Abstract
Background
Early gastric cancer is treated endoscopically, but patients require surveillance due to the risk of metachronous gastric lesions (MGLs). Epigenetic alterations, particularly aberrant DNA methylation in genes, such as MIR124-3, MIR34b/c, NKX6-1, EMX1, MOS and CDO1, have been identified as promising biomarkers for MGL in Asian populations. We aimed to determine whether these changes could predict MGL risk in intermediate-risk Caucasian patients.
Methods
This case–cohort study included 36 patients who developed MGL matched to 48 patients without evidence of MGL in the same time frame (controls). Multiplex quantitative methylation-specific PCR was performed using DNA extracted from the normal mucosa adjacent to the primary lesion. The overall risk of progression to MGL was assessed using Kaplan–Meier and Cox proportional hazards model analyses.
Results
MIR124-3, MIR34b/c and NKX6-1 were successfully analyzed in 77 samples. MIR124-3 hypermethylation was detected in individuals who developed MGL (relative quantification 78.8 vs 50.5 in controls, p = 0.014), particularly in females and Helicobacter pylori-negative patients (p = 0.021 and p = 0.0079, respectively). This finding was further associated with a significantly greater risk for MGL development (aHR = 2.31, 95% CI 1.03–5.17, p = 0.042). Similarly, NKX6-1 was found to be hypermethylated in patients with synchronous lesions (relative quantification 7.9 vs 0.0 in controls, p = 0.0026). A molecular-based methylation model incorporating both genes was significantly associated with a threefold increased risk for MGL development (aHR = 3.10, 95% CI 1.07–8.95, p = 0.037).
Conclusions
This preliminary study revealed an association between MIR124-3 and NKX6-1 hypermethylation and the development of MGL in a Western population. These findings may represent a burden reduction and a greener approach to patient care.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13148-024-01712-z.
Keywords: Gastric cancer, Epigenetics, MicroRNAs, Molecular markers, Precision medicine
Background
Each year, gastric cancer (GC) is the cause of more than one million new cancer cases, leading to hundreds of thousands of fatalities [1]. This figure accounts for one in every 13 global deaths, ranking it as the fourth most prevalent contributor to cancer-related mortality [1]. Endoscopic submucosal dissection (ESD) is the gold standard for the management of early GC patients with a low risk of lymph node metastasis and is associated with an improved quality of life compared to gastrectomy [2, 3]. Despite its advantages, there is a non-negligible rate (10–20%) of metachronous gastric lesions (MGLs) detected during surveillance, manifesting at least 1 year after the initial ESD [4, 5]. Although Helicobacter pylori (HP) eradication after ESD has the potential to reduce the likelihood of MGL development, the challenge lies in accurately identifying individuals at a greater risk of developing these lesions [6]. Current clinicopathological findings, including HP infection status, lack reliable predictions [7]. Although several risk factors have been identified, such as older age, male sex, severe mucosal precancerous changes, persistent HP infection and family history of GC, they do not comprehensively explain the overall risk associated with MGL development [8].
The definition of molecular panels for a better MGL risk assessment is highly important and will further impact future care toward a greener endoscopy [9]. Epigenetic alterations, namely aberrant DNA methylation, are deeply involved in carcinogenesis, particularly in chronic inflammation-associated tumors, such as GC [10]. Recently, there has been increasing interest in exploring the role of DNA methylation-based markers in the context of MGL development [11–15]. For instance, Kubota et al. [11] highlighted the potential utility of cysteine dioxygenase type 1 (CDO1) hypermethylation in tumor-adjacent noncancerous mucosa as a useful indicator of MGL risk. Another study by Asada and Maeda et al. [14, 15] revealed a correlation between higher methylation levels of microRNA (MIR)124–3, empty spiracles homeobox 1 (EMX1) and NK6 homeobox 1 (NKX6-1) and an increased incidence of MGL. Suzuki et al. [13] demonstrated a strong association between MIR34b/c methylation and the risk of MGL, with the cumulative incidence being significantly greater among patients exhibiting increased levels. Additionally, the MOS proto-oncogene, serine/threonine (MOS) hypermethylation has emerged as a promising marker for predicting metachronous recurrence after endoscopic resection [12].
Despite the valuable insights from these studies conducted in Asian populations, translating them to lower than high-GC-risk regions or other ethnic populations requires validation due to the intricate interplay between genetic background, environmental exposure and lifestyles that modulate molecular-based signatures or patterns [16–18]. While discovering studies using screening strategies, like methylation sequencing, are valuable, validation studies are crucial for confirming the applicability of findings across diverse populations. Validation ensures that biomarkers are robust, reliable and generalizable across various clinical settings, which is an essential step for developing effective clinical decision rules, guidelines that help clinicians make informed diagnostic and treatment decisions [19]. Furthermore, focusing on known candidates from previous studies allows for a more resource-efficient research process, directing efforts toward the most promising biomarkers.
This study bridges the gap regarding the applicability of these epigenetic markers across ethnically diverse populations, shedding light on their utility in predicting MGL risk in Western settings.
Materials and methods
We aimed to explore all the previously reported aberrant DNA methylation biomarkers (MIR124-3, MIR34b/c, NKX6-1, EMX1, MOS and CDO1) in an intermediate-risk Caucasian population and assess their feasibility as predictors for MGL.
Patients and sample selection
A case–cohort study was performed on a cohort of patients previously treated by ESD for a primary superficial lesion, with a minimum of 36 months of endoscopic surveillance (n = 263). All patients who developed MGL were included (n = 42), as well as 48 patients without MGL detected during the time frame of the cohort who were matched for age, sex, HP infection status, lesion histology and location.
The full cohort of patients is described in the original study by Rei et al. [20], and the data were reviewed up to December 2023. The inclusion criteria for MGL patients stipulated that patients must have undergone ESD for a primary superficial gastric lesion, local-risk resection not suitable for surgery, with a minimum of 3 years of subsequent endoscopic surveillance. MGL was defined as any lesion identified after the initial endoscopic surveillance that was located at a different site from the index lesion. The exclusion criteria encompassed patients with a primary lesion unfit for ESD, patients who required surgical resection following ESD due to noncurative resection and patients with an endoscopic follow-up period of less than 3 years after resection. Persistent HP infection was defined as the presence of histopathological evidence of HP in gastric biopsy samples from the gastric antrum and/or corpus persisting beyond 1 year after ESD, irrespective of eradication attempts. Synchronous gastric lesions were defined as those detected within 6 months of ESD, either at the initial endoscopy, during the ESD procedure itself or at the first follow-up endoscopy. Corpus intestinal metaplasia (IM) was considered present if documented in the histopathological reports and/or an endoscopic grading of gastric intestinal metaplasia (EGGIM) score of ≥ 5 was reported in endoscopic findings, assessed by an experienced gastroenterologist trained in chromoendoscopy with narrow-band imaging.
DNA extraction, bisulfite treatment and preamplification
All formalin-fixed paraffin-embedded (FFPE) samples were reviewed by an experienced pathologist. DNA extraction from the normal-appearing mucosa adjacent to the primary lesion was performed using an AllPrep DNA/RNA FFPE Kit (Qiagen, cat. no. 80234, Hilden Germany) following the manufacturer’s instructions, and the DNA was stored at − 80 °C until further use. DNA concentrations were determined using a Qubit 4 Fluorometer (Invitrogen, Waltham, Massachusetts, USA). The 100 ng of FFPE-derived DNA was subjected to bisulfite treatment whenever possible using the EZ DNA Methylation-Gold™ Kit (Zymo Research, cat. no. D5005, Orange, CA, USA) according to the manufacturer’s instructions. Eight μL of modified DNA was preamplified using SsoAdvanced™ PreAmp Supermix (Bio-Rad, cat. no. 1725160, Hercules, CA, USA). The final product was diluted at a ratio of 1:2 for FFPE-extracted DNA using sterile distilled water. DNA was successfully obtained from a total of 36 MGL patients, with exclusion criteria applied to patients with neuroendocrine tumors for this analysis.
Methylation analysis
A total of six genes were previously identified as methylation-based biomarkers for MGL development [11–15]. Specific methylation primers for four of the six genes, MIR124-3, MIR34b/c, EMX1 and NKX6-1, were designed according to the methods described by Lobo et al. [21] and are given in Table S1. Multiplex MIR124-3, MIR34b/c, NKX6-1 and actin beta (ACTB) and singleplex EMX1 reactions were performed using methylation-specific quantitative polymerase chain reaction (qMSP) with 1 μL of DNA as a template. ACTB was selected as the reference gene [22]. In brief, 5 µL of MasterMix Xpert Fast Probe with ROX (GRiSP, cat. no. GE30.0100, Porto, Portugal), 1 µL of bisulfite-treated DNA, a variable volume of primers and probe at 10 µM and sterile bidistilled water were added to each well in a total volume of 10 µL. Reactions were carried out in 96-well plates using an ABI 7500 Real-Time PCR System (Applied Biosystems, Waltham, Massachusetts, USA). All samples were run in triplicate. Human HCT116 DKO Non-Methylated DNA (Zymo Research, cat. no. D5014-1, Orange, CA, USA) was used as a negative control, and human HCT116 DKO Methylated DNA (Zymo Research, cat. no. D5013-2, Orange, CA, USA) was used as a positive control in a 1:5 dilution series (run in duplicate) to construct a standard curve for quantification and to determine the PCR efficiency of each plate. Two nontemplate controls were also included in every plate, assuring, together with the negative control, the absence of contamination and specificity for the methylated DNA template. All reactions were performed under the following thermal cycling conditions: 95 °C for 3 min and 45 cycles of 95 °C for 5 s and 64 °C for 30 s. Samples were excluded if the relative quantity of ACTB was < 800. For included samples where target methylation was not successfully detected, a quantity mean of 0 was assigned. Relative methylation levels were determined as the ratio of the mean quantity of each gene to the mean quantity of the reference gene (target/ACTB), multiplied by 1000 for easier tabulation [23].
Statistical analysis
Data processing and analysis were performed in RStudio (version 2023.9.1.494) using R software (version 4.3.2). Student’s test was performed to compare mean values between groups, and the corresponding nonparametric tests (Mann–Whitney) were applied when appropriate. Kaplan–Meier analysis was performed to assess the overall progression to MGL of patients based on the DNA methylation levels of the genes under study. Low or high expression was defined according to the median, followed by the optimal cutoff approach, which maximizes the difference in MGL development between the two groups and was determined by the maximally selected log-rank statistics from the “maxstat” R package. The log-rank test was used to compare curves, and progression to MGL estimates was visualized with the “survival” and “survminer” R packages [24, 25]. Cox regression analyses were carried out for each biomarker to quantify their individual associations with progression to MGL. Multivariate Cox regression was further performed to adjust for the presence or absence of synchronous lesions at baseline, which was identified as an independent variable of prognosis (Table 2). These analyses were conducted using the “finalfit” R package [26]. All p values were two-sided, and p values < 0.05 were considered to indicate statistical significance.
Table 2.
Univariate and multivariate Cox proportional hazards regression analyses for metachronous gastric lesion development according to methylated genes and clinical variables
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95%CI) | p value | aHR (95%CI) | p value | |
|
MIR124-3 [High expression vs Lowa] | ||||
| Median (59.80b) | 2.78 (1.26–6.12) | 0.011 | 2.31 (1.03–5.17) | 0.042 |
| Optimal cutoff (59.80b) | 2.78 (1.26–6.12) | 0.011 | 2.30 (1.03–5.13) | 0.042 |
|
NKX6-1 [High expression vs Lowa] | ||||
| Median (1.30b) | 2.02 (0.95–4.29) | 0.069 | 1.73 (0.80–3.75) | 0.162 |
| Optimal cutoff (1.37b) | 2.35 (1.11–4.99) | 0.026 | 2.01 (0.93–4.33) | 0.074 |
|
MIR34b/c [High expression vs Lowa] | ||||
| Median (26.70b) | 1.24 (0.59–2.60) | 0.564 | – | – |
| Optimal cutoff (23.75b) | 1.87 (0.87–4.04) | 0.111 | – | – |
| Synchronous lesions | ||||
| Absenta | – | |||
| Present | 3.56 (1.59–7.96) | 0.002 | 3.11 (1.38–7.01)c | 0.006 |
| 3.01 (1.33–6.79)d | 0.008 | |||
| Family history | ||||
| Noa | – | – | – | |
| Yes | 2.40 (0.83–6.91) | 0.106 | – | – |
| MI corpus | ||||
| Absenta | – | – | – | |
| Present | 2.33 (0.81–6.71) | 0.116 | – | – |
|
Molecular-based model [High expression* vs Lowa] | ||||
| Median | – | – | 3.10 (1.07–8.95) | 0.037 |
| Optimal cutoff | – | – | 3.10 (1.07–8.95) | 0.037 |
Values were considered statistically significant if p < 0.1 in univariate analysis and p < 0.05 in multivariate analysis
CI, Confidence interval; HR, Hazard Ratio; IM, Intestinal metaplasia; aHR, adjusted multivariate analysis including the independent prognostic factors from the univariate analysis
aReference group
bExact values of median/optimal cutoff
cMedian
dOptimal cutoff
*MIR124-3 and/or NKX6-1 high expression versus MIR124-3 and NKX6-1 low expression
Results
Population description
Table 1 presents a summary of the relevant clinical, demographic and pathological data of the patients enrolled in this matched case–cohort study. Variables identified as risk factors for MGL by Rei et al. [19], namely age (> 65 years old), male sex, family history of GC, persistent HP infection, synchronous gastric lesions and corpus IM, were included. It is worth noting that all cases included in this analysis had documented IM in the mucosa adjacent to the lesion. Focusing on the matched baseline characteristics, the group of patients who developed MGL had an average age of 71 years and were predominantly male (64%), with 8% showing persistent HP infection. High-grade dysplasia (HGD) and intramucosal or submucosal adenocarcinoma (ADC) were the most commonly diagnosed postresection pathological World Health Organization (WHO) classifications (44% and 38%, respectively), with more than 70% of primary superficial lesions being located in the antrum. Regarding nonmatched variables, a statistically significant difference was observed in the presence of synchronous lesions at baseline, occurring in 33% of patients with MGLs, which was 25% greater than that in the group without MGLs (p = 0.002). Although not statistically significant (p = 0.07), there was a 22% greater prevalence of IM in the corpus among patients with MGL than among those without MGL. Moreover, having a positive family history of GC was significantly different (MGL = 31%, without MGL = 23%, p = 0.03). Nevertheless, it is worth noting that a considerable percentage of patients in both groups lacked available information. After removing those missing data, the significance was lost.
Table 1.
Clinicopathological features of all patients enrolled in this study
| Baseline characteristics | With MGL (n = 36) | Without MGL (n = 48) | p value |
|---|---|---|---|
| Age, mean (SD) | 71.36 (7.76) | 68.90 (9.70) | 0.25 |
| ≤ 65 years | 8 (23) | 15 (31) | |
| > 65 years | 28 (77) | 33 (69) | |
| Sex, n (%) | 0.73 | ||
| Female | 13 (36) | 20 (42) | |
| Male | 23 (64) | 28 (58) | |
| HP status, n (%) | 0.43 | ||
| Persistent | 3 (8) | 3 (6) | |
| Negative/eradicated | 32 (89) | 43 (90) | |
| Unknown | 1 (3) | 2 (4) | |
| Synchronous at baseline, n (%) | 0.002 | ||
| Present | 12 (33) | 4 (8) | |
| Absent | 24 (67) | 44 (92) | |
| Family history GC, n (%) | 0.03* | ||
| Positive | 11 (31) | 11 (23) | |
| Negative | 8 (22) | 24 (50) | |
| Unknown | 17 (47) | 13 (27) | |
| Corpus IM, n (%) | 0.07 | ||
| Present | 32 (89) | 32 (67) | |
| Absent | 4 (11) | 16 (33) | |
| Samples** | n = 39 | n = 50 | |
|---|---|---|---|
| Primary gastric lesion location | 0.99 | ||
| Antrum | 28 (72) | 36 (72) | |
| Body | 6 (15) | 7 (14) | |
| Angular incisure | 4 (10) | 6 (12) | |
| Other | 1 (3) | 1 (2) | |
| Histology type | 0.84 | ||
| LGD | 7 (18) | 9 (18) | |
| HGD | 17 (44) | 24 (48) | |
| ADC | 15 (38) | 17 (34) | |
p values were calculated using the Student’s t-test for continuous variables or X2 test for categorical variables. p < 0.05 was considered statistically significant
ADC, Adenocarcinoma; GC, Gastric cancer; HGD, High-grade dysplasia; HP, Helicobacter pylori; LGD, Low-grade dysplasia; MGL, Metachronous gastric lesion
*After adjusting for missing values, the statistical significance was no longer evident (p = 0.174)
**In instances of synchronous gastric lesions, multiple samples from the same patient might have been used
MIR124-3 is hypermethylated in metachronous gastric lesions
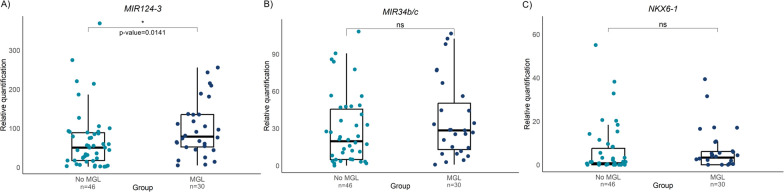
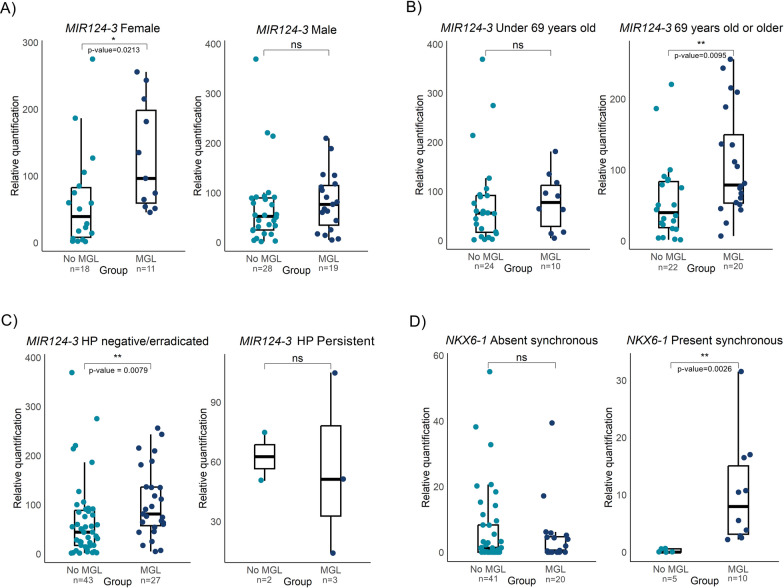
MIR124-3, MIR34b/c and NKX6-1 were successfully amplified and analyzed in a total of 77 samples. In instances where histological heterogeneity was reported between synchronous lesions, samples from each lesion were included. One sample was identified as an outlier and subsequently excluded from the analysis. Notably, hypermethylation of the MIR124-3 gene was detected in patients who developed MGL (median MGL = 78.8, N = 30; median no MGL = 50.5, N = 46, p = 0.014, Fig. 1), whereas no differences were detected in the MIR34b/c and NKX6-1 DNA methylation levels. Differential methylation status was further analyzed across subgroups. A statistically significant difference was observed in female patients, with higher levels of MIR124-3 methylation in the MGL group (median MGL = 96.3, N = 11; median no MGL = 39.3, N = 18, p = 0.02, Fig. 2A). Concerning age and HP status patterns, MIR124-3 methylation levels were significantly greater in older (median MGL = 78.8, N = 20; median no MGL = 39.8, N = 22, p = 0.0095) and HP-negative or HP-eradicated patients who developed MGL (median MGL = 81.1, N = 27; median no MGL = 44.3, N = 43, p = 0.0079, Fig. 2B, C), than in the non-MGL group. Regarding the presence of synchronous lesions, only NKX6-1 was hypermethylated in the MGL group (median MGL = 7.9, N = 10; median no MGL = 0, N = 5; p = 0.0026; Fig. 2D). No statistically significant differences were detected in the methylation patterns of the remaining subgroups (Figs. S1, S2, S3).
Fig. 1.
Overall A MIR124-3, B MIR34b/c and C NKX6-1 methylation levels between patients who developed metachronous gastric lesions (MGL) and patients who did not (No MGL)
Fig. 2.
Subgroup analyses of relative methylation levels of MIR124-3 and NKX6-1 stratified by demographic and clinical characteristics. Boxplots depicting the methylation levels of the MIR124-3 gene in A female and male patients, B patients grouped by age (younger than or older than 69 years) and C the patients with Helicobacter pylori (HP) infection. Similarly, D shows the NKX6-1 methylation levels in patients with and without synchronous gastric lesions
Aberrant MIR124-3 and NKX6-1 methylation is associated with the risk of developing metachronous gastric lesions
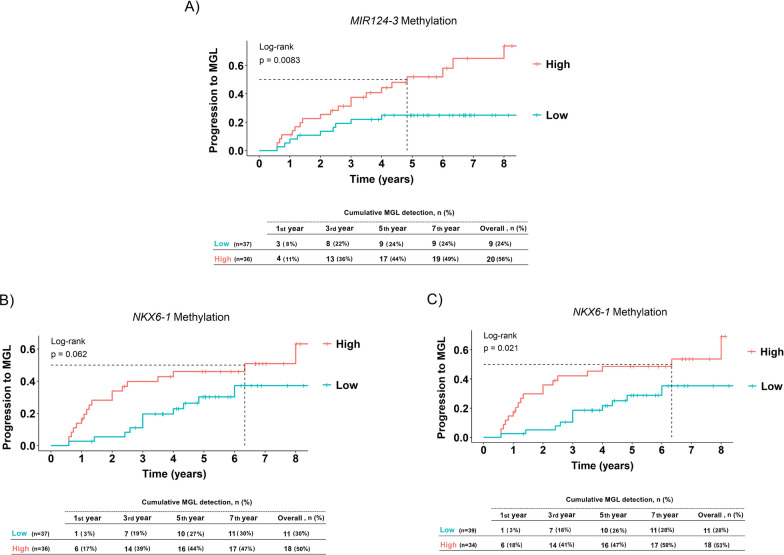
To assess the ability to predict MGL, we analyzed Kaplan–Meier curves depicting progression to MGL in groups stratified by low and high methylation levels of each gene, categorized based initially on their respective median values. These analyses were complemented by HR estimation and multivariate Cox regression modeling, adjusting for the presence of synchronous lesions, which was the only clinical factor identified as an independent prognostic variable in the univariate analysis (HR = 3.56, 95% CI 1.59–7.96, p = 0.002, Table 2).
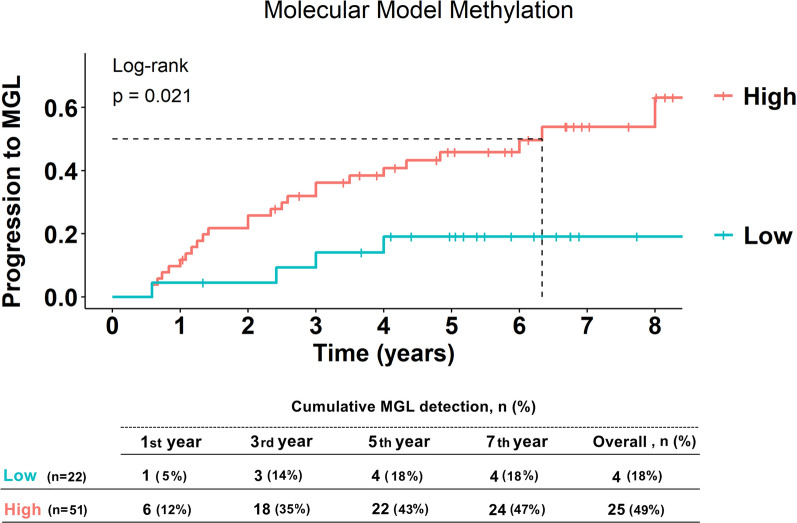
Notably, high methylation levels of MIR124-3 were significantly associated with progression to MGL (p = 0.0083, Fig. 3A), consistent with the univariate analysis (Table 2). Specifically, patients with elevated MIR124-3 methylation had an over twofold increased risk of developing MGL compared to those with lower levels, after adjusting for the presence of synchronous lesions (aHR = 2.31, 95% CI 1.03–5.17, p = 0.042). MIR34b/c did not exhibit a statistically significant difference in the progression to MGL between methylation levels (Fig. S4). On the other hand, there was a suggestive trend indicating that patients with high methylation levels of NKX6-1 might have an increased risk of developing MGL compared to those with lower methylation levels (p = 0.062, Fig. 3B), corresponding to a 1.7-fold increase in risk (HR = 1.73, 95% CI 0.80–3.75, p = 0.162, Table 2), which reached statistical significance considering the optimal cutoff value (p = 0.021, Fig. 3C, aHR = 2.01, 95% CI 0.93–4.33, p = 0.074, Table 2). Similar to the results observed for MIR124-3, 18 patients in the hypermethylated group were diagnosed with MGL (53%), nearly twice the number of individuals compared to the low-methylation group (28%). However, over the observed period, both groups exhibited a similar trend, with the majority of individuals developing lesions within 5-year follow-up (low-methylation group = 91%, high-methylation group = 89%). A DNA methylation model was constructed to further assess the impact of aberrant MIR124-3 and NKX6-1 methylation on MGL development. Patients with hypermethylation of MIR124-3 and/or NKX6-1 were more likely to develop MGL than those with a lower-methylation profile (p = 0.021, Fig. 4). This corresponded to a threefold increased risk of progression after adjusting for the presence of synchronous lesions (aHR = 3.10, 95% CI 1.08–8.95, p = 0.037, Table 2).
Fig. 3.
Kaplan–Meier curves showing progression to metachronous gastric lesions (MGL) according to risk group. Time to progression for A MIR124-3 with high versus low methylation levels and B NKX6-1 with high versus low methylation levels, using a median approach, and C for NKX6-1 using the optimal cutoff point approach. High-methylation groups were compared to low-methylation groups over a follow-up period of 8 years. The table inset in each panel displays the cumulative number and percentage of MGL detected at 1, 3, 5 and 7 years, as well as overall
Fig. 4.
Kaplan–Meier curves showing progression to metachronous gastric lesions (MGLs) according to risk group for the molecular-based methylation model, comprising the MIR124-3 and NKX6-1 genes
Discussion
In this preliminary study, we successfully validated one of the previously reported aberrantly methylated genes in Asian populations, MIR124-3, as a potential predictor of progression to MGL in Western Caucasians. Interestingly, in our intermediate-risk population, patients exhibiting hypermethylation of MIR124-3 and/or NKX6-1 at baseline accounted for more than 86% of all MGLs during the surveillance period.
The development of a predictive model for MGL has been a long-standing goal, and a recent meta-analysis by our group reported a greater risk of MGL after endoscopic resection (cumulative incidence at 5 years of 9.5%) and identified several risk factors for MGL development [8]. Subsequently, Rei et al. [20] established a predictive model, named FAMISH, integrating six clinical predictors, including positive family history, age older than 65 years, male sex, corpus IM, synchronous gastric lesions and persistent HP, to better estimate the risk of MGL development, which has been subsequently validated in other studies [27]. However, this scoring system may not capture 15% of MGL cases at 3-year follow-up, highlighting the relevance of additional research and validation of novel biomarkers [20].
A previous study showed that the methylation levels of the gastric mucosa could serve as a valuable predictor for GC development risk [28]. Furthermore, patients who have high methylation levels of specific marker genes tend to have greater MGL occurrence [14, 15]. Nevertheless, the identification of a marker gene that accurately indicates susceptibility to MGL remains elusive. Here, for the first time, we focused on the aberrant methylation profiles of CDO1, MOS, EMX1, MIR124-3, MIR34b/c and NKX6-1 genes and in a Caucasian population, as these genes have been previously associated with MGL exclusively in Asians [11–15]. To the best of our knowledge, those were the only studies that identified DNA methylation signatures for MGL development. However, due to the unsuccessful validation of the CDO1 and MOS primers and the lack of amplification of EMX1 in our samples, only the remaining three genes could be analyzed. A comprehensive analysis of MIR124-3 and NKX6-1 DNA methylation revealed interesting results. The MIR124-3 methylation level was significantly greater in patients who developed MGL, with the potential for predicting the risk of MGL development, as demonstrated by univariate and multivariate adjusted analyses and consistent with the findings of Asada et al. [14]. Moreover, there was significant MIR124-3 hypermethylation in females and HP-negative or HP-eradicated patients with MGL. This gene was reported to have a tumor-suppressive function and to be prone to methylation in several cancers [29–32]. Methylation of MIR124-3 leads to the silencing of its expression, which in turn reduces its ability to suppress tumorigenesis [29–32]. This epigenetic alteration may contribute to the development of MGL by facilitating an environment conducive to tumor growth and recurrence. Ando et al. [32] also suggested that high methylation levels of MIR124-3 in noncancerous tissue from GC patients could be associated with a predisposition to developing this type of cancer. There was a trend toward hypermethylation of NXK6-1 in individuals with MGL and this trend reached statistical significance, particularly in the presence of synchronous lesions. In fact, NKX6-1 hypermethylation was associated with an increased risk of developing MGL compared to low levels according to the univariate analysis. However, this association did not persist in the multivariate analysis, suggesting that the prognostic value of NKX6-1 may be confounded by other factors. These findings align with those of Asada et al. [14], who similarly reported significant associations between higher methylation levels of NKX6-1 and an increased risk of MGL development.
Despite the potential of MIR124-3 and NKX6-1 as biomarkers for MGL development, the results for MIR34b/c are less. Although there was a trend toward higher methylation levels in MGL patients, these results were not statistically significant. In a study by Suzuki et al. [13], patients with aberrant levels of MIR34b/c methylation had a greater incidence of MGL, suggesting its potential utility as a biomarker. However, our results do not replicate these findings, as our population did not exhibit significant differences in methylation levels between groups. The absence of significant findings for MIR34b/c, along with the lack of amplification of EMX1 in our cohort, may reflect differences between distinct ethnic groups. Various diseases have been associated with distinct DNA methylation patterns among different racial/ethnic groups [17]. For instance, disparities in methylation levels have been observed in cancers such as squamous cell carcinomas, prostate cancer and breast cancer, with variations contributing to disease onset, progression and clinical outcomes [17]. An exploratory study by Song et al. [33] focused on blood DNA methylation differences between Japanese-American and European-American women, identifying 174 differentially methylated sites in Japanese women, half of which were related to liver function and disease, aligning with the higher susceptibility of this ethnicity noted in previous studies. Eastern Asian countries are well documented to have a greater incidence of GC, which may be linked to significant levels of gene methylation in Asian populations [1]. However, these associations may not apply to other individuals, namely Caucasians. Given the intrinsic genetic variability and exposure to distinct environmental factors and lifestyle behaviors, expanding research to include larger and more diverse populations is essential to validate the findings and to explore their potential as MGL predictors in various ethnic groups. This expansion could uncover population-specific epigenetic markers, enhancing our understanding of disease progression in different demographic settings.
Currently, post-ESD surveillance guidelines recommend endoscopic examinations after 3–6 months, followed by annual screenings spanning a 5-year period [4]. Our analysis revealed that most patients who developed MGL in the low-MIR124-3 methylation group were diagnosed within 3 years, with only one case occurring thereafter. These findings suggest that patients with lower methylation levels of MIR124-3 might be suitable candidates for potentially less frequent surveillance assessments. Conversely, considering the significant increase in the risk of developing MGL among individuals with MIR124-3 hypermethylation, it is conceivable that these patients could benefit from a more rigorous surveillance regimen than the standard 5-year period. This assumption is supported by the fact that 15% of individuals developed MGL after the fifth year. These findings could guide clinicians in tailoring follow-up schedules and preventive strategies for patients based on their DNA methylation status. The construction of a DNA methylation model involving both the MIR124-3 and NKX6-1 genes represents a significant step toward understanding the synergistic effect of aberrant methylation on MGL development. The ability of the model to predict a threefold increased risk of progression, in contrast to the twofold increase associated with MIR124-3 hypermethylation alone, emphasizes the importance of considering a combination of molecular markers in clinical risk assessment. From a clinical perspective, obtaining biopsies next to the lesion or scar during baseline assessments, such as ESD or initial endoscopy for recurrence determination, could provide valuable information. The identification of significant methylation biomarkers may provide added value for confirming individualized management based solely on clinical factors. The predictive accuracy could be improved by epigenetic markers, leading to better use of healthcare resources, and enhancing the effectiveness of endoscopic surveillance [34].
While our study yielded promising results, a few limitations must be acknowledged. We used a relatively small sample size, although we included all patients who developed MGL from the original cohort study, restricting a more precise evaluation of biomarker performance. Additionally, it is important to recognize that the analysis was conducted on FFPE samples, which may undergo DNA damage caused by the paraffinization process, potentially affecting integrity and quality [35]. Despite our diligent approach in DNA extraction kit selection and primer design, these factors may hamper biomarker performance. Moreover, the need for a DNA preamplification step prior to qMSP analysis introduces the possibility of bias, while the multiplex approach enhances the probability of false positive results, which could impact cutoff points [36]. Additionally, challenges in primer design precluded the evaluation of the methylation profile of some genes previously identified as dysregulated in MGL, restricting our understanding of their specific contributions to our study population. Nevertheless, it should be noted that positive and negative DNA methylation controls were used to ensure primer specificity. Last, the lack of comprehensive clinical information, particularly concerning factors previously identified as risk factors, such as family history, may affect the interpretation of the biomarkers in a wider clinical context.
Future studies employing emerging techniques, such as digital PCR, may overcome the limitations faced in this study by enhancing the sensitivity of potential biomarkers, while maintaining specificity and omitting the need for DNA preamplification. Furthermore, integrating other methylated genes with MIR124-3 and NKX6-1 could contribute to the development of a more robust and precise methylation-based molecular model. Similarly, the incorporation of omics-based approaches may offer deeper insights into the molecular pathways involved in MGL development, paving the way for more personalized and effective diagnostic strategies. Additionally, considering that hypermethylation is observed in IM, studies focusing on both IM-positive and IM-negative mucosa could enhance the accuracy of the molecular model [37]. Nevertheless, in this study, the intent was to focus on the adjacent mucosa to the lesion as a proxy for the overall condition of the surrounding stomach tissue. The use of these data could further lead to management strategies during follow-up by endoscopic biopsies in noncancerous sites. Moreover, exploring the potential of liquid biopsies to monitor and manage MGL could significantly enhance personalized patient care, allowing for earlier detection and more precise treatment strategies. Finally, the validation of these biomarkers in larger patient cohorts and their integration with recently proposed clinical scores, namely FAMISH, could further enhance patient stratification through a better risk assessment for MGL development.
Conclusions
Overall, our study provides valuable insights for future research and clinical practice in the field of MGL and its association with epigenetic biomarkers. The identification of an aberrant methylation pattern in the MIR124-3 gene, as well as the impact of a DNA methylation model in MGL development, aligns with previous reports and contributes to the growing body of evidence on the importance of these genes. Nevertheless, further studies involving larger and diverse populations are warranted to validate these results. Additionally, the potential for reducing patient burden and transitioning toward more sustainable approach in patient care stands out as a significant implication of this study. By pinpointing high-risk individuals through molecular biomarkers, healthcare systems can streamline individualized surveillance efforts, advancing toward personalized medicine and sustainable strategies.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ACTB
Actin beta
- ADC
Adenocarcinoma
- CDO1
Cysteine dioxygenase type 1
- DNA
Deoxyribonucleic acid
- EMX1
Empty spiracles homeobox 1
- ESD
Endoscopic submucosal dissection
- FFPE
Formalin-fixed paraffin-embedded
- GC
Gastric cancer
- HP
Helicobacter pylori
- HR
Hazard ratio
- HGD
High-grade dysplasia
- IM
Intestinal metaplasia
- LGD
Low-grade dysplasia
- MIR
MicroRNA
- MGL
Metachronous gastric lesion
- MOS
MOS proto-oncogene serine/threonine
- NKX6-1
NK6 homeobox 1
- qMSP
Methylation-specific quantitative polymerase chain reaction
Author contributions
CP, M-DR and CJ assisted with conceptualization; CM-S and CJ helped with methodology; CL, TCA and CM-S carried out formal analysis and investigation; CL and TCA were involved in writing—original draft preparation; CM-S, JS, SP, CJ, DL, M-DR and CP contributed to writing—reviewing and editing; CP and M-DR took part in funding acquisition and supervision; and JC, SP and DL were responsible for resources.
Funding
This article results from the Project NORTE-01-0145-FEDER-000050, supported by the North Portugal Regional Operational Program (NORTE 2020), under the PORTUGAL 2020 partnership agreement, through the European Regional Development Fund (ERDF). Catarina Lopes (UI/BD/151488/2021) is a research fellowship holder supported by Fundação para a Ciência e Tecnologia (FCT), cofinanced by European Social Funds (ESF) and national funds of MCTES under the Human Strategic Reference Framework (POCH). Tatiana C. Almeida is a master research Grant holder under the core funding of the PRECAM group. Carina Pereira holds an Assistant Researcher position cofunded by the European Union (101095359 and 101101252 Grants) and by the UK Research and Innovation (10058099 Grant).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of IPO-Porto, CES IPO: 118/122.
Consent for publication
Written informed consent had been previously given by each patient authorizing the inclusion of surplus tissues in the institutional tumor biobank for future use in biomedical research.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Catarina Lopes and Tatiana C. Almeida contributed equally to this work and share first authorship.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Park E, Nishimura M, Simoes P. Endoscopic advances in the management of gastric cancer and premalignant gastric conditions. World J Gastrointest Endosc. 2023;15(3):114–21. 10.4253/wjge.v15.i3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libânio D, Braga V, Ferraz S, Castro R, Lage J, Pita I, et al. Prospective comparative study of endoscopic submucosal dissection and gastrectomy for early neoplastic lesions including patients’ perspectives. Endoscopy. 2019;51(1):30–9. 10.1055/a-0628-6601 [DOI] [PubMed] [Google Scholar]
- 4.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365–88. 10.1055/a-0859-1883 [DOI] [PubMed] [Google Scholar]
- 5.Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015;47(12):1113–8. 10.1055/s-0034-1392484 [DOI] [PubMed] [Google Scholar]
- 6.Salvatori S, Marafini I, Laudisi F, Monteleone G, Stolfi C. Helicobacter pylori and gastric cancer: pathogenetic mechanisms. Int J Mol Sci. 2023;24(3):2895. 10.3390/ijms24032895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung DH, Kim JH, Chung HS, Park JC, Shin SK, Lee SK, et al. Helicobacter pylori eradication on the prevention of metachronous lesions after endoscopic resection of gastric neoplasm: a meta-analysis. PLoS ONE. 2015;10(4):e0124725. 10.1371/journal.pone.0124725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortigão R, Figueirôa G, Frazzoni L, Pimentel-Nunes P, Hassan C, Dinis-Ribeiro M, et al. Risk factors for gastric metachronous lesions after endoscopic or surgical resection: a systematic review and meta-analysis. Endoscopy. 2022;54(9):892–901. 10.1055/a-1724-7378 [DOI] [PubMed] [Google Scholar]
- 9.Cunha Neves JA, Rodriguez de Santiago E, Pohl H, Lorenzo-Zúñiga V, Cunha MF, Voiosu AM, et al. Perspectives and awareness of endoscopy healthcare professionals on sustainable practices in gastrointestinal endoscopy: results of the LEAFGREEN survey. Endoscopy. 2024;56:355–63. 10.1055/a-2240-9414 [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Rong H, Xu J, Cao R, Li S, Gao Y, et al. DNA methylation: an important biomarker and therapeutic target for gastric cancer. Front Genet. 2022;13:823905. 10.3389/fgene.2022.823905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota Y, Tanabe S, Azuma M, Horio K, Fujiyama Y, Soeno T, et al. Predictive significance of promoter DNA methylation of Cysteine Dioxygenase Type 1 (CDO1) in metachronous gastric cancer. J Gastric Cancer. 2021;21(4):379–91. 10.5230/jgc.2021.21.e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin CM, Kim N, Yoon H, Choi YJ, Park JH, Park YS, et al. Aberrant DNA methylation maker for predicting metachronous recurrence after endoscopic resection of gastric neoplasms. Cancer Res Treat. 2022;54(4):1157–66. 10.4143/crt.2021.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki R, Yamamoto E, Nojima M, Maruyama R, Yamano HO, Yoshikawa K, et al. Aberrant methylation of microRNA-34b/c is a predictive marker of metachronous gastric cancer risk. J Gastroenterol. 2014;49(7):1135–44. 10.1007/s00535-013-0861-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asada K, Nakajima T, Shimazu T, Yamamichi N, Maekita T, Yokoi C, et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut. 2015;64(3):388–96. 10.1136/gutjnl-2014-307094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda M, Nakajima T, Oda I, Shimazu T, Yamamichi N, Maekita T, et al. High impact of methylation accumulation on metachronous gastric cancer: 5-year follow-up of a multicentre prospective cohort study. Gut. 2017;66(9):1721–3. 10.1136/gutjnl-2016-313387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64(11):1721–31. 10.1136/gutjnl-2014-308252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kader F, Ghai M. DNA methylation-based variation between human populations. Mol Genet Genomics. 2017;292(1):5–35. 10.1007/s00438-016-1264-2 [DOI] [PubMed] [Google Scholar]
- 18.Elliott HR, Burrows K, Min JL, Tillin T, Mason D, Wright J, et al. Characterisation of ethnic differences in DNA methylation between UK-resident South Asians and Europeans. Clin Epigenetics. 2022;14(1):130. 10.1186/s13148-022-01351-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heerink JS, Oudega R, Hopstaken R, Koffijberg H, Kusters R. Clinical decision rules in primary care: necessary investments for sustainable healthcare. Prim Health Care Res Dev. 2023;24: e34. 10.1017/S146342362300021X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rei A, Ortigão R, Pais M, Afonso LP, Pimentel-Nunes P, Dinis-Ribeiro M, et al. Metachronous lesions after gastric endoscopic submucosal dissection: first assessment of the FAMISH prediction score. Endoscopy. 2023;55(10):909–17. 10.1055/a-2089-6849 [DOI] [PubMed] [Google Scholar]
- 21.Lobo J, Constâncio V, Guimarães-Teixeira C, Leite-Silva P, Miranda-Gonçalves V, Sequeira JP, et al. Promoter methylation of DNA homologous recombination genes is predictive of the responsiveness to PARP inhibitor treatment in testicular germ cell tumors. Mol Oncol. 2021;15(4):846–65. 10.1002/1878-0261.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97. 10.1056/NEJMoa1311194 [DOI] [PubMed] [Google Scholar]
- 23.Macedo-Silva C, Constâncio V, Miranda-Gonçalves V, Leite-Silva P, Salta S, Lobo J, et al. DNA methylation biomarkers accurately detect esophageal cancer prior and post neoadjuvant chemoradiation. Cancer Med. 2023;12(7):8777–88. 10.1002/cam4.5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therneau T, Grambsch P. Modeling survival data: Extending the Cox model. 2000.
- 25.Therneau T. A package for survival analysis in R. 2023.
- 26.Harrison E, Drake T, Pius R. Quickly create elegant regression results tables and plots when modelling. R package version 1.0.8. 2024. https://github.com/ewenharrison/finalfit.
- 27.Niu Z, Liang D, Guan C, Zheng Y, Meng C, Sun X, et al. External validation of the FAMISH predicting score for early gastric cancer with endoscopic submucosal dissection. Eur J Gastroenterol Hepatol. 2024;36(1):26–32. 10.1097/MEG.0000000000002635 [DOI] [PubMed] [Google Scholar]
- 28.Yamashita S, Kishino T, Takahashi T, Shimazu T, Charvat H, Kakugawa Y, et al. Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues. Proc Natl Acad Sci U S A. 2018;115(6):1328–33. 10.1073/pnas.1717340115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y, et al. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014;392(1–2):153–9. 10.1007/s11010-014-2028-0 [DOI] [PubMed] [Google Scholar]
- 30.Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut. 2012;61(2):278–89. 10.1136/gut.2011.239145 [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Hu H, Zhao J, Zhang Z, Ai X, Tang L, et al. miR-124-3p acts as a potential marker and suppresses tumor growth in gastric cancer. Biomed Rep. 2018;9(2):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ando T, Yoshida T, Enomoto S, Asada K, Tatematsu M, Ichinose M, et al. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int J Cancer. 2009;124(10):2367–74. 10.1002/ijc.24219 [DOI] [PubMed] [Google Scholar]
- 33.Song MA, Seffernick AE, Archer KJ, Mori KM, Park SY, Chang L, et al. Race/ethnicity-associated blood DNA methylation differences between Japanese and European American women: an exploratory study. Clin Epigenetics. 2021;13(1):188. 10.1186/s13148-021-01171-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—update 2022. Endoscopy. 2022;54(6):591–622. 10.1055/a-1811-7025 [DOI] [PubMed] [Google Scholar]
- 35.Dietrich D, Uhl B, Sailer V, Holmes EE, Jung M, Meller S, et al. Improved PCR performance using template DNA from formalin-fixed and paraffin-embedded tissues by overcoming PCR inhibition. PLoS ONE. 2013;8(10):e77771. 10.1371/journal.pone.0077771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson D, Akrap N, Svec D, Godfrey TE, Kubista M, Landberg G, et al. Properties of targeted preamplification in DNA and cDNA quantification. Expert Rev Mol Diagn. 2015;15(8):1085–100. 10.1586/14737159.2015.1057124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi C, Yamashita S, Liu Y-Y, Takeshima H, Sasaki A, Fukuda M, et al. Precancerous nature of intestinal metaplasia with increased chance of conversion and accelerated DNA methylation. Gut. 2024;73(2):255–67. 10.1136/gutjnl-2023-329492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.