Abstract
The endocytosis of membrane receptors is a complex and tightly controlled process that is essential for maintaining cellular homoeostasis. The removal of receptors from the cell surface can be constitutive or ligand-induced, and occurs in a clathrin-dependent or -independent manner. The recruitment of receptors into specialized membrane domains, the formation of vesicles and the trafficking of receptors together with their ligands within endocytic compartments are regulated by reversible protein modifications, and multiple protein–protein and protein–lipid interactions. Recent reports describe a variety of multidomain molecules that facilitate receptor endocytosis and function as platforms for the assembly of protein complexes. These scaffold proteins typically act in a cargo-specific manner, recognizing one or more receptor types, or function at the level of endocytic cellular microcompartments by controlling the movement of cargo molecules and linking endocytic machineries to signalling pathways. In the present review we summarize present knowledge on endocytic scaffold molecules and discuss their functions.
Keywords: cargo, endocytosis, microcompartment, scaffold
Abbreviations: Alix, ALG-2 (apoptosis-linked gene 2)-interacting protein X; ANTH domain, AP180 N-terminal homology domain; AP-2, adaptor protein-2; ARH, autosomal recessive hypercholesterolaemia; BAR domain, Bin/amphiphysin/Rvs domain; CD2AP, CD2-associated protein; CIN85, Cbl-interacting protein of 85 kDa; Dab2, Disabled-2; EEA1, early endosome antigen 1; EGFR, epidermal growth factor receptor; EH domain, Eps15 homology domain; ENTH domain, epsin N-terminal homology domain; ESCRT, endosomal sorting complexes required for transport; FYVE, Fab1p, YOTB, Vac1p and EEA1; GAP, GTPase-activating protein; GPCR, G-protein-coupled receptor; Hrs, hepatocyte growth factor-regulated tyrosine kinase substrate; LBPA, lysobiphosphatidic acid; LDL, low-density lipoprotein; LNX, ligand of Numb protein X; MVB, multivesicular body; NAK, Numb-associated kinase; NSF, N-ethylmaleimide-sensitive fusion protein; PON, partner of Numb; PTB domain, phosphotyrosine-binding domain; RTK, receptor tyrosine kinase; SH3, Src homology 3; SNARE, soluble NSF attachment protein receptor; STAM, signal-transducing adaptor molecule; TCR, T-cell receptor
INTRODUCTION
Endocytosis ensures the rapid clearance of transmembrane proteins from the cell surface and influences the extent of cell responses to stimuli. Ligand-stimulated receptor internalization appears to be controlled by multimeric protein complexes that function both in desensitization and in signalling by activated receptors. In recent years, a large number of adaptor molecules have been identified that are required for the efficient assembly of complexes necessary for the endocytosis of plasma membrane receptors. The features of classical cytoplasmic adaptor proteins include the presence of protein binding domains, sites for inducible post-translational modifications, and a lack of catalytic domains. In a broader sense, scaffold molecules are characterized by their ability to bind simultaneously to multiple proteins and form higher-order complexes. Work by many research groups has indicated that molecular scaffolds play critical roles in the regulation of receptor endocytosis by binding to numerous endocytic accessory and regulatory proteins. In such protein clusters, increased local concentrations of proteins enable otherwise low-efficiency interactions and transient binding in a simultaneous or sequential manner. Dynamic changes in protein and lipid composition at discrete membrane microregions were initially shown to couple the fate of the cargo with membrane invagination and internalization. Similar mechanisms direct receptors to specific domains at the plasma membrane and in endosomes, leading to the endosomal retention and degradation of receptors. Moreover, similar principles appear to govern other trafficking processes in cells, including biosynthetic, recycling and secretory pathways. Several mechanisms underlying these processes have been proposed, including specific post-translational modifications of trafficking cargoes, such as phosphorylation and mono-ubiquitination, as well as functions of endocytic scaffold molecules that act in a cargo- and compartment-specific manner.
In this review, we will introduce and discuss the concept of receptor-specific adaptor proteins that bind to the cytoplasmic part of a class or family of receptors and connect it to the components of the endocytic machinery at the plasma membrane and in endosomal compartments. In addition, we will describe a class of scaffold proteins that are compartment-specific and participate in the recognition and sorting of trafficking cargoes along functional microdomains in the endosome.
RECEPTOR ENDOCYTOSIS – COMMON PRINCIPLES
The removal of receptors from the cell surface can be either a constitutive (ligand-independent) or an induced (ligand-dependent) process. The best studied examples are the transferrin receptor and the EGFR (epidermal growth factor receptor) respectively. Endocytosis can be regarded as a general desensitization mechanism that allows the system to reset its sensitivity to repeated stimulation. It also has a marked impact on signalling specificity by changing the location and composition of signalling complexes that are assembled at the active receptor. Transferrin and LDL (low-density lipoprotein) receptors, whose primary function is the transport of nutritional molecules into the cell, are internalized via clathrin-coated pits in a ligand-independent manner and are rapidly recycled back to the cell surface. On the other hand, signalling receptors, such as the EGFR, require ligand binding before they can be concentrated in clathrin-coated pits and enter the cells [1,2]. Unoccupied EGFRs also undergo constitutive endocytosis at the rate of 2–3% per min [1,2], which corresponds to the random entrapment of receptors in endocytic structures. Ligand-induced internalization is up to 10-fold faster, and appears to involve dynamic mechanisms that control distinct steps in receptor transport through the endosome.
The classical key components of clathrin-dependent endocytosis are clathrin, AP-2 (adaptor protein-2, the adaptor complex) and dynamin [3–5]. These proteins form part of the coat complex at a very early stage, and are recruited in various combinations in different receptor pathways. According to the current view, the recruitment of AP-2 to the plasma membrane is a complex process, which involves interactions with phospholipids, tyrosine- or dileucine-based endocytic sorting motifs present in the cytoplasmic tails of receptors [6], synaptotagmin, and accessory proteins such as Eps15 [7]. AP180/CALM (clathrin assembly protein lymphoid myeloid), epsins and HIP1/HIP1R (huntingtin-interacting proteins) may function as cargo adaptors in addition to AP-2, as they bind simultaneously to receptors and to clathrin and phospholipids, and can promote clathrin assembly in vitro [8–10]. Other proteins that are more specific for a particular receptor cargo, e.g. β-arrestin, can also recruit receptors to clathrin lattices, although these proteins do not promote clathrin assembly and work in combination with AP-2 [11]. After the clathrin lattice is formed, endophilin, epsin and amphiphysin are involved in membrane invagination and clathrin rearrangements. The GTPase dynamin has been convincingly implicated at the fission stage [12]. According to one hypothesis, dynamin functions as a forcegenerating mechanochemical motor protein. Another hypothesis implies that dynamin acts as a regulator of the fission event, possibly by recruiting other effector proteins [13]. Both mechanisms may also work simultaneously. Observation of clathrin-coated pit dynamics using total internal reflection microscopy has indicated that, during fission, dynamin recruitment to coated pits is followed rapidly by the recruitment of actin [14]. Moreover, perturbation of actin in the lamprey giant synapse disrupts the endocytic reaction, with the accumulation of coated pits with wide necks [15], suggesting a role for actin and dynamin-interacting accessory proteins in promoting constriction of the neck.
The basic endocytic machinery involves numerous accessory proteins that facilitate and modulate receptor internalization. However, it remains to be clarified how these proteins are co-ordinated to participate in sequential steps of the endocytic process in different cellular compartments. Another important and not yet understood topic deals with the specificity of transport of a particular receptor along the endosomal pathway. This pathway represents a major route for transporting receptors from the cell surface to different destinations in the cells. Endosomes are composed of a network of vesicular, tubular and lamellar structures that form morphologically distinct cellular compartments. Importantly, they are not only distinguished based on their shape, but also play different functional roles and are usually decorated by specific protein or lipid resident markers. Transmembrane receptors are removed from the cell membrane via clathrin-dependent and -independent pathways and are delivered to early endosomes. Early endosomes are characterized by a mildly acidic pH that favours dissociation of the ligand from the receptor. Molecules that are destined for recycling leave the early endosome, and either are translocated directly and very rapidly to the plasma membrane, or are initially sorted to the so-called endocytic recycling compartment and then returned back to the plasma membrane. The remaining receptors anchored in early endosomal vesicles are translocated together along microtubules to form a more acidic late endosomal compartment during the maturation process. Whereas early endosomes tend to be tubular and are located towards the cell periphery, late endosomes are more spherical and are often closer to the nucleus. In addition, a subset of late endosomes has a multivesicular appearance, and hence are called MVBs (multivesicular bodies). Late endosomes form a dynamic network together with lysosomal structures, which represents the end point of endocytosis and the site of protein degradation.
CARGO-SPECIFIC SCAFFOLD PROTEINS
Cargo-specific scaffold proteins can be defined as multifunctional proteins that are able to co-ordinate distinct steps in the internalization and sorting of selected types of cargos. While certain adaptors have been considered to be dedicated exclusively to the endocytosis of a particular receptor type, others have been shown to regulate the endocytosis of several kinds of cargo in a specific manner. They share a common mode of action via their ability to bind directly or indirectly to cargo and at the same time to interact with membrane lipids, coat components, endocytic accessory proteins and enzymes involved in the regulation of phospholipid metabolism. They are also commonly subject to dynamic post-translational modifications, including phosphorylation and ubiquitination, that regulate their scaffolding functions. Thus cargo-specific scaffolds act as major regulators of incoming signals that drive the endocytosis and sorting of cargoes along common pathways in the endosome. On the other hand, there is also a high degree of diversity among cargo-specific adaptors. This is exemplified by distinct means of recognizing cargoes either via direct binding to a receptor or by binding to receptor-associated proteins that are trafficking together with the receptor through the endosome. In addition, more evidence is emerging that a single adaptor, rather than being highly specific for a particular receptor type, can in fact regulate the endocytosis of several types of cargo. Moreover, cargo-specific adaptor proteins typically form families of structurally related proteins that are functionally redundant, such that the internalization of one class of receptors may employ several related adaptors (see below). Of note, the endocytic scaffolds often act at the nexus of trafficking and signalling, and in this way affects numerous cellular responses. Here we review several prototype cargo scaffolds, indicate their mode of action and discuss some recent evidence about their role in several receptor pathways.
β-Arrestin
β-Arrestins are clathrin adaptors that regulate the endocytosis of GPCRs (G-protein-coupled receptors) via clathrin-coated vesicles [16]. Arrestins were initially characterized as desensitizing agents that uncouple receptors from G-proteins. There are four known members of the arrestin family in mammals. Expression of the two visual arrestins is restricted to the retina, whereas β-arrestin 1 and 2 are expressed ubiquitously and are involved in the down-regulation of numerous GPCRs. Various aspects of arrestin function in the endocytosis and signalling of GPCRs has been discussed in earlier reviews [17,18], and here we focus on the specificity of action of β-arrestins in receptor-mediated endocytosis.
In response to receptor activation, β-arrestins are recruited to the plasma membrane and associate preferentially with activated and phosphorylated GPCRs. There are marked differences in the ability of arrestin isoforms to bind different GPCRs [19]. β-Arrestin 2 binds preferentially to class A GPCRs, such as the β2-adrenergic receptor, and dissociates from it before internalization. Class B GPCRs, such as the V2 vasopressin receptor, bind to β-arrestins 1 and 2 in a more prolonged fashion. While persistent association of β-arrestins targets receptors for lysosomal degradation, dissociation of β-arrestins from GPCRs favours receptor dephosphorylation and recycling back to the plasma membrane [20].
β-Arrestins, with exception of the visual arrestins, interact simultaneously with clathrin and with the β2 subunit of AP-2 through their C-terminus. It has been shown that the latter association is required for the efficient targeting of β-arrestins to the membrane [21]. Unlike other AP-2-binding adaptors, such as the epsins, arrestins do not induce clathrin polymerization, but target cargo to pre-existing clathrin-coated pits. In addition, β-arrestins bind the phosphoinositide InsP6, which mediates the recruitment of β-arrestin to phosphoinositide-rich plasma membrane microdomains, causing their concentration in clathrin-coated pits [22]. β-Arrestins also interact with a number of signalling molecules, including the kinases Src, JNK3 (c-Jun N-terminal kinase 3), ASK1 (apoptosis signal-regulating kinase 1), ERK1/2 (extracellular-signal-regulated kinase 1/2) and Raf, possibly recruiting these molecules to the activated GPCRs. Additionally, they associate with NSF (N-ethylmaleimide-sensitive fusion protein), an ATPase whose catalytic activity seems to promote the internalization of GPCRs [23], as well as with the GDP-bound form of ARF6 (ADP-ribosylation factor 6) and with ARNO (ARF nucleotide-binding-site opener), thus promoting receptor endocytosis [24,25].
Upon ligand stimulation, β-arrestins are rapidly and transiently ubiquitinated by Mdm2 E3 ligase. Usually β-arrestins associate transiently with receptors, but if the molecule is fused with ubiquitin it remains associated with the GPCR in the endosomes. Expression of such a chimaera increases the rate of receptor internalization, suggesting that the ubiquitination of arrestins affects receptor trafficking [26]. Interestingly, while ubiquitination of β-arrestins is critical for the internalization of GPCRs, ubiquitination of the receptor itself is required for its degradation [26]. In addition to ubiquitination, the phosphorylation of β-arrestins also regulates the clathrin-mediated internalization of β2-adrenergic receptors, as the ligand-induced phosphorylation at Ser-412 controls the binding of β-arrestin 1 to clathrin [27].
The binding site for β-arrestins is located either at the C-terminus of the GPCR (as in the case of the β2-adrenergic receptor) [28] or within the third cytoplasmic loop (as for the D1 dopamine receptor) [29]. Arrestins can also be recruited indirectly to GPCR complexes and mediate the internalization of heterologous receptor types. For example, Fz4 is a seven-transmembrane receptor that, upon activation by glycoproteins of the Wnt family, binds to Dvl2 (dishevelled), which initiates a signal transduction cascade that is involved in regulation of developmental processes [30,31]. It is phosphorylated Dvl2 that recruits β-arrestin 2 to the receptor complex, which is a critical step required for the endocytosis of Fz4 [32].
The specificity of β-arrestins for GPCRs is not absolute, since β-arrestin 2 is involved in the endocytosis of other types of membrane receptors, such as type II and type III transforming growth factor-β receptors, leading to the down-regulation of signalling [33]. The association is mediated by Thr-841 in the type III receptor that is phosphorylated by the type II receptor. It has also been shown that β-arrestins bind to the ligand-occupied insulin-like growth factor receptor, promoting its endocytosis, and enhancing ligand-dependent mitogen-activated protein kinase phosphorylation and DNA synthesis [34]. Finally, β-arrestin 2 associates with and enhances internalization of the LDL receptor [35].
Dab2 (Disabled-2), ARH (autosomal recessive hypercholesterolaemia) and Numb
Dab2 has been characterized as a specific adaptor protein that orchestrates the internalization of lipoprotein receptors. Dab2 contains an N-terminal PTB (phosphotyrosine-binding) domain that binds preferentially to non-phosphorylated NPXY (Asn-Pro-Xaa-Tyr) motifs. These sequences are found in the LDL receptor family that is represented by the LDL receptor, ApoER2 (apolipoprotein E receptor 2) and gp330/gp600 megalin, as well as amyloid precursor protein [36]. The same domain associates with PtdIns(4,5)P2 [37]. In addition, Dab2 contains two types of motifs commonly found in endocytic proteins, namely two DPF (Asp-Pro-Phe) motifs and five NPF (Asn-Pro-Phe) motifs. DPF/W modules are found in Eps15, epsin, amphiphysin, auxillin and AP180, and mediate binding to the α-adaptin ear domain of AP-2. NPF triplets, also present in epsin, intersectin, Numb, Rab, Pan1 and stonin 2, interact with the EH (Eps15 homology) domain of Eps15, intersectin, POB-1 and EHD1 [38]. Due to its ability to bind to specific internalization motifs in AP-2 as well as in EH domains, Dab2 links LDL receptors to the endocytic machinery and downstream signalling pathways.
Most reports describing the function of Dab proteins have been based on attempts to increase or decrease its expression level. Overexpression of Dab2 resulted in the redistribution of AP-2 into clusters that did not contain clathrin [36], while overexpression of the PTB domain of Dab2 specifically blocked LDL receptor uptake [37]. A conditional knockout of Dab2 in kidney proximal tubule cells resulted in a decreased number of apical coated pits and vesicles and the excretion of certain plasma proteins, transport of which is megalin-dependent [39]. In Caenorhabditis elegans, the corresponding protein Ce-DAB-1 is required for the efficient secretion of fibroblast growth factor, mediated by the lipoprotein receptor-related proteins [40].
Similarly to Dab2, ARH binds to the LDL receptor, clathrin and AP-2 [41]. It has been suggested that ARH and Dab2 act sequentially in endocytic cargo protein selection. Initially at the plasma membrane, ARH sequesters receptors at the rim of coated pits due to association with the β-appendage domain of AP-2. Binding of clathrin to AP-2 results in dissociation of ARH from the complex, and in this way cargo can be transferred on to Dab2, which is associated with the α-appendage of AP-2 [42]. A different model has been suggested for the co-operation of ARH and Dab2 in mediating endocytosis of the multi-ligand receptor megalin. This receptor has two FXNPXY motifs, and ARH interacts preferentially with the first [43], while Dab2 associates with the second [44]. Current data agree with a model whereby Dab2 associates initially with megalin in clathin-coated pits and then passes it on to ARH, which escorts the receptor all the way through early endosome and recycling endosome compartments [43]. The co-operation among these adaptors is supported by the observation that the importance of ARH for the efficient uptake of LDL varies between cell types. Patients with an autosomal recessive form of hypercholesterolaemia who bear mutations in the ARH gene display impaired degradation of LDL in hepatocytes and lymphocytes, but not in fibroblasts [45]. Some data suggest that Dab2 [43] or β-arrestin 2 [35] can compensate for the lack of ARH in fibroblasts.
Numb, the third PTB-domain-containing adaptor, induces the endocytosis and down-regulation of the transmembrane receptor Notch. The PTB domain of Numb interacts with the cytoplasmic domain of Notch, and associates with PON (partner of Numb), NAK (Numb-associated kinase), and LNX (ligand of Numb protein X). Numb interacts with the AP-2 complex via the DPF motif, and with Eps15 via the NPF motif. Numb and Eps15 are found in separate clathrin-coated pits, where both proteins associate with AP-2, while in endosomes Numb and Eps15 bind simultaneously and co-localize [46]. Notably, Numb is negatively regulated by the Ser/Thr kinase NAK [47] and the ubiquitin ligase LNX [48]. The adaptor protein PON directs the asymmetrical distribution of Numb in dividing neuroblasts [49]. The asymmetrical localization of Numb is also regulated by its association with the transmembrane protein NIP (Numb-interacting protein), which recruits Numb from the cytosol to the plasma membrane [50]. An asymmetrical distribution of Numb during cell division results in the polarized distribution of α-adaptin and thereby differentially regulates endocytosis in daughter cells [51]. This mechanism is involved in the development of the nervous system and muscles in Drosophila, where asymmetrical partitioning of Numb at mitosis defines Notch activity in daughter cells originating from sensory precursor cells, thus determining cell fate in several lineages [52].
CIN85 (Cbl-interacting protein of 85 kDa) and CD2AP (CD2-associated protein) as clustering scaffolds
The family of CIN85 and CD2AP/CMS [Cas ligand with multiple SH3 (Src homology 3) domains] adaptor proteins has attracted considerable interest due to recent findings that they act as scaffold molecules in the internalization and endocytic sorting of several RTKs (receptor tyrosine kinases) [53]. The down-regulation of RTKs is governed largely by the Cbl family of ubiquitin ligases, which mediates the ligand-dependent ubiquitination of RTKs and also recruits CIN85/CD2AP proteins in complexes with activated RTKs. Involvement of a Cbl–CIN85 or Cbl–CD2AP complex is vital for the internalization of EGFR [54], c-Met [55], the platelet-derived growth factor receptor, c-Kit [56] and Flt-1 [57]. Importantly, CIN85 appears to be critical for clustering of Cbl in these complexes, and additionally regulates the recruitment of endocytic accessory proteins such as endophilins, and may provide the link to the clathrin coat as well as to reorganization of the actin cytoskeleton. Similarly, Cbl associates with CD2AP that also links the receptor complex with endophilin, and additionally recruits cortactin, thus associating receptor endocytosis with Arp2/3 complex-mediated actin polymerization [58].
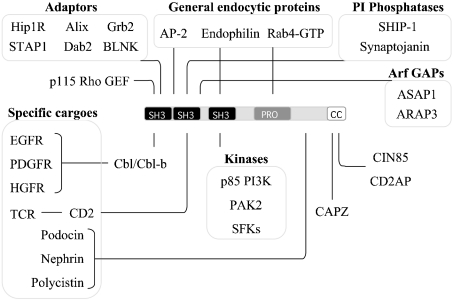
The scaffolding properties of CIN85 and CD2AP are clearly suggested by the multidomain composition of these ubiquitious adaptors, which are composed of three SH3 domains, polyproline motifs and a coiled-coil domain [59]. A number of independent groups identified these adaptors based on their binding to various partners. CD2AP has been independently characterized as a partner of CD2 [60] and p130Cas [61]. The adaptor CIN85 [62] is also known as Ruk (regulator of ubiquitous kinase) [63] and SETA (SH3 domain-containing gene expressed in tumorigenic astrocytes) [64]. CIN85 has been shown to associate with a number of signalling and adaptor proteins, including phosphoinositide 3-kinase, Src and Grb2 [59], the actin-capping protein CAPZ [65], endophilin [55] and Rab4 [66], which link this adaptor with the actin cytoskeleton, clathrin-coated pits and the early endosome compartment respectively (Figure 1). The coiled-coil is required for the localization of CIN85 to the plasma membrane [65], and mediates the homo- and hetero-typic oligomerization of CIN85 and CD2AP [67].
Figure 1. CIN85 is an endocytic scaffold.
CIN85 is an example of a multimeric adaptor that is involved in cargo internalizaton and sorting. Through its multiple domains, CIN85 associates with a number of proteins, which are divided here into functional groups. CIN85 oligomerizes and simultaneously binds various molecules, changing its conformation and binding partners upon cell stimulation, thus co-ordinating the trafficking of several cargo proteins. Abbreviations: STAP, stem-cell-specific adaptor protein containing pleckstrin homology and SH2 domains; BLNK, B cell linker protein; SHIP-1, SH2-containing inositol phosphatase-1; GEF, guanine nucleotide exchange factor; ASAP1, Arf-GTPase-activating protein 1; ARAP3, Arf GAP and Rho GAP with ankyrin repeat and pleckstrin homology domains protein 3; PDGFR, platelet-derived growth factor receptor; HGFR, hepatocyte growth factor receptor; PI3K, phosphoinositide 3-kinase; PAK, p21-activated kinase; SFK, Src family kinase; CC, coiled-coil.
The three SH3 domains of CIN85 and CD2AP are particularly important for their scaffolding functions in receptor endocytosis. They bind to an atypical proline–arginine (PXXXPR) motif present in Cbl, Cbl-b, synaptojanin 2B1, SHIP-1 (SH2-containing inositol phosphatase-1), Hip1R, p115RhoGEF (guanine nucleotide-exchange factor) and the Arf GTPase-activating proteins ASAP1 [Arf-GAP (GTPase-activating protein) 1] and ARAP3 (Arf GAP and Rho GAP with ankyrin repeat and pleckstrin homology domains protein 3) [68] (Figure 1). Importantly, the relatively low affinity of individual CIN85 SH3 domain–PXXXPR interactions [69] allows the rapid exchange of CIN85 binding partners, depending on their local concentration, cellular compartmentalization and/or post-translational modifications, in response to changes in the environment. Consistent with this hypothesis, CIN85 was shown to form high-molecular-mass complexes in native lysates of cells that could be dynamically exchanged following ligand stimulation [68]. These various complexes that assemble around CIN85 SH3 domains can differently influence the fate of internalized receptors. For example, Dab2 associates with the SH3 domains of CIN85 and links it to clathrin complexes [70]. Ligand stimulation of the EGFR leads to dissociation of the Dab2–CIN85 interaction and recruitment of CIN85 to the Cbl receptor complex [70]. All of this evidence suggests that CIN85 may serve as a platform that interacts simultaneously with multiple endocytic effectors and rapidly exchanges them along the endocytic pathway.
Recent studies on transgenic mice deficient in CD2AP revealed additional functions of CD2AP in the regulation of receptor endocytosis in T cells [71], where CD2AP is likely to facilitate TCR (T-cell receptor) patterning in the contact area by linking specific adhesion receptors to the cytoskeleton [60]. Since CD2 is associated with the TCR, clustering of CD2 can recruit TCR to the contact surface. Concentrating antigen, receptors and kinases in the stable central cluster of TCR enhances signalling and simultaneously induces receptor degradation. T cells deficient in CD2AP do not form this immunological synapse and show sustained tyrosine phosphorylation as well as enhanced proliferation [71]. This corresponds with reduced down-regulation of TCR, which normally occurs after T cell activation. CD2AP seems to participate in regulating the delivery of TCR to lysosomes for degradation, rather than removal of receptors from the plasma membrane [71]. Notably, Cbl and Cbl-b proteins, known to bind to CD2AP and CIN85, are also associated with TCR complexes and are essential for their targeting for degradation. This effect is observed only in Cbl/Cbl-b double knockout cells, whereas T cells lacking Cbl or Cbl-b did not show significant defects in the targeting of TCR for degradation [72].
COMPARTMENT-SELECTIVE SCAFFOLDS
Based on recent reports, the traditional view of the endocytic system being divided into distinct early, late and recycling endosomes seems to be giving way to the recognition of a set of functional membrane subdomains, or endocytic microcompartments, that are critical for the transport of cargoes [73]. These membrane regions exist in each endocytic compartment and are characterized by defined biophysical properties. At the critical points of transition between these distinct functional regions, several regulatory protein and lipids are specifically distributed. For example, functional regions of the early endosome contain membrane domains enriched in clathrin, Rab5 GTPases, raft components and annexin II, which are all important for the transport of cargo through this compartment. Usually, the recruitment of lipid kinases or phosphatases induces local lipid enrichment and leads to the exchange of lipid-binding proteins. It is thought that the presence of compartment-specific scaffolds might be essential for the coordination of these multiple effector–membrane interactions and preservation of the functional integrity of particular compartments. Based on this local assembly of multifunctional complexes, cargo sorting is also coupled functionally to the process of membrane invagination and biogenesis of endosomes [74].
Scaffolds in plasma membrane microcompartments
Several groups of molecular scaffolds have been implicated in the initial steps of endocytosis, forming distinct plasma membrane microcompartments. These include proteins involved in nucleation of the clathrin coat and in linking it to lipid membranes, proteins involved in bending of the lipid bilayer and recruiting molecules involved in fission, and large protein complexes that couple cargo recognition and vesicle tethering.
Scaffolds with a BAR (Bin/amphiphysin/Rvs) domain
Certain endocytic adaptors are able to sense and alter membrane curvature by modifying the lipid content or by deforming the membrane mechanically via their BAR domains [75]. The BAR domain has a coiled-coil structure that binds preferentially to negatively charged membranes [76]. This curvature-sensing and -inducing module is present in diverse groups of endocytic proteins, including amphiphysins, endophilins, nadrins, the Rab5 effectors APPL 1 and 2, sorting nexins, arfaptins, centaurins and oligophrenins [77].
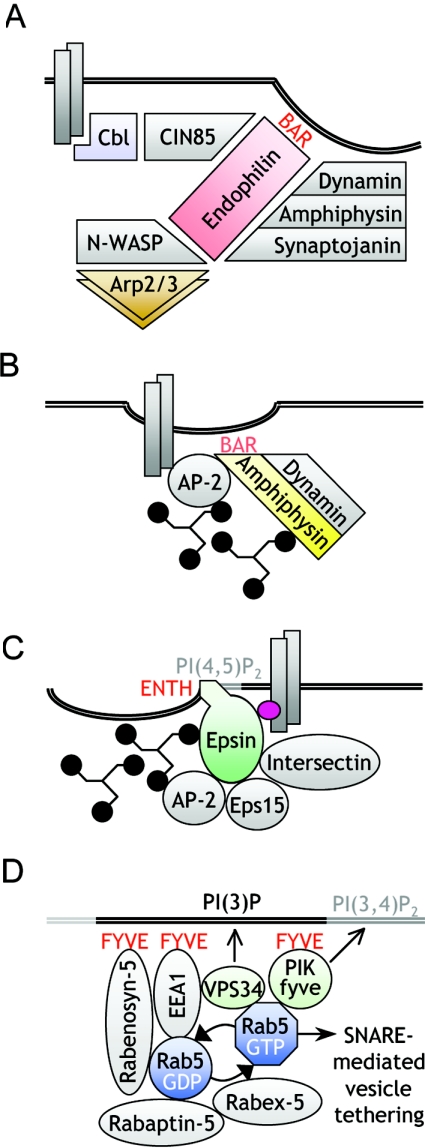
The endophilins participates in invagination, scission and vesicle recycling, representing a good example of a scaffold protein that orchestrates multiple activities at the nexus of signalling and internalization (Figure 2A). The SH3 domain at the C-terminal end of endophilin associates with synaptojanin, intersectin, dynamin and amphiphysin [78,79]. The coiled-coil BAR domain at the N-terminus is required for dimerization and membrane tubulation. Stimulation by EGF induces the association of endophilin with neural Wiskott–Aldrich syndrome protein, enhancing the activity of the Arp2/3 protein complex and leading to the accumulation of actin at the internalization sites, thus contributing to the fission of clathrin-coated vesicles [80]. In addition to co-ordinating cargo, membrane shape and cytoskeleton, endophilin may also participate in ubiquitin-dependent sorting, as it is mono-ubiquitinated upon ligand stimulation [81].
Figure 2. Examples of dynamic protein–lipid complexes that couple the cargo with the biogenesis and trafficking of vesicles in endocytic microcompartments.
(A) Endophilin co-ordinates cargo entry and actin polymerization at clathrin-coated pits. Its BAR domain senses and induces membrane curvature. (B) The BAR domain of amphiphysin binds to curved negatively charged phospholipids, while its other domains associate with AP-2, clathrin and dynamin. (C) Epsin interacts with clathrin, AP-2, Eps15, intersectin and ubiquitin (purple oval). Its ENTH domain binds PtdIns(4,5)P2 [PI(4,5)P2] and introduces membrane curvature. (D) The Rab domain at the level of the early endosome is a functional protein multimer that is coupled to the membrane phospholipid PtdIns3P via binding of FYVE-domain-containing proteins. For details, see the text.
The amphiphysins are another group of BAR domain proteins, which link the clathrin coat components to the membrane [82–84] (Figure 2B). Amphiphysins 1 and 2 are composed of three main binding regions. The N-terminal region forms the BAR domain that is involved in dimerization [76]. The central region binds the heavy chain of clathrin and the α subunit of AP-2 via distinct sites. In addition, it contains a proline-rich region, which binds efficiently to the SH3 domain of amphiphysin itself [83]. The C-terminal region comprises an SH3 domain, which interacts with dynamin and synaptojanin [4]. The interactions of this SH3 domain and those of the central region are both regulated by phosphorylation [85]. Experimental manipulations of living cells that disrupt the interactions of amphiphysin with either dynamin or clathrin and AP-2 lead to potent inhibition of clathrin-mediated endocytosis [85–87].
Scaffolds with an ENTH (epsin N-terminal homology) domain
Initially described as phospholipid-binding domains, ENTH domains are present in a variety of proteins involved in the formation of clathrin-coated pits and vesicles [88–91]. The interaction of the ENTH domain with the head of PtdIns(4,5)P2 results in the formation of an amphipathic helix, which may account for bilayer penetration and bending [9]. The related ANTH (AP180 N-terminal homology) domain lacks this ability, and instead regulates lattice size [9]. In a model mechanism, AP180 and AP-2 induce polymerization of the clathrin lattice at the plasma membrane, while epsins facilitate its invagination [9,91]. Epsins 1–3 were initially identified based on their ability to bind to the EH domains of Eps15 (‘epsin’ is derived from Eps15-interacting protein) [92]. In addition to the ENTH domain, epsin contains two ubiquitin-interacting motifs, a DPW (Asp-Pro-Trp) domain that binds to the α subunit of AP-2, a clathrin box, and three NPF motifs that bind Eps15. It promotes the assembly of clathrin in vitro and, although it is not a constitutive compound of clathrin-coated pits, it participates in dynamic rearrangements during the assembly, invagination and scission of clathrin-coated vesicles [92] (Figure 2C). The affinity of epsin for the α-appendage of AP-2 is greater than that of AP180 [93] and, similarly to Eps15, dephosphorylation of epsin enhances its association with AP-2 [94]. In addition, the ubiquitin-interacting motif of epsin is considered to play an important role in recognizing the ubiquitinated cargo and triggering internalization events [95].
Characteristically, the location of action of these scaffolds seems to be dependent on their specificity of binding to membrane lipids. The ENTH domain of epsin associates preferentially with PtdIns(4,5)P2 enriched at the plasma membrane, whereas the ENTH domain of the related protein enthoprotin shows a preference for PtdIns4P enriched in the trans-Golgi network [91]. Interestingly, enthoprotin, as well as the yeast proteins Ent3p and Ent5p, associate with the GGA family and AP-1 complex [96]. The ENTH domains of these proteins bind the SNARE (soluble NSF attachment protein receptor) Vti1b and are required for transport from the trans-Golgi network to late endosomes [97]. Ent5p localizes to endosomes and binds specifically to PtdIns(3,5)P2 via its ENTH domain. Ent3p and Ent5p are associated with Vps27p, the yeast homologue of Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate), and deletion of both Ent3p and Ent5p disrupts the ubiquitin-dependent sorting of biosynthetic and endocytic cargo into MVBs [98].
Scaffold proteins linked with Rab-decorated compartments
The classical case of functionally distinct microdomains is defined by the presence of specific Rab GTPases and their effectors. Based on the work of Zerial and colleagues [99,100], the concept of spatially restricted and functionally specialized regions defined by the presence of particular Rab proteins and their effectors has become a prototype for endocytic microcompartments. The family of Rab GTPases, as well as their regulators and adaptors, has been reviewed in detail elsewhere [99,101]. In a highly organized and sequential manner, they form oligomeric complexes involving several adaptor proteins that co-ordinate membrane fusion and budding, vesicle tethering, and the microtubule- and actin-dependent motility of vesicles. The activity of Rab proteins depends on the rate of nucleotide exchange and hydrolysis, and is regulated by positive feedback loops generated by Rab effectors. For example, Rab5 co-ordinates membrane traffic from the plasma membrane to early endosomes. Expression of the constitutively active, GTP-bound mutant Q79L-Rab5 stimulates endosome fusion and the enlargement of early endosome compartments (Figure 2D). This results in increased internalization of the transferrin receptor. Expression of the S34N mutant that preferentially binds GDP has the opposite effect [102]. Importantly, ligand-induced endocytosis seems to require specific adaptor proteins that modulate the function of Rab proteins and link them to various neighbouring compartments. Rabenosyn-5 contains a FYVE (Fab1p, YOTB, Vac1p and EEA1) finger binding PtdIns(3)P and five copies of the NPF tripeptide that can associate with EH-domain proteins. Rabaptin-5 and rabaptin-5b have oligomerizing coiled-coil domains and a DPF motif that interacts with the AP-2 complex. Co-operation and an ability to self-organize results in the formation of high-molecular-mass complexes at the membrane of early endosomes, including the Rab nucleotide exchange factor Rabex-5 as well as the tethering molecule EEA1 (early endosome antigen 1). Localization of EEA1 to Rab5 domains is dependent on an association with phospholipids via the FYVE domain and on direct binding to Rab5, resulting in NSF/SNARE-mediated early endosome docking and fusion. The lipid-modifying enzyme VPS34 is targeted to Rab5 domains on early endosomes and produces PtdIns3P, which in turn recruits FYVE-domain-containing proteins. One of these is PIKfyve, which converts PtdIns3P into PtdIns(3,5)P2 in the late endosome [103]. In addition, the trafficking of the EGFR is regulated specifically via Rin1, an exchange factor for Rab5 [104,105], and Eps8, which is a partner of the Rab5 GTPase-activating protein RN-tre [106].
Through the action of the rabenosyn-5, rabaptin-5 and rabaptin-5β adaptors, Rab5 is connected to Rab4 domains that are involved in the rapid recycling of cargo to the plasma membrane. Similar adaptors are predicted to link Rab4 and Rab11 in recycling endosomes, and probably constitute a common pattern of interactions that ensure sequential membrane traffic between interchanging Rab complexes.
Scaffold proteins controlling traffic of ubiquitinated cargo
In recent years, the modification of cargo and endocytic proteins by a single ubiquitin molecule has become a well recognized mechanism responsible for differential sorting at both the internalization and MVB formation steps. Internalization of a number of RTKs, GPCRs and immune receptors, as well as membrane transporters and channels, is mediated by conjugated ubiquitin that functions as a signal for cargo recruitment [107]. While monoubiquitination of the EGFR appears to be sufficient to trigger internalization, it does not appear to be essential, due to the presence of several redundant pathways [108]. Notably, the classical clathrin adaptor AP-2, which was thought to be the main adaptor that regulates the selection and recruitment of activated receptors into clathrin-coated pits, is not required for the internalization of ubiquitinated receptors [109–111]. Instead, epsin and Eps15 are accepted candidates for ubiquitin-specific scaffolds at the level of clathrin-coated vesicles. These pathways may substitute for each other under physiological conditions, and can also be involved in the regulation of multiple entry routes into the cell. On the other hand, modification by a single or multiple ubiquitin moieties is a common feature required for sorting of the majority of cargo into MVBs, being the crucial step for their degradation in lysosomes. It is currently believed that recognition of mono-ubiquitin by a set of ubiquitin receptors [proteins containing one or more ubiquitin-binding domain, i.e. UBA (ubiquitin-associated) domain, ubiquitin-interacting motif, CUE (coupling of ubiquitin conjugation to endoplasmic reticulum degradation) domain] present in the microdomains of endosomal membranes allows transfer of cargo from one compartment to another. In addition, numerous endocytic proteins involved in cargo transport are themselves subject to mono-ubiquitination, a process that is dependent on the presence of their ubiquitin-binding domains. Obviously, ubiquitination regulates the endocytic function of molecular scaffolds. According to one hypothesis, the ubiquitin-interacting motif may associate with ubiquitin appended on the same molecule (cis-interactions), which induces a structural change of the adaptor and the release of ubiquitinated cargo. In general, ubiquitin-binding domains have rather low affinity for mono-ubiquitin, thus ensuring readily reversible interactions, which therefore require associated protein complexes that will affect the avidity and duration of their interactions. In accordance with this, multifunctional scaffold proteins have been identified, which may regulate ubiquitin-dependent transport in a sequential and highly organized manner that is conserved from yeast to mammals (Figure 3).
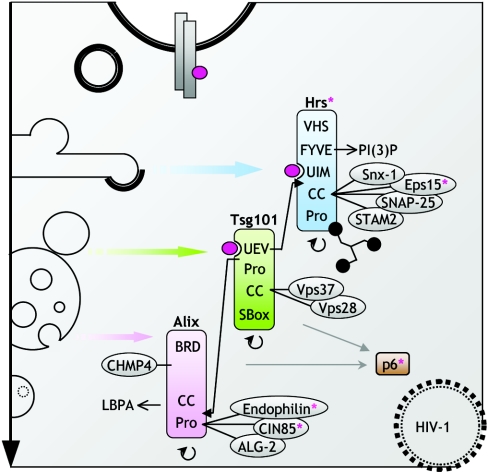
Figure 3. Ubiquitin traffic.
Lysosomal degradation of ubiquitinated receptors is directed by the sequential action of the ubiquitin-binding protein complex composed of Hrs, Tsg101 and Alix with associated proteins. All three molecules form oligomers. Hrs and Tsg101 bind ubiquitin directly via UIM (ubiquitin-interacting motif) and UEV (ubiquitin-conjugating enzyme E2 variant) domains respectively. Hrs binds PtdIns3P [PI(3)P] and sequesters cargo in clathrin-coated regions of early endosomes, preventing it from recycling. The UEV domain of Tsg101 associates with PSAP motifs in Hrs and Alix (block arrows). Tsg101 and Alix co-ordinate the function of ESCRT protein complexes involved in the biogenesis of MVBs. Alix associates with LPBA, a lipid that is necessary for the formation of inner vesicles of MVBs. Upon ligand stimulation, several accessory proteins, including Hrs, undergo mono-ubiquitnation (indicated by a purple asterisk). This has been implicated in co-ordinating vesicular transport. The endocytic sorting machinery is recruited during the budding of RNA viruses, whose Gag proteins are ubiquitinated and have motifs that bind Tsg101 and Alix. Abbreviations: VHS, Vsp27p, Hrs, STAM; CC, coiled-coil; SNAP-25, 25 kDa synaptosome-associated protein; BRD, Bro1-rhophilin conserved domain; CHMP4, charged MVB protein; ALG, apoptosis-linked gene 2.
Most of our current understanding of these multiprotein complexes has been revealed by genetic studies of the Vps class E proteins that are involved in MVB biogenesis and protein sorting in yeast. There are around 20 known class E mutants in yeast, which can be divided into three subgroups called ESCRTs (endosomal sorting complexes required for transport). ESCRT-I is recruited to the membrane of early endosomes by the ubiquitin- and clathrin-binding protein Vps27p. Vps23p in ESCRT-I and Vps36p in ESCRT-II contain ubiquitin-binding motifs and may interact directly with ubiquitinated cargo. The sequential actions of ESCRT-I, -II and -III result in inward budding and sorting of cargo to the inner vesicles of endosomes [112–114].
Hrs
Hrs is a mammalian homologue of the yeast vacuolar sorting protein Vps27p. It is localized in endosomes, where it recognizes ubiquitinated cargo, sequesters it to the clathrin-coated microdomains, and recruits the ESCRTs [115]. Hrs has a phospholipid-binding FYVE domain and is therefore concentrated in clathrin-coated regions of endosomal membranes that contain low levels of PtdIns3P. This is in contrast with other FYVE domain-containing proteins, such as EEA1, SARA (Smad anchor for receptor activation) and CISK (cytokine-independent survival kinase), which co-localize in domains rich in PtdIns3P and Rab5 [116]. Hrs is a part of a large 550 kDa complex [117] and binds to the adaptor molecules STAM (signal-transducing adaptor molecule), STAM2 and Smad2, SNAP-25 (25 kDa synaptosome-associated protein) and Eps15 [118]. The VHS (Vsp27p, Hrs, STAM) domain of Hrs is structurally similar to E/ANTH domains and determines its membrane localization. In the early endosome, a clathrin-associated complex containing Hrs, STAM2 and Eps15 binds ubiquitinated cargo, promoting its retention in the endocytic compartments, and prevents recycling [119]. All three proteins seem to participate in a multivalent complex that sorts ubiquitinated membrane proteins into the MVB pathway. In addition, Hrs contains the tetrapeptide motif PSAP (Pro-Ser-Ala-Pro), similar to the PTAP motif present in Tsg101 and the HIV Gag protein, which mediates association with the UEV (ubiquitin-conjugating enzyme E2 variant) domain of Tsg101 [120].
In Vps27-deficient yeast cells, proteins normally transported to the vacuole, such as the α-factor Ste3p, accumulate in prevacuolar endosomal compartments [121]. In mammalian cells, both deletion and overexpression of Hrs lead to enlargement of the early endosome compartment [119,122]. With respect to constitutive endocytosis, some reports found that overexpression of Hrs inhibited the internalization of transferrin [122], while the others stated that, under conditions when Hrs overexpression blocked the trafficking of receptor-bound (EGF) and fluid phase marker (dextran), transferrin uptake and recycling was not affected [123]. Stimulation by EGF, hepatocyte growth factor or platelet-derived growth factor, as well as interleukin-2 or granulocyte/macrophage colony-stimulating factor, induced phosphorylation of Hrs [124,125] and its partner STAM [126]. This leads to dissociation of Hrs from endosomes, and is prevented under conditions that inhibit endocytosis [127]. Depletion of Hrs by small interfering RNA impairs the degradation of internalized EGFR and inhibits MVB biogenesis, resulting in a decreased number of internal vesicles [119]. In accordance with its importance for maintaining functional endocytic processing, Hrs−/− mouse embryos die at day 11 [128].
Alix [ALG-2 (apoptosis-linked gene 2)-interacting protein X]
Alix, also known as AIP1 (ALG-2-interacting protein 1), is a 95 kDa protein with a coiled-coil motif and a proline-rich region. It interacts with Tsg101 (a component of ESCRT-I) and several CHMP4 (charged MVB protein; component of ESCRT-III) [129,130], thus linking these two protein complexes [131] and co-ordinating late steps of MVB sorting. Subsequent recruitment of the ATPase Vps4 induces dissociation of Alix and the sorting complexes from endosome membranes [114]. The polyproline motifs of Alix bind to CIN85 [132] and endophilin [133], further suggesting an involvement of this protein in endocytosis processes. The yeast orthologue Bro1/Vps31p is required for the ubiquitin-dependent vacuolar sorting of Gap1 amino acid permease, and a deficiency of Bro1 results in recycling and de-ubiquitination of Gap1 [134]. Bro1 is also required for endosomal sorting of the uracil permease Fur4, the maltose permease Mal61, the hexose permease Hxt6/7 [135], Ste2 and carboxypeptidase S [136]. In addition, data obtained with yeast, fungal and mammalian homologues of Alix suggest a role in the recruitment of cargo possessing the YPXL/I motif to the late steps of endocytosis. Intriguingly, this motif is present in the p9 Gag protein of the equine infectious anaemia virus and in p6 Gag of HIV-1, both of which require Alix for efficient budding through endosomal and plasma membranes [129,131]. Apparently, the process of budding of enveloped viruses resembles the vesicle budding inside MVBs and recruits the same cellular compounds [131]. The late stages of retroviral budding are ubiquitin-dependent and can be blocked by the use of proteasome inhibitors [137,138], with the retroviral Gag proteins mimicking the endocytic adaptor Hrs [120,139].
In addition, Alix was recently shown to bind to LBPA (lysobiphosphatidic acid), a resident phospholipid of internal vesicles of the late endosome, thus being localized in subdomains of internal membranes of the MVBs [140]. LBPA is a fusogenic lipid that has an intrinsic ability to induce the formation of vesicles within acidic liposomes. Interestingly, the addition of a recombinant Alix to LBPA-containing liposomes leads to inhibition of MVB formation, whereas its depletion by specific antibodies stimulates MVB formation [140]. In vivo, down-regulation of Alix by small interfering RNA decreases the number of acidic late endosomal compartments and reduces the level of LBPA [140]. Thus Alix may regulate endosome biogenesis and receptor sorting in the late endosome via its interactions with sorting complexes and LBPA.
FINAL COMMENTS AND PERSPECTIVES
In the past few years we have seen great progress in understanding the complexity of interactions at the membrane–cytosol interface. Studies in yeast, Drosophila and mammalian cells have revealed common themes underlying membrane traffic in the cell. The specificity of cargo transport is co-ordinated by a class of multifunctional scaffold proteins that act in a cargo- or compartment-selective manner. Owing to numerous interactions and often overlapping mechanisms of action, classification of endocytic adaptors is currently far from being complete. In this review we have tried to emphasize the interconnection and importance of scaffold protein functions in the trafficking and signalling of various receptors. Dissecting the cross-linked pathways by studying the role of a particular adaptor must be placed in the global context of the endocytic machinery that co-ordinates cargo sorting, membrane fission, lipid metabolism, signalling pathways and local modification of the cytoskeleton. Detailed mechanistic analysis of the roles of scaffold networks on the molecular level, particularly under physiological and pathological conditions, remains a challenge for future research.
Acknowledgments
We apologize to investigators whose important contributions were not included in this review due to space limitations. We thank Daniela Hoeller and Mirko Schmidt for critical reading of the manuscript.
References
- 1.Herbst J. J., Opresko L. K., Walsh B. J., Lauffenburger D. A., Wiley H. S. Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J. Biol. Chem. 1994;269:12865–12873. [PubMed] [Google Scholar]
- 2.Wiley H. S., Herbst J. J., Walsh B. J., Lauffenburger D. A., Rosenfeld M. G., Gill G. N. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- 3.Mousavi S. A., Malerod L., Berg T., Kjeken R. Clathrin-dependent endocytosis. Biochem. J. 2004;377:1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy V. N., De Camilli P. Cell biology of the presynaptic terminal. Annu. Rev. Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- 5.Gundelfinger E. D., Kessels M. M., Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 2003;4:127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., Wakeham D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 8.Ford M. G., Pearse B. M., Higgins M. K., Vallis Y., Owen D. J., Gibson A., Hopkins C. R., Evans P. R., McMahon H. T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 9.Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. Curvature of clathrin-coated pits driven by epsin. Nature (London) 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 10.Wendland B. Epsins: adaptors in endocytosis? Nat. Rev. Mol. Cell Biol. 2002;3:971–977. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 11.Goodman O. B., Jr, Krupnick J. G., Gurevich V. V., Benovic J. L., Keen J. H. Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J. Biol. Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 12.Hinshaw J. E. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Praefcke G. J., McMahon H. T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 14.Merrifield C. J., Feldman M. E., Wan L., Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 15.Shupliakov O., Bloom O., Gustafsson J. S., Kjaerulff O., Löw P., Tomilin N., Pieribone V. A., Greengard P., Brodin L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claing A., Laporte S. A., Caron M. G., Lefkowitz R. J. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 17.Shenoy S. K., Lefkowitz R. J. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem. J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald P. H., Lefkowitz R. J. Beta-Arrestins: new roles in regulating heptahelical receptors' functions. Cell. Signalling. 2001;13:683–689. doi: 10.1016/s0898-6568(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 19.Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 20.Luttrell L. M., Lefkowitz R. J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 21.Laporte S. A., Miller W. E., Kim K. M., Caron M. G. beta-Arrestin/AP-2 interaction in G protein-coupled receptor internalization: identification of a beta-arrestin binding site in beta 2-adaptin. J. Biol. Chem. 2002;277:9247–9254. doi: 10.1074/jbc.M108490200. [DOI] [PubMed] [Google Scholar]
- 22.Gaidarov I., Krupnick J. G., Falck J. R., Benovic J. L., Keen J. H. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald P. H., Cote N. L., Lin F. T., Premont R. T., Pitcher J. A., Lefkowitz R. J. Identification of NSF as a beta-arrestin1-binding protein. Implications for beta2-adrenergic receptor regulation. J. Biol. Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 24.Claing A., Chen W., Miller W. E., Vitale N., Moss J., Premont R. T., Lefkowitz R. J. beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J. Biol. Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- 25.Delaney K. A., Murph M. M., Brown L. M., Radhakrishna H. Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis. J. Biol. Chem. 2002;277:33439–33446. doi: 10.1074/jbc.M205293200. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy S. K., Lefkowitz R. J. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J. Biol. Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 27.Lin F. T., Krueger K. M., Kendall H. E., Daaka Y., Fredericks Z. L., Pitcher J. A., Lefkowitz R. J. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J. Biol. Chem. 1997;272:31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 28.Kovoor A., Celver J., Abdryashitov R. I., Chavkin C., Gurevich V. V. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J. Biol. Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- 29.Kim O. J., Gardner B. R., Williams D. B., Marinec P. S., Cabrera D. M., Peters J. D., Mak C. C., Kim K. M., Sibley D. R. The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J. Biol. Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wodarz A., Nusse R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 31.Malbon C. C. Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front. Biosci. 2004;9:1048–1058. doi: 10.2741/1308. [DOI] [PubMed] [Google Scholar]
- 32.Chen W., ten Berge D., Brown J., Ahn S., Hu L. A., Miller W. E., Caron M. G., Barak L. S., Nusse R., Lefkowitz R. J. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 33.Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., Blobe G. C. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 34.Lin F. T., Daaka Y., Lefkowitz R. J. Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J. Biol. Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 35.Wu J. H., Peppel K., Nelson C. D., Lin F. T., Kohout T. A., Miller W. E., Exum S. T., Freedman N. J. The adaptor protein beta-arrestin2 enhances endocytosis of the low density lipoprotein receptor. J. Biol. Chem. 2003;278:44238–44245. doi: 10.1074/jbc.M309450200. [DOI] [PubMed] [Google Scholar]
- 36.Morris S. M., Cooper J. A. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- 37.Mishra S. K., Keyel P. A., Hawryluk M. J., Agostinelli N. R., Watkins S. C., Traub L. M. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santolini E., Salcini A. E., Kay B. K., Yamabhai M., Di Fiore P. P. The EH network. Exp. Cell Res. 1999;253:186–209. doi: 10.1006/excr.1999.4694. [DOI] [PubMed] [Google Scholar]
- 39.Morris S. M., Tallquist M. D., Rock C. O., Cooper J. A. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 2002;21:1555–1564. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamikura D. M., Cooper J. A. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in C. elegans. Genes Dev. 2003;17:2798–2811. doi: 10.1101/gad.1136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He G., Gupta S., Yi M., Michaely P., Hobbs H. H., Cohen J. C. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J. Biol. Chem. 2002;277:44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 42.Mishra S. K., Watkins S. C., Traub L. M. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16099–16104. doi: 10.1073/pnas.252630799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagai M., Meerloo T., Takeda T., Farquhar M. G. The adaptor protein ARH escorts megalin to and through endosomes. Mol. Biol. Cell. 2003;14:4984–4996. doi: 10.1091/mbc.E03-06-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oleinikov A. V., Zhao J., Makker S. P. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem. J. 2000;347:613–621. [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia C. K., Wilund K., Arca M., Zuliani G., Fellin R., Maioli M., Calandra S., Bertolini S., Cossu F., Grishin N., et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 2001;292:1394–1398. doi: 10.1126/science.1060458. [DOI] [PubMed] [Google Scholar]
- 46.Santolini E., Puri C., Salcini A. E., Gagliani M. C., Pelicci P. G., Tacchetti C., Di Fiore P. P. Numb is an endocytic protein. J. Cell Biol. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chien C. T., Wang S., Rothenberg M., Jan L. Y., Jan Y. N. Numb-associated kinase interacts with the phosphotyrosine binding domain of Numb and antagonizes the function of Numb in vivo. Mol. Cell. Biol. 1998;18:598–607. doi: 10.1128/mcb.18.1.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nie J., McGill M. A., Dermer M., Dho S. E., Wolting C. D., McGlade C. J. LNX functions as a RING type E3 ubiquitin ligase that targets the cell fate determinant Numb for ubiquitin-dependent degradation. EMBO J. 2002;21:93–102. doi: 10.1093/emboj/21.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu B., Rothenberg M., Jan L. Y., Jan Y. N. Partner of Numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell. 1998;95:225–235. doi: 10.1016/s0092-8674(00)81753-5. [DOI] [PubMed] [Google Scholar]
- 50.Qin H., Percival-Smith A., Li C., Jia C. Y., Gloor G., Li S. S. A novel transmembrane protein recruits Numb to the plasma membrane during asymmetric cell division. J. Biol. Chem. 2004;279:11304–11312. doi: 10.1074/jbc.M311733200. [DOI] [PubMed] [Google Scholar]
- 51.Berdnik D., Torok T., Gonzalez-Gaitan M., Knoblich J. A. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- 52.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 53.Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem. Soc. Trans. 2003;31:1178–1181. doi: 10.1042/bst0311178. [DOI] [PubMed] [Google Scholar]
- 54.Soubeyran P., Kowanetz K., Szymkiewicz I., Langdon W. Y., Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature (London) 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 55.Petrelli A., Gilestro G. F., Lanzardo S., Comoglio P. M., Migone N., Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature (London) 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- 56.Szymkiewicz I., Kowanetz K., Soubeyran P., Dinarina A., Lipkowitz S., Dikic I. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J. Biol. Chem. 2002;277:39666–39672. doi: 10.1074/jbc.M205535200. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi S., Sawano A., Nojima Y., Shibuya M., Maru Y. The c-Cbl/CD2AP complex regulates VEGF-induced endocytosis and degradation of Flt-1 (VEGFR-1) FASEB J. 2004;18:929–931. doi: 10.1096/fj.03-0767fje. [DOI] [PubMed] [Google Scholar]
- 58.Lynch D. K., Winata S. C., Lyons R. J., Hughes W. E., Lehrbach G. M., Wasinger V., Corthals G., Cordwell S., Daly R. J. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 2003;278:21805–21813. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 59.Dikic I. CIN85/CMS family of adaptor molecules. FEBS Lett. 2002;529:110–115. doi: 10.1016/s0014-5793(02)03188-5. [DOI] [PubMed] [Google Scholar]
- 60.Dustin M. L., Olszowy M. W., Holdorf A. D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P. A., Allen P. M., Shaw A. S. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 61.Kirsch K. H., Georgescu M. M., Ishimaru S., Hanafusa H. CMS: an adapter molecule involved in cytoskeletal rearrangements. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6211–6216. doi: 10.1073/pnas.96.11.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Take H., Watanabe S., Takeda K., Yu Z. X., Iwata N., Kajigaya S. Cloning and characterization of a novel adaptor protein, CIN85, that interacts with c-Cbl. Biochem. Biophys. Res. Commun. 2000;268:321–328. doi: 10.1006/bbrc.2000.2147. [DOI] [PubMed] [Google Scholar]
- 63.Gout I., Middleton G., Adu J., Ninkina N. N., Drobot L. B., Filonenko V., Matsuka G., Davies A. M., Waterfield M., Buchman V. L. Negative regulation of PI 3-kinase by Ruk, a novel adaptor protein. EMBO J. 2000;19:4015–4025. doi: 10.1093/emboj/19.15.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogler O., Furnari F. B., Kindler-Roehrborn A., Sykes V. W., Yung R., Huang H. J., Cavenee W. K. SETA: a novel SH3 domain-containing adapter molecule associated with malignancy in astrocytes. Neuro-oncology. 2000;2:6–15. doi: 10.1093/neuonc/2.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutchings N. J., Clarkson N., Chalkley R., Barclay A. N., Brown M. H. Linking the T cell surface protein CD2 to the actin-capping protein CAPZ via CMS and CIN85. J. Biol. Chem. 2003;278:22396–22403. doi: 10.1074/jbc.M302540200. [DOI] [PubMed] [Google Scholar]
- 66.Cormont M., Meton I., Mari M., Monzo P., Keslair F., Gaskin C., McGraw T. E., Le Marchand-Brustel Y. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic. 2003;4:97–112. doi: 10.1034/j.1600-0854.2003.40205.x. [DOI] [PubMed] [Google Scholar]
- 67.Verdier F., Valovka T., Zhyvoloup A., Drobot L. B., Buchman V., Waterfield M., Gout I. Ruk is ubiquitinated but not degraded by the proteasome. Eur. J. Biochem. 2002;269:3402–3408. doi: 10.1046/j.1432-1033.2002.03031.x. [DOI] [PubMed] [Google Scholar]
- 68.Kowanetz K., Husnjak K., Holler D., Kowanetz M., Soubeyran P., Hirsch D., Schmidt M. H., Pavelic K., De Camilli P., Randazzo P. A., Dikic I. CIN85 associates with multiple effectors controlling intracellular trafficking of EGF receptors. Mol. Biol. Cell. 2004;15:3155–3166. doi: 10.1091/mbc.E03-09-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kowanetz K., Szymkiewicz I., Haglund K., Kowanetz M., Husnjak K., Taylor J. D., Soubeyran P., Engstrom U., Ladbury J. E., Dikic I. Identification of a novel proline-arginine motif involved in CIN85-dependent clustering of Cbl and down-regulation of epidermal growth factor receptors. J. Biol. Chem. 2003;278:39735–39746. doi: 10.1074/jbc.M304541200. [DOI] [PubMed] [Google Scholar]
- 70.Kowanetz K., Terzic J., Dikic I. Dab2 links CIN85 with clathrin-mediated receptor internalization. FEBS Lett. 2003;554:81–87. doi: 10.1016/s0014-5793(03)01111-6. [DOI] [PubMed] [Google Scholar]
- 71.Lee K. H., Dinner A. R., Tu C., Campi G., Raychaudhuri S., Varma R., Sims T. N., Burack W. R., Wu H., Wang J., et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 72.Naramura M., Jang I. K., Kole H., Huang F., Haines D., Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 73.Gruenberg J. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 74.Gruenberg J., Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 75.Zimmerberg J., McLaughlin S. Membrane curvature: how BAR domains bend bilayers. Curr. Biol. 2004;14:R250–R252. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 76.Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 77.Habermann B. The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 2004;5:250–255. doi: 10.1038/sj.embor.7400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huttner W. B., Schmidt A. A. Membrane curvature: a case of endofeelin'. Trends Cell Biol. 2002;12:155–158. doi: 10.1016/s0962-8924(02)02252-3. [DOI] [PubMed] [Google Scholar]
- 79.Micheva K. D., Ramjaun A. R., Kay B. K., McPherson P. S. SH3 domain-dependent interactions of endophilin with amphiphysin. FEBS Lett. 1997;414:308–312. doi: 10.1016/s0014-5793(97)01016-8. [DOI] [PubMed] [Google Scholar]
- 80.Otsuki M., Itoh T., Takenawa T. Neural Wiskott-Aldrich syndrome protein is recruited to rafts and associates with endophilin A in response to epidermal growth factor. J. Biol. Chem. 2003;278:6461–6469. doi: 10.1074/jbc.M207433200. [DOI] [PubMed] [Google Scholar]
- 81.Stamenova S. D., Dunn R., Adler A. S., Hicke L. The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J. Biol. Chem. 2004;279:16017–16025. doi: 10.1074/jbc.M313479200. [DOI] [PubMed] [Google Scholar]
- 82.Wigge P., McMahon H. T. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci. 1998;21:339–344. doi: 10.1016/s0166-2236(98)01264-8. [DOI] [PubMed] [Google Scholar]
- 83.Farsad K., Slepnev V., Ochoa G., Daniell L., Hauke V., De Camilli P. A putative role for intramolecular regulatory mechanisms in the adaptor function of amphiphysin in endocytosis. Neuropharmacology. 2003;45:787–796. doi: 10.1016/s0028-3908(03)00306-x. [DOI] [PubMed] [Google Scholar]
- 84.Evergren E., Marcucci M., Tomilin N., Löw P., Slepnev V., Andersson F., Gad H., Brodin L., De Camilli P., Shupliakov O. Amphiphysin is a component of clathrin coats formed during synaptic vesicle recycling at the lamprey giant synapse. Traffic. 2004;5:514–528. doi: 10.1111/j.1398-9219.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 85.Slepnev V. I., Ochoa G. C., Butler M. H., Grabs D., Camilli P. D. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 86.Evergren E., Tomilin N., Vasylieva E., Sergeeva V., Bloom O., Gad H., Capani F., Shupliakov O. A pre-embedding immunogold approach for detection of synaptic endocytic proteins in situ. J. Neurosci. Methods. 2004;135:169–174. doi: 10.1016/j.jneumeth.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 87.Shupliakov O., Löw P., Grabs D., Gad H., Chen H., David C., Takei K., De Camilli P., Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- 88.De Camilli P., Chen H., Hyman J., Panepucci E., Bateman A., Brunger A. T. The ENTH domain. FEBS Lett. 2002;513:11–18. doi: 10.1016/s0014-5793(01)03306-3. [DOI] [PubMed] [Google Scholar]
- 89.Nossal R., Zimmerberg J. Endocytosis: curvature to the ENTH degree. Curr. Biol. 2002;12:R770–R772. doi: 10.1016/s0960-9822(02)01289-7. [DOI] [PubMed] [Google Scholar]
- 90.Hurley J. H., Wendland B. Endocytosis: driving membranes around the bend. Cell. 2002;111:143–146. doi: 10.1016/s0092-8674(02)01044-9. [DOI] [PubMed] [Google Scholar]
- 91.Legendre-Guillemin V., Wasiak S., Hussain N. K., Angers A., McPherson P. S. ENTH/ANTH proteins and clathrin-mediated membrane budding. J. Cell Sci. 2004;117:9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- 92.Chen H., Fre S., Slepnev V. I., Capua M. R., Takei K., Butler M. H., Di Fiore P. P., De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature (London) 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 93.Kalthoff C., Alves J., Urbanke C., Knorr R., Ungewickell E. J. Unusual structural organization of the endocytic proteins AP180 and epsin 1. J. Biol. Chem. 2002;277:8209–8216. doi: 10.1074/jbc.M111587200. [DOI] [PubMed] [Google Scholar]
- 94.Chen H., Slepnev V. I., Di Fiore P. P., De Camilli P. The interaction of epsin and Eps15 with the clathrin adaptor AP-2 is inhibited by mitotic phosphorylation and enhanced by stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 1999;274:3257–3260. doi: 10.1074/jbc.274.6.3257. [DOI] [PubMed] [Google Scholar]
- 95.Umebayashi K. The roles of ubiquitin and lipids in protein sorting along the endocytic pathway. Cell Struct. Funct. 2003;28:443–453. doi: 10.1247/csf.28.443. [DOI] [PubMed] [Google Scholar]
- 96.Duncan M. C., Costaguta G., Payne G. S. Yeast epsin-related proteins required for Golgi-endosome traffic define a gamma-adaptin ear-binding motif. Nat. Cell Biol. 2003;5:77–81. doi: 10.1038/ncb901. [DOI] [PubMed] [Google Scholar]
- 97.Chidambaram S., Mullers N., Wiederhold K., Haucke V., von Mollard G. F. Specific interaction between SNAREs and epsin N-terminal homology (ENTH) domains of epsin-related proteins in trans-Golgi network to endosome transport. J. Biol. Chem. 2004;279:4175–4179. doi: 10.1074/jbc.M308667200. [DOI] [PubMed] [Google Scholar]
- 98.Eugster A., Pecheur E. I., Michel F., Winsor B., Letourneur F., Friant S. Ent5p is required with Ent3p and Vps27p for ubiquitin-dependent protein sorting into the multivesicular body. Mol. Biol. Cell. 2004;15:3031–3041. doi: 10.1091/mbc.E03-11-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 100.Miaczynska M., Zerial M. Mosaic organization of the endocytic pathway. Exp. Cell Res. 2002;272:8–14. doi: 10.1006/excr.2001.5401. [DOI] [PubMed] [Google Scholar]
- 101.Stenmark H., Olkkonen V. M. The Rab GTPase family. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews3007. REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stenmark H., Parton R. G., Steele-Mortimer O., Lutcke A., Gruenberg J., Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sbrissa D., Ikonomov O. C., Shisheva A. Phosphatidylinositol 3-phosphate-interacting domains in PIKfyve. Binding specificity and role in PIKfyve endomenbrane localization. J. Biol. Chem. 2002;277:6073–6079. doi: 10.1074/jbc.M110194200. [DOI] [PubMed] [Google Scholar]
- 104.Barbieri M. A., Kong C., Chen P. I., Horazdovsky B. F., Stahl P. D. The SRC homology 2 domain of Rin1 mediates its binding to the epidermal growth factor receptor and regulates receptor endocytosis. J. Biol. Chem. 2003;278:32027–32036. doi: 10.1074/jbc.M304324200. [DOI] [PubMed] [Google Scholar]
- 105.Tall G. G., Barbieri M. A., Stahl P. D., Horazdovsky B. F. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 106.Lanzetti L., Rybin V., Malabarba M. G., Christoforidis S., Scita G., Zerial M., Di Fiore P. P. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature (London) 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 107.Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 108.Holler D., Dikic I. Receptor endocytosis via ubiquitin-dependent and -independent pathways. Biochem. Pharmacol. 2004;67:1013–1017. doi: 10.1016/j.bcp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Huang K. M., D'Hondt K., Riezman H., Lemmon S. K. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 1999;18:3897–3908. doi: 10.1093/emboj/18.14.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nesterov A., Carter R. E., Sorkina T., Gill G. N., Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. Effect of clathrin heavy chain- and alpha-adaptin specific small interfering RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- 112.Katzmann D. J., Babst M., Emr S. D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 113.Conibear E. An ESCRT into the endosome. Mol. Cell. 2002;10:215–216. doi: 10.1016/s1097-2765(02)00601-9. [DOI] [PubMed] [Google Scholar]
- 114.Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 115.Bache K. G., Brech A., Mehlum A., Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gillooly D. J., Raiborg C., Stenmark H. Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem. Cell Biol. 2003;120:445–453. doi: 10.1007/s00418-003-0591-7. [DOI] [PubMed] [Google Scholar]
- 117.Chin L. S., Raynor M. C., Wei X., Chen H. Q., Li L. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 2001;276:7069–7078. doi: 10.1074/jbc.M004129200. [DOI] [PubMed] [Google Scholar]
- 118.Komada M., Kitamura N. Hrs and hbp: possible regulators of endocytosis and exocytosis. Biochem. Biophys. Res. Commun. 2001;281:1065–1069. doi: 10.1006/bbrc.2001.4441. [DOI] [PubMed] [Google Scholar]
- 119.Bache K. G., Raiborg C., Mehlum A., Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 2003;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- 120.Garrus J. E., von Schwedler U. K., Pornillos O. W., Morham S. G., Zavitz K. H., Wang H. E., Wettstein D. A., Stray K. M., Cote M., Rich R. L., et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 121.Piper R. C., Cooper A. A., Yang H., Stevens T. H. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bean A. J., Davanger S., Chou M. F., Gerhardt B., Tsujimoto S., Chang Y. Hrs-2 regulates receptor-mediated endocytosis via interactions with Eps15. J. Biol. Chem. 2000;275:15271–15278. doi: 10.1074/jbc.275.20.15271. [DOI] [PubMed] [Google Scholar]
- 123.Raiborg C., Bache K. G., Mehlum A., Stang E., Stenmark H. Hrs recruits clathrin to early endosomes. EMBO J. 2001;20:5008–5021. doi: 10.1093/emboj/20.17.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Komada M., Kitamura N. Growth factor-induced tyrosine phosphorylation of Hrs, a novel 115-kilodalton protein with a structurally conserved putative zinc finger domain. Mol. Cell. Biol. 1995;15:6213–6221. doi: 10.1128/mcb.15.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Asao H., Sasaki Y., Arita T., Tanaka N., Endo K., Kasai H., Takeshita T., Endo Y., Fujita T., Sugamura K. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J. Biol. Chem. 1997;272:32785–32791. doi: 10.1074/jbc.272.52.32785. [DOI] [PubMed] [Google Scholar]
- 126.Takeshita T., Arita T., Asao H., Tanaka N., Higuchi M., Kuroda H., Kaneko K., Munakata H., Endo Y., Fujita T., Sugamura K. Cloning of a novel signal-transducing adaptor molecule containing an SH3 domain and ITAM. Biochem. Biophys. Res. Commun. 1996;225:1035–1039. doi: 10.1006/bbrc.1996.1290. [DOI] [PubMed] [Google Scholar]
- 127.Urbe S., Mills I. G., Stenmark H., Kitamura N., Clague M. J. Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol. Cell. Biol. 2000;20:7685–7692. doi: 10.1128/mcb.20.20.7685-7692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Komada M., Soriano P. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev. 1999;13:1475–1485. doi: 10.1101/gad.13.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Strack B., Calistri A., Craig S., Popova E., Gottlinger H. G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 130.Katoh K., Shibata H., Suzuki H., Nara A., Ishidoh K., Kominami E., Yoshimori T., Maki M. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J. Biol. Chem. 2003;278:39104–39113. doi: 10.1074/jbc.M301604200. [DOI] [PubMed] [Google Scholar]
- 131.von Schwedler U. K., Stuchell M., Muller B., Ward D. M., Chung H. Y., Morita E., Wang H. E., Davis T., He G. P., Cimbora D. M., et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 132.Chen B., Borinstein S. C., Gillis J., Sykes V. W., Bogler O. The glioma-associated protein SETA interacts with AIP1/Alix and ALG-2 and modulates apoptosis in astrocytes. J. Biol. Chem. 2000;275:19275–19281. doi: 10.1074/jbc.M908994199. [DOI] [PubMed] [Google Scholar]
- 133.Chatellard-Causse C., Blot B., Cristina N., Torch S., Missotten M., Sadoul R. Alix (ALG-2-interacting protein X), a protein involved in apoptosis, binds to endophilins and induces cytoplasmic vacuolization. J. Biol. Chem. 2002;277:29108–29115. doi: 10.1074/jbc.M204019200. [DOI] [PubMed] [Google Scholar]
- 134.Nikko E., Marini A. M., Andre B. Permease recycling and ubiquitination status reveal a particular role for Bro1 in the multivesicular body pathway. J. Biol. Chem. 2003;278:50732–50743. doi: 10.1074/jbc.M306953200. [DOI] [PubMed] [Google Scholar]
- 135.Springael J. Y., Nikko E., Andre B., Marini A. M. Yeast Npi3/Bro1 is involved in ubiquitin-dependent control of permease trafficking. FEBS Lett. 2002;517:103–109. doi: 10.1016/s0014-5793(02)02586-3. [DOI] [PubMed] [Google Scholar]
- 136.Odorizzi G., Katzmann D. J., Babst M., Audhya A., Emr S. D. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell Sci. 2003;116:1893–1903. doi: 10.1242/jcs.00395. [DOI] [PubMed] [Google Scholar]
- 137.Patnaik A., Chau V., Wills J. W. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13069–13074. doi: 10.1073/pnas.97.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ott D. E., Coren L. V., Sowder R. C., II, Adams J., Schubert U. Retroviruses have differing requirements for proteasome function in the budding process. J. Virol. 2003;77:3384–3393. doi: 10.1128/JVI.77.6.3384-3393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pornillos O., Higginson D. S., Stray K. M., Fisher R. D., Garrus J. E., Payne M., He G. P., Wang H. E., Morham S. G., Sundquist W. I. HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. J. Cell Biol. 2003;162:425–434. doi: 10.1083/jcb.200302138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N. S., Matile S., Dubochet J., Sadoul R., et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]