Abstract
Introduction
India grapples with a formidable health challenge, with an estimated 315 million adults afflicted with hypertension and 100 million living with diabetes mellitus. Alarming statistics reveal rates for poor treatment and control of hypertension and diabetes. In response to these pressing needs, the Community Control of Hypertension and Diabetes (CoCo-HD) program aims to implement structured lifestyle interventions at scale in the southern Indian states of Kerala and Tamil Nadu.
Aims
This research is designed to evaluate the implementation outcomes of peer support programs and community mobilisation strategies in overcoming barriers and maximising enablers for effective diabetes and hypertension prevention and control. Furthermore, it will identify contextual factors that influence intervention scalability and it will also evaluate the program’s value and return on investment through economic evaluation.
Methods
The CoCo-HD program is underpinned by a longstanding collaborative effort, engaging stakeholders to co-design comprehensive solutions that will be scalable in the two states. This entails equipping community health workers with tailored training and fostering community engagement, with a primary focus on leveraging peer supportat scale in these communities. The evaluation will undertake a hybrid type III trial in, Kerala and Tamil Nadu states, guided by the Institute for Health Improvement framework. The evaluation framework is underpinned by the application of three frameworks, RE-AIM, Normalisation Process Theory, and the Consolidated Framework for Implementation Research. Evaluation metrics include clinical outcomes: diabetes and hypertension control rates, as well as behavioural, physical, and biochemical measurements and treatment adherence.
Discussion
The anticipated outcomes of this study hold immense promise, offering important learnings into effective scaling up of lifestyle interventions for hypertension and diabetes control in low- and middle-income countries (LMICs). By identifying effective implementation strategies and contextual determinants, this research has the potential to lead to important changes in healthcare delivery systems.
Conclusions
The project will provide valuable evidence for the scaling-up of structured lifestyle interventions within the healthcare systems of Kerala and Tamil Nadu, thus facilitating their future adaptation to diverse settings in India and other LMICs.
Keywords: Diabetes, Hypertension, Scale-up, India, Structured lifestyle intervention, Peer support groups
Introduction
The burden of non-communicable diseases (NCDs) continues to rise in India. Globally, India had the highest number of people with cardiometabolic diseases in 2021: type 2 diabetes at 100 million, and hypertension at 315 million. The overall weighted prevalence of diabetes was 11.0%, and hypertension was 35.5% in 2016 [1]. The broader societal and economic impacts arising from this situation in India are now very substantial, with $3.55 trillion in productivity loss predicted by 2030 [2]. It is now critical for India’s health system to adopt explicit preventive and control strategies to address this problem.
It has been very challenging for state governments in India to control diabetes and hypertension, and the recent COVID-19 pandemic has exacerbated this further. About 59% of people with diabetes and 45% of people with hypertension in India are receiving treatment. Of these, 65% of people with diabetes and 52% of people with hypertension achieved control [3, 4]. As in most other LMIC settings, the efforts to improve outcomes for hypertension and diabetes in India are still mostly facility-based and curative. To address the issue, the Governments of Kerala and Tamil Nadu are implementing India’s comprehensive ‘National Program for Prevention & Control of Non-Communicable Diseases (NP-NCD), including programs such as Patient Support Groups (PSG) and Makkalai Thedi Maruthuvam (“drugs on doorsteps”), to combat NCDs. PSGs involve a community-based intervention to strengthen community-level efforts for better NCD control. The Makkalai Thedi Maruthuvam scheme screens those over 45 years and those with disabilities through routine door-to-door check-ups to detect NCDs [5].
The original Kerala Diabetes Prevention Program (K-DPP) was an evidence-based program that was carefully adapted from the Good Ageing in Lahti (GOAL) Lifestyle Implementation Trial in Finland [6] based on the earlier Finnish Diabetes Prevention Study [7] and the US Diabetes Prevention Program. It was conducted as a cluster-randomised controlled trial in the Trivandrum district of Kerala in 2012–2014 [8]. This trial demonstrated that lifestyle interventions involving community engagement, mobilisation, and peer support can significantly improve cardiovascular risk factors and health-related quality of life (QoL) [9]. Furthermore, it was adapted for broader implementation in Kerala in collaboration with the Kudumbashree Mission [10]. This approach to diabetes prevention has been recently adapted for improving the management of diabetes and hypertension along with other evidence-based programs in India, including the Control of Hypertension in Rural India (CHIRI) program for hypertension control, involving ASHA workers in three rural communities in India [11].
By integrating findings from these and other community-based trials in India and by leveraging the two states’ recent initiatives, this study now aims to create a scalable program model – called Community Control of Hypertension and Diabetes (CoCo-HD) - that aims to improve both behavioural and clinical outcomes related to hypertension and diabetes [11]. Currently, there is limited evidence about how to do this beyond research trials and at scale.
Objectives
Evaluate the implementation outcomes of a peer support program and community mobilisation strategy to improve the control of diabetes and hypertension.
Identify and address contextual factors within the community and health systems that act as enablers and barriers to scale-up in Kerala and Tamil Nadu.
Determine the program’s value and return on investment by assessing program cost and cost-effectiveness in Kerala and Tamil Nadu.
Study methods
Study context
The study is being conducted in the Southern states of India, specifically Kerala and Tamil Nadu, chosen based on local evidence highlighting significant gaps in care [3, 4]. According to recent data from the Indian Council of Medical Research–India Diabetes (ICMR-INDIAB) study, Kerala, with an estimated population of 35 million (with an average population size per district of about 2.5 million), exhibits a diabetes prevalence of 25.5%, significantly higher than the national prevalence of 11.4%, alongside a hypertension prevalence of 47.6% (compared to the national prevalence of 35.5%). Meanwhile, Tamil Nadu, with an estimated total population of 72 million, reports a diabetes prevalence of 14.4% and a hypertension prevalence of 38.3% among adults [12].
For the implementation of the CoCo-HD program, we selected two districts in Tamil Nadu: Villupuram with a population size of about 2.2 million, and Cuddalore with an estimated population size of about 2.7 million. Conversely, in Kerala, the intervention will be carried out across all 14 districts, as decided by the state government to ensure comprehensive coverage.
Scale-up approach
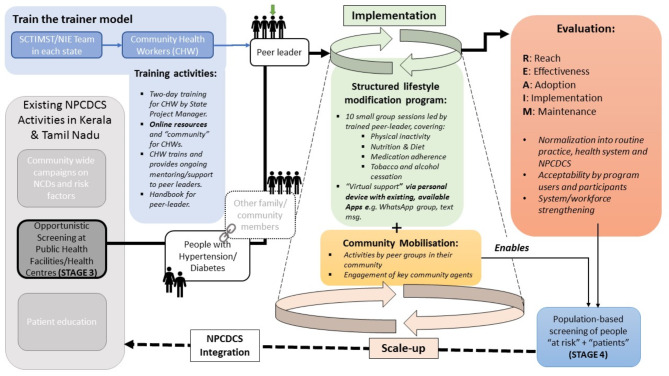
The total study period will be four years and will be conducted in three stages (summarized in Fig. 1 below).
Fig. 1.
Multi-level program delivery and iterative evaluation model
In the first stage, the two research teams from the National Institute of Epidemiology (NIE) and Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) have worked in close collaboration with state health departments of Tamil Nadu and Kerala, respectively, other relevant stakeholders in the respective states, in the co-design of the program. The advisory board of the program, chaired by the Director General of the Indian Council for Medical Research, guided the development of the program. Through various technical consultations with state health departments and using the lessons learned from K-DPP and other related programs, the team developed the broad components of the program.
In the second stage, we are co-designing context-specific implementation strategies. The co-design process involves a range of stakeholders. The stakeholders in this process are people with hypertension and diabetes in the community, residents of the included community, CHWs, primary health centre care providers, including doctors, and district program officials. We are conducting a series of consultations and technical discussions with the above stakeholders. We are visiting the community to observe the community-level activities. We are performing formative assessments to understand the needs and contextual factors associated with implementing the Peer Support Group (PSG) intervention. The target population for this assessment are people with hypertension and diabetes, community residents, and CHWs. We are prioritising the strategies using the APEASE (Acceptability, Practicability, Effectiveness, Affordability, Side-effects, Equity) criteria [13].
In the third stage, the intervention model will be implemented and evaluated in both states. We will conduct a baseline assessment of the control status of individuals with diabetes and hypertension before implementing the intervention model at community-level using CHWs. After 12 months of intervention delivery, we will evaluate implementation and effectiveness outcomes. In the fourth stage, we will propose refinements in the delivery of the program at scale, based on the learnings from the evaluation.
Needs assessment and development of the program
This goal-based intervention program focuses on adopting a healthy lifestyle and self-care behaviours related to improving hypertension and diabetes control. Individual-level goals for the components such as physical activity (PA), healthy diet, blood pressure/sugar control, body weight, and medication adherence will be set through a co-design approach in collaboration with the health system.
Training material is being developed for CHWs/Mid-Level Service Providers (MLSPs) to deliver community-based interventions for PSGs. We are reviewing the existing training materials and program guidelines in each state in developing the specific training material. Input has been received from subject matter experts, healthcare providers, and relevant stakeholders on the content, applicability, and suitability to the local context.
The training materials incorporate visualisation, information on disease, medication adherence, treatment outcomes, community mobilisation skills, and conducting PSG sessions. In addition to the training material, role specifications are being developed for CHWs to deliver the intervention. To facilitate the program delivery, we will also develop job aid that will consist of activities and discussion topics to be delivered through PSG sessions.
Implementation of CoCo-HD program
Intervention
PSGs will be formed with two PLs, one male and one female, to organize PSG session, faciliate dialogues and support within a group. Trained CHWs or MLSPs will deliver the structured intervention in the PSG sessions. These sessions will be conducted once a month per PSG. In a year, we aim to conduct 12–15 sessions, each lasting 60–90 min. There will be group discussions and group activities as part of each session. Each PSG will consist of 20–25 individuals with 10–14 individuals having hypertension and diabetes in Tamil Nadu and Kerala, respectively. Emphasis on medication adherence, lifestyle modification, and peer support will be delivered in the sessions to improve the control of hypertension and diabetes. Two peer leaders (PLs) will be selected among the individuals with hypertension and diabetes. The PLs will support the CHWs/MLSPs in conducting these sessions. The PSGs will also have their own WhatsApp groups to connect, interact, and share experiences.
Training for MLSPs in Kerala
The project team will conduct a two-day training program for MLSPs with 60–70 participants in each training group. The training will be conducted at the district level using customised study modules. During district-level meetings, the medical officers will be briefed about the program components and how they can support their implementation and evaluation. In coordination with the state health departments, continuous technical support will be provided to the district medical officers and MLSPs by the project teams.
Training of trainers in Kerala
The Kerala project team will initiate the training of selected MLSPs as trainers in all 14 districts They will introduce the pedagogy in 2–3 training sessions where the participants will get the opportunity to get involved in training under the supervision of the research team. Our research team will formally evaluate each trainer, and a graduation certificate will be issued upon completing the training. The graduated trainers will be officially inducted into the training team and conduct further training sessions within their respective districts.
Training of trainers in Tamil Nadu
This has involved a comprehensive needs assessment and evaluation which will be based on the Analysis, Design, Development, Implementation, and Evaluation (ADDIE) standards. Adopting the Training of Trainers model, our implementation team will prepare Program Officers, Medical Officers, Senior Staff Nurses, and selected CHWs to ensure effective scale-up. These trained trainers will then conduct training sessions for CHWs across the districts. Additionally, facilitator manuals for trainers and training manuals for participants will be developed to maintain training quality.
Training of PLs in Kerala
The CHWs/MLSPs will select and train the PLs. The training component will impart skills of group facilitation, communication, goal setting, and monitoring of lifestyle change. The MLSPs and PLs will receive a handbook of procedures to follow. The manual will highlight the roles and describe the contents of each session and the procedures they need to follow during the sessions to achieve the behavioural targets. The list of training and intervention materials for the program are shown in Table 1 below.
Table 1.
List of training and intervention materials for the CoCo-HD program
| SN | Type | Purpose | Users |
|---|---|---|---|
| 1 | Training materials and manuals | Guide the training of CHWs/MLSPs on the intervention and its implementation. | Trainers & CHWs/MLSPs; supervisors |
| 2 | Job Aids | Assist the CHWs in the facilitation of PSG sessions and activities. | CHWs & PLs in Tamil Nadu |
| 3 | Flipbooks | Directs CHWs to explain lifestyle modification to patients in a pictorial format | PLs & participants in Tamil Nadu |
| 4 | Standard operating procedures | Standardise the process of conducting patient group sessions | CHWs & PLs in Tamil Nadu |
| 5 | Participant Handbook and Factsheets | Guides participants and PLs to set, monitor, and evaluate goals. It also includes essential information about the selected topics for people with diabetes and hypertension. | PLs & participants |
| 6 | Monitoring checklists | To document measurements of fasting blood sugar (FBS), blood pressure, waist circumference, and medication adherence; and adoption of lifestyle modification measures; also includes provisions to monitor and record attendance and key parameters about the sessions. | CHWs/MLSPs |
Evidence-based interventions
The CoCo-HD program will implement evidence-based structured lifestyle interventions that will improve (1) the awareness, knowledge, and skills of people with diabetes and hypertension on disease management; (2) participation in PA; (3) the consumption of healthy eating and salt reduction; (4) medication adherence and self-management of disease; and (5) early detection and management of complications.
Implementation strategies
The CoCo-HD program implementation strategy has three elements. The first comprises PSG sessions and activities led by trained PLs and facilitated by CHWs/MLSPs. These will focus on PA, nutrition, diet, medication adherence, and tobacco and alcohol cessation. The second will be virtual support to CHWs/MLSPs and PLs via personal devices with existing, available apps, e.g., WhatsApp group, text message, etc. The third element will consist of supportive interventions such as monitoring blood pressure, blood glucose, waist circumference, body mass index (BMI), and medication adherence. Regular feedback on structured lifestyle modification (SLM) strategies and goals will be provided to the participants based on the values of the above measurements. The project teams in the two states will provide support in the implementation of these strategies.
Formation of PSGs
CHWs (Women health volunteers (WHV), Health Inspector, Block Health Supervisors, and Village Health Nurses)/MLSPs will identify the patients from the respective area; pre-inform the patients before the day of PSG, mobilise the patient to the venue, and facilitate the PSG. Two PLs will be selected from among the participants in each PSG. We will explore the usage of digital health interventions to improve the engagement of participants in PSGs.
Peer group sessions
The CHWs/MLSPs will choose a suitable venue and time each month. CHWs/MLSPS will deliver the structured intervention in the PSG sessions. Each session will have (1) a brief education by CHWs/MLSPs on a particular topic; (2) repetitive slogans; (3) group discussion about the planned topics with the use of case scenarios, myths, and facts on disease process and secondary prevention; (4) at least one group PA session; (5) measurement and documentation in follow-up register; and (6) a gift or appreciation expressed to a patient who has exhibited better control. These sessions will be conducted once a month. We will aim to conduct 12 sessions in a year. The participants will receive a factsheet on myths and facts about hypertension and diabetes. A participant card will be maintained for each participant in which monthly participation details, blood pressure, body weight, waist circumference, and random capillary blood sugar will be recorded by the CHWs/MLSPs in each session.
The topics for the PSG sessions, as co-developed and agreed with the respective state health are shown in Table 2 below. As outlined in the table, the topics for PSG sessions co-designed with the state health departments of the two states are similar except for inclusion of a topic on mental health and sleep in Tamil Nadu and Insulin in Kerala.
Table 2.
List of topics to be covered in the PSG sessions
| PSG topic | Tamil Nadu | Kerala |
|---|---|---|
| Introductory session | √ | √ |
| Preparatory phase | √ | √ |
| Goal setting | √ | √ |
| Diabetes and Hypertension | √ | |
| Healthy eating and salt reduction | √ | √ |
| Physical Activity | √ | √ |
| Tobacco and alcohol | √ | √ |
| Medication adherence | √ | √ |
| diabetes and hypertension complications | √ | √ |
| Mental Health and the importance of sleep | √ | |
| Self-monitoring and self-care | √ | √ |
| Insulin | √ | |
| Community resources | √ | √ |
| Goal review and planning for maintenance | √ |
While the contents of the intervention are largely the same between the two states, the implementation strategies will be context-specific. Accordingly, we will tailor our implementation strategies to the specific contexts of the two states. Table 3 summarises the contextualisation of the implementation strategies in the two states.
Table 3.
Contextualization of implementation strategies of the CoCo-HD program
| Implementation strategies | Tamil Nadu | Kerala |
|---|---|---|
| Training of CHWs | ||
| The actor | ICMR-NIE team and District Program Officers/ Medical Officers | SCTIMST team and District Programme Officers + ToT by selected MLSPs for further training under supervision. |
| The action | Face-to-face training | Face-to-face + online |
| Action target | MLSP and WHV | MLSPs in the entire state of Kerala |
| Temporality | August 2023 – Sept 2023 | December 2023 – March 2024 |
| Dose | 2 days (1 + 1); 6 h/ day | 1.5 days; 6 + 3 h |
| Outcome | Improved knowledge and skills in conducting PSG sessions | Empowered MLSPs in leading Peer group sessions in a structured and organized manner. |
| Justification | Training of the trainers and cascading to CHWs has been proven to be effective | A hybrid approach of face-to-face and online training mode is the preferred approach of the state health department. |
| PSG Sessions | ||
| The actor | MLHP and WHV | MLSPs |
| The action |
Conducting PSG sessions and activities: Repetitive slogan Special topic Activities Measurements |
Conducting PSG Sessions and activities: Set and review of goals Discussion on the core topics Initiate activities specific to the concerned sessions Plan for the forthcoming session Conduct study-specific measurements |
| Action target | People with diabetes and hypertension | People with diabetes and hypertension |
| Temporality | Oct 2023 – Sept 2024 | December 2023 – March 2025 (As the implementation is conceived in a phased manner across the state, with concurrent training and initiation of implementation overlapping). |
| Dose | 12 sessions; 60–90 min | 12 sessions; 60–90 min |
| Outcome | Medication compliance, Achieve treatment targets of hypertension (Blood pressure control) and diabetes (Blood sugar control) |
Improved adoption of self-care practices. Improved medication adherence. Weight and waist circumference reduction. Improvement in consumption of fruits and vegetables. Reduction in daily salt use. Improved control rates of hypertension and diabetes. |
| Justification | Monthly sessions have been used and proven to be effective in other settings. | Monthly sessions have been used and proven to be effective in other settings. |
| Community engagement activities | ||
| The actor | MLHP and WHV | MLSPs, Medical Officers and Supervisors |
| The action | Community Mobilisation during outreach activities, Information, Education, Communication (IEC) Provisions, Advertisements such as announcements, elevation of banners/posters, Peer Selection, and Reminders | Activities to engage and ensure more community participation in building awareness of the need for lifestyle modifications. |
| Action target | People with diabetes and hypertension and the community | Adults of all age groups |
| Temporality | 18 months | 18 months |
| Dose | Monthly once | One session: In a phased manner |
| Outcome | Community sessions conducted | Improved community awareness. Improved readiness to participate in structured lifestyle modification intervention sessions. |
| Justification | Community engagement is critical for effectively implementing PSG sessions and activities. | Community engagement will improve the acceptability of MLSPs and PSG in managing hypertension and diabetes. |
Evaluation of CoCo-HD program
Study design
We will u undertake a hybrid type III trial focusing on implementation outcomes. Given that the CoCo-HD program is a scale-up of evidence-based lifestyle interventions, this is the most appropriate design. Pre-post design (baseline and end-line assessments) with a mixed-method approach will be used to evaluate implementation outcomes. A template for the Intervention Description and Replication (TIDR) checklist will be used to describe the intervention’s structure [14].
Study population
The CoCo-HD program is developed on the background of both states putting major emphasis on diabetes and hypertension control and how they are planning to addressthese public health issues, especially following Covid-19. Both states have issued a Government Order regarding the details of the CoCo-HD program. In line with this, Individuals diagnosed with diabetes and hypertension by the health systems of Tamil Nadu and Kerala will be our target population.
Inclusion criteria
Individuals with diabetes and hypertension irrespective of gender.
Aged 18 years and above.
Residents of selected study districts for at least one year.
Exclusion criteria
Individuals with terminal, debilitating illnesses will be excluded due to differences in their care-seeking behavior, treatment outcomes, and low possibility of participating in the proposed intervention.
Individuals who were bedridden due to illness.
Operational definitions
Based on the NP-NCD, we define key terms as follows:
People with diabetes
Anyone aged 18 years and above reported FBS ≥ 126 mg/dl & PPBS ≥ 200 mg/dl or receiving medication for diabetes.
People with hypertension
Anyone aged 18 years and above with Systolic BP ≥ 140 mm Hg or Diastolic BP ≥ 90 mm Hg on two different occasions or receiving medication for hypertension.
Control of blood pressure
Hypertensive patients who achieved systolic blood pressure less than 140 mm Hg and diastolic blood pressure less than 90 mm Hg.
Control of blood glucose
DM patients who achieved FBS less than 126 mg/dl.
Regular follow-up
An individual registered for hypertension, diabetes, or both treatments with the State Government health facilities and visited the Primary Health Centre at least once in the previous three months at the time of reporting (as per the guideline, NP-NCD) [11].
Study participant selection and sampling technique
One administrative block will be selected randomly from each district of Tamil Nadu. Administrative blocks are geographical divisions under each district (coverage of population). We will select health sub-centres in each block by a multi-stage cluster random sampling along with probability proportional to the size of hypertension and diabetes patients from population-based screening registers.
Health sub-centres are the first point of contact of the public primary care system, which caters to a population of 3,000 to 5,000 on average. Each health sub-centre is operated by a CHW who maintains the line list of individuals diagnosed with diabetes and hypertension in their catchment area. The CHWs will select 20–25 individuals with diabetes, hypertension, or both from their line list.
In Kerala, all health sub-centres under the public health care system where MLSPs are posted will be included in the study. The MLSPs in Kerala are trained nurses. Approximately 4,400-4,500 MLSPs are posted in health sub-centres across Kerala. Each MLSP will form a PSG with at least 10 participants (maximum 15) as per inclusion criteria. Participants are patients with hypertension, diabetes, or both residing in the respective health sub-centre area. Approximately 40,000–45,000 people will participate in the PSG intervention across Kerala.
Sample size estimation
In estimating the sample size, we have considered multiple factors. The baseline control rates of hypertension and diabetes are 34% and 40%, respectively. To detect 20% improvements in both by the end of the program (12 months), these baseline control rates need to increase to 41% and 48%, respectively. We used the diabetes control rate in the power calculations as it provides a higher power. For those measured, the evaluation component will have 90% power to detect relative improvement of over 20% (ICC = 0.05, cluster size = 100, number of clusters = 82, alpha = 0.05). This will lead to an estimated 4,100 participants (100 participants each in 41 separate sub-centers) for Tamil Nadu.
In Kerala, the intervention component will be integrated with routine service delivery at the health sub-centre level. Our implementation model, with 40,000–50,000 participants from over 4,000 health sub-centres, will have more than 90% power to detect even an incremental increase in control rates (5%) in the pre-post evaluation.
Evaluation of implementation outcomes
We will use RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance). The RE-AIM dimensions of the CoCo-HD program evaluation will be computed from the data collected at community levels by CHWs. RE-AIM dimensions and indicators are summarized in Table 4 below.
Table 4.
RE-AIM dimensions & indicators for CoCo-HD program evaluation
| SN | Dimensions | Key indicators |
|---|---|---|
| 1 | Reach |
Tamil Nadu: The proportion of people with diabetes and hypertension enrolled in PSG in their respective communities. The proportion of CHWs trained on CoCo-HD program delivery. Kerala: Number of individuals with hypertension /diabetes or both approached and enrolled in the PSG; number of PSGs with a minimum of 10 participants; proportion or the number of individuals in absolute terms dropped-out from the PSG. The qualitative reach dimension will identify the influence of personal and contextual factors that contributed to the participation and non-participation in the intervention, the question of Why and Why not. |
| 2 | Effectiveness |
The proportion of people with diabetes and hypertension who achieved adequate control of blood pressure and blood glucose at the end of the intervention. The proportion of people with diabetes and hypertension who attended at least five PSG sessions. Qualitatively, we will explore factors that affected the effectiveness of the intervention from the perspectives of the provider and the beneficiaries, and whether they find the outcome of the intervention meaningful. |
| 3 | Adoption |
The proportion of people with diabetes and hypertension who have set at least one lifestyle goal. The proportion of CHWs/MLSPs who facilitated PSGs as part of the community health program. The proportion of CHWs/MLSPs who conducted at least nine (75%) PSG sessions. Qualitatively, we will explore the system factors that influence the adoption of PSG interventions and the enabling and challenging factors towards the adoption or initiation of the implementation. |
| 4 | Implementation |
The proportion of PSG sessions and activities delivered per the protocol. The proportion of PSG members who attended all 12 sessions. Qualitatively, we will explore adaptations and modifications in the intervention delivery. |
| 5 | Maintenance |
The proportion of people with diabetes and hypertension who maintained blood pressure and blood glucose within recommended ranges for six months after intervention. The proportion of PSGs who meet monthly after six months of intervention. The proportion of health sub-centres included in the study where the PSG intervention became part of routine care. Qualitatively, we will explore factors that facilitated the institutionalisation of PSG interventions in the routine management of hypertension and diabetes in the health sub-centres. At the individual-level, we will explore reasons for adherence and non-adherence to the intervention six months after implementation of the PSG. |
Outcome measures
Individual level outcomes
The primary outcome measures among the included individuals will be the proportion of individuals participating in the PSGs who achieve adequate control of blood pressure and FBS. CHWs will document the proportion of individuals who visited health facilities for regular follow-up. The proportion/actual number of individuals who participated in the peer group sessions will be documented and analysed. Other secondary outcome measures include behavioural variables (tobacco use, alcohol use, diet, PA, sedentary behaviour) and physical measurements (weight, waist, blood pressure) that will be collected using structured questionnaires at baseline and end-line.
Program level outcomes
Glasgow’s RE-AIM framework [15] will be used to evaluate the implementation outcomes of the program.
Data collection methods
We will train CHWs through the trainer of trainers’ model for measuring physical parameters and behavioural patterns of the individuals registered and followed up in the PSGs. CHWs will record the measurements and attendance in the PSG on the information system adopted by the state health department. Everyone in a PSG will receive a participant card where measurements will be recorded during the PSG. Data recorded at baseline, monthly observations, and at end evaluation will be converted into digital records for analysis.
The research team will train the MLSPs in data collection, including physical and behavioural measurements. The date will be entered manually during or after each PSG session into a monitoring and evaluation booklet maintained by the MLSPs and in the participant’s handbook. Data collected at baseline, regular intervals, and at the end of sessions will be transferred to a digital platform with access to the concerned stakeholders at the state health department and the research team.
The data elements to be collected at baseline and follow-up assessments will include sociodemographic characteristics, medical history/chronic conditions, Lifestyle/behavioral factors [Diet, physical activity, tobacco use, alcohol use, sleep], medication adherence (using Morisky scale), Health service utilisation (short version), health-related quality of life (EQ-5D-5 L), and biochemical and anthropometric measurements (FBS, Blood pressure, waist circumference, BMI). In addition, PSG Monitoring data and unit costs of the intervention & health services will be collected at regular intervals throughout the program.
Assessing contextual factors – enablers and barriers
We will explore contextual factors, such as those within the health system and at the participant-level, that facilitate or hinder the implementation and scale-up of the program.
The research team will conduct semi-structured, in-depth interviews, key informant interviews, and focus group discussions (FGDs) with multiple stakeholders such as PSG participants, patients who did not show interest in participation in PSG interventions, dropped-out participants, MLSPs, the medical officers at primary health centres, other cadres of non-physician health workers, and the district health program managers during the implementation of the CoCo-HD program. The tools for the study will be conceived in an inductive manner with necessary iterations and corrections made as required. The queries in the qualitative research tools will be sequenced inductively to understand the perspectives about factors that act as enablers and barriers at different implementation phases. The interview guide, which has been built inductively, will be extended to the constructs of identified frameworks in implementation research, such as NPT, CFIR [16], and components of RE-AIM [17].
Key questions
The identification of enablers and barriers to implementation will be guided by the CFIR [16]. Using CFIR, we will formulate key questions on five domains of contextual factors that affect program implementation: intervention characteristics, outer setting, inner setting, characteristics of individuals, and process of implementation.
Enablers and barriers to further scaling-up of the program are based on NPT. NPT focuses on what groups or individuals do, rather than what they believe [18]. NPT provides a framework to understand how interventions are implemented, embedded, and integrated in healthcare settings [19]. By focusing on the interactions between contexts, actors, practices, and procedures, NPT can help facilitate the exploration of translational gaps between evidence, policy, and practice [20]. We have operationalised the constructs and sub-constructs of NPT for the evaluation of the CoCo-HD program in Table 5 as follows:
Table 5.
Operationalisation of NPT constructs & sub-constructs for the CoCo-HD program
| SN | Constructs | Operationalisation of the CoCo-HD program |
|---|---|---|
| 1 |
Coherence (What is the process? ) Data source: In-depth interviews and FGDs |
Definition: Enablers and barriers of how various actors make sense, had an explicit knowledge and understanding of the program and its associated elements when initiating the intervention. Experience by the actors (CHWs/MLSPs, Peer supporters, participants) who found it valuable to them and agreed to the usefulness and purpose of the CoCo-HD program. Key questions: How does the program differ from current practice? How does the program fit with current practice? To what extent do various actors share a common understanding of the program? What are the perceived benefits of the program to patients and the health system? What are factors inhibit the routine practice of the CoCo-HD program? |
| 2 |
Cognitive participation (Who performs the process? ) Data source: IDI and FGDs |
Definition: Enablers and barriers of buy-in, engagement, and commitment of the various actors in implementing the CoCo-HD program. Key questions: To what extent have the various actors bought into it? To what extent have the various actors engaged with it? To what extent have the various actors committed to it? To what extent have the various actors legitimized it? To what extent have the various actors supported the program over time? |
| 3 |
Collective action (How does the process get performed? ) Data source: IDI and FGDs |
Definition: Enablers and barriers to implementing and integrating the intervention into the health system. Key questions: What health system resources have been allocated to the program? How was the program operationalised during implementation in the existing context? Are there clear definitions of the roles and responsibilities? To what extent did the program get integrated into community health? |
| 4 |
Reflexive monitoring (How is the process understood? ) Data source: IDI and FGDs |
Definition: Enablers and barriers of formal and informal monitoring of the progress, benefits, and costs of the intervention by the various actors. How did the actors evaluate the program and its supportive tools, either individually or collectively? Key questions: How much feedback did the various actors provide during the program’s implementation? What are the various actors’ judgments about the effectiveness and usefulness of the program? What are the various actors’ judgments about the costs and benefits of the program? What are the suggestions to modify/improve the program? |
Study informants
To explore enablers and barriers to implementation of the CoCo-HD program from the health system, community, and participants’ perspective, our research team will select informants from five categories of respondents: (1) participants (people with diabetes and hypertension); (2) PLs; (3) CHWs (MLSPs, WHVs, and health inspects); (4) District Medical Officers and District Programme Managers; and (5) Health Program managers, State level stakeholders, and policymakers.
Data collection methods
We will conduct in-depth interviews and FGDs to identify barriers and facilitators of implementing the CoCo-HD program in the two states. The information saturation will determine the final number of in-depth interviews and FGDs. However, we will aim to conduct at least three 18–20 in-depth interviews with participants (including participants who continued and discontinued participation in the PSGs), 14–17 key informant interviews with MLSPs (including MLSPs providing interventions in different contexts; e.g. Rural, urban, coastal, terrain, etc.), 6–7 key informant interviews medical officers and district program managers, and 2–3 key informant interviews with each of state-level implementing officers, policymakers, and other Gatekeepers.
Economic evaluation of the CoCo-HD program
The economic evaluation of the CoCo-HD program will assess the cost of the interventions and estimate the program’s cost-effectiveness to draw inferences about the returns on investment associated with scale-up.
Costs of interventions
Estimation of costs of interventions
The cost analysis will assess the (added) costs of integrating the intervention into ongoing government delivery systems (such as NCD clinics, primary health centres, and sub-centres). These will include costs of adaptation of the intervention, training activities, structured lifestyle programs, and community engagement activities.
Variation of costs across contexts
The costing analysis will also explore how costs are likely to vary between the two states, and within the states based on geographic areas (rural versus urban), the categories of health workers and peers involved, and any economies of scale and scope that might occur with program scale-up. The scale-up costs will be adjusted for variations across geographical settings and population groups.
Data sources for unit costs
Our data sources for unit costs of the major activities will include program accounts/registers, financial accounts data from government departments in the two states, global data (e.g., WHO-CHOICE estimates for the region), and evidence from previous studies in Kerala and Tamil Nadu.
Estimation of cost-effectiveness
In this pre-post evaluation, the costs of implementing the intervention as described above plus any changes in the costs of healthcare service use (relative to baseline), including both public subsidies and out-of-pocket expenses during the intervention period, will be used to capture the incremental costs of the intervention. Data on follow-up, outpatient visits, admission, and days lost due to illness will be collected at baseline and follow-up using the main questionnaire. We will estimate the costs of these outcomes using unit costs from the health system and international estimates. We will compute the change in costs of these outcomes between baseline and follow-up to estimate any cost savings associated with the implementation of the program.
Outcomes will be reported as Quality-Adjusted Life-Years (QALYs) gained measured with EQ-5D-5 L converted into an index score using time-trade-off-based weights from the Indian population [21]. The EQ-5D-5 L comprises five dimensions: mobility, self-care, usual activities, pain, discomfort, and anxiety/depression. Each dimension has five levels of response categories ranging from ‘no problems’ to ‘severe problems.’ The QALYs will combine time lived and QoL into a single index number where ‘1’ corresponds to 1 year of full health and ‘0’ corresponds to being dead. We will estimate the program’s cost effectiveness as the ratio of program costs per participant to changes in QoL between baseline and follow-up assessments.
Data management and analysis
The collection of baseline data, monthly monitoring data, and follow-up data will be led by NIE and SCTIMST in collaboration with the state health departments of Tamil Nadu and Kerala. The original data will be owned by and stored in the state health departments as per their respective data management standards. Based on data sharing agreements with the respective state health departments, de-identified data will be shared to the collaborating research institutions. We will apply the following methods in the analysis of data.
Evaluation of outcomes
Using R programming, we will employ descriptive statistics, paired t-test, and repeated measure ANOVA to examine the changes in outcome variables between baseline and follow-up assessments. Mixed-effects models will be used to assess the program’s effects on diabetes and hypertension control, lifestyle factors, and medication adherence. The model will include the participants’ baseline status of blood glucose and blood pressure, medical treatment, participation in PSG interventions, and other behavioural characteristics. For monthly monitoring data, we will apply a time-series analysis to describe trends in blood glucose, blood pressure, waist circumference and BMI across the 12 months. Any skewed continuous outcome variable may be transformed before fitting this model. RE-AIM indicators will be described using adjusted proportions.
Identification of contextual factors
After transcription and translation of scripts from qualitative techniques, we will use Nvivo 14 to code, sort and summarize the data. We will develop a coding algorithm based on the constructs and sub-constructs of CFIR and NPT to code the same. Key findings on barriers and enablers will be presented based on the dimensions of NPT and CFIR.
Economic evaluation
We will collect data on program costs, direct costs (for health service utilisation, medications, and diagnostics), and indirect costs (loss of wages due to illness, transportation, food, and accommodation costs to seek health care). Unit costs for these components will be obtained from State Health Departments, National and International estimates, and previous studies. Cost-effectiveness analysis will be conducted from a health system perspective. Incremental Cost-Effectiveness Ratios [22] will be estimated by dividing the program + direct costs with the changes in QALYs between baseline and follow-up. All adjusted measures will be estimated using a generalised estimating equation gamma regression model for repeated measures. Sensitivity analyses will use different assumptions concerning program effectiveness, service delivery costs, and discount rates.
Discussion
The findings of this study will inform the future scale-up of community-based structured lifestyle interventions in in India and other LMIC to improve the control of hypertension and diabetes in LMICs by generating strong evidence on implementation outcomes and identifying the contextual factors that affect the scaling up of the interventions. It will also determine the costs and cost-effectiveness of the program. We will disseminate these findings and learnings to the State and National Government policymakers to inform future policy recommendations and public health action. One of the limitations of this research is that we will not be able to assess directly the community-level control of hypertension and diabetes. Since we are identifying individuals who are already diagnosed with hypertension and diabetes, we will not have access to data regarding the total number of people with diabetes and hypertension in the community, nor their control rates.
Acknowledgements
The Community Control of Hypertension and Diabetes Program is funded by the Australian National Health and Medical Research Council (NHMRC) through the Global Alliance for Chronic Diseases (GACD) (Grant ID:1169766). We acknowledge the State Health Departments of Tamil Nadu and Kerala, ICMR-National Institute of Epidemiology and Sree Chitra Institute for Medical Sciences and Technology, Baker Heart and Diabetes Institute, and the University of Melbourne for their support at different stages of the project. We also extend our thanks to Kai Wallens for his contributions to the preparation of this protocol manuscript.
Abbreviations
- ADDIE
Analysis, Design, Development, Implementation, and Evaluation
- BMI
Body Mass Index
- CFIR
Consolidated Framework for Implementation Research
- CHIRI
Control of Hypertension in Rural India
- CHW
Community Health Worker
- CoCo-HD
Community Control of Hypertension and Diabetes
- FBS
Fasting Blood Sugar
- FGD
Focus Group Discussion
- GOAL
Good Ageing in Lahti
- K-DPP
Kerala Diabetes Prevention Program
- LMIC
Low- and Middle-Income Country
- MLSP
Mid-Level Service Provider
- NCD
Non-Communicable Disease
- NP-NCD
National Program for Prevention & Control of Non-Communicable Diseases
- NPT
Normalisation Process Theory
- PA
Physical Activity
- PL
Peer Leader
- PSG
Peer Support Group
- QALY
Quality-Adjusted Life-Year
- QoL
Quality of Life
- RE-AIM
Reach, Effectiveness, Adoption, Implementation, and Maintenance
- SLM
Structured Lifestyle Modification
- TIDR
Template for the Intervention Description and Replication
- WHV
Women Health Volunteers
Author contributions
GP, PJ, KRT, TH and BO wrote the first version of the manuscript. Other authors made substantial contributions. All authors have read and approved the final version of the manuscript for submission.
Funding
The study was supported by the Australian National Health and Medical Research Council through the Global Alliance of Chronic Diseases (Grant ID: 1169766) to the Baker Heart and Diabetes Institute (Administering Institution) and ICMR-National Institute for Epidemiology and Sree Chitra Tirunal Institute for Medical Sciences and Technology (the collaborating research institutions in Tamil Nadu and Kerala) to inform the scaling-up of structured intervention for control of hypertension and diabetes led by the State Governments of Kerala and Tamil Nadu.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical considerations
The study was approved by the Institutional Human Ethics Committee of the National Institute of Epidemiology [ID: NIE/IHEC/2010-02], the Institutional Ethics Committee of the Shree Chitra Tirunal Institute of Medical Sciences and Technology, Thiruvananthapuram [ID: SCT/IEC/1423/SEPTEMBER-2019] and the Ethics Committee of the University of Melbourne [ID: 1955651]. The research has also obtained ethics clearance from Alfred Health [ID: 465/21]. Written informed consent will be obtained from the study participants. The project was also approved by India’s national Health Ministry Screening Committee (HMSC) based on ICMR Guidelines. Written informed consent will be obtained from those participants from whom additional information is to be collected.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Global Burden of Disease Group. The increasing burden of diabetes and variations among the States of India: the global burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6(12):e1352–62. 10.1016/S2214-109X(18)30387-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelgau MM, Karan A, Mahal A. The economic impact of non-communicable diseases on households in India. Globalization Health. 2012;8(1):9. 10.1186/1744-8603-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varghese JS, Anjana RM, Geldsetzer P, Sudharsanan N, Manne-Goehler J, Thirumurthy H, et al. National estimates of the adult Diabetes Care Continuum in India, 2019–2021. JAMA Intern Med. 2023;183(9):963–72. 10.1001/jamainternmed.2023.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varghese JS, Venkateshmurthy NS, Sudharsanan N, Jeemon P, Patel SA, Thirumurthy H, et al. Hypertension diagnosis, treatment, and control in India. JAMA Netw Open. 2023;6(10):e2339098–e. 10.1001/jamanetworkopen.2023.39098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Special Correspondent. TN CM launches Makkalai Thedi Maruthuvam scheme The Hindu. 2021.
- 6.Absetz P, Valve R, Oldenburg B, Heinonen H, Nissinen A, Fogelholm M, et al. Type 2 diabetes prevention in the real world: one-year results of the GOAL implementation trial. Diabetes Care. 2007;30(10):2465–70. 10.2337/dc07-0171 [DOI] [PubMed] [Google Scholar]
- 7.Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. The Finnish diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26(12):3230–6. 10.2337/diacare.26.12.3230 [DOI] [PubMed] [Google Scholar]
- 8.Mathews E, Thomas E, Absetz P, D’Esposito F, Aziz Z, Balachandran S, et al. Cultural adaptation of a peer-led lifestyle intervention program for diabetes prevention in India: the Kerala diabetes prevention program (K-DPP). BMC Public Health. 2018;17(1):974. 10.1186/s12889-017-4986-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sathish T, Williams ED, Pasricha N, Absetz P, Lorgelly P, Wolfe R, et al. Cluster randomised controlled trial of a peer-led lifestyle intervention program: study protocol for the Kerala diabetes prevention program. BMC Public Health. 2013;13(1):1035. 10.1186/1471-2458-13-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravindranath R, Oldenburg B, Balachandran S, Mini GK, Mahat K, Sathish T, et al. Scale-up of the Kerala Diabetes Prevention Program (K-DPP) in Kerala, India: implementation evaluation findings. Transl Behav Med. 2020;10(1):5–12. 10.1093/tbm/ibz197 [DOI] [PubMed] [Google Scholar]
- 11.Riddell MA, Mini GK, Joshi R, Thrift AG, Guggilla RK, Evans RG et al. ASHA-Led Community-based groups to Support Control of Hypertension in Rural India are feasible and potentially scalable. Front Med. 2021;8. [DOI] [PMC free article] [PubMed]
- 12.Anjana RM, Unnikrishnan R, Deepa M, Pradeepa R, Tandon N, Das AK, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474–89. 10.1016/S2213-8587(23)00119-5 [DOI] [PubMed] [Google Scholar]
- 13.Michie S, Atkins L, West R. The behaviour change wheel. A guide to designing interventions 1st ed Great Britain. Silverback Publishing. 2014;1003:1010. [Google Scholar]
- 14.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 15.Glasgow ER. ea. Evaluating the Public Health Impact of health promotion Interventions: The RE-AIM framework. Americal Journal of Public Health. 1999;89(9):1322-7. [DOI] [PMC free article] [PubMed]
- 16.Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022;17(1):75. 10.1186/s13012-022-01245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM planning and evaluation Framework: adapting to New Science and Practice with a 20-Year review. Front Public Health. 2019;7:64. 10.3389/fpubh.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci. 2018;13(1):80. 10.1186/s13012-018-0758-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huddlestone L, Turner J, Eborall H, Hudson N, Davies M, Martin G. Application of normalisation process theory in understanding implementation processes in primary care settings in the UK: a systematic review. BMC Fam Pract. 2020;21(1):52. 10.1186/s12875-020-01107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res. 2006;6:86. 10.1186/1472-6963-6-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amegah AK. Slum decay in Sub-saharan Africa: Context, environmental pollution challenges, and impact on dweller’s health. Environ Epidemiol. 2021;5(3):e158. 10.1097/EE9.0000000000000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaya AB, Caceres CF, Spicer N, Balabanova D. After the Global Fund: who can sustain the HIV/AIDS response in Peru and how? Glob Public Health. 2014;9(1–2):176–97. 10.1080/17441692.2013.878957 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.