Abstract
We report the co-ordinated fine-tune of mRNA molecules that takes place in yeast (Saccharomyces cerevisiae) in response to diverse environmental stimuli. We performed a systematic and refined quantification of the absolute expression patterns of 16 genes coding for thioredoxin- and glutathione-dependent redox system components. Quantifications were performed to examine the response to oxidants, to sudden temperature upshifts and in association with metabolic changes accompanying culture growth and to explore the contribution of mRNA decay rates to the differences observed in basal expression levels. Collectively, these quantifications show (i) vast differences in the steady-state amounts of the investigated transcripts, cTPxI being largely overexpressed compared with GPX1 during the exponential phase and GPX2 beyond this growth stage; (ii) drastic changes in the relative abundance of the transcripts in response to oxidants and heat shock; and (iii) a unique temporal expression profile for each transcript as cells proceed from exponential to stationary growth phase, yet with some general trends such as maximal or near-maximal basal amounts of most mRNA species at early growth stages when glucose concentration is high and cells are actively growing. Moreover, the results indicate that (i) the half-lives of the investigated transcripts are longer and distributed within a narrower range than previously reported global mRNA half-lives and (ii) transcriptional initiation may play an important role in modulating the significant alterations that most mRNAs exhibit in their steady-state levels along with culture growth.
Keywords: absolute mRNA level, gene expression, glutathione, growth phase, thioredoxin, yeast
Abbreviations: GPx, glutathione peroxidase; MD, menadione; MPCR, multiplexed PCR; ROS, reactive oxygen species; TPx, thioredoxin peroxidase
INTRODUCTION
The survival of living cells depends on their ability to sense changes in the environment and to respond appropriately to the new situation. The imbalance in the redox status of cells towards an oxidized state is known as oxidative stress. Saccharomyces cerevisiae has evolved an effective and intricate network of enzymic as well as non-enzymic defences to protect itself from the harmful effects of ROS (reactive oxygen species) [1].
Glutathione and thioredoxin are two of the most important and abundant cellular antioxidants, which play key roles in maintaining the redox homoeostasis of the cell (see [2] for a review). In S. cerevisiae, the GSH1 gene product (γ-glutamylcysteine synthetase) catalyses the first and rate-limiting step for GSH biosynthesis. The second enzyme (glutathione synthase), encoded by GSH2, is dispensable for normal growth, confirming an important role for the γ-glutamylcysteine dipeptide in replacing GSH in essential functions [3]. Glutathione reductase (the product of GLR1) catalyses an NADPH-dependent reaction for the regeneration of GSH. Two thioredoxins (encoded by TRX1 and TRX2), one thioredoxin reductase (TRR1) and NADPH as reductant, operate in yeast cytoplasm as a general disulphide reductase pathway [2]. Additional mitochondrial species (TRX3 and TRR2) have also been identified, which offer protection against oxidative stress generated during mitochondrial metabolism [4].
Thioredoxin and GSH are also required for the detoxification of ROS by providing the reducing equivalents for TPxs [thioredoxin (or thiol) peroxidases] and GPxs (glutathione peroxidases). Quite a large number of TPxs have been found in yeast, including cytoplasmic (coded for by the cTPxI, cTPxII and cTPxIII genes), mitochondrial (mTPx) and nuclear (nTPx) species [5]. The mitochondrial protein appears to use the mitochondrial thioredoxin pathway as electron donor [6]. So far, three gene products (GPX1, GPX2 and GPX3) with significant homology to mammalian GPxs have been identified from the S. cerevisiae genome [7]. Although these three enzymes are required for yeast resistance against phospholipid and non-phospholipid hydroperoxides [7,8], the GPX3 product seems to serve the predominant role of hydroperoxide receptor and redox transducer in gene activation mediated by the transcription factor Yap1p [9]. Because of this regulatory function in Yap1p activation, Gpx3p has been renamed recently as ‘oxidant receptor peroxidase 1 (Orp1p)’ [9].
Control of mRNA levels is crucial in regulating protein production in eukaryotes. Current knowledge on transcript amounts of the 16 yeast genes mentioned above has emerged from experiments using methods that generate relative data. Given that modulation of transcription can be much better understood in absolute quantitative terms, we present here, for the first time, valuable new information on the basis of actual mRNA molecule numbers. To this end, we used a quantitatively rigorous approach based on reverse transcription followed by a combination of multiplexed PCR (MPCR) and real-time PCR amplifications [10]. We performed a systematic and comprehensive investigation of absolute expression patterns in response to oxidizing agents, to sudden temperature upshifts and in association with the metabolic changes accompanying culture growth. Since the stability of individual mRNAs contributes significantly to the differential control of gene expression, decay rates of the investigated transcripts were also determined. Aside from its inherent value, the information given here provides a more complete picture of how a co-ordinated fine-tune of mRNA levels might be important for the cell to respond effectively to diverse environmental stimuli.
EXPERIMENTAL
Yeast strains and growth conditions
S. cerevisiae Y00000 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) was used as the wild-type. Strain Y262 (MATα ura3-52 his4-539 rpb1-1), with a temperature-sensitive mutation in the gene encoding the largest subunit of RNA polymerase II [11], was used for mRNA decay quantifications. Cells were grown in YPD medium [1% (w/v) yeast extract/2% (w/v) peptone/2% (w/v) glucose] or SD medium [bacto yeast nitrogen base without amino acids (6.7 g/l) and glucose (20 g/l)] [12]. Minimal medium was supplemented with appropriate amino acids and bases (20 mg/l L-histidine, 100 mg/l L-leucine, 20 mg/l L-methionine and 20 mg/l uracil). Growth was monitored by measuring the absorbance A at 600 nm (A600). Cells were inoculated into YPD medium and incubated for 20 h. These overnight cultures were then diluted into fresh YPD or minimal SD medium (A600 0.05) and incubated until they reached the absorbance specified in individual Figures. Unless otherwise indicated, incubations were performed at 30 °C with constant agitation (125 rev./min).
RNA isolation and reverse transcription
Total RNA was extracted using the hot-phenol method described previously [13]. The RNA sample was then treated for 90 min at 37 °C with RNase-free DNase I (≥5 units/μg of RNA) to remove contaminating DNA. The quality of RNA sample was checked electrophoretically, and quantification was performed spectrophotometrically. Standard RNA and cDNA were synthesized as detailed in [14]. Absence of genomic DNA contamination was checked by PCR amplification of RNA samples without prior cDNA synthesis. At least two independent RNA preparations were isolated for each experimental condition, each RNA sample being retrotranscribed on three separate occasions.
Primers
Sequences of genes for primer design were obtained from Saccharomyces Genome Database (www.standford.edu). Primers with high Tm values (≥82 °C) and optimal 3′-ΔG values (≥−9.5 kcal/mol; 1 kcal≈4184 J) were prepared with Oligo 6.1.1/98 (Molecular Biology Insights, Plymouth, MN, U.S.A.) program, as detailed in [15]. Primers for amplification of the target mRNAs (total of 16) were distributed into two sets, because of the large number of sequences and the restricted parameters used in primer selection [15]. Set A included primer pairs for amplification of TRX1 (YLR043C), TRX2 (YGR209C), TRX3 (YCR083W), TRR1 (YDR353W), TRR2 (YHR106W), cTPxIII (YLR109W), GSH1 (YJL101C) and GLR1 (YPL091W), whereas set B included primer pairs for amplification of cTPxI (YML028W), cTPxII (YDR453C), mTPx (YBL064C), nTPx (YIL010W), GPX1 (YKL026C), GPX2 (YBR244W), GPX3 (YIR037W) and GSH2 (YOL049W). Sets A and B also included primer pairs for the amplification of ACT1 (YFL039C) and PDA1 (YER178W) mRNAs. ACT1, which codes for actin (a cytoskeleton component), and PDA1, which codes for the E1α subunit of the pyruvate dehydrogenase complex, are commonly used as internal standards for quantifying mRNA in S. cerevisiae [16]. Forward primers were labelled with 4,7,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein for MPCR. The primer sequences are available from the authors on request.
Real-time PCR and MPCR
Real-time PCRs were performed in quadruplicate as detailed in [14]. No primer dimers were detected, and the investigated transcripts showed optimal PCR efficiencies. An absolute standard curve was constructed with an external standard in the range of 102–109 RNA molecules. The number of mRNA molecules was calculated from the linear regression of the standard curve, as described previously [14]. MPCR conditions were optimized as detailed in [15] to ensure that the amplifications were in the exponential phase and the efficiencies remained constant in the course of the PCR. Components of the reaction mixture were described previously [15]. For both set A and set B, 25 cycles of amplification were performed. Relative quantification of the MPCR products was performed as described in [15]. MPCR data were from an average of six MPCR amplifications. Absolute measurements (mRNA molecules) were inferred from the relative expression ratios given by MPCR, as we have described recently [10].
Statistical analysis
Results are the means±S.E.M. for n≥6 determinations. Statistical significance was evaluated using ANOVA followed by post-hoc multiple comparison according to the Student–Newman–Keuls method. P≤0.05 was considered significant.
RESULTS
Quantification of the response to oxidative stress
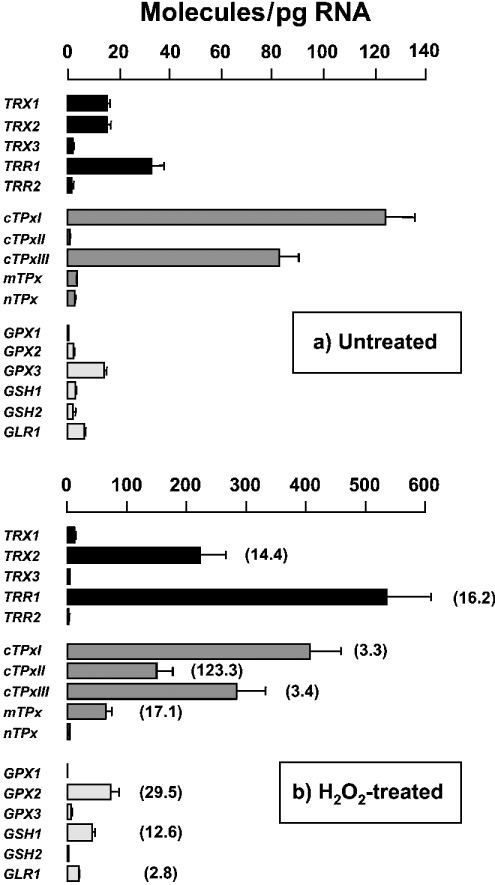
A key requirement of the current analysis is the ability to detect reproducibly, identify and quantify accurately both high- and low-abundance mRNAs. Hence, we first determined the steady-state amounts of the investigated transcripts in wild-type cells cultured in minimal SD medium to reach an A600 1. Under this particular growth condition, vast differences in abundance were detected (Figure 1a). For instance, cTPxI mRNA was largely overexpressed compared with GPX1 mRNA (124 molecules/pg versus 0.21 molecule/pg). The former was as abundant as the house-keeping ACT1 mRNA but more abundant than the PDA1 mRNA (results not shown).
Figure 1. Induction of gene expression by oxidative stress.
Wild-type cells were grown in minimal SD medium to reach an absorbance A600 1 and then treated for 10 min with 100 μM H2O2 (b). Untreated cells served as reference (a). The means±S.E.M. of mRNA molecules/pg of total RNA were plotted for the different genes. Some error bars are not visible because of small S.E.M. values. To facilitate the comparison of relative and absolute quantifications, the data from H2O2-treated cells were divided by those of untreated cells. These fold increments are given in parentheses. Only statistically significant increments are indicated.
Our second goal was to quantify the abundance of the investigated transcripts under an oxidizing environment. To this end, wild-type cells were challenged with H2O2 through both dosage and time-course experiments (results not shown). Quantifications made at 100 μM H2O2 for 10 min are shown in Figure 1(b). The steady-state amounts of nine transcripts were readily up-regulated by H2O2. In contrast, no significant variation of other mRNA levels was detected under any of the H2O2 exposure conditions investigated herein.
The relative increments calculated in Figure 1(b) highlight the importance of the absolute measurements reported in the present study. For instance, although a relative increment of approx. 3-fold in both cTPxI and cTPxIII mRNA levels might look insignificant compared with the 123-fold increase in cTPxII mRNA, the actual scenario is that, after H2O2 exposure, cTPxI and cTPxIII mRNAs are still present in higher amounts compared with cTPxII mRNA (406 and 283 molecules/pg versus 148 molecules/pg). In short, absolute quantifications make us realize that even small increments in highly abundant transcripts may have more influence on the antioxidative capacity of the cell when compared with huge increments of poorly transcribed genes.
To compare the response of yeast to H2O2 with its response to other oxidants, the expression profiles of the investigated genes were quantified in response to challenges posed by t-butylhydroperoxide or the superoxide anion generated by the redox cycling agent MD (menadione). With both oxidants (results not shown), the responding genes were the same as those in Figure 1(b). However, whereas similar up-regulation levels were obtained with t-butylhydroperoxide, the order of mRNA abundance for the upregulated genes, after exposure to MD, changed to cTPxI>cTPxIII>TRX2∼TRR1>GPX2>cTPxII∼mTPx>GLR1>GSH1, compared with that in Figure 1(b).
The lack of both cytoplasmic thioredoxins (Δtrx1Δtrx2) increased (relative to the parental wild-type) the steady-state levels of the nine transcripts that were up-regulated under oxidative stress conditions (results not shown). Of note is the observation that basal transcript amounts quantified in Δtrx1Δtrx2 mutant cells (single mutants showed no significant effect) were strikingly similar to the induced amounts quantified in the wild-type after exposure to MD (100 μM for 10 min).
Transcript levels along with culture growth
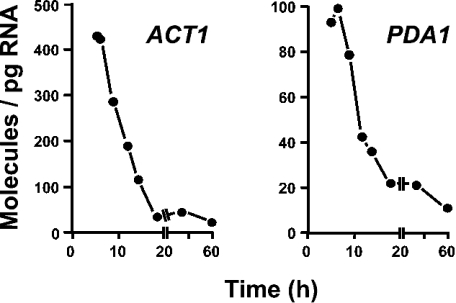
Regulation of gene expression is an integral and dynamic component of the yeast life cycle [1]. In the present study, we quantified the absolute amounts of the investigated transcripts throughout the growth curve in YPD medium. As indicated in Figure 2, quantifications were performed in samples collected at different stages of the exponential fermentative growth phase; next, when the glucose has become limiting and the cells have to reset their metabolism from fermentation to respiration (diauxic shift); subsequently, when the cells resume growth at a much lower rate using ethanol and other by-products from the former glucose catabolism (post-diauxic phase); and later, during the stationary phase. ACT1 and PDA1 mRNAs have been used as internal standards for relative quantification of transcript levels under a wide range of growth stages and conditions (see e.g. [16,17]). Quantifications of both mRNAs demonstrated that their levels do not remain constant along the growth curve but decrease significantly (Figure 3). It follows that, under these experimental conditions, the use of ACT1 or PDA1 transcript as reference would seriously overestimate the quantification of any investigated mRNA that would be present at detectable levels. In this context, it is noteworthy that the absolute expression profiles reported in Figure 4 are not normalized and, therefore, do not assume that a reference is steadily expressed.
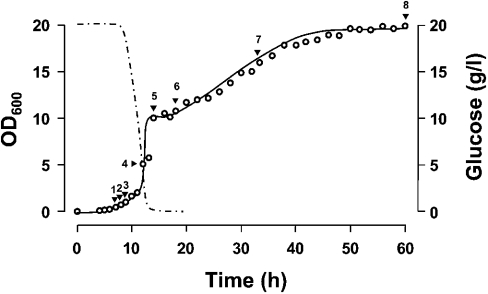
Figure 2. Growth curve of wild-type yeast in YPD medium.
Wild-type cells from an overnight culture (late-exponential phase) in YPD were diluted in fresh medium (starting with A600 0.05) and incubated at 30 °C with shaking at 125 rev./min. Culture growth was monitored by measuring the A600. The glucose content of the medium was determined using the glucose (hexokinase) assay kit (Sigma–Aldrich, St. Louis, MO, U.S.A.). Samples were collected at the indicated time intervals (indicated by arrowheads) for total RNA isolation. Growth phases: early exponential (1–2), mid-exponential (3), late-exponential (4), diauxic shift (5), post-diauxic (6–7) and stationary (8). Doubling time during the exponential phase of growth was estimated in 1.56 h based on A600 measurements. Glucose concentration decreased to 0.1% at the onset of the diauxic shift. The diauxic arrest lasted from 14 to 17 h after inoculation.
Figure 3. ACT1 and PDA1 mRNA levels at different stages of growth.
Samples were collected at the growth stages mentioned in the legend to Figure 2. Results are the mean values of mRNA molecules/pg of total RNA (n≥6).
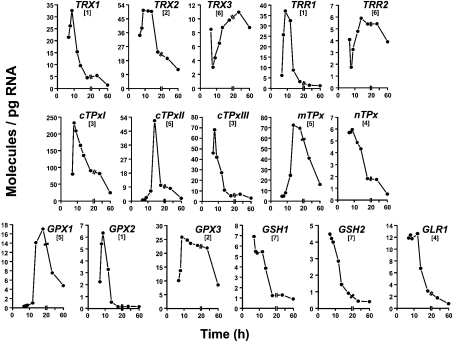
Figure 4. Levels of 16 target mRNAs at different stages of growth.
Samples were collected at the growth stages mentioned in the legend to Figure 2. Results are the mean values of mRNA molecules/pg of total RNA (n≥6). Arbitrary expression patterns referred to in the text are given in brackets.
All of the 16 target mRNAs show detectable alterations in their steady-state levels throughout the different stages of growth. For simplicity, seven distinct temporal patterns of expression were distinguished, although no two profiles were identical in terms of magnitude and the choreography of expression. In pattern 1 (TRX1, TRR1 and GPX2), the mRNA accumulated from early to mid-exponential phase and then decreased to show the lowest expression level at the stationary phase. In pattern 2, the accumulated mRNA remained at a high level after mid-exponential phase, i.e. to the onset of the diauxic shift (TRX2) or, even later, during the post-diauxic phase (GPX3). In pattern 3 (cTPxI and cTPxIII), the mRNA level was maximal somewhat earlier than mid-exponential phase. In pattern 4, maximal mRNA amount was sustained during early and mid-exponential phases (nTPx) and even during late-exponential phase (GLR1). In pattern 5, the mRNA accumulation took place from early exponential to the diauxic shift (cTPxII and mTPx) and took place even further, till early post-diauxic (GPX1). In pattern 6 (TRX3 and TRR2), this upregulation was preceded by a decrease. Finally, in pattern 7 (GSH1 and GSH2), the mRNA level decreased throughout all the growth stages investigated in the present study, from early exponential to stationary phase.
The absolute quantitative results presented in this study are largely in disagreement with previous studies that monitored relative transcript abundances or activities of reporter fusion constructs at different growth stages. One illustrative discrepancy is as follows: TRX1 mRNA increased 1.5-fold (21.5 molecules/pg versus 32.6 molecules/pg) from early to mid-exponential phase and then decreased 24-fold to show the lowest amount (1.4 molecules/pg) at the stationary phase (Figure 4). Contrary to this absolute mRNA profile, TRX1 expression remained largely unchanged according to microarray hybridization [18,19] or increased 6-fold after entry into the stationary phase according to a TRX1::lacZ construct [20].
Culturing of S. cerevisiae on minimal or rich media [Figure 1(a) versus 9 h data in Figure 4] had little effect (≤2-fold variation) on the steady-state levels of the investigated transcripts and therefore on their relative abundance. In contrast, the relative abundance of the mRNAs quantified in the present study showed significant changes along the growth curve (Figure 4). For instance, the order of mRNA abundance for the related TPx isoenzymes was cTPxI>cTPxIII>nTPx∼mTPx>cTPxII during early and mid-exponential phases, but cTPxI>mTPx>cTPxII>cTPxIII>nTPx at the diauxic transition. Remarkably, cTPxI mRNA was the most abundant of all studied target transcripts under any of the investigated growth conditions (media and growth phase), in agreement with the current idea that cTPxIp is an abundant (as it constitutes 0.7% of total soluble protein of yeast grown aerobically) and critical antioxidant in vivo [5,21]. In contrast, the less abundant transcript was GPXI, throughout the exponential growth, and GPX2 during the rest of the monitored growth period.
mRNA decay profiles
The main determinants of mRNA concentration in S. cerevisiae are transcriptional initiation and mRNA decay. The half-life of an mRNA species determines the number of times an individual transcript can be translated, which, in turn, affects the total amount of protein that can be produced by a gene at a given rate of transcriptional initiation. Current procedures for determining the half-lives of individual yeast mRNAs include inhibition of transcription by specific drugs or thermal inactivation of a temperature-sensitive RNA polymerase II [22,23].
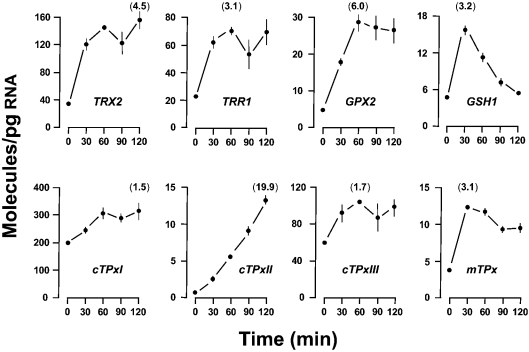
We first inhibited total mRNA synthesis by the use of thiolutin, an effective inhibitor of all yeast RNA polymerases both in vitro and in vivo [24,25]. Surprisingly, the levels of eight (Figure 5) out of nine mRNAs that were up-regulated under oxidizing conditions (Figure 1b) increased (not decreased) after the addition of the transcriptional inhibitor. In contrast, the rest of the mRNAs decayed with half-lives ranging from 72.9 min (TRX1) to 12.0 min (GPX1) (Table 1). These results suggest that thiolutin induces the oxidative stress response under conditions in which the transcription of other genes is blocked. In this way, we hypothesize that the high half-life value (Table 1) calculated for GLR1 mRNA (up-regulated in response to oxidants, e.g. Figure 1b) is overestimated. The moderate decrease in GLR1 mRNA levels in the presence of thiolutin is most probably the result of a modest induction paralleling the blocking transcription.
Figure 5. Time-course effect of thiolutin on mRNA levels.
Wild-type cells were grown in YPD medium to reach an A600 0.5. Cells were then treated with 87 μM thiolutin dissolved in DMSO. Samples were collected at the indicated times after drug addition. Results are the means±S.E.M. of mRNA molecules/pg of total RNA. Data at 0 min represent the steady-state levels of solvent control cells. No time-related effect was noted in these control cells. Some error bars are not visible because of small S.E.M. values. All transcripts were quantified, but only those mRNAs for which statistically significant increases were observed at a given thiolutin exposure time are shown. The remaining genes were transcriptionally inhibited by thiolutin, and their mRNAs exhibited the half-life values shown in Table 1. For each transcript, the maximal fold increment is given in parentheses.
Table 1. Decay rates of mRNAs.
Wild-type (wt) or rpb1-1 mutant cells were grown in YPD to reach an A600 0.5 (exponentially growing, expG) or to the onset of the diauxic arrest (0.1% glucose). Transcription was shut-off as detailed in Figure 5 (thiolutin) or Figure 6 (temperature upshift). Each mRNA decay profile (see e.g. Figure 7) was plotted as the log10 (relative concentration of mRNA) versus time. The half-life of mRNA was calculated from the equation of the decay line for the full 90 min of chase. Half-life values of mRNA that might be overestimated or underestimated (as discussed in the text) are given in parentheses. The arrow (↑) indicates that the mRNA level increased (not decreased) in the presence of thiolutin or at the non-permissive temperature; n.d., not determined. The half-lives and the 95% percentage confidence intervals (in parentheses) previously obtained by using DNA microarray [23] are included for comparison. The microarray data should be in agreement with the half-life values quantified in expG-phase cells.
| Temperature upshift (rpb1-1) | ||||
|---|---|---|---|---|
| Transcript | Thiolutin (wt) ExpG | ExpG | Diauxic | Microarray |
| TRX1 | 72.9 | 69.0 | n.d. | Poor signal |
| TRX2 | ↑ | ↑ | 51.1 | 68 (50–100) |
| TRX3 | 29.6 | 27.6 | 27.8 | Poor signal |
| TRR1 | ↑ | 18.7 | n.d. | 15 (14–17) |
| TRR2 | 34.9 | 30.4 | 32.4 | 34 (29–42) |
| cTPxI | ↑ | 43.5 | n.d. | 34 (30–38) |
| cTPxII | ↑ | ↑ | (39.0) | 37 (33–43) |
| cTPxIII | ↑ | 39.5 | n.d. | 78 (68–92) |
| mTPx | ↑ | ↑ | (20.4) | Poor signal |
| nTPx | 12.7 | 12.6 | n.d. | 22 (17–29) |
| GPX1 | 12.0 | ↑ | (11.1) | Poor signal |
| GPX2 | ↑ | (4.8) | 38.9 | 12 (10–15) |
| GPX3 | 32.5 | 34.3 | n.d. | 60 (48–76) |
| GSH1 | ↑ | (9.6) | (5.3) | 15 (12–19) |
| GSH2 | 16.0 | 20.1 | n.d. | 20 (16–23) |
| GLR1 | (87.6) | 33.5 | n.d. | 31 (26–36) |
| PDA1 | 44.3 | 43.9 | n.d. | 46 (37–50) |
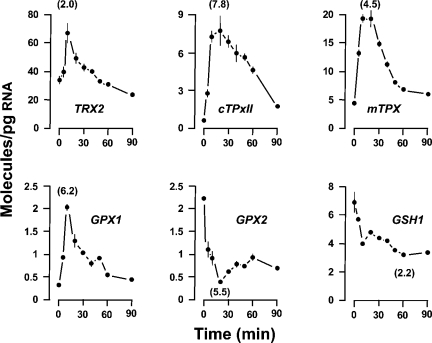
In our effort to quantify accurately the decay rates of the investigated mRNAs, we next considered the possibility of using the RNA polymerase II mutant rpb1-1 for a rapid cessation of mRNA synthesis in exponentially growing cells [11]. Given the thiolutin effect on the genes responding to oxidizing conditions, our first step was to assess the consequences that the temperature upshift might have on the mRNA levels of wild-type cells. As shown in Figure 6, shifting cells from 24 to 37 °C resulted in the altered expression of six genes. Partial overlap with the thiolutin (i.e. oxidative stress) response (Figure 5) was detected. Differences were the up-regulation of the GPX1 gene (not responding to thiolutin) and the down-regulation of GPX2 and GSH1 genes (up-regulated by thiolutin exposure). Distinctions in the upregulation kinetics were also observed. Heat shock resulted in transcript abundance increments within the first 10 min of the shock. Then the mRNA levels gradually adjust to the levels seen in unstressed cells (Figure 6). In contrast, the thiolutin response lasted longer (Figure 5).
Figure 6. Time-course effect of temperature upshift on mRNA levels.
Wild-type cells were grown in YPD medium at 24 °C to reach an A600 0.5. The temperature was then abruptly shifted to 37 °C by transferring the culture to a new flask containing an equal volume of YPD medium that had been prewarmed to 49 °C. Results are the means±S.E.M. of mRNA molecules/pg of total RNA. Data at 0 min represent the steady-state levels in cells before the temperature upshift. No time-related effect was noted in these control cells for the full 90 min of chase. The Figure shows those mRNAs for which statistically significant increases or decreases were observed at a given time after the temperature upshift. For each transcript, the maximal fold variation is given in parentheses.
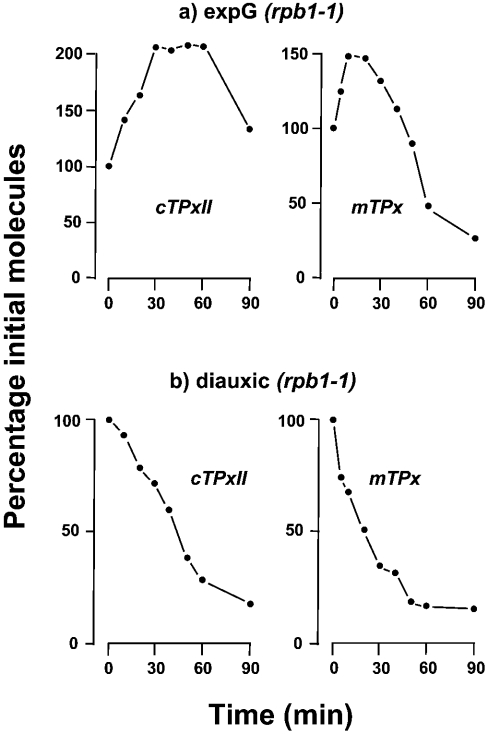
The heat-shock response quantified in wild-type cells (Figure 6) raised the question as to how the temperature upshift can influence the decay profiles of mRNAs in the conditional mutant strain. For four genes (TRX2, cTPxII, mTPx and GPX1), the mRNA level underwent a slight (≤2-fold) increase immediately after the rpb1-1 mutant cells were transferred to the non-permissive temperature and, therefore, no mRNA decay rate could be accurately determined (exemplified in Figure 7a by cTPxII and mTPx). In contrast, the rest of the mRNAs decreased immediately after the temperature upshift, and their half-lives were readily calculated (Table 1).
Figure 7. Examples of mRNA decay curves determined by absolute quantification.
rpb1-1 mutant cells were grown in YPD medium to reach an A600 0.5 (exponentially growing, expG) or to the onset of the diauxic arrest (0.1% glucose). Transcription was shut-off as detailed in Figure 6. The percentage of cTPxII or mTPx molecules remaining after the temperature upshift was plotted against time. Earlier works [11,22] have shown that RNA polymerase II-dependent transcription is reduced in rpb1-1 mutant cells to less than 10% within 2 min after the shift to 37 °C.
The half-lives of mRNA determined in temperature-shifted rpb1-1 cells were in good agreement with those of six mRNAs (TRX1, TRX3, TRR2, nTPx, GPX3 and GSH2) previously approached by thiolutin inhibition. The GLR1 exception further supports the notion that the half-life value of mRNA calculated in thiolutin-treated cells is most probably overestimated. Besides, the half-lives of five (TRR1, cTPxI, cTPxIII, GPX2 and GSH1) out of eight mRNAs that were up-regulated by thiolutin could be determined by means of thermal inactivation of RNA polymerase II in rpb1-1 mutant cells. Nevertheless, given that the increase in temperature triggers in wild-type cells (Figure 6), a reduction in the steady-state levels of GPX2 and GSH1 mRNAs, we cannot completely rule out the possibility of some underestimation in the decay rates of 4.8 and 9.6 min quantified for these transcripts in rpb1-1 cells (Table 1).
Since S. cerevisiae displays the heat-shock response essentially as actively growing cells [26], one further question was whether yeast cells transiently arrested at the diauxic shift would show the same rapid response to the temperature upshift as cells in the exponential phase. We approached this question by following the heat-shock response of wild-type cells grown to the onset of the diauxic phase. Overall, the magnitude of the response of cells in diauxic arrest (results not shown) was lower compared with the response seen in rapidly growing cells (Figure 6). Hence, the amounts of TRX2 and GPX2 mRNAs did not vary significantly after shifting the wild-type cells to 37 °C, and the maximal up-regulation of cTPxII, mTPx and GPX1 mRNAs was less than 2-fold. Only GSH1 mRNA was reduced in abundance to a similar degree as seen in exponential cells.
The slight increase in cTPxII, mTPx and GPX1 mRNAs quantified in wild-type cells after the temperature shift was not observed in the conditional rpb1-1 mutant cells (illustrated by cTPxII and mTPx transcripts in Figure 7b); the mRNA levels decayed with characteristic half-lives (Table 1). Nevertheless, given the slight effect quantified in wild-type cells, the possibility of some bias in the decay rates calculated for these transcripts in rpb1-1 mutant cells cannot be ruled out completely. An interesting observation is that the half-life of 11.1 min calculated for GPX1 mRNA is in very good agreement with that previously determined in wild-type by thiolutin inhibition. In any case, we have to keep in mind that the half-life of individual mRNAs might change with the growth phase, i.e. in response to change in the physiological demands of cells. In this context, previous research [27] concluded that, whereas nutrient limitation at diauxic shift does not affect the stability of some mRNAs, others show accelerated turnover via TOR (target of rapamycin) pathway inhibition.
Recently, Wang et al. [23] have coupled the global transcriptional shut-off assay, based on the rpb1-1 mutation, with DNA microarray analysis to determine half-lives of yeast mRNA on a genomic scale. As shown in Table 1, our data are in reasonably good agreement with data determined by DNA microarray in four (TRR1, TRR2, GSH2 and GLR1) out of 16 transcripts investigated. For most mRNAs, however, the half-lives reported in the present study (based on refined absolute quantifications) were longer (cTPxI) or shorter (e.g. cTPxIII) or impossible to determine in exponentially growing cells (e.g. cTPxII; see Figure 7a).
DISCUSSION
The response to oxidants
Quantifications reported in the present study demonstrate that the transcript levels of nine out of 16 genes investigated were increased in response to oxidative stress. Regarding the thioredoxin-dependent redox system, up-regulated genes code for one (TRX2) of the two cytoplasmic thioredoxins and the corresponding reductase (TRR1) and for four (cTPxI, cTPxII, cTPxIII, mTPx) of the five TPxs. Large amounts of transcripts, ranging from >500 to approx. 50 copies/total RNA, were quantified under oxidizing conditions, in agreement with the central role attributed to this system in defence against oxidative stress [2]. Concerning the GSH-dependent system, elevated transcripts code for one (GPX2) of the three GPxs and for two (GSH1, GLR1) of the three enzymes for the synthesis and reduction of glutathione. Upregulated levels of these transcripts were, in general, lower (≤50 copies/total RNA) compared with those quantified for the thioredoxin system. Overall, the relative induction levels reported in previous studies [2] are much lower than the relative increments calculated in the present study from absolute measurements, and they were typically obtained under more stringent conditions, i.e. higher oxidant dose and/or longer exposure time. For instance, maximal increments of 7-fold were quantified for cTPxII under oxidative conditions [5]. Regarding their regulation, our results are consistent with the idea that these nine transcripts are primarily up-regulated by Yap1p in response to mild ROS stress [2,18], and our results also agree with the observation that this transcriptional factor is constitutively activated in Δtrx1Δtrx2 double-null mutant [28].
The transcript levels of the remaining seven genes (TRX1, TRX3, TRR2, nTPx, GPX1, GPX3 and GSH2) were not affected by the oxidants; this result shows some discrepancies with previous observations. For instance, up-regulation by H2O2 of nTPx and GSH2 expressions has been reported previously [29,30], even though the increments were small in both cases (≤2-fold) and were generated under acute conditions (400 μM, ≥30 min). On the basis of the refined quantifications determined in the present study and the fact that they were obtained at relatively low oxidant concentrations and short exposure time to distinguish primary from putative secondary responses to the given drug, we conclude that these nine transcripts are actually not induced in response to mild oxidative stress. Furthermore, our observation that GSH1 but not GSH2 is induced by oxidant challenge is consistent with γ-glutamylcysteine synthetase being the bottleneck for de novo synthesis of GSH and with the observation that GSH2 disruption results in the accumulation of γ-glutamylcysteine, which is as good a yeast antioxidant as GSH [3].
Crosstalk between heat-shock and oxidative stress responses
Mild heat shock causes expression to be induced in four cases (TRX2, cTPxII, mTPx and GPX1) and to be repressed or lowered in two cases (GPX2 and GSH1), the level of the remaining ten transcripts being unaffected after shifting the cells from 24 to 37 °C. Expression of the genes responsible for ROS detoxification (such as catalase or superoxide dismutase) is induced by mild heat shock (see e.g. [31,32]), thus connecting both stress responses. In the present study, we show that the transcript levels of TRX2, cTPxII and mTPx increase also in response to oxidative stress. Nonetheless, they were superinduced by oxidants (Figure 1) relative to the response to mild heat shock (Figure 6). This finding further supports the idea that these three components of the thioredoxin system are regulated by different transcription factors depending on the specific stressful environmental condition, i.e. through Yap1p in response to oxidants and through Msn2p/Msn4p in response to heat shock [18]. In contrast, the three components of the GSH-dependent redox system (GPX1, GPX2 and GSH1) show unique responses to the above-mentioned environmental changes (Figures 1 and 6), a result that is mostly in disagreement with previous studies [2]. For instance, contrary to the quantifications given here, Northern blotting and lacZ transcriptional fusion have indicated that the expression of GPX1 [7] and GSH1 [29] is induced by both heat-shock and oxidative stress conditions. Nonetheless, in our studies, these two genes appear to play distinct roles in both stress responses.
Growth phase-related gene expression
Data given in the present study show that each transcript exhibits a unique temporal expression profile in YPD as yeast cells proceed from exponential to stationary growth phase. Nonetheless, some general trends are observed, as follows.
Most mRNAs (TRX1, TRX2, TRR1, cTPxI, cTPxIII, nTPx, GPX2, GPX3, GSH1, GSH2 and GLR1) reached maximal or near-maximal steady-state levels a few hours after the inoculum, when glucose concentration is high and cells are actively growing. After this early up-regulation, the mRNA levels decrease to reach minimal or near-minimal expression levels in the stationary phase. This temporal expression profile contrasts with the following ideas: (i) gene expression is basically stable during the exponential growth phase in glucose-rich medium; (ii) changes in gene expression are mostly associated with the metabolic shift from fermentation to respiration; and (iii) the expression of genes coding for antioxidant defences, especially if they can be induced by ROS (e.g. TRX2 or cTPxIII) are largely up-regulated during and after the diauxic shift and in the stationary phase (see e.g. [5,19,20,30,33] for the investigated genes). In contrast, our results as a whole are more in line with the following observations: (i) outstanding transcriptional changes take place early after the inoculation of cells into a complete fermentable growth medium [17]; (ii) the up-regulation of antioxidant-defensive functions does not depend on the post-diauxic onset of respiration [33]; and (iii) the high overall transcriptional capacity of cells at the early growth stage decreases progressively to be almost completely blocked at the end of the exponential phase [34].
Exceptions to the most common temporal expression profile were cTPxII, mTPx and GPX1. For these genes, the number of transcript molecules was at the minimal level at high glucose concentrations, reaching maximal or near-maximal value during the diauxic shift (Figure 4). This variation ranged from 81-fold (cTPxII) to 17-fold (mTPx), depending on the transcript. These outstanding increments in transcript levels, together with their up-regulation in response to mild heat shock (Figure 6), agree with the key role attributed to the transcription factors Msn2p and Msn4p in mediating the transactivation of cTPxII and mTPx during the diauxic transition [35–37] and also agree with the identification by computer search of GPX1 among putative STRE (stress-response element)-controlled genes [19,38,39].
Genes (TRX3 and TRR2) encoding mitochondrial thioredoxin and its specific reductase show other noteworthy exceptions, since their mRNAs were at considerably high levels at both high and low glucose concentrations in the growth medium. Nonetheless, when compared with the rest of the investigated transcripts, basal amounts of TRX3 and TRR2 mRNAs were quite constant; the rather small variations of approx. 3.5-fold observed along the growth curve was most probably connected to mitochondrial development [4]. It is also noteworthy that, although none of the investigated mRNAs were specifically up-regulated or maintained at maximal amounts in stationary-phase cells, the transcripts (TRX3, TRR2 and mTPx) encoding the three mitochondrial proteins together with the transcript encoding Gpx1p exceptionally showed, in stationary phase, levels that were higher than or similar to those quantified in actively proliferating cells. This finding suggests that these four proteins might offer a first line of defence against endogenous ROS generated during the stationary-phase arrest under non-stressed physiological conditions.
mRNA decay in yeast
In S. cerevisiae, transcription is commonly inhibited by specific drugs, such as thiolutin, or by thermal inactivation of a temperature-sensitive RNA polymerase II [22,23]. In the present study, we report that these procedures bring the oxidative stress and heat-shock responses into play. We have also evaluated how these methods interfere with the analysis of the decay rate of individual mRNAs, and we have emphasized the difficulties in precisely measuring the half-lives of those yeast mRNAs that are particularly sensitive to environmental changes. Keeping these experimental uncertainties in mind, as a whole our results satisfied the criterion that the decay rates of individual yeast mRNAs are related to the physiological function of the proteins they encoded [23]. Hence, the half-lives of the investigated mRNAs were distributed within a narrower range (from approx. 70 to ≤10 min) and were significantly longer (with a mean of 31 min and a median of 33 min) when compared with previously reported global mRNA half-lives [23]. This may underlie the need for a prolonged translational window to reach high levels of expression, which seems to be a property of the mRNAs involved in central physiological functions and working in the same biological process [23].
Moreover, in agreement with previous results in mouse liver [10], we find that (i) transcripts (TRX1 and TRX2) encoding cytoplasmic thioredoxins persist ≥3-fold longer than the transcripts (TRR1) encoding the reductase that, in turn, control the redox state and activity of these molecules; (ii) the transcript (TRR2) for mitochondrial reductase turns over more slowly than the transcript (TRR1) for the cytosolic isoenzyme; and (iii) the transcript (GLR1) encoding the enzyme for recycling GSH displays a decay rate approx. 2-fold higher than that of the transcript (TRR1) encoding the enzyme for recycling reduced thioredoxins. Although a positive significant correlation was found between the decay rates of liver mRNAs and their steady-state amounts [10], no simple correlation could be appreciated in yeast. This lack of correspondence highlights the importance of the regulation of transcriptional initiation rates in modulating the significant alterations that most yeast mRNAs exhibit in their steady-state levels throughout the different stages of growth.
Rates of transcriptional initiation
Given that the steady-state levels of any mRNA depend on its rate of degradation as well as its rate of initiation, our measurements of mRNA decay rates (Table 1) and the number of mRNA molecules (Figure 4) make it possible to estimate the rates of transcriptional initiation [40]. As a whole, these calculations (not shown) indicate that, in S. cerevisiae, the rate of in vivo transcriptional initiation for individual genes may vary over a range of at least two orders of magnitude. For instance, we found that, whereas cTPxI transcripts would be initiated every 47 s at early exponential phase in YPD medium, the rate of GPX1 transcriptional initiation would be one transcript every 77 min. Remarkably, however, this latter transcript would be much more frequently initiated at diauxic phase, for which an initiation rate of one GPX1 transcript every 68 s could be estimated.
Similarly, on the basis of the number of mRNA molecules quantified in H2O2-stressed cells (Figure 1) and assuming that the decay rate is not affected by the treatment (only a few examples of regulated mRNA decay in response to external cues have been described in yeast), we now calculate that, for instance, the rate of TRR1 transcriptional initiation would be one transcript every 4 s in the presence of the oxidant. It is noteworthy that this value is in excellent agreement with the maximal transcriptional initiation in vivo calculated for yeast his3 mRNA (one transcript every 6–8 s) [40] and with the maximal initiation rate of E. coli RNA polymerase in vivo (one transcript every 2–3 s) [41].
Acknowledgments
This work was supported by Ministerio de Ciencia y Tecnología (grants PB98-1627 and BMC2002-00179). F. M.-C. was a recipient of a predoctoral fellowship from Junta de Andalucía and C. M. was supported by a postdoctoral contract from Junta de Andalucía. We thank J. Madrid-Rísquez for technical support and P. O. Brown (Department of Biochemistry, Stanford University School of Medicine, Stanford, CA, U.S.A.) for providing Y262 strain.
References
- 1.Hohmann S., Mager W. H. Heidelberg: Springer-Verlag; 1997. Yeast Stress Responses. [Google Scholar]
- 2.Grant C. M. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 2001;39:533–541. doi: 10.1046/j.1365-2958.2001.02283.x. [DOI] [PubMed] [Google Scholar]
- 3.Grant C. M., MacIver F. H., Dawes I. W. Glutathione synthetase is dispensable for growth under both normal and oxidative stress conditions in the yeast Saccharomyces cerevisiae due to an accumulation of the dipeptide γ-glutamylcysteine. Mol. Biol. Cell. 1997;8:1699–1707. doi: 10.1091/mbc.8.9.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedrajas J. R., Kosmidou E., Miranda-Vizuete A., Gustafsson J. A., Wright A. P., Spyrou G. Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:6366–6373. doi: 10.1074/jbc.274.10.6366. [DOI] [PubMed] [Google Scholar]
- 5.Park S. G., Cha M. K., Jeong W., Kim I. H. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:5723–5732. doi: 10.1074/jbc.275.8.5723. [DOI] [PubMed] [Google Scholar]
- 6.Pedrajas J. R., Miranda-Vizuete A., Javanmardy N., Gustafsson J. A., Spyrou G. Mitochondria of Saccharomyces cerevisiae contain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J. Biol. Chem. 2000;275:16296–16301. doi: 10.1074/jbc.275.21.16296. [DOI] [PubMed] [Google Scholar]
- 7.Inoue Y., Matsuda T., Sugiyama K., Izawa S., Kimura A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:27002–27009. doi: 10.1074/jbc.274.38.27002. [DOI] [PubMed] [Google Scholar]
- 8.Avery A. M., Avery S. V. Saccharomyces cerevisiae expresses three phospholipid hydroperoxide glutathione peroxidases. J. Biol. Chem. 2001;276:33730–33735. doi: 10.1074/jbc.M105672200. [DOI] [PubMed] [Google Scholar]
- 9.Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell (Cambridge, Mass.) 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 10.Jurado J., Prieto-Alamo M. J., Madrid-Rísquez J., Pueyo C. Absolute gene expression patterns of thioredoxin and glutaredoxin redox systems in mouse. J. Biol. Chem. 2003;278:45546–45554. doi: 10.1074/jbc.M307866200. [DOI] [PubMed] [Google Scholar]
- 11.Nonet M., Scafe C., Sexton J., Young R. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol. Cell. Biol. 1987;7:1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke D., Dawson D., Stearns T. Plainview, NY: Cold Spring Harbor Laboratory Press; 2000. Methods in Yeast Genetics. [Google Scholar]
- 13.Collart M. A., Oliviero S. Saccharomyces cerevisiae. Preparation of yeast RNA. In: Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K., editors. Current Protocols in Molecular Biology, vol. 2. Indianapolis, IN: John Wiley & Sons; 1993. pp. 13.12.1–13.12.2. [Google Scholar]
- 14.Prieto-Álamo M. J., Cabrera-Luque J. M., Pueyo C. Absolute quantitation of normal and ROS-induced patterns of gene expression: an in vivo real-time PCR study in mice. Gene Expr. 2003;11:23–34. doi: 10.3727/000000003783992315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pueyo C., Jurado J., Prieto-Álamo M. J., Monje-Casas F., López-Barea J. Multiplex reverse transcription-polymerase chain reaction for determining transcriptional regulation of thioredoxin and glutaredoxin pathways. Methods Enzymol. 2002;347:441–451. doi: 10.1016/s0076-6879(02)47044-9. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel T. J., Teunissen A. W., de Steensma H. Y. PDA1 mRNA: a standard for quantitation of mRNA in Saccharomyces cerevisiae superior to ACT1 mRNA. Nucleic Acids Res. 1995;23:883–884. doi: 10.1093/nar/23.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brejning J., Jespersen L., Arneborg N. Genome-wide transcriptional changes during the lag phase of Saccharomyces cerevisiae. Arch. Microbiol. 2003;179:278–294. doi: 10.1007/s00203-003-0527-6. [DOI] [PubMed] [Google Scholar]
- 18.Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeRisi J. L., Iyer V. R., Brown P. O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 20.Garrido E. O., Grant C. M. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol. Microbiol. 2002;43:993–1003. doi: 10.1046/j.1365-2958.2002.02795.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim I. H., Kim K., Rhee S. G. Induction of an antioxidant protein of Saccharomyces cerevisiae by O2, Fe3+, or 2-mercaptoethanol. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6018–6022. doi: 10.1073/pnas.86.16.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker R., Herrick D., Peltz S. W., Jacobson A. Measurement of mRNA decay rates in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:415–423. doi: 10.1016/0076-6879(91)94032-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Liu C. L., Storey J. D., Tibshirani R. J., Herschlag D., Brown P. O. Precision and functional specificity in mRNA decay. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5860–5865. doi: 10.1073/pnas.092538799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tipper D. J. Inhibition of yeast ribonucleic acid polymerases by thiolutin. J. Bacteriol. 1973;116:245–256. doi: 10.1128/jb.116.1.245-256.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez A., Tipper D. J., Davies J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1973;3:729–738. doi: 10.1128/aac.3.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirk N., Piper P. W. The determinants of heat-shock element-directed lacZ expression in Saccharomyces cerevisiae. Yeast. 1991;7:539–546. doi: 10.1002/yea.320070602. [DOI] [PubMed] [Google Scholar]
- 27.Albig A. R., Decker C. J. The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 2001;12:3428–3438. doi: 10.1091/mbc.12.11.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izawa S., Maeda K., Sugiyama K., Mano J., Inoue Y., Kimura A. Thioredoxin deficiency causes the constitutive activation of Yap1, an AP-1-like transcription factor in Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:28459–28465. doi: 10.1074/jbc.274.40.28459. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama K., Izawa S., Inoue Y. The Yap1p-dependent induction of glutathione synthesis in heat shock response of Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:15535–15540. doi: 10.1074/jbc.275.20.15535. [DOI] [PubMed] [Google Scholar]
- 30.Cha M. K., Choi Y. S., Hong S. K., Kim W. C., No K. T., Kim I. H. Nuclear thiol peroxidase as a functional alkyl-hydroperoxide reductase necessary for stationary phase growth of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:24636–24643. doi: 10.1074/jbc.M302628200. [DOI] [PubMed] [Google Scholar]
- 31.Sakaki K., Tashiro K., Kuhara S., Mihara K. Response of genes associated with mitochondrial function to mild heat stress in yeast Saccharomyces cerevisiae. J. Biochem. (Tokyo) 2003;134:373–384. doi: 10.1093/jb/mvg155. [DOI] [PubMed] [Google Scholar]
- 32.Boy-Marcotte E., Lagniel G., Perrot M., Bussereau F., Boudsocq A., Jacquet M., Labarre J. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol. Microbiol. 1999;33:274–283. doi: 10.1046/j.1365-2958.1999.01467.x. [DOI] [PubMed] [Google Scholar]
- 33.Maris A. F., Assumpção A. L., Bonatto D., Brendel M., Henriques J. A. Diauxic shift-induced stress resistance against hydroperoxides in Saccharomyces cerevisiae is not an adaptive stress response and does not depend on functional mitochondria. Curr. Genet. 2001;39:137–149. doi: 10.1007/s002940100194. [DOI] [PubMed] [Google Scholar]
- 34.Jona G., Choder M., Gileadi O. Glucose starvation induces a drastic reduction in the rates of both transcription and degradation of mRNA in yeast. Biochim. Biophys. Acta. 2000;1491:37–48. doi: 10.1016/s0167-4781(00)00016-6. [DOI] [PubMed] [Google Scholar]
- 35.Hong S. K., Cha M. K., Choi Y. S., Kim W. C., Kim I. H. Msn2p/Msn4p act as a key transcriptional activator of yeast cytoplasmic thiol peroxidase II. J. Biol. Chem. 2002;277:12109–12117. doi: 10.1074/jbc.M111341200. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro G., Pereira G. A., Netto L. E. Regulation of mitochondrial thioredoxin peroxidase I expression by two different pathways: one dependent on cAMP and the other on heme. Free Radical Biol. Med. 2002;32:278–288. doi: 10.1016/s0891-5849(01)00801-2. [DOI] [PubMed] [Google Scholar]
- 37.Wong C. M., Ching Y. P., Zhou Y., Kung H. F., Jin D. Y. Transcriptional regulation of yeast peroxiredoxin gene TSA2 through Hap1p, Rox1p, and Hap2/3/5p. Free Radical Biol. Med. 2003;34:585–597. doi: 10.1016/s0891-5849(02)01354-0. [DOI] [PubMed] [Google Scholar]
- 38.Treger J. M., Schmitt A. P., Simon J. R., McEntee K. Transcriptional factor mutations reveal regulatory complexities of heat shock and newly identified stress genes in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:26875–26879. doi: 10.1074/jbc.273.41.26875. [DOI] [PubMed] [Google Scholar]
- 39.Moskvina E., Schuller C., Maurer C. T., Mager W. H., Ruis H. A search in the genome of Saccharomyces cerevisiae for genes regulated via stress response elements. Yeast. 1998;14:1041–1050. doi: 10.1002/(SICI)1097-0061(199808)14:11<1041::AID-YEA296>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Iyer V., Struhl K. Absolute mRNA levels and transcriptional initiation rates in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5208–5212. doi: 10.1073/pnas.93.11.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennell D., Riezman H. Transcription and translation initiation frequencies of the Escherichia coli lac operon. J. Mol. Biol. 1977;114:1–21. doi: 10.1016/0022-2836(77)90279-0. [DOI] [PubMed] [Google Scholar]