Abstract
Background
Clonorchiasis has significant socioeconomic importance in endemic areas; however, studies investigating the disease burden in specific sub-regions are lacking. This study aims to address the gap by quantifying the current disease burden caused by clonorchiasis in Guangdong province and assessing its distribution characteristics.
Methods
Comprehensive measures, including prevalence rates, disability-adjusted life years (DALYs), and direct medical costs, were used to assess the disease burden of clonorchiasis. To estimate the prevalence rate, the number of infections was divided by the examined population, based on the annual surveillance data on clonorchiasis cases during 2016–2021. The calculation of DALYs was based on the epidemiological parameters according to the definition issued by the World Health Organization. Cost data of clonorchiasis were utilized to quantify the direct medical costs. The distribution characteristics of disease burden were assessed through comparisons of groups of population defined by geographic area, time, and characteristics of people.
Results
In 2021, clonorchiasis posed a significant disease burden in Guangdong Province. The prevalence rate was found to be 4.25% [95% CI (4.02%, 4.49%)], with an associated burden of DALYs of 406,802.29 [95% CI (329,275.33, 49,215,163.78)] person-years. The per-case direct medical costs of patients with clonorchiasis were estimated to be CNY 7907.2 (SD = 5154.4). Notably, while the prevalence rate and DALYs showed a steady decrease from 2016 to 2020, there was a rising trend in 2021. Spatial clustering of clonorchiasis cases and DALYs was also observed, particularly along the Pearl River and Han River. This suggests a concentration of the disease in these regions. Furthermore, significant differences in prevalence rates were found among various demographic groups, including sex, age, occupation, and education level. Additionally, patients with longer hospital stays were more likely to incur higher direct medical costs.
Conclusions
The burden of clonorchiasis in Guangdong Province remains high, despite significant progress achieved through the implementation of the prevention and control programs. It is suggested that measures should be taken based on the distribution characteristics to maximize the effectiveness of prevention and control, with a primary focus on key populations and areas.
Graphical Abstract
Keywords: Clonorchiasis, Disease burden, Prevalence, DALY, Cost of illness
Background
Clonorchiasis, caused by Clonorchis sinensis, is a significant neglected tropical disease with features of low mortality and high disability [1, 2]. This liver fluke infection is predominantly prevalent in areas with habits of eating raw fish and carries significant socioeconomic implications in endemic areas [3, 4]. It has been estimated that the total economic burden of liver and biliary diseases caused by C. sinensis infection in Guangdong Province amounts to 1.6 billion Chinese yuan (CNY) [5]. This economic burden includes the direct economic burden of the disease, direct non-medical costs, and indirect economic burden, which cover expenditures from medical insurance, out-of-pocket payments, public funding, and other sources.
Clonorchiasis is a food-borne parasitic disease primarily transmitted through the ingestion of raw or undercooked freshwater fish that contain encysted metacercariae of C. sinensis [4, 6–8]. Following infection, most of the individuals remain asymptomatic or experience mild symptoms, while some turn into substantial clinical or subclinical liver and biliary diseases, with cholangiocarcinoma (CCA) being the severe and fatal complication [9]. In 2009, C. sinensis was classified as a definite human carcinogenic (Group 1) by the International Agency for Research on Cancer (IARC) [10, 11]. Currently, the main treatment for clonorchiasis is chemotherapy [12]. Considering the high economic burden caused by symptomatic therapy for sequelae, the prevention strategy, which includes preventive and curative measures, was proposed [13, 14].
According to conservative estimation, approximately 15 million people worldwide have been infected with C. sinensis, with 85% of the cases attributed to China [15]. However, the degree of clonorchiasis endemicity in China varies across regions, with higher concentrations in the southeast and northeast [16]. Guangdong Province was found to be one of the most severely infected provinces according to the three large-scale nationwide surveys conducted in 1990, 2003, and 2015 [16, 17]. The natural conditions, such as abundant river bodies and a climate suitable for the parasite, combined with the local dietary culture of eating raw fish, have contributed to the endemicity of clonorchiasis in Guangdong. The prevalence of the disease has been steadily increasing in Guangdong since 1990 [16, 17]. In response, the Guangdong government issued the Prevention and Control Program for Key Parasitic Diseases (2016–2020) in 2016, implementing a series of prevention strategies were adopted [18]. It was assumed that high infection intensity and number of re-infections among hosts in endemic areas were associated with chronic infection, which might result in more severe health problems and produce a higher disease burden. Thus, it is of great importance to evaluate the current disease burden caused by clonorchiasis and investigate its temporal trends and distribution characteristics in Guangdong to facilitate the development of control strategies.
As defined by the World Health Organization (WHO), the disease burden was not only limited to incidence, mortality, and disability-adjusted life years (DALYs) [19] but also included the economic burden. Several studies have investigated the disease burden of clonorchiasis, among which most researchers followed the Global Burden of Disease (GBD) study and selected disability-adjusted life years as the measurement, and only one study evaluated the economic burden measured by currency. Furst et al. [20] found that the global disease burden caused by clonorchiasis in 2005 was approximately 275,370 DALYs, with China accounting for 84.1% (231,547 DALYs) and Vietnam, South Korea and Russia accounting for 15.9% (43,823 DALYs). Another study reported a higher figure with the DALYs owing to clonorchiasis were 489,174.04 person-years in China [21]. As for the economic burden, it was calculated that 1.3 billion CNY was created by C. sinensis infection in Guangdong [5]. Although research has been carried out on the estimation of the disease burden of clonorchiasis, only a few studies focused on endemic regions, and there was a lack of studies using comprehensive indicators.
Therefore, the primary objective of this study is to evaluate the current disease burden of clonorchiasis in Guangdong Province by utilizing synthesized indexes such as prevalence, DALYs, and cost of illness. Furthermore, the study aims to analyze the trends and distribution characteristics after the implementation of an integrated preventive strategy.
Methods
Study areas
Guangdong was one of the seven high clonorchiasis prevalence settings and was chosen to be the disease surveillance province [22]. Thus, an annual survey in fixed monitoring stations and mobile monitoring stations was carried out to investigate the current epidemic status of clonorchiasis from 2016 to 2021. The study included 16 fixed national surveillance sites, which were categorized according to their prevalence rate into low prevalence regions (≤ 10%), middle prevalence regions (> 10% and ≤ 30%), and high prevalence regions (> 30%). In each region, five villages or communities were selected from five different directions, including north, south, east, west, and middle. Subsequently, 200 villagers or citizens aged > 3 years were included in the survey from each unit. The study used a stratified random sampling method to select participants from each of the 16 fixed national surveillance sites. The sample size was determined based on the estimated prevalence rate of clonorchiasis in each region. The same sampling method was adopted for mobile surveillance sites.
Data collection
The prevalence survey data from 2016 to 2021 was collected from the Guangdong Provincial Center for Disease Control and Prevention, using methods such as reporting cases and active disease surveillance. The annual survey included fixed monitoring stations and mobile monitoring stations, which were used to collect data from the sampled individuals. Guangdong Province has established six national monitoring sites for liver fluke, two national comprehensive prevention and control demonstration areas, and eight provincial mobile monitoring sites. Among them, there are three fixed national monitoring sites, each with an annual budget of 45,000 CNY (6690.2 United States dollars, USD), totaling 135,000 CNY (20,070.5 USD); three mobile national monitoring sites, each with an annual budget of 50,000 CNY (7,433.5 USD), totaling 150,000 CNY (22,300.5 USD); two comprehensive prevention and control project counties, each with an annual budget of 150,000 CNY (22,300.5 USD), totaling 300,000 CNY (44,601.0 USD); and eight provincial monitoring sites, each with an annual budget of 50,000 CNY (7433.5 USD), totaling 400,000 CNY (59,468.1 USD). The total budget amounts to 985,000 CNY (146,440.1 USD). In addition, the study also utilized data from community health centers, hospitals, and clinics to ensure a comprehensive collection of prevalence data for clonorchiasis.
The sample size for the infection rate is specifically based on the 'Monitoring Plan for Clonorchiasis and Soil-Transmitted Helminthiases in Guangdong Province (Trial).' This study employed the modified Kato-Katz thick smear technique to examine and report egg counts. According to infection intensity classifications, > 98% of the infected individuals in the annual monitoring data had light infections (< 1000 eggs per gram of feces). The data included information on the number of examined persons, the number of persons infected with C. sinensis, and the general demographic information (e.g. age, sex, occupation, and education) of each examined individual. The crucial epidemiological disease parameters and demographic information, which were used to calculate DALYs, were obtained from literature and Guangdong Statistic Yearbooks or official documents. The cost data for treating clonorchiasis were acquired from a tertiary hospital in Guangdong Province, which was selected based on its reputation as a leading provider of healthcare services in the region. The hospital is known for its expertise in the treatment of infectious diseases, including clonorchiasis. While the hospital may not be representative of all healthcare facilities in Guangdong Province, it was chosen due to its status as a tertiary hospital and its extensive experience in treating clonorchiasis. The direct medical cost data were sourced from the medical information system of the hospital. Using International Classification of Diseases ten version (ICD-10) coding, the names of major diagnosed diseases between 2008 and 2022 (Clonorchiasis, Clonorchiasis (Chinese liver fluke disease), Clonorchiasis infection, parasitic infection, trematode infection, and Chinese liver fluke disease) were identified. Sociodemographic information (e.g. age, sex, ethnic and marital status), cost information, and healthcare utilization (length of stay) information of each case was included in the database.
A total of 124 patient medical records were selected for analysis. The costs were adjusted to 2022 CNY levels using the consumer price index, and extreme values (n = 114) were excluded before conducting the calculations. Considerations for the inclusion criteria of certain studies were as follows The C. sinensis infection group consisted of patients discharged with diagnoses of C. sinensis infection complicated by obstructive jaundice, cholecystitis, cholangitis, gallstones, cirrhosis, or malignant liver tumors. The non-C. sinensis infection group comprised patients discharged without diagnoses of C. sinensis infection but with complications such as obstructive jaundice, cholecystitis, cholangitis, gallstones, cirrhosis, or malignant liver tumors. Therefore, in this study, we considered the secondary disease burdens of obstructive jaundice, cholecystitis, cholangitis, gallstones, cirrhosis, and malignant liver tumors resulting from C. sinensis infection. Consequently, only patients with uncomplicated C. sinensis infections were included in the analysis for this study, excluding any cases with complications arising from the infection.
Data analysis
Calculation of DALYs
DALY is an index used to quantify health loss caused by a disease, and one DALY can be considered as one lost year of healthy life due to morbidity [22–25]. There are two methods for the calculation of DALY. One is based on incidence, and the other is based on prevalence [25]. DALY is calculated by adding years of life lost (YLLs) due to premature death from disease and years lived with disability (YLDs) owing to morbidity [25]. The formula was as follows:
In this study, the calculation of DALYs used a prevalence approach. YLDs were calculated by multiplying the number of the infected (number of population*prevalence rate of clonorchiasis) with corresponding disability weights (DW). The disability weights range from zero to one, and one represents the most severe health outcome of death. The specific calculation process is listed in formula (a).
The calculation of YLLs followed the method adopted by previous studies [20, 21]. Premature death from C. sinensis was thought to be the attributable case of CCA. The odds ratio between C. sinensis infection and CCA and the incidence of CCA was used to compute the incidence rate of CCA, which was attributable to C. sinensis (I*(OR-1)). Considering the poor prognosis and short disease duration of CCA [12], the mortality rate was replaced by its incidence rate. Finally, YLLs were calculated by the summation of all fatal populations multiplied by the life span at the age of death (Le-Ld). The detailed procedure is expressed in formula (b).
The parameters used for evaluation were listed as follows. An estimation of the infection rate was obtained by dividing the number of infected by the number of examined persons. The provincial and municipal population was derived from the Guangdong Statistic Yearbooks [26]. The weighted disability weight due to C. sinensis in the Chinese population was 0.075 [95% confidential interval (95% CI): (0.060, 0.091)] [9]. The incidence rate of CCA among patients infected with C. sinensis was 1.5 per 100,000 population [27], and the odds ratio between C. sinensis and CCA was 4.47 [95% CI (2.61, 7.66)] [15]. The average life expectancy of the Guangdong population was 79.6 years old. As for the age of death in patients with CCA, it was replaced by the average age at the time of diagnosis. The figure used in the evaluation was 62.6 [95% CI (62.4, 62.8)] years old [28].
Calculation of cost of illness
The electronic medical records provided data for calculating direct medical costs from the healthcare system’s perspective. The direct calculation approach was used to evaluate the direct medical costs caused by clonorchiasis. In terms of composition regulated by the government, direct medical costs were made up of bed fees, diagnostic fees, treatment fees, and other fees. Considering the infection characteristics and high cure rate of clonorchiasis [29], the direct medical costs per case were calculated based on each record. Patients with re-infection were counted as multiple cases. Considering inflation, costs were adjusted to 2021 CNY value using the Consumer Price Index (CPI).
The prevalence rate of C. sinensis infection was calculated for each municipality in Guangdong Province, with the definition of positive infection being the presence of at least one egg. The 95% CI was calculated using a one-sample t-test. Comparisons of prevalence rates among people with different characteristics were achieved through the chi-square test. The significance level was set at 0.05. The main examined variables, DALYs and DALYs per 1000 population, were calculated by definition issued by WHO. The propagating imprecision approach was used to produce a 95% CI of DALYs [30]. The spatial distributions of DALYs and DALYs per 1000 population were demonstrated through maps, while the temporal trends were illustrated by line chart. As for cost data, descriptive statistics, including mean, standard deviation, median, percentiles, and proportion, were used to depict sociodemographic characteristics and direct medical costs. Due to the skewed distribution of medical costs, comparisons of costs for groups with different characteristics were made through the rank sum test (Mann-Whitney U test for two groups and Kruskal-Wallis H test for three and above groups). Statistical analyses were performed with Microsoft Excel 2019 ((Microsoft, Albuquerque, NM, USA) and R language (version 4.2.2). *P < 0.05 is considered statistically significant.
Results
A total of 129,582 people were examined to determine the infection level of C. sinensis in Guangdong Province from 2016 to 2021, with 10,270 in 2016, 17,332 in 2017, 21,577 in 2018, 22,879 in 2019, 28,880 in 2020, and 28,644 in 2021. The total number of infections was 4210, and the annual number of infections from 2016 to 2021 was 773, 856, 692, 417, 254, and 1218, respectively. The overall prevalence rate of clonorchiasis was 3.25% [95% CI 3.15%, 3.35%].
The infection rate of C. sinensis in Guangdong Province showed a decreasing trend between 2016 and 2020 and then staged a recovery in 2021. The infection rates from 2016 to 2021 were 7.53 [95% CI (7.02, 8.05)], 4.94 [95% CI (4.62, 5.27)], 3.21 [95% CI (2.98, 3.45)], 1.82 [95% CI (1.65, 2.00), 0.88 [95% CI (0.78, 0.99)], and 4.25 [95% CI (4.02, 4.49)], respectively (Table 1). The chi-square test indicated that the difference in infection rates in different years was remarkable (χ2 = 1511.1, P < 0.001).
Table 1.
Infection rate (%) of Clonorchis sinensis in Guangdong Province and its prefecture-level cities
| City | In 2016 | In 2017 | In 2018 | In 2019 | In 2020 | In 2021 |
|---|---|---|---|---|---|---|
| Chaozhou | / | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | / | 0.00 (0.00, 0.37) |
| Dongguan | 0.00 (0.00, 0.37) | / | / | / | 0.00 (0.00, 0.32) | 0.00 (0.00, 0.32) |
| Foshan | 7.33 (5.80, 9.12) | 17.25 (14.96, 19.73) | 34.77 (31.91, 37.71) | 8.57 (6.92, 10.48) | 1.73 (1.03, 2.71) | 11.13 (9.26, 13.23) |
| Guangzhou | 12.23 (10.27, 14.40) | 3.04 (2.06, 4.31) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.36) | 0.10 (0.00, 0.56) | 2.90 (1.95, 4.14) |
| Heyuan | / | 30.40 (27.56, 33.36) | 0.25 (0.05, 0.72) | 0.32 (0.13, 0.66) | 0.79 (0.45, 1.28) | 37.15 (34.57, 39.79) |
| Huizhou | 0.13 (0.00, 0.70) | 0.31 (0.06, 0.92) | 0.00 (0.00, 0.37) | 0.10 (0.00, 0.55) | 0.30 (0.11, 0.65) | 0.00 (0.00, 0.36) |
| Jiangmen | 23.10 (20.55, 25.80) | 15.52 (13.34, 17.91) | 13.03 (11.02, 15.25) | 6.94 (5.88, 8.13) | 7.74 (6.18, 9.54) | 12.83 (11.65, 14.07) |
| Jieyang | / | 0.00 (0.00, 0.37) | 0.09 (0.00, 0.50) | 0.00 (0.00, 0.36) | 0.00 (0.00, 0.35) | 0.00 (0.00, 0.37) |
| Maoming | / | 0.00 (0.00, 0.37) | 0.10 (0.00, 0.54) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) |
| Meizhou | / | 10.07 (8.35, 12.01) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.36) | 7.01 (5.51, 8.78) | 0.00 (0.00, 0.37) |
| Qingyuan | 0.35 (0.10, 0.90) | 2.38 (1.56, 3.47) | 2.99 (2.03, 4.25) | 0.79 (0.34, 1.54) | 0.58 (0.21, 1.25) | 0.19 (0.02, 0.67) |
| Shantou | / | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.36) | 0.00 (0.00, 0.36) | 0.00 (0.00, 0.36) |
| Shanwei | / | / | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) |
| Shaoguan | 0.18 (0.02, 0.64) | 0.53 (0.27, 0.95) | 0.22 (0.07, 0.50) | 0.04 (0.00, 0.25) | 0.00 (0.00, 0.16) | 0.38 (0.16, 0.74) |
| Shenzhen | 0.21 (0.03, 0.76) | 0.30 (0.06, 0.87) | 0.89 (0.41, 1.68) | 0.88 (0.40, 1.66) | 0.50 (0.24, 0.91) | 1.08 (0.71, 1.58) |
| Yangjiang | / | (0.00, 0.00) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.37) | 0.00 (0.00, 0.18) | 0.00 (0.00, 0.30) |
| Yunfu | / | 2.40 (1.54, 3.55) | 0.10 (0.00, 0.57) | 0.00 (0.00, 0.34) | 0.09 (0.00, 0.50) | 0.38 (0.10, 0.97) |
| Zhanjiang | 0.00 (0.00, 0.36) | 0.00 (0.00, 0.35) | 0.00 (0.00, 0.34) | 0.00 (0.00, 0.33) | 0.00 (0.00, 0.34) | 0.00 (0.00, 0.36) |
| Zhaoqing | / | / | 6.55 (5.10, 8.26) | / | 0.37 (0.21, 0.61) | 1.28 (0.91, 1.75) |
| Zhongshan | 27.43 (24.9230.06) | / | 8.91 (6.92, 11.26) | 8.08 (6.49, 9.90) | 2.89 (1.95, 4.13) | 9.33 (7.62, 11.29) |
| Zhuhai | / | 1.28 (0.70, 2.14) | 0.89 (0.41, 1.68) | 7.64 (6.10, 9.43) | 0.20 (0.02, 0.72) | 1.06 (0.53, 1.88) |
| Guangdong | 7.53 (7.02, 8.05) | 4.94 (4.62, 5.27) | 3.21 (2.98, 3.45) | 1.82 (1.65, 2.00) | 0.88 (0.78, 0.99) | 4.25 (4.02, 4.49) |
As for spatial distribution, C. sinensis infection appeared in area clustering in Guangdong Province, concentrating in the Pearl River and Han River basins. Of 21 prefecture-level cities, Jiangmen, Foshan, Zhongshan, and Heyuan had the highest infection rates. In 2021, the figures respectively reached 12.8%, 11.1%, 9.3%, and 37.2%. The prevalence situation was similar to that in other years (Table 1).
In terms of population distribution, people of different sex, age, occupation, and education showed significant differences in prevalence rates. Table 2 shows the infection rate of C. sinensis, which was stratified according to sex, age group, occupation, education, and years. Men had a significantly higher prevalence rate (4.27%) than women (2.26%) for infections with C. sinensis (χ2 = 413.3, P < 0.001). People aged < 18 years old were less likely to have C. sinensis infection (0.54%) compared with individuals in other age groups (χ2 = 1860.1, P < 0.001). With increase in age, the prevalence rose at the beginning but then it decreased, and the prevalence peak appeared in the 41–50-year-old group. Students had the lowest prevalence rate (0.69%) among all occupations (χ2 = 1041.0, P < 0.001). The C. sinensis infection rate was the highest in people with a college degree (5.21%), followed by those with high school degrees (5.18%), illiteracy (4.63%), junior school degrees (3.36%), and elementary school education (1.80%). A significant difference was observed in people with different education levels (χ2 = 818.4, P < 0.001).
Table 2.
Population distribution of Clonorchis sinensis infection rate in Guangdong Province
| Characteristics | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Examined population | Infections (infection rate %) | Examined population | Infections (infection rate %) | Examined population | Infections (infection rate %) | Examined population | Infections (infection rate %) | Examined population | Infections (infection rate %) | Examined population | Infections (infection rate %) | Examined population | Infections (infection rate%) | |
| Sex | ||||||||||||||
| Male | 4924 | 468 (9.50) | 8701 | 589 (6.77) | 10,774 | 407 (3.78) | 11,159 | 252 (2.26) | 13,869 | 163 (1.18) | 14,190 | 837 (5.90) | 63,617 | 2716 (4.27) |
| Female | 5346 | 305 (5.71) | 8631 | 267 (3.09) | 10,803 | 285 (2.64) | 11,720 | 165 (1.41) | 15,011 | 91 (0.61) | 14,454 | 381 (2.64) | 65,965 | 1494 (2.26) |
| Age groups | ||||||||||||||
| < 18 | 3119 | 67 (2.15) | 5644 | 41 (0.73) | 7139 | 26 (0.36) | 8525 | 31 (0.36) | 10,106 | 11 (0.11) | 8622 | 57 (0.66) | 43,155 | 233 (0.54) |
| 18–30 | 1239 | 73 (5.89) | 1728 | 67 (3.88) | 2483 | 59 (2.38) | 2582 | 49 (1.90) | 2654 | 24 (0.90) | 2651 | 101 (3.81) | 13,337 | 373 (2.80) |
| 31–40 | 1610 | 168 (10.43) | 2813 | 136 (4.83) | 3496 | 145 (4.15) | 3173 | 95 (2.99) | 4198 | 40 (0.95) | 5343 | 249 (4.66) | 20,633 | 833 (4.04) |
| 41–50 | 1511 | 138 (9.13) | 2736 | 218 (7.97) | 2870 | 135 (4.70) | 2821 | 86 (3.05) | 3352 | 52 (1.55) | 3830 | 376 (9.82) | 17,120 | 1005 (5.87) |
| 51–60 | 1397 | 167 (11.95) | 2097 | 222 (10.59) | 2545 | 131 (5.15) | 2545 | 84 (3.30) | 3743 | 58 (1.55) | 3717 | 272 (7.32) | 16,044 | 934 (5.82) |
| 61–70 | 932 | 123 (13.20) | 1471 | 120 (8.16) | 2040 | 147 (7.21) | 2175 | 54 (2.48) | 3085 | 42 (1.36) | 2901 | 112 (3.86) | 12,604 | 598 (4.74) |
| > 70 | 461 | 37 (8.03) | 842 | 52 (6.18) | 1004 | 49 (4.88) | 1058 | 18 (1.70) | 1742 | 27 (1.55) | 1580 | 51 (3.23) | 6687 | 234 (3.50) |
| Occupations | ||||||||||||||
| Student | 1824 | 68 (3.73) | 4261 | 40 (0.94) | 4620 | 15 (0.32) | 6355 | 37 (0.58) | 7265 | 8 (0.11) | 5647 | 40 (0.71) | 29,972 | 208 (0.69) |
| Worker | 1306 | 91 (6.97) | 1481 | 67 (4.52) | 2528 | 202 (7.99) | 2004 | 73 (3.64) | 2855 | 57 (2.00) | 2602 | 129 (4.96) | 12,776 | 619 (4.85) |
| Peasant | 3055 | 380 (12.44) | 5963 | 295 (4.95) | 6288 | 193 (3.07) | 8188 | 206 (2.52) | 9175 | 110 (1.20) | 6491 | 263 (4.05) | 39,160 | 1447 (3.70) |
| Public official | 835 | 34 (4.07) | 441 | 49 (11.11) | 840 | 80 (9.52) | 668 | 45 (6.74) | 1437 | 19 (1.32) | 2398 | 173 (7.21) | 6619 | 400 (6.04) |
| Teacher and medical staff | 380 | 31 (8.16) | 750 | 71 (9.47) | 878 | 26 (2.96) | 727 | 14 (1.93) | 1113 | 13 (1.17) | 1494 | 151 (10.11) | 5342 | 306 (5.73) |
| Other | 2870 | 169 (5.89) | 4436 | 334 (7.53) | 6423 | 176 (2.74) | 4937 | 42 (0.85) | 7035 | 47 (0.67) | 10,012 | 462 (4.61) | 35,713 | 1230 (3.44) |
| Education | ||||||||||||||
| Illiterate and semiliterate | 425 | 31 (7.29) | 634 | 87 (13.72) | 596 | 16 (2.68) | 660 | 9 (1.36) | 531 | 7 (1.32) | 721 | 15 (2.08) | 3567 | 165 (4.63) |
| Preschool and primary school | 4161 | 300 (7.21) | 7308 | 191 (2.61) | 9028 | 196 (2.17) | 9745 | 84 (0.86) | 12,803 | 63 (0.49) | 11,460 | 148 (1.29) | 54,505 | 982 (1.80) |
| Junior | 2466 | 167 (6.77) | 4630 | 257 (5.55) | 6233 | 178 (2.86) | 6789 | 149 (2.19) | 8486 | 90 (1.06) | 6879 | 352 (5.12) | 35,483 | 1193 (3.36) |
| Senior | 1714 | 141 (8.23) | 3271 | 223 (6.82) | 3760 | 177 (4.71) | 3816 | 122 (3.25) | 3950 | 67 (1.70) | 5398 | 404 (7.48) | 21,909 | 1134 (5.18) |
| College | 1504 | 134 (8.91) | 1489 | 98 (6.58) | 1960 | 125 (6.38) | 1867 | 53 (2.84) | 3110 | 27 (0.87) | 4186 | 299 (7.14) | 14,116 | 736 (5.21) |
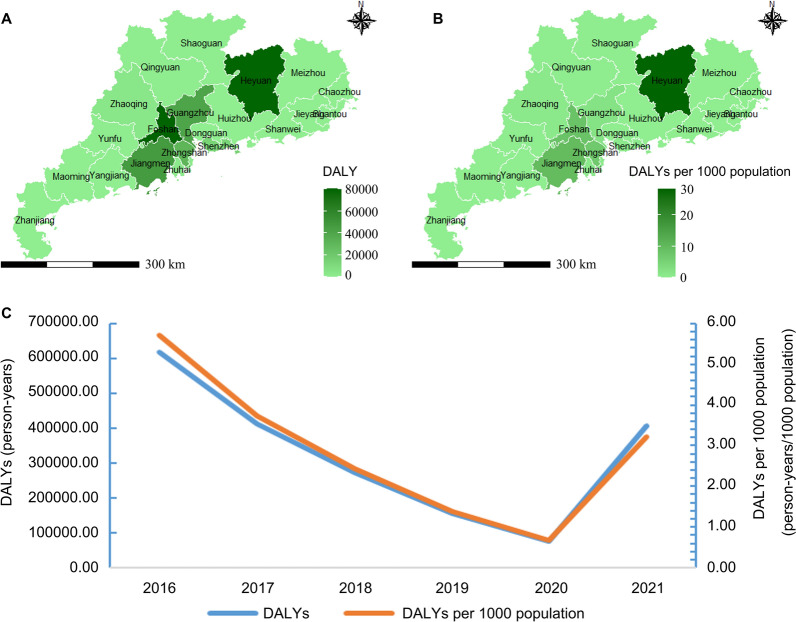
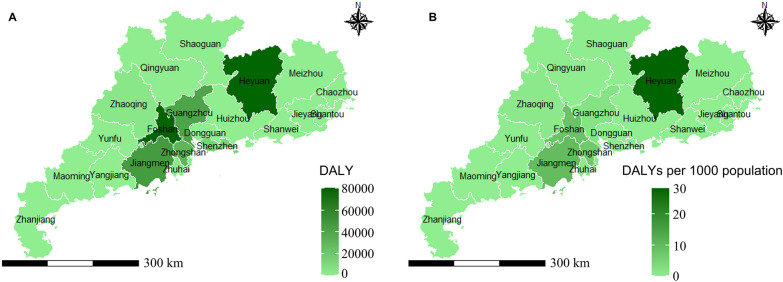
The overall disease burden resulting from clonorchiasis in Guangdong was 406,802.29 [95% CI (329,275.33, 4,9215,163.78)] person-years in 2021, consisting of 402,390.00 [95% CI (324,984.35, 4,8761,687.27)] YLDs and 4,412.29 [95% CI (2,046.96, 847,007.02)] YLLs. The DALYs per 1000 population were 3.22 [95% CI (2.61, 389.85)] person-years. Among the 21 prefecture-level cities in Guangdong Province, Foshan, Heyuan, and Zhongshan showed the highest disease burden measured by DALYs (Table 3). The spatial distribution of DALYs caused by C. sinensis infection in each municipality across Guangdong is displayed in Fig. 1.
Table 3.
DALY and its 95% confidential interval of clonorchiasis in Guangdong Province in 2021
| City | Infection rate (%) | No. population in 2020 (person) | YLLs (person-years) | YLDs (person-years) | DALYs (person-years) | DALYs per 1000 population (person-year/1000 persons)) |
|---|---|---|---|---|---|---|
| Chaozhou | 0.00 | 2,566,600 | 0.00 (0.00, 315.25) | 0.00 (0.00, 28,334.49) | 0.00 (0.00, 28,595.20) | 0.00 (0.00, 11.14) |
| Dongguan | 0.00 | 10,483,600 | 0.00 (0.00, 1121.94) | 0.00 (0.00, 100,839.09) | 0.00 (0.00, 101,766.91) | 0.00 (0.00, 9.71) |
| Foshan | 11.13 | 9,518,800 | 871.27 (403.72, 167,741.56) | 79,458.18 (61,689.57, 10,050,717.92) | 80,329.46 (62,505.74, 10,143,964.25) | 8.44 (6.57, 1065.68) |
| Guangzhou | 2.90 | 18,740,300 | 446.94 (206.02, 87,081.77) | 40,760.15 (29,839.38, 5,543,173.73) | 41,207.10 (30,235.02, 5,594,478.08) | 2.20 (1.61, 298.53) |
| Heyuan | 37.15 | 2,835,600 | 866.33 (401.86, 166,323.72) | 79,006.91 (63,472.16, 9,619,644.69) | 79,873.23 (64,310.47, 9,709,076.54) | 28.17 (22.68, 3423.99) |
| Huizhou | 0.00 | 6,057,200 | 0.00 (0.00, 726.59) | 0.00 (0.00, 65,305.22) | 0.00 (0.00, 65,906.09) | 0.00 (0.00, 10.88) |
| Jiangmen | 12.83 | 4,804,100 | 506.89 (235.03, 97,325.58) | 46,227.45 (36,848.72, 5,675,174.73) | 46,734.35 (37,335.60, 5,727,907.02) | 9.73 (7.77, 1192.30) |
| Jieyang | 0.00 | 5,578,700 | 0.00 (0.00, 683.18) | 0.00 (0.00, 61,403.30) | 0.00 (0.00, 61,968.27) | 0.00 (0.00, 11.11) |
| Maoming | 0.00 | 6,180,000 | 0.00 (0.00, 759.08) | 0.00 (0.00, 68,225.34) | 0.00 (0.00, 68,853.08) | 0.00 (0.00, 11.14) |
| Meizhou | 0.00 | 3,871,000 | 0.00 (0.00, 475.47) | 0.00 (0.00, 42,734.67) | 0.00 (0.00, 43,127.88) | 0.00 (0.00, 11.14) |
| Qingyuan | 0.19 | 3,974,000 | 6.21 (2.68, 1572.90) | 566.30 (321.68, 123,773.24) | 572.50 (325.97, 124,913.92) | 0.14 (0.08, 31.43) |
| Shantou | 0.00 | 5,503,700 | 0.00 (0.00, 652.56) | 0.00 (0.00, 58,651.63) | 0.00 (0.00, 59,191.28) | 0.00 (0.00, 10.75) |
| Shanwei | 0.00 | 2,669,400 | 0.00 (0.00, 327.88) | 0.00 (0.00, 29,469.37) | 0.00 (0.00, 29,740.52) | 0.00 (0.00, 11.14) |
| Shaoguan | 0.38 | 2,855,300 | 8.92 (4.01, 1812.40) | 813.76 (535.07, 126,677.38) | 822.68 (542.18, 127,847.11) | 0.29 (0.19, 44.78) |
| Shenzhen | 1.08 | 17,633,800 | 156.62 (72.00, 30,533.13) | 14,283.38 (10,364.43, 1,958,427.39) | 14,440.00 (10,501.88, 1,976,549.15) | 0.82 (0.60, 112.09) |
| Yangjiang | 0.00 | 2,605,900 | 0.00 (0.00, 259.68) | 0.00 (0.00, 23,340.12) | 0.00 (0.00, 23,554.88) | 0.00 (0.00, 9.04) |
| Yunfu | 0.38 | 2,383,700 | 7.45 (3.34, 1656.26) | 679.35 (422.06, 122,919.14) | 686.80 (427.68, 124,052.95) | 0.29 (0.18, 52.04) |
| Zhanjiang | 0.00 | 6,980,700 | 0.00 (0.00, 829.28) | 0.00 (0.00, 74,535.29) | 0.00 (0.00, 75,221.09) | 0.00 (0.00, 10.78) |
| Zhaoqing | 1.28 | 4,116,900 | 43.34 (20.03, 8417.06) | 3952.22 (2939.46, 527,988.12) | 3995.56 (2978.41, 532,877.25) | 0.97 (0.72, 129.44) |
| Zhongshan | 9.33 | 4,431,100 | 339.99 (157.48, 65,499.81) | 31,006.62 (23,934.33, 3,947,990.51) | 31,346.62 (24,251.07, 3,984,607.79) | 7.07 (5.47, 899.24) |
| Zhuhai | 1.06 | 2,449,600 | 21.35 (9.73, 4287.72) | 1947.43 (1329.96, 291,821.89) | 1968.79 (1347.62, 294,518.04) | 0.80 (0.55, 120.23) |
| Guangdong | 4.25 | 126,240,000 | 4412.29 (2046.96, 847,007.02) | 402,390.00 (324,984.35, 48,761,687.27) | 406,802.29 (329,275.33, 49,215,163.78) | 3.22 (2.61, 389.85) |
Fig. 1.
Distribution of the disease burden of clonorchiasis in Guangdong Province in 2021. A DALY. B DALY per 1000 persons
From 2016 to 2021, the disease burden indicators, including DALYs and DALYs per 1000, gradually decreased at the beginning of 5 years and then rose suddenly (Fig. 2), consistent with the temporal trends of prevalence rate. However, the overall trends of DALYs and DALYs per 1000 presented a downward trend, decreasing from 618,862.85 and 5.70 in 2016 to 406,802.29 and 3.22 in 2021 (Table 4).
Fig. 2.
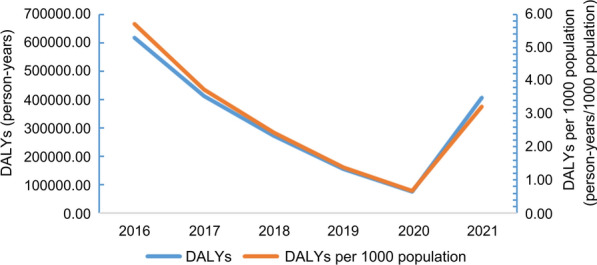
Temporal trends of DALYs of clonorchiasis in Guangdong Province
Table 4.
DALYs of clonorchiasis in Guangdong Province in 2016–2021
| Year | Infection rate (%) | No. population in 2020 (person) | YLL (person-year) | YLD (person-year) | DALY (person-year) | DALY per 1000 population (person-year/1000 persons)) |
|---|---|---|---|---|---|---|
| 2016 | 7.53 | 108,490,000 | 6165.57 | 612,697.28 | 618,862.85 | 5.70 |
| 2017 | 4.94 | 109,990,000 | 4129.08 | 407,512.95 | 411,642.03 | 3.74 |
| 2018 | 3.21 | 111,690,000 | 2724.54 | 268,893.68 | 271,618.21 | 2.43 |
| 2019 | 1.82 | 113,460,000 | 1569.23 | 154,872.90 | 156,442.13 | 1.38 |
| 2020 | 0.88 | 115,210,000 | 802.12 | 76,038.60 | 76,840.72 | 0.67 |
| 2021 | 4.25 | 126,240,000 | 4412.29 | 402,390.00 | 406,802.29 | 3.22 |
In total, 124 patients with the primary diagnosis of clonorchiasis were identified. After excluding extreme values, 114 patients were included for cost analysis. The basic characteristics are displayed in Table 5. Out of the total cases, males accounted for 80.7%. The mean age was 44.9 (SD = 12.0) years old. Among all age groups, more cases were classified into the age group 40 to 60 years (47.4%), followed by the group < 40 years (37.7%) and > 60 years (14.9%). Of all the ethnic groups, the Han patients comprised the majority, with a proportion of 99.1%. As for marital status, 87.7% of patients were married. The average length of stay was 8.4 (SD = 4.4) days.
Table 5.
Demographic characteristics of patients with clonorchiasis
| Characteristics | Total sample (n = 114) |
|---|---|
| Sex | |
| Female, n (%) | 22.0 (19.3) |
| Male, n (%) | 92.0 (80.7) |
| Age | |
| Mean ± sd | 44.9 ± 12.0 |
| Median (25th–75th) | 43.0 (36.0–54.0) |
| Age groups | |
| < 40, n (%) | 43.0 (37.7) |
| 40–60, n (%) | 54.0 (47.4) |
| > 60, n (%) | 17.0 (14.9) |
| Ethnic group | |
| Han, n (%) | 113.0 (99.1) |
| Other, n (%) | 1.0 (0.9) |
| Marital status | |
| Married, n (%) | 100.0 (87.7) |
| Other, n (%) | 14.0 (12.3) |
| Length of stay | |
| Mean ± sd | 8.4 ± 4.4 |
| Median (25th–75th) | 7.0 (5.0–11.0) |
| < 8 days | 62 (54.4) |
| ≥ 8 days | 52 (45.6) |
Overall, the direct medical costs of patients with clonorchiasis per case were CNY 7907.2 (SD = 5154.4) (equivalent to 1175.7 USD ± 766.3) (Table 6). Costs did not show significant differences among patients with different sex, age, ethnicity, and marital status, while the costs for patients having different lengths of stay were significantly different (P < 0.001).
Table 6.
Direct medical costs of clonorchiasis for patients with different characteristics
| Characteristics | Direct medical costs (CNY) Mean ± SD |
Direct medical costs (USDa) Mean ± SD |
P value |
|---|---|---|---|
| Direct medical costs | 7907.2 ± 5154.4 | 1175.7 ± 766.3 | |
| Sex | 0.085 | ||
| Female | 6960.9 ± 5951.8 | 1.34.9 ± 88.49 | |
| Male | 8133.5 ± 4954.6 | 1209.2 ± 736.6 | |
| Age groups (years) | 0.980 | ||
| < 40 | 7916.5 ± 5115.6 | 1176.9 ± 760.5 | |
| 40–60 | 7688.6 ± 4690.1 | 1143.1 ± 697.3 | |
| > 60 | 8578.0 ± 6740.8 | 1275.3 ± 1002.2 | |
| Ethnic group | 0.202 | ||
| Han | 7845.9 ± 5135.5 | 1166.5 ± 763.5 | |
| Other | / | ||
| Marital status | 0.839 | ||
| Married | 7983.1 ± 5229.1 | 1186.8 ± 777.4 | |
| Other | 7365.2 ± 4727.0 | 1095.0 ± 702.8 | |
| Length of stay (days) | < 0.001* | ||
| < 8 days | 5033.4 ± 2987.0 | 748.3 ± 444.1 | |
| ≥ 8 days | 11,333.7 ± 5117.0 | 1685.0 ± 760.7 |
*Statistically significant
aBased on the average exchange rate of 1 USD to 6.7263 CNY in 2022
Discussion
In Guangdong, C. sinensis infections are highly prevalent, and the occurrence of repeated and chronic infection is common [13, 31, 32]. Notably, few studies have previously explored the disease burden of clonorchiasis in endemic areas using synthesized indexes. This study aimed to evaluate the disease burden caused by C. sinensis infection through comprehensive indicators and assess its trends from 2016 to 2021 in Guangdong.
It was found that the burden of disease associated with clonorchiasis in Guangdong was overwhelming, with a prevalence rate of 4.25%, DALYs of 406,802.29 [95% CI (329,275.33, 49,215,163.78)] person-years, and direct medical costs of CNY 7907.2 (USD 1175.7) in 2021. The prevalence rate, DALYs, and DALYs per 1000 population all experienced a downward trend between 2016 and 2020, then an upward trend in 2021, while in general, the situation in 2021 was better than in 2016. Moreover, the distribution of disease burden also showed spatial clustering, with infection cases concentrated in the Pearl River and Han River basins, such as Foshan, Zhongshan, and Heyuan. As for population distribution, people with different characteristics tend to have different prevalence rates and direct medical costs.
Based on the 2021 provincial clonorchiasis surveillance survey results, the prevalence rate of C. sinensis infection was 4.25% [95% CI (4.02, 4.49)]. The DALYs and DALYs per 1000 population due to clonorchiasis were 406,802.29 [95% CI (329,275.33, 49,215,163.78)] person-years and 3.22 [95% CI (2.61, 389.85)], respectively. Zhao et al. [20] found that the burden of clonorchiasis in Guangdong was 157,245.48 [95% CI (120,532.89, 199,807.40)] DALYs in 2015, and the global burden of clonorchiasis reported by Furst et al. [20] was 231,547 person-years. Compared with previous research, this study reported a much higher figure. The different computation methods and study time might explain the condition [21]. In the former study, a standardized prevalence rate was used for calculation, and thus the results might not accurately reflect the reality, while in the latter study, only severe disability due to infection was considered, which might lead to the result of lower disease burden. Different from these two studies, this research considered all severity levels of disability due to C. sinensis infection and adopted the latest raw prevalence rate for calculation. Therefore, to some extent, the results in this study could precisely reflect the current disease burden caused by clonorchiasis in Guangdong.
Through cost analysis of clonorchiasis, we found that the average direct medical costs per case in Guangdong were CNY 7,907.2 (SD = 5154.4) (equivalent to 1175.7 USD ± 766.3), lower than the previous research findings [5]. In that study, the calculated direct medical costs of clonorchiasis-induced gallbladder cholangitis, cholelithiasis, liver cirrhosis, and malignant liver tumor were respectively CNY 5015.52, CNY 9971.76, CNY 13,168.83, and CNY 17,638.28 in 2009 (CNY 7034.32, CNY 13,985.50, CNY 18,469.42, and CNY 24,737.87 in 2022) [33]. Different selection criteria for the study population might partly explain the situation. In this study, patients whose primary diagnosis was clonorchiasis were chosen, and the symptoms were relatively mild, while in that study, patients with liver and biliary disease were selected. Therefore, the costs in our study were relatively lower and closer to the real world’s true values.
From 2016 to 2021, the prevalence rate and DALYs of clonorchiasis decreased steadily first and then increased in 2021. The continued decline in the first 5 years might be attributed to the implementation of the Clonorchiasis Prevention and Control Project in Guangdong Province. Since 2016, the relevant departments have taken a series of measures such as propaganda and education, surveillance, and deworming treatment to prevent clonorchiasis [18]. Thus, the main source of contagion and transmission route were controlled, and the prevalence rate and DALYs decreased correspondingly. For the rise in 2021, one possible explanation was that, owing to the pandemic of COVID-19, there was a lack of human, physical, and financial resources. It was indicated that the shortage of resources was an obstacle to the prevention of clonorchiasis [34]. Therefore, the prevalence rate and DALYs showed a slight rebound in 2021.
The results revealed that the clonorchiasis cases were distributed in most areas of Guangdong Province, with the prevalence rate and DALYs primarily located alongside the Pearl River and Han River, consistent with the results of previous prevalence surveys conducted in Guangdong [35–37]. There were several reasons for the occurrence of spatial clustering. First, this might be partly due to the livable environments for C. sinensis [32]. Sufficient freshwater resources and suitable climate conditions in these regions make sure that different growth states of C. sinensis and the competence of infection exist, thus facilitating the spread of clonorchiasis. Second, this fluke develops in two intermediate hosts in fresh water and one definitive host, giving it a clear survival advantage [29, 38]. In these settings, the existence of abundant intermediate hosts and definitive hosts laid good foundations for the formation of a relatively suitable food chain for predation and prey, thus making it possible for C. sinensis to develop a complete life history and resulting in clonorchiasis prevalence [3]. Moreover, studies showed that the well-developed fish breeding in these regions and the dietary habit of eating “sashimi” collaboratively promote the transmission of clonorchiasis [32, 39]. In certain areas, poor management of human and animal feces results in their discharge into water sources, potentially containing eggs of C. sinensis, which can release miracidia and thus lead to the formation of an endemic disease [3, 29]. Additionally, the high accessibility to infection sources was an important contributor that should not be ignored [3]. It was shown that habits such as catching fish without washing hands, holding fish with the mouth, and mixed use of the kitchen chopping boards could also increase the risk of infection.
This research, consistent with the previous study [17], found that the prevalence rate of clonorchiasis in males was significantly higher than that of females in Guangdong. Males have more chances to eat raw fish and are more indulged in this diet [40]. Although health education has been carried out for a long time, these dietary habits in men were hard to change [3, 17]. Moreover, public officials, medical staff, and teachers had been reported to have higher infection rates among all occupations in this study, which further indicated the paradox between knowledge and behavior where the knowledge of clonorchiasis could not prevent people from consuming raw fish [32]. Additionally, the findings that the infection rate increased with age (0−50 years) and people aged between 40 to 60 years had the largest proportion of C. sinensis infection, in line with a previous study [3], indicated that the frequency of raw fish consumption was associated with clonorchiasis. As for education level, people with college degrees reported the highest rate. The confounding effect of age might explain the situation. The direct medical costs for patients with clonorchiasis significantly differed according to the length of stay. Generally, patients with severe clinical symptoms are more likely to have longer stays and higher direct medical costs. As previous findings showed, patients with different complications of C. sinensis infection had quite different costs [5].
This study quantified the current disease burden of clonorchiasis in Guangdong by using indexes of prevalence rate, DALYs, and direct medical costs and demonstrated its distribution characteristics and temporal trends. To the best of our knowledge, this was the first study having evaluated the disease burden of clonorchiasis in endemic areas from diverse dimensions and explained the sub-regional and temporal variation.
However, several limitations need to be acknowledged. First, due to the inaccessibility of data on clonorchiasis deaths in Guangdong, this study followed the approach of previous studies [12, 21] and extracted the key parameters from the literature. The parameters used to calculate DALYs were the best available data we could find. Second, in the cost of illness study, because measurement of direct non-medical and indirect costs is not feasible with current data sources, only direct medical costs were included in this analysis. Thus, the economic burden reported in our analysis may be underestimated. It was recommended that the recording and archiving of relevant data should be strengthened in the future to provide more precise information.
Conclusions
Although effective measures have been taken to prevent and control clonorchiasis, the disease burden caused by clonorchiasis in Guangdong is still remarkable and showed a brief rebound in 2021. This situation highlights the urgent need for sustainable prevention and control strategies. Tailored preventive recommendations should be formulated based on an evidence-based scientific approach, considering the distribution characteristics of the disease burden. In endemic areas, it is crucial to prioritize efforts such as strengthening fish farming management, improving food safet,y and enhancing awareness, which plays a vital role in effectively combating clonorchiasis. It is important to pay particular attention to men, adults, and individuals with lower levels of education during the clonorchiasis prevention campaign.
Acknowledgements
We would like to express our sincere gratitude to all the units and individuals who participated in the study of disease burden analysis and health economic evaluation of clonorchiasis. We also thank the Key Laboratory of Tropical Disease Control, Ministry of Education, Sun Yat-sen University, for their valuable assistance.
Abbreviations
- CNY
Chinese Yuan
- YLLs
Adding years of life lost
- CPI
Consumer Price Index
- WHO
World Health Organization
- GBD
Global burden of disease
- DALY
Aisability-adjusted life year
- CI
Confidence interval
Author contributions
ZDW, XS, ZHD, LG, and DTL conceived and designed the research. LG, DTL, and ZBC were responsible for drafted the manuscript. LG, DTL, ZBC, QMZ, and QXZ were responsible for study selection and data extraction. DTL, KFJ, WJZ, LG, ZHD, JL, and XS revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Key Research and Development Program of Guangdong Province (no. 2022B1111030002), the National Key Research and Development Program of China (nos. 2021YFC2300800 and 2021YFC2300801), the National Natural Science Foundation of China (nos. 82272361, 81902081, 82202560, and 82161160343), the Natural Science Foundation of Guangdong Province (nos. 2024A1515010615 and 2021A1515010976), the Science and Technology Planning Project of Guangdong Province (no. 2021B1212040017), the Science and Technology Projects in Guangzhou (no. 2024A04J4314), the National Parasitic Resource Center of China (no. NPRC-2019–194-30), and the 111 Project (no. B12003). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Sun Yat-sen University (2023–096).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Datao Lin, Zhuohui Deng and Zebin Chen contributed equally to this work.
Contributor Information
Zhuohui Deng, Email: tracydzh@163.com.
Lan Guo, Email: guolan3@mail.sysu.edu.cn.
Xi Sun, Email: sunxi2@mail.sysu.edu.cn.
References
- 1.Qian MB. Neglected tropical diseases and global burden of disease in China. Infect Dis Poverty. 2017;6:25. 10.1186/s40249-017-0237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Working to overcome the global impact of neglected tropical diseases—summary. Wkly Epidemiol Rec. 2011;86:113–20. [PubMed] [Google Scholar]
- 3.Tang ZL, Huang Y, Yu XB. Current status and perspectives of Clonorchissinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect Dis Poverty. 2016;5:71. 10.1186/s40249-016-0166-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. 10.1016/S1473-3099(04)01252-6 [DOI] [PubMed] [Google Scholar]
- 5.Huang J. Study on disease economic burden of liver and gallbladder disease caused by Clonorchissinensis infected in Guangdong province (In Chinese). Master's thesis. Guangdong Pharmaceutical University. 2011;6.
- 6.Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–83. 10.1128/CMR.00012-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507–14. 10.3201/eid1110.050614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song LG, Zheng XY, Lin DT, Wang GX, Wu ZD. Parasitology should not be abandoned: data from outpatient parasitological testing in Guangdong, China. Infect Dis Poverty. 2017;6:119. 10.1186/s40249-017-0332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian MB, Chen YD, Fang YY, Xu LQ, Zhu TJ, Tan T, et al. Disability weight of Clonorchis sinensis infection: captured from community study and model simulation. PLoS Negl Trop Dis. 2011;5:e1377. 10.1371/journal.pntd.0001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iarc L. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 11.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El GF, et al. A review of human carcinogens–part B: biological agents. Lancet Oncol. 2009;10:321–2. 10.1016/S1470-2045(09)70096-8 [DOI] [PubMed] [Google Scholar]
- 12.Qian MB, Zhou CH, Zhu HH, Chen YD, Zhou XN. Cost yield of different treatment strategies against Clonorchissinensis infection. Infect Dis Poverty. 2021;10:136. 10.1186/s40249-021-00917-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi MH, Park SK, Li Z, Ji Z, Yu G, Feng Z, et al. Effect of control strategies on prevalence, incidence and re-infection of clonorchiasis in endemic areas of China. PLoS Negl Trop Dis. 2010;4:e601. 10.1371/journal.pntd.0000601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YD, Li HZ, Xu LQ, Qian MB, Tian HC, Fang YY, et al. Effectiveness of a community-based integrated strategy to control soil-transmitted helminthiasis and clonorchiasis in the People’s Republic of China. Acta Trop. 2021;214:105650. 10.1016/j.actatropica.2020.105650 [DOI] [PubMed] [Google Scholar]
- 15.Qian MB, Chen YD, Liang S, Yang GJ, Zhou XN. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty. 2012;1:4. 10.1186/2049-9957-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yingdan C, Changhai Z, Huihui Z, Jilei H, Lei D, Tinjun Z, et al. National survey on the current status of important human parasitic diseases in China in 2015. Chin J Parasitol Parasitic Dis. 2020;38:5–16. [Google Scholar]
- 17.Chen YD, Zhou CH, Xu LQ. Analysis of the results of two nationwide surveys on Clonorchis sinensis infection in China. Biomed Environ Sci. 2012;25:163–6. [DOI] [PubMed] [Google Scholar]
- 18.Notification issued by 12 departments including Health commission of Guangdong Province on the prevention and control plan of key parasitic diseases in Guangdong Province (2016–2020). 2017. https://wsjkw.gd.gov.cn/gkmlpt/content/2/2130/mpost_2130867.html#2531 (accessed on 17 june 2024).
- 19.Call for experts—WHO initiative to estimate the global burden of foodborne diseases. 2020. https://www.who.int/news-room/articles-detail/call-for-experts-who-initiative-estimate-global-burden-of-foodborne-diseases (accessed on 17 june 2024).
- 20.Furst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–21. 10.1016/S1473-3099(11)70294-8 [DOI] [PubMed] [Google Scholar]
- 21.Zhao T, Fang Y, Lai Y. Assessment of the burden of clonorchiasis and its temporal changes in China. Chin J Schistosomiasis Control. 2021;33:162–8. [DOI] [PubMed] [Google Scholar]
- 22.Qian MB, Chen YD, Zhu HH, Zhu TJ, Zhou CH, Zhou XN. Establishment and role of national clonorchiasis surveillance system in China. Chin J Epidemiol. 2018;39:1496–500. [DOI] [PubMed] [Google Scholar]
- 23.Kuchenmuller T, Abela-Ridder B, Corrigan T, Tritscher A. World Health Organization initiative to estimate the global burden of foodborne diseases. Rev Sci Tech. 2013;32:459–67. 10.20506/rst.32.2.2249 [DOI] [PubMed] [Google Scholar]
- 24.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. 10.1371/journal.pmed.1001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organization WH. WHO estimates of the global burden of foodborne diseases: foodborne diseases burden epidemiology reference group 2007–2015. 2015. https://www.who.int/publications/i/item/9789241565165. Accessed 17 Jun 2024..
- 26.Bureau GS. Guangdong Statistic Yearbook 2021. 2021. http://stats.gd.gov.cn/gdtjnj/content/post_3557537.html. Accessed 17 Jun 2024
- 27.Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99:315–35. 10.1016/j.suc.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 28.Kamsa-Ard S, Luvira V, Suwanrungruang K, Kamsa-Ard S, Luvira V, Santong C, et al. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand from 1989 through 2013: a population-based cancer registry study. J Epidemiol. 2019;29:197–204. 10.2188/jea.JE20180007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian MB, Utzinger J, Keiser J, Zhou XN. Clonorchiasis. Lancet. 2016;387:800–10. 10.1016/S0140-6736(15)60313-0 [DOI] [PubMed] [Google Scholar]
- 30.Newcombe RG. Propagating imprecision : combining confidence intervals from independent sources. Commun Stat Theory Methods. 2011;40:3154–80. 10.1080/03610921003764225 [DOI] [Google Scholar]
- 31.Huang SY, Chen JD, Zeng QS, Lai YS. High-resolution mapping of age- and gender-specific risk of Clonorchis sinensis infection risk in Guangdong, China: a geostatistical modeling study. Parasit Vectors. 2024;17:67. 10.1186/s13071-024-06166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng ZH, Fang YY, Zhang QM, Mao Q, Pei FQ, Liu MR. The control of clonorchiasis in Guangdong province, southern China. Acta Trop. 2020;202:105246. 10.1016/j.actatropica.2019.105246 [DOI] [PubMed] [Google Scholar]
- 33.CCEMG-EPPI-Centre cost converter. 2023. http://eppi.ioe.ac.uk/costconversion/default.aspx. Accessed 17 Jun 2024.
- 34.Zhuo-Hui D, Yue-Yi F. Epidemic situation and prevention and control strategy of clonorchiasis in Guangdong Province, China. Chin J Schistosomiasis Control. 2016;28:229–33. [DOI] [PubMed] [Google Scholar]
- 35.Yueyi F, Xianchang Z, Jun W, Caiwen R, Qimin Z, Shayu H, et al. Survey and analysis on the current status of human major parasitic diseases in Guangdong Province. J Pathogen Biol. 2008;3:1673–5234. [Google Scholar]
- 36.Yueyi F, Jun W, Qing L, Shaoyu H, Rongxing L, Qiming Z, et al. Investigation and analysis on epidemic status of clonorchiasis in Guangdong Province, China. J Pathogen Biol. 2007;2:1673–5234. [Google Scholar]
- 37.Yueyi F, Bo P, Xiaochu S, Zuze C, Honored L, Shaoyu H, et al. Comparative anlysis of two surveys of distribution of human parasites in Guangdong Province (In Chinese). Strait Journal of Preventive Medicine. 2000(02):32-3. doi: 10.3969/j.issn.1007-2705.2000.02.013
- 38.Solodovnik DA, Tatonova YV, Burkovskaya PV. The geographical vector in distribution of genetic diversity for Clonorchis sinensis. Parasitol Res. 2018;117:335–8. 10.1007/s00436-017-5687-4 [DOI] [PubMed] [Google Scholar]
- 39.Xianchang Z, Fuquan P, Qiming Z, Rongsheng L, Shaoyu H, Jinlong W, et al. Current status of environmental sanitation and clonorchis sinensis intermediate host infection of freshwater aquaculture in partial areas of Guangdong Province. South China J Preventive Med. 2010;36(03):9-13.
- 40.Qian MB, Zhou CH, Jiang ZH, Yang YC, Lu MF, Wei K, et al. Epidemiology and determinants of Clonorchissinensis infection: a community-based study in southeastern China. Acta Trop. 2022;233:106545. 10.1016/j.actatropica.2022.106545 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.