Abstract
Objectives
Hepatitis C virus (HCV) infection poses a global health challenge. By the end of 2021, the WHO estimated that less than a quarter of global HCV infections had been diagnosed. There is a need for a public health tool that can facilitate the identification of people with HCV infection and link them to testing and treatment, and that can be customised for each country.
Methods
We derived and validated a risk score to identify people with HCV in Egypt and demonstrated its utility. Using data from the 2008 and 2014 Egypt Demographic and Health Surveys, two risk scores were constructed through multivariable logistic regression analysis. A range of diagnostic metrics was then calculated to evaluate the performance of these scores.
Results
The 2008 and 2014 risk scores exhibited similar dependencies on sex, age and type of place of residence. Both risk scores demonstrated high and similar areas under the curve of 0.77 (95% CI: 0.76 to 0.78) and 0.78 (95% CI: 0.77 to 0.80), respectively. For the 2008 risk score, sensitivity was 73.7% (95% CI: 71.5% to 75.9%), specificity was 68.5% (95% CI: 67.5% to 69.4%), positive predictive value (PPV) was 27.8% (95% CI: 26.4% to 29.2%) and negative predictive value (NPV) was 94.1% (95% CI: 93.5% to 94.6%). For the 2014 risk score, sensitivity was 64.0% (95% CI: 61.5% to 66.6%), specificity was 78.2% (95% CI: 77.5% to 78.9%), PPV was 22.2% (95% CI: 20.9% to 23.5%) and NPV was 95.7% (95% CI: 95.4% to 96.1%). Each score was validated by applying it to a different survey database than the one used to derive it.
Conclusions
Implementation of HCV risk scores is an effective strategy to identify carriers of HCV infection and to link them to testing and treatment at low cost to national programmes.
Keywords: epidemiology, risk factors, public health
Strengths and limitations of this study.
This study derived a risk score that provides a non-invasive and easily administered method to identify hepatitis C virus carriers and link them to treatment and care.
The risk score was based on and validated using two rounds of population-based, high-quality national surveys in Egypt.
The derivation of the risk score used only a few variables and thus may not adequately capture the complex epidemiology of hepatitis C virus infection.
The derived risk score is specific to Egypt and may not be applicable to populations in other countries.
Introduction
Hepatitis C virus (HCV) infection is a global public health challenge1 2 and a major cause of morbidity and mortality, resulting in liver cancer, fibrosis and cirrhosis.3 By the end of 2021, the WHO estimated that 58 million people were infected with HCV, but only 15 million of them were diagnosed and only 9 million received treatment.4 Direct-acting antivirals (DAA) offer highly effective treatment to cure this infection and to prevent progression toward severe forms of liver disease,5 as well as an opportunity to reduce HCV transmission through treatment as prevention.6 7 Accordingly, the WHO has set a global target to eliminate HCV infection as a public health problem by 2030.2 8
While DAAs are becoming accessible globally, it has been challenging to identify carriers of this infection so as to treat them, especially in the Middle East and North Africa (MENA), the region most affected by HCV infection and where most people with HCV infection remain undiagnosed.9 10 Limited resources have made it challenging for viral hepatitis programmes to find low-cost and cost-effective approaches to identify people with HCV. While mass testing and treatment programmes may be relevant in high-prevalence countries, other countries have relatively low HCV prevalence making such programmes less cost-effective.10–16 While low-cost point-of-care tests have been beneficial in some countries, such as Egypt,17 they remain relatively expensive for countries like Pakistan, which bear a substantial share of the global burden.18–20 There is a need for a public health tool that can assist in identifying persons potentially living with HCV, to link them to testing and treatment.
One such tool is the use of risk scores to identify individuals potentially living with HCV. A risk score comprises a small set of simple questions that can be used to assess the likelihood that an individual has a specific health condition,21–24 in this case, HCV infection. Such risk scores have proven influential as public health tools for a range of health conditions, such as diabetes.21–24
In this study, we demonstrate the application of this public health tool for HCV infection in Egypt, aiming to illustrate the public health value and practical utility of developing HCV risk scores in various countries. The risk score derived here is not intended for universal application across diverse settings; it is specifically designed for Egypt. However, the concept and analytical approach can be adapted to other countries by considering the local HCV epidemiology to determine the relevant factors and their respective weights for inclusion in a score tailored to each specific context.
Methods
Egypt Demographic and Health Surveys
The Egypt Demographic and Health Survey (EDHS) is a national survey that collected data pertaining to the health and demographics of a nationally representative sample of the resident population of Egypt, including HCV infection.25 26 The EDHS that included HCV biomarkers was conducted in 2008 and 2014 and used rigorous sampling methods.27 Details on study design, data collection and laboratory methods can be found in El-Zanaty and Way.25 26
HCV antibody testing was done using a third-generation ELISA, the Enzyme Immunoassay Adlatis EIAgen HCV Ab test (Adaltis, Montreal, Canada).25 26 All samples that were positive in the ELISA assay and 5% of the negative samples were then retested using a more specific assay, the chemiluminescent microplate immunoassay (CMIA ARCHITECT plus i1000SR, Abbott Diagnostic, USA).25 26 If a sample was positive in both the ELISA and the CMIA testing, it was also tested for current active infection, using real-time, reverse-transcription polymerase chain reaction testing to detect HCV RNA.25 26 Samples were further retested for internal and external quality assurance.25 26 Here we restrict our analyses to the HCV antibody results.
Data from the EDHS 2008 and EDHS 2014 were downloaded with permission from Measure DHS.28 The data can be accessed through an application to the DHS Program at https://dhsprogram.com. For purposes of this study, the EDHS individual database was merged with the HCV biomarker database, based on established guidelines for managing DHS data.27 All individuals with results for HCV antibody testing were included in the analysis.
Patient and public involvement
None.
Risk score derivation
Associations of HCV antibody positivity (seropositivity) with a priori variables that are easy to evaluate in a primary healthcare setting, and that can be included in a risk score, were investigated. These variables included sex (male vs female), age (5-year age strata) and type of place of residence (urban vs rural). Frequency distributions were generated to describe the demographic and clinical profiles of tested individuals.
X2 tests and univariable logistic regression were implemented to investigate associations. Participants younger than 15 years of age were excluded as this age group was not included in the EDHS 2008 and has low HCV prevalence (online supplemental table S1).6 29–31 ORs, 95% CIs and p values were reported. Covariates with p values≤0.1 in the univariable regression analysis were considered possibly associated with HCV seropositivity. These were included in the multivariable analysis for estimation of adjusted ORs and associated 95% CIs and p values. No other forward or backward elimination for variable selection was used. Covariates with p values≤0.05 in the multivariable model were considered predictors of HCV seropositivity. Univariable and multivariable analyses were adjusted for sampling weights.
bmjopen-2024-085506supp001.pdf (113.6KB, pdf)
A risk score was constructed based on the β-coefficients obtained from the multivariable regression model. β-coefficients were multiplied by a factor of 10 and then rounded to the nearest integer. The total risk score was calculated by adding the individual scores. To keep the score simple enough for use in primary healthcare and other general population settings, we did not consider any interaction terms.
Performance and validation of the risk score
A receiver operating characteristics (ROC) curve was plotted to investigate the performance of the risk score in predicting HCV seropositivity at different score cut-offs. A larger area under the curve (AUC), also called the c-index, indicates better performance of the risk score. The cut-off for the score was determined by maximising the sum of the sensitivity and specificity. Sensitivity is the probability that the risk score will yield a positive diagnosis in a subject who is truly HCV antibody-positive. Specificity is the probability that the risk score will yield a negative diagnosis in a subject who is truly HCV antibody-negative.
The performance of the risk score was also investigated by estimating the positive predictive value (PPV) and the negative predictive value (NPV) of the risk score. PPV is the probability that a subject with a positive diagnosis per the risk score is truly HCV antibody-positive. NPV is the probability that a subject with a negative diagnosis per the risk score is truly HCV antibody-negative. The proportion of subjects who have scores greater than or equal to the cut-off of the risk score was estimated to determine the proportion of individuals who need to be biochemically tested for HCV antibodies.
To validate the performance of the EDHS 2008 risk score, it was applied to the EDHS 2014 data, providing an independent validation with a data set different from the one used for its derivation. Performance diagnostics were subsequently assessed. Given the pronounced cohort effect in the epidemiology of HCV infection in Egypt,6 29–31 the age variable was adjusted to reflect the 6-year interval between the surveys. For example, individuals who were 11 years old in 2008 would have been 17 years old at the time of the second survey in 2014. The same approach was also used to validate the EDHS 2014 risk score—it was applied to the EDHS 2008 database and performance diagnostics were assessed.
While the cut-off for the score was determined by maximising the sum of sensitivity and specificity, this cut-off can be adjusted as needed from a programmatic standpoint to optimise a specific diagnostic metric, such as sensitivity instead of specificity. To illustrate this flexibility, an additional analysis was incorporated featuring a variety of score cut-offs, resulting in diverse values of sensitivity, specificity, PPV and NPV. Such additional analysis enables programme managers and readers to discern the trade-offs among these diagnostic metrics and observe the implications of selecting an alternative programmatic approach, such as prioritising the optimisation of sensitivity over specificity.
Analyses were conducted in Stata V.16.1 (Stata Corporation, College Station, Texas, USA). The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (online supplemental table S2).
Results
In the 2008 EDHS, 11 126 individuals 15–59 years of age were tested, of whom 1571 were antibody-positive.25 The 2014 EDHS included children 1–14 years of age in addition to adults 15–59 years of age.26 In this latter survey, 26 047 individuals were tested of whom 1456 were antibody-positive.26
Characteristics of individuals who were tested for HCV antibodies and the proportion of each population stratum that was HCV antibody-positive are shown in online supplemental table S1 for both of the EDHS surveys. Results of both surveys were consistent, taking into account the age shift in the national cohort with the passage of 6 years between the EDHS 2008 and EDHS 2014.
HCV seropositivity was strongly associated with sex, age and place of residence in both national surveys (table 1 and online supplemental table S3). Male sex and rural residence were associated with higher seropositivity. Seropositivity increased rapidly with age.
Table 1.
Results of the multivariable regression analyses to derive the Egypt Hepatitis C Risk Score using data from EDHS 2008 and EDHS 2014
| EDHS 2008 | EDHS 2014 | |||||||
| aOR*† (95% CI) | P value | β‡ | Risk score§ | aOR*† (95% CI) | P value | β‡ | Risk score§ | |
| Sex | ||||||||
| Female | 1.00 | Ref | 0 | 1.00 | Ref | 0 | ||
| Male | 1.52 (1.34 to 1.73) | <0.001 | 0.42 | 4 | 1.62 (1.40 to 1.87) | <0.001 | 0.48 | 5 |
| Age group (years) | ||||||||
| 15–19 | 1.00 | 0 | 1.00 | 0 | ||||
| 20–24 | 1.23 (0.89 to 1.69) | 0.213 | 0.20 | 2 | 3.30 (1.88 to 5.81) | <0.001 | 1.19 | 12 |
| 25–29 | 1.60 (1.15 to 2.23) | 0.005 | 0.47 | 5 | 4.50 (2.60 to 7.79) | <0.001 | 1.51 | 15 |
| 30–34 | 3.21 (2.35 to 4.39) | <0.001 | 1.17 | 12 | 7.41 (4.35 to 12.65) | <0.001 | 2.00 | 20 |
| 35–39 | 3.89 (2.84 to 5.34) | <0.001 | 1.36 | 14 | 8.74 (5.13 to 14.88) | <0.001 | 2.17 | 22 |
| 40–44 | 7.36 (5.47 to 9.90) | <0.001 | 1.99 | 20 | 13.03 (7.79 to 21.81) | <0.001 | 2.57 | 26 |
| 45–49 | 10.34 (7.71 to 13.85) | <0.001 | 2.34 | 23 | 19.23 (11.66 to 31.69) | <0.001 | 2.96 | 30 |
| 50–54 | 16.43 (12.29 to 21.96) | <0.001 | 2.80 | 28 | 41.11 (25.05 to 67.46) | <0.001 | 3.71 | 37 |
| 55–59 | 17.05 (12.50 to 23.26) | <0.001 | 2.84 | 28 | 55.31 (33.59 to 91.06) | <0.001 | 4.01 | 40 |
| Type of place of residence | ||||||||
| Urban | 1.00 | Ref | 0 | 1.00 | Ref | 0 | ||
| Rural | 2.34 (2.0 to 2.7) | <0.001 | 0.85 | 9 | 2.15 (1.78 to 2.59) | <0.001 | 0.76 | 8 |
*The analysis applied the EDHS sampling weights.
†The OR was adjusted for sex, age and type of place of residence.
‡β-coefficients were based on the multivariable regression analysis.
§The risk score was calculated by multiplying the β coefficient by 10 and then rounding the result to the nearest integer.
aOR, adjusted OR; EDHS, Egypt Demographic and Health Survey; Ref, reference category.
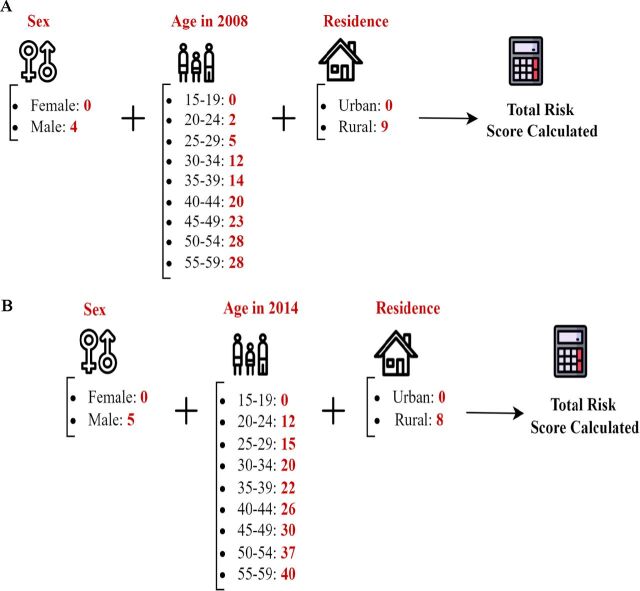
The 2008 and 2014 Egypt Hepatitis C Risk Scores derived using the EDHS 2008 and EDHS 2014 data, respectively, are shown in figure 1. The 2008 risk score had a range of 0–41. The 2014 risk score had a range of 0–53. Both showed similar dependence on sex, age and type of place of residence. Both demonstrated high and similar AUCs (figure 2). The AUC was 0.77 (95% CI: 0.76 to 0.78) for the 2008 risk score and 0.78 (95% CI: 0.77 to 0.80) for the 2014 risk score. The highest sum of sensitivity and specificity was obtained at a score cut-off value of 22 for the 2008 risk score and at a cut-off of 34.5 for the 2014 risk score.
Figure 1.
Mathematical formula of the derived Egypt Hepatitis C Risk Score. (A) Egypt Hepatitis C Risk Score using the EDHS 2008. (B) Egypt Hepatitis C Risk Score using the EDHS 2014. The two scores exhibited comparable structures and diagnostic performances, with minor differences attributed to sampling variation of the same population across two distinct rounds of the EDHS surveys. Details on the derivation of these scores are provided in the Methods and Results sections. EDHS, Egypt Demographic and Health Survey.
Figure 2.
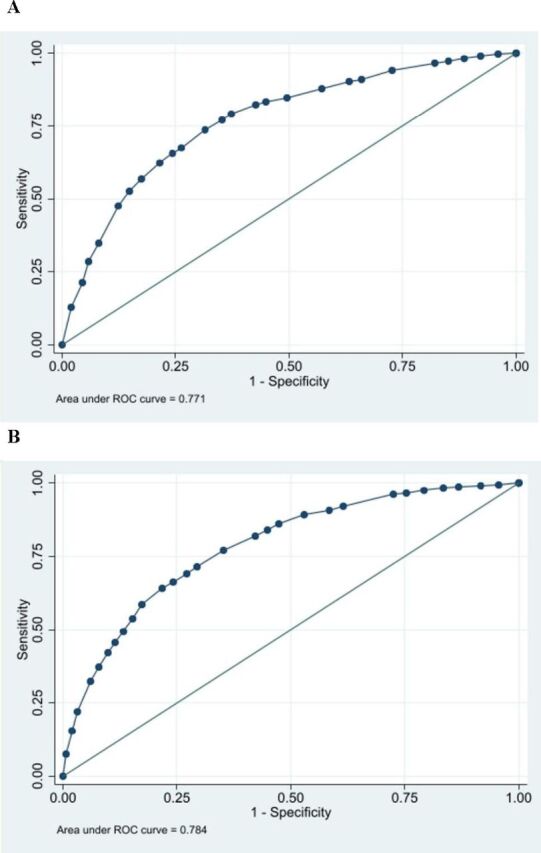
Diagnostic performance of the Egypt Hepatitis C Risk Score using the area under the (ROC) curve. (A) Using the EDHS 2008 data. (B) Using the EDHS 2014 data. EDHS, Egypt Demographic and Health Survey; ROC, receiver operating characteristics.
For the 2008 risk score, sensitivity was 73.7% (95% CI: 71.5% to 75.9%), specificity was 68.5% (95% CI: 67.5% to 69.4%), PPV was 27.8% (95% CI: 26.4% to 29.2%) and NPV was 94.1% (95% CI: 93.5% to 94.6%) (table 2). For the 2014 risk score, sensitivity was 64.0% (95% CI: 61.5% to 66.6%), specificity was 78.2% (95% CI: 77.5% to 78.9%), PPV was 22.2% (95% CI: 20.9% to 23.5%) and NPV was 95.7% (95% CI: 95.4% to 96.1%). The proportion of the population 15–59 years of age that needed to be biochemically tested for HCV antibodies was 37.2% (95% CI: 36.3% to 38.1%) using the 2008 risk score and 25.5% (95% CI: 24.9% to 26.2%) using the 2014 risk score. Of all people with HCV in the EDHS samples, application of this score would have diagnosed (that is identified; sensitivity) 73.7% (95% CI: 71.5% to 75.9%) and 64.0% (95% CI: 61.5% to 66.6%) of all these persons in samples of the EDHS 2008 and 2014, respectively.
Table 2.
Performance of the Egypt Hepatitis C Risk Score
| AUC (95% CI) | Risk score cut-off* | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Proportion needing testing (95% CI) | |
| Derived risk scores | |||||||
| Risk score derived using the EDHS 2008 | 0.77 (0.76 to 0.78) |
22.0 | 73.7 (71.5 to 75.9) |
68.5 (67.5 to 69.4) |
27.8 (26.4 to 29.2) |
94.1 (93.5 to 94.6) |
37.2 (36.3 to 38.1) |
| Risk score derived using the EDHS 2014 | 0.78 (0.77 to 0.80) |
34.5 | 64.0 (61.5 to 66.6) |
78.2 (77.5 to 78.9) |
22.2 (20.9 to 23.5) |
95.7 (95.4 to 96.1) |
25.5 (24.9 to 26.2) |
| Validation of risk scores | |||||||
| 2008 risk score applied to the EDHS data 2014† | 0.75 (0.74 to 0.77) |
22.0 | 66.1 (63.5 to 68.6) |
72.3 (71.5 to 73.1) |
21.9 (20.6 to 23.2) |
94.8 (94.3 to 95.2) |
31.7 (30.9 to 32.5) |
| 2014 risk score applied to the EDHS data 2008‡ | 0.76 (0.74 to 0.77) |
33.5 | 70.0 (67.5 to 72.6) |
70.0 (69.0 to 70.9) |
24.7 (23.3 to 26.1) |
94.3 (93.7 to 94.9) |
34.6 (33.7 to 35.5) |
*The optimal cut-off for the score was determined by maximising the sum of the sensitivity and specificity.
†The risk score assumes the age of the individuals in 2008 in order to account for the age shift.
‡The risk score assumes the age of the individuals in 2014 in order to account for the age shift.
AUC, area under the curve; EDHS, Egypt Demographic and Health Survey; NPV, negative predictive value; PPV, positive predictive value.
When the 2008 risk score was applied to the EDHS 2014 data, the AUC was 0.75 (95% CI: 0.74 to 0.77), the sensitivity was 66.1% (95% CI: 63.5% to 68.6%) and the specificity was 72.3% (95% CI: 71.5% to 73.1%) (table 2). These performance indicators were similar to the original performance indicators generated using the EDHS 2008 data, as well as to the performance indicators of the 2014 risk score on the EDHS 2014 data. Therefore, this application validates this risk score. A similar outcome was found when the 2014 risk score was applied to the EDHS 2008 data, also providing a validation of the 2014 risk score.
Figure 3 displays the proportion of HCV infections in the population that are diagnosed as a function of the proportion of the population that needs to be tested to identify these infections, using each of EDHS 2008 and EDHS 2014 data. The figure shows the effect of prioritisation of testing for those with higher to lower risk scores. This provides a demonstration of the utility of using the risk score: a large proportion of HCV infections can be diagnosed by testing only a small proportion of the population. It is most efficient programmatically to start testing individuals with the highest risk score and progressively moving on to those with lower and lower risk scores. As testing is expanded to those with low-risk scores, the yield in identifying more HCV infections is very limited.
Figure 3.
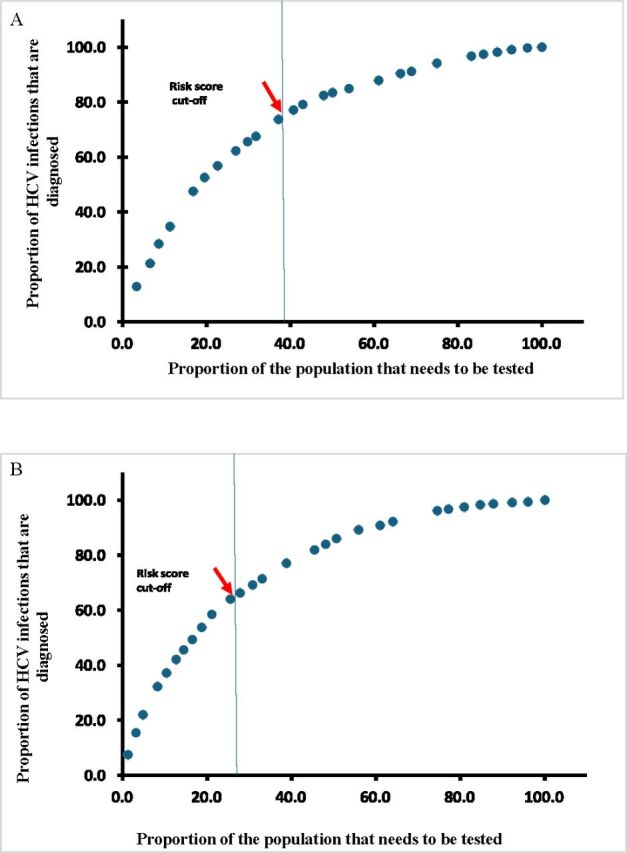
Proportion of HCV infections in the population that are diagnosed as a function of the proportion of the population that needs to be tested to identify these infections. The figure shows the effect of prioritisation of testing for those with higher to lower risk score. (A) Using the EDHS 2008 data. (B) Using the EDHS 2014 data. EDHS, Egypt Demographic and Health Survey; HCV, hepatitis C virus.
Table 3 illustrates the implications of selecting various score cut-offs, providing insight into the trade-offs among different diagnostic metrics, as well as the proportion of the population requiring biochemical testing and the proportion of all individuals with HCV identified through the application of this score. For instance, by enhancing the specificity of the risk score, the PPV increases, and the proportion of the population necessitating testing decreases. This reduction in testing requirements helps alleviate costs and streamline the logistics of the test-and-treat programme. However, this enhanced programme efficiency comes at the expense of lower NPV and sensitivity, implying a smaller proportion of individuals with HCV in the population being identified through the risk score.
Table 3.
Implications of selecting various score cutoffs on the different diagnostic metrics.
| Risk score cut-off | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Proportion needing testing (95% CI) | Proportion that are diagnosed (95% CI) |
| EDHS 2008 risk score* | ||||||
| ≥2.0 | 99.8 (99.4 to 100.0) | 3.9 (3.5 to 4.3) | 14.6 (13.9 to 15.3) | 99.2 (97.7 to 99.8) | 96.5 (96.2 to 96.8) | 99.8 (99.4 to 100.0) |
| ≥4.0 | 99.1 (98.5 to 99.5) | 7.8 (7.3 to 8.4) | 15.0 (14.3 to 15.7) | 98.2 (96.9 to 99.0) | 92.8 (92.8 to 93.2) | 99.1 (98.1 to 99.5) |
| ≥5.0 | 98.2 (97.4 to 98.8) | 11.4 (10.8 to 12.1) | 15.4 (14.7 to 16.1) | 97.5 (96.4 to 98.3) | 89.3 (88.8 to 89.9) | 98.2 (97.4 to 98.8) |
| ≥6.0 | 97.5 (69.5 to 98.2) | 14.8 (14.1 to 15.6) | 15.8 (15.1 to 16.6) | 97.3 (96.3 to 98.0) | 86.1 (85.5 to 86.7) | 97.5 (96.6 to 98.2) |
| ≥9.0 | 96.8 (95.8 to 97.6) | 17.9 (17.1 to 18.7) | 16.2 (15.5 to 17.0) | 97.1 (96.2 to 97.8) | 83.2 (82.5 to 83.9) | 96.8 (95.8 to 97.6) |
| ≥11.0 | 94.2 (92.9 to 95.3) | 27.3 (26.4 to 28.2) | 17.6 (16.7 to 18.4) | 96.6 (95.6 to 97.3) | 74.9 (74.1 to 75.7) | 94.2 (92.9 to 95.3) |
| ≥12.0 | 91.2 (89.6 to 92.5) | 34.0 (33.1 to 35.0) | 18.5 (17.6 to 19.4) | 95.9 (95.2 to 96.5) | 68.9 (68.0 to 69.7) | 91.2 (89.6 to 92.5) |
| ≥13.0 | 90.5 (88.9 to 91.9) | 36.8 (35.8 to 37.7) | 19.0 (18.2 to 20.0) | 95.9 (95.2 to 96.5) | 66.3 (65.4 to 67.1) | 90.5 (88.9 to 91.9) |
| ≥14.0 | 87.9 (86.2 to 89.5) | 42.8 (41.8 to 43.8) | 20.2 (19.2 to 21.1) | 95.6 (94.9 to 96.2) | 61.0 (60.1 to 61.9) | 87.9 (86.2 to 89.5) |
| ≥15.0 | 84.8 (82.9 to 86.5) | 50.5 (49.5 to 51.5) | 22.1 (21.0 to 23.1) | 95.3 (94.7 to 95.8) | 54.0 (53.1 to 54.9) | 84.8 (82.9 to 86.5) |
| ≥16.0 | 83.5 (81.5 to 85.3) | 55.1 (54.0 to 56.1) | 23.74 (22.6 to 24.9) | 95.3 (94.7 to 95.8) | 50.1 (49.2 to 50.9) | 83.5 (81.5 to 85.3) |
| ≥18.0 | 82.4 (80.5 to 84.3) | 57.3 (56.3 to 58.3) | 24.1 (23.0 to 25.3) | 95.2 (94.6 to 95.7) | 47.9 (47.0 to 48.8) | 82.4 (80.5 to 84.3) |
| ≥20.0 | 79.1 (77.0 to 81.1) | 62.7 (61.7 to 63.7) | 25.8 (24.6 to 27.1) | 94.8 (94.2 to 95.3) | 43.0 (42.1 to 43.9) | 79.1 (77.0 to 81.1) |
| ≥21.0 | 77.1 (75.0 to 79.2) | 64.7 (63.8 to 65.7) | 26.5 (25.2 to 27.8) | 94.5 (93.9 to 95.0) | 40.8 (39.9 to 41.6) | 77.2 (75.0 to 79.2) |
| ≥23.0 | 73.7 (71.5 to 78.9) | 68.5 (67.5 to 69.4) | 27.8 (26.4 to 29.2) | 94.1 (93.5 to 94.6) | 37.2 (36.3 to 38.1) | 73.7 (71.5 to 75.9) |
| ≥24.0 | 67.5 (65.1 to 69.8) | 73.7 (72.8 to 74.6) | 29.7 (28.2 to 31.2) | 93.3 (92.6 to 93.8) | 31.8 (30.9 to 32.6) | 67.5 (65.1 to 69.8) |
| ≥25.0 | 65.6 (63.2 to 67.9) | 75.7 (74.8 to 76.5) | 30.7 (29.1 to 32.3) | 93.0 (92.4 to 93.6) | 29.8 (29.0 to 30.6) | 65.6 (63.2 to 67.9) |
| ≥27.0 | 62.3 (59.8 to 64.7) | 78.5 (77.7 to 73.3) | 32.3 (30.6 to 34.0) | 92.7 (92.1 to 93.2) | 27.0 (26.2 to 27.8) | 62.3 (59.8 to 64.7) |
| ≥28.0 | 56.7 (54.2 to 59.2) | 82.6 (81.8 to 83.3) | 34.9 (33.0 to 36.8) | 92.1 (91.5 to 92.6) | 22.7 (21.9 to 23.4) | 56.7 (54.2 to 59.2) |
| ≥29.0 | 52.5 (50.0 to 55.0) | 85.1 (84.4 to 85.8) | 36.7 (34.8 to 38.8) | 91.6 (91.0 to 92.2) | 19.6 (18.9 to 20.3) | 52.5 (50.0 to 55.0) |
| ≥32.0 | 47.6 (45.1 to 50.1) | 87.6 (86.9 to 88.3) | 38.7 (36.5 to 40.9) | 91.0 (90.4 to 91.6) | 16.8 (16.2 to 17.5) | 47.6 (45.1 to 50.1) |
| ≥33.0 | 34.7 (32.3 to 37.1) | 92.0 (91.4 to 92.5) | 41.5 (38.8 to 44.2) | 89.5 (88.9 to 90.1) | 11.3 (10.7 to 11.9) | 34.7 (32.3 to 37.1) |
| ≥36.0 | 28.4 (26.2 to 30.7) | 94.2 (93.7 to 94.7) | 44.6 (41.4 to 47.7) | 88.9 (88.3 to 89.5) | 8.6 (8.1 to 9.1) | 28.4 (26.2 to 30.7) |
| ≥37.0 | 21.3 (19.3 to 23.4) | 95.6 (95.2 to 96.0) | 44.3 (40.7 to 47.9) | 88.1 (87.4 to 88.7) | 6.5 (6.1 to 7.0) | 21.3 (19.3 to 23.4) |
| ≥41.0 | 12.7 (11.1 to 14.5) | 98.1 (97.8 to 98.3) | 51.8 (46.7 to 56.9) | 87.2 (86.6 to 87.9) | 3.3 (3.0 to 3.6) | 12.7 (11.1 to 14.5) |
| EDHS 2014 risk score† | ||||||
| ≥5.0 | 99.4 (98.8 to 99.7) | 4.3 (4.0 to 4.7) | 9.1 (8.7 to 9.6) | 98.6 (97.3 to 99.4) | 96.0 (95.7 to 96.3) | 99.4 (98.8 to 99.7) |
| ≥8.0 | 99.2 (98.5 to 99.6) | 8.3 (7.9 to 8.9) | 9.5 (9.0 to 10.0) | 99.0 (98.3 to 99.5) | 92.3 (91.9 to 92.7) | 99.2 (98.5 to 99.6) |
| ≥12.0 | 98.7 (97.9 to 99.2) | 13.2 (12.6 to 13.7) | 9.9 (9.4 to 10.4) | 99.0 (98.5 to 99.4) | 87.9 (87.3 to 88.3) | 98.7 (97.9 to 99.2) |
| ≥13.0 | 98.3 (97.5 to 98.9) | 16.5 (15.9 to 17.1) | 10.2 (9.7 to 10.8) | 99.0 (98.5 to 99.4) | 84.7 (84.2 to 85.3) | 98.3 (97.5 to 98.9) |
| ≥15.0 | 97.5 (96.6 to 98.3) | 20.7 (20.1 to 21.4) | 10.7 (10.1 to 11.2) | 98.9 (98.4 to 99.2) | 80.9 (80.3 to 81.5) | 97.5 (96.6 to 98.3) |
| ≥17.0 | 96.7 (65.6 to 97.5) | 24.7 (24.0 to 25.4) | 11.1 (10.5 to 11.6) | 98.7 (98.3 to 99.1) | 77.2 (76.5 to 77.8) | 96.7 (95.6 to 97.5) |
| ≥20.0 | 96.2 (95.0 to 97.1) | 27.5 (26.7 to 28.2) | 11.4 (10.8 to 12.0) | 98.7 (98.3 to 99.0) | 74.5 (73.9 to 75.2) | 96.2 (95.0 to 97.1) |
| ≥22.0 | 92.2 (90.7 to 93.6) | 38.5 (37.7 to 39.2) | 12.7 (12.0 to 13.3) | 98.1 (97.7 to 98.4) | 64.0 (63.3 to 64.7) | 92.2 (90.7 to 93.4) |
| ≥23.0 | 90.8 (89.2 to 92.3) | 41.5 (40.7 to 42.4) | 13.1 (12.4 to 13.8) | 97.9 (97.5 to 98.2) | 61.0 (60.3 to 61.8) | 90.8 (89.2 to 92.3) |
| ≥25.0 | 89.2 (87.4 to 90.7) | 47.1 (46.3 to 47.9) | 14.0 (13.3 to 14.8) | 97.8 (97.5 to 98.2) | 55.9 (55.2 to 56.7) | 89.2 (87.4 to 90.7) |
| ≥26.0 | 86.1 (84.1 to 87.8) | 52.6 (51.8 to 53.4) | 15.0 (14.2 to 15.8) | 97.5 (97.1 to 97.8) | 50.6 (49.9 to 51.4) | 86.1 (84.1 to 87.8) |
| ≥27.0 | 83.9 (81.9 to 85.8) | 55.1 (54.3 to 55.9) | 15.3 (14.5 to 16.2) | 97.3 (96.9 to 97.6) | 48.1 (47.3 to 48.9) | 83.9 (81.3 to 85.8) |
| ≥28.0 | 81.9 (79.8 to 84.0) | 57.8 (57.0 to 58.6) | 15.8 (15.0 to 16.7) | 97.1 (96.7 to 97.4) | 45.5 (44.7 to 46.2) | 81.9 (79.8 to 83.9) |
| ≥30.0 | 77.1 (74.8 to 79.2) | 64.8 (64.0 to 65.6) | 17.5 (16.6 to 18.5) | 96.7 (96.3 to 97.0) | 38.8 (38.0 to 39.5) | 77.1 (74.8 to 79.2) |
| ≥31.0 | 71.4 (69.0 to 73.8) | 70.6 (69.8 to 71.3) | 19.0 (18.0 to 20.1) | 96.2 (95.8 to 96.6) | 33.1 (32.3 to 33.8) | 71.4 (69.0 to 73.8) |
| ≥33.0 | 69.1 (66.7 to 71.5) | 72.8 (72.1 to 73.5) | 19.8 (18.7 to 20.9) | 96.1 (95.7 to 96.4) | 30.8 (30.1 to 31.5) | 69.1 (66.7 to 71.5) |
| ≥34.0 | 66.2 (63.7 to 68.7) | 75.9 (75.2 to 76.6) | 21.0 (19.8 to 22.2) | 95.9 (95.5 to 96.2) | 27.8 (27.1 to 28.5) | 66.2 (63.7 to 68.7) |
| ≥35.0 | 64.1 (61.5 to 66.6) | 78.2 (77.5 to 78.9) | 22.2 (20.9 to 23.5) | 95.7 (95.4 to 96.1) | 25.5 (24.9 to 26.2) | 64.1 (61.5 to 66.6) |
| ≥37.0 | 58.5 (55.9 to 61.1) | 82.7 (82.0 to 83.3) | 24.6 (23.2 to 26.1) | 95.4 (95.0 to 95.7) | 21.1 (20.5 to 21.7) | 58.5 (55.9 to 61.1) |
| ≥38.0 | 53.7 (51.1 to 56.3) | 84.7 (84.1 to 85.2) | 25.3 (23.8 to 26.9) | 95.0 (94.6 to 95.3) | 18.7 (18.1 to 19.3) | 53.7 (51.1 to 56.3) |
| ≥39.0 | 49.3 (46.7 to 52.0) | 86.7 (86.1 to 87.2) | 26.4 (24.7 to 28.1) | 94.5 (94.2 to 95.0) | 16.5 (15.9 to 17.1) | 49.3 (46.7 to 52.0) |
| ≥40.0 | 45.6 (43.0 to 48.2) | 88.6 (88.1 to 89.1) | 27.9 (26.1 to 29.8) | 94.4 (94.0 to 94.8) | 14.4 (13.1 to 15.0) | 45.6 (43.0 to 48.2) |
| ≥42.0 | 42.1 (39.5 to 44.7) | 90.1 (89.6 to 90.6) | 29.2 (27.3 to 31.3) | 94.1 (93.7 to 94.5) | 12.7 (12.2 to 13.2) | 42.1 (39.5 to 44.7) |
| ≥43.0 | 37.2 (34.6 to 39.7) | 92.1 (91.7 to 92.6) | 31.4 (29.1 to 33.6) | 93.8 (93.4 to 94.2) | 10.4 (9.9 to 10.8) | 37.2 (34.6 to 39.7) |
| ≥45.0 | 32.3 (29.8 to 34.8) | 94.0 (93.6 to 94.4) | 34.3 (31.7 to 36.9) | 93.5 (93.1 to 93.9) | 8.3 (7.9 to 8.7) | 32.3 (29.8 to 34.8) |
| ≥48.0 | 22.0 (19.9 to 24.3) | 96.8 (96.5 to 97.1) | 40.0 (36.6 to 43.6) | 92.8 (92.2 to 93.2) | 4.8 (4.5 to 5.1) | 22.0 (19.9 to 24.3) |
| ≥50.0 | 15.4 (13.6 to 17.4) | 98.1 (97.8 to 98.3) | 43.7 (39.3 to 48.2) | 92.3 (91.9 to 92.7) | 3.1 (2.8 to 3.4) | 15.4 (13.6 to 17.4) |
| ≥53.0 | 7.4 (6.1 to 8.9) | 99.3 (99.2 to 99.4) | 51.5 (44.4 to 58.5) | 91.8 (91.4 to 92.3) | 1.3 (1.1 to 1.4) | 7.4 (6.1 to 8.9) |
*The AUC for the derived risk score using the EDHS 2008 was 0.77 (95% CI: 0.76 to 0.78).
†The AUC for the derived risk score using the EDHS 2014 was 0.78 (95% CI: 0.77 to 0.80).
AUC, area under the curve; EDHS, Egypt Demographic and Health Survey; HCV, Hepatitis C virus; NPV, negative predictive value; PPV, positive predictive value.
Discussion
We demonstrated that a risk score that consists of a few simple questions that are easy to evaluate in a primary healthcare setting or implemented through a website or an application that helps persons identify their risk of being HCV infected, provides an effective and non-invasive public health tool to identify carriers of HCV infection and to link them to testing and treatment. Biochemical testing methods to identify people with HCV are invasive and time-consuming and require human and financial resources, as well as complex logistics, making them less scalable, particularly in resource-limited settings. In contrast, initial screening using a risk score can be easily administered or self-administered, is non-invasive and requires minimal resources and logistics. Therefore, HCV risk scores can be an indispensable strategy for the global response to attain the target of HCV elimination as a public health problem by 2030.
While the concept of a risk score shares similarities with risk-based testing, which has been implemented in some countries, predominantly in higher-income nations,32–34 the risk score approach transcends mere risk-based testing. It enables a broader application across various settings and situations and can significantly contribute to raising awareness of the infection among the general population. The risk score approach represents a tool that addresses several public health needs simultaneously, extending the application of risk-based testing beyond conventional healthcare settings. Moreover, it entails minimal costs and logistics, making it feasible even in resource-limited settings.
Remarkably, the risk score, comprising three simple questions, demonstrated considerable diagnostic accuracy, as evidenced by the values of various diagnostic metrics, including AUC, sensitivity, specificity, PPV and NPV. Of particular note is the high NPV, ensuring that a negative result is highly unlikely to be a false negative, thereby obviating the need for individuals with a negative outcome using the score to undergo testing for HCV antibodies. The score also identified 73.7% and 64.0% of all HCV infections in the EDHS 2008 and EDHS 2014 samples, respectively. Thus, the score fulfils its objective of facilitating the efficient identification of individuals with HCV infection while minimising the necessity for conducting biochemical testing. This underscores the value of this approach in identifying as many people with HCV as early as possible and initiating treatment before progression to serious clinical disease.
This approach was demonstrated for Egypt, considering the availability of two EDHS surveys to derive and validate the score. The two scores exhibited comparable structures and diagnostic performances, with minor differences attributed to sampling variation of the same population across two distinct rounds of the EDHS surveys. Each score was validated by applying it to a database other than the one used to derive it. The latter application yielded a diagnostic performance that was comparable to the original diagnostic performance against the database used to originate it. This highlights how a single national survey for HCV infection may be sufficient to develop an effective risk score for this infection, and that can become an integral component of the national response to eliminate HCV infection.
The approach demonstrated in this study can be applied in other countries, including those in the MENA region. In countries where nationally representative population-based surveys have been conducted, these surveys can serve as the basis for deriving the risk score, as was done in this study. However, only three MENA countries—Egypt, Libya and Pakistan—have conducted such surveys.25 26 35 36 For countries where such surveys are not available,10–16 the risk score can still be derived using data from available regional surveys. Alternatively, if regional surveys are not available, the effects of risk factors for infection can be pooled, either in terms of ORs or relative risks, using data from analytical studies.37 These effects can also be derived from meta-regression analyses applied to all available HCV prevalence studies for each country.38–45
While this study focused on demonstrating the utility of this concept as a public health tool, the actual application of this approach to different countries can be enhanced for even higher diagnostic accuracy. One extension could be adding more variables to the score in a manner tailored to the local epidemiology of each country. For instance, province or city of birth and/or current residence, prior exposure to an HCV mode of transmission37 or history of HCV infection in the family, could be added, among others. Given that the risk of exposure to HCV infection varies immensely by at-risk population type and shows a distinctive hierarchy,46 an additional component to the score could be to integrate the at-risk population type as a variable,41 46 thereby further enhancing the diagnostic accuracy of the score. Testing strategies, therefore, could be highly efficient in identifying people with HCV at a modest cost.
However, caution must be exercised to prevent the creation of stigma associated with HCV infection or the use of an HCV risk score. For instance, it may not be feasible to include questions about stigmatised behaviours in the MENA context, such as injecting drug use or specific sexual practices, when the score is applied in general population settings like primary healthcare. However, such questions may be appropriate in other settings, such as voluntary counselling and testing centres or outreach efforts by community organisations working with the most at-risk populations.47 It is important also for the risk score to factor community acceptance in its design and implementation, ensuring it addresses the specific needs of certain groups, such as women of childbearing age in contexts where the risk of HCV vertical transmission is not negligible.48–50
The application of HCV risk scores can be influenced by programmatic considerations and variations in context. This may necessitate prioritising specific diagnostic metrics, such as sensitivity over specificity. The approach presented here demonstrates an inherent flexibility of the score, allowing adjustments to address specific programmatic needs, as illustrated by the analysis using different cut-off points (table 3). However, it is critical to acknowledge the inherent trade-offs between diagnostic metrics. Optimising one metric, such as sensitivity, will inevitably impact others, like specificity. Therefore, careful consideration is essential to align the score’s cut-off with the specific programmatic context and its corresponding needs.
This study has limitations. For ease of use in primary healthcare and more broadly by the public, a risk score has to be simple. Accordingly, it cannot fully represent the complex epidemiology of HCV infection, such as interactions among risk factors. This risk score was derived for Egypt, which may not benefit from this risk score, given that this country has opted for mass testing of its entire population.17 Derivation of a risk score typically requires at least one round of a population-based survey, ideally at the national level, but many countries may not have such survey data to be able to easily derive a risk score. The risk score was derived for a high-burden country, and the utility of this approach still needs to be demonstrated for countries with low HCV prevalence. Nonetheless, this approach may prove to have higher utility in countries with low HCV prevalence than in countries with high HCV prevalence, as HCV epidemiology shows a clearer hierarchy of infection exposure risk in countries with concentrated HCV epidemics compared with those with generalised HCV epidemics.46
Conclusions
An HCV risk score can be derived using only one round of a population-based survey and offers an effective, simple, non-invasive strategy to identify carriers of HCV infection and to link them to testing and treatment, at low cost. This public health tool can be implemented and used for prioritising populations for interventions with minimal logistical complexity and cost, especially in resource-limited countries.
Supplementary Material
Acknowledgments
The authors are grateful for the administrative support of Adona Canlas.
Footnotes
@rayaneelkhoury2
Contributors: RE-K conducted the data analyses with HC and NN. RE-K, NN and LJA-R co-wrote the first draft of the article. LJA-R conceived and led the design of the study, analyses and drafting the article. All authors contributed to drafting and revising the manuscript. All authors have read and approved the final manuscript. LJA-R is the guarantor of the study. ChatGPT was exclusively used to verify grammar and refine the English phrasing in our text. No other functionalities or applications of ChatGPT were employed beyond this specific scope. Following the use of this tool, the authors thoroughly reviewed and edited the content as necessary and take full responsibility for the accuracy and quality of the publication.
Funding: This work was supported by the National Priorities Research Program (NPRP) (grant number 12S-0216-190094) from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors. The authors are also grateful for infrastructure support provided by the Biostatistics, Epidemiology and Biomathematics Research Core at Weill Cornell Medicine-Qatar.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. All data analysed in this study can be accessed through application to the DHS Program at https://dhsprogram.com/ or by contacting archive@dhsprogram.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet 2016;388:1081–8. 10.1016/S0140-6736(16)30579-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief. World Health Organization, 2016. [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41–52. 10.1056/NEJM200107053450107 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Hepatitis C. 2021. Available: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- 5.Calvaruso V, Craxì A. Hepatic benefits of HCV cure. J Hepatol 2020;73:1548–56. 10.1016/j.jhep.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Ayoub HH, Abu-Raddad LJ. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: A case for treatment as prevention. J Viral Hepat 2017;24:486–95. 10.1111/jvh.12671 [DOI] [PubMed] [Google Scholar]
- 7.Ayoub HH, Abu-Raddad LJ. Treatment as prevention for hepatitis C virus in Pakistan: mathematical Modelling projections. BMJ Open 2019;9:e026600. 10.1136/bmjopen-2018-026600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . World health organization, global health sector strategy on viral hepatitis 2016-2021. towards ending viral hepatitis. 2016.
- 9.World Health Organization . Global Hepatitis Report 2017. World Health Organization, 2017. [Google Scholar]
- 10.World Health Organization . Epidemiology of hepatitis C virus in the WHO Eastern Mediterranean region: implications for strategic action. 2020.
- 11.Chemaitelly H, Mahmud S, Rahmani AM, et al. The epidemiology of hepatitis C virus in Afghanistan: systematic review and meta-analysis. Int J Infect Dis 2015;40:54–63. 10.1016/j.ijid.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 12.Mohamoud YA, Riome S, Abu-Raddad LJ. Epidemiology of hepatitis C virus in the Arabian Gulf countries: systematic review and meta-analysis of prevalence. Int J Infect Dis 2016;46:116–25. 10.1016/j.ijid.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Chemaitelly H, Chaabna K, Abu-Raddad LJ. The epidemiology of hepatitis C virus in the fertile crescent: systematic review and meta-analysis. Plos One 2015;10:e0135281. 10.1371/journal.pone.0135281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadlalla FA, Mohamoud YA, Mumtaz GR, et al. The epidemiology of hepatitis C virus in the Maghreb region: systematic review and meta-analyses. PLoS One 2015;10:e0121873. 10.1371/journal.pone.0121873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaabna K, Kouyoumjian SP, Abu-Raddad LJ. Hepatitis C virus epidemiology in Djibouti, Somalia, Sudan, and Yemen: systematic review and meta-analysis. PLoS One 2016;11:e0149966. 10.1371/journal.pone.0149966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmud S, Akbarzadeh V, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Iran: systematic review and meta-analyses. Sci Rep 2018;8:150. 10.1038/s41598-017-18296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waked I, Esmat G, Elsharkawy A, et al. Screening and treatment program to eliminate hepatitis C in Egypt. N Engl J Med 2020;382:1166–74. 10.1056/NEJMsr1912628 [DOI] [PubMed] [Google Scholar]
- 18.Al Kanaani Z, Mahmud S, Kouyoumjian SP, et al. The epidemiology of hepatitis C virus in Pakistan: systematic review and meta-analyses. R Soc Open Sci 2018;5:180257. 10.1098/rsos.180257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayoub HH, Al Kanaani Z, Abu-Raddad LJ. Characterizing the temporal evolution of the hepatitis C virus epidemic in Pakistan. J Viral Hepat 2018;25:670–9. 10.1111/jvh.12864 [DOI] [PubMed] [Google Scholar]
- 20.Mahmud S, Al Kanaani Z, Abu-Raddad LJ. Characterization of the hepatitis C virus epidemic in Pakistan. BMC Infect Dis 2019;19:809. 10.1186/s12879-019-4403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble D, Mathur R, Dent T, et al. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343:d7163. 10.1136/bmj.d7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins GS, Mallett S, Omar O, et al. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med 2011;9:1–14. 10.1186/1741-7015-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown N, Critchley J, Bogowicz P, et al. Risk scores based on self-reported or available clinical data to detect Undiagnosed type 2 diabetes: a systematic review. Diabetes Res Clin Pract 2012;98:369–85. 10.1016/j.diabres.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 24.Awad SF, Dargham SR, Toumi AA, et al. A diabetes risk score for Qatar utilizing a novel mathematical modeling approach to identify individuals at high risk for diabetes. Sci Rep 2021;11:1811. 10.1038/s41598-021-81385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo: Egypt Ministry of Health, El Zanaty and Associates, and Macro International, 2009. [Google Scholar]
- 26.El-Zanaty F. Egypt Health Issue Survey 2015. Cairo, Egypt and Rockville, Maryland, USA: Ministry of Health and Population and ICF International, 2015. [Google Scholar]
- 27.Rutstein SO, Rojas G. Guide to DHS Statistics. Calverton, MD: ORC Macro, 2006:38. [Google Scholar]
- 28.MEASURE DHS . The DHS program demographic and health surveys. 2021. Available: https://dhsprogram.com
- 29.Ayoub HH, Chemaitelly H, Kouyoumjian SP, et al. Characterizing the historical role of parenteral Antischistosomal therapy in hepatitis C virus transmission in Egypt. Int J Epidemiol 2020;49:798–809. 10.1093/ije/dyaa052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamoud YA, Mumtaz GR, Riome S, et al. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013;13:1–21. 10.1186/1471-2334-13-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-Regressions. Sci Rep 2018;8:1661. 10.1038/s41598-017-17936-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic MR, Miljatovic A, Puskas L, et al. Does the strategy of risk group testing for hepatitis C hit the target. Front Pharmacol 2017;8:437. 10.3389/fphar.2017.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smart A, Geboy A, Basch P, et al. Identification of risk factors for testing of hepatitis C in non-birth cohort patients: is universal screening necessary J Addict Med 2021;15:109–12. 10.1097/ADM.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 34.Jordan AE, Perlman DC. The shift in emphasis from risk-based to age-based hepatitis C virus (HCV) testing in the US tends to remove injection drug use from discourse on HCV. Subst Use Misuse 2017;52:340–50. 10.1080/10826084.2016.1225767 [DOI] [PubMed] [Google Scholar]
- 35.Qureshi H, Bile KM, Jooma R, et al. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures [French]. East Mediterr Health J 2010;16:S15–23. [PubMed] [Google Scholar]
- 36.Daw MA, El-Bouzedi A, In association with Libyan Study Group of Hepatitis & HIV . Prevalence of hepatitis B and hepatitis C infection in Libya: results from a national population based survey. BMC Infect Dis 2014;14:14–7. 10.1186/1471-2334-14-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmud S, Kouyoumjian SP, Al Kanaani Z, et al. Individual-level key associations and modes of exposure for hepatitis C virus infection in the Middle East and North Africa: a systematic synthesis. Ann Epidemiol 2018;28:452–61. 10.1016/j.annepidem.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 38.Harfouche M, Chemaitelly H, Kouyoumjian SP, et al. Hepatitis C virus Viremic rate in the Middle East and North Africa: systematic synthesis, meta-analyses, and meta-Regressions. PLoS One 2017;12:e0187177. 10.1371/journal.pone.0187177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harfouche M, Chemaitelly H, Mahmud S, et al. Epidemiology of hepatitis C virus among Hemodialysis patients in the Middle East and North Africa: systematic syntheses, meta-analyses, and meta-Regressions. Epidemiol Infect 2017;145:3243–63. 10.1017/S0950268817002242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heijnen M, Mumtaz GR, Abu-Raddad LJ. Status of HIV and hepatitis C virus infections among prisoners in the Middle East and North Africa: review and synthesis. J Int AIDS Soc 2016;19:20873. doi:20873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmud S, Chemaitelly H, Al Kanaani Z, et al. Hepatitis C virus infection in populations with liver-related diseases in the Middle East and North Africa. Hepatol Commun 2020;4:577–87. 10.1002/hep4.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahmud S, Chemaitelly H, Alaama AS, et al. Characterizing trends and associations for hepatitis C virus antibody prevalence in the Middle East and North Africa: meta-regression analyses. Sci Rep 2022;12:20637. 10.1038/s41598-022-25086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmud S, Mumtaz GR, Chemaitelly H, et al. The status of hepatitis C virus infection among people who inject drugs in the Middle East and North Africa. Addiction 2020;115:1244–62. 10.1111/add.14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamoud YA, Miller FD, Abu-Raddad LJ. Potential for human immunodeficiency virus parenteral transmission in the Middle East and North Africa: an analysis using hepatitis C virus as a proxy biomarker. World J Gastroenterol 2014;20:12734–52. 10.3748/wjg.v20.i36.12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahmud S, Chemaitelly HS, Kouyoumjian SP, et al. Key associations for hepatitis C virus Genotypes in the Middle East and North Africa. J Med Virol 2020;92:386–93. 10.1002/jmv.25614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chemaitelly H, Mahmud S, Kouyoumjian SP, et al. Who to test for hepatitis C virus in the Middle East and North Africa?: pooled analyses of 2,500 prevalence measures, including 49 million tests. Hepatol Commun 2019;3:325–39. 10.1002/hep4.1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumtaz GR, Chemaitelly H, AlMukdad S, et al. Status of the HIV epidemic in key populations in the Middle East and North Africa: Knowns and unknowns. Lancet HIV 2022;9:e506–16. 10.1016/S2352-3018(22)00093-5 [DOI] [PubMed] [Google Scholar]
- 48.Benova L, Awad SF, Abu-Raddad LJ. Estimate of vertical transmission of hepatitis C virus in Pakistan in 2007 and 2012 birth cohorts. J Viral Hepat 2017;24:1177–83. 10.1111/jvh.12748 [DOI] [PubMed] [Google Scholar]
- 49.Benova L, Awad SF, Miller FD, et al. Estimation of hepatitis C virus infections resulting from vertical transmission in Egypt. Hepatology 2015;61:834–42. 10.1002/hep.27596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014;59:765–73. 10.1093/cid/ciu447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-085506supp001.pdf (113.6KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. All data analysed in this study can be accessed through application to the DHS Program at https://dhsprogram.com/ or by contacting archive@dhsprogram.com.