Abstract
We found that neurocan, a major brain chondroitin sulphate proteoglycan, interacts with HSPGs (heparan sulphate proteoglycans) such as syndecan-3 and glypican-1. Binding of these HSPGs to neurocan was prevented by treatment of the HSPGs with heparitinases I and II, but not by treatment of neurocan with chondroitinase ABC. Scatchard plot analysis indicated that neurocan has two binding sites for these HSPGs with different affinities. It is known that neurocan in the rodent brain is proteolytically processed with aging into N- and C-terminal fragments. When a mixture of whole neurocan and N- and C-terminal fragments prepared from neonatal mouse brains or recombinant N- and C-terminal fragments was applied to a heparin column, the whole molecule and both the N- and C-terminal fragments bound to heparin. A centrifugation cell adhesion assay indicated that both the N- and C-terminal neurocan fragments could interact with these HSPGs expressed on the cell surface. To examine the biological significance of the HSPG–neurocan interaction, cerebellar granule cells expressing these HSPGs were cultured on the recombinant neurocan substrate. A significant increase in the rate of neurite outgrowth was observed on the wells coated with the C-terminal neurocan fragment, but not with the N-terminal one. Neurite outgrowth-promoting activity was inhibited by pretreatment of neurocan substrate with heparin or the addition of heparitinase I to culture medium. These results suggest that HSPGs such as syndecan-3 and glypican-1 serve as the cell-surface receptor of neurocan, and that the interaction of these HSPGs with neurocan through its C-terminal domain is involved in the promotion of neurite outgrowth.
Keywords: heparan sulphate proteoglycan, neurocan, neurite outgrowth, brain development
Abbreviations: HRP, horseradish peroxidase; HSPG, heparan sulphate proteoglycan
INTRODUCTION
It is known that syndecan-3 is a membrane-bound HSPG (heparan sulphate proteoglycan) and has a unique mucin-like domain unlike other syndecans [1]. It has been reported that syndecan-3 interacts with some extracellular matrix components and heparin-binding growth factors through its heparan sulphate chains and that these interactions are probably involved in some biological functions such as cell adhesion, cell migration, neurite outgrowth and long-term potentiation [2–5].
In the present study, we performed a ligand overlay assay with biotinylated soluble syndecan-3 to detect binding proteins for syndecan-3. Neurocan was identified as a new binding protein for syndecan-3. The binding was prevented by the treatment of syndecan-3 with heparitinases I and II, suggesting that neurocan could bind to syndecan-3 through its heparan sulphate chains, which is consistent with the recent report that neurocan binds to heparin [6]. Similarly, we found that glypican-1 is also a neurocan-binding protein. Glypican-1, one of the GPI (glycosylphosphatidylinositol)-anchored HSPGs, is significantly expressed in the developing rodent brain similar to glypican-2 and syndecan-3 [7]. It is known that neurocan is capable of binding to various ligands including cell-surface molecules [8–10], extracellular matrix components [11–15] and growth-associated molecules [15,16], indicating that neurocan is a multi-functional component of the extracellular matrix of the brain. Syndecan-3 is expressed on the growing axonal surface during brain development [17–19]. Glypican-1 is detected on axonal projections and synaptic terminal fields in developing and adult brains [20–22]. It is well known that these cell-surface HSPGs act as co-receptors for various morphogenesis factors [23]. A recent report showed that the disruption of heparan sulphate synthesis leads to severe patterning defects in the developing mouse brain [24]. Since the HSPGs may also play some roles in the interaction between the cell and extracellular matrix [23], it is very important to determine the biological significance of cell-surface HSPG–neurocan interaction. To address this issue, cerebellar granule cells prepared from neonatal mouse cerebellum were examined, since immunohistochemical studies have revealed that syndecan-3, glypican-1 and neurocan are co-localized in the molecular layer of neonatal rodent cerebellum [17,21,25]. In the present study, we also found that the interaction of cell-surface HSPGs with neurocan substrate promoted neurite outgrowth from the cerebellar granule cells. Pretreatment of neurocan substrate with heparin or addition of heparitinase I to culture medium abolished the neurocan-induced neurite outgrowth, indicating that the heparan sulphate chain is essential for the activity. Since both soluble and cell-surface HSPGs could bind to neurocan, soluble HSPGs shed from the cell surface are probably involved in the regulation of neurocan-induced neurite outgrowth. Such a possibility will be considered in the present study.
MATERIALS AND METHODS
Animals and cells
Mice (ICR strain) and rats (Wistar strain) were purchased from SLC (Shizuoka, Japan). The animals were kept according to the ethical guidelines of the Kyoto Sangyo University animal committee. Neonatal mice aged 5–10 days were used throughout the experiments. N18TG-2 mouse neuroblastoma and PC12 rat pheochromocytoma cells were donated by Dr H. Higashida (Kanazawa University, Japan) and Dr M. Kurokawa-Seo (Kyoto Sangyo University, Japan) respectively.
Antibodies
An anti-mouse neurocan monoclonal antibody, designated as 3A11 (IgG2b), was generated by immunizing a female rat aged 6 weeks with proteoglycans prepared from neonatal mouse brains as described by Oohira et al. [26]. The specificity of the 3A11 monoclonal antibodies was confirmed by immunoblot analysis using purified neurocan. Goat anti-syndecan-3 and anti-glypican-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). An antibody against rabbit cell adhesion molecule L1 was donated by Dr C. Lagenaur (University of Pittsburgh, U.S.A.). The monoclonal antibodies F69-3G10 (anti-ΔHS monoclonal antibodies) and M2 (anti-FLAG monoclonal antibodies) were obtained from Seikagaku Corp. (Tokyo, Japan) and Sigma (St. Louis, MO, U.S.A.) respectively.
Purification and identification of syndecan-3-binding protein (neurocan)
A soluble extract of neonatal mouse brains was prepared and precipitated by ammonium sulphate as described previously [27,28]. The precipitate was dissolved in a solution containing 25 mM Tris/HCl (pH 7.5), 0.1 M NaCl and 7 M urea and then dialysed against the same solution. The dialysed material was applied to a column of DEAE-Sephacel (3 cm×15 cm; Amersham Biosciences, Piscataway, NJ, U.S.A.) equilibrated with the same solution. After washing with 25 mM Tris/HCl (pH 7.5), 0.3 M NaCl and 7 M urea, the bound proteins were eluted with 25 mM Tris/HCl (pH 7.5), 1.5 M NaCl and 7 M urea. After dialysis against 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl, the eluate was applied to a column of heparin–Sepharose (1 cm×6 cm; Amersham Biosciences), washed with 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl and then eluted with 25 mM Tris/HCl (pH 7.5) and 0.7 M NaCl. The eluate was further fractionated by gel filtration on Sepharose CL-4B (1 cm×110 cm; Amersham Biosciences) equilibrated with 50 mM Tris/HCl (pH 7.5), 5 mM EDTA, 0.1% Chaps and 4 M guanidinium chloride, and then eluted with the same solution.
Syndecan-3-binding proteins were detected by the ligand overlay assay as follows. Proteins contained in each fraction were subjected to SDS/PAGE under non-reducing conditions and then transferred to a membrane. After blocking with 25 mM Tris/HCl (pH 7.5), 0.15 M NaCl, 5 mM CaCl2, 5 mM MgCl2 and 5% (w/v) BSA, the membrane was incubated overnight at 4 °C with biotinylated soluble syndecan-3 (0.8 μg of protein/ml) in the blocking solution. After incubation with HRP (horse-radish peroxidase)-conjugated streptavidin (Zymed, South San Francisco, CA, U.S.A.), syndecan-3-binding protein was detected with an enhanced chemiluminescence reagent (ECL®; Amersham Biosciences).
Purification of syndecan-3 and glypican-1
Soluble glypican-1 from the culture medium of PC12 cells was purified essentially by the method of Williamson et al. [29].
Soluble syndecan-3 was purified from neonatal mouse brains as described previously [28].
Neurocan–Sepharose affinity chromatography
Neurocan (450 μg of protein) purified from neonatal mouse brains was coupled with CNBr-activated Sepharose CL-4B (dry weight, 0.28 g; Amersham Biosciences) according to the manufacturer's instructions. An extract of neonatal mouse brains was fractionated first by ammonium sulphate precipitation and then on DEAE-Sephacel and heparin–Sepharose as described above. The fractions that did not bind to heparin–Sepharose were dialysed against 25 mM Tris/HCl (pH 7.5), 0.15 M NaCl, 2 mM CaCl2 and 2 mM MgCl2 and applied to a column of neurocan–Sepharose (0.7 cm×2.6 cm). After extensive washing with the above buffer, the bound proteins were eluted with 25 mM Tris/HCl (pH 7.5), 0.15 M NaCl and 10 mM EDTA and then with 25 mM Tris/HCl (pH 7.5), 1.0 M NaCl and 10 mM EDTA.
Binding of labelled HSPGs to neurocan
Radioiodination of syndecan-3 and glypican-1 was performed using IODO-GEN (Pierce Biotechnology). Neurocan–Sepharose (equivalent to 0.18 μg of protein of neurocan) was incubated overnight at 4 °C with increasing concentrations (0–8 nM) of 125I-syndecan-3 (2.5×106 c.p.m./μg) or 125I-glypican-1 (5×106 c.p.m./μg) in 50 μl of 25 mM Tris/HCl (pH 7.5), 0.15 M NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.5% BSA and 0.05% Tween 20. After washing the neurocan–Sepharose three times with the above buffer, the bound radioactivity was measured with an ARC-1000M gamma counter (Aloka Co., Tokyo, Japan). Non-specific binding was examined by incubation of 125I-syndecan-3 or 125I-glypican-1 with neurocan–Sepharose in the presence of a 200-fold excess of unlabelled syndecan-3 or glypican-1. 125I-syndecan-3 or 125I-glypican-1 (10000 c.p.m.) was also treated with heparitinases I and II (5 m-units/ml) as described previously [28]. Neurocan–Sepharose (equivalent to 0.9 μg of protein of neurocan) was treated with protease-free chondroitinase ABC (20 m-units/ml) as described previously [28]. These materials were used for the binding experiments; for a control experiment, the radiolabelled proteoglycans were incubated with Sepharose CL-4B.
Preparation of recombinant N- and C-terminal fragments and syndecan-3 and glypican-1 cDNA transfectants
Based on the sequences of mouse neurocan (GenBank® accession no. X84727), syndecan-3 (GenBank® accession no. XM_124395) and glypican-1 (GenBank® accession no. AF185613), four pairs of primers were synthesized. Polyadenylated RNA was prepared from neonatal mouse brains using a QuickPrep® mRNA purification kit (Amersham Biosciences). First-strand cDNA, synthesized using a random primer, was amplified with four pairs of specific primers: 5′-128GTGGCTGCTTCTCCTAGTCG-3′ and 5′-2058CAAGTGTAGAGCGTGGCAGA-3′ and 5′-1942CAGAGGCCCTAAGTGCTGTC-3′ and 5′-3943GAAACGCTCTGGAGAAGGTG-3′ to produce recombinant N- and C-terminal fragments of mouse neurocan respectively and 5′-14ACAAAGGCCGCCATGAAG-3′ and 5′-1369GGCACTGTGGCTCTGCTAAG-3′ and 5′-156ACCTTGGCTCTGCCCTTC-3′ and 5′-1949AGACAGTCCTTGGGGCTAGG-3′ to obtain full-length cDNAs encoding mouse syndecan-3 and glypican-1 respectively. cDNAs encoding the N- and C-terminal fragments of neurocan were subcloned into the pFLAG-CTC expression vector (Sigma), with which BL21-CodonPlus® competent cells (Stratagene, Heidelberg, Germany) were transformed to generate C- and N-terminally FLAG-tagged recombinant fragments. Full-length cDNA encoding mouse syndecan-3 or glypican-1 was subcloned into the pcDNA 3.1 expression vector (Invitrogen, Carlsbad, CA, U.S.A.), with which N18TG-2 neuroblastoma cells were transformed using NeuroPORTER™ transfection reagent (Gene Therapy System, San Diego, CA, U.S.A.). As a control, cells were transfected with the vehicle vector only. These cells were cultured in Dulbecco's modified Eagle's medium and 10% (v/v) fetal calf serum in the presence of G418 (600 μg/ml). After cloning by limiting dilution, the cloned transfectants were designated as Syn3-N18TG-2 (syndecan-3 cDNA transfectants), Gly1-N18TG-2 (glypican-1 cDNA transfectants) and Mock-N18TG-2 (vehicle vector transfectants).
Binding of whole neurocan and its fragments to heparin
A mixture of whole neurocan and its fragments was prepared from neonatal mouse brains as described above. It was applied to a column of TSKgel Heparin-5PW (0.75 cm×7.5 cm; Tosoh, Tokyo, Japan) equilibrated with 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl and eluted with a linear gradient of NaCl, from 0.15 to 1.0 M.
N- and C-terminal neurocan fragments were prepared as follows and their binding to heparin was also examined. After induction with isopropyl β-D-thiogalactoside, BL21-CodonPlus® competent cells, transformed with pFLAG CTC expression vector, were lysed by sonication in 25 mM Tris/HCl (pH 7.5), 0.15 M NaCl, 7 M urea and 1 mM PMSF and then centrifuged at 165000 g for 30 min. The proteins obtained from the supernatant were mixed with heparin–Sepharose or anti-FLAG M2 monoclonal antibody-conjugated agarose (Sigma) or Sepharose CL-4B and kept overnight at 4 °C. After washing with 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl, the bound proteins were subjected to SDS/PAGE followed by electroblotting; subsequently, FLAG-tagged recombinant neurocan fragments were detected with HRP-conjugated anti-FLAG M2 monoclonal antibodies. Lysates of the transformed cells were applied to a column of TSKgel Heparin-5PW and the FLAG-tagged recombinant neurocan fragment in the eluted fractions was detected by dot-blot analysis with HRP-conjugated anti-FLAG M2 monoclonal antibodies. To confirm the binding of the N-terminal neurocan fragment to heparin, the FLAG-tagged recombinant protein was purified by affinity chromatography. The protein was applied to a column of anti-FLAG M2 monoclonal antibody-conjugated agarose (0.7 cm×2.6 cm). After washing with 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl, the bound protein was eluted with 0.1 M glycine/HCl, pH 3.5. The wells of a Maxisorp 96-well plate (Nalge Nunc, Rochester, NY, U.S.A.) were coated with syndecan-3 or glypican-1 (0.25 μg/50 μl) overnight at 4 °C. After blocking with 30 mM NaHCO3 (pH 8.0), containing 1% BSA, purified FLAG-tagged recombinant protein (5 μg/ml) in PBS containing 1% BSA was added to the wells, followed by incubation overnight at 4 °C. After washing with 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl containing 0.05% Tween 20, a bound N-terminal neurocan fragment was detected with HRP-conjugated anti-FLAG M2 monoclonal antibodies using the ELISA TMB kit (Nacalai tesque, Kyoto, Japan).
Attachment of N18TG-2 cells to recombinant neurocan substrate
Cell attachment to recombinant neurocan-coated wells was examined by means of the centrifugation cell adhesion assay [30,31]. First, each well of a U-shaped 96-well plate (Nalge Nunc) was coated with 50 μl of anti-FLAG M2 monoclonal antibodies (20 μg/ml) overnight at 4 °C. After washing with 30 mM NaHCO3 (pH 8.0) and blocking with 30 mM NaHCO3 (pH 8.0) containing 1% BSA, lysates of transformed Escherichia coli cells expressing the FLAG-tagged recombinant N- or C-terminal neurocan fragment were added to the wells, followed by incubation overnight at 4 °C. To examine the effect of heparin, wells coated with FLAG-tagged recombinant neurocan fragments were further incubated with heparin (5 μg/50 μl) in PBS containing 1% BSA. After washing with serum-free Dulbecco's modified Eagle's medium containing 1% BSA, Syn3-, Gly1- or Mock-N18TG-2 cells (1×104 cells) were added to each well and the plate was immediately centrifuged at 250 g for 2 min. The wells were photographed under a phase-contrast microscope. The diameter of the area to which the cells attached uniformly was measured as an index of the attachment strength by the method of Grumet et al. [31].
Neurite outgrowth assay of cerebellar granule cells
Neurite outgrowth assay was performed by using the wells of a Maxisorp 96-well plate (Nalge Nunc) treated as described for the centrifugation cell adhesion assay in the previous section. Cerebellar granule cells were prepared from neonatal mice aged 6 days by the method of Fushiki et al. [32]. The cells were planted on to the wells at a density of 5×103 cells/well in 100 μl of culture medium. In some experiments, heparitinase I (10 m-units) was added to the culture medium to remove cell-surface heparan sulphate chains. After culturing for 48 h, cells bearing neurites longer than the diameter of a cell body were counted as neurite-bearing cells under a phase-contrast microscope. To confirm the expression of syndecan-3, glypican-1 and cell adhesion molecule L1, the granule cells were immunostained with specific antibodies against each molecule.
Other methods
Amino acid sequencing was performed using an Applied Biosystems sequencer (Procise 492). Biotinylation of proteins was performed using EZ-Link™ Sulfo-NHS-Biotin (Pierce Biotechnology, Rockford, IL, U.S.A.). The protein concentration was determined using a BCA protein assay kit (Bio-Rad, Hercules, CA, U.S.A.) using BSA as the standard. Student's t test was used for statistical analysis of the experiments. P<0.05 was taken as the level of significance.
RESULTS
Identification of new ligand for syndecan-3
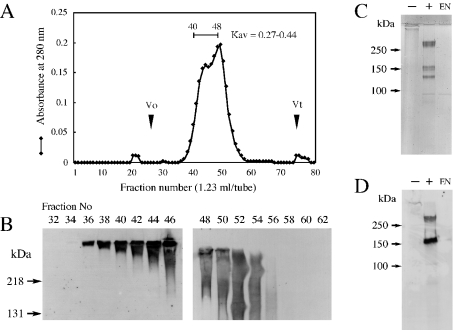
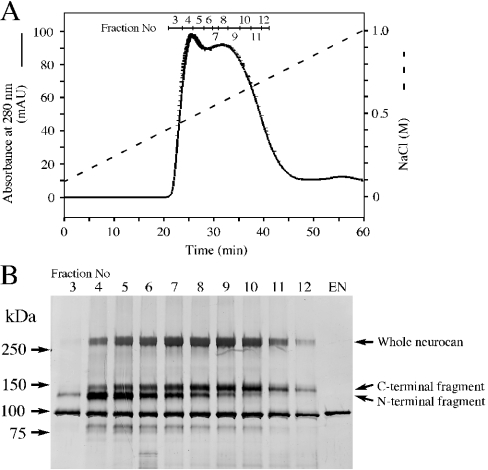
Using the ligand overlay assay, the syndecan-3-binding protein was detected through its purification as described in the Materials and methods section. As shown in Figures 1(A) and 1(B), the syndecan-3-binding protein was recovered mainly in fractions [Kav=(Ve−Vo)/(Vt−Vo)=0.27–0.44, where Ve is the elution volume, Vo the void volume and Vt the maximum elution volume] of gel filtration on Sepharose CL-4B. A control experiment using biotinylated BSA instead of syndecan-3 did not give any detectable band (results not shown). The above fractions were collected and analysed by SDS/PAGE. Treatment of the binding protein with chondroitinase ABC produced three molecular species exhibiting molecular masses of 260, 150 and 130 kDa on SDS/PAGE (Figure 1C). It was revealed that the 260 and 130 kDa components had the same N-terminal amino acid sequence, i.e. DQDTQDTTA, which coincided with that of neurocan, indicating that these core proteins correspond to the whole molecule and the N-terminal fragment of neurocan respectively. The N-terminal amino acid sequence of the 150 kDa core protein was LRAPKLWLLP, which coincided with the N-terminal sequence of the C-terminal fragment of neurocan. Anti-mouse neurocan monoclonal antibody 3A11 reacted with the 260 and 150 kDa core proteins, but not with the 130 kDa core protein (Figure 1D).
Figure 1. Purification and identification of syndecan-3-binding protein.
(A) Syndecan-3-binding protein was fractionated by chromatography on Sepharose CL-4B and the elution profile and elution volume Ve were analysed. The void volume Vo and the maximum elution volume Vt were measured with respect to the elution positions of Blue Dextran and p-nitrophenol respectively. (B) Aliquots of each fraction were subjected to ligand overlay assay using biotinylated soluble syndecan-3. (C, D) Positive fractions [Kav=(Ve−Vo)/(Vt−Vo)=0.27–0.44] were collected, treated with (+) or without (–) chondroitinase ABC and then separated by SDS/PAGE (6% gel), followed by either visualization by CBB staining (C) or immunochemical detection using anti-neurocan monoclonal antibody 3A11 (D). EN, the same amount of chondroitinase ABC used in (C, D) was subjected to SDS/PAGE followed by the same procedure as described above.
Identification of the neurocan-binding proteins
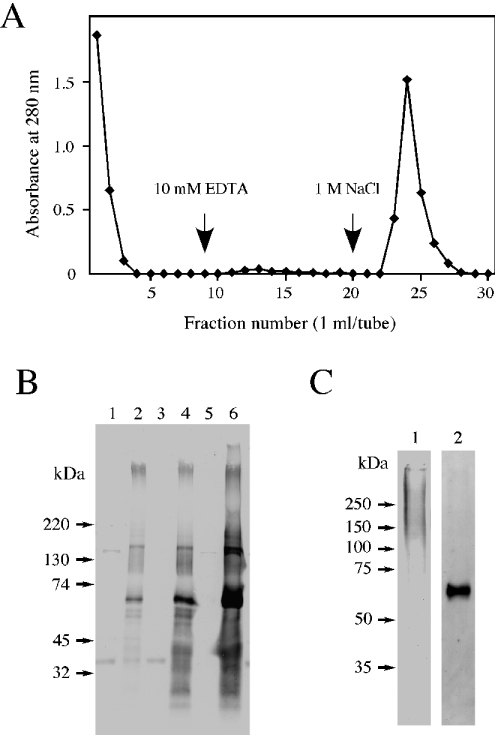
As shown in Figure 1, we found that neurocan could bind to syndecan-3. Next, we tried to confirm this binding ability using purified neurocan and asked whether neurocan is capable of binding to other HSPGs. An extract of neonatal mouse brains was fractionated by ammonium sulphate precipitation and ion-exchange chromatography on a column of DEAE-Sephacel as described in the Materials and methods section. The fractions that eluted with 0.3–1.5 M NaCl from DEAE-Sephacel were collected. Neurocan contained in these fractions was excluded using a heparin column. The pass-through fraction from the heparin column was applied to a column of neurocan–Sepharose. After extensive washing, the bound proteins were eluted with the same solution containing 10 mM EDTA and then 1 M NaCl (Figure 2A). After treatment with or without heparitinase I, eluates were subjected to SDS/PAGE followed by electroblotting. Immunoblot analysis with F69-3G10 monoclonal antibodies revealed several core proteins of HSPGs in the eluate from neurocan–Sepharose (Figure 2B). Among these bands, the core protein having a molecular mass of 62 kDa showed strong reactivity to this antibody. In the eluate with 1 M NaCl, syndecan-3 was detected on immunoblot analysis with anti-syndecan-3 antibodies (Figure 2C, lane 1). In addition, the 62 kDa core protein was identified to be glypican-1 by its immunoreactivity to anti-glypican-1 antibodies (Figure 2C, lane 2). At present, the other HSPGs that bound to neurocan–Sepharose are yet to be identified.
Figure 2. Identification of neurocan-binding proteins.
An extract of neonatal mouse brains was fractionated by ammonium sulphate precipitation and ion-exchange chromatography on a column of DEAE-Sephacel as described in the Materials and methods section. The fractions eluted with 0.3–1.5 M NaCl from DEAE-Sephacel were applied to a column of heparin–Sepharose. (A) The pass-through fraction from the heparin column was applied to a column of neurocan–Sepharose. After washing, the bound components were eluted with 10 mM EDTA and then with 1.0 M NaCl. (B) After treatment with (lanes 2, 4 and 6) or without (lanes 1, 3 and 5) heparitinase I, aliquots of each fraction were subjected to SDS/PAGE (2–15% gel), followed by immunoblot analysis with F69-3G10 monoclonal antibodies. Lanes 1 and 2, pass-through fraction from the heparin column; lanes 3 and 4, 10 mM EDTA eluate; lanes 5 and 6, 1 M NaCl eluate. (C) The eluate obtained with 1.0 M NaCl, before and after treatment with heparitinase I, was subjected to SDS/PAGE (7% gel) followed by immunoblot analysis with anti-syndecan-3 antibodies (lane 1) and anti-glypican-1 (core protein) antibodies (lane 2) respectively.
Binding of syndecan-3 and glypican-1 to neurocan
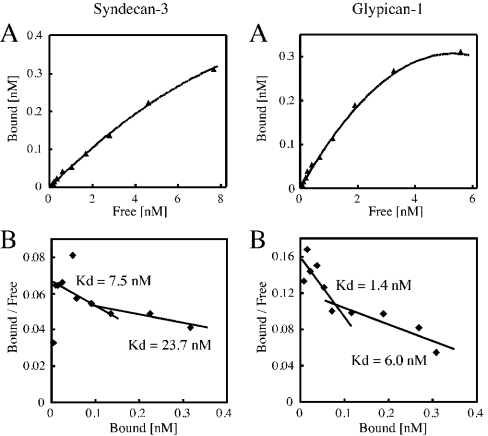
To examine binding properties, neurocan–Sepharose was incubated with increasing amounts of 125I-syndecan-3 or 125I-glypican-1. The binding of these HSPGs to neurocan was saturated (Figure 3A), and Scatchard plot analysis revealed the presence of two binding sites (Figure 3B). The calculated Kd values were 7.5 and 23.7 nM for syndecan-3 and 1.4 and 6.0 nM for glypican-1.
Figure 3. Binding of neurocan to syndecan-3 or glypican-1.
(A) Neurocan–Sepharose was incubated with increasing amounts of 125I-syndecan-3 (left panel) or 125I-glypican-1 (right panel) as described in the Materials and methods section. Specific binding was calculated by subtracting the non-specific binding from the total binding, where total binding represents the binding with increasing concentrations of 125I-syndecan-3 or 125I-glypican-1 and non-specific binding represents the binding in the presence of a 200-fold excess of unlabelled syndecan-3 or glypican-1. The bound radioactivity was measured with a gamma counter. Results are expressed as the means for duplicate analyses. (B) Results of Scatchard plot analysis.
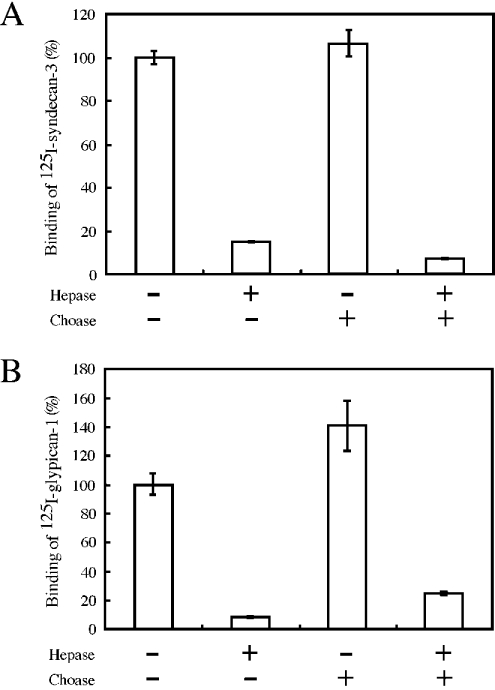
We examined whether the heparan sulphate chains of syndecan-3 or glypican-1 are relevant to the binding of neurocan. 125I-syndecan-3 or 125I-glypican-1 treated with or without heparitinases I and II was incubated with neurocan–Sepharose or Sepharose CL-4B as a control. Removal of the heparan sulphate chains of syndecan-3 and glypican-1 decreased the binding to neurocan by approx. 85 and 92% respectively, indicating that their heparan sulphate chains were actually essential for the binding (Figure 4). A similar experiment was performed to examine the effect of the chondroitin sulphate chains of neurocan on the binding to syndecan-3 or glypican-1. As shown in Figure 4(A), removal of the chondroitin sulphate chains of neurocan had no effect on the binding to syndecan-3, and the binding of glypican-1 to neurocan increased 1.4-fold after treatment with chondroitinase ABC (Figure 4B), indicating that the neurocan core protein is responsible for the binding to the heparan sulphate chains of syndecan-3 or glypican-1. Binding of labelled HSPGs to Sepharose CL-4B was less than 1% of the maximum binding to neurocan–Sepharose (results not shown).
Figure 4. Involvement of glycosaminoglycans in the interaction between neurocan and syndecan-3 or glypican-1.
(A) 125I-syndecan-3 (10000 c.p.m.) was treated with (+) or without (–) heparitinases I and II (Hepase) and then mixed with neurocan–Sepharose treated with (+) or without (–) chondroitinase ABC (Choase). As a control, Sepharose CL-4B was used instead of neurocan–Sepharose CL-4B. After washing, the bound radioactivity was measured with a gamma counter. (B) Binding of neurocan to glypican-1 was examined similarly using 125I-glypican-1 (10000 c.p.m.). Results are expressed as the averages for duplicate determinations. Error bars represent the S.E.M.
Binding of neurocan fragments to heparin
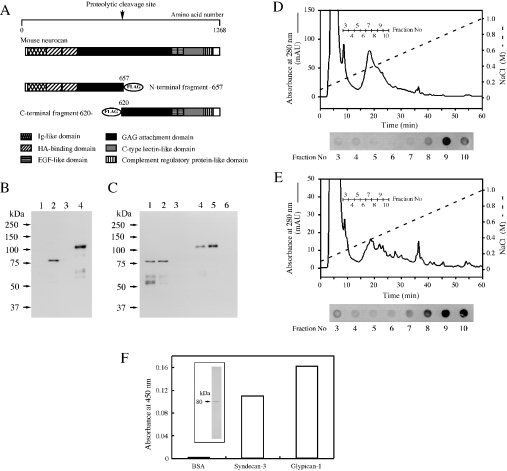
As shown in Figure 1(C), the syndecan-3-binding protein is a mixture of the whole neurocan and its N- and C-terminal fragments, which is consistent with the report that neurocan is proteolytically processed with aging and cleaved its N- and C-terminal fragments [25,33]. To analyse the binding of whole neurocan and its fragments to heparin, this mixture was fractionated on a column of TSKgel Heparin-5PW (Figure 5A). After digestion with chondroitinase ABC, the eluted proteins were separated by SDS/PAGE and detected by silver staining (Figure 5B). Analysis of the N-terminal amino acid sequence revealed that the 260 kDa band was the core protein of the whole neurocan, and the 150 and 130 kDa bands corresponded to its C- and N-terminal fragments respectively. The N-terminal fragment was eluted first with 0.41 M NaCl from a column of TSKgel Heparin-5PW, followed by elution of the whole neurocan and C-terminal fragment with 0.45 M NaCl. These results suggest that both the N- and C-terminal fragments of neurocan can bind to heparin and a binding site with higher affinity for heparin was located in its C-terminus, which is consistent with the finding that neurocan has two binding sites for both syndecan-3 and glypican-1, as described above. However, this result contradicts a recent report that the C-terminal fragment, but not the N-terminal one, could bind to heparin [6]. To confirm the binding of both the N- and C-terminal fragments of neurocan to heparin, we attempted to produce FLAG-tagged recombinant N- and C-terminal neurocan fragments, as shown in Figure 6(A). FLAG-tagged recombinant N- and C-terminal fragments were detected as major bands corresponding to molecular masses of 80 and 110 kDa respectively (Figure 6B, lanes 2 and 4). The molecular masses of these recombinant fragments were higher than those expected on the basis of their amino acid contents and this is probably due to the characteristic rod-like structure of neurocan, as reported by Retzler et al. [12] and Li et al. [34]. Cell lysates containing each fragment were mixed with anti-FLAG monoclonal antibody-conjugated agarose, heparin–Sepharose or Sepharose CL-4B. After washing with 25 mM Tris/HCl (pH 7.5) and 0.15 M NaCl, the bound proteins were subjected to SDS/PAGE followed by immunoblot analysis. The FLAG-tagged recombinant N- and C-terminal neurocan fragments were co-precipitated with anti-FLAG monoclonal antibody-conjugated agarose and heparin–Sepharose, but not with Sepharose CL-4B (Figure 6C). When these cell lysates were fractionated on TSKgel Heparin-5PW, both FLAG-tagged recombinant neurocan fragments bound to the heparin column (Figures 6D and 6E). To confirm further the binding of the N-terminal fragment to syndecan-3 or glypican-1, the FLAG-tagged recombinant N-terminal neurocan fragment was isolated as described in the Materials and methods section. As shown in Figure 6(F), the purified N-terminal fragment could bind to these HSPGs.
Figure 5. Elution profile of neurocan and its fragments on TSKgel Heparin-5PW.
An extract of neonatal mouse brains was fractionated by ammonium sulphate precipitation and DEAE-Sepharose and heparin-affinity chromatographies as described in the Materials and methods section. (A) The fractions eluted from heparin–Sepharose with 0.1–0.7 M NaCl were refractionated with a linear gradient of NaCl from 0.1 to 1.0 M on TSKgel Heparin-5PW. (B) After digestion with chondroitinase ABC, aliquots of each fraction were subjected to SDS/PAGE (2–15% gel), with detection by silver staining. EN, chondroitinase ABC was used for the same procedure.
Figure 6. Binding of recombinant N- and C-terminal neurocan fragments to heparin or HSPGs.
(A) FLAG-tagged recombinant neurocan fragments, as represented schematically, were prepared as described in the Materials and methods section. EGF, epidermal growth factor; GAG, glycosaminoglycan; HA, hyaluronic acid; Ig, immunoglobulin. (B) BL21 competent cells were transformed using plasmids constructed with cDNA encoding the N-terminal (lanes 1 and 2) or C-terminal fragment (lanes 3 and 4) of mouse neurocan. Before (lanes 1 and 3) and after (lanes 2 and 4) induction with isopropyl β-D-thiogalactoside, lysates of each type of cell were subjected to SDS/PAGE (7% gel) followed by immunoblot analysis. FLAG-tagged recombinant neurocan fragments were detected with HRP-conjugated anti-FLAG monoclonal antibodies. (C) Cell lysates containing either the FLAG-tagged recombinant N-terminal (lanes 1–3) or C-terminal (lanes 4–6) recombinant neurocan fragment were mixed with anti-FLAG monoclonal antibody-conjugated agarose (lanes 1 and 4), heparin–Sepharose (lanes 2 and 5) or Sepharose CL-4B (lanes 3 and 6). The bound proteins were subjected to SDS/PAGE and immunoblot analysis as described above. Additional bands corresponding to lower molecular masses, which were detected in (B) (lane 4) and (C) (lanes 1 and 2), were probably produced by proteolysis. (D, E) Cell lysates containing the FLAG-tagged recombinant N- (D) or C-terminal (E) neurocan fragment were applied to a column of TSKgel Heparin-5PW. The bound proteins were eluted with a linear gradient of NaCl from 0.1 to 1.0 M. FLAG-tagged recombinant neurocan fragments were detected by dot-blot analysis with HRP-conjugated anti-FLAG monoclonal antibodies. (F) Purified N-terminal neurocan fragment was subjected to SDS/PAGE and stained with Coomassie Brilliant Blue (inset). This fragment was added to the wells coated with syndecan-3 or glypican-1. Bound fragment was detected as described in the Materials and methods section.
Binding of neurocan to HSPGs expressed on N18TG-2 cells
Full-length cDNA encoding mouse syndecan-3 or glypican-1 was transfected into N18TG-2 mouse neuroblastoma cells as described in the Materials and methods section. We confirmed a high level of expression of syndecan-3 or glypican-1 at mRNA and protein levels in Syn3- and Gly1-N18TG-2 cells (results not shown).
We investigated the molecular interaction between cell-surface HSPGs and neurocan using the centrifugation assay described by Friedlander et al. [30]. To determine the degree of attachment, we measured the diameter of the cell-attachment area. As shown in Table 1, the diameters of the attachment area for Syn3-N18TG-2 cells on the wells coated with the recombinant N- and C-terminal neurocan fragments were 4.71±0.36 and 5.14±0.11 mm respectively. These areas were significantly greater than those of Mock-N18TG-2 cells. Similar results were obtained with the centrifugation assay using Gly1-N18TG-2 cells. The diameters were 4.94±0.16 and 5.49±0.15 mm in the wells coated with the recombinant N- and C-terminal neurocan fragments respectively. These results suggest that cell-surface HSPGs play a significant role in cell adhesion to the neurocan substrate. In the control wells coated with anti-FLAG monoclonal antibodies and blocked with BSA, the diameters of the cell-attachment areas were significantly smaller compared with those of recombinant neurocan-coated wells. The attachment of Mock-N18TG-2 cells to recombinant neurocan-coated wells was slightly promoted, probably due to other cell adhesion molecules expressed in Mock-N18TG-2 cells. The cell-attachment area in the wells incubated with heparin after coating with recombinant neurocan fragments was significantly decreased. The addition of heparin to the neurocan-uncoated wells had no effect on the binding of these transfectants.
Table 1. Measurement of the cell-attachment area by centrifugation assay.
The centrifugation assay was performed as described in the Materials and methods section. The diameter (in mm) of the area to which the cells attached was measured as an index of the attachment strength using an internal calibrate graticule. Coating with various substrates and blocking with 1% BSA were performed as described in the Materials and methods section. Results are expressed as the means±S.E.M. for triplicate determinations. *P<0.05, **P<0.001 compared with Mock-N18TG-2 on the recombinant N- or C-terminal neurocan fragment-coated wells. †P<0.01, ††P<0.001 compared with each transfectant on the recombinant N- or C-terminal neurocan fragment-coated wells. FLAG Ab, anti-FLAG monoclonal antibody; N-Neuro, FLAG-tagged recombinant N-terminal neurocan fragment; C-Neuro, FLAG-tagged recombinant C-terminal neurocan fragment; Hep, heparin.
| Diameter (mm) | |||
|---|---|---|---|
| Substrate | Mock-N18TG-2 cells | Syn3-N18TG-2 cells | Glyp1-N18TG-2 cells |
| FLAG Ab+BSA | 2.77±0.11 | 2.91±0.10 | 2.72±0.12 |
| FLAG Ab+BSA+Hep | 2.77±0.10 | 2.96±0.10 | 2.71±0.07 |
| FLAG Ab+BSA+N-Neuro | 3.51±0.10 | 4.71±0.36* | 4.94±0.16** |
| FLAG Ab+BSA+C-Neuro | 3.96±0.08 | 5.14±0.11** | 5.49±0.15** |
| FLAG Ab+BSA+N-Neuro+Hep | 2.92±0.15 | 3.29±0.16† | 3.33±0.11†† |
| FLAG Ab+BSA+C-Neuro+Hep | 2.86±0.13 | 3.10±0.17†† | 2.92±0.14†† |
Effect of HSPG–neurocan interaction on neurite outgrowth from cerebellar granule cells
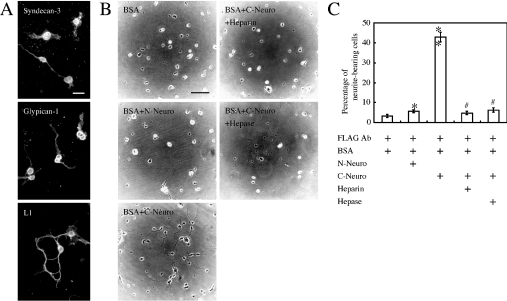
Biological effect of neurocan on HSPG-expressing cells was investigated by using cerebellar granule cells prepared from the neonatal mouse cerebellum, since it has been reported that neurocan, syndecan-3 and glypican-1 are co-localized in the molecular layer of neonatal mouse cerebellum [17,21,25]. In the developing cerebellum, the cerebellar granule cells extend their neurites horizontally forming the parallel fibres in the molecular layer [35] where neurocan is highly expressed [25]. Syndecan-3 and glypican-1 were also detected on the growing parallel fibres in the neonatal rodent cerebellar cortex [17,21]. Approx. 80% of the cells prepared from neonatal mouse (aged 6 days) cerebellum expressed syndecan-3 and glypican-1 in addition to cell adhesion molecule L1, a marker for cerebellar granule cells (Figure 7A). The granular cells prominently extended their neurites on the wells coated with FLAG-tagged recombinant C-terminal neurocan fragment via anti-FLAG antibody, but not on the wells coated with only anti-FLAG antibodies (Figure 7B). To examine the involvement of cell-surface HSPGs, the recombinant C-terminal neurocan fragment-coated wells were further treated with heparin before cell planting. Neurite outgrowth was significantly decreased by pretreatment with heparin (Figure 7B). In addition, neurite outgrowth was also suppressed when heparitinase I was added to culture medium (Figure 7B). These treatments with heparin or heparitinase I did not affect the cell viability (results not shown). To determine the level of neurite outgrowth-promoting activity, the neurite-bearing cells cultured under various conditions as described above were counted (Figure 7C). The C-terminal neurocan fragment increased the percentage of neurite-bearing cells by 42.8%. In contrast, the N-terminal neurocan fragment showed little promotion of neurite outgrowth (Figures 7B and 7C).
Figure 7. Neurite outgrowth from mouse cerebellar granule cells on recombinant neurocan substrate.
(A) Cerebellar granule cells from neonatal mice aged 6 days were cultured on poly-D-lysine-coated glass slides for 48 h. The cells were immunostained with anti-syndecan-3 antibodies or anti-glypican-1 antibodies. The cells were also immunostained with antibodies against cell adhesion molecule L1, as a marker for cerebellar granule cells. As a control, the cells treated with normal goat IgG did not exhibit any positive reactivity (results not shown). Scale bar, 20 μm. (B) For the neurite outgrowth assay, the wells were coated with anti-FLAG antibodies only or coated with FLAG-tagged recombinant N- or C-terminal neurocan fragment via anti-FLAG antibodies. To examine further the effect of the heparan sulphate chains, the granule cells were cultured on the C-terminal neurocan fragment-substrate treated with heparin (10 μg/ml) or on the same substrate in the presence of heparitinase I (0.1 unit/ml). The granule cells from neonatal mice aged 6 days were cultured on these substrates for 48 h. These experiments were repeated three times and images from a typical experiment are shown. Scale bar, 100 μm. (C) The percentage of neurite-bearing cells is presented as the means for triplicate determinations. Error bars represent the S.E.M. *P<0.05, **P<0.001, compared with the neurocan-uncoated wells. #P<0.001, compared with recombinant C-terminal neurocan fragment-coated wells. FLAG Ab, anti-FLAG monoclonal antibody; N-Neuro, FLAG-tagged recombinant N-terminal neurocan fragment; C-Neuro, FLAG-tagged recombinant C-terminal neurocan fragment; Hepase, heparitinase I.
DISCUSSION
The mechanisms that control the establishment of neuronal connection are very crucial for development of the nervous system. It is generally agreed that syndecan-3, similar to three other syndecans, plays an important role in tissue morphogenesis and differentiation by virtue of its ability to bind to a number of extracellular matrix components and growth factors [1,23]. In the present study, we demonstrated that both soluble and cell-surface syndecan-3 and glypican-1 could bind to neurocan. Scatchard plot analysis showed that these HSPGs bound to neurocan with significantly high affinity (Kd=7.5 and 23.7 nM for syndecan-3 and Kd=1.4 and 6.0 nM for glypican-1). Digestion of these HSPGs with heparitinases I and II decreased their binding abilities to neurocan by 85–92%. On the other hand, the binding of these HSPGs did not decrease by the treatment of neurocan with chondroitinase ABC. These results suggest that these HSPGs bind to the neurocan core protein through their heparan sulphate chains, which is consistent with the recent report that neurocan binds to heparin [6]. It has been reported that neurocan binds to some heparin-binding factors such as pleiotrophin/HB-GAM [15], amphoterin [15] and fibroblast growth factor-2 [16]. The binding of neurocan to these molecules is mainly mediated through its chondroitin sulphate chains [15,16]. Thus there is a possibility that the binding of neurocan to HSPGs is mediated by these heparin-binding factors, which may bridge between heparan sulphate and chondroitin sulphate chains. However, for the binding of neurocan to syndecan-3 and glypican-1, this is not the case, because treatment of neurocan with chondroitinase ABC did not cause any decrease in the binding ability.
It is known that in adult rodent brain, most neurocan is cleaved proteolytically into its C- and N-terminal fragments [25,33]. Recently, Feng et al. [6] reported that, when an extract of adult mouse brains was applied to a heparin column, the C-terminal fragment, but not the N-terminal one, could be detected in the eluate on immunoblot analysis with anti-neurocan antiserum. However, in the present study, we demonstrated that the N-terminal fragment of neurocan prepared from neonatal mouse brains, in addition to the C-terminal fragment and whole molecule, could bind to a heparin column. To confirm this, we prepared recombinant N- and C-terminal fragments of neurocan. Using these recombinant fragments, we clearly demonstrated that both the N- and C-terminal neurocan fragments could bind to heparin, as shown in Figure 6. In addition, a higher concentration of NaCl was necessary to elute the C-terminal fragment when compared with the N-terminal fragment (Figure 5B), which is consistent with our finding that neurocan has two binding sites with different affinities for syndecan-3 and glypican-1. N-CAM (neural cell-adhesion molecule) could also bind to the N-terminal, central and C-terminal parts of neurocan [36]. Thus, by possessing multi-binding sites for ligands, neurocan may form stable molecular complexes with various extracellular and cell-surface molecules.
To examine whether neurocan could also bind to these HSPGs on the cell surface, we performed the centrifugation cell adhesion assay using N18TG-2 mouse neuroblastoma cells transfected with syndecan-3 or glypican-1 cDNA. When the wells were coated with the FLAG-tagged recombinant N- or C-terminal neurocan fragment via anti-FLAG monoclonal antibodies, the diameters of the attachment areas of Syn3- and Gly1-N18TG-2 cells were greater than those of Mock-N18TG-2 cells. These results suggest that neurocan could bind not only to soluble syndecan-3 and glypican-1 in the extracellular matrix, but also to membrane-bound ones on the neuronal cell surface.
Some experiments in vitro have shown that neurocan inhibits cell adhesion and neurite outgrowth and this property depends on its chondroitin sulphate chains and/or core proteins [34,37]; hence, we performed an experiment to see if the interaction between neurocan and cell-surface HSPGs has a biological effect. We examined neurite outgrowth from mouse cerebellar granule cells cultured on neurocan substrate, since immunohistochemical studies have shown that syndecan-3, glypican-1 and neurocan are co-localized in the molecular layer of neonatal rodent cerebellum [17,21,25]. When the granule cells were cultured on the wells coated with the recombinant C-terminal neurocan fragment, neurite outgrowth was significantly promoted. This neurite outgrowth-promoting activity was suppressed by pretreatment of neurocan substrate with heparin. Addition of heparitinase I into culture medium also inhibited neurite outgrowth, indicating that cell-surface HSPGs mediate this activity. Thus these results suggest that the interaction of cell-surface syndecan-3 and glypican-1 with neurocan substrate could promote neurite outgrowth. These results are consistent with the fact that the granule cells extend their neurites into the neurocan-rich molecular layer in the developing cerebellum [25,35]. In contrast with the C-terminal neurocan fragment, we could find little effect of the N-terminal fragment on neurite outgrowth, even though the cell adhesion assay showed that the N-terminal fragment could bind to cell-surface HSPGs. One possibility is that neurocan-induced neurite outgrowth needs the co-operative interactions of cell-surface HSPGs and other unidentified molecules, which could bind to the C-terminal domain of neurocan but not to the N-terminal one. As reported previously, the cerebellar granule cells express cell adhesion molecule L1, which can bind to neurocan [8] and the L1 molecule itself [38], and the L1–L1 homophilic binding promotes cell adhesion and neurite outgrowth [38]. Thus neurocan may prevent the L1–L1 homophilic binding, resulting in the inhibition of cell adhesion and neurite outgrowth [8]. However, how and what kind of effect is produced by neurocan–L1 interaction has not been demonstrated. It also remains unclear whether this interaction is co-operatively involved in the neurite outgrowth through the neurocan–cell-surface HSPG interaction. Further studies will be necessary to elucidate in detail the mechanism behind neurocan-induced neurite outgrowth.
It should also be noted that considerable amounts of these HSPGs are shed from the cell surface in the developing brain [7,18,19,28]. Soluble HSPGs shed from the cell surface may inhibit the cell-surface HSPG–neurocan interactions competitively and may regulate neurocan-induced neurite outgrowth. This possibility is currently under investigation.
Acknowledgments
This work was supported by the Foundation for Bio-venture Research Center (Ministry of Education, Culture, Sports, Science and Technology, Japan).
References
- 1.Carey D. J. N-syndecan: structure and function of a transmembrane heparan sulfate proteoglycan. Perspect. Dev. Neurobiol. 1996;3:331–346. [PubMed] [Google Scholar]
- 2.Chernousov M. A., Stahl R. C., Carey D. J. Schwann cells secrete a novel collagen-like adhesive protein that binds N-syndecan. J. Biol. Chem. 1996;271:13844–13853. doi: 10.1074/jbc.271.23.13844. [DOI] [PubMed] [Google Scholar]
- 3.Kinnunen T., Kaksonen M., Saarinen J., Kalkkinen N., Peng H. B., Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- 4.Lauri S. E., Kaukinen S., Kinnunen T., Ylinen A., Imai S., Kaila K., Taira T., Rauvala H. Regulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. J. Neurosci. 1999;19:1226–1235. doi: 10.1523/JNEUROSCI.19-04-01226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdman R., Stahl R. C., Rothblum K., Chernousov M. A., Carey D. J. Schwann cell adhesion to a novel heparan sulfate binding site in the N-terminal domain of alpha 4 type V collagen is mediated by syndecan-3. J. Biol. Chem. 2002;277:7619–7625. doi: 10.1074/jbc.M111311200. [DOI] [PubMed] [Google Scholar]
- 6.Feng K., Arnold-Ammer I., Rauch U. Neurocan is a heparin binding proteoglycan. Biochem. Biophys. Res. Commun. 2000;272:449–455. doi: 10.1006/bbrc.2000.2823. [DOI] [PubMed] [Google Scholar]
- 7.Lander A. D., Stipp C. S., Ivins J. K. The glypican family of heparan sulfate proteoglycans: major cell-surface proteoglycans of the developing nervous system. Perspect. Dev. Neurobiol. 1996;3:347–358. [PubMed] [Google Scholar]
- 8.Friedlander D. R., Milev P., Karthikeyan L., Margolis R. K., Margolis R. U., Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J. Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balsamo J., Ernst H., Zanin M. K., Hoffman S., Lilien J. The interaction of the retina cell surface N-acetylgalactosaminylphosphotransferase with an endogenous proteoglycan ligand results in inhibition of cadherin-mediated adhesion. J. Cell Biol. 1995;129:1391–1401. doi: 10.1083/jcb.129.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milev P., Maurel P., Haring M., Margolis R. K., Margolis R. U. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and N-CAM. J. Biol. Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- 11.Grumet M., Milev P., Sakurai T., Karthikeyan L., Bourdon M., Margolis R. K., Margolis R. U. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J. Biol. Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- 12.Retzler C., Wiedemann H., Kulbe G., Rauch U. Structural and electron microscopic analysis of neurocan and recombinant neurocan fragments. J. Biol. Chem. 1996;271:17107–17113. doi: 10.1074/jbc.271.29.17107. [DOI] [PubMed] [Google Scholar]
- 13.Aspberg A., Miura R., Bourdoulous S., Shimonaka M., Heinegard D., Schachner M., Ruoslahti E., Yamaguchi Y. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein–protein interactions independent of carbohydrate moiety. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10116–10121. doi: 10.1073/pnas.94.19.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauch U., Clement A., Retzler C., Frohlich L., Fassler R., Gohring W., Faissner A. Mapping of a defined neurocan binding site to distinct domains of tenascin-C. J. Biol. Chem. 1997;272:26905–26912. doi: 10.1074/jbc.272.43.26905. [DOI] [PubMed] [Google Scholar]
- 15.Milev P., Chiba A., Haring M., Rauvala H., Schachner M., Ranscht B., Margolis R. K., Margolis R. U. High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-zeta/beta with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J. Biol. Chem. 1998;273:6998–7005. doi: 10.1074/jbc.273.12.6998. [DOI] [PubMed] [Google Scholar]
- 16.Milev P., Monnerie H., Popp S., Margolis R. K., Margolis R. U. The core protein of the chondroitin sulfate proteoglycan phosphacan is a high-affinity ligand of fibroblast growth factor-2 and potentiates its mitogenic activity. J. Biol. Chem. 1998;273:21439–21442. doi: 10.1074/jbc.273.34.21439. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe E., Matsui F., Keino H., Ono K., Kushima Y., Noda M., Oohira A. A membrane-bound heparan sulfate proteoglycan that is transiently expressed on growing axons in the rat brain. J. Neurosci. Res. 1996;44:84–96. doi: 10.1002/(SICI)1097-4547(19960401)44:1<84::AID-JNR11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Carey D. J., Conner K., Asundi V. K., O'Mahony D. J., Stahl R. C., Showalter L., Cizmeci-Smith G., Hartman J., Rothblum L. I. cDNA cloning, genomic organization, and in vivo expression of rat N-syndecan. J. Biol. Chem. 1997;272:2873–2879. doi: 10.1074/jbc.272.5.2873. [DOI] [PubMed] [Google Scholar]
- 19.Hsueh Y. P., Sheng M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J. Neurosci. 1999;19:7415–7425. doi: 10.1523/JNEUROSCI.19-17-07415.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwack E. D., Stipp C. S., Kumbasar A., Lander A. D. Neuronal expression of glypican, a cell-surface glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan, in the adult rat nervous system. J. Neurosci. 1994;14:3713–3724. doi: 10.1523/JNEUROSCI.14-06-03713.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karthikeyan L., Flad M., Engel M., Meyer-Puttlitz B., Margolis R. U., Margolis R. K. Immunocytochemical and in situ hybridization studies of the heparan sulfate proteoglycan, glypican, in nervous tissue. J. Cell Sci. 1994;107:3213–3222. doi: 10.1242/jcs.107.11.3213. [DOI] [PubMed] [Google Scholar]
- 22.Litwack E. D., Ivins J. K., Kumbasar A., Paine-Saunders S., Stipp C. S., Lander A. D. Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev. Dyn. 1998;211:72–87. doi: 10.1002/(SICI)1097-0177(199801)211:1<72::AID-AJA7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Bernfield M., Gotte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 24.Inatani M., Irie F., Plump A. S., Tessier-Lavigne M., Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- 25.Rauch U., Gao P., Janetzko A., Flaccus A., Hilgenberg L., Tekotte H., Margolis R. K., Margolis R. U. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified with monoclonal antibodies. J. Biol. Chem. 1991;266:14785–14801. [PubMed] [Google Scholar]
- 26.Oohira A., Matsui F., Matsuda M., Takida Y., Kuboki Y. Occurrence of three distinct molecular species of chondroitin sulfate proteoglycan in the developing rat brain. J. Biol. Chem. 1988;263:10240–10246. [PubMed] [Google Scholar]
- 27.Akita K., Fushiki S., Fujimoto T., Inoue M., Oguri K., Okayama M., Yamashina I., Nakada H. Developmental expression of a unique carbohydrate antigen, Tn antigen, in mouse central nervous tissues. J. Neurosci. Res. 2001;65:595–603. doi: 10.1002/jnr.1190. [DOI] [PubMed] [Google Scholar]
- 28.Akita K., Fushiki S., Fujimoto T., Munesue S., Inoue M., Oguri K., Okayama M., Yamashina I., Nakada H. Identification of the core protein carrying the Tn antigen in mouse brain: specific expression on syndecan-3. Cell Struct. Funct. 2001;26:271–278. doi: 10.1247/csf.26.271. [DOI] [PubMed] [Google Scholar]
- 29.Williamson T. G., Mok S. S., Henry A., Cappai R., Lander A. D., Nurcombe V., Beyreuther K., Masters C. L., Small D. H. Secreted glypican binds to the amyloid precursor protein of Alzheimer's disease (APP) and inhibits APP-induced neurite outgrowth. J. Biol. Chem. 1996;271:31215–31221. doi: 10.1074/jbc.271.49.31215. [DOI] [PubMed] [Google Scholar]
- 30.Friedlander D. R., Hoffman S., Edelman G. M. Functional mapping of cytotactin: proteolytic fragments active in cell-substrate adhesion. J. Cell Biol. 1988;107:2329–2340. doi: 10.1083/jcb.107.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grumet M., Flaccus A., Margolis R. U. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J. Cell Biol. 1993;120:815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fushiki S., Matsumoto K., Nagata A. Neurite outgrowth of murine cerebellar granule cells can be enhanced by aniracetam with or without alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) Neurosci. Lett. 1995;199:171–174. doi: 10.1016/0304-3940(95)12054-8. [DOI] [PubMed] [Google Scholar]
- 33.Matsui F., Watanabe E., Oohira A. Immunological identification of two proteoglycan fragments derived from neurocan, a brain-specific chondroitin sulfate proteoglycan. Neurochem. Int. 1994;25:425–431. doi: 10.1016/0197-0186(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Leung T. C., Hoffman S., Balsamo J., Lilien J. Coordinate regulation of cadherin and integrin function by the chondroitin sulfate proteoglycan neurocan. J. Cell Biol. 2000;149:1275–1288. doi: 10.1083/jcb.149.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson M. 3rd edn. New York and London: Plenum Press; 1991. Developmental Neurobiology; pp. 430–441. [Google Scholar]
- 36.Retzler C., Gohring W., Rauch U. Analysis of neurocan structures interacting with the neural cell adhesion molecule N-CAM. J. Biol. Chem. 1996;271:27304–27310. doi: 10.1074/jbc.271.44.27304. [DOI] [PubMed] [Google Scholar]
- 37.Talts U., Kuhn U., Roos G., Rauch U. Modulation of extracellular matrix adhesiveness by neurocan and identification of its molecular basis. Exp. Cell. Res. 2000;259:378–388. doi: 10.1006/excr.2000.4987. [DOI] [PubMed] [Google Scholar]
- 38.Lemmon V., Farr K. L., Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]