Abstract
Pyroptosis, a lytic form of cell death mediated by the gasdermin family, is characterized by cell swelling and membrane rupture. Inducing pyroptosis in cancer cells can enhance antitumor immune responses and is a promising strategy for cancer therapy. However, excessive pyroptosis may trigger the development of inflammatory diseases due to immoderate and continuous inflammatory reactions. Nanomaterials and nanobiotechnology, renowned for their unique advantages and diverse structures, have garnered increasing attention owing to their potential to induce pyroptosis in diseases such as cancer. A nano-delivery system for drug-induced pyroptosis in cancer cells can overcome the limitations of small molecules. Furthermore, nanomedicines can directly induce and manipulate pyroptosis. This review summarizes and discusses the latest advancements in nanoparticle-based treatments with pyroptosis among inflammatory diseases and cancer, focusing on their functions and mechanisms and providing valuable insights into selecting nanodrugs for pyroptosis. However, the clinical application of these strategies still faces challenges owing to a limited understanding of nanobiological interactions. Finally, future perspectives on the emerging field of pyroptotic nanomaterials are presented.
Graphical abstract
Keywords: Pyroptosis, Nanoparticle, Tumor immunotherapy, Nano-delivery system, Nanodrugs, Gasdermin
Introduction
Cell death is an essential part of the life cycle. Based on functionality and controllability, cell death can be categorized into uncontrolled accidental cell death (ACD) and controlled regulated cell death (RCD), also known as programmed cell death (PCD). ACD occurs when cells are exposed to harmful stimuli that surpass their adaptable capacity, ultimately leading to cellular fatality. In contrast, RCD typically involves a signaling cascade comprising distinctive morphological characteristics, biochemical properties, and immunological implications.
RCD, which includes apoptosis, necroptosis, autophagy, and ferroptosis, has been extensively studied [1]. Advancements in understanding novel types of RCD and their implications in immune responses and tumor development have spurred the progress of therapeutic approaches against cancer. Pyroptosis, an inflammatory form of PCD, has emerged as a promising avenue for antitumor treatments. Pyroptosis was first described in 1986. Friedlander et al. treated primary mouse macrophages with the anthrax toxin and found that the macrophages rapidly underwent cytolytic death with a non-specific release of cellular contents [2]. Owing to the limited knowledge of cell death at the time, this phenomenon was mistaken for a specific process of apoptosis. At the turn of the century, a handful of researchers became aware that cell pyroptosis represents an alternative form of cell death. Morphologically, cell death is characterized by cellular enlargement followed by rupture of the plasma membrane, resulting in the liberation of interleukin 1β (IL-1β), IL-18, and the intracellular contents into the surrounding environment while triggering a robust inflammatory response. Compared to apoptosis, pyroptosis occurs more swiftly and entails the release of various pro-inflammatory factors. This process exhibits similarities with apoptosis, such as chromatin condensation, nuclear pyknosis, and vesicle exocytosis [3] but also shares resemblances with necrosis, including cellular enlargement, rupture, and the discharge of inflammatory components [4].
Currently, the approach to combating tumors has shifted from using drugs or radiation to kill them to activating the body’s natural immune systems to eliminate cancer cells and create a lasting antitumor immune response. Tumor cell death can either be immunogenic or non-immunogenic. It is essential to induce immunogenic cell death (ICD) in tumors as a prerequisite for establishing effective antitumor immunity [5]. Pyroptosis, a highly immunogenic type of cell death, initiates local inflammation and attracts inflammatory cells for infiltration. This offers an opportunity to alleviate the immune suppression within the tumor microenvironment (TME) and stimulate systemic immune responses during the treatment of solid tumors [6, 7]. Wang et al. demonstrated that even if pyroptosis occurs in less than 15% of cancer cells, it is still effective in eliminating the 4T1 mouse breast tumor graft. However, when mice are immunodeficient or T cells are depleted, it does not lead to tumor regression, suggesting that the antitumor effect is related to an enhanced antitumor immune response [8]. Inflammation-induced pyroptosis can stimulate a strong antitumor immune response, and pyroptosis makes tumors more sensitive to immunotherapy. This shows that inducing pyroptosis effectively treats tumors and provides a new idea for developing tumor immunotherapy drugs.
Typically, Moderate pyroptosis typically functions in host defense against pathogen infections. However, excessive pyroptosis can lead to uncontrolled inflammatory reactions, extensive cell death, and severe tissue damage, resulting in the development of inflammatory or autoimmune disorders such as nervous system diseases, cardiovascular diseases, and liver diseases. Therefore, numerous research efforts have been dedicated to inhibiting pyroptosis for therapeutic interventions aimed at managing these conditions.
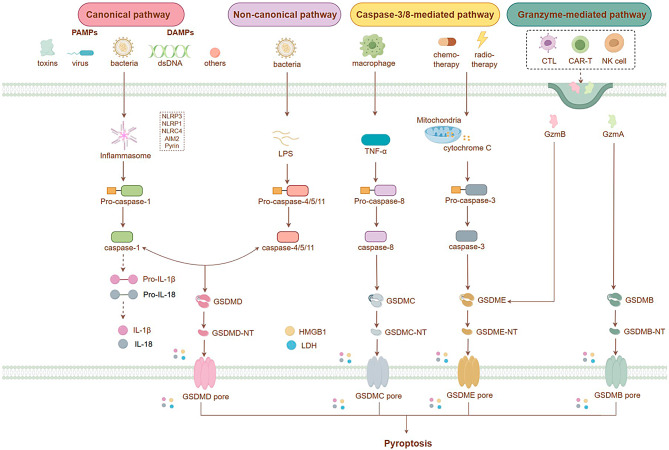
Pyroptosis can be triggered through four different pathways, categorized as inflammasome-dependent or -independent (Fig. 1). The inflammasome-mediated pathway includes both the conventional caspase-1-dependent pathway and the alternative caspase-4/5/11-dependent pathway. In contrast, the inflammasome-independent focal death pathway involves caspase-3/8 and granzyme-mediated pathways [9]. Intracellular clusters of multiple proteins known as inflammasomes are generated following exposure to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [10]. The classical inflammasome pathway involves receptor proteins such as NLRP1, NLRP3, NLRC4, AIM2, and pyrin. When PAMP or DAMP signals are recognized, pro-caspase-1 is recruited, which activates caspase-1. This activated form cleaves pro-IL-1β and pro-IL-18, forming active mature IL-1β and IL-18 [11, 12]. Gasdermin (GSDM) D, the first executor of pyroptosis, is a direct substrate of inflammatory caspases and the main executor of macrophage pyroptosis [13–15]. Caspase-1 activation leads to the splitting of GSDMD into two domains. The N-terminal domain can disrupt cell membrane integrity by creating non-selective pores, resulting in the release of IL-1β and IL-18 [16].
Fig. 1.
Diagram illustrating the cellular pathway of pyroptosis
Pyroptosis can also be triggered through an alternative pathway, wherein intracellular LPS directly binds to pro-caspases-4/5 or mouse pro-caspase-11, leading to caspase oligomerization and activation [17]. Chemotherapeutic drugs induce pyroptosis by causing caspase-3-mediated GSDME cleavage [18, 19]. TNF-α stimulation leads to activated caspase-3 cleaving human/mouse GSDME at specific amino acid residues. Although this type of pyroptosis does not follow classical or non-classical inflammasome pathways, it still promotes the maturation/release of IL-1β/IL-18 when GSDME-N is produced by activated caspase-3. Additionally, the granzyme-mediated cleavage of GSDMB by natural killer/cytotoxic T lymphocytes can lead to tumor pyroptosis through pore formation activity [20, 21].
The GSDM family, consisting of GSDMA, GSDMB (absent in mice), GSDMC, GSDMD, GSDME (also known as DFNA5), and PJVK (Pejvakin, also known as DFNB59), comprises pore-forming proteins that can create pores on the plasma membrane and inner layer of organelles. These proteins play a crucial role in pyroptosis [22]. Notably, humans possess six homologous collateral genes within the GSDM family; triploid GSDMA (GSDMA1-3) and tetraploid GSDMC (GSDMC1-4) are present [23]. GSDM family members share 46% sequence homology. Except for PJVK, all contain pore-forming N-terminal and self-inhibiting C-terminal domains connected by a flexible linker. Full-length GSDM molecules cannot induce apoptosis. Active caspases shear and release their cytotoxic N-terminal domains, which can polymerize on the cell membrane to form pores and trigger pyroptosis [13, 19, 24].
As a suppressor of tumor growth, GSDME primarily inhibits tumors by facilitating pyroptosis [21]. GSDME is resistant to cleavage by caspases 1, 4, 6, 7, 8, and 9 but can be specifically cleaved by activated caspase-3. The activation of caspase-3 (19 kDa) results in the generation a fragment called GSDME-N (residues 1-270, weighing approximately 34 kDa). This GSDME-N fragment is crucial in mediating pyroptosis and promoting the secretion of IL-1β [25, 26]. By inducing apoptosis and pyroptosis through caspase-3 activation, GSDME determines the type of cell death. In human primary and tumor cells treated with chemotherapeutic drugs, cells expressing positive levels of GSDME undergo pyroptosis, while those lacking GSDME expression undergo apoptosis. Knocking down GSDME expression using RNA interference in cells that initially expressed it resulted in conversion from chemotherapy-induced pyroptosis to apoptosis [19]. Therefore, caspase-3 plays an important role during both apoptosis and pyroptosis involving biological functions mediated by substrate cleavage.
Numerous chemical target drugs, reagents, and natural products have been found to initiate GSDM family-induced pyroptosis in various malignant cancers. Small-molecule drugs, including traditional chemotherapy drugs and photodynamic agents, are among the primary initiators of pyroptosis. Their small molecular weight allows for easy loading into carriers for accurate and targeted delivery. Additionally, small molecules can form specific compounds, exhibiting unique chemical structures and biological activities, through covalent bonding with other molecules. Some chemotherapeutic drugs, such as metformin, docosahexaenoic acid, cisplatin, paclitaxel, arsenic trioxide, and doxorubicin, can trigger pyroptosis during cancer therapy through the involvement of GSDMD/GSDME-mediated pathways. Among them, disulfiram (DSF), a drug approved by the Food and Drug Administration (FDA) for the treatment of alcohol addiction, has been found to inhibit pyroptosis by blocking the formation of GSDMD pores [27]. However, these small molecules often face several delivery challenges, such as rapid clearance from the bloodstream, non-specific biodistribution, intracellular trafficking barriers, and systemic side effects caused by high drug doses. Emerging nanotechnology can effectively overcome these limitations and is promising to improve disease diagnosis and treatment specificity [28]. Nanotechnology is continuously transforming the world by harnessing the intricate principles, approaches, and methodologies established in nanomedicine to offer unparalleled prospects for altering or potentially revolutionizing our lifestyles [29, 30].
While the initial purpose of nanotechnology was to improve the delivery of diagnostic and therapeutic agents more efficiently and safely, researchers have now developed various nanomaterials and nanobiotechnologies that are specifically designed based on the characteristics and causes of lesions (Fig. 2). These advancements have demonstrated significant benefits in both disease diagnosis and treatment [31]. Furthermore, clinical applications have seen the development of nanodiagnostics and nanotherapeutics. Nanomaterials possess unique physicochemical properties that make them highly compatible with biological systems, allowing for excellent tumor accumulation and real-time feedback. This potential opens up possibilities for enhancing the stability and solubility of encapsulated cargoes, improving transport across membranes, and prolonging circulation time for increased safety and efficacy [32, 33]. By leveraging these advances, the limitations associated with small-molecule pyroptosis agents can be addressed while gaining insights into therapeutic mechanisms to facilitate precise treatment.
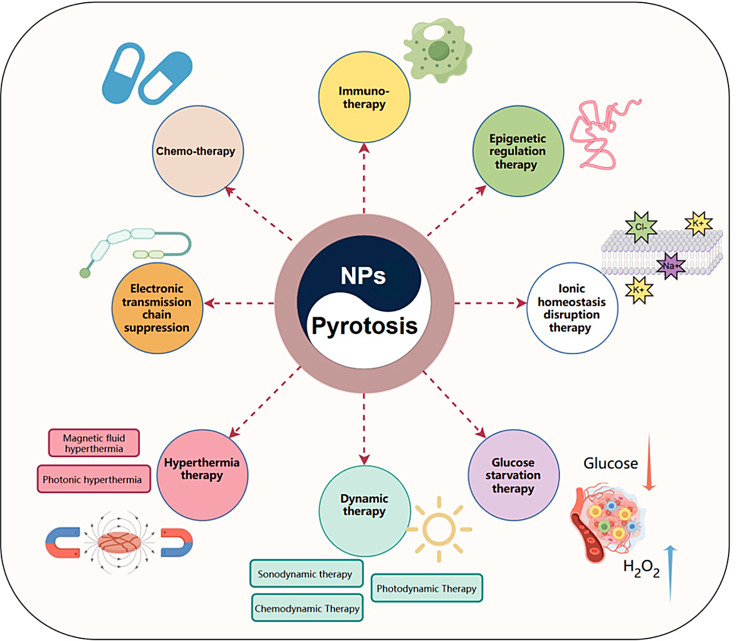
Fig. 2.
Schematic diagram of nanomaterial structures with different action modes on pyroptosis
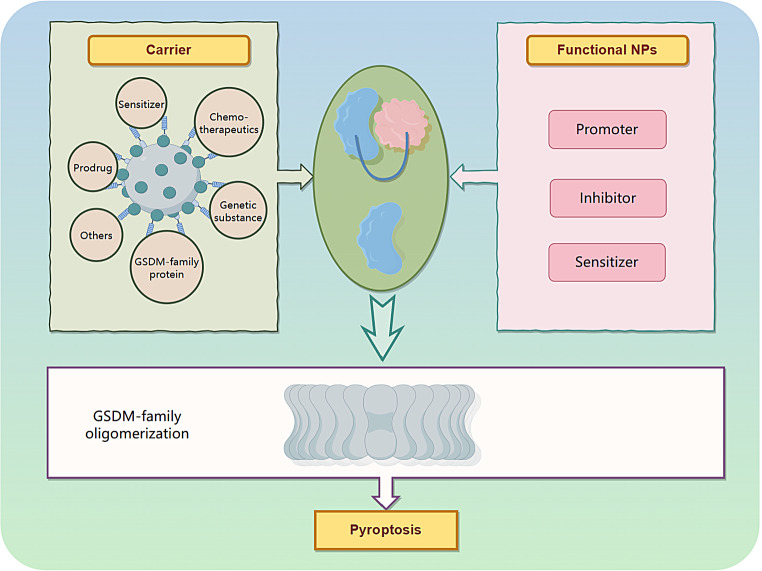
Nanocarriers have unique advantages in disease and cancer therapy, such as reduced drug toxicity, improved drug bioavailability and specificity, increased tumor accumulation via the enhanced permeability and retention effect, reduced non-specific reactions with proteins or the reticuloendothelial system, active tumor targeting through simple and appropriate modifications, and high clinical potential [18, 34, 35].
To overcome the challenges in delivering therapeutic agents, recent advancements in controlled synthesis strategies have been utilized to create complex architectures, incorporate bioresponsive components, and include targeting agents within nanoparticle (NP) designs [36, 37]. As a result, the utilization of NPs can be extended to more complex systems like nanocarrier-mediated combination therapies. These strategies aim to modify numerous pathways, optimize the efficacy of treatments against specific macromolecules, target particular stages of cell cycles, and overcome mechanisms that cause resistance to drugs. Nanobiotechnology offers possibilities for precise intracellular delivery and the retention of pro- or anti-pyroptosis agents by modifying carriers with appropriate ligands. Tailored nanobiology and nanomaterials have been specifically designed to address the requirements related to tumors and inflammatory conditions, aiming to enhance or inhibit pyroptosis. Various methods involving favorable internal conditions such as the TME, physiological state, exosomes, and external stimuli such as light or sound have been explored to develop different interference techniques or nanobiotechnologies (Fig. 3) [38, 39]. These methods enable the spatiotemporally controllable activation of pyroptosis to enhance precision and efficiency in anti-inflammatory and antitumor treatments [40]. By employing rational design principles, nanoplatforms loaded with pyroptotic drugs or functional agents regulated by pyroptosis are anticipated to induce or inhibit this process. Pyroptosis-engineered nanoplatforms can modulate caspase protein expression and alter the levels of pyroptotic proteins in vivo upon exposure to endogenous or exogenous triggers.
Fig. 3.
Schematic illustration of NPs combined with pyroptosis therapy
Therefore, this review aims to summarize and discuss the latest advancements in NP-based treatments with pyroptosis among inflammatory diseases and cancer, focusing on their functions and mechanisms (Table 1).
Table 1.
Summary of the discussed nanoparticle-mediated pyroptosis mechanisms
| Categories | Nanoparticles | Gasdermin | Cells | Mechanism | Refs |
|---|---|---|---|---|---|
| Deliver genetic substances | mRNA/LNPs | GSDMB | HEK 293, HeLa, 4T1, and B16F10-Luc cells | - | [41] |
| Plasmid expressing GSDME and MnCO/cRGD-modified liposome | GSDME | 4T1 cells | Caspase-3 | [42] | |
| ICG + GSDME plasmid DNA/ PLGA | GSDME | CT26 cells | Caspase-3 | [43] | |
| Deliver chemotherapeutics |
ICG + DAC/PLGA + cell membrane |
GSDME | 4T1 cells | Caspase-3 | [6] |
| DAC/LipoDDP | GSDME | 4T1 cells | Caspase-3 | [44] | |
| DAC/Bovine serum albumin NPs + IR820 + anti-CD11b | GSDME | 4T1 cells | Caspase-3 | [45] | |
| Deliver Prodrug | CyNH2/PEG-b-PLGA | GSDME | 4T1 cells | Caspase-3 | [46] |
| MPNPs/NI/mPEG-b-P(MTE-co-PDA) | GSDME | 4T1 cells | Caspase-3 | [47] | |
| PTX + P18/MCPP | GSDME | CT26 cells | Caspase-3 | [48] | |
| Deliver GSDM family protein | Phe-BF3/NP-GA3 | GSDMA3 | 4T1 cells | - | [8] |
| Deliver sensitizers | 17-AAG + Ce6/CANPs | GSDME | 4T1 cells | Caspase-3 | [49] |
| Fe3O4/MMSN-cRGD@Ce6 | GSDMD | MDA-MB-231 cells | Caspase-1, Caspase-3 | [50] | |
| Ce6 + TPZ/ZTC@M | GSDMD | AGS cells | Caspase-1 | [51] | |
| Deliver others | Tea polyphenols/TPNs | GSDMD | HEK293T Cells | - | [52] |
|
GOx/ROS-responsive polyion complex |
- | 4T1 cells |
Oxidative DNA damage |
[53] | |
| OA@IR820 | GSDMD | B16 cells | Caspase-1, Caspase-3 | [54] | |
| RGD-OA@FeMOF NPs | GSDME | B16 cells | Caspase-3 | [55] | |
| Tf-LipoMof@PL | GSDMD | 4T1 cells | Caspase-1 | [56] | |
| Promotors | NaHCO3 NPs | GSDMD | 4T1 cells | Caspase 1 | [57] |
| MIL-100(Fe) MOF/DOPC | GSDMD | Hela cells | Caspase-3 | [38] | |
| CaNMs | GSDME | 4T1 cells | Caspase-3 | [58] | |
| GOx-Mn | GSDMD | 4T1 cells | Caspase 1 | [59] | |
| LCO | GSDMD | H838 cells | Caspase-1 | [60] | |
| As2O3 NPs | GSDME | Huh7 cells | Caspase-3 | [61] | |
| CB NPs | - | RAW264.7 cells | Caspase-1 | [62] | |
| Inhibitors | PBzyme | GSDMD | BV2 cells | Caspase-1 | [63] |
| tFNAs | GSDMD | HaCaT cells | Caspase-1 | [64] | |
| Sensitizers | Au@AgBiS2 | GSDME | 4T1 cells | Caspase-3 | [65] |
Carriers
Significant progress has been made in studying chemotherapy-induced pyroptosis in tumor. However, several challenges need to be overcome before these drugs can effectively reach the tumor site, including drug insolubility, rapid clearance from the bloodstream, non-specific biodistribution, and systemic side effects caused by high drug doses. To broaden the scope of pyroptosis-related drugs, more studies are exploring nano-delivery systems (NDS) to mitigate drug toxicity and achieve efficient drug accumulation in tumors through targeted modifications [41]. This approach improves drug solubility and potency, enhancing the efficacy of pyroptosis in killing cancer cells or inhibiting pyroptosis to alleviate inflammatory diseases. Owing to their ability to improve drug pharmacokinetics, enable tumor-targeted delivery, and facilitate site-directed drug release in response to the TME, NDS has been used to deliver chemotherapeutics and immunotherapeutics to maximize their therapeutic effects. Consequently, NDS offers advantages that make the targeted induction of tumor pyroptosis more feasible. Therefore, combining these two approaches represents a promising strategy for cancer treatment.
Delivery of genetic substances
The involvement of the GSDM family is crucial in the occurrence of pyroptosis. Following cleavage, the N-terminal region can initiate pyroptosis. However, numerous cancer types exhibit reduced levels of GSDM expression and encounter challenges regarding the intricate process of cleavage, impeding the release of proteases that trigger pyroptosis and hindering its antitumor immune response. Taking GSDME as an example, its function is deficient in tumor tissues owing to promoter methylation, lack of gene expression, or gene mutations. Therefore, importing exogenous genetic material, such as DNA and RNA, to improve the expression levels of the GSDM family loaded with NPs is an effective strategy for inducing pyroptosis.
Li et al. developed an innovative approach utilizing mRNA-based nanomedicine, where the AA3-Dlin lipid NPs (LNPs) formulation acts as a carrier with exceptional safety and high efficacy in translating mRNA in laboratory settings and living organisms [42]. This formulation encapsulates GSDMB-NT mRNA responsible for encoding the N-terminal domain of GSDMB within LNPs formed by combining AA3-Dlin, phospholipid (DOPE), cholesterol, and polyethylene glycol (PEG). Incorporating electrostatic interactions facilitates the encapsulation of GSDMB-NT mRNA within LNPs. The findings indicate that utilizing mRNA/LNPs to induce pyroptosis can transform “cold” tumors into “hot” ones, establishing a positive feedback loop to enhance the immune response against tumors in female mouse models. Furthermore, activating pyroptosis through mRNA/LNPs sensitizes tumors to anti-programmed cell death 1 (PD-1) immunotherapy, suppressing tumor growth. Notably, this LNP formulation can be directly administered at the tumor site, triggering pyroptosis without requiring protease cleavage for activation.
Through investigating targeted therapy for triple-negative breast cancer, Zhong et al. devised a PEG-cyclic RGDfK (cRGD)-modified liposome with a positive charge that efficiently transported a plasmid expressing GSDME and manganese carbonyl (MnCO) into 4T1 cells [43]. The liposomal surface was enveloped by a PEG chain attached to cRGD targeting peptides. This nanodrug exhibited enhanced stability and specifically accumulated at the tumor site, upregulating GSDME in tumors. Within the 4T1 cells, encapsulated MnCO decomposed into Mn2+ and CO upon exposure to high levels of endogenous H2O2. Consequently, caspase-3 activation occurred, resulting in the cleavage of full-length GSDME and pyroptosis induction. Pyroptosis led to the release of significant amounts of DAMPs. In addition, the presence of Mn2+ enhanced the immune response against tumors by activating both cyclic GMP-AMP synthase and stimulating the interferon gene pathway in dendritic cells that infiltrate the TME. These combined effects suppressed the growth of breast tumors, whether they were located orthotopically or had spread to the liver.
Jiang et al. developed a lipid-coated poly(lactic-co-glycolic acid) (PLGA) NPs containing the photosensitizer indocyanine green (ICG) and heat-inducible mHSP70 promoter-regulated GSDME plasmid DNA to achieve the specific expression of the pyroptosis-implementing protein GSDME in tumors [44]. By utilizing the photothermal effect induced by the photosensitizer, GSDME plasmid DNA expression was enhanced by using a heat-inducible HSP70 promoter. This allowed for the temperature-dependent activation of GSDME expression at the tumor site when exposed to local irradiation mediated by ICG. In this manner, oxaliplatin, a chemotherapeutic agent, initiated caspase-3 and cleaved GSDME-induced pyroptosis, specifically within tumors. Consequently, DAMPs were released, and adaptive immunity was boosted without harming normal tissues. This approach successfully achieved controlled spatiotemporal GSDME expression in tumors to avoid damage to normal tissues.
Delivery of chemotherapeutics
Pyroptosis is induced by chemotherapeutics such as cisplatin, doxorubicin, simvastatin and paclitaxel, which have limited biomedical applications owing to drug resistance and severe side effects [19, 45, 46]. Many chemotherapeutics activate caspase-3-mediated apoptosis to kill tumors, meaning chemotherapy can trigger pyroptosis based on GSDME and an immunological response [47]. However, the primary reason for the lack of GSDME in caspase-3-induced pyroptosis is the excessive methylation of GSDME in most malignant cells [48].
Epigenetic therapy, specifically DNA demethylation, is important for treating DNA hypermethylation in tumors. In brief, the deregulation of methylated DNA modifications and the restoration of normal functional protein expression can be achieved by inhibiting DNA methyltransferase (DNMT). Decitabine (DAC) is a widely used DNMT inhibitor that treats hematological malignancies. DAC also enhances the expression of methylated genes, facilitating GSDME expression for caspase-3 cleavage. This ultimately leads to potent cancer pyroptosis through demethylation within tumors [49].
Zhao et al. developed a novel approach for cancer treatment using biomimetic NPs (BNPs) loaded with ICG and DAC, designed to have minimal systemic toxicity by incorporating a breast cancer cell membrane onto a PLGA polymer core [6]. By leveraging the tumor-homing properties and low immunogenicity of the cancer cell membrane, the BNPs effectively accumulate in solid tumors. Upon exposure to low-dose near-infrared (NIR) light, which induces local hyperthermia in the particles, cytochrome c is released, followed by caspase-3 activation. The presence of ICG within the BNP-punctured cancer cell membranes rapidly increases the cytoplasmic Ca2+ concentration. Additionally, DAC enhances GSDME expression by inhibiting DNA methylation, promoting caspase-3 cleavage, and inducing cancer pyroptosis. Ultimately, this photoactivated pyroptosis mediated by BNPs triggers systemic antitumor immunity that can inhibit both primary and distant tumors.
Fan et al. proposed a novel approach to enhance the immunological impact of chemotherapy by combining DAC with chemotherapeutic nanodrugs, resulting in pyroptosis induction in tumors through epigenetic mechanisms [48]. To achieve this, DAC was specifically targeted to tumor-bearing mice, upregulating DFNA5 and demethylating DFNA5 in tumors overexpressing GSDME. Chemotherapeutics were delivered into tumor regions using a commonly used tumor-targeting nanoliposome loaded with cisplatin (LipoDDP), activating the caspase-3 pathway and triggering pyroptosis within the tumors. This process resulted in the rapid release of various intracellular components such as IL-1β, HMGB1, and tumor antigens, leading to robust inflammation and the activation of antigen-specific T cells. Both in vitro and in vivo, this combined strategy elicits a potent immune response capable of preventing tumor metastasis and recurrence.
Yu et al. developed a nano-vehicle with stealth capabilities, utilizing neutrophil camouflage to achieve precise delivery and tumor immunotherapy by inducing pyroptosis with minimal toxicity from nanomaterials [50]. The nano-vehicle consisted of bovine serum albumin NPs conjugated with IR820 and anti-CD11b, loaded with DAC for drug storage. By disguising themselves as neutrophils, the nano-vehicles efficiently delivered the drugs to tumors through their natural affinity for cancer cells. A fluorescent signaling molecule, IR820, was used as a navigation tracker to monitor the delivery of the nano-vehicle. Upon systemic administration, activated neutrophils in the bloodstream were targeted by anti-CD11b to capture the nano-vehicles. Once the nano-vehicle reached the tumor site, the photothermal control system facilitated its release from the cellular carrier using IR820. Consequently, DAC was released, and GSDME expression was increased while laser irradiation activated caspase-3. This resulted in pyroptosis and enhanced the adaptive immune response within the system. Using this approach, the immunosuppressive microenvironment was effectively modulated to enhance the efficacy of tumor immunotherapy and inhibit metastasis to the lungs.
Prodrug delivery
Currently, the induction of pyroptosis is limited to a few cytotoxic drugs and photosensitizers. Insufficient water solubility, restricted tumor-penetration capability, and limited bioavailability challenge the efficacy of small molecules in triggering pyroptosis. Administering these drugs systemically leads to severe toxicity as it triggers pyroptosis in normal cells. Fortunately, advancements in nano-prodrugs combined with alternative catalysts have addressed this issue. Prodrugs are modified compounds that are biologically inactive but can be converted into active components within the body. They offer improved solubility, reduced adverse effects, enhanced chemical stability, and easier uptake by cells compared to their original drug counterparts [51].
Wang et al. developed a theranostic agent called NCyNH2, designed to target cancer cells that overexpress the enzyme NAD(P)H: quinone oxidoreductase isozyme 1 (NQO1). This enhanced the selectivity of amin-containing hemicyanine fluorophore (CyNH2) towards tumors, as NQO1 is substantially overexpressed in various cancer cells and remains non-fluorescent and minimally toxic until activated [52]. To facilitate systemic administration and the real-time detection of CyNH2 activation, NCyNH2 was encapsulated within an FDA-approved amphiphilic polymer known as PEG-b-PLGA. The fluorescence imaging system enabled the monitoring of CyNH2 activation in vivo. Additionally, combining NCyNH2 with the immune checkpoint inhibitor αPD-1 proved effective in inhibiting tumor growth and achieving long-term memory efficacy.
Acting as a prodrug, NCyNH2 exhibits a high specificity for cancer cells that overexpress NQO1. The real-time monitoring of CyNH2 activation can be achieved by observing changes in its near-infrared (NIR) fluorescence signal. Prior to activation, intramolecular charge transfer causes the fluorescence of CyNH2 to remain off. However, upon encountering elevated levels of intracellular NQO1 within cancer cells, the NIR fluorescence signal becomes activated and restores the fluorescence state of CyNH2. This selective reactivation initiates pyroptosis, specifically within cancer cells. By selectively accumulating within energized mitochondria, CyNH2 damages mitochondrial membranes, releasing cytochrome c into the cytoplasm and subsequently activating caspase-3. Activated caspase-3 then cleaves GSDME, producing a GSDME-N fragment capable of perforating cell membranes and inducing pyroptosis. This novel fluorogenic NIR dye is promising as a theranostic agent for selectively inducing tumor pyroptosis while exhibiting potent memory effects against tumor recurrence.
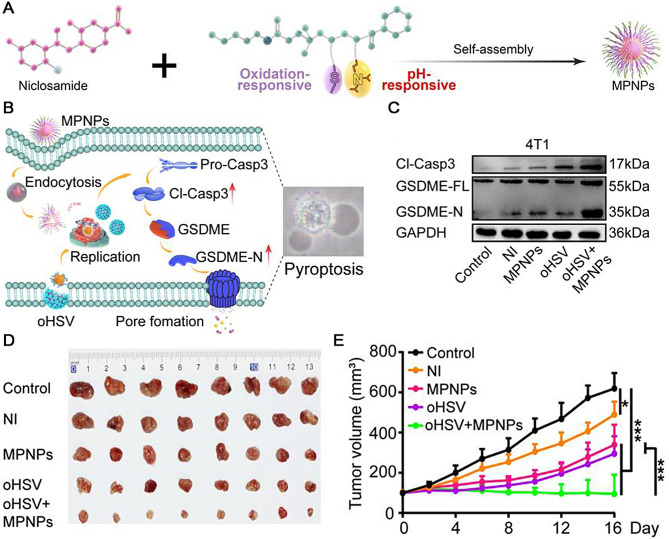
The combination of oncolytic viruses (OVs) and nano-prodrugs has been reported as a therapeutic strategy to target solid tumors [53]. The therapy using oncolytic herpes simplex virus 1 induces pyroptosis synergistically and enhances immunotherapy. To accomplish this, Su et al. utilized modified prodrug known as magnetic polymer NPs (MPNPs) that were stably loaded through self-assembly with the aid of niclosamide (NI), an inhibitor responsive to both reactive oxygen species (ROS) and pH signals capable of activating transcription 3 (STAT3). By employing reversible addition-fragmentation chain transfer (RAFT) polymerization, a nanocarrier called mPEG-b-P(MTE-co-PDA) was successfully constructed. Given the overexpression of STAT3 in tumors and myeloid-derived suppressor cells (MDSCs), the nanocarrier is crucial in maintaining tumor stemness, facilitating the formation of pre-metastatic niches, evading immune responses, and promoting the expansion and recruitment of MDSCs. MPNPs considerably accumulated within tumors while concurrently reducing tumor cell stemness and enhancing immune responses against tumors. By utilizing MPNPs, the systemic toxicity was minimized compared to that of traditional non-targeted oral NI, which significantly reduced the adverse off-target reactions. Additionally, when Ovs (particularly oHSV)were applied simultaneously along with MPNPs, they reduced the tumor burden and induced ROS production, resulting in higher tumor penetration capacity for MPNPs and significant GSDME-mediated pyroptosis (Fig. 4A, B). In the in vitro experiments, the levels of caspase-3 cleavage and GSDME-N in the oHSV + MPNPs group were higher than those in the control group, as detected using western blot (Fig. 4C). In 4T1 tumor-bearing mice, treatment with oHSV + MPNPs showed the most pronounced antitumor effect (Fig. 4D, E). This approach overcomes obstacles presented by dense solid tumors, enabling precise intratumor drug penetration and efficient T cell infiltration. As a result, pyroptosis is triggered, enhancing tumor immunogenicity and promoting adaptive immune responses that generate a significant T cell-dependent antitumor immune memory effect against recurrence and pulmonary metastasis.
Fig. 4.
(A) Schematic representation of MPNP synthesis process. (B) Schematic illustration of mechanism underlying treatment involving oHSV combined with MPNPs to induce tumor cell pyroptosis. (C) Western blots of pyroptosis-related protein expression. (D) Images of 4T1 tumors after different treatments. (E) 4T1 tumor volumes in different groups (n = 8). Reproduced with permission [53]. Copyright 2023, Wiley-VCH
Xiao et al. developed a nano-prodrug (MCPP) that responds to two stimuli and contains a substantial amount of paclitaxel (PTX) and the photosensitizer purpurin 18 (P18). This innovative nano-prodrug enables drug visualization [54]. A drug-delivery system responsive to ROS was synthesized through RAFT polymerization of a thioether functional monomer, facilitating controlled drug release. The NPs were utilized for the co-encapsulation of PTX-SS-PTX (SPTX), which is sensitive to glutathione (GSH), together with disulfate linker and P18 photosensitizer methoxy polyethylene glycols-4-cyano-4-(phenylcarbonothioylthio) pentanoic acid block-P(M4)@SPTX/P18, MPEG-CPPA-b-P(M4)@SPTX/P18, MCPP, serving as the inner core of the NPs. These self-assembled chemo-photodynamic NPs exhibit high drug-loading capacity, controlled release in the TME, deep penetration into tumors, a significant ability to induce pyroptosis, and minimal systemic side effects.
The ROS/GSH system, which responds to dual stimuli, enables a targeted response within the TME and the optimal release of drugs in tumors. By utilizing laser irradiation, P18 triggers the generation of ROS, leading to the controlled release of PTX through chemo-photodynamic therapy (PDT). The pyroptotic tumor cells then release DAMPs, initiating adaptive immunity and enhancing the efficiency of immune checkpoint blockades. This process promotes tumor regression, generates immunological memory, and prevents tumor recurrence. Through a synergistic effect between chemo-PDT and controlled-release PTX, GSDME-related pyroptosis is induced. Using MCPP-triggered chemo-PDT presents an innovative approach for treating tumors while also having the potential as an immune adjuvant to enhance anti-PD-1 efficacy due to its remarkable ability to induce pyroptosis.
Delivery of GSDM family proteins
The GSDM family of proteins is the major effector of pyroptosis with membrane pore-forming activity. The pore-forming N-terminal domain (N-domain) of GSDM translocates to the plasma membrane and induces pyroptosis. Therefore, pyroptosis can be induced by activating intrinsic GSDM family proteins in tumors. Simultaneously, GSDMs can be directly delivered via the NDS.
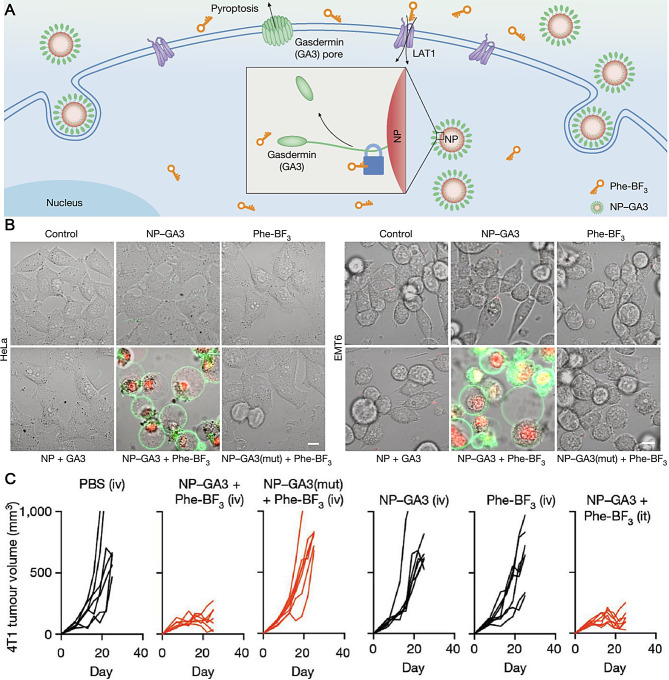
Wang and colleagues devised a bioorthogonal chemical system that employs a cancer imaging probe known as phenylalanine trifluoroborate (Phe-BF3) [8]. This probe can penetrate cells and selectively release a client protein, such as an active GSDM, from an NP conjugate into tumors in mice. By introducing mutations to specific cysteine residues within the C domain of GSDM, only the N domain of GSDM was utilized for attachment to 60 nm NPs via a triethylsilyl ether linker, resulting in the formation of NP-GSDMA3 (GA3). Upon exposure to Phe-BF3, GA3 (N + C) was liberated from NP-GA3 and triggered pyroptosis by creating pores on liposome membranes (Fig. 5A). Remarkably, inducing pyroptosis in less than 15% of the tumors effectively eliminated the 4T1 breast tumor graft. When HeLa or EMT6 cells were treated with NP-GA3 and Phe-BF3, they exhibited a classical pyroptotic morphology (Fig. 5B). Additionally, treatment with NP-GA3 and Phe-BF3 caused tumor shrinkage in the 4T1 tumor model (Fig. 5C). These results strongly confirm the antitumor effect caused by GA3 released from the NPs. This innovative approach of directly activating GSDM through desilylation mediated by Phe-BF3 aids the understanding of antitumor immunity stimulated by ICD-induced pyroptosis.
Fig. 5.
(A) Schematic illustration of NP-GA3 for inducing pyroptotic therapy. (B) HeLa and EMT6 were treated as indicated. Scale bars: 20 μm. (C) Tumor volume curves in 4T-1 tumor-bearing mice treated with different groups. Reproduced with permission [8]. Copyright 2020, Springer Nature
Delivery of sensitizers
In addition to conventional pyroptosis enhancers or nanomaterials, several emerging nanostructures facilitate novel anticancer treatments that trigger extensive tumor pyroptosis and substantial immune reactions. Consequently, this results in tumor regression and prevents tumor metastasis or recurrence. PDT and sonodynamic therapy (SDT) are novel tumor treatment methods. Compared with radiotherapy, chemotherapy, and conventional surgery, PDT/SDT has better selectivity and effectiveness and fewer side effects. They can effectively relieve patients’ pain and improve their quality of life to cope with skin, esophageal, bronchial, and bladder cancers, especially for those with advanced cancers that do not respond to radiotherapy and chemotherapy. Therefore, combining the targeting effects of photosensitizers and sonosensitizers with pyroptosis to suppress tumor growth has become important.
Photosensitizer
PDT kills tumors using photosensitizers that produce many ROS when exposed to a specific laser wavelength. Photosensitizers transfer absorbed laser energy to molecules that cannot absorb light energy and promote the occurrence of photoreactions but do not participate in the reaction, only acting as carriers of energy transfer [55]. Photosensitizers are divided into three generations: the first is dominated by hematoporphyrin derivatives. The second is dominated by porphyrins and chlorins, and the third is based on second-generation photosensitizers and combines substances with biological properties to improve the targeting of PDT.
The application of nanomaterials in the biomedical field has been constantly expanding. Nanomaterials, which can easily combine with many substances to form complexes, can increase the specific surface areas of photosensitizers and improve their chemical activities. Chlorine, a degradation product of chlorophyll a, has a significant light-absorption capacity and can be used as a good photosensitizer. The maximum energy absorption range of chlorine is between 650 nm and 700 nm, where it can produce a large amount of singlet oxygen to induce pyroptosis [56].
Zhou and colleagues have successfully developed a novel nanodrug (CANPs) that effectively targets tumors. This innovative drug combines HSP90 inhibitor tanespimycin (17-AAG) with the photosensitizer chlorin e6 (Ce6) [57]. To achieve the controlled activation of the prodrug in response to GSH, which is abundant in the TMEs, a covalent crosslinking strategy was employed. Importantly, this approach eliminates the need for additional agents to induce pyroptosis. Utilizing laser irradiation at 660 nm, Ce6 generates ROS, resulting in ROS-mediated pyroptosis and subsequent tumor cell death through PDT. Additionally, including 17-AAG enhances PDT efficacy by facilitating caspase-9 processing, activating caspase-3, and promoting ROS accumulation. Notably, it also sensitizes tumors to anti-PD-1 therapy while simultaneously reducing MDSCs. The CANP system efficiently delivers both Ce6 and 17-AAG to tumor sites due to their specific accumulation within tumors. Once released from the NPs, Ce6 and 17-AAG generate ROS upon laser stimulation, triggering GSDME-mediated pyroptosis. This strategy also significantly improves drug-loading capacity, tumor targeting, penetration depth, and overall drug accumulation within tumors. Inducing targeted pyroptosis in tumors using this prodrug approach inhibits tumor growth while enhancing responses to anti-PD-1 therapy and prolonging survival in mice bearing 4T1 mammary tumors.
Chen et al. developed a theranostic agent called PETA by modifying Fe3O4-embedded magnetic mesoporous silica NPs (MMSN) with cRGD and engineering pyroptosis, which accommodated Ce6 photosensitizers (MMSN-cRGD@Ce6), resulting in increased ROS levels, pyroptosis induction, and malignancy suppression through Ce6-mediated photodynamic processes [58]. The tumor-targeting polypeptide ligand cRGD, which binds to integrin αVβ3 highly expressed on tumors, facilitated the accumulation of PETAs in tumors and regressed malignancy through pyroptosis. In addition, Fe3O4 facilitated precise tumor localization using contrast-enhanced magnetic resonance and particle-imaging techniques, making it suitable for targeted diagnosis and prognostic evaluation. The integration of ultrafine Fe3O4 NPs within mesoporous silica NPs produced hydroxyl radicals and released oxygen via a chemodynamic process activated by the Fenton reaction. The released oxygen alleviated hypoxia and generated ROS, as hypoxia significantly inhibits ROS production. Moreover, the photodynamic process mediated by Ce6 with a 660 nm laser generated singlet oxygen ROS that enhanced the chemodynamic process. By mitigating hypoxia and inducing ROS production, pyroptosis pathways were also activated, overcoming resistance to apoptosis caused by hypoxia and maximizing therapeutic outcomes against breast tumors both in laboratory settings and animal models. This occurred through the increased accumulation of intratumoral ROS via synergistic effects between the photodynamic process, Fe3O4-mediated nanocatalytic medicine, and concomitant mechanisms enhancing O2 release to alleviate hypoxia.
Sonosensitizers
SDT has gained significant popularity in cancer research due to its potential as a noninvasive therapeutic approach. SDT generates ROS using acoustic sensitizers via the ultrasonic stimulation of acoustic apertures, sonochemistry, and sonoluminescence [59]. Effective acoustic sensitizers are important prerequisites for SDT. Although SDT is superior and has good application prospects, ROS production is limited by the insufficient concentration of O2 in the TME, which leads to decreased ROS yield and unsatisfactory SDT treatment effects. This key scientific issue must be addressed when using SDT to treat tumors. Organic acoustic sensitizers, mainly porphyrins and related derivatives, usually derived from photosensitizers, were applied early in this field.
Ce6 exhibits low toxicity as an organic sonosensitizer that selectively accumulates within tumor tissues while being efficiently eliminated from organisms. It possesses remarkable sensitivity and ROS generation capabilities [60]. Activating Ce6 through light or ultrasound leads inhibits tumor growth [61]. Nevertheless, its hydrophobic nature poses challenges as it can aggregate into larger crystals, impeding its optimal functionality. Moreover, prolonged circulation may result in rapid clearance and degradation. Henceforth, exploring suitable delivery strategies for achieving the desired antitumor effect of Ce6 remains crucial.
Yu et al. developed a pH-responsive and ultrasound-activated nanoplatform (ZTC@M) using ZIF-8. This nanoplatform was loaded with hydrophobic Ce6 and hydrophilic tirapazamine (TPZ) and then coated with the tumor cytomembrane of gastric cancer cells to enhance its targeting ability for improved synergistic sonodynamic chemotherapy [62]. The biomimetic properties of ZTC@M enable precise delivery. The ZTC@M NPs exhibited excellent biocompatibility, targeted delivery, and therapeutic efficacy. Upon ultrasound irradiation, activated Ce6 selectively released ROS to effectively eliminate tumors while exacerbating hypoxia in the TME. This enhanced activation of TPZ resulted in efficient tumor eradication and potentiated chemotherapeutic effects, augmenting overall therapeutic outcomes. The NLRP3 inflammasome was activated in AGS cells when exposed to ZTC@M and ultrasound, activating caspase-1. As a result, GSDMD and IL-1β were cleaved by the activated caspase-1, generating GSDMD-N and mature IL-1β, respectively. This process ultimately triggered inflammatory PCD. These findings imply that ultrasound, along with ZTC@M, can trigger pyroptosis in AGS cells, enabling its potential antitumor effect.
Delivery of other substances
Tea polyphenols
Tea polyphenols, also known as tea tannins, are the most important and characteristic chemical constituents of tea, accounting for 18–36% of its dry mass. Tea polyphenols have multiple effects on tumors, including anti-oxidation, scavenging free radicals, inhibiting the formation of nitroso groups, regulating the expression of key enzymes and related genes in the carcinogenic process, inhibiting information transfer between cancer cells, inhibiting cell proliferation, killing tumor cells, inducing tumor cell apoptosis, inhibiting telomerase activity, increasing anticancer drug sensitivity, and inhibiting tumor cell angiogenesis.
Chen et al. successfully created self-assembled NPs (TPNs) using tea polyphenols [63]. These TPNs show great potential as therapeutic candidates for treating endotoxin-induced sepsis. They eliminate ROS and nitrogen species (RONS) while inhibiting pyroptosis, demonstrating a dual mechanism of action. The RONS-scavenging activities of TPNs have been observed both in solution and within cells. Using epigallocatechin-3-gallate as the starting material, TPNs with a wide range of RONS-scavenging activity were prepared through a simple polymerization and self-assembly process. The ability to scavenge RONS is attributed to the structure derived from polyphenols, whereas the inhibition of pyroptosis is achieved through blocking GSDMD, which plays a crucial role in pore formation and membrane rupture. This highlights the multifunctionality of sepsis therapy provided by TPNs.
Significantly, mechanistic investigations have demonstrated that TPNs exhibit a potent ability to hinder pyroptosis by impeding the oligomerization of GSDMD-NT. This attribute complements their capacity to scavenge RONS and enhances the efficacy of sepsis treatment through synergistic effects. In vivo, TPNs offer significant protection against lethal sepsis induced by endotoxins in mice, leading to improvements in hypothermia, reducing pro-inflammatory cytokine production and oxidative damage, and preventing multi-organ failure. Notably, TPNs exhibited excellent therapeutic efficacy in a mouse model of sepsis with enhanced survival rates, the alleviation of hypothermia symptoms, and protection against organ damage.
Glucose oxidase (GOx)
A highly desirable endogenous oxidoreductase for cancer starvation therapy, GOx can interact with glucose and oxygen within cells, producing H2O2 and gluconic acid. This process deprives cancer cells of their essential nutrients, inhibiting their growth and proliferation [64, 65]. This reaction can also increase acidity and worsen hypoxia, further regulating the TME. Therefore, designing a multifunctional nanocomposite based on GOx for the multimodal treatment of tumors is possible. According to the specific differences between the tumor and normal physiological environments, designing a multimodal intelligent release-targeted drug-treatment system can realize the mutual coordination and promotion of multiple single treatment modes. Using GOx with biocompatible inorganic silicon materials can safely deliver drugs to the tumor site and trigger multimodal therapy that can treat cancer.
A general method was suggested by Li et al. for the production of responsive nanoreactors utilizing polyion complex vesicles (PICsomes) that contained a covalently crosslinked membrane network with thioketal linkers. These PICsomes were utilized to encapsulate GOx [66]. The resulting ROS-responsive nanoreactor exhibited self-sustaining catalytic glucose oxidation activity, preserving the long-term functionality of GOx, and achieving cytotoxicity by inducing oxidative stress and glucose deprivation. This effect was attributed to the stimuli-responsive expansion of the vesicles without rupture and selective cargo release based on size. Upon entering the tumor, the loaded GOx decomposed intratumoral glucose into H2O2, leading to energy and nutrient depletion within the TME. Notably, the accumulated H2O2 triggered the swelling of PICsomes through interaction with thioketal linkers, enabling the controlled release of GOx while maintaining structural integrity. Furthermore, the presence of H2O2 and glucose deprivation in stimulation-induced reactions increased the generation of ROS and heightened the permeability of PICsomes. This increased ROS generation within cells, ultimately activating the pyroptosis signaling pathways characterized by the formation of large bubbles on cell membranes, morphological changes in cells, and the upregulation of calcium network proteins and HMGB1 expression as indicative markers for tumor pyroptosis initiation. By harnessing their inherent ability for self-enhancing catalytic ROS production along with sustained cytocidal function over time, GOx-loaded PICsomes are promising therapeutic nanoreactors capable of improving cancer treatment by inducing oxidative stress and glucose deprivation.
Oligomycin A (OA)
The mitochondrial electron transport chain (ETC) is the primary generator of ROS in living organisms. The premature release of high-energy electrons from complexes I and III leads to the formation of highly oxidative superoxide anion radicals when they react with intracellular oxygen. Additionally, by inhibiting downstream ATPases, it is possible to reduce ATP synthesis while slowing down electron flow within the ETC. This increases the likelihood of electron leakage and the accumulation of bursts of ROS [67]. By acting as a mitochondrial respiratory inhibitor, OA can hinder ATP synthase activity and impede the flow of electrons within the ETC. This leads to the premature interaction between high-energy electrons and intracellular O2, ultimately causing significant oxidative stress at an intracellular level.
Ji et al. developed a pyroptosis nanoagonist, OA@IR820, which is biocompatible and specifically targets mitochondria. This nanoagonist was designed to treat malignant melanoma by combining OA (an inhibitor of ATP synthase) and IR820 through self-assembly [68]. Upon exposure to NIR laser, IR820 undergoes disassembly and releases OA. This inhibits ATP synthesis in the ETC and disrupts mitochondrial function. The prepared OA@IR820 nanoagonists exhibit exceptional biocompatibility, water solubility, high drug-loading capacity, and strong absorption in the NIR range. These nanoagonists disintegrate upon NIR stimulation at the tumor site, inducing pyroptosis while also providing mild photothermal therapy (PTT) to eliminate malignant melanoma cells. This process induces electron leakage within mitochondria and triggers the rapid release of ROS, activating downstream signaling pathways associated with pyroptosis. In the group treated with OA@IR820 and NIR irradiation, a significant increase was observed in the expression levels of NLRP3, cleaved caspase-1, GSDMD-N, cleaved caspase-3, and IL-1β. This suggests that pyroptosis occurs along with the potential activation of antitumor immunity. Western blot analysis demonstrates that this mechanism primarily relies on pyroptosis for its antitumor effects in vivo. The assembled nanoagonist OA@IR820 actively induces oxidative-stress-mediated pyroptosis as a therapeutic approach against malignant tumors in vitro and in vivo. This approach holds promise as a strategy for cancer therapy while minimizing adverse effects on healthy tissues or organs.
Wang et al. developed iron-based metal-organic framework particles (FeMOF) decorated with cyclic arginine-glycine-aspartic acid peptides that specifically target the αvβ3 integrin overexpressed on melanoma cell membranes [69]. These NPs were then loaded with OA, a compound known for its ability to disrupt mitochondrial function and induce oxidative stress within cells. This resulted in the formation of RGD-OA@FeMOF NPs (FeOA). FeMOF acted as a targeted drug delivery system that could be selectively degraded under conditions found in tumors, releasing iron species for nanocatalytic reactions and OA to inhibit mitochondrial respiration within cancer cells. The FeOA NPs induced melanoma-specific oxidative stress and pyroptosis while also enhancing the effectiveness of immunotherapy through PD-L1 immune checkpoint blockades. Molecular studies revealed that this cell death switch was triggered by the caspase-3-mediated GSDME cleavage pathway. In vivo experiments using subcutaneous tumor and lung metastasis models confirmed that FeOA NPs induce pyroptosis and enhance immunotherapy against melanoma, highlighting their potential for treating malignant tumors.
Piperlongamide (PL)
Unlike exogenous trigger-motivated PDT and SDT, the concept of catalytic drug therapy (CDT) is based on the decomposition of intratumoral H2O2 to ROS. This process is facilitated by transition metal nanomaterials in an acidic TME through Fenton or Fenton-like reactions, leading to tumor cell death [70]. CDT does not rely on endogenous oxygen levels, allowing it to target hypoxic solid tumors regardless of their depth. PL, a natural alkaloid derived from plants belonging to the Piperaceae family, exhibits potent cytotoxic effects against various malignant tumors, including colon, breast, liver, and ovarian cancers, while sparing normal cells.
Xu et al. developed a novel nanodrug called Tf-LipoMof@PL, which combines a pH-sensitive lipid coated with transferrin and a PL-loaded MOF. This nanodrug aims to achieve anticancer effects by inducing ferroptosis/pyroptosis [71]. Iron levels were significantly increased due to the combined effects of an iron-containing MOF and transferrin-mediated iron endocytosis. Additionally, an enhanced Fenton reaction resulted in a synergistic increase in ROS, facilitated by the high amount of H2O2 provided by PL (a potent inducer of ferroptosis) and elevated intracellular iron levels. Ferroptosis can be further induced by PL as it downregulates GSH, leading to the depletion of glutathione peroxidase 4 (GPX4). This deterioration impairs the cellular ability to reduce lipid peroxidation, resulting in the accumulation of lipid peroxide (LPO). In vitro and in vivo experiments using Tf-LipoMof@PL demonstrated the successful induction of both ferroptosis and pyroptosis. Ferroptosis was confirmed by GPX4 depletion and the elevation of LPO levels, while pyroptosis was evidenced by the increased cleavage of GSDMD and the elevated IL-1β and lactate dehydrogenase (LDH) levels. These findings highlight the potential efficacy and future prospects for dual-inductive nanodrugs as promising modalities for cancer treatment with superior performance capabilities.
Functional particles
Nanomaterials can activate or inhibit the pyroptotic pathway, highlighting their intricate associations with pyroptosis. Furthermore, nanomaterials can act as sensitizers with other chemoradiotherapy approaches to enhance antitumor immune responses via pyroptosis. Consequently, the assessment of the biosafety of nanomaterials and the advancement of nanomedicine development substantially rely on pyroptosis, which serves as a vital indicator. Exploring the connection between pyroptosis and nanomaterials could potentially lead to improved treatments for tumors or inflammatory diseases.
Promotors
Regarding tumor treatment, pyroptosis can enhance antitumor immune activation and improve therapeutic outcomes, highlighting the need for efficient pyroptosis promoters. Using nanomedicine-induced pyroptosis has gained significant attention, focusing on using nanobiology or nanomaterials to suppress tumor progression [72].
Adjusting ion concentrations
Ion balance is crucial for cellular processes and biological events because it plays a role in activating internal signaling pathways, regulating enzyme function, maintaining osmotic pressure, balancing redox potentials, and stabilizing pH levels [73]. Reversing ion concentrations within the cytoplasm or other cellular components can potentially initiate PCD. Imbalances in ion levels facilitate the controlled regulation of cell destiny but may face obstacles due to spontaneous alterations in ion transportation. To counteract the natural restoration of cellular equilibrium after disturbances, therapies have been devised that disturb ion balance by introducing ions incapable of being expelled from cells [40]. Manipulating ion balance through ingested NPs can disturb the equilibrium between intra- and extracellular ion concentrations and induce intracellular pyroptosis via distinct pathways associated with different ions. Specific ions can induce pyroptosis by activating GSDMD and GSDME in certain circumstances. While ferroptosis is commonly induced by elevated iron levels, the liberation of iron can alternatively result in pyroptosis, a form of cell death involving an immune response. The liberation of iron specifically occurs within mildly acidic extracellular surroundings, confining cell death to acidic microenvironments and facilitating external regulation. This release mechanism relies on the lipid-coated NPs taken up by cells through endocytosis and subsequently degraded in lysosomes via cysteine-mediated reduction, which is highly efficient in mildly acidic extracellular environments.
Calcium storage is crucial for cell survival and proliferation. Normally, there is a dynamic balance between free and bound calcium ions within mitochondria [74]. However, in pathological or toxicological conditions, the equilibrium can be disturbed, releasing cytochrome C within mitochondria and subsequently activating caspase-3, resulting in apoptosis [75]. However, these small ions may experience systemic effects due to rapid blood circulation, non-specific biodistribution, and adverse reactions. The rapid advancements in nanotechnology and nanocarriers have opened up new possibilities for cancer treatment by reducing drug toxicity while improving drug bioavailability.
Ding et al. developed an innovative approach to cancer immunotherapy by creating NaHCO3 NPs, a drug-free inorganic nanomaterial, using a rapid microemulsion method. These NPs act as immunoadjuvants for pyroptosis and metabolism, enhancing the immune response against cancer cells [76]. By neutralizing the acidic environment within tumors through acid-base regulation and inhibiting tumor growth and metastasis, NaHCO3 reverses the immunosuppressive effects caused by acidity. Moreover, NaHCO3 triggers pyroptosis/ICD-induced immune activation by releasing high levels of Na+ ions inside tumors and increasing intracellular osmolarity. This leads to the release of DAMP and inflammatory factors, further boosting immune responses. The findings from this study offer a promising new direction for tumor treatment that focuses on lactic acid metabolism and pyroptosis-mediated approaches with potential applications in tumor immunotherapy.
Ploetz et al. demonstrated the successful development of a hybrid MOF NPs composed of Fe3+ and trimesic acid coated with lipids to enhance its cellular uptake [38]. The coating process involved mixing MIL-100(Fe) NPs with liposomes containing 1,2-dioleoyl-sn-glycero-3-phosphocholine, followed by fusion of the lipid layers. This lipid coating enabled endolysosomal uptake while allowing the controlled release of the MOF NPs components within cells. Combining the unique advantages of both systems, these MOF NPs bypassed cellular regulation and delivered substantial amounts of iron ions into cells. Furthermore, adjusting the extracellular pH facilitated the intracellular degradation of the MOF NPs and the subsequent release of iron ions. The activation of caspases induces pyroptosis, leading to GSDMD cleavage and cell lysis. Notably, this induction was triggered by changes in extracellular pH levels as an external stimulus for pyroptosis initiation. Such dependence on extracellular pH offers opportunities for further investigation into degradation kinetics, quantity, and specific types of released ions through the adjustable properties inherent in MOF NPs.
Zheng et al. researched the application of biodegradable nanomodulators containing Ca2+ ions (CaNMs) for cancer immunotherapy by triggering pyroptosis through excessive mitochondrial Ca2+ accumulation [77]. The CaNMs comprise CaCO3 and curcumin (CUR), serving as pyroptosis inducers. By utilizing CaCO3 as a carrier to deliver CUR, these nanomodulators generate an increased concentration of Ca2+, specifically within tumors. CUR, recognized for its capacity to regulate calcium levels, induces the liberation of Ca2+ from the endoplasmic reticulum into the cytoplasm while impeding its outward flow from the cytoplasm to the extracellular fluid. Under low pH conditions, the CaNMs degrade and release additional calcium ions. As a result, curcumin causes a sudden increase in levels of calcium within the mitochondria, leading to an overload of calcium that leads to higher levels of ROS, release of cytochrome C, activation of caspase-3, cleavage of GSDME protein, cellular bubbling, and ultimately pyrolysis. This innovative method not only induces pyroptosis through overwhelming mitochondrial calcium using CaNMs but also stimulates strong immune responses. In vivo experiments further confirmed the compatibility with living organisms and the safety profile of these nanomodulators by effectively suppressing tumor growth and preventing lung metastasis. This study expands upon the potential biomedical applications of calcium-based nanomodulators while offering novel strategies and inspiration for cancer treatments mediated by pyroptosis.
Nanozymes
Nanozymes are a type of nanomaterial that possess catalytic functions similar to natural enzymes but with superior structural stability and catalytic activity. Due to the association between dysregulated biological enzymes and the development of diseases, there is a high demand for nanozymes with more stable structures and lower sensitivity to environmental factors compared to natural enzymes. In tumor catalytic therapy, nanozymes have been extensively utilized as they generate cytotoxic ROS [78, 79]. Therefore, utilizing nanozymes as exogenous suppliers of ROS could be an effective approach to inducing cell death through pyroptosis. Over the past decade, artificial nanozymes have garnered significant attention due to their remarkable advantages, such as adjustable catalytic activities, multiple enzyme-like functionalities, and exceptional stability. Currently, over 900 instances of nanozyme utilization with various materials have been reported.
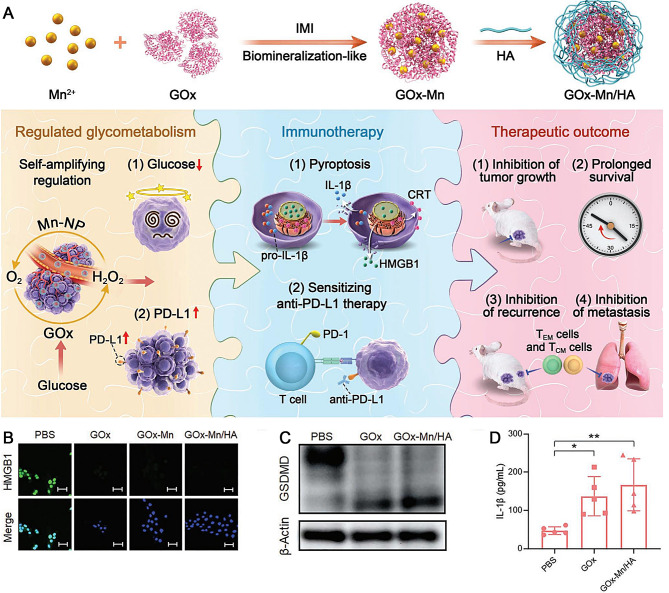
Zhang et al. developed a dual enzymatic activity GOx-Mn NPs by merging nanozymes and GOx through a biomineralization-like technique [80]. The enzyme GOx facilitates the conversion of glucose into gluconic acid and H2O2, enabling the regulation of glycometabolism at tumor sites. On the contrary, nanozymes containing Mn act as catalysts to selectively produce O2 from H2O2 at the location of the tumor. This resultant O2 significantly boosts the glucose-consumption capability of GOx in the NPs, regulating glycometabolism specifically at tumor sites. Additionally, H2O2 plays a beneficial role in catalytic reactions involving nanoenzymes. By combining Mn-containing nanozymes with GOx, two catalytic reactions are synergistically achieved, cyclically enhancing glucose consumption. As a result, the utilization of glucose by GOx-Mn NPs leads to pyroptosis and an increase in PD-L1 expression in cancer cells. This subsequently triggers a notable immune response against tumors and enhances the sensitivity of cells to PD-L1/PD-1 immune-checkpoint-blockade therapy. To specifically target mice with breast cancer through intravenous administration, GOx-Mn was combined with hyaluronic acid (HA) to form GOx-Mn/HA complexes. To achieve highly effective treatment for antitumor immunity, Mn-GOx/HA complexes were further combined with anti-PD-L1 antibodies, significantly inhibiting tumor development and prolonging survival time for mice (Fig. 6A). In vitro experiments showed that the levels of HMGB1 released extracellularly, GSDMD, and IL-1β were markedly increased in the GOx and GOx-Mn/HA groups, as measured using the immunofluorescence assay, western blot, and ELISA, respectively (Fig. 6B-D). This indicates that GOx-Mn/HA can efficiently induce cell pyroptosis and initiate a series of antitumor immune responses, providing a distinct paradigm for cancer treatment. The integration of regulating tumor glycometabolism with immunotherapy suppresses tumor growth and prevents recurrence and metastasis.
Fig. 6.
(A) Scheme of GOx-Mn/HA synthesis and biomineralized two-enzyme NPs that regulate tumor glycometabolism inducing tumor cell pyroptosis and robust antitumor immunotherapy. (B) IF staining of HMGB1. Scale bars: 50 μm. (C) Western blots of GSDMD. (D) Release of IL-1β. Reproduced with permission [80]. Copyright 2022, Wiley-VCH
Xu et al. conducted research on lanthanide-based nanocrystals, particularly LaCoO3 (LCO), to manipulate the TME, generating cytotoxic ROS and releasing metal ions to induce pyroptosis in lung cancer cells [81]. The NPs containing Co ions possess various enzyme-like properties due to the high redox potential of Co3+/Co2+, resembling intrinsic peroxidase, catalase (CAT), and superoxide dismutase. As a result, this leads to the production of ROS. The LCO nanoenzyme exhibits activities similar to CAT and GPX, altering the hypoxic conditions within tumors by disrupting the activated antioxidant system of cancer cells and enhancing their susceptibility toward ROS. Reducing intracellular GSH levels through its CAT-like activity improves the hypoxic environment in lung cancer cells. Ultrasound acts as an external energy source that compensates for the high activation energy needed by Co3+ in terms of chemical kinetics while simultaneously expediting dynamic enzymatic reactions. The disruption of lysosomal membranes is a result of the release of La3+ from LCO crystals, which triggers canonical pyroptotic cell death characterized by caspase-1 activation, GSDMD cleavage, and IL-1β/LDH release both in vitro and in vivo. By inducing programmed cell pyroptosis through LCO nanocrystals, therapeutic effects are enhanced both in vitro and in vivo, inhibiting lung cancer growth and metastasis.
Nanodrugs
Numerous chemotherapeutic agents are currently utilized in clinical settings. Arsenic trioxide is employed as a standalone treatment or combined with other drugs for acute promyelocytic leukemia. Furthermore, notable advancements have been made in utilizing arsenic trioxide to combat solid tumors, such as hepatocellular carcinoma (HCC). The antitumor properties of As2O3 are manifested through diverse mechanisms, which encompass the initiation of G2/M arrest and apoptosis [82]. Moreover, As2O3 promotes the differentiation of the cancer cells that survive. This distinctive attribute allows As2O3 to reduce the malignant behavior and metastatic potential of these resilient cancer cells during chemotherapy. Consequently, it enhances treatment responses characterized by decreased rates of metastasis and recurrence in comparison to conventional anticancer medications [83]. However, due to its rapid elimination from the bloodstream, achieving the effective accumulation of As2O3 within solid tumors presents a challenge [84].
Hu et al. employed mPEG-PLGA-PLL triblock copolymers comprising cationic amine groups and hydrophobic/hydrophilic chains to encapsulate As2O3 NPs, which were administered intratumorally [85]. The growth of tumors was effectively suppressed by As2O3 NPs by downregulating PCNA and DNMT-related proteins and upregulating GSDME-N. In vitro experiments indicated that As2O3 NPs exhibited superior inhibitory effects compared to free As2O3, resulting in increased LDH release and the induction of cell morphology indicative of pyroptosis. Moreover, Huh7 cells treated with As2O3 NPs displayed elevated expression levels of GSDME-N while reducing the expression levels of Dnmt3a, Dnmt3b, and Dnmt1 compared to those under treatment with the unencapsulated drug. Significantly reduced expression levels of Dnmt3a, Dnmt3b, and Dnmt1 were observed upon the in vivo administration of As2O3 NPs, while GSDME-N expression increased. Notably, caspase-3 activation is crucial in chemically induced pyroptosis, triggering downstream GSDME activation and subsequent cleavage to induce pyroptotic cell death. These discoveries offer fresh perspectives on the mechanism by which As2O3 affects HCC cells and present promising therapeutic possibilities for further investigation.
Inorganic NPs (Carbon black [CB], silica, two-dimensional graphene oxide [GO])
Inorganic NPs are a class of pyroptosis inducers. Various inorganic NPs, including metal oxide, CB, mesoporous silica, and MOF NPs, induce pyroptosis in different cell types in an uncontrolled manner. Unlike bioinert bulk carbon materials, nanocarbons cause inflammatory reactions and cytotoxic damage. Inhaling nano-silica particles can also induce macrophage pyroptosis, which plays an important role in lung inflammation pathogenesis. Two-dimensional GO nanomaterials are another candidate for pyroptosis inducers whose physical size directly affects the activation level of pyroptosis [86].
Reisetter et al. demonstrated that inflammasome activation and subsequent pyroptosis occurred in macrophages upon exposure to CB NPs [87]. The induction of oxidative stress in alveolar macrophages by CB NPs is attributed to the functionality of the particle surface. The inflammation caused by CB particles may result from the production of ROS through interactions between the particles and cells. This is due to the high reactivity of CB NPs, which triggers the activation of pro-inflammatory genes sensitive to redox signaling. The findings demonstrated that exposure to CB NPs led to caspase-1 activation, increased the release of IL-1 after LPS priming, and induced pyroptosis with inflammatory characteristics.
Inhibitors
In normal physiological circumstances, the controlled activation of pyroptosis is a crucial mechanism for the body to defend against harmful microorganisms. Nevertheless, excessive pyroptosis leading to unregulated inflammatory responses and cellular death is associated with the pathological advancement of various diseases, particularly those characterized by inflammation. Consequently, inhibiting pyroptosis is a promising therapeutic strategy for treating inflammatory diseases because it can trigger inflammation and exacerbate disease progression. The significant advances in pyroptosis-engineered nanobiotechnologies offer valuable insights into potential treatments for inflammatory diseases by targeting and suppressing pyroptotic pathways.
Ma et al. employed Prussian blue nanozyme (PBzyme) to inhibit pyroptosis and suppress the activation of microglial NLRP3 inflammasome in a mouse model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [88]. PBzyme possesses nanomaterial properties and exhibits an exceptional intrinsic catalytic function similar to that of enzymes that scavenge ROS, such as •OH, •OOH, and H2O2. Due to its exceptional ability to scavenge ROS and its catalytic activity, PBzyme effectively hinders the assembly and activation of the NLRP3 inflammasome in microglia. This suppression reduces the cleavage of GSDMD by caspase-1 and the subsequent liberation of inflammatory cytokines such as IL-1β and IL-18. Additionally, PBzyme mitigates mitochondrial dysfunction caused by exogenous MPTP or MPP+, reducing microglial pyroptosis and damage to dopaminergic neurons. These findings indicate that targeting the microglial NLRP3 inflammasome with PBzyme could be a potential therapeutic approach for slowing down the progression of Parkinson’s disease.
Recently, there have been advancements in the development of innovative nucleic acid materials, such as nanoscale tetrahedral framework nucleic acids (tFNAs), with applications across various domains [89]. tFNAs possess anti-inflammatory and antioxidant properties and facilitate the healing process of skin wounds [90]. Due to their distinctive and stable three-dimensional spatial structures, tFNAs can permeate different cell types without requiring carriers. This enables them to induce cell proliferation, migration, and differentiation while maintaining specificity in their functions. The exceptional biosafety and biocompatibility of tFNAs make them a promising therapeutic option for patients with spinal cord injury, Alzheimer’s disease, depression, multiple sclerosis, and glioblastoma multiforme. Furthermore, tFNAs exhibit significant potential in tissue regeneration and gene and drug delivery applications.
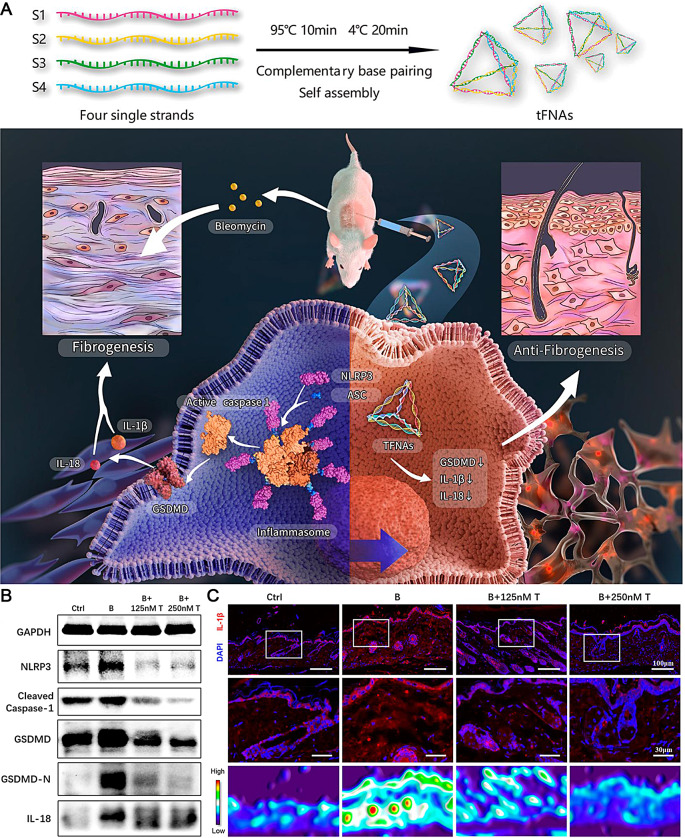
Jiang et al. conducted experiments on HaCaT cells and BALB/c mice exposed to substances promoting fibrosis to explore the potential of tFNAs for treating skin fibrosis, a highly dynamic disease process [91]. tFNAs are DNA nanomaterials renowned for their distinctive spatial structure and exceptional biosafety. They possess diverse advantageous characteristics such as anti-inflammatory, antioxidant, antifibrotic, angiogenic, and dermal wound-healing properties without inducing significant toxicity. When administered to cells or co-injected with profibrogenic molecules in mice, tFNAs hinder the process of epithelial-mesenchymal transition, lower levels of inflammatory factors, reduce skin collagen content, and suppress the expression of proteins involved in the pyroptosis pathway. Specifically, treatment with tFNAs decreased NLRP3 and procaspase-1 levels, which indicated a reduction in the inflammasome packaging and active caspase-1 form, decreasing N-terminal GSDMD levels (Fig. 7A). In vitro findings showed that pyroptosis-related proteins (NLRP3, pro-caspase-1, and GSDMD-N) were significantly downregulated upon tFNA treatment, while the expression of IL-1β was markedly increased, as measured using western blot and immunofluorescence assays, respectively (Fig. 7B, C). The examination of the correlation between tFNAs and the pyroptosis pathway has uncovered a fresh approach to tackling skin fibrosis. These discoveries underscore the potential therapeutic uses of tFNAs in conditions linked to pathways associated with pyroptosis.
Fig. 7.
(A) tFNAs reduce skin fibrosis by inhibiting pyroptosis pathway. (B) Western blots of pyroptosis-related protein expression. (C) IF detection and thermal diagrams of IL-1β expression. Scale bars: 100 and 30 μm. Reproduced with permission [91]. Copyright 2022, American Chemical Society
Sensitizers
Precious metal nanomaterials (such as gold, silver, platinum, and palladium) are among the most studied nanomaterials owing to their mature preparation methods, simple surface modification processes, good biocompatibility, and surface plasmon resonance properties. Compared with the most common nanogold, nanosilver has a stronger glioma-killing ability when it interacts with megavolt X-rays, and its main signaling pathway in radiotherapy produces ROS and induces autophagy in cancer cells. Bismuth-based nanomaterials are emerging owing to their good X-ray and infrared absorption capacities and biological safety. Several nanomaterials, such as bismuth sulfide, selenide, and copper sulfide, have been used for PTT and radiotherapy sensitization.
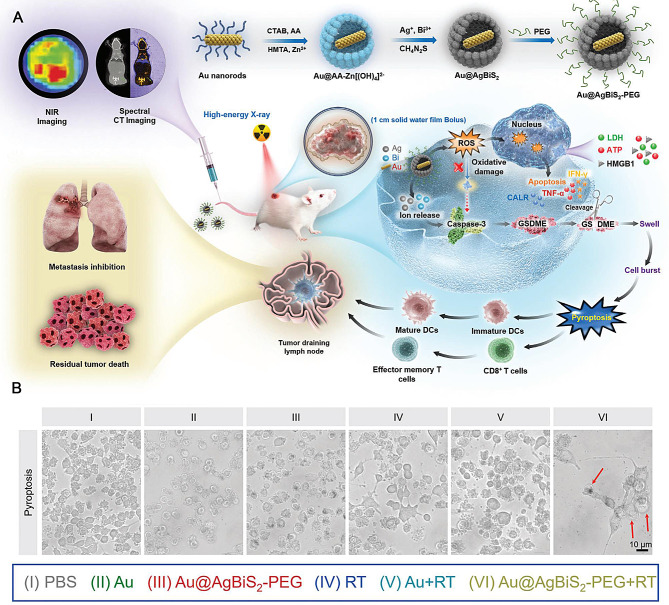
Xiao et al. developed a metal-semiconductor core-shell nanostructure of Au@AgBiS2 using the polyol method with a hard template, as shown in Fig. 8A, which demonstrated excellent compatibility and safety for biomedical applications [92]. This innovative approach aimed to enhance the effectiveness of radiotherapy in TMEs by acting as an exceptional radiosensitizer. The Au nanorods served as a stable inner core, continuously sensitizing the TME to radiotherapy. To improve the biocompatibility further, PEG-C18PMH was used to modify the obtained Au@AgBiS2 NPs (denoted as Au@AgBiS2-PEG). Upon exposure to high-energy X-rays, the Bi shell effectively absorbed radiation energy due to its high atomic number, while Ag ions were released in minimal amounts. This process facilitated charge separation, increasing the generation of ROS and enhancing DNA damage caused by radiotherapy. Consequently, cancer cells became more susceptible to radiotherapy due to their heightened sensitivity induced by Au@AgBiS2-PEG treatment triggered by irradiation. As shown in Fig. 8B, noticeable cell swelling with large bubbles was observed in the Au@AgBiS2-PEG group treated with radiotherapy (RT), whereas the other groups displayed almost no balloon-like cells. Au@AgBiS2 under radiation conditions can induce pyroptosis, providing an opportunity to stimulate the immune response at the tumor site. In vitro experiments revealed that upon activation through caspase-3 cleavage, GSDME mediated pyroptosis induction and the release of DAMPs. Furthermore, combining Au@AgBiS2-PEG with radiotherapy in vivo significantly augmented ROS production and elicited a robust antitumor immune response while preventing lung and systemic metastases.
Fig. 8.
(A) Schematic illustration of Au@AgBiS2 -PEG design and antitumor application. (B) Cell morphology images depicting diverse treatments. Scale bars: 10 μm. Reproduced with permission [92]. Copyright 2023, Wiley-VCH
Conclusions and future perspectives
Pyroptosis, a recently identified type of programmed cellular death, initiates the liberation of inflammatory substances, tumor-specific antigens, and adaptive immune reactions. Additionally, it stimulates ICD within malignant cells while enhancing the body’s defense against tumors. Inducing pyroptosis in even a small number of tumor cells can effectively eliminate tumors and regulate the TME to produce potent antitumor immune effects. Therefore, activating pyroptosis to stimulate immune responses is an effective approach for treating tumors. To optimize the effectiveness of pyroptosis therapy, a range of nanobiotechnologies, nanomaterials, and treatment approaches have been devised utilizing agents associated with pyroptosis. Nanotechnology holds immense potential in modulating pyroptosis by either stimulating or inhibiting it, bolstering the immune response, and enhancing therapeutic outcomes for cancer and inflammatory ailments. This article presents an overview of recent advancements in nanomaterials and nanotechnology aimed at regulating pyroptosis as a viable strategy for addressing cancer and inflammation-related diseases. Despite the significant progress made using cell pyroptosis-based nanotechnology for cancer therapy, several critical challenges must be addressed before the clinical applications can be fully realized.
Further exploration of nanomedicines as inducers or inhibitors of pyroptosis
In the context of tumor-related pyroptosis, undiscovered GSDM family members may exist in addition to those already associated with the pyroptosis pathway that could serve as promising therapeutic targets for intervention. The cleavage of GSDMs is not solely attributed to caspases, indicating the need for additional research to uncover the essential processes and proteases implicated in pyroptosis.
Exploring new applications and mechanisms of existing inducers is necessary. However, based on the discovered caspase and GSDM family proteins, searching for upstream targets and developing new and efficient small-molecule pyroptosis inducers that can be loaded via the NDS to induce tumor pyroptosis is promising. In addition, few cancer cells exhibit anti-pyroptosis mechanisms. To address this issue, antisense nucleic acids or siRNAs can be delivered through NDS to target and silence related genes, achieving precise tumor gene editing. NDS can also synergistically deliver pyroptosis inducers and other cell death inducers (such as ferroptosis and cuproptosis) to enhance the antitumor effects.
NP-uptake efficiency of tumors
An ideal and efficient anti-tumor NP should possess five key characteristics, namely long duration of blood circulation, high tumor enrichment, deep tumor penetration, high tumor cell uptake efficiency, and controlled drug release. The effectiveness of NPs in inducing pyroptosis and exerting an anti-tumor effect relies on their efficient uptake by tumor cells. NPs are often functionalized with targeting ligands, such as antibodies, peptides, aptamers, and small molecules, to specifically recognize tumor cells and enhance their uptake through receptor-mediated endocytosis. In addition, for non-actively targeted NPs, cellular uptake efficiency can be improved by adjusting their physicochemical properties such as size, shape, charge, surface ratio, and mechanical properties. Through the enhanced permeability and retention effect, NPs can accumulate in tumor tissue, exert stronger effects on tumor cells, and reduce damage to normal tissues. Therefore, developing and designing NPs that can be more efficiently and specifically taken up by tumor cells to induce pyroptosis and thereby kill tumors is an important direction in the field of nanotechnology.
Controllability of NPs inducing pyroptosis
Pyroptosis is typically beneficial in helping the host defend against pathogen invasion. However, excessive pyroptosis can lead to heightened inflammatory reactions, widespread cell death, and severe tissue damage, which can contribute to the development of inflammatory or autoimmune disorders. In addition, the unintended effects of biomaterials on tumor immunity can potentially impact treatment outcomes. In recent years, there has been a growing interest in utilizing drug delivery systems to develop more effective and controlled approaches for pyroptosis. The ongoing investigation of drug delivery systems, comprising nanorobots and various stimulation therapies, such as PTT therapy, electromagnetic thermal therapy, and acoustic therapy, exhibits promising prospects. These nanorobots are miniature machines capable of performing tasks within the human body, including drug delivery, immune system stimulation, diagnosis, and detection. Although they are primarily in experimental and early clinical stages, they have garnered considerable interest. Photothermal, electromagnetic, and acoustic stimulation response delivery integrates different physical properties to control and release drugs with features such as noninvasive operation, spatiotemporal controllability, and minimal side effects. PDT employs light-controlled activation of pyroptosis to enhance systemic immunity through photoirradiation. The potential of this method to stimulate the immune response through pyroptosis is noteworthy. The approach utilized in SDT involves the use of sonosensitizers and focused ultrasound to target tumors, with the ability to deliver precise doses of ultrasound for effective treatment. In the future, the development of drug delivery systems offers a promising opportunity for formulating more regulated and effective strategies for pyroptosis.
Biosafety and biocompatibility of pyroptosis nanoinducers
The acceleration of clinical translation heavily relies on the biosafety and biocompatibility exhibited by nanoinducers for pyroptosis. While the preliminary results have suggested the impressive biosafety of specific pyroptosis nanoinducers, their potential for long-term toxicity and their biological consequences remain largely uninvestigated. The stability and binding efficiency of NPs with cell-surface-targeted molecules in the physiological environment are not fully understood. However, when nanomedicine targets tumor stem cells, normal cells may be damaged during treatment because of the many common signaling pathways between tumor and normal stem cells. The toxicological and biological effects of nanomaterials have shown that materials that are initially nontoxic or relatively less toxic can become toxic or more toxic once their particle size reduces to an extent. NPs can also overload bacteriophage and reduce immunity, and, owing to their large surface areas, they can easily adsorb large molecules such as enzymes and other proteins in tissues or body fluids, interfering with biological processes. In addition, the current pyroptosis nanoinducers predominantly rely on inorganic nanosystems such as metal peroxide NPs, which lack sufficient evidence for their biosafety in clinical translation. Moreover, there are potential concerns associated with other types of nanomaterials, such as dendritic nanomaterials, which induce permeability damage and the rupture of cell membranes. It is crucial to conduct comprehensive and systematic assessments in vitro and in vivo to obtain reliable data regarding biocompatibility and biosafety aspects.
Promotion of clinical translation
Excluding carriers such as liposomes and polymer micelles, most NDS are still in the experimental stage. The main reason is that the mechanism of interaction between nanomedicines and the complex in vivo environment is not fully understood, including how to break through biological barriers, avoid phagocytosis by mononuclear phagocytic cell systems, and improve uneven distribution within tumors. Further research is required to address the side effects of nanotechnology in treating malignant tumors. Therefore, while developing new smart nanocarriers, investigating the mechanisms of interaction between nanomaterials and biological systems to improve their clinical safety and efficacy is necessary.
In conclusion, the exploration and utilization of pyroptosis present a continuous journey that brings forth fresh challenges and prospects in the field of nanomaterial development. It is imperative for future research to focus on refining and tailoring pyroptotic treatments with enhanced precision, specifically targeting tumors. Additionally, more clinical trials should be conducted to investigate the potential applications of cancer therapy based on pyroptosis. We anticipate that these advancements will greatly enhance the effectiveness of treating tumors and other pyroptosis-related diseases, ultimately benefiting patient well-being.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82303258), the Natural Science Foundation of Hunan Province (Nos. 2023JJ40807, 2023JJ40874, 2024JJ5478), and the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University (Nos. QH20230262, QH20230202).
Author contributions
L. Z. and J. C. proposed the conception and wrote the orginal draft, L.Z., H. C. and J. C. wrote the main manuscript text, Z. L. prepared Figs. 1, 2 and 3 and Z. T prepared Figs. 4, 5, 6, 7 and 8. All authors reviewed the manuscript.
Funding
Open access funding provided by The Second Xiangya Hospital, Central South University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Lin Zhao is the independent first author.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–6. 10.1016/S0021-9258(17)38364-3 [DOI] [PubMed] [Google Scholar]
- 3.Ye Z, Zhang L, Li R, Dong W, Liu S, Li Z, et al. Caspase-11 mediates pyroptosis of tubular epithelial cells and septic acute kidney Injury. Kidney Blood Press Res. 2019;44:465–78. 10.1159/000499685 [DOI] [PubMed] [Google Scholar]
- 4.Liang F, Zhang F, Zhang L, Wei W. The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflamm Res. 2020;69:159–66. 10.1007/s00011-020-01315-3 [DOI] [PubMed] [Google Scholar]
- 5.Hongda Z, Kai M, Rui R, Chaobo Y, Aqin Y, Jing L, et al. Tumor-targeted self-assembled micelles reducing PD-L1 expression combined with ICIs to enhance chemo-immunotherapy of TNBC. Chin Chem Lett. 2024;35:108536. 10.1016/j.cclet.2023.108536 [DOI] [Google Scholar]
- 6.Zhao P, Wang M, Chen M, Chen Z, Peng X, Zhou F, et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142. 10.1016/j.biomaterials.2020.120142 [DOI] [PubMed] [Google Scholar]
- 7.Tu L, Li C, Ding Q, Sharma A, Li M, Li J, et al. Augmenting Cancer Therapy with a supramolecular immunogenic cell death inducer: a lysosome-targeted NIR-Light-activated ruthenium(II) metallacycle. J Am Chem Soc. 2024;146:8991–9003. 10.1021/jacs.3c13224 [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421–6. 10.1038/s41586-020-2079-1 [DOI] [PubMed] [Google Scholar]
- 9.Tan Y, Chen Q, Li X, Zeng Z, Xiong W, Li G, et al. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40:153. 10.1186/s13046-021-01959-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamkanfi M, Dixit VM. In retrospect: the inflammasome turns 15. Nature. 2017;548:534–5. 10.1038/548534a [DOI] [PubMed] [Google Scholar]
- 11.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–21. 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 14.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 15.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–98. 10.1038/cr.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin N, Wang B, Liu X, Yin C, Li X, Wang Z, et al. Mannose-doped metal-organic frameworks induce tumor cell pyroptosis via the PERK pathway. J Nanobiotechnol. 2023;21:426. 10.1186/s12951-023-02175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–92. 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 18.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. 10.1126/science.aaz7548 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–20. 10.1038/s41586-020-2071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57. 10.1038/s41577-019-0228-2 [DOI] [PubMed] [Google Scholar]
- 23.Kovacs SB, Miao EA, Gasdermins. Effectors of Pyroptosis. Trends Cell Biol. 2017;27:673–84. 10.1016/j.tcb.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6. 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- 25.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in Monocytes and macrophages. Cell Chem Biol. 2017;24:507–e14504. 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BL, Mirrashidi KM, Stowe IB, Kummerfeld SK, Watanabe C, Haley B, et al. ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci Rep. 2018;8:3788. 10.1038/s41598-018-21998-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736–45. 10.1038/s41590-020-0669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery. 2021;20:101–24. 10.1038/s41573-020-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang B, Chen Y, Shi J. Nanocatalytic Medicine. Adv Mater. 2019;31:e1901778. 10.1002/adma.201901778 [DOI] [PubMed] [Google Scholar]
- 30.Hu H, Yu L, Qian X, Chen Y, Chen B, Li Y. Chemoreactive Nanotherapeutics by Metal Peroxide based Nanomedicine. Adv Sci (Weinh). 2020;8:2000494. 10.1002/advs.202000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Wang S, Yu G, Chen X. Cell death mediated by the pyroptosis pathway with the aid of nanotechnology: prospects for Cancer Therapy. Angew Chem Int Ed Engl. 2021;60:8018–34. 10.1002/anie.202010281 [DOI] [PubMed] [Google Scholar]
- 32.Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41:2656–72. 10.1039/C2CS15261D [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Wang M, Hu H, Yang X, Wen B, Wang Z, et al. Multimodal-imaging-guided Cancer Phototherapy by Versatile Biomimetic Theranostics with UV and γ-Irradiation Protection. Adv Mater. 2016;28:3273–9. 10.1002/adma.201505700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu G, Yang Z, Fu X, Yung BC, Yang J, Mao Z, et al. Polyrotaxane-based supramolecular theranostics. Nat Commun. 2018;9:766. 10.1038/s41467-018-03119-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Q, Luo Y, Xu L, Du C, Zhang W, Xu J, et al. Smart responsive Fe/Mn nanovaccine triggers liver cancer immunotherapy via pyroptosis and pyroptosis-boosted cGAS-STING activation. J Nanobiotechnol. 2024;22:95. 10.1186/s12951-024-02354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clegg JR, Irani AS, Ander EW, Ludolph CM, Venkataraman AK, Zhong JX, et al. Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications. Sci Adv. 2019;5:eaax7946. 10.1126/sciadv.aax7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner AM, Gran MP, Peppas NA. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharmacol Sin B. 2018;8:147–64. 10.1016/j.apsb.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploetz E, Zimpel A, Cauda V, Bauer D, Lamb DC, Haisch C, et al. Metal-Organic Framework nanoparticles induce pyroptosis in cells controlled by the Extracellular pH. Adv Mater. 2020;32:e1907267. 10.1002/adma.201907267 [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Xie W, Li Y, Ni Q, Timashev P, Lyu M, et al. Biomimetic Nanocarriers Guide Extracellular ATP homeostasis to Remodel Energy Metabolism for activating Innate and adaptive immunity system. Adv Sci (Weinh). 2022;9:e2105376. 10.1002/advs.202105376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Fang C, Zhang W, Zhang K. Emerging pyroptosis-engineered nanobiotechnologies regulate cancers and inflammatory diseases: a double-edged sword. Matter. 2022;5:3740–4. 10.1016/j.matt.2022.08.026 [DOI] [Google Scholar]
- 41.Kou L, Bhutia YD, Yao Q, He Z, Sun J, Ganapathy V. Transporter-guided delivery of nanoparticles to Improve Drug Permeation across Cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018;9:27. 10.3389/fphar.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li F, Zhang XQ, Ho W, Tang M, Li Z, Bu L, et al. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat Commun. 2023;14:4223. 10.1038/s41467-023-39938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong H, Chen G, Li T, Huang J, Lin M, Li B, et al. Nanodrug Augmenting Antitumor Immunity for Enhanced TNBC Therapy via Pyroptosis and cGAS-STING activation. Nano Lett. 2023;23:5083–91. 10.1021/acs.nanolett.3c01008 [DOI] [PubMed] [Google Scholar]
- 44.Jiang A, Wang M, Liu H, Liu S, Song X, Zou Y, et al. Gasdermin E plasmid DNA/indocyanine green coloaded hybrid nanoparticles with spatiotemporal controllability to induce pyroptosis for colon cancer treatment. MedComm – Oncol. 2023;2:e33. 10.1002/mog2.33 [DOI] [Google Scholar]
- 45.Zhang X, Zhang T, Tuo W, Liu Y, Umar T, Chen Y, et al. Rising interest in the accurate and controllable anticancer strategy: based on photon-evoked pyroptosis engineering perspective. Coord Chem Rev. 2024;501:215588. 10.1016/j.ccr.2023.215588 [DOI] [Google Scholar]
- 46.Li C, Tu L, Xu Y, Li M, Du J, Stang PJ et al. A NIR-Light-activated and lysosomal-targeted pt(II) metallacycle for highly potent evoking of immunogenic cell death that Potentiates Cancer Immunotherapy of Deep-seated tumors. Angew Chem Int Ed Engl. 2024:e202406392. [DOI] [PubMed]
- 47.Ma Y, Mou Q, Zhu X, Yan D. Small molecule nanodrugs for cancer therapy. Mater Today Chem. 2017;4:26–39. 10.1016/j.mtchem.2017.01.004 [DOI] [Google Scholar]
- 48.Fan JX, Deng RH, Wang H, Liu XH, Wang XN, Qin R, et al. Epigenetics-based Tumor cells pyroptosis for enhancing the Immunological Effect of Chemotherapeutic Nanocarriers. Nano Lett. 2019;19:8049–58. 10.1021/acs.nanolett.9b03245 [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Yao Y, Shen Q, Li G, Hu L, Zhang X. Demethylating agent decitabine disrupts tumor-induced immune tolerance by depleting myeloid-derived suppressor cells. J Cancer Res Clin Oncol. 2017;143:1371–80. 10.1007/s00432-017-2394-6 [DOI] [PubMed] [Google Scholar]
- 50.Yu X, Xing G, Sheng S, Jin L, Zhang Y, Zhu D, et al. Neutrophil camouflaged Stealth Nanovehicle for Photothermal-Induced Tumor Immunotherapy by triggering pyroptosis. Adv Sci (Weinh). 2023;10:e2207456. 10.1002/advs.202207456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P, Jin X, Cai J, Chen J, Ji M. Recent advances in small molecule prodrugs for cancer therapy. Anti-Cancer Agents Med Chem. 2014;14:418–39. 10.2174/18715206113139990317 [DOI] [PubMed] [Google Scholar]
- 52.Wang K, Xiao X, Jiang M, Li J, Zhou J, Yuan Y. An NIR-Fluorophore-based theranostic for selective initiation of Tumor Pyroptosis-Induced Immunotherapy. Small. 2021;17:e2102610. 10.1002/smll.202102610 [DOI] [PubMed] [Google Scholar]
- 53.Su W, Qiu W, Li SJ, Wang S, Xie J, Yang QC, et al. A dual-responsive STAT3 inhibitor Nanoprodrug Combined with Oncolytic Virus elicits synergistic Antitumor Immune responses by igniting pyroptosis. Adv Mater. 2023;35:e2209379. 10.1002/adma.202209379 [DOI] [PubMed] [Google Scholar]
- 54.Xiao Y, Zhang T, Ma X, Yang QC, Yang LL, Yang SC, et al. Microenvironment-responsive Prodrug-Induced pyroptosis boosts Cancer Immunotherapy. Adv Sci (Weinh). 2021;8:e2101840. 10.1002/advs.202101840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. 10.1038/nrc1071 [DOI] [PubMed] [Google Scholar]
- 56.Luo T, Wang D, Liu L, Zhang Y, Han C, Xie Y, et al. Switching reactive oxygen species into reactive Nitrogen species by Photocleaved O2 -Released nanoplatforms favors hypoxic Tumor repression. Adv Sci (Weinh). 2021;8:e2101065. 10.1002/advs.202101065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou J, Ma X, Li H, Chen D, Mao L, Yang L, et al. Inspired heat shock protein alleviating prodrug enforces immunogenic photodynamic therapy by eliciting pyroptosis. Nano Res. 2022;15:3398–408. 10.1007/s12274-021-3946-2 [DOI] [Google Scholar]
- 58.Chen M, Liao H, Bu Z, Wang D, Fang C, Liang X, et al. Pyroptosis activation by photodynamic-boosted nanocatalytic medicine favors malignancy recession. Chem Eng J. 2022;441:136030. 10.1016/j.cej.2022.136030 [DOI] [Google Scholar]
- 59.Son S, Kim JH, Wang X, Zhang C, Yoon SA, Shin J, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49:3244–61. 10.1039/C9CS00648F [DOI] [PubMed] [Google Scholar]
- 60.Rengeng L, Qianyu Z, Yuehong L, Zhongzhong P, Libo L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagn Photodyn Ther. 2017;19:159–66. 10.1016/j.pdpdt.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 61.Bhatta A, Krishnamoorthy G, Marimuthu N, Dihingia A, Manna P, Biswal HT, et al. Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery. Int J Biol Macromol. 2019;136:951–61. 10.1016/j.ijbiomac.2019.06.127 [DOI] [PubMed] [Google Scholar]
- 62.Yu Z, Cao W, Han C, Wang Z, Qiu Y, Wang J, et al. Biomimetic Metal-Organic Framework nanoparticles for Synergistic Combining of SDT-Chemotherapy Induce pyroptosis in gastric Cancer. Front Bioeng Biotechnol. 2022;10:796820. 10.3389/fbioe.2022.796820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Luo R, Li J, Wang S, Ding J, Zhao K, et al. Intrinsic radical species scavenging activities of tea polyphenols nanoparticles Block Pyroptosis in Endotoxin-Induced Sepsis. ACS Nano. 2022;16:2429–41. 10.1021/acsnano.1c08913 [DOI] [PubMed] [Google Scholar]
- 64.Fan W, Lu N, Huang P, Liu Y, Yang Z, Wang S, et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew Chem Int Ed Engl. 2017;56:1229–33. 10.1002/anie.201610682 [DOI] [PubMed] [Google Scholar]
- 65.Yu Z, Zhou P, Pan W, Li N, Tang B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun. 2018;9:5044. 10.1038/s41467-018-07197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Anraku Y, Kataoka K. Self-boosting Catalytic Nanoreactors Integrated with Triggerable Crosslinking membrane networks for initiation of immunogenic cell death by Pyroptosis. Angew Chem Int Ed Engl. 2020;59:13526–30. 10.1002/anie.202004180 [DOI] [PubMed] [Google Scholar]
- 67.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–21. 10.1038/nrm3801 [DOI] [PubMed] [Google Scholar]
- 68.Ji P, Zhang S, Liu P, Li X, Bao W, Cui X, et al. Modulation of mitochondrial electron transport chain by pyroptosis for tumor destruction. Nano Today. 2022;44:101511. 10.1016/j.nantod.2022.101511 [DOI] [Google Scholar]
- 69.Wang L, Lu D, Huo M, Xu H. Oligomycin a induces apoptosis-to-pyroptosis switch against Melanoma with Sensitized Immunotherapy. Adv Funct Mater. 2022;32:2106332. 10.1002/adfm.202106332 [DOI] [Google Scholar]
- 70.Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like reactions. Angew Chem Int Ed Engl. 2019;58:946–56. 10.1002/anie.201805664 [DOI] [PubMed] [Google Scholar]
- 71.Xu R, Yang J, Qian Y, Deng H, Wang Z, Ma S, et al. Ferroptosis/pyroptosis dual-inductive combinational anti-cancer therapy achieved by transferrin decorated nanoMOF. Nanoscale Horiz. 2021;6:348–56. 10.1039/D0NH00674B [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Zhou Y, Zhao S, Ding L, Chen B, Chen Y. Nanomedicine-Enabled/Augmented cell pyroptosis for efficient Tumor Nanotherapy. Adv Sci (Weinh). 2022;9:e2203583. 10.1002/advs.202203583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell. 2017;168:344–61. 10.1016/j.cell.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pathak T, Trebak M. Mitochondrial Ca2+ signaling. Pharmacol Ther. 2018;192:112–23. 10.1016/j.pharmthera.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu L, Tong G, Song Q, Zhu C, Zhang H, Shi J, et al. Enhanced intracellular Ca2+ nanogenerator for tumor-specific synergistic therapy via disruption of mitochondrial Ca2+ homeostasis and Photothermal Therapy. ACS Nano. 2018;12:6806–18. 10.1021/acsnano.8b02034 [DOI] [PubMed] [Google Scholar]
- 76.Ding B, Zheng P, Tan J, Chen H, Meng Q, Li J, et al. Sodium bicarbonate nanoparticles for Amplified Cancer Immunotherapy by inducing pyroptosis and regulating Lactic Acid Metabolism. Angew Chem Int Ed Engl. 2023;62:e202307706. 10.1002/anie.202307706 [DOI] [PubMed] [Google Scholar]
- 77.Zheng P, Ding B, Zhu G, Li C, Lin J. Biodegradable Ca2+ nanomodulators activate pyroptosis through mitochondrial Ca2+ overload for Cancer Immunotherapy. Angew Chem Int Ed Engl. 2022;61:e202204904. 10.1002/anie.202204904 [DOI] [PubMed] [Google Scholar]
- 78.Lei S, Zhang J, Blum NT, Li M, Zhang DY, Yin W, et al. In vivo three-dimensional multispectral photoacoustic imaging of dual enzyme-driven cyclic cascade reaction for tumor catalytic therapy. Nat Commun. 2022;13:1298. 10.1038/s41467-022-29082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang M, Wang Z, Dong C, Zhou R, Chen L, Huang H, et al. Ultrasound-amplified enzyodynamic tumor therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis. Adv Mater. 2023;35:e2208817. 10.1002/adma.202208817 [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, Zhang Y, Feng Y, Wu J, Hu Y, Lin L, et al. Biomineralized two-enzyme nanoparticles regulate Tumor Glycometabolism Inducing Tumor Cell pyroptosis and Robust Antitumor Immunotherapy. Adv Mater. 2022;34:e2206851. 10.1002/adma.202206851 [DOI] [PubMed] [Google Scholar]
- 81.Xu K, Chang M, Wang Z, Yang H, Jia Y, Xu W, et al. Multienzyme-mimicking LaCoO3 nanotrigger for Programming Cancer-Cell pyroptosis. Adv Mater. 2023;35:e2302961. 10.1002/adma.202302961 [DOI] [PubMed] [Google Scholar]
- 82.Zhang X, Jia S, Yang S, Yang Y, Yang T, Yang Y. Arsenic trioxide induces G2/M arrest in hepatocellular carcinoma cells by increasing the tumor suppressor PTEN expression. J Cell Biochem. 2012;113:3528–35. 10.1002/jcb.24230 [DOI] [PubMed] [Google Scholar]
- 83.Zhao Z, Zhang H, Chi X, Li H, Yin Z, Huang D, et al. Silica nanovehicles endow arsenic trioxide with an ability to effectively treat cancer cells and solid tumors. J Mater Chem B. 2014;2:6313–23. 10.1039/C4TB00874J [DOI] [PubMed] [Google Scholar]
- 84.Fei W, Zhang Y, Han S, Tao J, Zheng H, Wei Y, et al. RGD conjugated liposome-hollow silica hybrid nanovehicles for targeted and controlled delivery of arsenic trioxide against hepatic carcinoma. Int J Pharm. 2017;519:250–62. 10.1016/j.ijpharm.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 85.Hu J, Dong Y, Ding L, Dong Y, Wu Z, Wang W, et al. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct Target Ther. 2019;4:28. 10.1038/s41392-019-0062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J, Wang X, Mei KC, Chang CH, Jiang J, Liu X, et al. Lateral size of graphene oxide determines differential cellular uptake and cell death pathways in Kupffer cells, LSECs, and hepatocytes. Nano Today. 2021;37:101061. 10.1016/j.nantod.2020.101061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reisetter AC, Stebounova LV, Baltrusaitis J, Powers L, Gupta A, Grassian VH, et al. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem. 2011;286:21844–52. 10.1074/jbc.M111.238519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma X, Hao J, Wu J, Li Y, Cai X, Zheng Y. Prussian Blue Nanozyme as a pyroptosis inhibitor alleviates neurodegeneration. Adv Mater. 2022;34:e2106723. 10.1002/adma.202106723 [DOI] [PubMed] [Google Scholar]
- 89.Tian T, Xiao D, Zhang T, Li Y, Shi S, Zhong W, et al. A Framework Nucleic Acid based robotic nanobee for active targeting therapy. Adv Funct Mater. 2021;31:2007342. 10.1002/adfm.202007342 [DOI] [Google Scholar]
- 90.Zhang M, Zhang X, Tian T, Zhang Q, Wen Y, Zhu J, et al. Anti-inflammatory activity of curcumin-loaded tetrahedral framework nucleic acids on acute gouty arthritis. Bioact Mater. 2022;8:368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang Y, Li S, Zhang T, Zhang M, Chen Y, Wu Y, et al. Tetrahedral Framework nucleic acids inhibit skin fibrosis via the Pyroptosis Pathway. ACS Appl Mater Interfaces. 2022;14:15069–79. 10.1021/acsami.2c02877 [DOI] [PubMed] [Google Scholar]
- 92.Xiao L, Chen B, Wang W, Tian T, Qian H, Li X, et al. Multifunctional Au@AgBiS2 nanoparticles as high-efficiency Radiosensitizers to induce pyroptosis for Cancer Radioimmunotherapy. Adv Sci (Weinh). 2023;10:e2302141. 10.1002/advs.202302141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.