Abstract
Neurodegenerative diseases, encompassing conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, prion disease, and Huntington’s disease, present a growing health concern as human life expectancy increases. Despite this, effective treatments to halt disease progression remain elusive due to various factors, including challenges in drug delivery across physiological barriers like the blood–brain barrier and patient compliance issues leading to treatment discontinuation. In response, innovative treatment approaches leveraging noninvasive techniques with higher patient compliance are emerging as promising alternatives. This Review aims to synthesize current treatment options and the challenges encountered in managing neurodegenerative diseases, while also exploring innovative treatment modalities. Specifically, noninvasive strategies such as intranasal administration and nanosized drug delivery systems are gaining prominence for their potential to enhance treatment efficacy and patient adherence. Nanosized drug delivery systems, including liposomes, polymeric micelles, and nanoparticles, are evaluated within the context of outstanding studies. The advantages and disadvantages of these approaches are discussed, providing insights into their therapeutic potential and limitations. Through this comprehensive examination, this Review contributes to the ongoing discourse surrounding the development of effective treatments for neurodegenerative diseases.
1. Introduction
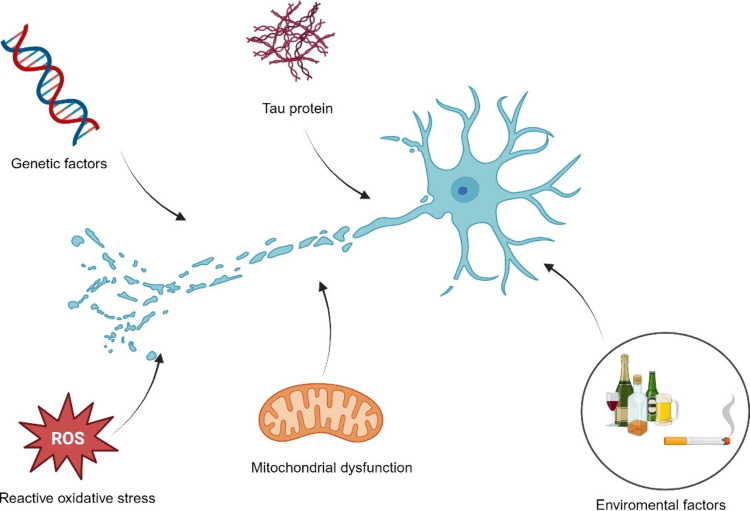
Neurodegenerative disease is an inclusive term for a range of disorders characterized by progressive loss of nervous system cells and consequent loss of nervous system functions.(1) Many different diseases, such as Alzheimer’s disease, Parkinson’s disease, Multiple sclerosis, Amyotrophic Lateral Sclerosis disease, Prion disease and Huntington’s disease, are included in this group. With the increase in the average human life expectancy worldwide, “neurodegenerative diseases” are seen in increasing prevalence today due to the increased oxidative stress in nerve cells and brain cells, free radical accumulation, and intensified immune response resulting from pro-inflammatory cytokine release, tau protein accumulation, mitochondrial dysfunction, genetic factors, environmental factors such as smoking and alcohol consumption (Figure 1) Since all causes and mechanisms of neurodegenerative diseases have not been fully elucidated and the clinical response obtained in treating these diseases is not at the desired level, studies on treating diseases in this group are still up to date.
Figure 1.
Factors that trigger neuron damage and lead to neurodegenerative disease. Internal and external contributors lead to complex series of events that result in neurodegeneration. Environmental toxins, pollution, heavy metals, genetic predisposition, gene mutations, protein misfolding, oxidative stress in cells, and lack of energy production in mitochondria are among several factors. (This figure was created with BioRender.com).
In these researches, nanosized drug delivery systems come to the fore in providing effective treatment of neurodegenerative diseases, thanks to their intrinsic properties. Nanosized drug delivery systems have significant advantages due to their mechanical, electrical, optical, and magnetic superiority compared to conventional dosage forms, especially in the treatment of all neurodegenerative diseases that require bypassing the blood–brain barrier (BBB).1,2
These advantages include their nanoscale size, high surface area to volume ratio, high drug loading capacity, ability to escape from the reticuloendothelial system and long residence time in the blood circulation, suitability for surface modifications, active and passive targeting, biosafety, protect the active substance from enzymatic degradation, targeting ability, suitable for surface modification.1,3
Especially in the treatment of neurodegenerative diseases that require long-term treatment, nanosized drug delivery systems provide controlled drug release, increase bioavailability and biocompatibility properties come to the fore.4,5
Within the scope of this Review, neurodegenerative diseases will be defined, current treatment options and challenges encountered in these treatments will be summarized and nanodrug carrier-based treatment approaches to create the desired therapeutic effect will be discussed.
2. Neurodegenerative Disease
In this section, the most common neurodegenerative diseases will be defined, and treatment approaches will be discussed as a foundational framework for further discussion.
2.1. Alzheimer’s Disease
Today, Alzheimer’s disease (AD) is the most common neurodegenerative disease, affecting 24 million people worldwide. This disease is characterized by memory loss, decreased cognitive functions, and various psychiatric and behavioral disorders.6 Risk factors for AD include age, gender, genetic factors (presence of APOE ϵ 4 allele), smoking, diabetes, hypertension, and high serum cholesterol levels. Extracellular beta-amyloid plaque formation, neurofibrillary tangle formation containing tau-protein, glial dysfunction, neuron damage, and synapse damage in different central nervous system (CNS) regions are the main distinguishing factors in the histopathological examinations of this disease.7,8
Different treatment strategies are being developed for the treatment of specified pathologies. Cholinesterase inhibitors and N-Methyl d-Aspartate (NMDA) receptor antagonists are clinically approved for AD treatment. Although recent studies suggest the use of tacrine, estradiol, curcumin and peptides, their use is limited due to the limited passage of these components the BBB and potential serious side effects.7
2.2. Parkinson’s Disease
Parkinson’s disease (PD) was first described as “shaking palsy” by British physician James Parkinson in 1817. In Parkinson’s disease, which is a progressive neurological disease in which dopaminergic neuron loss in the substantia nigra part of the brain and Lewy bodies are detected, individuals experience tremors, bradykinesia, rigidity, postural instability and deterioration of other motor functions, and loss of sensation.
According to the data published by The Global Burden of Disease Study, Parkinson’s disease is the neurological disease with the fastest incidence, and it is predicted that approximately 13 million people will be diagnosed with this disease in 2040.9
PD causes complex disorders that require an individualized treatment approach. Currently, treatment is carried out with levodopa/carbidopa, dopamine agonists (rotigotine, apomorphine, pramipexole, ropinirole), catechol-O-methyl transferase inhibitors (entacapone, tolcapone, opicapone), monoamine oxidase inhibitors. Studies continue to reduce side effects and develop targeted treatment strategies.9
2.3. Multiple Sclerosis
Multiple Sclerosis (MS), an autoimmune CNS disease characterized by inflammation, demyelination, and axonal damage, was first described by Jean-Martin Charcot in 1868 as “sclerose en plaques”.10
After MS attacks, together with the deterioration of electrical signal transmission in nerve cells, numbness in the limbs, paralysis, visual disturbances, and other neurological disorders occur. In this disease, the diagnosis is made by detecting scars, plaques, and lesions in the brain, spinal cord, and cerebrospinal fluid with magnetic resonance imaging. The risk factors for this disease, are genetic factors, especially gender, metabolism, virus, etc., and immunological response to infections caused by other pathogens.10,11
There are different treatment options for MS treatment to relieve symptoms and prevent disease progression. In order to prevent disease progression, immunosuppressant (like fingolimod, natalizumab, ocrelizumab) or immunomodulatory (interferon beta, glatiramer acetate, teriflunomide, etc.) agents are frequently used in treatment. Treatment for relieve symptoms is often planned to improve the patient’s quality of life. For this purpose, tricyclic antidepressants, painkillers, or anticholinergic agents are oftenly used.11
2.4. Prion Diseases
Prion diseases are rare transmissible neurodegenerative diseases. This disease, which occurs due to the accumulation of misfolded prion proteins, progresses rapidly and can lead to death. Because of their infectious properties, these diseases are considered infectious diseases of the central nervous system and are also called ’Transmissible Spongiform Encephalopathies’. This disease can be transmitted to humans through medical devices, blood, and from animals. Although there is no definitive treatment yet for this disease, which is divided into different subgroups as sporadic, hereditary, and acquired, treatment development studies are continuing.12,13
2.5. Huntington’s Disease
Huntington’s disease (HD), is a rare disease of inherited origin that causes progressive degeneration of neurons in the CNS. In this disease, which was first described by George Huntginton in 1872, the presence of the 350 kDa “Huntingtin” protein is observed due to the constant repetition of cytosine-adenine-guanine nucleotides in the human genome. Progressive destruction begins in the CNS due to excessive production and accumulation of huntingtin protein by neurons. The hereditary transmission of this disease is seen at the same level in men and women.14
Different motor, cognitive, and psychiatric symptoms are observed in HD. Cachexia, dysarthria, dysphagia, dystonia, movement disorders, loss of coordination, muscle stiffness, seizures, cognitive impairment, and insomnia occur in patients. There is no radical cure for HD yet or a treatment that will slow disease progression. Current treatment options include symptomatic treatment with haloperidol, tetrabenzaine, and amantadine.15
2.6. Amyotrophic Lateral Sclerosis Disease
ALS (Amyotrophic Lateral Sclerosis Disease) is a neurodegenerative disease in which damage occurs primarily in the motor neurons responsible for the movement of the muscles in the brain and spinal cord.
Pathologically, loss of neuromuscular connections, axonal retraction, and death in motor neurons due to these, together with ubiquitin-positive inclusion formations, are observed. As a result, progressive weakness, muscle wasting, and coordination disorders occur in the extremities and trunk, which can significantly affect the quality of life of individuals. Risk factors for this disease include age, genetic factors, gender, and exposure to environmental toxins.
In addition to the treatment with two FDA-approved (Food and Drug Administration) drugs, Rilozule (1993) and Edaravone (2017), symptomatic and palliative treatment is applied to improve the quality of life of ALS patients.16
3. Challenges with Current Treatment Options
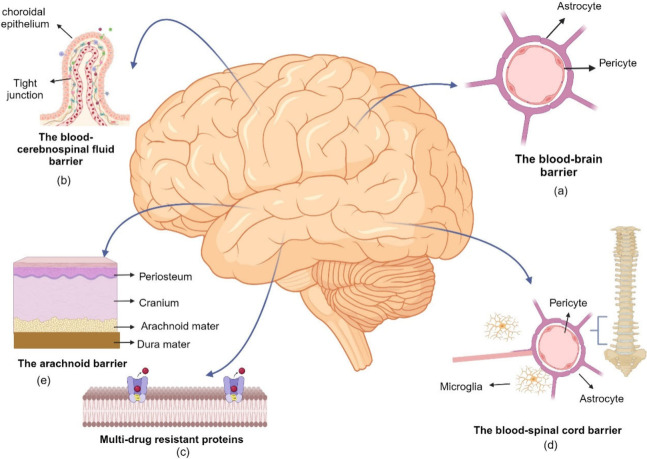
Although there are limited treatment options for neurodegenerative diseases, current treatment strategies mainly aim to slow the progression of the disease and alleviate its symptoms. A crucial obstacle in establishing the desired therapeutic efficacy is the presence of protective barriers surrounding the central nervous system. Most of the drugs with clinical potential cannot reach the targeted area at adequate concentration due to various physiological barriers, especially the blood–brain barrier.17 Blood-cerebrospinal fluid barrier, arachnoid barrier, blood-spinal cord barrier, and multidrug resistant proteins (P-glycoprotein) are other significant physiological barriers that limit the passage of drugs to the CNS. (Figure 2)
Figure 2.
Physiological barriers that protect the CNS from harmful toxins and provide a stable environment for optimal function limit the passage of drugs to the CNS. The passage of substances into the CNS is tightly protected by various physiological barriers: (a) the blood–brain barrier, (b) the blood–cerebrospinal barrier, (c) multidrug resistant proteins, (d) the blood–spinal cord barrier, and (e) the arachnoid barrier. (This figure was created with BioRender.com).
3.1. The Blood–Brain Barrier
The blood–brain barrier (BBB) is the most critical barrier in the transition to the CNS and basically acts as a border between blood circulation and the brain’s interstitial fluid. The functions of this barrier in the membranous structure include maintaining the homeostasis of the central nervous system, obtaining necessary nutrients from the blood, providing neuroprotection, and preventing the passage of neurotoxic metabolites and xenobiotics.17 The BBB consists of endothelial cells, pericytes, astrocytes (astroglia), and the basement membrane.
There are tight connections called “tight junctions” between endothelial cells that form the microvascular structure in the brain parenchyma.18 These connections create a high electrical resistance, resulting in a low transit rate by transcytosis and a limited rate of transcellular and paracellular transport.19 Endothelial cells have a high mitochondrial content, creating high metabolic activity.20,21 Pericytes, as another component of the blood–brain barrier, contribute to the formation of the barrier and are involved in regulating cerebral flow, regulating permeability from blood walls, controlling the entry of immune cells, and clearing residues from cell death.22 The passage of drugs through the blood–brain barrier is affected by several physicochemical properties such as the concentration difference between the compartments, molecular weight of the active substance, lipophilic character, partition coefficient, and degree of ionization. In parallel, the presence of pathological conditions due to the neurodegenerative disease and the binding affinity of the drugs to plasma proteins are critical. Based on these factors, the passage through the blood–brain barrier occurs through different mechanisms including passive paracellular transition, passive transcellular transition, passage through carrier proteins, receptor-mediated transcytosis, adsorptive transcytosis, and active efflux pumps.23
3.1.1. Passive Paracellular Transition
Primarily the passage of water-soluble and low molecular weight substances between endothelial cells is mediated via this route.24,25 Tight junction connections between endothelial cells have a major role in limiting the passage of molecules in this way. Therefore, using this transition is not an ideal strategy in cases where it is aimed to increase the passage through the blood–brain barrier.26
3.1.2. Passive Transcellular Transition
The passage of lipophilic molecules with low molecular weight predominantly occurs through this route. However, in this transition, due to the high lipophilic character of drugs, involvement in the microvascular area can be observed in the brain; this may lower the rate of drug passing to the targeted area in the treatment.27
3.1.3. Passage Through Carrier Proteins
The high electrical resistance and low permeability of the blood–brain barrier, restrict the passage of hydrophilic substances to an almost negligible extent. The passage of substances of this character takes place in the barrier through stereospecific carrier proteins located in bidirectional transport systems. In this way, the passage of more vitamins, nucleosides, glucose, and amino acids takes place, and it is in an exciting position in cases where it is aimed to increase the passage through the blood–brain barrier.24,25,28,29
3.1.4. Receptor-Mediated Transcytosis
Specific receptors on endothelial cell membranes are involved in this transition, which is based on energy use and occurs through endocytotic mechanisms. As the drug/ligand binds to its specific receptor, vesicle formation occurs and after passing through the endothelial membranes, the drug leaves the receptor and becomes free. This passage is achieved through endocytosis.30,31 The transport of substances in a peptide–protein structure, such as immunoglobulin G (IgG), transferrin, leptin, and low-density lipoprotein (LDL), which have a high molecular weight, mainly occurs in this way.31
3.2.5. Adsorptive Transcytosis
This transition occurs through adsorptive mechanisms due to the electrostatic interaction between the positively charged ligand/active substance and the negatively charged endothelial cell wall.32,33 Compared to receptor-mediated transcytosis, it has lower specificity but higher transport capacity.34 The transition of molecules such as oligopeptide and protamine, cationic albumin, cationic peptides, and avidin occurs through this transition mechanism.35,36
3.2.6. Active Efflux Pumps
The blood–brain barrier also has efflux transporters that allow passage from the brain parenchyma to the bloodstream. These transporters not only ensure the removal of xenobiotics, substances that cause neurotoxicity but also cause the removal of drugs with high lipophilic character.37,38 Located in the endothelial cell membrane, these transporters belong to 2 large gene families, ATP-binding Casette (ABC) Family and Solute Carrier (SLC) Family. P-glycoprotein (P-gp), the best-known member of the ABC transporters, was first detected in drug-resistant tumor cells. In the presence of neurodegenerative disease, a significant increase in p-glycoproteins is observed.38 Since this leads to a decrease in the amount of the active substance reaching the targeted area, it significantly limits the generation of the intended therapeutic effect.
3.2. The Blood–Cerebrospinal Fluid Barrier
The second significant barrier that limits the passage of active substances to the CNS in systemic drug administration is the blood-cerebrospinal fluid barrier, which consists of choroid plexus epithelial cells. It plays a crucial role in maintaining the homeostasis of the cerebrospinal fluid.39
3.3. The Arachnoid Barrier
The arachnoid barrier is the part that separates the dura mater, where the passage of nanoparticles takes place, from the subarachnoid space, facilitated by the lymphatic channels embedded within its structure. The main component forming this barrier, which has an average thickness of 200 μm in a healthy individual, is leptomeningeal cells. A study was carried out to elucidate the structure of the arachnoid barrier and revealed the high numbers of P-gp and breast cancer resistance protein (BCRP) transporters.40,41
3.4. The Blood–Spinal Cord Barrier
The blood-spinal cord (spinal cord) barrier, which provides a unique microenvironment for the cellular components of the spinal cord, consists of morphological components like the blood–brain barrier. Its structure includes specialized endothelial cells, surrounding basal lamina, astrocytes, pericytes, and tight junctions. The blood-spinal cord (spinal cord) barrier has a protective and regulating function for the spinal cord parenchyma.42,43
4. Treatment Approaches to Increase Drug Transition to the Central Nervous System
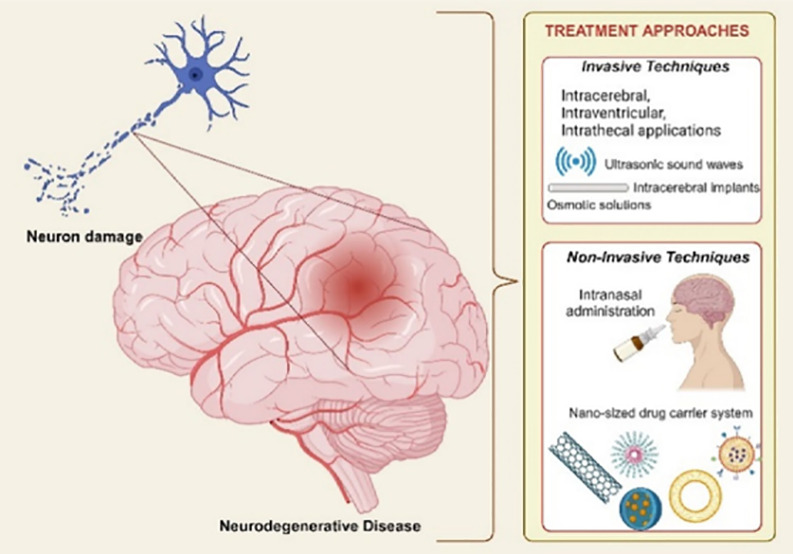
As stated earlier, one of the most important factors limiting success in treating neurodegenerative diseases is the existence of various physiological barriers that are responsible for protecting the sensitive structure of the central nervous system. The impeded drug transport to the targeted area; obstructs the achievement of the desired therapeutic effect. In order to overcome this problem different treatment strategies have been developed based on increasing the passage through the blood–brain barrier or bypassing the blood–brain barrier. Developed treatment strategies are grouped under two headings by evaluating whether they involve direct intervention in the central nervous system. (Table 1)
Table 1. Treatment Approaches to Increase Transition to the Central Nervous Systema.
| treatment approach | advantages | disadvantages |
|---|---|---|
| invasive techniques | ||
| intracerebral, intrathecal, intraventricular administration | the required drug concentration in the target tissue is provided | high risk of CNS infection |
| intracerebral implants | applicable to different molecule size | catheter obstruction |
| ultrasounds | low systemic exposure | limited distribution of therapeutic agents |
| hyperosmotic solutions | controlled or extended drug release | trauma risk |
| vasoactive substances | ||
| non-invasive techniques | ||
| alternative drug administrations routes (e.g., intranasal) | high patient compliance | scale-up process |
| chemical modification | easy application | the limited volume of nasal cavity |
| nanosized drug delivery systems | rapid absorption | the presence of mucociliary clearance |
| controlled or extended drug release | damage of nasal mucosa | |
| high risk of oxidative metabolism | ||
Although each treatment approach has advantages and disadvantages, noninvasive methods are featured in the overall evaluation.
4.1. Invasive Techniques
These treatment methods, which involve a direct intervention to the central nervous system, include intracerebral, intraventricular, and intrathecal applications, intracerebral implants, different treatment techniques using ultrasonic sound waves, vasoactive components, and osmotic solutions.17,23,44,45 For example, the blood–brain barrier is bypassed in intrathecal applications by direct injection into the lumbar subarachnoid region. In intracerebral and intraventricular applications, direct applications to the brain parenchyma or ventricles allow the drugs to reach the targeted area by bypassing the blood–brain barrier.23 The intracerebral implants prepared from biodegradable polymeric materials, provide treatment by controlled drug release in the targeted area over a predetermined period. Particularly, with applications aimed at deliberately damaging the physiological barriers, which have been revealed in studies on Parkinson’s disease, a high level of penetration of drugs into the brain parenchyma occurs. For example, by performing a temporary structural deterioration at “tight junctions,″ specific openings are created in the blood–brain barrier in a time-controlled and reversible manner.
Other applications in this context are the application of osmotic solutions (for example, hyperosmotic mannitol solution), vasoactive components (for example, bradykinin), and ultrasonic sound waves. Similarly, in these applications, conscious disruptions are formed in the blood–brain barrier’s structure, and the active drug transition to the brain parenchyma is ensured.46
The advantages of invasive techniques with different treatment strategies include reaching the optimum drug concentration in the targeted area, the applicability of the treatment for molecules of different sizes, low levels of systemic exposure, and controlled drug release (for intracerebral implants).
However, in addition to these advantages, patient compliance is deficient in invasive treatment strategies, and these applications can cause significant complications. Vascular pathological disorders and other chronic neuropathological disorders can be observed. The infection risk, catheter obstruction, and trauma situations are other possible complications.17
4.2. Noninvasive Techniques
For noninvasive treatment methods, where patient compliance is higher than invasive techniques, there are strategies based on increasing the passage through the blood–brain barrier with lipid-mediated transport mechanisms, the use of alternative administration routes, and the use of nanosized drug delivery systems. There are several strategies based on increasing lipid-mediated transport mechanisms and crossing the blood–brain barrier which include chemical modification with various functional groups and the pro-drug approach.44
The basic principle in chemical modification is to increase the lipophilicity of the drug molecule. In case of drugs with hydrophilic character, the modification is achieved by adding a functional group that increases the lipophilic character of the drug, so that the low level of passage through the blood–brain barrier can be increased. In the pro-drug approach, the inactive form of the original molecule is metabolized in the brain parenchyma as it passes through the blood–brain barrier. A bioactivation step is needed in the pro-drug approach.47 While evaluating the effectiveness of these treatment strategies, the increased sensitivity to oxidation due to the increased lipophilic character of the drug, its distribution to other tissues, and the need for a metabolic bioactivation step in the pro-drug approach must be taken into consideration.47,48 Due to the unique physiological characteristics of the brain, studies have been carried out on alternative administration routes to achieve the desired treatment outcomes. Among the alternative routes of administration, intranasal administration stands out for its ability to bypass the blood–brain barrier via the neuron axons responsible for the sense of smell in the olfactory mucosa region.49 The olfactory and trigeminal nerve pathways, which are located in the nasal cavity and have a direct connection with the central nervous system, play a role in this route of administration, first discovered by Frey in 1989. The recent studies have revealed that the trigeminal nerve pathway is crucial in targeting the brain’s frontal region.50,51 This route of administration, which is preferred especially for drugs with low oral bioavailability, has significant advantages such as high patient compliance, ease of application, and rapid absorption. However, the limited volume of the nasal cavity and the presence of mucociliary clearance in the nasal cavity significantly limit the use of this route of administration.
Among noninvasive techniques, nanosized drug delivery systems hold significant promise in treating neurodegenerative diseases, attributed to their small particle size at the nanometer scale which provides many advantages such as increased permeability, stability, and therapeutic activity.44
5. the Use of Nanoscale Drug Delivery Systems in the Treatment for Neurodegenerative Diseases
The term “nanoparticle” is defined by the National Nanotechnology Initiative (NNI) as a particle with dimensions ranging from 1 to 100 nm in at least one dimension. Nanosized drug delivery systems, on the other hand, are colloidal drug carrier systems that can be prepared from natural or synthetic polymers and inorganic elements, whose dimensions are several hundred nanometers.4,51
Nanosized drug delivery systems appear to be quite successful in targeting, creating the desired pharmacological effect, and reducing side effects, thanks to their ability to interact with cells. The success of these systems in passing through the blood–brain barrier and reducing side effects is associated with their effects on pharmacokinetic properties such as absorption, biodistribution, and bioavailability. In addition, nanosized drug delivery systems protect therapeutic active compounds from enzymatic degradation, enabling greater passage through the blood–brain barrier.
For nanosized drug delivery systems to be successful in targeting the central nervous system, they must have ideal properties such as physicochemical stability, permeability to blood vessels, appropriate circulation time in the blood circulation, specificity to the targeted cells, controlled release profile, high receptivity to the transported molecules, biocompatibility and biodegradable properties. These ideal properties are directly related to the intrinsic properties of nanosized drug delivery systems such as size and polydispersity index, zeta potential, composition, hydrophobicity, stiffness, mucoadherence, and permeability.52
Nanosized drug delivery systems have significant advantages, especially in treating neurodegenerative diseases due to their mechanical, electrical, optical, and magnetic advantages when compared to conventional dosage forms (Table 2).53 They have adjustable nanoscale dimensions, their surface area/volume ratio and drug loading capacity is high, due to their small size they can escape from the reticuloendothelial system and prolong the residence time in the blood circulation, they are suitable for surface modifications, active and passive targeting can be done, they protect the drug from enzymatic degradation, target to specific region and provide controlled drug release.4,5,53−55 In addition, nanosized drug delivery systems are considered as promising systems for improving the bioavailability of drugs with solubility problems or short biological half-lives.55,56
Table 2. Summarized Examples of Nanosized Drug Delivery Systems’ Advantages and Studies.
| advantages | studies | |
|---|---|---|
| liposomes | increase drug efficacy | Mengke Qu and co-workers designed N-3,4-bis(pivaloyloxy)-dopamine-loaded- 29 amino-acid peptide (RVG29) functionalized liposomes for Parkinson’s disease. In this study, this liposome formulation provided better brain targeting comparing free N-3,4-bis(pivaloyloxy)-dopamine. In in vivo studies, this liposome formulation demonstrated selective distribution to brain endothelial cells.71 |
| increase stability via encapsulation | A leptin-functionalized liposome formulation was developed as a drug carrier for resveratrol and epigallocatechin gallate for Parkinson’s disease. In this study, they demonstrated that liposome formulation has enhanced BBB permeability, and cellular uptake into cloned cell line derived from the SK-N-SH neuroblastoma cell line (SH-SY5Y) cells.72 | |
| good delivery vehicle for both hydrophobic and hydrophilic drugs | In a study conducted by Gailard et al., glutathione-conjugated PEGylated liposome formulations were prepared to increase the therapeutic availability of methylprednisolone in the treatment of MS. The prepared formulations were administered intravenously to rats for which an EAE model was developed. Prolonged plasma circulation and increased brain uptake were obtained in rats administered glutathione-conjugated PEGylated liposome formulations compared to rats administered free methylprednisolone. In addition, thanks to the liposome formulations prepared in the study, treatment effectiveness was achieved at lower doses, thus increasing the therapeutic availability of methylprednisolone.73 | |
| high compatibility | ||
| reduce the toxicity of the encapsulated drug | ||
| reduce side effects | ||
| nontoxic, biocompatible, biodegradable, and nonimmunogenic for systemic administrations | ||
| polymeric nanoparticles | ease of production process | Yang et al. prepared brain-targeted peptide-functionalized chitosan nanoparticles for resveratrol delivery to brain. The Morris water maze test was used to compare the effectiveness of the formulations. The cognitive improvement was more evident in mice that received treatment with functionalized chitosan nanoparticle formulation.74 |
| biocompatible | Gonzalez et al. investigated intranasal polymeric nanoparticles containing Interferon-β (IFN-β) which has a short half-life, and limited CNS access for multiple sclerosis. In mice with the experimental autoimmune encephalomyelitis (EAE) model, the same dose of intranasal and systemic free interferon administration had no effect. The improvement in clinical symptoms, reduction of inflammation, and the demyelinated area were observed in the mice receiving the nanoparticle formulations containing IFN-β.75 | |
| biodegradable | si-RNA loaded chitosan nanoparticles were prepared for gene delivery in HD treatment. Si-RNA treatment reduces the expression of genes encoding the huntingtin protein, which shows excessive accumulation in HD. However, intracerebral and intrathecal applications, which are invasive methods in chronic treatment, negatively affect patient compliance. si-RNA loaded chitosan nanoparticles were prepared in the study conducted by Sava et al. to increase patient compliance and treatment effectiveness. After the prepared formulations were administered intranasally to transgenic mice, it was determined that huntingtin protein expression in the brain tissues of the mice was significantly reduced.76 | |
| targetable carrier systems | In a study conducted by Saleh and colleagues, berberine-loaded PLGA nanoparticle formulations conjugated with Tet-1 peptide were designed for the treatment of AD. Berberine is a natural compound known for its effects in mitigating Tau hyperphosphorylation and reducing fibril formation; however, its bioavailability is extremely low, hindering its ability to reach the brain adequately. The formulated Tet-1 peptide-conjugated PLGA nanoparticles aimed to achieve brain targeting of berberine and alleviate AD symptoms. When administered to rats with an AD model, it was observed that the group treated with berberine-loaded Tet-1 peptide-conjugated PLGA nanoparticles exhibited significantly reduced fibril formation compared to other groups. Additionally, substantial improvement in synaptic activity and higher success rates in cognitive function tests were observed in this group.77 | |
| protection of encapsulated drug from chemical and enzymatic degradation | ||
| solid lipid nanoparticles | protection of encapsulated drug from chemical and enzymatic degradation | Dimethyl fumarate-loaded solid lipid nanoparticles were evaluated for multiple sclerosis treatment. Dimethyl fumarate has serious side effects like lymphopenia. The same dose of oral dimethyl fumarate and subcutaneous dimethyl fumarate-loaded solid lipid nanoparticles were compared for clinical outcomes. The dimethyl fumarate-loaded solid lipid nanoparticles prevented disease progression without causing any harm to lymphocytes.78 |
| low cytotoxicity | Piperine, an antioxidant natural alkaloid, mimics acetylcholine due to its similarity in structure, blocks the acetylcholinesterase enzyme, and increases cholinergic activity in the brain. When applied alone, the amount reaching the brain is quite low due to the solubility problem and the hepatic first-pass effect. To overcome these problems related to piperine, in a study conducted by Yusuf et al., Polysorbate-80 coated solid lipid nanoparticle formulations containing piperine were prepared. Positive effects were obtained from these formulations in vitro and in vivo tests. A decrease in cholinergic degradation, immobilization, and the presence of amyloid plaque was observed.79 | |
| increasing the bioavailability of active substances with low solubility in aqueous media | Chitosan coated- dopamine loaded-solid lipid nanoparticle formulations were developed aiming to achieve brain targeting for the treatment of PD. The studies evaluating the in vitro uptake of the prepared formulations confirmed a strong interaction between the developed solid lipid nanoparticle formulation and brain endothelial cells, affirming the potential of solid lipid nanoparticles as drug carriers.80 | |
| reducing serious side effects | ||
| biocompatible | ||
| dendrimers | high drug loading | In a study conducted by Gothwal et al., PAMAM dendrimer formulation and lactoferrin-conjugated PAMAM dendrimer formulations were prepared to increase the therapeutic effectiveness of memantine used in the treatment of AD. When the prepared formulations were applied to rats in which the AD model was developed, more successful results and improving memory were obtained in behavioral tests with the extended-release effect in the group in which memantine-loaded lactoferrin-conjugated PAMAM dendrimers were applied.81 |
| improve bioavailability | The BBB is an important barrier that tightly controls the passage to the brain for drug and gene delivery. In a study conducted by Zarebkohan et al., PAMAM dendrimers were designed as gene carrier systems that carry Serine–Arginine–Leucine peptide targeting the brain. Carrier systems were prepared by binding Serine–Arginine–Leucine peptide to bifunctional PEG–PAMAM dendrimers. When the prepared systems were administered to rats by i.v. injection, it was observed that the amount of Serine–Arginine–Leucine peptide that crossed the BBB and reached the brain parenchyma was higher in the group to which Serine–Arginine–Leucine peptide bifunctional PEG–PAMAM dendrimers were applied, compared to the control groups. Study results show that PAMAM dendrimers are suitable and effective nanocarriers for brain gene delivery.82 | |
| protection of encapsulated drug from chemical and enzymatic degradation | In a study conducted by Singh et al., donepezil-loaded-PAMAM dendrimer formulations were prepared to increase the effectiveness of donepezil used in the treatment of AD. Evaluation of treatment effectiveness was carried out in vivo and in vitro studies. In in vitro acetylcholine esterase inhibition activity measurement, it was determined that donepezil-loaded-PAMAM dendrimer formulations provided a higher level of inhibition compared to donepezil alone. In in vivo studies, the prepared formulations were administered intravenously to rats. It was observed that the amount of donepezil reaching the brain was 4 times higher in the group where PAMAM dendrimer formulations were applied compared to the group receiving free donepezil.83 | |
| ease of production process | ||
| demonstrating controlled released effect | ||
| nanogels | improving solubility of hydrophobic drugs | A nano gel carrier system was developed for the delivery of albiflorin to enhance BBB permeability. The intranasal application of nano gel system was compared to the free drug and enhanced brain distribution and improved stability was achieved via the nanogel system.84 |
| enhancing the stability of therapeutic agents against chemical/enzymatic degradation | Dopamine-loaded PEGylated nanogel formulations were prepared for the treatment of AD, and the prepared formulations were modified with transferrin receptor ligand, aiming to increase the passage through the BBB. In vivo studies conducted on rats showed that the amount of dopamine reaching the brain was 9 times higher in the group in which nanogel formulations were applied, compared to the group in which free dopamine was administered.85 | |
| increasing stability | ||
| demonstrating sustained released effect | ||
| good delivery vehicle for both hydrophilic and hydrophobic drugs | ||
| nanoemulsions | enhanced long-term stability | Pterostilbene is a molecule with low bioavailability that has neuroprotective activity with its antioxidant and anti-inflammatory properties. In a study conducted by Liu et al., they prepared pterostilbene-loaded nanoemulsion formulations to increase the bioavailability of pterostilbene for use in the treatment of AD. When the prepared formulations were treated in mice, it was observed that its bioavailability increased, the NRF2 pathway was stimulated more strongly, and higher neuroprotective activity was obtained in the group in which pterostilbene-loaded nanoemulsion formulations were treated, compared to the group in which free pterostilbene was treated.86 |
| simplicity for production | In a study conducted by Friedman-Levi et al., nanoemulsion formulations containing grape seed oil were prepared to increase the neuroprotective activity of grape seed oil in the treatment of HD. It was determined that when the prepared formulations were applied to transgenic mice, disease progression was delayed.87 | |
| improved solubility | ||
| good delivery vehicle for both hydrophilic and hydrophobic drugs | ||
| protection of encapsulated drug from enzymatic degredation | ||
| polymeric micelles | increasing the solubility and bioavailability of drugs with low water solubility | A study was conducted to develop galantamine-loaded 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-73 (DSPE-PEG2000) based micelles. Galantamine is an approved drug for the treatment of Alzheimer’s disease. It has poor brain penetration, low intestinal permeation. Lohan et. al compared galantamine-loaded micelle formulation to galantamine suspension in in vivo studies. They revealed improved bioavailability in pharmacokinetic studies, improved cognitive improvement and good clinical outcomes in pharmacodynamics studies.88 |
| prevention of interaction with serum proteins | Dexamethasone-loaded polymeric micelle formulations for intranasal administration was prepared to reduce the presystemic elimination of dexamethasone. Parallel artificial membrane permeability assay and other in vitro test results showed that this formulation has high permeability values for BBB.89 | |
| prevention the degradation of the drug | A polydopamine-coated micelle formulation was prepared with the aim of increasing the bioavailability of the phosphodiesterase inhibitor ibudilast and targeting the brain for the treatment of MS. The micelles were administered intranasally to mice with an experimental autoimmune encephalomyelitis (EAE) model at different doses. Through the micelle formulations, it was demonstrated that even in low-dose treatment regimens, a higher level of ibudilast reached the brain compared to oral and nasal administration of free ibudilast, leading to higher neuroprotective efficacy.90 | |
| targeting specific tissues | Apolipoprotein-modified micelle formulations were prepared to enhance the bioavailability of the plant-derived compounds oridonin and phillyrin, which possess antioxidant, anti-inflammatory, and neuroprotective effects, for the AD treatment. When these formulations were administered to mice with an AD model, it was found that they achieved a higher level of brain targeting compared to mice treated with free oridonin and phillyrin. Additionally, an increased compound uptake by nerve cells, higher levels of cognitive improvement, and decreased astrocyte-microglia activity were observed.91 | |
| inorganic nanoparticles | compatible with various surface modifications | Hyperforin-loaded gold nanoparticles were evaluated in the treatment of multiple sclerosis. This formulation reduced inflammatory cells in the spinal cord and regulated transcription factors. The study revealed that hyperforin-loaded gold nanoparticles had a distinguishable effect on the EAE model in terms of clinical and laboratory outcomes.92 |
| relatively low cytotoxicity | Formulations of transmembrane peptide-modified chondroitin sulfate gold nanoparticles were prepared for AD treatment. Chondroitin sulfate is a glycosaminoglycan derivative that inhibits tau protein accumulation and Aβ fibril formation, but its passage through the BBB is limited due to its high molecular weight. Formulations were prepared to enhance BBB permeability, and evaluations of their anti-AD efficacy were conducted on SH-SY5Y cells. The usage of gold nanoparticles increased the antioxidant activity of chondroitin sulfate, the cellular uptake and transport ability were also enhanced.93 | |
| ability to cross the blood–brain barrier | Formulations of glucosamine-conjugated gold nanoparticles were used to evaluate the neuroprotective efficacy of glucosamine in the treatment of neurodegenerative diseases. Studies conducted on SH-SY5Y cells demonstrated that glucosamine-conjugated gold nanoparticle formulations significantly reduced protein aggregation and substantially prevented cell death in nerve cells.94 | |
| optical properties suitable for conjugation of diagnostic and imaging agents | ||
| exosomes | low risk of immunogenicity | The intranasal administration of resveratrol-loaded exosome formulations for multiple sclerosis treatment was evaluated. The formulation reduced inflammatory responses in CNS and augmented clinical outcomes in vivo.95 |
| high biocompatibility | The intranasal administration of exosome-based carrier system carrying catalase, a natural potent antioxidant for Parkinson’s disease was investigated. In in vivo studies, this formulation accumulated remarkably in the PD mouse brain and accomplished neuroprotective effects.96 | |
| good delivery vehicle for both hydrophobic and hydrophilic drugs | Plasma-derived exosomes loaded with quercetin were prepared aiming to achieve brain targeting and enhance the bioavailability of quercetin for the treatment of AD. When these formulations were administered to mice with an AD model, it was observed that the improvement in cognitive functions was higher compared to the administration of free quercetin, indicating an increased bioavailability of quercetin.97 | |
| targeting specific tissues | Silibinin encapsulated in macrophage-derived exosomes was prepared to enhance the bioavailability of silibinin while simultaneously reducing Aβ aggregation and astrocyte deactivation in Alzheimer’s disease (AD) treatment. The in vivo studies revealed that the administration of silibinin-loaded macrophage-derived exosomes significantly reduced Aβ plaque formation compared to mice treated with silibinin alone. Moreover, astrocyte activation and the release of inflammatory cytokines were markedly inhibited. Mice treated with drug loaded exosomes showed better improvements in neuronal recovery and cognitive functions compared to those treated with free silibinin.98 | |
| provide neural regeneration | Formulations of coenzyme Q10 loaded adipose stem cell-derived exosomes were evaluated for enhancing the therapeutic efficacy of coenzyme Q10 in the neuroprotective activity for AD treatment. These formulations were administered intraperitoneally to rats with AD models. Evaluation of changes in memory and cognition was performed using passive avoidance and Morris water maze tests, revealing that rats treated with coenzyme Q10 loaded exosome formulations achieved more successful outcomes compared to those treated with free coenzyme Q10.99 | |
| nanoparticular systems coated with cell membrane | targeting spesific tissue | In a study by Rittchen et al., PLGA nanoparticles containing leukemia inhibitory factor (LIF) were surrounded by oligodendrocyte precursor cell membrane vesicles. The prepared formulas were applied to mice with focal demyelination in the corpus callosum region. When the efficacy of the treatment was evaluated in terms of the region where remyelination was achieved and the axon thicknesses, it was observed that the remyelination was higher in the group treated with biomimetic nanoparticles and the axon thicknesses in the mice in this group were close to the healthy mice.100 |
| high biocompatibility | Cell membrane-coated biomimetic formulations containing Cu2-xSe were designed for the treatment of Parkinson’s disease (PD). In PD mice model, the biomimetic formulations successfully promoted neurite outgrowth via the p-Akt/p-CREB signaling pathway and facilitated improvement in synaptic function.101 | |
| low immunogenicity | Erythrocyte membrane-coated nanoparticle formulations modified with TGNYKALHPHN peptide were designed to enhance the bioavailability of curcumin for the treatment of AD. The biomimetic character was imparted, and functional peptide conjugation was utilized to increase the passage of curcumin across BBB and target the brain. After administration to rats with an AD model for a 4-week treatment period, the amount of curcumin reaching the brain was significantly higher in the group treated with the biomimetic formulation compared to the group treated with free curcumin. Moreover, the group treated with the biomimetic formulation achieved more successful results in the evaluation of cognitive functions using the new object recognition test.102 | |
| ability to cross the blood–brain barrier | ||
| good delivery vehicle for both hydrophobic and hydrophilic drugs | ||
| increased stability via encapsulation |
Many approved drugs and natural origin compounds (polyphenols such as curcumin, quercetin, resveratrol, genistein, cyanidin) with clinical potential in the treatment of neurodegenerative diseases could not reach their target site due to physiological barriers such as the BBB. The use of natural origin compounds in the treatment of neurodegenerative diseases is promising, because of increasing brain plasticity, improving cognitive functions, neutralization of reactive oxygen species, cell protection, and redox homeostasis effects. However, they could not reach the target area due to reasons such as degradation, low stability, and low passage through the BBB. It is thought that the effectiveness of these compounds can be increased by encapsulating them into nanosized drug delivery systems.57−59
At the same time, nanosized drug delivery systems also enable the delivery of viral vectors, siRNA, peptide–protein, and oligonucleotides for gene therapy. Studies on the transport of these factors, especially for the treatment of AD and PD, have increased the effectiveness of treatment.6061
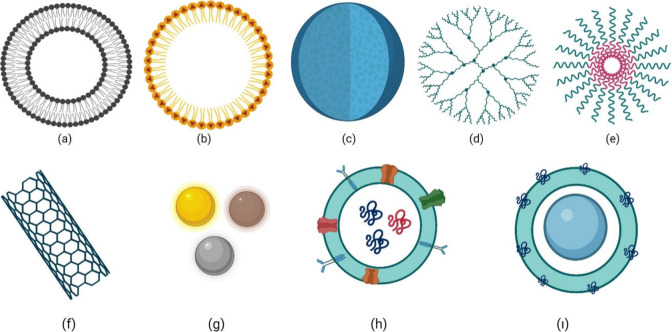
Various nanosized drug delivery systems can be formulated by considering their sizes, production methodologies, and compositions. (Figure 3)
Figure 3.
Various nanosized drug delivery systems: (a) liposomes, (b) solid lipid nanoparticles, (c) polymeric nanoparticle, (d) dendrimer, (e) polymeric micelles, (f) carbon nanotubes, (g) inorganic nanoparticles, (h) exosomes, (i) cell membrane coated nanoparticles. (This figure was created with BioRender.com).
5.1. Liposomes
Liposomes are closed vesicular systems consisting of one or more lipid bilayers with an aqueous phase between them. The basic building blocks of these systems are phospholipids, both hydrophilic and hydrophobic drugs can be loaded into liposomes with high encapsulation efficiency. Due to the similarity of the double-layered phospholipid structure to cell membranes, the drugs can pass through the blood–brain barrier more easily. Because of its ability to cross the blood–brain barrier, low intrinsic toxicity, low immunogenicity, and biodegradability properties; Liposomes are very suitable nanosized delivery in neurodegenerative diseases.62
In a study by Yang et al., liposome formulations containing riluzole and verapamil were prepared to increase the bioavailability of riluzole for the treatment of ALS. The significant increase in the synthesis of P-glycoproteins in the CNS cells in ALS limits the passage of riluzole through the blood–brain barrier as it is a P-glycoprotein substrate. Yang et al. designed liposome formulations containing riluzole and the P-glycoprotein inhibitor verapamil to increase the bioavailability of riluzole by thin lipid film hydration method. The effectiveness of liposomes was evaluated on healthy and ALS cell culture models prepared with brain endothelial Bend3 type cells and compared with free drug. In both cell groups higher riluzole uptake was achieved with liposome formulations containing riluzole and verapamil combination compared to riluzole formulation alone.63
The phospholipids are prone to degradation and rapid systemic elimination of liposomes limits their use. Ligand-mediated liposome/multifunctional liposome formulations are prepared by various surface modifications to eliminate the in vivo and in vitro stability problems. Glutathione-modified liposomes, PEGylated liposomes, and cationic liposomes are several examples of this approach.64
5.2. Polymeric Nanoparticles
Polymeric nanoparticles are colloidal systems prepared from biocompatible and biodegradable polymers of natural or synthetic origin. In the preparation of these systems, chitosan, alginate, gelatin, polyacrylate, PLGA (Poly lactic-co-glycolic acid), PACA (Polyalkylcyanoacrylate), PCL (Polycaprolactone), PLA (Polylactic acid), PEG (Polyethylene glycol) polymers are used. The advantages of these systems are that they are biocompatible and biodegradable, have fewer stability problems, low production costs, low immunogenicity, and toxicity. Nanoparticles containing mucoadhesive polymers, increase the residence time in the nasal cavity in the intranasal application and provide a significant advantage for developing different treatment strategies for neurodegenerative diseases.
Polyphenols provide an antioxidant effect by inducing inflammatory pathways encoding nuclear factor erythroid 2 related factor (NRF2)-mediated protein and cytoprotective vitamins. Antioxidant activity is provided by the transcription factor NRF2 up-regulation of genes called vitagenes, thereby inducing intracellular antioxidant and phase II detoxification enzymes. In this way, polyphenols reduce reactive oxidative stress, ensure redox homeostasis and provide neural cell protection.
Considering these properties, polyphenols can be used in the treatment of neurodegenerative diseases.
They are thought to be effective. However, due to their enzymatic degradation, low stability and low passage through the BBB, the amounts reaching the brain are very low.65,66
In a study conducted by Naeimi et al., formulation design was carried out by encapsulating chitosan alginate nanoparticles to increase the bioavailability of curcumin, which has potent antioxidant and anti-inflammatory activity. Initially, curcumin and chitosan alginate nanoparticles containing curcumin were applied to rats for 10 days. On the 10th day, a demyelination model was conducted by injecting lysolecithin (LPC) into the corpus callosum regions of the rats. In order to evaluate the efficacy of chitosan alginate nanoparticles containing curcumin, inflammation level, myelin repair, and glial activation levels were evaluated in rats for 1 week and 2 weeks after LPC injection. The glial activation and inflammation levels were lower in rats that received chitosan alginate nanoparticles containing curcumin compared to the plain curcumin administered group. Similarly, when evaluated in terms of myelin repair, it was determined that the level of demyelinating area was decreased significantly in rats treated with curcumin-containing chitosan alginate nanoparticles.67
In a study conducted by Sun et al., quercetin loaded PLGA nanoparticles were prepared to increase the effectiveness of quercetin in reducing Aβ fibril formation that occurs in AD. Quercetin, a natural polyphenolic compound, has low water solubility, low stability, and problems with the BBB barrier during passage to the CNS. To increase its effectiveness in the treatment of AD, PLGA nanoparticles containing quercetin were prepared and the prepared formulations were applied to mice with 7 days of treatment. Following the treatment, behavioral tests performed on mice showed improvement in cognitive functions and positive effects on memory.68
5.3. Solid Lipid Nanoparticles
Solid lipid nanoparticles, which have been developed as an alternative to polymeric nanoparticular systems, are colloidal drug carrier systems prepared using lipid matrices and surfactants that are in solid state at room temperature.69 Due to the biocompatible/biodegradable nature of the lipid matrices in the formulation, these systems have essential advantages such as low cytotoxicity, high biocompatibility, protection of the encapsulated drug from chemical and enzymatic degradation, and increasing the bioavailability of drugs with low aqueous solubility.
A study was conducted to increase the amount of erythropoietin (EPO) in the brain as it is considered as a promising neuroprotective agent for Alzheimer’s disease. EPO, which has low bioavailability due to its high molecular weight, hydrophilicity, and rapid clearance, was loaded into solid lipid nanoparticles prepared by the double emulsion solvent evaporation method. In the formulation, glycerin monostearate was used as the lipid phase component, Span 80/Span 60 as the internal phase surfactants, and Tween 80 as the external phase surfactant. The prepared formulations were injected intraperitoneally to the rats, in which the Alzheimer’s model was developed using beta-amyloid protein to evaluate the in vivo efficacy. After the treatment, oxidative stress levels and beta-amyloid plaque depositions in the hippocampus were measured in rats, and the Morris water tank test was performed. Higher treatment efficacy was obtained in terms of these parameters in rats to which EPO-loaded solid lipid nanoparticles were administered.70
5.4. Dendrimers
The term dendrimer is derived from the Greek word “dendron” meaning “tree”. Dendrimers are drug carrier systems with a three-dimensional structure consisting of a core, branching units around the core, and active terminal groups called functional groups. While branching units enable dendrimers to grow repeatedly, the diversity of dendrimers is provided by functional groups. Controlled drug release can be achieved by encapsulating the active substance into the space inside the dendrimers. In addition, targeting the CNS can be achieved by conjugating different active substances and various molecules to the many-branched structures and functional groups in its structure.
Dendrimers are nanosized drug carrier systems that are biocompatible and enable increased permeation through the blood–brain barrier. There are different types of dendrimers depending on their composition: polyamidoamine (PAMAM), polypropyleneimine, chiral, peptide, and polyester dendrimers. PAMAM-type dendrimers are frequently used as drug carrier systems, thanks to the high amount of primary amines and highly charged surface groups for proper conjugation.103,104
Kumar and his colleagues prepared PAMAM (polyamidoamine) dendrimer formulations loaded with galantamine hydrobromide in order to improve the pharmacokinetic properties of galantamine hydrobromide and increase its uptake into the brain. In order to increase the amount of galantamine hydrobromide reaching the brain, bifunctional PAMAM dendrimeric formulations were prepared by conjugating vitamin C/PEG to the surface of PAMAM dendrimers. When the prepared formulations were applied to AD model mice, it was observed that amyloid beta fibril formation and acetylcholine esterase enzyme activity were significantly reduced compared to the group administered free galantamine hydrobromide. In pharmacokinetic-based studies, it was observed that the amounts of galantamine hydrobromide were 2.3 times higher in the group where the prepared dendrimer formulations were applied compared to the application of free galantamine hydrobromide.105
5.5. Nanogels
“Nanogel” systems, an essential alternative to macroscopic hydrogels have essential properties attributed to their size at nanoscale (2–250 nm), are polymeric drug carrier systems prepared by physical or chemical cross-linking. With their nanosized network structure, high encapsulation efficiency, and targetability with various surface modifications they can overcome the blood–brain barrier and further prevent drug degradation.106
In a study by Azadi et al., chitosan nanogels loaded with methotrexate were prepared. To ensure the passage through receptor-mediated endocytosis, the prepared nanogels were coated with polysorbate 80. When drug delivery systems prepared with an average size of 118,4 nm were administered to rats by intravenous administration, a higher transition to the brain parenchyma was observed.107
5.6. Nanoemulsion
Nanoemulsions are drug carrier systems where the oil and water phases, which are immiscible, are formed in the presence of surfactant and the dispersed phase is in the form of droplets at the nanometer level. Nanoemulsions, which can be prepared in the form of oil in water and water in oil, have important advantages such as increasing solubility, improving bioavailability, increasing stability, reducing toxicity, and providing targeting to the central nervous system. Studies continue to use these systems in the treatment of various neurodegenerative diseases, especially AD and PD.108
Empagliflozin, an anti-inflammatory and antioxidant drug apart from an antidiabetic agent, is a neuroprotective agent that has the potential to be used in the treatment of AD and PD-related neuroinflammation. The amount of empagliflozin crossing the BBB is limited due to solubility and permeability problems. In a study conducted by Alhakamy et al., empagliflozin-loaded nanoemulsion formulations were prepared and when the prepared formulations were treated to mice, it was found that empagliflozin-loaded nanoemulsion formulation showed higher anti-inflammatory and neuroprotective activity compared to groups administered free empagliflozin.109
5.7. Polymeric Micelles
Polymeric micelles are formed by the self-assembly of amphiphilic polymers into a core–shell type of nanodrug carrier system. The hydrophilic polar blocks surround the hydrophobic blocks in the core.106 These systems, whose sizes range from 5 to 100 nm, have essential advantages in eliminating solubility problems of poorly water-soluble drugs and improving their bioavailability. In addition, they have significant advantages such as preventing the interaction with serum proteins, preventing the release of the active substance before reaching to the targeted area, preventing the degradation of the active substance, providing targeting through a specific ligand, and having easy and reproducible production processes.110,111
Recently, there have been many studies on target-specific functionalization of polymeric micelles in neurodegenerative diseases. In this context, there are many different studies on the functionalization of polymeric micelles with active ligands, cargo agents, and smart linkers.112
Bi et al., designed a rotigotine-loaded polyethylene glycol-polylactic acid–based micelle formulation for the treatment of Parkinson’s disease. They functionalized the polymeric micelles by coating with lactoferrin. In cell culture studies on the human bronchial epithelial cell line (16HBE) and SH-SY5Y cell line, cellular uptake of rotigotine was higher in lactoferrin-coated micelles compared to uncoated ones. The in vivo studies conducted in mice, revealed higher rotigotine accumulation in brain upon intranasal administration of lactoferrin-coated micelles compared to uncoated micelles.113
5.8. Carbon Nanotubes
First discovered by S. Iijima in 1991, carbon nanotubes are cylindrical systems formed due to rolling carbon layers with sp’2 hybridization between C–C atoms. These systems have high chemical stability due to their electronic, structural, and thermal properties, provide high drug encapsulation, large surface area, and high penetration to the biological membranes.114 In addition to their use as drug delivery systems, studies were carried out on the formation of scaffolds within the scope of tissue engineering to regulate impaired electrical stimulation in the CNS and provide neuro-regeneration.115
Genistein is a molecule that can inhibit amyloid precursor protein synthesis in the treatment of Alzheimer’s disease. Due to its low solubility and problems binding to biological membranes, its therapeutical efficiency is low. Researchers have developed genistein-loaded fullerene and multiwall carbon nanotube formulations. Upon intranasal application to Wistar rats, genistein bioavailability was improved and the side effects were reduced.116
5.9. Carbon Nano-onions
Carbon nano-onions are a more recently emerged class of carbon fillers with significant potential in the pharmaceutical field. Following the first discovery of fullerenes as carbon nanomaterials, carbon nanotubes, graphene, and finally carbon nano-onions emerged. Carbon nano-onions are defined as multishell fullerenes consisting of several graphene layers with inner layer spacing of 0.34 nm. Carbon nano-onions, which have a lattice-like shape, consist of polyhedral- and quasi-spherical-shaped layers. These systems, whose dimensions are below 100 nm, have the potential to be used as a drug carrier system, thanks to their low density, high surface area, high biocompatibility, and low toxicity properties. Existing studies have shown that carbon nano-onions provide controlled drug release and improve bioavailability in molecules with low bioavailability problems.1,117
Carbon nano-onions, with their large surface area and surface properties, can be functionalized by attaching different ligands to the surface. Studies have revealed that functionalized carbon nanobulbs have a very high potential to pass through the blood–brain barrier and interact with brain endothelial cells.118,119
To date, there is no study on the use of carbon nano-onions in the treatment of neurodegenerative diseases. However, considering the characteristics of these systems, they are thought to have significant potential for their use in the treatment of neurodegenerative diseases and are an interesting research area by researchers.
5.10. Nanowires
Nanowires are nanosized drug carrier systems that have the potential to be used in the treatment of neurodegenerative diseases and are relatively newly discovered compared to other systems. Nanowires are anisotropic nanocrystal systems with a high length/diameter ratio. Nanowires are used in sensor devices thanks to their high chemical reactivity and electronic properties. However, because of their unique properties (mechanical, electronic, and length/diameter ratios, etc.), their use as drug carrier systems is attracting increasing attention.
They are potential drug delivery systems to provide controlled drug release, especially in the treatment of neurodegenerative diseases. In the studies carried out, thanks to titanium-dioxide-based nanowires, a higher level of penetration through the blood–brain barrier and an increase in the amount of drug reaching the CNS were achieved. Studies on the use of nanowires in the diagnosis and treatment of neurodegenerative diseases, especially AD and PD, continue.120,121
5.11. Inorganic Nanoparticles
Compared to polymer and lipid-based systems, inorganic-based nanoparticles (e.g., gold, iron oxide, silica, silver, cerium oxide nanoparticles) can pass through the BBB, interact with CNS components, and reduce oxidative stress. They are compatible with various surface modifications and can be conjugated with diagnostic and imaging agents. They have recently attracted attention due to their optical properties.122
Gold nanoparticles have high biocompatibility; silver nanoparticles have a high level of transition to the brain parenchyma. Conversely, cerium oxide nanoparticles have essential advantages for treating neurodegenerative diseases with their regenerative antioxidant and neuroprotective effects.
One of the first striking issues in neurodegenerative diseases is the increased oxidative stress levels in the CNS. Considering the physicochemical properties and oxygen storage capacity of cerium oxide nanoparticles, it is predicted that they can reduce the level of reactive oxidative stress and protect neural survival.123
In a study by Eitan et al., researchers investigated the potent anti-inflammatory molecule (lenalidomide) and antioxidant cerium oxide nanoparticles synergistic effects in the EAE mouse model of Multiple Sclerosis. Mice developed in the EAE model were divided into various treatment groups that received lenalidomide treatment, cerium oxide nanoparticles, and combined treatment with lenalidomide and cerium oxide nanoparticles. While the onset of disease symptoms was delayed in the group receiving lenalidomide treatment alone, no change in symptom onset or disease severity was observed in the group receiving cerium oxide nanoparticles alone. However, more successful results were obtained in the group receiving combined treatment with lenalidomide and cerium oxide nanoparticles in terms of white matter pathology and inflammatory cell responses.124
5.12. Exosomes
Exosomes are miniature membranous vesicular systems, ranging in size from 40 to 100 nm. These vesicles, which have different characteristics according to the tissues and cells they originate from (such as neurons, tumor cells, and kidney cells), mediate the transport of different peptides and proteins.125
Due to their natural origin, their low rate of retention by mononuclear phagocyte cells and, accordingly, their high rate of passage through the BBB is a feature that makes them very advantageous in terms of targeting. These systems, which have essential advantages because they can carry different peptides and proteins in targeting, have low immunogenicity, escape from reticuloendothelial system components, and circulate in the bloodstream for a long time, are up-and-coming systems in immunotherapy and RNAi treatment.125,126
5.13. Nanoparticular Systems Coated with Cell Membrane
Nanosized drug delivery systems can contain many reactive groups on their surfaces, depending on their large surface area. Therefore, exposure to light, UV, and transition metals can cause an increase in the existing oxidative stress with the formation of prooxidants.127 In order to prevent this from happening, “ghost (nano ghost) nanocarriers” were prepared by coating the particulate systems with hydrophilic polymers (polysorbate-80, polyethylene glycol). In recent years, there has been growing interest in biomimetic drug delivery systems prepared by coating particulate systems with cell membrane fragments. These systems carry innate cells’ features and the advantages of synthetic nanoparticles.128
Avoiding immune system elements, circulating in the bloodstream for a longer time, and tissue targeting can be achieved by cell membrane coated biomimetic systems. Based on this idea, in a study conducted by Hu et al., the coating process was performed around PLGA nanoparticles using erythrocyte membrane vesicles. These biomimetic systems had higher in vivo circulation times compared to pegylated PLGA nanoparticles.129 Other studies conducted on erythrocyte cell membrane-coated synthetic nanoparticles also revealed the prolonged presence in blood circulation, low immunogenicity and high treatment effectiveness.130−134 Although the first studies on this subject were carried out with “nuclear-free” erythrocyte cells due to their more straightforward production process, today, different systems are designed using neutrophils, platelet cells, stem cells, and tumor cells.135
One of the critical steps in designing biomimetic nanoparticle systems is obtaining the nanoghost (isolated cell membrane) structure. For this purpose, after the proliferation of the selected cell type under in vitro conditions, isolation of the cell membrane is provided by hypotonic applications or homogenization methods. The second stage is coating the polymeric nanoparticular systems with the nanoghost structure. For this purpose, cell membrane vesicles are extruded several times with the help of a porous polymeric membrane around the polymeric nanoparticles in the core in the “mechanical coextraction” method, which is the oldest and preferred method.128 Along with the developing technology, electroporation and sonication methods, which provide higher efficiency in the coating process, are also used.128
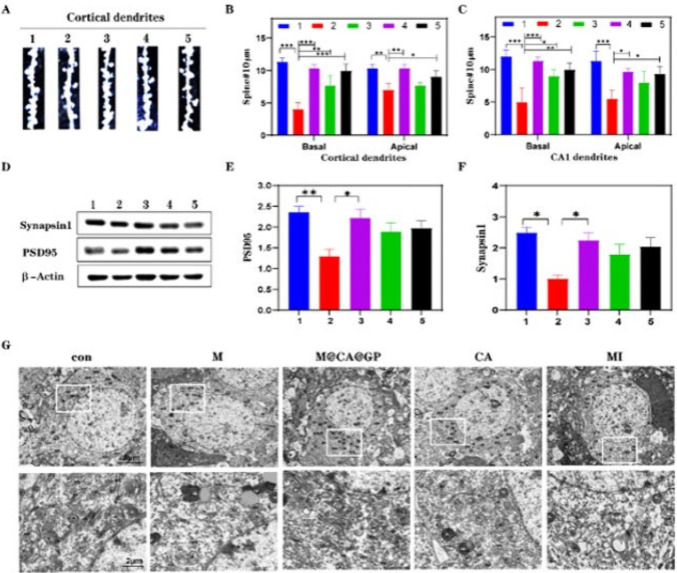
Li and co-workers designed an aspirin-curcumin ester (CA) loaded graphene oxide quantum dots nanosize carrier with the modification of a hybrid cell membrane (MCAGP NPs) for Alzheimer’s disease. They aimed to transfer drugs to the targeted area, regulate microglia activity, provide synaptic elasticity, and improve memory. For evaluating neuroprotection, they analyzed synaptic protein, synapse morphology, and Golgi staining. In the group treated with MCAGP NPs, the mice had an important synaptic protection score. (Figure 4) The microglia activity was assessed by performing fluorescence Aβ deposition and microglia in mouse brain. The MCAGP NPs treatment remarkably reduced Aβ deposition, repolarization of microglia, and augmented inflammatory environment in brain tissue. In the Morris Waster maze test and nest behavior test, changes in cognitive abilities were observed. The group treated with MCAGP NPs was compared to other groups and the subjects had shorter escape latencies and swimming speeds and crossed over the platform in the Morris Waster maze test.136
Figure 4.
Reprinted with permission from ref (136). Copyright 2022 Elsevier. (A) The study includes representative images of dendritic segments from cortical layer II/III pyramidal neurons, visualized using Golgi staining on brain sections. (B, C) Quantitative analysis was conducted to determine the density of apical and basal dendritic segments in cortical layer II/III pyramidal neurons, as well as spine density in hippocampus CA1 pyramidal neurons. (D) Western blotting analysis was performed to detect specific protein expression levels. (E, F) The study involved quantifying the levels of Synapsin1 and PSD95 proteins in the cortex, providing insights into synaptic protein dynamics. (G) Representative images obtained through Transmission Electron Microscopy (TEM) illustrate the ultrastructure of dorsal hippocampus neurons. The experimental conditions or treatments mentioned (M, RBC-MIC hybrid membrane; CA, curcumin-aspirin esterl; MI, minocycline; MCAGP, CA loading the graphene oxide quantum dots nanosize carrier with the modification of a hybrid cell membrane) were applied to investigate their effects on the observed parameters, such as dendritic morphology and protein levels.
Han and colleagues developed a genistein (GS) loaded solid lipid nanoparticle (SLN) formulation coated with a macrophage membrane (MA). The macrophage membrane coating enabled the escape of nanocarrier from the reticuloendothelial system compounds. Rabies virus glycoprotein (RVG29) was added to the macrophage membrane surface to facilitate passage through BBB and to provide neuronal targeting and triphenylphosphine (TPP) was added to the MA surface to ensure mitochondrial targeting. The effectiveness of genistein (GS)-loaded biomimetic nanoparticle (RVG/TPP-MASLNs) formulations designed with all the synergistic effects provided by macrophage membrane (MA), RVG29, and TPP was demonstrated by in vitro and in vivo studies.
In vitro antioxidative stress effect tests and other cell culture studies showed that genistein (GS) loaded biomimetic nanoparticle (RVG/TPP-MASLNs) formulation had a high antioxidant effect and free radical binding capacity compared to other formulations. For formulation evaluation of behavioral effects, the Morris Water tank test was performed on 9-month-old APP/PS1 mice. The escape latency was significantly reduced by RVG/TPP-MASLNs, the presence time in the target area was noticeably longer and spatial learning was effectively restored. These findings showed that cognitive learning levels in the RVG/TPP-MASLNs received group developed at a higher level compared to all groups.137
A biomimetic drug delivery system was developed for curcumin. In this carrier, human serum albumin nanoparticles encapsulating curcumin were coated with red blood cell membranes (RBC). Triphenylphosphine (TPP) and positron emission tomography (PET) agent (T807), which can easily pass the blood–brain barrier and bind to nerve cells, were conjugated to the red blood cell membranes (CUR-loaded T807/TPP-RBC-NPs). By combining appropriate physicochemical and biological properties, biocompatibility was increased, long-term circulation was ensured, and nerve cells were directly targeted via TPP and T807 conjugation. In vitro and in vivo studies revealed that CUR-loaded T807/TPP-RBC-NPs could significantly reduce oxidative stress and reduce neuronal death. To observe the change in learning levels in behavioral tests, the Morris Water Maze test was performed on mice in which Alzheimer’s model was developed by okadaic acid injection. According to the test results, a significant improvement in cognitive abilities was observed in the group treated with CUR-loaded T807/TPP-RBC-NPs compared to the groups treated with free curcumin (free CUR), curcumin loaded RBC-coated human serum albumin nanoparticle (CUR-loaded RBC-NP), curcumin loaded RBC-coated TPP-conjugated human serum albumin nanoparticle (CUR-loaded TPP-RBC-NPs). While a shortening in escape latency was observed in the group receiving CUR-loaded T807/TPP-RBC-NPs compared to the other groups, an increase was observed in the frequency of across the platform and prolonging the time spent in a targeted quadrant after removing the platform. (Figure 5)138
Figure 5.

Repair of the spatial learning abilities of AD model mice. Adapted with permission from ref (138). Copyright 2020 Elsevier. (A) The time taken to reach the escape point for each group. (B) Recorded swimming paths representing different groups. (C) The number of times the platform was crossed on the final day after its removal. (D) Proportionate time spent in the target quadrant. The data are shown as mean values with standard deviations (n = 5). *Signifies statistical significance at P < 0.05. **Signifies statistical significance at P < 0.01..
The preliminary studies on biomimetic systems are promising in the advanced treatment of neurodegenerative diseases and they are leading toward the concept of personalized drug therapy. However, much research is necessary to evaluate the effectiveness and safety of these systems. Their safety properties must be proven, especially through clinical studies.
6. Future Prospects
With the increasing average lifespan worldwide, the frequency of neurodegenerative diseases is also increasing day by day. The primary problem encountered in the treatment of neurodegenerative diseases such as AD, PD, MS, ALS, Prion, and Huntington’s disease is the inability of drugs to reach the CNS at the desired level. The CNS is well protected by various physiological barriers, and the passage of substances into the CNS is tightly regulated by these barriers.
Ensuring effective treatment in the management of neurodegenerative diseases is crucial for slowing disease progression and improving patients’ quality of life. Considering the increasing prevalence of these diseases, developing an effective treatment remains an open area of research, drawing the interest of many researchers.
In this regard, in addition to increasing passage through the BBB, patient compliance must also be taken into account in developing different treatment strategies. Among the developed strategies, noninvasive methods stand out for increasing BBB passage and significantly increasing drug levels reaching the brain parenchyma, given the high patient compliance in treatment. One alternative route of administration, intranasal delivery, offers a significant advantage in treatment by directly delivering the drug to the CNS via the olfactory region and trigeminal nerves. However, in this strategy where patient compliance is high, the short residence time of the drug in the nasal mucosa and mucociliary clearance significantly affect treatment efficacy. To overcome these issues, nanosized drug carrier systems prepared with various polymeric systems hold importance.
Another noninvasive treatment strategy, nanosized drug delivery systems, possess ideal properties for overcoming the BBB due to their intrinsic characteristics (nanoscale dimensions, composition, mucoadhesion, etc.). Particularly in the treatment of neurodegenerative diseases requiring long-term therapy, nanosized drug delivery systems stand out for providing controlled drug release, increasing bioavailability, and exhibiting biocompatibility properties. These systems have significant advantages in targeting the brain, such as high drug-loading capacities, the ability to evade the reticuloendothelial system, and long circulation times in the bloodstream, which protect the drug from enzymatic degradation.
These systems attract researchers’ attention in the development of different treatment strategies due to their suitability for surface modifications, enabling active and passive targeting, biocompatibility, providing controlled drug release, and increasing drug bioavailability. Studies have been conducted on the use of various drug carrier systems such as polymeric systems, dendrimers, liposomes, and solid lipid nanoparticles for treatment, demonstrating not only their biocompatibility but also their significant enhancement of treatment efficacy.
One of the most significant drawbacks of these systems is their potential to increase existing reactive oxidative stress levels due to exposure to UV, light, and metals, depending on their large surface area.127 Researchers have developed strategies such as PEG coating around nanoparticles to overcome this issue; however, the development of biomimetic systems has opened a new door in treatment. Studies have shown that biomimetic drug carrier systems stay in the bloodstream longer and have higher access to the CNS compared to PEG-coated nanoparticles.129 To achieve this, initially, coatings were made around nanoparticles using cell membranes obtained from erythrocytes, leading to the design of biomimetic systems. Nowadays, different cell types, especially membranes obtained from stem cells, are being used to develop new systems.139 Developed biomimetic systems not only enhance treatment efficacy but also reduce reactive oxidative stress, promising great hope in the treatment of neurodegenerative diseases due to their biocompatibility and safety.100,140
7. Conclusion
Neurodegenerative diseases, which have had an increasing prevalence in recent years, remain current because they significantly reduce patients’ quality of life, and effective treatment has not yet been provided. Compared to invasive techniques, noninvasive techniques can provide higher patient compliance and lower complication and trauma probability in treatment. Among the noninvasive techniques, nano drug delivery systems are considered ideal properties such as physicochemical stability, permeability to blood vessels, appropriate circulation time in the blood circulation, specificity to the targeted cells, controlled release profile, high receptivity to the transported molecules, biocompatibility and biodegradable properties. These ideal properties are directly related to the intrinsic properties of nanosized drug delivery systems such as size and polydispersity index, zeta potential, composition, hydrophobicity, stiffness, mucoadherence, and permeability. Nanosized drug delivery systems enable the transportation of substances approved for clinical use as well as polyphenolic compounds with anti-inflammatory activity, viral vectors, siRNA, peptide–protein complexes, and oligonucleotides to the brain.
Among these systems, “biomimetic drug delivery systems” have emerged as novel systems demonstrating superior features. Higher therapeutic efficacy compared to conventional systems has been achieved with these systems, due to the synergetic contributions of natural cells characteristics and the advantages of synthetic nanoparticles.
There are numerous studies on nano drug delivery systems for the treatment of neurodegenerative diseases, including liposomes, polymeric nanoparticles, dendrimers, exosomes, solid lipid nanoparticles, etc. This article provides a broad perspective on different systems and various studies. Research is ongoing for the development of new systems or modifications to existing ones. In addition to the currently developed nanosized drug delivery systems, current studies continue on the usage of various drug carrier systems such as nanosponges, carbon dots, quantum dots, nanozymes, nanotransfersomes for the treatment of these diseases. Additionally, further studies are needed to evaluate the suitability of the developed systems for industrial production and their possible toxicity.
Acknowledgments
This study was supported by TUBITAK (The Scientific and Technological Research Council of Türkiye) under Grant SBAG-121S478. Figures 1–3 and the TOC graphic were created with BioRender.com.
Glossary
ABBREVATIONS
- ABC
ATP-binding casette family
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- BBB
blood–brain barrier
- BCRP
breast cancer resistance protein
- CNS
central nervous system
- DSPE-PEG2000
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycerol)-2000
- EAE
experimental autoimmune encephalomyelitis
- EPO
erythropoietin
- GS
genistein
- FDA
Food and Drug Administration
- HD
Huntington’s disease
- IFN-β
interferon-β
- IgG
immunoglobulin G
- LDL
low-density lipoprotein
- LIF
leukemia inhibitory factor
- LPC
lysolecithin
- MA
macrophage membrane
- MS
multiple sclerosis
- NMDA
N-methyl d-Aspartate
- NNI
National Nanotechnology Initiative
- NRF2
nuclear factor erythroid 2 related factor
- PACA
polyalkylcyanoacrylate
- PCL
polycaprolactone
- PD
Parkinson’s disease
- PEG
polyethylene glycol
- PET
positron emission tomography
- PLA
polylactic acid
- PLGA
poly lactic-co-glycolic acid
- RVG29
Rabies virus glycoprotein
- SH-SY5Y
SK-N-SH neuroblastoma cell line
- SLC
solute carrier family
- SLN
solid lipid nanoparticle
- TPP
triphenylphospine
- 16HBE
human bronchial epithelial cell line
The authors declare no competing financial interest.
References
- Mamidi N.; Zuníga A. E.; Villela-Castrejón J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Materials Science and Engineering: C 2020, 112, 110928. 10.1016/j.msec.2020.110928. [DOI] [PubMed] [Google Scholar]
- Mamidi N.; Delgadillo R. M. V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf., B 2021, 204, 111819. 10.1016/j.colsurfb.2021.111819. [DOI] [PubMed] [Google Scholar]
- Mamidi N.; Delgadillo R. M. V.; Barrera E. V.; Ramakrishna S.; Annabi N. Carbonaceous nanomaterials incorporated biomaterials: The present and future of the flourishing field. Composites Part B: Engineering 2022, 243, 110150. 10.1016/j.compositesb.2022.110150. [DOI] [Google Scholar]
- Modi G.; Pillay V.; Choonara Y. E. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann. N.Y. Acad. Sci. 2010, 1184 (1), 154–172. 10.1111/j.1749-6632.2009.05108.x. [DOI] [PubMed] [Google Scholar]
- Migliore L.; Uboldi C.; Di Bucchianico S.; Coppedè F. Nanomaterials and neurodegeneration. Environmental and molecular mutagenesis 2015, 56 (2), 149–170. 10.1002/em.21931. [DOI] [PubMed] [Google Scholar]
- Re F.; Gregori M.; Masserini M. Nanotechnology for neurodegenerative disorders. Maturitas 2012, 73 (1), 45–51. 10.1016/j.maturitas.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Hettiarachchi S. D.; Zhou Y.; Seven E.; Lakshmana M. K.; Kaushik A. K.; Chand H. S.; Leblanc R. M. Nanoparticle-mediated approaches for Alzheimer’s disease pathogenesis, diagnosis, and therapeutics. Journal of controlled release 2019, 314, 125–140. 10.1016/j.jconrel.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 2021 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2021, 17, 327–406. 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- Jankovic J.; Tan E. K. Parkinson’s disease: Etiopathogenesis and treatment. Journal of Neurology, Neurosurgery & Psychiatry 2020, 91 (8), 795–808. 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- Dobson R.; Giovannoni G. Multiple sclerosis-a review. European journal of neurology 2019, 26 (1), 27–40. 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- Hauser S. L.; Cree B. A. C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133 (12), 1380–1390. 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans M. Prion diseases. Anaesthesia & Intensive Care Medicine 2020, 21 (1), 56–59. 10.1016/j.mpaic.2019.10.014. [DOI] [Google Scholar]
- Banerjee P.; Adhikary K.; Sarkar R.; Chakraborty S.; Jana S.. Prion diseases: A rare group of neurodegenerative disorders. In Viral, Parasitic, Bacterial, and Fungal Infections; Elsevier, 2023; pp 651–666. [Google Scholar]
- Rana N.; Kapil L.; Singh C.; Singh A. Modeling Huntington’s disease: An insight on in-vitro and in-vivo models. Behav. Brain Res. 2024, 459, 114757. 10.1016/j.bbr.2023.114757. [DOI] [PubMed] [Google Scholar]
- Ersoy N.; Basak A. N. Huntington Hastalığı’nın Moleküler Biyolojisi. Turk. J. Neurol. 2005, 11, 27–44. [Google Scholar]
- Masrori P.; Van Damme P. Amyotrophic lateral sclerosis: a clinical review. European journal of neurology 2020, 27 (10), 1918–1929. 10.1111/ene.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. I.; Lopes C.; Amaral M. H.; Costa P. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. 10.1016/j.ejpb.2020.01.005. [DOI] [PubMed] [Google Scholar]
- Hladky S. B.; Barrand M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B.; Daneman R.; Ransohoff R. M. Development, maintenance and disruption of the blood-brain barrier. Nature medicine 2013, 19 (12), 1584–1596. 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge M. A.; Fetterman J. L.; Vita J. A. Mitochondria and endothelial function. Circulation research 2013, 112 (8), 1171–1188. 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. M.; Mejia E. M.; Chang W.; Wang Y.; Watson E.; On N.; Miller D. W.; Hatch G. M. Reduction in cardiolipin decreases mitochondrial spare respiratory capacity and increases glucose transport into and across human brain cerebral microvascular endothelial cells. Journal of neurochemistry 2016, 139 (1), 68–80. 10.1111/jnc.13753. [DOI] [PubMed] [Google Scholar]
- McConnell H. L.; Kersch C. N.; Woltjer R. L.; Neuwelt E. A. The translational significance of the neurovascular unit. J. Biol. Chem. 2017, 292 (3), 762–770. 10.1074/jbc.R116.760215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur I. P.; Bhandari R.; Bhandari S.; Kakkar V. Potential of solid lipid nanoparticles in brain targeting. Journal of Controlled release 2008, 127 (2), 97–109. 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Barar J.; Rafi M. A.; Pourseif M. M.; Omidi Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts: BI 2016, 6 (4), 225. 10.15171/bi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjevic I.; Steinbusch H. W.; Schmitz C.; Martinez-Martinez P.; Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87 (4), 212–251. 10.1016/j.pneurobio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Tajes M.; Ramos-Fernández E.; Weng-Jiang X.; Bosch-Morató M.; Guivernau B.; Eraso-Pichot A.; Salvador B.; Fernandez-Busquets X.; Roquer J.; Munoz F. J. The blood-brain barrier: structure, function and therapeutic approaches to cross it. Molecular membrane biology 2014, 31 (5), 152–167. 10.3109/09687688.2014.937468. [DOI] [PubMed] [Google Scholar]
- Deli M. A.Drug transport and the blood-brain barrier. In Solubility, Delivery, and ADME Problems of Drugs and Drug-Candidates; Bentham Science Publishers Ltd., 2011; pp 144–165.
- Lawther B. K.; Kumar S.; Krovvidi H. Blood-brain barrier. Continuing Education in Anaesthesia, Critical Care & Pain 2011, 11 (4), 128–132. 10.1093/bjaceaccp/mkr018. [DOI] [Google Scholar]
- Serlin Y.; Shelef I.; Knyazer B.; Friedman A.. Anatomy and physiology of the blood-brain barrier. In Seminars in cell & developmental biology; Elsevier, 2015; Vol. 38, pp 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikitsh J. L.; Chacko A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Med. Chem. 2014, 10.4137/PMC.S13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlieghe P.; Khrestchatisky M. Peptide-based vectors for blood-brain barrier targeting and delivery of drugs to the central nervous system. Therapeutic delivery 2010, 1 (4), 489–494. 10.4155/tde.10.44. [DOI] [PubMed] [Google Scholar]
- Correale J.; Villa A. Cellular elements of the blood-brain barrier. Neurochemical research 2009, 34, 2067–2077. 10.1007/s11064-009-0081-y. [DOI] [PubMed] [Google Scholar]
- Jouyban A.; Fakhree M.. Experimental and Computational Methods Pertaining to Drug Solubility. In Toxicity and Drug Testing; Acree W., Jr., Ed.; IntechOpen, 2012. [Google Scholar]
- Mandal A.; Bisht R.; Pal D.; Mitra A. K.. Diagnosis and drug delivery to the brain: Novel strategies. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier, 2017; pp 59–83. [Google Scholar]
- Chen Y.; Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Advanced drug delivery reviews 2012, 64 (7), 640–665. 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Lu W. Adsorptive-mediated brain delivery systems. Current pharmaceutical biotechnology 2012, 13 (12), 2340–2348. 10.2174/138920112803341851. [DOI] [PubMed] [Google Scholar]
- Strazielle N.; Ghersi-Egea J.-F. Potential pathways for CNS drug delivery across the blood-cerebrospinal fluid barrier. Current pharmaceutical design 2016, 22 (35), 5463–5476. 10.2174/1381612822666160726112115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal M.; Fischer K.; Gentile C.; Gitto S.; Zhang Y.-Z.; McDannold N. Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS One 2017, 12 (1), e0166061 10.1371/journal.pone.0166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A. Development of the choroid plexus and blood-CSF barrier. Front. Neurosci. 2015, 9, 32. 10.3389/fnins.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Y.; Leach A. G.; Smith J.; Pluchino S.; Coxon C. R.; Sivakumaran M.; Downing J.; Fatokun A. A.; Teixidò M.; Ehtezazi T. Physiological and pathological factors affecting drug delivery to the brain by nanoparticles. Adv. Sci. 2021, 8 (11), 2002085. 10.1002/advs.202002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K.; Cline C.; Vogel P.; Onciu M.; Fatima S.; Sorrentino B. P.; Thirumaran R. K.; Ekins S.; Urade Y.; Fujimori K.; et al. Drug transporters on arachnoid barrier cells contribute to the blood-cerebrospinal fluid barrier. Drug Metab. Dispos. 2013, 41 (4), 923–931. 10.1124/dmd.112.050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh D. S.; Wadajkar A. S.; Roberts N. B.; Perez J. G.; Connolly N. P.; Frenkel V.; Winkles J. A.; Woodworth G. F.; Kim A. J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22 (9), 1177–1193. 10.2174/1381612822666151221150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartanusz V.; Jezova D.; Alajajian B.; Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Annals of neurology 2011, 70 (2), 194–206. 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- Antimisiaris S.; Mourtas S.; Papadia K.. Brain targeting with lipidic nanocarriers. In Design of nanostructures for versatile therapeutic applications; Elsevier, 2018; pp 255–324. [Google Scholar]
- Grabrucker A. M.; Chhabra R.; Belletti D.; Forni F.; Vandelli M. A.; Ruozi B.; Tosi G. Nanoparticles as blood-brain barrier permeable CNS targeted drug delivery systems. Blood Brain Barrier (BBB) 2013, 10, 71–89. 10.1007/7355_2013_22. [DOI] [Google Scholar]
- Alyautdin R.; Khalin I.; Nafeeza M. I.; Haron M. H.; Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int. J. Nanomed. 2014, 0, 795–811. 10.2147/IJN.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio J.; Laine K.; Gynther M.; Savolainen J. Prodrug approaches for CNS delivery. AAPS journal 2008, 10, 92–102. 10.1208/s12248-008-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M. C. B. A.; Huchting J. Prodrugs: Overcoming Drugs’ Obstacles by Inactive Precursors. Rev. Virtual Quim. 2016, 8 (5), 1485–1509. 10.21577/1984-6835.20160105. [DOI] [Google Scholar]
- Hanson L. R.; Frey W. H. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (S3), S5. 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadur S.; Pardhi D. M.; Rautio J.; Rosenholm J. M.; Pathak K. Intranasal nanoemulsions for direct nose-to-brain delivery of actives for CNS disorders. Pharmaceutics 2020, 12 (12), 1230. 10.3390/pharmaceutics12121230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe T. P.; Greenlee M. H. W.; Kanthasamy A. G.; Hsu W. H. Mechanism of intranasal drug delivery directly to the brain. Life sciences 2018, 195, 44–52. 10.1016/j.lfs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- de Lima L. S.; Mortari M. R. Therapeutic nanoparticles in the brain: A review of types, physicochemical properties and challenges. Int. J. Pharm. 2022, 612, 121367. 10.1016/j.ijpharm.2021.121367. [DOI] [PubMed] [Google Scholar]
- Barbu E.; Molnàr É.; Tsibouklis J.; Górecki D. C. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrier. Expert opinion on drug delivery 2009, 6 (6), 553–565. 10.1517/17425240902939143. [DOI] [PubMed] [Google Scholar]
- Kabanov A.; Gendelman H. E. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog. Polym. Sci. 2007, 32 (8–9), 1054–1082. 10.1016/j.progpolymsci.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V. P. Nanobased nano drug delivery: a comprehensive review. Applications of targeted nano drugs and delivery systems 2019, 69–92. 10.1016/B978-0-12-814029-1.00004-1. [DOI] [Google Scholar]
- Naik J.Nano based drug delivery; IAPC: Zagreb, Croatia, 2015. [Google Scholar]
- Uddin M. S.; Al Mamun A.; Kabir M. T.; Ahmad J.; Jeandet P.; Sarwar M. S.; Ashraf G. M.; Aleya L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. European journal of pharmacology 2020, 886, 173412. 10.1016/j.ejphar.2020.173412. [DOI] [PubMed] [Google Scholar]
- Nayab D. E.; Din F. u.; Ali H.; Kausar W. A.; Urooj S.; Zafar M.; Khan I.; Shabbir K.; Khan G. M. Nano biomaterials based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: an up-to-date perspective. J. Nanobiotechnol. 2023, 21, 477. 10.1186/s12951-023-02250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabska-Kobyłecka I.; Szpakowski P.; Król A.; Książek-Winiarek D.; Kobyłecki A.; Głąbiński A.; Nowak D. Polyphenols and their impact on the Prevention of neurodegenerative Diseases and Development. Nutrients 2023, 15 (15), 3454. 10.3390/nu15153454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S.; Harashima H. Non-invasive gene delivery across the blood-brain barrier: present and future perspectives. Neural regeneration research 2022, 17 (4), 785–787. 10.4103/1673-5374.320981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male D.; Gromnicova R. Nanocarriers for Delivery of Oligonucleotides to the CNS. International journal of molecular sciences 2022, 23 (2), 760. 10.3390/ijms23020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P.; Goyal K.; Kumar S. G. V.; Singh A.; Katare O. P.; Mishra D. N. Liposomal drug delivery systems-clinical applications. Acta Pharm. 2005, 55 (1), 1–25. [PubMed] [Google Scholar]
- Yang T.; Ferrill L.; Gallant L.; McGillicuddy S.; Fernandes T.; Schields N.; Bai S. Verapamil and riluzole cocktail liposomes overcome pharmacoresistance by inhibiting P-glycoprotein in brain endothelial and astrocyte cells: A potent approach to treat amyotrophic lateral sclerosis. European Journal of Pharmaceutical Sciences 2018, 120, 30–39. 10.1016/j.ejps.2018.04.026. [DOI] [PubMed] [Google Scholar]
- Vieira D. B.; Gamarra L. F. Getting into the brain: liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. 10.2147/IJN.S117210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuto M.; Salinaro A. T.; Caligiuri I.; Ontario M. L.; Greco V.; Sciuto N.; Crea R.; Calabrese E. J.; Rizzolio F.; Canzonieri V.; et al. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021, 199, 111551. 10.1016/j.mad.2021.111551. [DOI] [PubMed] [Google Scholar]
- Scuto M.; Ontario M. L.; Salinaro A. T.; Caligiuri I.; Rampulla F.; Zimbone V.; Modafferi S.; Rizzolio F.; Canzonieri V.; Calabrese E. J.; et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free Radical Biol. Med. 2022, 179, 59–75. 10.1016/j.freeradbiomed.2021.12.267. [DOI] [PubMed] [Google Scholar]
- Naeimi R.; Safarpour F.; Hashemian M.; Tashakorian H.; Ahmadian S. R.; Ashrafpour M.; Ghasemi-Kasman M. Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neuroscience letters 2018, 674, 1–10. 10.1016/j.neulet.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Sun D.; Li N.; Zhang W.; Zhao Z.; Mou Z.; Huang D.; Liu J.; Wang W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf., B 2016, 148, 116–129. 10.1016/j.colsurfb.2016.08.052. [DOI] [PubMed] [Google Scholar]
- Cacciatore I.; Ciulla M.; Fornasari E.; Marinelli L.; Di Stefano A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert opinion on drug delivery 2016, 13 (8), 1121–1131. 10.1080/17425247.2016.1178237. [DOI] [PubMed] [Google Scholar]
- Dara T.; Vatanara A.; Sharifzadeh M.; Khani S.; Vakilinezhad M. A.; Vakhshiteh F.; Meybodi M. N.; Malvajerd S. S.; Hassani S.; Mosaddegh M. H. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol. Learn. Mem. 2019, 166, 107082. 10.1016/j.nlm.2019.107082. [DOI] [PubMed] [Google Scholar]
- Qu M.; Lin Q.; He S.; Wang L.; Fu Y.; Zhang Z.; Zhang L. A brain targeting functionalized liposomes of the dopamine derivative N-3, 4-bis (pivaloyloxy)-dopamine for treatment of Parkinson’s disease. Journal of controlled release 2018, 277, 173–182. 10.1016/j.jconrel.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Kuo Y.-C.; Wang I.-H.; Rajesh R. Use of leptin-conjugated phosphatidic acid liposomes with resveratrol and epigallocatechin gallate to protect dopaminergic neurons against apoptosis for Parkinson’s disease therapy. Acta Biomaterialia 2021, 119, 360–374. 10.1016/j.actbio.2020.11.015. [DOI] [PubMed] [Google Scholar]
- Gaillard P. J.; Appeldoorn C. C.; Rip J.; Dorland R.; van der Pol S. M.; Kooij G.; de Vries H. E.; Reijerkerk A. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. Journal of controlled release 2012, 164 (3), 364–369. 10.1016/j.jconrel.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Yang L.; Wang Y.; Li Z.; Wu X.; Mei J.; Zheng G. Brain targeted peptide-functionalized chitosan nanoparticles for resveratrol delivery: Impact on insulin resistance and gut microbiota in obesity-related Alzheimer’s disease. Carbohydr. Polym. 2023, 310, 120714. 10.1016/j.carbpol.2023.120714. [DOI] [PubMed] [Google Scholar]
- González L. F.; Acuña E.; Arellano G.; Morales P.; Sotomayor P.; Oyarzun-Ampuero F.; Naves R. Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Controlled Release 2021, 331, 443–459. 10.1016/j.jconrel.2020.11.019. [DOI] [PubMed] [Google Scholar]
- Sava V.; Fihurka O.; Khvorova A.; Sanchez-Ramos J. Enriched chitosan nanoparticles loaded with siRNA are effective in lowering Huntington’s disease gene expression following intranasal administration. Nanomedicine: Nanotechnology, Biology and Medicine 2020, 24, 102119. 10.1016/j.nano.2019.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh S. R.; Abd-Elmegied A.; Madhy S. A.; Khattab S. N.; Sheta E.; Elnozahy F. Y.; Mehanna R. A.; Ghareeb D. A.; Abd-Elmonem N. M. Brain-targeted Tet-1 peptide-PLGA nanoparticles for berberine delivery against STZ-induced Alzheimer’s disease in a rat model: Alleviation of hippocampal synaptic dysfunction, Tau pathology and amyloidogenesis. Int. J. Pharm. 2024, 658, 124218. 10.1016/j.ijpharm.2024.124218. [DOI] [PubMed] [Google Scholar]
- da Silva G. B. R. F.; das Neves S. P.; Oliveira S. C. R.; Marques F.; de Oliveira A. G.; de Lima Leite F.; Cerqueira J. J. Comparative effectiveness of preventive treatment with dimethyl fumarate-loaded solid lipid nanoparticles and oral dimethyl fumarate in a mouse model of multiple sclerosis. J. Autoimmun. 2022, 132, 102893. 10.1016/j.jaut.2022.102893. [DOI] [PubMed] [Google Scholar]
- Yusuf M.; Khan M.; Khan R. A.; Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J. Drug Targeting 2013, 21 (3), 300–311. 10.3109/1061186X.2012.747529. [DOI] [PubMed] [Google Scholar]
- Martínez E. O.; Hernández M. E. M.; Castillo-González J.; González-Rey E.; Martínez M. A. R. Dopamine-loaded chitosan-coated solid lipid nanoparticles as a promise nanocarriers to the CNS. Neuropharmacology 2024, 249, 109871. 10.1016/j.neuropharm.2024.109871. [DOI] [PubMed] [Google Scholar]
- Gothwal A.; Kumar H.; Nakhate K. T.; Ajazuddin; Dutta A.; Borah A.; Gupta U. Lactoferrin coupled lower generation PAMAM dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer’s disease in mice. Bioconjugate Chem. 2019, 30 (10), 2573–2583. 10.1021/acs.bioconjchem.9b00505. [DOI] [PubMed] [Google Scholar]
- Zarebkohan A.; Najafi F.; Moghimi H. R.; Hemmati M.; Deevband M. R.; Kazemi B. Synthesis and characterization of a PAMAM dendrimer nanocarrier functionalized by SRL peptide for targeted gene delivery to the brain. European Journal of Pharmaceutical Sciences 2015, 78, 19–30. 10.1016/j.ejps.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Singh A. K.; Gothwal A.; Rani S.; Rana M.; Sharma A. K.; Yadav A. K.; Gupta U. Dendrimer donepezil conjugates for improved brain delivery and better in vivo pharmacokinetics. ACS Omega 2019, 4 (3), 4519–4529. 10.1021/acsomega.8b03445. [DOI] [Google Scholar]
- Chen Y.-B.; Qiao T.; Wang Y.-Q.; Cui Y.-L.; Wang Q.-S. Hydrogen bond-enhanced nanogel delivery system for potential intranasal therapy of Parkinson’s disease. Materials & Design 2022, 219, 110741. 10.1016/j.matdes.2022.110741. [DOI] [Google Scholar]
- Zhang Y.; Zou Z.; Liu S.; Miao S.; Liu H. Nanogels as novel nanocarrier systems for efficient delivery of CNS therapeutics. Frontiers in Bioengineering and Biotechnology 2022, 10, 954470. 10.3389/fbioe.2022.954470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Xu J.; Jia L.; Zhou Y.; Fu Q.; Wang Y.; Mu D.; Wang D.; Li N.; Hou Y. Pterostilbene nanoemulsion promotes Nrf2 signaling pathway to downregulate oxidative stress for treating Alzheimer’s disease. Int. J. Pharm. 2024, 655, 124002. 10.1016/j.ijpharm.2024.124002. [DOI] [PubMed] [Google Scholar]
- Friedman-Levi Y.; Meiner Z.; Canello T.; Frid K.; Kovacs G. G.; Budka H.; Avrahami D.; Gabizon R. Fatal prion disease in a mouse model of genetic E200K Creutzfeldt-Jakob disease. PLoS pathogens 2011, 7 (11), e1002350 10.1371/journal.ppat.1002350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lohan S.; Sharma T.; Saini S.; Swami R.; Dhull D.; Beg S.; Raza K.; Kumar A.; Singh B. QbD-steered development of mixed nanomicelles of galantamine: Demonstration of enhanced brain uptake, prolonged systemic retention and improved biopharmaceutical attributes. Int. J. Pharm. 2021, 600, 120482. 10.1016/j.ijpharm.2021.120482. [DOI] [PubMed] [Google Scholar]
- Sipos B.; Csóka I.; Budai-Szűcs M.; Kozma G.; Berkesi D.; Kónya Z.; Balogh G. T.; Katona G. Development of dexamethasone-loaded mixed polymeric micelles for nasal delivery. European Journal of Pharmaceutical Sciences 2021, 166, 105960. 10.1016/j.ejps.2021.105960. [DOI] [PubMed] [Google Scholar]
- Sharifian A.; Varshosaz J.; Aliomrani M.; Kazemi M. Nose to brain delivery of ibudilast micelles for treatment of multiple sclerosis in an experimental autoimmune encephalomyelitis animal model. Int. J. Pharm. 2023, 638, 122936. 10.1016/j.ijpharm.2023.122936. [DOI] [PubMed] [Google Scholar]
- Yu Y.; He S.-y.; Kong L.; Shi N.-n.; Liu Y.; Zang J.; Guo R.-b.; Zhang L.; Li X.-y.; Li X.-t. Brain-targeted multifunctional micelles delivering Oridonin and Phillyrin for synergistic therapy of Alzheimer’s disease. Journal of Drug Delivery Science and Technology 2023, 87, 104794. 10.1016/j.jddst.2023.104794. [DOI] [Google Scholar]
- Nosratabadi R.; Rastin M.; Sankian M.; Haghmorad D.; Mahmoudi M. Hyperforin-loaded gold nanoparticle alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Nanomedicine: Nanotechnology, Biology and Medicine 2016, 12 (7), 1961–1971. 10.1016/j.nano.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Li X.; Ji D.; Tian J.; Peng Q.; Shen Y.; Xiao Y. Functionalised penetrating peptide-chondroitin sulphate-gold nanoparticles: Synthesis, characterization, and applications as an anti-Alzheimer’s disease drug. Int. J. Biol. Macromol. 2023, 230, 123125. 10.1016/j.ijbiomac.2022.123125. [DOI] [PubMed] [Google Scholar]
- Randhawa S.; Dar A. I.; Saini T. C.; Bathla M.; Acharya A. Glucosamine conjugated gold nanoparticles modulate protein aggregation induced autophagic neuronal cell death via regulation of intracellular Parkin homeostasis. Nano Today 2024, 56, 102243. 10.1016/j.nantod.2024.102243. [DOI] [Google Scholar]
- Zheng X.; Sun K.; Liu Y.; Yin X.; Zhu H.; Yu F.; Zhao W. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J. Controlled Release 2023, 353, 675–684. 10.1016/j.jconrel.2022.12.026. [DOI] [PubMed] [Google Scholar]
- Haney M. J.; Klyachko N. L.; Zhao Y.; Gupta R.; Plotnikova E. G.; He Z.; Patel T.; Piroyan A.; Sokolsky M.; Kabanov A. V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Controlled Release 2015, 207, 18–30. 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y.; Guo L.; Jiang Y.; Shi Y.; Sui H.; Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Delivery 2020, 27 (1), 745–755. 10.1080/10717544.2020.1762262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Q.; Shi Y.; Qi Y.; Huang L.; Sui H.; Zhao L. Biomimetic silibinin-loaded macrophage-derived exosomes induce dual inhibition of Aβ aggregation and astrocyte activation to alleviate cognitive impairment in a model of Alzheimer’s disease. Materials Science and Engineering: C 2021, 129, 112365. 10.1016/j.msec.2021.112365. [DOI] [PubMed] [Google Scholar]
- Sheykhhasan M.; Amini R.; Asl S. S.; Saidijam M.; Hashemi S. M.; Najafi R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomed. Pharmacother. 2022, 152, 113224. 10.1016/j.biopha.2022.113224. [DOI] [PubMed] [Google Scholar]
- Rittchen S.; Boyd A.; Burns A.; Park J.; Fahmy T. M.; Metcalfe S.; Williams A. Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials 2015, 56, 78–85. 10.1016/j.biomaterials.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.; Xu L.; Han Y.; Jiang Z.; Zheng Q.; Gao Y.; Xing W.; Li Z. Boosting neurite outgrowth and anti-oxidative stress for treatment of Parkinson’s disease by biomimetic ultrasmall nanoparticles. Sustainable Materials and Technologies 2024, 39, e00807 10.1016/j.susmat.2023.e00807. [DOI] [Google Scholar]
- Gu J.; Yan C.; Yin S.; Wu H.; Liu C.; Xue A.; Lei X.; Zhang N.; Geng F. Erythrocyte membrane-coated nanocarriers modified by TGN for Alzheimer’s disease. J. Controlled Release 2024, 366, 448–459. 10.1016/j.jconrel.2023.12.030. [DOI] [PubMed] [Google Scholar]
- Marangoz Ö.; Yavuz O. Nano-drug delivery systems and their toxicological assessment. Turk. Hij. Den. Biyol. Derg. 2020, 77, 509. 10.5505/TurkHijyen.2020.37790. [DOI] [Google Scholar]
- Nair A.; Bu J.; Bugno J.; Rawding P. A.; Kubiatowicz L. J.; Jeong W.-j.; Hong S. Size-dependent drug loading, gene complexation, cell uptake, and transfection of a novel dendron-lipid nanoparticle for drug/gene co-delivery. Biomacromolecules 2021, 22 (9), 3746–3755. 10.1021/acs.biomac.1c00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Rana M.; Sharma A. K.; Sinha S.; Ajazuddin; Gupta U. Vitamin-C decorated PEGylated PAMAM dendrimers for effective inhibition of amyloid aggregation and improved in vivo pharmacokinetics. J. Drug Deliv. Sci. Technol. 2023, 89, 105058. 10.1016/j.jddst.2023.105058. [DOI] [Google Scholar]
- Vissers C.; Ming G.-l.; Song H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Advanced drug delivery reviews 2019, 148, 239–251. 10.1016/j.addr.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi A.; Hamidi M.; Rouini M.-R. Methotrexate-loaded chitosan nanogels as ‘Trojan Horses’ for drug delivery to brain: preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. 10.1016/j.ijbiomac.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Nirale P.; Paul A.; Yadav K. S. Nanoemulsions for targeting the neurodegenerative diseases: Alzheimer’s, Parkinson’s and Prion’s. Life sciences 2020, 245, 117394. 10.1016/j.lfs.2020.117394. [DOI] [PubMed] [Google Scholar]
- Alhakamy N. A.; Aljehani E. A.; Abdel-Naim A. B.; Shaik R. A.; Iqubal M. K.; Asfour H. Z.; Bazuhair M. A.; Md S. Development, optimization, and evaluation of Empagliflozin nanoemulsion for the management of neuroinflammation associated Alzheimer’s disease. Journal of Drug Delivery Science and Technology 2024, 93, 105425. 10.1016/j.jddst.2024.105425. [DOI] [Google Scholar]
- Kahraman E.; Özsoy Y. Polymeric Micelles And Its Nasal Applications. Istanbul J. Pharm. 2011, 41, 121–139. [Google Scholar]
- Sezgin Z.; Yüksel N.; Baykara T. Preparation And Characterization Of Polymeric Micelles. J. Fac. Pharm. Ankara Univ. 2003, 32 (2), 125–142. 10.1501/Eczfak_0000000201. [DOI] [Google Scholar]
- Kaur J.; Gulati M.; Kapoor B.; Jha N. K.; Gupta P. K.; Gupta G.; Chellappan D. K.; Devkota H. P.; Prasher P.; Ansari M. S.; et al. Advances in designing of polymeric micelles for biomedical application in brain related diseases. Chem.-Biol. Interact. 2022, 361, 109960. 10.1016/j.cbi.2022.109960. [DOI] [PubMed] [Google Scholar]
- Bi C.; Wang A.; Chu Y.; Liu S.; Mu H.; Liu W.; Wu Z.; Sun K.; Li Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. 10.2147/IJN.S120939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafa H.; Wang J. T.-W.; Rubio N.; Klippstein R.; Costa P. M.; Hassan H. A.; Sosabowski J. K.; Bansal S. S.; Preston J. E.; Abbott N. J.; et al. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood-brain barrier in vitro and in vivo. J. Controlled Release 2016, 225, 217–229. 10.1016/j.jconrel.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro E.; Kumar A. Nanoparticles in drug delivery systems. Nanomedicine in drug delivery 2013, 1, 1–22. 10.1201/b14802-2. [DOI] [Google Scholar]
- Mehta P.; Shende P. Collation of fullerenes and carbon nanotubes with genistein for synergistic anti-Alzheimer’s activity by amyloid-β deaggregation. Journal of Drug Delivery Science and Technology 2024, 91, 105205. 10.1016/j.jddst.2023.105205. [DOI] [Google Scholar]
- Mamidi N.; Velasco Delgadillo R. M.; Gonzáles Ortiz A.; Barrera E. V. Carbon nano-onions reinforced multilayered thin film system for stimuli-responsive drug release. Pharmaceutics 2020, 12 (12), 1208. 10.3390/pharmaceutics12121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhira B.; Ghosh M.; Allam A.; Sarkar S. Carbon nano onions cross the blood brain barrier. RSC Adv. 2016, 6 (35), 29779–29782. 10.1039/C5RA23534K. [DOI] [Google Scholar]
- Majumder R.; Pal T.; Basumallick A.; Das Mukhopadhyay C. Functionalized carbon nano onion as a novel drug delivery system for brain targeting. J. Drug Deliv. Sci. Technol. 2021, 63, 102414. 10.1016/j.jddst.2021.102414. [DOI] [Google Scholar]
- Inal O.; Badilli U.; Ozkan A. S.; Mollarasouli F.. Bioactive hybrid nanowires for drug delivery. In Hybrid Nanomaterials for Drug Delivery; Elsevier, 2022; pp 269–301. [Google Scholar]
- Sharma A.; Menon P. K.; Patnaik R.; Muresanu D. F.; Lafuente J. V.; Tian Z. R.; Ozkizilcik A.; Mössler H.; Sharma H. S. Novel treatment strategies using TiO2-nanowired delivery of histaminergic drugs and antibodies to tau with cerebrolysin for superior neuroprotection in the pathophysiology of Alzheimer’s disease. International Review of Neurobiology 2017, 137, 123–165. 10.1016/bs.irn.2017.09.002. [DOI] [PubMed] [Google Scholar]
- DeCoteau W.; Heckman K. L.; Estevez A. Y.; Reed K. J.; Costanzo W.; Sandford D.; Studlack P.; Clauss J.; Nichols E.; Lipps J.; et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine: Nanotechnology, Biology and Medicine 2016, 12 (8), 2311–2320. 10.1016/j.nano.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Rzigalinski B. A.; Carfagna C. S.; Ehrich M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. WIREs Nanomed. Nanobiotechnol. 2017, 9 (4), e1444 10.1002/wnan.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E.; Hutchison E. R.; Greig N. H.; Tweedie D.; Celik H.; Ghosh S.; Fishbein K. W.; Spencer R. G.; Sasaki C. Y.; Ghosh P.; et al. Combination therapy with lenalidomide and nanoceria ameliorates CNS autoimmunity. Exper. Neurol. 2015, 273, 151–160. 10.1016/j.expneurol.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A.; Tyagi A.; Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Molecular neurobiology 2014, 49, 590–600. 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmalavičiu̅tė A.; Pivoriu̅nas A. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacological research 2016, 113, 816–822. 10.1016/j.phrs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Dash P.; Piras A. M.; Dash M. Cell membrane coated nanocarriers-an efficient biomimetic platform for targeted therapy. Journal of controlled release 2020, 327, 546–570. 10.1016/j.jconrel.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Wang M.; Xin Y.; Cao H.; Li W.; Hua Y.; Webster T. J.; Zhang C.; Tang W.; Liu Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomaterials science 2021, 9 (4), 1088–1103. 10.1039/D0BM01164A. [DOI] [PubMed] [Google Scholar]
- Hu C.-M. J.; Zhang L.; Aryal S.; Cheung C.; Fang R. H.; Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (27), 10980–10985. 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Kundrat L.; Pishesha N.; Bilate A.; Theile C.; Maruyama T.; Dougan S. K.; Ploegh H. L.; Lodish H. F. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (28), 10131–10136. 10.1073/pnas.1409861111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhateria M.; Rachumallu R.; Singh R.; Bhatta R. S. Erythrocytes-based synthetic delivery systems: transition from conventional to novel engineering strategies. Expert opinion on drug delivery 2014, 11 (8), 1219–1236. 10.1517/17425247.2014.927436. [DOI] [PubMed] [Google Scholar]
- Doshi N.; Zahr A. S.; Bhaskar S.; Lahann J.; Mitragotri S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (51), 21495–21499. 10.1073/pnas.0907127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Sun X.; Cheng L.; Yin S.; Yang G.; Li Y.; Liu Z. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer. Adv. Mater. 2014, 26 (28), 4794–4802. 10.1002/adma.201400158. [DOI] [PubMed] [Google Scholar]
- Fu Q.; Lv P.; Chen Z.; Ni D.; Zhang L.; Yue H.; Yue Z.; Wei W.; Ma G. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale 2015, 7 (9), 4020–4030. 10.1039/C4NR07027E. [DOI] [PubMed] [Google Scholar]
- Cerqueira S. R.; Ayad N. G.; Lee J. K. Neuroinflammation treatment via targeted delivery of nanoparticles. Frontiers in cellular neuroscience 2020, 14, 576037. 10.3389/fncel.2020.576037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Song Z.; He C.; Fan J.; Yu W.; Yang M.; Li P.; Luo R.; Zhou J.; Xu S.; et al. Aspirin curcumin ester loaded biomimetic nanodrug improves cognitive deficits in a mouse model of Alzheimer’s disease by regulating M1/M2 microglial polarization. Mater. Today Adv. 2022, 16, 100321. 10.1016/j.mtadv.2022.100321. [DOI] [Google Scholar]
- Han Y.; Gao C.; Wang H.; Sun J.; Liang M.; Feng Y.; Liu Q.; Fu S.; Cui L.; Gao C.; et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioactive Mater. 2021, 6 (2), 529–542. 10.1016/j.bioactmat.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C.; Wang Y.; Sun J.; Han Y.; Gong W.; Li Y.; Feng Y.; Wang H.; Yang M.; Li Z.; et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020, 108, 285–299. 10.1016/j.actbio.2020.03.029. [DOI] [PubMed] [Google Scholar]
- Liu L.; Bai X.; Martikainen M.-V.; Kårlund A.; Roponen M.; Xu W.; Hu G.; Tasciotti E.; Lehto V.-P. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat. Commun. 2021, 12, 5726. 10.1038/s41467-021-26052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Z.; Hu X.; Wei X.; Zhan C.; Lu L.; Jiang K.; Su B.; Ruan H.; Ran D.; Fang R. H.; et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J. Controlled Release 2017, 264, 102–111. 10.1016/j.jconrel.2017.08.027. [DOI] [PubMed] [Google Scholar]