures. In contrast, the curing profiles at 100 and 80 °C are broader, with incomplete curing after 15.50 and 38.65 min, respectively, which is indicative of a slower reaction. No curing is observed at 60 °C even after 180 min. The enthalpy values obtained in isothermal DSC tests are provided in Table 1. Although higher enthalpy values are observed at and above 100 °C, the enthalpy value is lower at 80 °C and absent at 60 °C. In isothermal DSC runs, the conversions depend on the cure temperature. At lower temperatures, if the isothermal DSC test time is not long enough, uncured resin will remain as a residue, which is the case at 60 °C. To determine the amount of residual unreacted epoxy resin, a dynamic DSC run is performed after the isothermal DSC tests.
Figure 7.
Isothermal curing profile of OCER formulated with 5% PUPI.
Table 1. Isothermal and Dynamic DSC Curing Analysis of PUPI-DGEBA.
| isothermal temperature (°C) | normalized curing enthalpy (J/g) | residual enthalpy (J/g) | conversion (%) |
|---|---|---|---|
| 60 °C | 0.0 | 149.5 | 0.0 |
| 80 °C | 334.8 | 59.0 | 85.0 |
| 100 °C | 429.4 | 25.6 | 94.4 |
| 120 °C | 411.5 | 10.3 | 97.6 |
| 140 °C | 406.3 | 2.9 | 99.3 |
3.4.3. Dynamic DSC Results of the OCER Formulated with 5% PUPI after Isothermal Curing
Dynamic DSC tests are conducted following the isothermal tests to determine residual curing enthalpy values at each temperature, as shown in Figure 8.
Figure 8.
Dynamic DSC tests performed after isothermal curing to obtain residual curing data.
The residual curing enthalpy values are summarized in Table 1 along with conversion values. The residual curing enthalpy values increased for the samples cured at lower isothermal temperatures. The total enthalpy value for the curing of DGEBA with PUPI at each temperature is taken as the sum of the enthalpy value from the isothermal DSC test and the residual enthalpy obtained in the follow-up dynamic DSC. The conversions in isothermal tests are found by dividing the enthalpy obtained in the isothermal DSC tests with the total enthalpy (Table 1). Isothermal curing at 140 °C for 3 h exhibits a conversion of 99.3%, while the conversion at 80 °C for 3 h drops to 85.0%. No curing is observed at 60 °C, and the dynamic DSC curing following the isothermal curing at 60 °C shows a residual enthalpy value of 149.52 J/g. After the isothermal DSC tests, the samples are cooled to room temperature, and a dynamic DSC scan is performed to cure the unreacted resin. In a dynamic DSC test, a sample is heated at a specific heating rate (typically 2–10 °C/min) from a low temperature (usually ambient temperature) up to a higher temperature (typically up to 300 °C for an epoxy resin). Thus, the curing behavior of the resin with respect to the temperature is determined. As shown in Figure 8 and Table 1, the residual peaks at lower temperatures have higher enthalpy values, indicating that there is more unreacted epoxy resin.
3.5. Optical Microscopy Images of the OCER
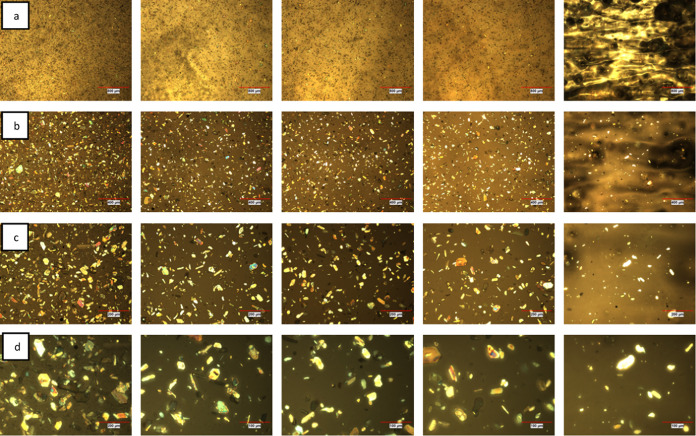
Optical microscopy images of the OCER (5% PUPI in DGEBA) are captured at 25, 60, and 80 °C to observe the distribution of PUPI in DGEBA. The initial image is taken at 25 °C immediately after mixing PUPI and DGEBA, while the image at 80 °C is acquired after heating the OCER on a hot plate until the thermocouple reads 80 °C. At 80 °C, the mixture loses its fluidity, and the formation of a film is observed, indicating the onset of curing (Figure 9).
Figure 9.
Optical microscopy images (25x) of the OCER (5% PUPI in DGEBA) (A, 25 °C; B, 80 °C, 2 min; C, 60 °C, 6 h).
In the image captured at 80 °C, there appears to be no significant decrease in the amount of PUPI, suggesting that the reaction is still in its initial stages. The black spots observed in the optical microscopy images at room temperature may be due to the voids present in the resin. At 80 °C, the resin is more mobile and less viscous; hence, the voids are filled with the resin but trapped air may leave bubbles as they are burst, thus leaving black spots at 80 °C. The bright spots observed at room temperature correspond to the DGEBA crystals and the curing agent. As the temperature is increased to 80 °C, DGEBA crystals melt (at 35–50 °C range as shown in Figure 6); thus, only PUPI crystals are observed as the bright spots at 80 °C. This is one of the reasons for observing less bright spots at 80 °C. The other reason is the dissolution of PUPI at 80 °C and its curing reaction with DGEBA. The DGEBA crystals dissolve and react with DGEBA; thus, bright spots gradually turn to darker spots as they dissolve and eventually disappear as they react with DGEBA. PUPI is insoluble in DGEBA at room temperature, but it dissolves as the temperature increases. To observe the dissolution behavior and homogeneity of PUPI in DGEBA, the optical images are obtained at 80 °C (not shown); however, since the curing reaction is fast at 80 °C, it is difficult to observe the dissolution and homogeneity before the curing reaction takes place. To better observe the dissolution and homogeneity, the mixture is monitored at 60 °C at varying times (Figures 9 and 10). Figure 10 illustrates the morphological change of the OCER at 60 °C over a period of 5 h. As the heating time at 60 °C increases from 1 to 5 h, less PUPI remains in the mixture, indicating its gradual dissolution and involvement in the curing of DGEBA. However, complete dissolution of PUPI is not achieved at this temperature within this period. The image on the far right side in Figure 10 exhibits a sticky substance resulting from the curing process, making it challenging to spread across the microscope slide. This observation aligns with the viscosity data presented in Figure 11, where a notable surge in viscosity is recorded at 60 °C after 250 min, indicating the progression of curing. In Figure 9, the optical image of the mixture held at 60 °C for 6 h is shown, which illustrates that complete dissolution of PUPI in DGEBA is achieved along with a possible partial curing of the OCER. In fact, this temperature-dependent solubility of PUPI in DGEBA imparts latency to the curing process of DGEBA, which we exploit in this study.
Figure 10.
Optical microscopy images of the OCER (5% PUPI in DGEBA) at 60 °C at different magnifications a: 25×, b: 50×, c: 100×, d: 200× (left to right: 1, 2, 3, 4, 5 h).
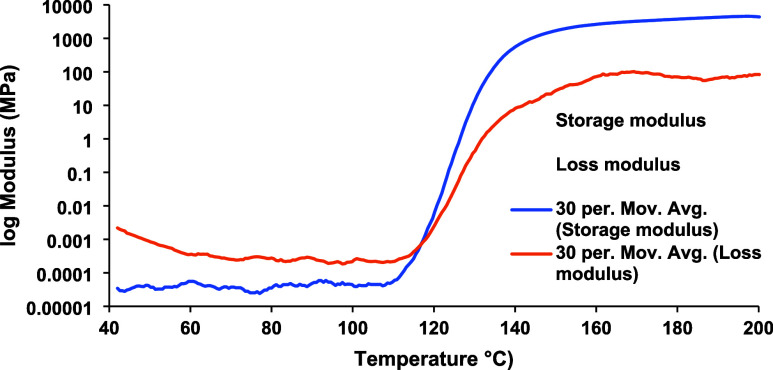
Figure 11.
Storage and loss moduli in temperature sweep rheology test of the OCER formulated with 5% PUPI.
3.6. Rheology Results
3.6.1. Storage and Loss Moduli of PUPI:DGEBA
In the temperature sweep rheology test of the OCER (5% PUPI in DGEBA), as shown in Figure 11, the storage and loss modulus intersect each other around 110 °C, indicative of the onset of the curing reaction. The fluctuations observed in the storage modulus prior to curing are attributed to the low viscosity of the liquid mixture (4.5 Pa·s at 25 °C). The viscosity data is smoothed to get rid of the noisy appearance through a moving average method with 30 smoothing steps.
3.6.2. Viscosity Profile of the OCER Formulated with 5% PUPI
Figure 12 illustrates the isothermal viscosity data at various temperatures, revealing distinct gel times corresponding to each temperature. Additionally, both dynamic DSC and temperature sweep rheology studies exhibit the onset of curing around 110 °C, observed as the initiation of exothermic peaks in dynamic DSC and the crossover point in the rheological temperature sweep study. Furthermore, rheology data obtained at 40 °C suggest that the OCER remains stable for up to 20 h. Viscosity increases exponentially with temperature increase in isothermal rheology studies, especially after 80 °C where the dynamic DSC data confirm the left limit of curing is near 80 °C.
Figure 12.
Complex viscosity profile of the OCER at isothermal rheology tests.
Gel times (process times) and shelf life times (times at which complex viscosity doubles) are obtained from complex viscosity data in rheology tests (Figure 13). The conversions corresponding to these times are found in the isothermal DSC tests. Applying the first-order reaction kinetics for epoxy curing with PUPI and using the Arrhenius equation, the rate constants (k values) at different temperatures are determined. The following equations were used to find k values
ln([A]t/[A]0) = ln (1 – α) = −kt (Arrhenius equation for the first-order reaction in natural logarithmic form)
where α is the conversion calculated using isothermal DSC data, [A]t and [A]0 are concentrations at time = t and time = 0, respectively, k is the rate constant, and t is either gel time or shelf life time.
Figure 13.

Estimation of process times (upper data and graphs) and shelf life times (lower data and graphs) at lower temperatures (shown in blue) using rheology and isothermal DSC data obtained at higher temperatures (shown in green color).
lnk values are then calculated, and ln k vs 1/T graphs (green-color graphs) are plotted to obtain an equation using a linear fit. Using this equation, ln k values at lower temperatures are estimated and plotted against 1/T (blue-color graphs). k values are then calculated and used to estimate process times and shelf life times at lower temperatures (average of the −kt values obtained at higher temperatures are used here, which means the average of the conversions obtained at higher temperatures are utilized here). Using the equation t = −ln(1 – α)/k, the t values (process times and shelf life times) at lower temperatures are found.
3.7. Room-Temperature Stability Tests
Stability tests are conducted with a Brookfield viscometer at 40 °C at 3-day intervals. The initial absolute viscosity was measured at 132.1 mPa·s. Over a span of 6 days of storage at room temperature, this value increased to 493.6 mPa·s. The results are depicted in Figure 14, indicating that the OCER maintained a pot life of at least 3 days.
Figure 14.
Complex viscosity results at room temperature storage.
3.8. PUPI as a Catalyst for DICY
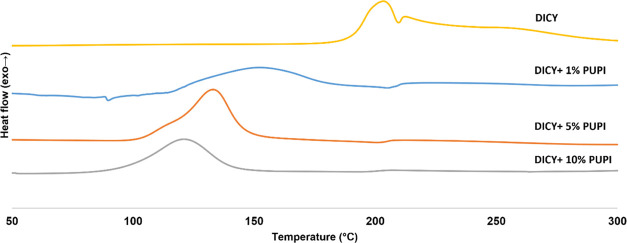
PUPI is combined with DICY to observe its effect on the reaction kinetics of DICY at varying ratios, as depicted in Figure 15. DICY’s functionality is selected as 4, resulting in a 1:2 mol ratio of DICY/DGEBA. PUPI:DGEBA ratios are adjusted to 1, 5, and 10% mol of PUPI relative to the epoxy groups in DGEBA. For 1% of PUPI, the reaction left-limit and peak temperatures are measured at 111 and 155 °C, respectively. In the case of 5% PUPI + DICY, the onset and peak temperatures of curing are observed at 97 and 135 °C, respectively. With 10% mol of PUPI + DICY, the onset and peak temperatures of curing were slightly lower, at 72 and 122 °C, respectively, owing to the increased presence of imidazole groups compared to the epoxy groups. The corresponding reaction enthalpies are 503.54 J/g for 1% PUPI, 417.22 J/g for 5% PUPI, and 291.96 J/g for 10% PUPI.
Figure 15.
Dynamic DSC results of DICY-DGEBA mixture and the OCERs formulated with 1% PUPI + DICY (blue), 5% PUPI + DICY (red), and 10% PUPI + DICY (green).
The left-, peak-, and right-limit temperatures as well as enthalpy values are listed in Table 2. The dynamic DSC curing data for the OCERs formulated with 1% PUPI + DICY, 5% PUPI + DICY, and 10% PUPI + DICY
Table 2. Enthalpy, Limit, and Peak Temperature Values of DICY:PUPI Cured DGEBA Formulations.
| % PUPI in DGEBA | reaction left-limit temperature (°C) | reaction peak temperature (°C) | reaction right-limit temperature (°C) | enthalpy (J/g) |
|---|---|---|---|---|
| 1 | 111 | 155 | 205 | 503.54 |
| 5 | 97 | 135 | 179 | 417.22 |
| 10 | 72 | 122 | 161 | 291.96 |
Isothermal curing of OCER formulated with 5% PUPI + DICY indicates that curing is completed in 9 min with no residual enthalpy peak showing a Tg of 152 °C, which is consistent with the Tg’s obtained with DICY+DGEBA resin.26
Imidazole and its derivatives are used as catalysts to accelerate the reaction between epoxy groups and DICY. The imidazole group functions as both a nucleophile and a base, facilitating the ring-opening of the epoxy resin by attacking the less sterically hindered carbon atom in the oxirane ring, forming an imidazole-epoxy adduct. The imidazole-activated epoxy can react with the active hydrogen atoms of the DICY, leading to the formation of cross-linked polymer networks. The catalytic action of imidazole (or imidazole derivatives) allows curing to take place at lower temperatures and increases the rate of curing, which reduces curing temperature and time as shown in Figure 15 and Table 2.
Dareh et al. studied the acceleration effect of imidazole derivatives for epoxy curing with DICY and found out that methyl imidazole accelerated DICY curing most, whereas regarding pot life and highest Tg, carbonyldiimidazole displayed the best results.27 Reuther et al. investigated the effect of imidazole derivatives on the acceleration of DICY curing of DGEBA resin. Tertiary amines such as imidazole can attack to the energy-rich secondary carbon of oxirane of epoxy groups.28 This results in chain oligomerization which produces positively charged amine groups and negatively charged alkoxylate end groups. Further, alkoxylate can deprotonate DICY, which increases its reactivity at lower temperatures. Reuther et al. concluded that above a critical temperature, the polymeric imidazole derivatives became soluble in epoxy and displayed high reactivities.28 Rodosek et al. researched three different accelerators to observe the effect on Tg of the cured epoxy with DICY. They found that the imidazole compound that they used, 2-methyl imidazole, decreased the cured epoxy Tg whereas other compounds such as N,N-benzyl dimethylamine (BDMA) and a commercial product Curezol increased the Tg of the cured epoxy.29 Olson filed a patent for a composition which included imidazole, DICY, and a reactivity controller for imidazole to achieve a one-component epoxy composition. He claims that the cured epoxy has a Tg of 135 °C.30 Gilbert stated that tertiary amines have been used to accelerate DICY curing; however, it refutes the room-temperature latency.26
4. Conclusions
In conclusion, phenylurea propyl imidazole (PUPI) was successfully synthesized using a one-pot, easy, and scalable synthesis method and characterized for its role as a TLC in OCER formulations with DGEBA. PUPI effectively delayed the onset of curing in the OCER, allowing precise control over the process. Dynamic DSC showed high conversion rates at 120 and 140 °C, with PUPI providing stability to the OCER for up to 3 days at room temperature. Furthermore, PUPI acted as an accelerator for DICY, significantly lowering its curing temperature. Overall, PUPI is a promising TLC for OCERs, offering control over curing kinetics and stability. Further research could optimize PUPI formulations for specific applications and explore their compatibility with other resins.
Acknowledgments
The authors thank Saeed Salamatgharamaleki for his contributions.
The authors declare no competing financial interest.
References
- Pouladvand A. R.; Mortezaei M.; Fattahi H.; Amraei I. A. A novel custom-tailored epoxy prepreg formulation based on epoxy-amine dual-curable systems. Composites, Part A 2020, 132, 105852 10.1016/j.compositesa.2020.105852. [DOI] [Google Scholar]
- Hassan M. H. A mini review on manufacturing defects and performance assessments of complex shape prepreg-based composites. Int. J. Adv. Des. Manuf. Technol. 2021, 115 (11), 3393–3408. 10.1007/s00170-021-07421-8. [DOI] [Google Scholar]
- Rangappa S. M.; Parameswaranpillai J.; Siengchin S.; Thomas S.. Handbook of Epoxy/Fiber Composites; Springer, 2022. [Google Scholar]
- Parameswaranpillai H.; Pulikkalparambil S. M.; Siengchin S.. Epoxy Composites: Fabrication, Characterization and Applications; Wiley, 2021. [Google Scholar]
- Jee S. M.; Ahn C.-H.; Park J. H.; Kim T. A.; Park M. Solvent-free encapsulation of curing agents for high performing one-component epoxy adhesives. Composites, Part B 2020, 202, 108438 10.1016/j.compositesb.2020.108438. [DOI] [Google Scholar]
- Banks R.; Mouritz A. P.; John S.; Coman F.; Paton R. Development of a new structural prepreg: characterisation of handling, drape and tack properties. Compos. Struct. 2004, 66 (1–4), 169–174. 10.1016/j.compstruct.2004.04.034. [DOI] [Google Scholar]
- Li C.; Tan J.; Gu J.; Xue Y.; Qiao L.; Zhang Q. Facile synthesis of imidazole microcapsules via thiol-click chemistry and their application as thermally latent curing agent for epoxy resins. Compos. Sci. Technol. 2017, 142, 198–206. 10.1016/j.compscitech.2017.02.014. [DOI] [Google Scholar]
- Chen J.; Chu N.; Zhao M.; Jin F.-L.; Park S.-J. Synthesis and application of thermal latent initiators of epoxy resins: A review. J. Appl. Polym. Sci. 2020, 137 (48), 49592 10.1002/app.49592. [DOI] [Google Scholar]
- Yen W.-P.; Chen K.-L.; Yeh M.-Y.; Uramaru N.; Lin H.-Y.; Wong F. F. Investigation of soluble PEG-imidazoles as the thermal latency catalysts for epoxy-phenolic resins. J. Taiwan Inst. Chem. Eng. 2016, 59, 98–105. 10.1016/j.jtice.2015.08.007. [DOI] [Google Scholar]
- Hesabi M.; Salimi A.; Beheshty M. H. Development of amine-based latent accelerator for one-pot epoxy system with low curing temperature and high shelf life. Eur. Polym. J. 2019, 112, 736–748. 10.1016/j.eurpolymj.2018.10.044. [DOI] [Google Scholar]
- Tomuta A. M.; Ramis X.; Ferrando F.; Serra A. The use of dihydrazides as latent curing agents in diglycidyl ether of bisphenol A coatings. Prog. Org. Coat. 2012, 74 (1), 59–66. 10.1016/j.porgcoat.2011.10.004. [DOI] [Google Scholar]
- Lee J. H. Using Dihydrazides as Thermal Latent Curing Agents in Epoxy-Based Sealing Materials for Liquid Crystal Displays. Polymers 2021, 13 (1), 109 10.3390/polym13010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Ali Shah S. A.; Gao F.; et al. Latent curing epoxy systems with reduced curing temperature and improved stability. Thermochim. Acta 2019, 676, 130–138. 10.1016/j.tca.2019.03.022. [DOI] [Google Scholar]
- Yu Z.; Ma S.; Tang Z.; et al. Amino acids as Latent Curing Agents and their Application in Fully Bio-based Epoxy Resins. Green Chem. 2021, 23, 6566–6575. 10.1039/D1GC02126E. [DOI] [Google Scholar]
- Budelmann D.; Schmidt C.; Meiners D. Prepreg tack: A review of mechanisms, measurement, and manufacturing implication. Polym. Compos. 2020, 41, 3440–3458. 10.1002/pc.25642. [DOI] [Google Scholar]
- Dubois O.; Le Cam J. B.; Beakou A. Experimental Analysis of Prepreg Tack. Exp. Mech. 2010, 50, 599–606. 10.1007/s11340-009-9236-7. [DOI] [Google Scholar]
- Gilbert M. D.; Schneider N. S.; MacKnight W. J. Mechanism of the Dicyandiamide/epoxide reaction. Macromolecules 1991, 24 (2), 360–369. 10.1021/ma00002a004. [DOI] [Google Scholar]
- Shi K.; Shen Y.; Zhang Y.; Wang T. A Modified Imidazole as a Novel Latent Curing Agent with Toughening Effect for Epoxy. Eng. Sci. 2018, 5, 66–72. 10.30919/es8d639. [DOI] [Google Scholar]
- Ham Y. R.; Kim S. H.; Shin Y. J.; et al. A comparison of some imidazoles in the curing of epoxy resin. J. Ind. Eng. Chem. 2010, 16, 556–559. 10.1016/j.jiec.2010.03.022. [DOI] [Google Scholar]
- Chen K.-L.; Shen Y.-H.; Yeh M.-Y.; Wong F. F. Complexes of imidazole with poly(ethylene glycol)s as the thermal latency catalysts for epoxy–phenolic resins. J. Taiwan Inst. Chem. Eng. 2012, 43 (2), 306–312. 10.1016/j.jtice.2011.08.007. [DOI] [Google Scholar]
- Yang B.; Wei Y.; Liu Y.; Qiu Y. A thermal latent imidazole complex containing copper (II) as the curing agent for an epoxy-based glass fiber composite. Text. Res. J. 2022, 92 (11–12), 1867–1875. 10.1177/00405175211069870. [DOI] [Google Scholar]
- Kohlan T. B.; Atespare A. E.; Yildiz M.; Menceloglu Y. Z.; Unal S.; Dizman B. Amphiphilic Polyoxazoline Copolymer–Imidazole Complexes as Tailorable Thermal Latent Curing Agents for One-Component Epoxy Resins. ACS Omega 2023, 8 (49), 47173–47186. 10.1021/acsomega.3c07177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T.; Rong M. Z.; Zhang M. Q.; Yang G. C. Self-healing epoxy composites – Preparation and effect of the healant consisting of microencapsulated epoxy and latent curing agent. Compos. Sci. Technol. 2007, 67 (2), 201–212. 10.1016/j.compscitech.2006.07.028. [DOI] [Google Scholar]
- Manivannan M.; Rajendran S. Investigation of inhibitive action of urea-Zn2+ system in the corrosion control of carbon steel in sea water. Int. J. Eng. Sci. Res. Technol. 2011, 3, 8048–8060. [Google Scholar]
- Attaei M.; Vale M.; Shakoor A.; Kahraman R.; Montemor M. F.; Marques A. C. Hybrid shell microcapsules containing isophorone diisocyanate with high thermal and chemical stability for autonomous self-healing of epoxy coatings. J. Appl. Polym. Sci. 2020, 137 (22), 48751 10.1002/app.48751. [DOI] [Google Scholar]
- Gilbert M. D.Mechanism and Kinetics of the Dicyandiamide Cure of Epoxy Resins 1988. https://api.semanticscholar.org/CorpusID:135935474.
- Dareh M.; Beheshty M. H.; Bazgir S. Effect of Type and Amount of Accelerator on Reactivity and Curing Behavior of Epoxy/Dicyandiamide/Accelerator System. Iran. J. Polym. Sci. Technol. 2020, 33 (4), 329–338. 10.22063/jipst.2020.1751. [DOI] [Google Scholar]
- Reuther P.; Dünnwald P.; Tabatabai M.; Schuh C.; Hartmann L.; Ritter H. Thermally Controlled Acceleration of Epoxy Resin Curing through Polymer-Bound Imidazole Derivatives with High Latency. ACS Appl. Polym. Mater. 2022, 4 (2), 1150–1158. 10.1021/acsapm.1c01568. [DOI] [Google Scholar]
- Rodošek M.; Mihelčič M.; Čolović M.; et al. Tailored Crosslinking Process and Protective Efficiency of Epoxy Coatings Containing Glycidyl-POSS. Polymers 2020, 12 (3), 591 10.3390/polym12030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. D.Curing Epoxy Resins Using Dicy, Imidazole and Acid. U.S. Patent US5,508,328, 1994.