Abstract
The NGF (nerve growth factor) from Naja sputatrix has been purified by gel filtration followed by reversed-phase HPLC. The protein showed a very high ability to induce neurite formation in PC12 cells relative to the mouse NGF. Two cDNAs encoding isoforms of NGF have been cloned and an active recombinant NGF, sputa NGF, has been produced in Escherichia coli as a His-tagged fusion protein. Sputa NGF has been found to be non-toxic under both in vivo and in vitro conditions. The induction of neurite outgrowth by this NGF has been found to involve the high-affinity trkA–p75NTR complex of receptors. The pro-survival mechanism of p75NTR has been mediated by the activation of nuclear factor κB gene by a corresponding down-regulation of inhibitory κB gene. Real-time PCR and protein profiling (by surface-enhanced laser-desorption–ionization time-of-flight) have confirmed that sputa NGF up-regulates the expression of the endogenous NGF in PC12 cells. Preliminary microarray analysis has also shown that sputa NGF is capable of promoting additional beneficial effects such as the up-regulation of arginine vasopressin receptor 1A, voltage-dependent T-type calcium channel. Hence, sputa NGF forms a new and useful NGF.
Keywords: Naja sputatrix, nerve growth factor (NGF), neurite outgrowth, PC12 cells, sputa NGF
Abbreviations: AKAP, A-kinase anchoring protein; ATF3, activating transcription factor 3; BAD, Bcl-2/Bcl-XL-antagonist, causing cell death; CaM, calmodulin; CE, cathepsin E; CNS, central nervous system; Egr 1, early growth response 1; IκB, inhibitory κB; IL-12, interleukin-12; NF-κB, nuclear factor κB; NGF, nerve growth factor; NT-3, neurotrophin-3; RT, reverse transcription; SELDI-TOF, surface-enhanced laser-desorption–ionization-time-of-flight; SHP-1, Src homology 2 domain-containing tyrosine phosphatase-1; Trk, tropomyosin-related kinase; V1a, vasopressin receptor 1A
INTRODUCTION
In the past decade, the neurotrophin family has gathered much interest as potentially useful therapeutic agents against neurological disorders. The neurotrophin family is made up of NGF (nerve growth factor), BNDF (brain-derived growth factor), NT-3 (neurotrophin-3), NT-4 and a newly discovered NT-6. Neurotrophins are made up of small, homodimeric proteins and they use two different receptors (i) Trk (tropomyosin-related kinase) receptor tyrosine kinase and (ii) p75 neurotrophin receptor [a member of the TNF (tumour necrosis factor) receptor superfamily]. The binding of neurotrophin to its receptors has been shown to meditate the survival, differentiation, growth or apoptosis of neurons. Both receptors have been found in the same cell and are known to act synergistically to bring about the targeted response of neurotrophins from the neurons during the development of the nervous system [1]. NGF is required for the survival of the differentiated cells. Both p75NTR and TrkA receptors act synergistically to mediate the trophic effects of NGF in cells that produce them (e.g. PC12 cells). Co-expression of p75NTR has also been found to enhance the biological response of cells expressing TrkA [2]. Interestingly, the TrkA receptor activates positive signals such as increased survival and growth, whereas the p75NTR receptor is known to activate both positive and negative signals. Complexes of p75NTR with TrkA have been found to enhance the neurotrophin-binding affinity and TrkA-associated signalling pathway [3]. Neuroprotection by NGF and activation of NF-κB (nuclear factor κB, a pro-survival transcription factor) have also been shown to be dependent on the expression of p75NTR in PC12 cells [4].
NGF has been detected in various animals including snakes [5–7]. NGFs from human placental tissues and body fluids have been documented by Goldstein et al. [8] and Lipps [7]. However, the most extensively studied NGF has been obtained from the male mouse submaxillary glands (mouse NGF; [9]). NGF from snakes, particularly from cobras, has been reported to be superior in inducing neurite outgrowth on PC12 cells when compared with NGFs derived from other types of snakes [9]. Server et al. [10] reported that NGFs from cobra, Naja naja, and mouse submaxillary glands could also elicit neurite outgrowth from chick embryonic dorsal root ganglion.
NGF is normally produced as a pre-pro-precursor, which is then processed to a mature protein by endopeptidases [11]. The NGF from snakes has also been produced as a pre-pro-NGF molecule with 241 amino acids, which is later processed to form a mature NGF comprising 119 amino acids. Approx. 60% homology has been seen between the NGF from snakes and rodents. cDNAs encoding mouse, human, bovine, chick and snake NGFs have also been cloned and sequenced [5,6]. In the present study, we describe the cloning and sequencing of two new cDNAs encoding the NGF in the venom glands of a spitting cobra, Naja sputatrix. The expressed and refolded recombinant protein, sputa NGF, has been found to be equally active as the mouse NGF in forming neurites in PC12 cells. Functional studies have shown that the sputa NGF promotes neurite outgrowth via the TrkA and p75NTR receptors. Activation of NF-κB gene showed that the pro-survival mechanism of p75NTR-mediated signalling is initiated after the addition of sputa NGF. Furthermore, the endogenous NGF in the PC12 cells has been found to be up-regulated only after treatment with sputa NGF. Oligoprobe microarray analysis also showed that the sputa NGF, although acting through similar mechanisms to mouse NGF, may be capable of bringing about additional beneficial effects.
MATERIALS AND METHODS
Venom, venom glands and PC12 cells
An adult snake (N. sputatrix) obtained from Singapore Zoological Gardens (Singapore), kept undisturbed, was milked and the venom was freeze-dried and stored at −20 °C. The venom glands from the same snake were frozen in liquid nitrogen immediately after killing the animal and stored at −85 °C. PC12 cells were obtained from A.T.C.C. (Manassas, VA, U.S.A.) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum and 1% antibiotics (penicillin and streptomycin; [12]) at 37 °C in 5% CO2. The ability of the sputa NGF to elicit neurite outgrowth on PC12 cells was determined as described by Greene and Tishler [13].
Purification of native cobra NGF
Freeze-dried crude venom (50 mg) was reconstituted in 1 ml of water and subjected to gel filtration followed by reversed-phase HPLC (Vision system; Applied Biosystems, Foster City, CA, U.S.A.) using a Sephasil C18 column as described by Armugam et al. [14]. Fractions showing neurite outgrowth on PC12 cells were used for further studies.
Cloning and sequencing of cDNAs
Total RNA was isolated from the venom glands using the guanidine isothiocyanate method [15], and the integrity of the total RNA was analysed by denaturing formaldehyde agarose electrophoresis. Oligonucleotide primers were synthesized by Oswel DNA Services (Southampton, U.K.) based on the sequence at the 5′- and 3′-untranslated regions of cDNA encoding NGF in Agkistrodon halys Pallas [6]. Total RNA (3 μg) was reverse-transcribed and amplified by PCR as described by Armugam et al. [14].
The PCR products were cloned into pT7Blue (R) vector (Novagen, Madison, WI, U.S.A.) and transformed into Escherichia coli DH5α: supE44 ΔlacU169 (Φ80lacZΔm15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 [16]. The recombinant plasmids from the transformants were subjected to Sanger dideoxy DNA sequencing using M13/pUC forward and reverse primers [14,17] on an automated DNA sequencer (Model 3100; Applied Biosystems).
Expression and purification of recombinant sputa NGF
The cDNA-encoding NGF was amplified using sense and antisense primers, 5′-cctggatccgaagatcatcctgtg-3′ and 5′-ctcaagctttcagtttccagtt-3′ respectively, and subcloned into pQE-30 expression vector (Qiagen, Chatsworth, CA, U.S.A.) between BamHI and HindIII sites.
The expression of the sputa NGF was induced by isopropyl β-D-thiogalactoside (1 mM) at 37 °C for 3 h and the protein was purified under denaturing conditions (8 M urea) on a Ni2+-nitrilotriacetate Sepharose resin (Qiagen) column. The purified fractions were analysed by Tris/Tricine SDS/10%-(w/v)-PAGE [18]. In vitro renaturation [19] of the denatured proteins was performed overnight at room temperature (25 °C) in 1×PBS (pH 8.0), containing 4 mM GSH and 2 mM GSSG.
CD spectral analysis
CD spectra for both the native NGF and recombinant sputa NGF were recorded on a Jasco spectropolarimeter (J715) equipped with an interfaced personal computer. It was calibrated with ammonium d-10-camphorsulphonate. Data collected and averaged from at least five recordings were considered acceptable. Spectral measurements were performed at appropriate concentrations using cells of 0.1 cm path-length [19].
Western-blot analysis
Western blotting was performed as described by Cher et al. [20], using either monoclonal anti-phosphotyrosine (AG10; Upstate Biological, Lake Placid, NY, U.S.A.) or polyclonal anti-TrkA and affinity-purified polyclonal anti-p75NTR (Advanced Targeting Systems, San Diego, CA, U.S.A.) at 1 μg/ml or monoclonal anti-β-actin (Sigma Chemicals, St. Louis, MO, U.S.A.) at a dilution of 1:2500. Anti-β-actin was used as a reference for quantification.
Real-time quantitative PCR
Total RNA from PC12 cells was extracted by a single-step method using TRIzol® reagent (Invitrogen, Carlsbad, CA, U.S.A.). Gene-specific primers for TrkA, p75NTR, endogenous NGF, NF-κB and IκB (inhibitory κB) were designed using the PrimerExpress 2.0 software (Applied Biosystems) and their corresponding mRNA sequences. The primers were designed to yield amplicons of <100 bp and synthesized by Alpha-DNA (Montreal, Quebec, Canada). β-Actin was used as an internal calibrator. The primer sequences were as follows: TrkA, 5′-catggagaacccacagtacttcag-3′ (sense) and 5′-ctagctcccacttgagaatgatgtc-3′ (antisense); p75NTR, 5′-gtggtcgtgggccttgtg-3′ (sense) and 5′-gcgccttgtttattttgtttgc-3′ (antisense); rat endogenous NGF, 5′-catggtacaatccctttcaac-3′ (sense) and 5′-ccaacccacacactgacactg-3′ (antisense); NF-κB, 5′-gaagagtcctttcaatggaccaa-3′ (sense) and 5′-tcgggaaggcacagcaata-3′ (antisense); IκB, 5′-gctgcccgagagtgcggat-3′ (sense) and 5′-cagtcatcgtagggcaactcatc-3′ (antisense); β-actin 5′-agggaaatcgtgcgtgaca-3′ (sense) and 5′-gccatctcctgctcgaagtc-3′ (antisense).
In the present study, a two-step RT (reverse transcription)–PCR was used. RT was performed as described in [20]. PCR mixtures contained RT products, forward and reverse primers each at 125 nM and SYBR® Green master mixture (Applied Biosystems). The PCR amplification was then performed for 1 cycle at 50 °C for 2 min and 95 °C for 10 min followed by 40 cycles with each cycle at 94 °C for 15 s and 60 °C for 1 min. All reactions were performed in triplicate using the ABI Prism 7000 SDS (Applied Biosystems).
Protein profiling [SELDI-TOF (surface-enhanced laser-desorption–ionization time-of-flight)]
PC12 cells showing neurite outgrowth were rinsed with 0.9% NaCl and lysed with lysis buffer (50 mM Tris/HCl, pH 7.4, 0.4 mM NaCl, 0.25 M sucrose and 1 mM EDTA, containing 8 M urea). The supernatant was fractionated on hydrophobic reverse-phase chip (H4 Protein Chip TM) using SELDI-TOF MS (Protein Biology System II) both from Ciphergen (Palo Alto, CA, U.S.A.) according to the manufacturer's instructions.
Microarray GeneChip™ analysis
Microarray analysis was performed as described by Cher et al. [20] with the following modifications. Total RNA isolated from control and treated (mouse NGF or sputa NGF) PC12 cells were processed and hybridized to each array of the rat expression 230A GeneChip™ Array set according to the methods described in the GeneChip™ expression analysis package (Affymetrix, Santa Clara, CA, U.S.A.). Relative mRNA expression levels were expressed as positive or negative fold changes when compared with untreated controls based on the Microarray Suite software 5.0 (Affymetrix). All genes showing a change of 2-fold or more were included in subsequent analysis. Members in each cluster were classified according to their biological functions as described in the NetAffix database (Affymetrix).
RESULTS
Bioassay of NGF on PC12 cells
Venoms from several elapids, N. sputatrix, Bungarus candidus, Pseudonaja textilis and Austrelaps superbus, as well as venoms from Chinese and Indian scorpions (Buthus martensi Karsch and Mesobuthus tumulus) and honey bee (Apis mellifera) were tested for neurite outgrowth in PC12 cells. β-NGF (7Sβ-NGF) from the mouse submaxillary gland was used as a positive control. The highest neurite outgrowth was observed for N. sputatrix crude venom at 250 ng/ml. Mouse NGF (7S) showed a comparable activity at 100 ng/ml (Table 1).
Table 1. Neurite outgrowth in PC12 cells.
Protein concentrations at 100 ng/ml were used unless otherwise stated. ‘+’ denotes neurite outgrowth observed.
| Sample | Neurite outgrowth |
|---|---|
| Mouse NGF (positive control; 100 ng/ml) | +++ |
| B. candidus (250 ng/ml) | ++ |
| N. sputatrix (250 ng/ml) | ++++ |
| A. superbus (250 ng/ml) | ++ |
| P. textilis (250 ng/ml) | +++ |
| Chinese scorpion (250 ng/ml) | ++ |
| Indian scorpion (250 ng/ml) | + |
| Bee venom (250 ng/ml) | ++ |
| Biogel P10 gel fractions of N. sputatrix (Figure 1A) | |
| Fraction 27 | ++ |
| Fraction 28 | +++ |
| Fraction 29 | ++ |
| Fraction 30 | +++ |
| Fraction 31 (1 ng/ml) | +++++ |
| Fraction 33 | ++ |
| Fraction 34 | Lysis |
| Fraction 35 | Lysis |
| Fraction 36 | Lysis |
| HPLC fractions (Figure 1B) | |
| Fraction 3 | − |
| Fraction 4 | − |
| Fraction 5 | + |
| Fraction 6 | ++ |
| Fraction 7 | +++++ |
| Fraction 8 (1 ng/ml) | ++++++ |
| Fraction 9 | +++ |
| Fraction 10 | + |
| Denatured and refolded recombinant NGF | |
| Denatured recombinant NGF (100 ng/ml) | ++ |
| Refolded recombinant NGF (100 ng/ml) | ++++ |
Identification of a NGF in N. sputatrix venom
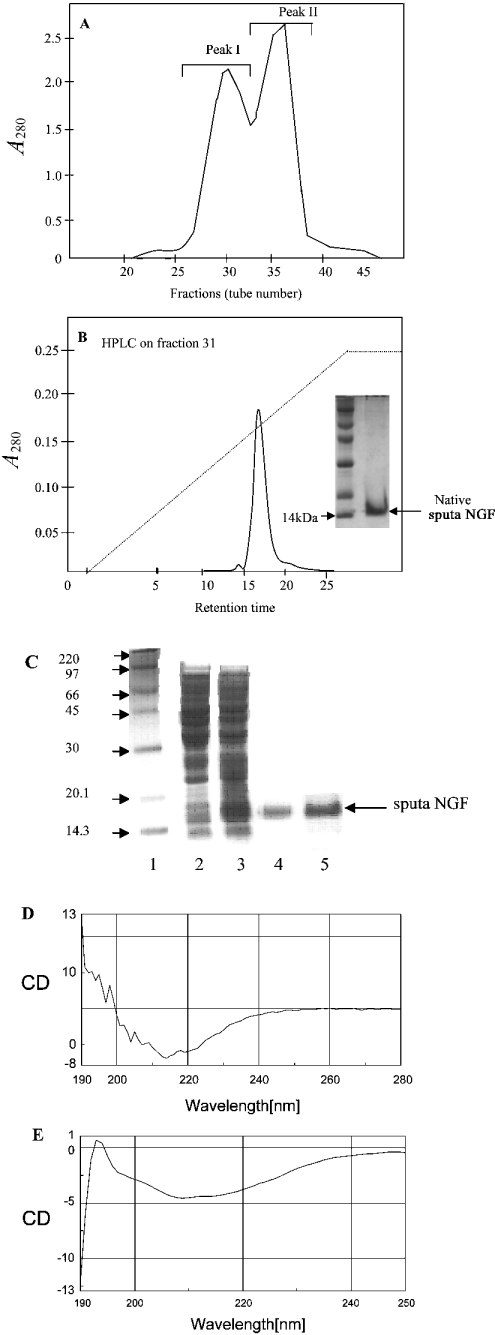
The N. sputatrix venom was chromatographed on Biogel P10 (Figure 1A). The first peak, Peak I, consisted of high-molecular-mass proteins (14–50 kDa), whereas Peak II contained the low-molecular-mass proteins (4–10 kDa). Each fraction (100 ng/ml) was tested for its ability to promote neurite growth (Table 1). The corresponding fraction was also subjected to SDS/PAGE analysis to verify their homogeneity and to determine the molecular mass. The fraction showing the highest neurite outgrowth was purified further on HPLC (Figure 1B). One homogeneous protein peak was obtained, which showed neurite outgrowth at 1 ng/ml (Table 1). This active protein was 14 kDa in mass (Figure 1B, inset). The N-terminal amino acid sequence of this protein was found to be -ETHPVHNRGEYSV- and the protein was found to be non-lethal up to 1 μg/g in mouse.
Figure 1. Purification of NGF.
(A) Gel-filtration chromatography of crude N. sputatrix venom. (B) Reversed-phase chromatography of the active fraction obtained from gel filtration. Inset shows the protein (native sputa NGF fraction) analysed by SDS/PAGE. (C) Purification and refolding of recombinant NGF (sputa NGF). Lane 1, protein molecular mass standards (sizes indicated in kDa); lane 2, E. coli cell lysate before induction; lane 3, E. coli cell lysate (inclusion bodies) after 3 h induction; lane 4, purified sputa NGF; lane 5, refolded sputa NGF. (D) CD spectrum obtained from refolded recombinant sputa NGF and (E) CD spectrum of native NGF protein.
Cloning and expression of the NGF from N. sputatrix
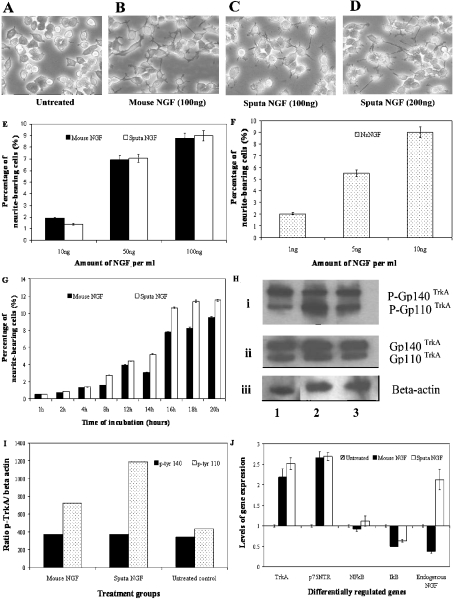
The cDNA encoded for a 22-amino-acid signal peptide, a 112-amino-acid pre-pro-domain of NGF and a mature NGF protein consisting of 117 amino acid residues. Analysis of the sequences indicated the presence of two isoforms (nsNGFI and nsNGFII) of venom NGF (Figure 2). The cDNA encoding the mature protein of nsNGFI (identical with the NGF isolated from the venom, Figure 1B) was expressed as a His6-tagged fusion protein (sputa NGF) and analysed on an SDS/12% (w/v) polyacrylamide gel (Figure 1C, lane 3). The sputa NGF protein was purified by affinity chromatography using a Ni2+-nitrilotriacetate column (Figure 1C, lane 4) and was renatured in the presence of GSSG and GSH (Figure 1C, lane 5). The yield of the refolded protein was found to be approx. 3 mg/l. Both the denatured and refolded recombinant NGFs varied in their potency to induce neurite outgrowth on PC12 cells (Table 1). Both the mouse and sputa NGF were found to exhibit comparable levels of biological activity (Figures 3A–3D). The CD spectrum of the refolded recombinant sputa NGF has been found to correlate well with that of the native NGF protein (Figures 1D and 1E). Quantification of neurite outgrowth in the presence of either mouse or sputa NGF (i) with increasing concentration (Figure 3E) and (ii) with increasing duration of NGF treatment (Figure 3G) has shown that the refolded sputa NGF exhibits a comparable activity with that of mouse NGF. The native NsNGF has also been found to induce neurite outgrowth in a dose-dependent manner but at relatively lower protein concentrations (1–10 ng/ml; Figure 3F).
Figure 2. Comparison of amino acid sequences of various NGF.
Sputa nsngfI and nsngfII refer to the two isoforms of NGF from N. sputatrix; bmNGF is from Bungarus multicinctus (S56212); agpngf is from Agkistrodon halys Pallas [6]; mousengf (K01759) and human ngf (humNGF, X52599). The pro-NGF region is underlined and the mature peptides are shown in boldface letters. Identical amino acids are shown by (*) and the proteolytic cleavage site (KR) for the mature peptide is indicated by ∧∧. The variant residues between nsngfI and nsngfII are shown by +.
Figure 3. Neurite extension and receptor protein expression after treatment with mouse and sputa NGF.
PC12 cells at 1×106 were plated and grown overnight. On the following day, cells were treated with either mouse or sputa NGF, at 200 ng for 16–18 h unless otherwise stated. (A, B) Neurite extensions observed after treatment with mouse NGF. (C, D) Neurites induced by sputa NGF. (E) Neurite extensions quantified for mouse (black bars) and sputa (white bars) NGF (10, 50 and 100 ng). (F) Neurite outgrowth observed for native nsNGF (stippled bars) at 1, 5 and 10 ng. (G) Time-course induction of neurite outgrowth by mouse (black bars) and sputa (white bars) NGF. (H) Activation of TrkA receptor determined by Western-blot analysis. Cells were treated for 15 min and total protein was extracted. Equal amounts of protein were separated on a SDS/7.5% polyacrylamide gel and subsequently blotted on to nitrocellulose membrane. (i) Phosphorylated TrkA receptors (gp110 and gp140) were detected using monoclonal anti-phosphotyrosine antibody. The same membrane was stripped and probed with (ii) anti-TrkA and (iii) β-actin antibodies. Lane 1, mouse NGF-treated cells; lane 2, sputa NGF-treated cells and lane 3, untreated control cells. (I) Ratios of the phosphorylated TrkA and β-actin (internal control) levels measured by densitometry. (J) Quantitative gene analysis via SYBR Green assay. The RNAs from treated and untreated samples were used to study the expression of five genes: TrkA, p75NTR, NF-κB and IκB.
Expression of TrkA and p75 receptors
Western-blot analysis of the PC12 cells treated separately with the mouse and sputa NGF for 15 min showed that the sputa NGF increased the production of the precursor gp110trkA receptors when compared with the mouse NGF. However, the mature gp140trkA receptor was found in equal amounts for both treatments (Figures 3H and 3I). Neurite extensions can be observed only after 12 h of treatment (optimal at 16–18 h). Under this condition, the cells showed higher concentrations of TrkA and p75NTR receptors than the untreated control. These results were also confirmed by real-time PCR (Figure 3J). Figure 3(J) also shows the expression patterns of endogenous NGF, NF-κB and IκB. The expression of IκB (inhibitor of NF-κB gene) has been found to be negatively regulated. Interestingly, an increased expression of the endogenous NGF gene has been observed only after treatment with the sputa NGF (Figure 3J).
Protein profiling (SELDI-TOF) analysis
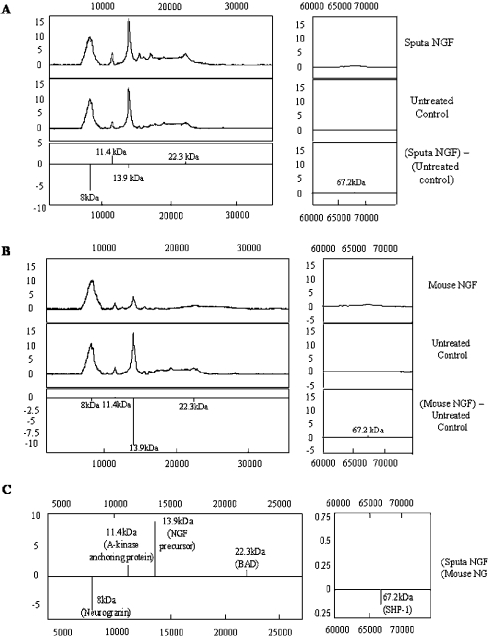
Protein profiling identified five proteins that showed significant changes after NGF treatment (Figure 4). The analysis showed changes in expression of both growth-related (NGF precursor and neurogranin) and an apoptotic protein (BAD, Bcl-2/Bcl-XL-antagonist, causing cell death). Cells treated with sputa NGF (Figure 4A) showed a decrease (approx. 6-fold) in an 8 kDa protein and an increase (approx. 2-fold) in an 11.4 kDa protein. Cells treated with mouse NGF (Figure 4B) showed a down-regulation (approx. 10-fold) of a 13.9 kDa protein. Comparison between sputa NGF and mouse NGF treatments (Figure 4C) showed that sputa NGF caused an increase in the growth-associated proteins similar to AKAP (A-kinase anchoring protein, 11.4 kDa) and the endogenous NGF precursor (13.9 kDa) when compared with pro-apoptotic protein (BAD). Expression of protein at 8 kDa (possibly neurogranin) was greatly decreased (approx. 5-fold), whereas proteins at 22.3 kDa (BAD) and 67.2 kDa [SHP-1 (Src homology 2 domain-containing tyrosine phosphatase-1)] showed a marginal up and down-regulation respectively (Figure 4C).
Figure 4. Protein profiling analysis.
Total protein extracts from cells treated with either mouse NGF or sputa NGF for 16–18 h were used. (A) Protein profiles obtained from cells treated with sputa NGF versus untreated control. (B) Protein profiles obtained from cells treated with mouse NGF versus untreated control. (C) Protein profiles obtained for sputa NGF versus mouse NGF.
Microarray analysis of NGF-treated PC12 cells
The global gene expression after treatment with both NGFs was studied using oligoprobe microarrays. The genes whose expression is altered by 2-fold or more were taken as significant from approx. 730 genes examined in our study. These probe sets were subjected to a self-organizing map (Data Mining Tool; Affymetrix) clustering and were grouped according to their expression patterns. The genes were also further assigned to categories according to their biological functions. Some of them are shown in Table 2. Most of these genes were found to be involved in the cell cycle and growth pathways.
Table 2. Classification of genes from microarray.
Genes were grouped based on their biological functions. Notable changes in gene expression are shown in boldface.
| Category of genes | Description | Mouse NGF (fold change) | Sputa NGF (fold change) |
|---|---|---|---|
| Cell cycle | Growth arrest and DNA-damage-inducible 45α | 3.63 | 3.18 |
| Proliferating cell nuclear antigen | −2.07 | −1.74 | |
| ret proto-oncogene | 2.87 | 2.91 | |
| ret proto-oncogene | 4.06 | 4.41 | |
| Cyclin-dependent kinase inhibitor 1A | 4.99 | 4.26 | |
| Growth-associated protein 43 | 2.27 | 2.10 | |
| Apoptosis | Apoptotic death agonist BID | 3.36 | 2.85 |
| Bcl-2-like-1 | 2.22 | 1.77 | |
| Eicosanoid synthesis | Prostaglandins endoperoxide synthase 1 | 4.59 | 2.36 |
| IL-12 p35 subunit | 6.19 | 4.72 | |
| Transcription factors | Core promoter element-binding protein | 2.53 | 1.57 |
| fos-like antigen 1 | 8.57 | 3.51 | |
| Nuclear transcriptional factor-Y-β | 2.01 | 1.14 | |
| ISL1 transcriptional factor, LIM/homeodomain 1 | 2.75 | 1.74 | |
| Global ischaemia-induced protein GIIG15B | −2.38 | −2.07 | |
| DNA polymerase β | 2.20 | 1.88 | |
| GATA-binding protein 4 | 1.39 | 2.17 | |
| Myelin transcription factor 1-like | −2.20 | −1.33 | |
| Signal transducer and activator of transcription 3 | 2.04 | 1.66 | |
| ATF3 | 2.2 | −1.54 | |
| Egr 1 | 15.14 | 12.91 | |
| Translational factors | Eukaryotic initiation factor-5 | 2.03 | 1.60 |
| Eukaryotic initiation factor-2B, subunit 3 | 2.04 | 1.82 | |
| Others | Meprin 1α | 2.55 | 2.10 |
| CE | 11.08 | 8.22 | |
| Mast cell protease 7 | 88.65 | 71.51 | |
| Plysia ras-related homologue A2 | 2.69 | 2.33 | |
| Small GTP-binding protein rab5 | 2.20 | 1.79 | |
| GTP cyclohydrolase 1 | 3.20 | 2.10 | |
| Phospholipase D | 2.41 | 2.55 | |
| Kinesin family member 1B | 1.45 | 2.01 | |
| Procollagen, type XII, α1 | 2.30 | 1.41 | |
| Integrin β1 | 2.31 | 2.39 | |
| Insulin receptor substrate 1 | 2.06 | 1.27 | |
| Double cortin and calcium/CaM-dependent protein kinase-like 1 | 2.69 | 1.84 | |
| Gap junction membrane channel protein β5 | 2.79 | 2.04 | |
| Jun-B oncogene | 2.28 | 2.39 | |
| Opioid receptor-like | 2.13 | 2.73 | |
| Arginine V1a | 5.43 | 20.25 | |
| Purinergic receptor P2X, ligand-gated ion channel 2 | −2.91 | −1.59 | |
| Purinergic receptor P2X, G-protein coupled 2 | 2.06 | 2.11 | |
| Adenosine monophosphate deaminase 3 | 2.48 | 1.85 | |
| Myelin basic protein | 1.04 | 2.35 | |
| Prolactin receptor | 2.17 | 1.54 | |
| Serum-inducible kinase | 7.26 | 3.12 | |
| Syntaxin | 2.71 | 1.45 | |
| Dynamin 1 | −2.07 | −1.39 | |
| Guanosine diphosphate dissociation inhibitor 3 | 2.17 | 2.39 | |
| Annexin 5 | 3.03 | 2.77 | |
| Neural receptor protein-tyrosine kinase | 9.19 | 3.16 | |
| Moesin | 2.97 | 2.48 | |
| Vesicle-associated membrane protein, associated proteins B and C | 1.71 | 2.10 | |
| ADP-ribosylation-like 4 | 2.71 | 2.28 | |
| Solute carrier family 1, member 3 | 6.28 | 4.99 | |
| Solute carrier family 9, member 4 | −1.44 | 2.23 | |
| Syndecan 1 | 2.95 | 2.39 | |
| Myosin IXA | 2.22 | 1.99 | |
| Potassium inwardly rectifying channel, subfamily J, member 6 | 2.20 | 1.95 | |
| Protein kinase, cAMP-dependent regulatory, type I, α | 2.53 | 2.08 | |
| Coated vesicle membrane protein | 2.00 | 1.89 | |
| Regulator of G-protein signalling protein 2 | 2.25 | 1.38 | |
| Cold-inducible RNA-binding protein | 1.97 | 2.30 | |
| Microtubule-associated protein 1b | 2.31 | 1.29 | |
| Acid phosphatase 5 | 2.01 | 1.91 | |
| Profilin II | 2.38 | 2.06 | |
| Glutamate receptor, ionotrophic, NMDA2C | −2.50 | −1.91 | |
| Lysosomal membrane glycoprotein 2 | −1.97 | −2.20 | |
| NGF receptor-associated protein | −2.03 | −1.55 | |
| Calcium-related pathway | Very-low-density lipoprotein receptor | 2.16 | 1.83 |
| Very-low-density lipoprotein receptor | 2.57 | 2.00 | |
| Very-low-density lipoprotein receptor | 2.22 | 2.10 | |
| Calcium-binding protein p22 | 3.66 | 2.19 | |
| S-100-related protein, clone 42C | 3.36 | 2.71 | |
| S-100-related protein A4 | 2.06 | 1.95 | |
| Cadherin | −3.14 | −2.33 | |
| Calcium channel, voltage-dependent, T-type, αII subunit | 5.54 | 7.21 | |
| Protein phosphatase 3, regulatory subunit B, α isoform, type 1 | 2.23 | 1.78 |
DISCUSSION
Cloning and expression of a new recombinant NGF protein
A widely studied and commercially available NGF, β-NGF, is from the mouse submaxillary gland. Lipps [9] reported that the NGFs from venomous sources are of the highest potency and particularly the NGF from snakes is approx. 1000-fold more active than the commercially available mouse NGF. The crude venom (250 ng/ml) from four untested snakes when assayed for neurite outgrowth on PC12 cells showed that the venom of N. sputatrix, Malayan spitting cobra, exhibited the highest activity. This was followed by the venom from an Australian elapid, P. textilis. Thus the NGF from N. sputatrix venom was purified, and its activity and properties were compared with those of mouse NGF. The molecular mass of the native sputa NGF was found to be 14 kDa, thus indicating that it is a monomer belonging to β-NGF family as suggested by Inoue et al. [21].
The cDNA encoding NGF from Agkistrodon halys Pallas has been reported previously [6]. In the present study, we report the cloning of cDNAs encoding two isoforms of sputa NGF from N. sputatrix. Each cDNA encoded a signal peptide, pre-pro-domain and the mature protein. The mature protein-coding region for N. sputatrix was identified from its N-terminal amino acid sequence as well as from the amino acid sequence of Naja atra NGF [21,22]. Comparison of sequences from the venom-derived NGFs with those of mouse NGF showed distinct variations at their N-terminal ends. The snake NGF shows higher homology (approx. 80%) with the mammalian-derived NGFs, thus suggesting similar function to the mammalian homologue. The pro-region of the cobra NGF however shows approx. 50% homology to the mammalian counterpart. Pro-NGF has long been postulated to contain a biologically active peptide that could induce phosphorylation of the tyrosine residue of the TrkA receptor protein [23]. On the other hand, secreted pro-NGF has been reported to activate cell death in brain following an injury via p75 and sortilin receptors [24], thus indicating a pathophysiological role for the pro-NGF during CNS (central nervous system) injury. In contrast, Fahnestock et al. [25] have shown that the pro-NGF is in abundance in CNS tissues where mature NGF is not detected. The researchers have demonstrated that this pro-NGF is responsible for the biological activity normally attributed by mature NGF, in the CNS tissues. The pro-NGF in snakes shows approx. 50% homology to the mammalian pro-NGF. Furthermore, a deletion of five amino acids can be observed in the mammalian pro-NGF sequence. Hence, their functions may be different. The snake pro-NGF could possibly be involved in the positioning of NGF moiety for proper proteolytic processing and activation of proper in vitro folding of the mature peptide [26]. The death-inducing property, if any, of the pro-NGF from snakes remains to be elucidated.
The venom-derived NGFs showed approx. 80–90% homology among them, and the two isoforms of N. sputatrix NGF (nsNGFI and nsNGFII) showed approx. 80% homology between them. nsNGFI and nsNGFII showed 70 and 60% homology to the mammalian NGF respectively. Whereas the mature protein region for both nsNGFI and nsNGFII showed high homology, the pro-region showed considerable variation among them. Owing to its higher homology to the mammalian homologue, nsNGFI was selected for the expression of recombinant protein in E. coli and termed sputa NGF. This recombinant molecule appears to have two pairs of β-sheets that are linked by three disulphide bonds. We were able to refold it in redox solution containing 2 mM GSSG and 4 mM GSH. The refolded recombinant protein showed a CD spectrum similar to that of the native protein, and it showed enhanced activity when compared with the unfolded NGF (Table 1). This refolded sputa NGF was also found to be non-toxic to PC12 cells at 1 μg/ml. It was able to bring about enhanced neurite outgrowth in a dose-dependent manner from 100 to 500 ng/ml.
Expression of TrkA and p75 receptors
The sputa NGF has been found to elicit neurite outgrowth via the TrkA–p75NTR complex. Two protein forms of TrkA that predominate in cell extracts are: (i) the 110 kDa N-glycosylated precursor form, gp110TrkA and (ii) the 140 kDa fully matured form, gp140TrkA [27]. PC12 cells are known to respond rapidly to NGF within minutes, with a reproducible burst of tyrosine phosphorylation of many cellular proteins and include the TrkA receptors. We observed that the ratio of gp140TrkA to gp110 TrkA decreased on both mouse and sputa NGF treatments for the purpose of replenishing the mature gp140TrkA at the cell surface as described by Jullien et al. [28]. TrkA and p75NTR receptors work in concert to co-ordinate and modulate responses of neurons to NGF. A competitive study using specific receptor antibodies on the percentage of neurite outgrowth showed that sputa NGF is more sensitive to receptor inhibition compared with that of mouse NGF. Reduced TrkA activation in both PC12 cells and sympathetic neurons has been observed after the addition of p75NTR-specific antibodies [29]. The researchers have also shown that any alteration in the optimal TrkA–p75NTR interaction or direct activation of p75NTR at the expense of TrkA, will result in an inhibition of NGF-dependent neurite outgrowth in adult sensory neurons. In the present study, inhibition by TrkA and p75NTR receptor-specific antibodies caused a reduction in neurite outgrowth for both mouse and sputa NGF by 34 and 70% for TrkA and 10 and 40% for p75NTR respectively. Thus the greater reduction in neurite formation on sputa NGF treatment implicates that the sputa NGF is more specific to TrkA and p75NTR, and stimulates signalling using the TrkA–p75NTR high-affinity complex as for mouse NGF. This antibody-based competitive study is in agreement with the quantitative analysis of the receptor (TrkA and p75NTR) genes examined via real-time PCR. The expression of both receptors was higher with sputa NGF when compared with mouse NGF. Activation of TrkA and p75NTR has been reported to induce the expression of NF-κB, which in turn is known to cause neurite extension in PC12 cells [30]. Real-time PCR (Figure 3C) showed a down-regulation of the NF-κB inhibitor, IκB, in both NGF treatments, implying that NF-κB will be made available to activate and promote neurite extension in PC12 cells. However, an increase in NF-κB expression is observed for sputa NGF treatment compared with mouse NGF treatment and such an NGF-induced increase in NF-κB is beneficial for the survival of neurons [31]. Similarly, the endogenous NGF level was up-regulated after treatment with sputa NGF in contrast with mouse NGF. Several studies have shown that neurotrophic factors, including NGF, constitute an important class of endogenous modulators of excitotoxicity, and are known to protect neurons against injury from several causes [32]. NGF has caused increase in glutathione levels of PC12 cells [33], and hence up-regulation (of NF-κB and endogenous NGF genes) by sputa NGF may have potential neuroprotective roles.
Global gene and protein analysis
Microarray analysis (Table 2) showed for the first time the global changes in gene expression after treatment with either mouse NGF or sputa NGF on PC12 cells. This study also yielded comparable data between the two NGFs. The differentiation pathway after NGF treatment is characterized by expression of immediate early genes [such as Egr 1 (early growth response 1) and serum-inducible kinase] and delayed response genes (such as Fos-like antigen, Fra-1 and prostaglandin-endoperoxide synthase 1). It was shown that Egr 1 acts via the extracellular-signal-regulated kinase pathway to produce eventually neurite outgrowth in PC12 cells [34]. Liby et al. [35] have shown that serum-inducible kinase has a role in cell proliferation. Expression of transcription factors, e.g. Fra-1, may participate in long-term neural responses [36], whereas overexpression of ATF3 (activating transcription factor 3) inhibited apoptosis [37]. Enhanced expression of transcription factors (Fra-1, ATF3 and Egr 1) may also cause subsequent increase in CE (cathepsin E) expression [38]. This explains the increase in CE observed in the present study (Table 2). CE is an aspartic protease, which has been implicated in antigen processing in the class II MHC pathway and is known to be up-regulated late in B-cell activation [39]. The concurrent up-regulation of mast cell protease 7 in the present study is in agreement with the previously reported effects of NGF on mast-cell development and differentiation [40].
Arginine V1a (vasopressin receptor 1A) was observed to be highly expressed by sputa NGF treatment when compared with mouse NGF. V1a is widely distributed and known to regulate nearly all the physiological actions of the neuropeptide, vasopressin (AVP), except antidiuresis (V2) and corticotrophin secretion (V1b; [41]). In addition to these, channel transporter genes (solute carrier family 1, member 3; SLC1A3, voltage-dependent calcium channel and purinergic P2X receptors) were found to be affected by the NGF treatment. Mouse NGF activated both the expression of SLC1A3 and P2X receptor genes, whereas sputa NGF activated the voltage-dependent calcium channel gene. The SLC1A3 gene encodes the glutamate receptor GLAST (glutamate/aspartate transporter) responsible for the removal of glutamate to terminate neurotransmission and prevent neuronal cell death [42], whereas the expression of voltage-dependent calcium channel subunit II is restricted to neuronal tissues for the maintenance of the neuronal physiology [43]. In the present study, the down-regulation of P2X receptor was observed in both NGF treatments. This proved to be beneficial as P2X is postulated to be involved in astrocyte-mediated inflammation and neurodegeneration [44]. Up-regulation of IL-12 (interleukin-12) and prostaglandin-endoperoxide synthase 1 seen in both cases also supports their beneficial roles. Prostaglandin-endoperoxide synthase 1 is believed to have a physiological role in neuronal differentiation of PC12 cells [45]. Similarly, IL-12 is known to play an important role in neuronal regeneration [46]. Hence, both NGFs showed similar activities, except for V1a expression. Arginine vasopressin that acts mainly through the V1a receptor is known to enhance NGF secretion in vascular smooth muscles and rat brain cells [47,48].
Protein profiling and real-time PCR results confirmed that an endogenous NGF synthesis is up-regulated after treatment with sputa NGF. Hence, sputa NGF could possibly be acting through V1a receptor to enhance the endogenous NGF production, an effect that will prove to be useful under conditions that require neuronal regeneration and differentiation.
The sputa NGF is also found to enhance the production of growth-associated proteins such as AKAP. The AKAP (11.4 kDa) belongs to a family of scaffolding proteins that bind to the regulatory subunits of protein kinase A, which regulates the phosphorylation of various proteins including those involved in synaptic plasticity. AKAP induction is known to stimulate protein kinase A-dependent phosphorylation of the pro-apoptotic protein BAD (protein at 22.3 kDa), thereby inhibiting the release of cytochrome c from mitochondria and protecting the cells from apoptosis [49]. Although sputa NGF showed a marginal increase in BAD protein when compared with mouse NGF, with AKAP up-regulated approx. 2-fold more than BAD, the pro-apoptotic property of BAD could be suppressed. This interpretation was confirmed experimentally by measuring for caspase activity on PC12 cells treated with sputa NGF. No caspase activity could be determined in these cells.
The mechanisms by which NGF exerts its neuroprotective effects are currently unknown. However, in vitro studies have shown that NGF can stabilize intracellular calcium by preventing accumulation of intracellular calcium and subsequent neuronal damage [50]. The protein profiling results showed a down-regulation in neurogranin (8 kDa). Neurogranin is a neural-specific Ca2+-sensitive CaM (calmodulin)-binding protein. It is believed that phosphorylated neurogranin has the potential to modulate neuronal free-Ca2+ and CaM levels [51]. Gene knock-out experiments leading to a total absence of neurogranin have resulted in deficiency of hippocampal synaptic plasticity and hippocampus-dependent spatial learning [51]. McNamara et al. [52] predicted that an increase in neurogranin expression would perturb Ca2+-CaM signalling that may in turn impair the formation and/or maintenance of synapses. Thus a decreased level of free neurogranin detected after treatment with sputa NGF indicates that a basal level of intracellular Ca2+ is maintained for neuronal differentiation and function.
Protein tyrosine phosphatase (SHP-1; 67.2 kDa) was identified as a phosphotyrosine phosphatase, which negatively regulates the expression of TrkA. It was shown in sympathetic neurons that expression of SHP-1 induces apoptosis and TrkA dephosphorylation [53]. Hence, a down-regulation of SHP-1 expression is also in agreement with TrkA activation after treatment with both NGFs.
In conclusion, sputa NGF seems to exert potentially life-sustaining and neuroprotective (NF-κB and endogenous NGF genes) effects in a manner similar to that of mouse NGF. The life-sustaining effects have been observed at both gene (microarray) and protein (SELDI-TOF) levels. Sputa NGF has also been found to increase the expression of V1a and endogenous NGF genes as well as the NGF precursor and the AKAPs. However, both neurogranin and SHP-1 proteins have been found to be down-regulated after treatment with sputa NGF. Real-time PCR analysis has revealed additional pro-survival effects of sputa NGF such as enhanced expression of NF-κB and the endogenous NGF genes. These effects could have potential neuroprotective roles.
Acknowledgments
This work was supported by a National Medical Research Council (Singapore) grant (grant number R-183-000-062-213). D. C.-I. K. acknowledges the receipt of research scholarship from the National University of Singapore.
References
- 1.Eide F. F., Lowenstein D. H., Reichardt L. F. Neurotrophins and their receptors – current concepts and implications for neurologic disease. Exp. Neurol. 1993;121:200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- 2.Culmsee C., Gerling N., Lehmann M., Nikolova-Karakashian M., Prehn J. H., Mattson M. P., Krieglstein J. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience. 2002;115:1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 3.Casaccia-Bonnefil P., Gu C., Chao M. V. Neurotrophins in cell survival/death decisions. Adv. Exp. Med. Biol. 1999;468:275–282. doi: 10.1007/978-1-4615-4685-6_22. [DOI] [PubMed] [Google Scholar]
- 4.Bui N. T., Konig H. G., Culmsee C., Bauerbach E., Poppe M., Krieglstein J., Prehn J. H. p75 neurotrophin receptor is required for constitutive and NGF-induced survival signalling in PC12 cells and rat hippocampal neurones. J. Neurochem. 2002;81:594–605. doi: 10.1046/j.1471-4159.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 5.Kashima S., Soares A. M., Roberto P. G., Pereira J. O., Astolfi-Filho S., Cintra A. O., Fontes M. R., Giglio J. R., de Castro Franca S. cDNA sequence and molecular modeling of a nerve growth factor from Bothrops jararacussu venomous gland. Biochimie. 2002;84:675–680. doi: 10.1016/s0300-9084(02)01429-3. [DOI] [PubMed] [Google Scholar]
- 6.Guo L. Y., Zhu J. F., Wu X. F., Zhou Y. C. Cloning of a cDNA encoding a nerve growth factor precursor from the Agkistrodon halys Pallas. Toxicon. 1999;37:465–470. doi: 10.1016/s0041-0101(98)00177-9. [DOI] [PubMed] [Google Scholar]
- 7.Lipps B. V. Isolation of nerve growth factor (NGF) from human body fluids; saliva, serum and urine: comparison between cobra venom and cobra serum NGF. J. Nat. Toxins. 2000;9:349–356. [PubMed] [Google Scholar]
- 8.Goldstein L. D., Reynolds C. P., Perez-Polo J. R. Isolation of human nerve growth factor from placental tissue. Neurochem. Res. 1978;3:175–183. doi: 10.1007/BF00964058. [DOI] [PubMed] [Google Scholar]
- 9.Lipps B. V. Biological and immunological properties of nerve growth factor from snake venoms. J. Nat. Toxins. 1998;7:121–130. [PubMed] [Google Scholar]
- 10.Server A. C., Herrup K., Shooter E. M., Hogue-Angeletti R. A., Frazier W. A., Bradshaw R. A. Comparison of the nerve growth factor proteins from cobra venom (Naja naja) and mouse submaxillary gland. Biochemistry. 1976;15:35–39. doi: 10.1021/bi00646a006. [DOI] [PubMed] [Google Scholar]
- 11.Kostiza T., Meier J. Nerve growth factors from snake venoms: chemical properties, mode of action and biological significance. Toxicon. 1996;34:787–806. doi: 10.1016/0041-0101(96)00023-2. [DOI] [PubMed] [Google Scholar]
- 12.Koike T. Nerve growth factor-induced neurite outgrowth of rat pheochromocytoma PC 12 cells: dependence on extracellular Mg2+ and Ca2+ Brain Res. 1983;289:293–303. doi: 10.1016/0006-8993(83)90030-6. [DOI] [PubMed] [Google Scholar]
- 13.Greene L. A., Tishler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armugam A., Earnest L., Chung M. C. M., Gopalakrishnakone P., Tan C. H., Tan N. H., Jeyaseelan K. Cloning and characterization of cDNAs encoding three isoforms of phospholipase A2 in Malayan spitting cobra (Naja naja sputatrix) venom. Toxicon. 1997;35:27–37. doi: 10.1016/s0041-0101(96)00071-2. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J., Fritsch E. F., Maniatis T. 2nd edn. Plainview, NY: Cold Spring Harbor Laboratory; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 17.Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schagger H., Von-Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 19.Ma D., Armugam A., Jeyaseelan K. Cytotoxic potency of cardiotoxin from Naja sputatrix: development of a new cytolytic assay. Biochem. J. 2002;366:35–43. doi: 10.1042/BJ20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cher C. D. N., Armugam A., Lachumanan R., Coghlan M.-W., Jeyaseelan K. Pulmonary inflammation and edema induced by phospholipase A2: global gene analysis and effects on aquaporins and Na+/K+-ATPase. J. Biol. Chem. 2003;278:31352–31360. doi: 10.1074/jbc.M302446200. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S., Oda T., Koyama J., Ikeda K., Hayashi K. Amino acid sequences of nerve growth factors derived from cobra venoms. FEBS Lett. 1991;279:38–40. doi: 10.1016/0014-5793(91)80244-w. [DOI] [PubMed] [Google Scholar]
- 22.Kostiza T., Dahinden C. A., Rihs S., Otten U., Meier J. Nerve growth factor from the venom of the Chinese cobra Naja naja atra: purification and description of non-neuronal activities. Toxicon. 1995;33:1249–1261. doi: 10.1016/0041-0101(95)00086-2. [DOI] [PubMed] [Google Scholar]
- 23.Dicou E., Pflug B., Magazin M., Lehy T., Djakiew D., Ferrara P., Nerriere V., Harvie D. Two peptides derived from the nerve growth factor precursor are biologically active. J. Cell Biol. 1997;136:389–398. doi: 10.1083/jcb.136.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nykjaer A., Lee R., Teng K. K., Jansen P., Madsen P., Nielsen M. S., Jacobsen C., Kliemannel M., Schwarz E., Willnow T. E., et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature (London) 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 25.Fahnestock M., Yu G., Michalski B., Mathew S., Colquhoun A., Ross G. M., Coughlin M. D. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J. Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 26.Suter U., Heymach J. V., Jr, Shooter E. M. Two conserved domains in the NGF propeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J. 1991;10:2395–2400. doi: 10.1002/j.1460-2075.1991.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol. Cell. Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jullien J., Guili V., Reichardt L. F., Rudkin B. B. Trafficking of TrkA-green fluorescent protein chimerae during nerve growth factor-induced differentiation. J. Biol. Chem. 2002;277:38700–38708. doi: 10.1074/jbc.M202401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimpinski K., Jelinski S., Mearow K. The anti-p75 antibody, MC192, and brain-derived neurotrophic factor inhibit nerve growth factor-dependent neurite growth from adult sensory neurons. Neuroscience. 1999;93:253–263. doi: 10.1016/s0306-4522(99)00156-6. [DOI] [PubMed] [Google Scholar]
- 30.Foehr E. D., Lin X., O'Mahony A., Geleziunas R., Bradshaw R. A., Greene W. C. NF-κ B signaling promotes both cell survival and neurite process formation in nerve growth factor-stimulated PC12 cells. J. Neurosci. 2000;20:7556–7563. doi: 10.1523/JNEUROSCI.20-20-07556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggirwar S. B., Ramirez S., Tong N., Gelbard H. A., Dewhurst S. Functional interplay between nuclear factor-κB and c-Jun integrated by coactivator p300 determines the survival of nerve growth factor-dependent PC12 cells. J. Neurochem. 2000;74:527–539. doi: 10.1046/j.1471-4159.2000.740527.x. [DOI] [PubMed] [Google Scholar]
- 32.Mattson M. P., Cheng B., Smith-Swintosky V. L. Mechanisms of neurotrophic factor protection against calcium- and free radical-mediated excitotoxic injury: implications for treating neurodegenerative disorders. Exp. Neurol. 1993;124:89–95. doi: 10.1006/exnr.1993.1178. [DOI] [PubMed] [Google Scholar]
- 33.Palmer C., Roberts R. L., Bero C. Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke. 1994;25:1039–1045. doi: 10.1161/01.str.25.5.1039. [DOI] [PubMed] [Google Scholar]
- 34.Harada T., Morooka T., Ogawa S., Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat. Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 35.Liby K., Wu H., Ouyang B., Wu S., Chen J., Dai W. Identification of the human homologue of the early-growth response gene Snk, encoding a serum-inducible kinase. DNA Seq. 2001;11:527–533. doi: 10.3109/10425170109041337. [DOI] [PubMed] [Google Scholar]
- 36.Paratcha G., de Stein M. L., Szapiro G., Lopez M., Bevilaqua L., Cammarota M., de Iraldi A. P., Izquierdo I., Medina J. H. Experience-dependent decrease in synaptically localized Fra-1. Mol. Brain Res. 2000;78:120–130. doi: 10.1016/s0169-328x(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 37.Nakagomi S., Suzuki Y., Namikawa K., Kiryu-Seo S., Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J. Neurosci. 2003;23:5187–5196. doi: 10.1523/JNEUROSCI.23-12-05187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook M., Caswell R. C., Richards R. J., Kay J., Tatnell P. J. Regulation of human and mouse procathepsin E gene expression. Eur. J. Biochem. 2001;268:2658–2668. doi: 10.1046/j.1432-1327.2001.02159.x. [DOI] [PubMed] [Google Scholar]
- 39.Sealy L., Mota F., Rayment N., Tatnell P., Kay J., Chain B. Regulation of cathepsin E expression during human B cell differentiation in vitro. Eur. J. Immunol. 1996;26:1838–1843. doi: 10.1002/eji.1830260826. [DOI] [PubMed] [Google Scholar]
- 40.Welker P., Grabbe J., Gibbs B., Zuberbier T., Henz B. M. Nerve growth factor-β induces mast-cell marker expression during in vitro culture of human umbilical cord blood cells. Immunology. 2000;99:418–426. doi: 10.1046/j.1365-2567.2000.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawtin S. R., Davies A. R., Matthews G., Wheatley M. Identification of the glycosylation sites utilized on the V1a vasopressin receptor and assessment of their role in receptor signalling and expression. Biochem. J. 2001;357:73–81. doi: 10.1042/0264-6021:3570073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takai S., Yamada K., Kawakami H., Tanaka K., Nakamura S. Localization of the gene (SLC1A3) encoding human glutamate transporter (GluT-1) to 5p13 by fluorescence in situ hybridization. Cytogenet. Cell Genet. 1995;69:209–210. doi: 10.1159/000133965. [DOI] [PubMed] [Google Scholar]
- 43.Chemin J., Monteil A., Dubel S., Nargeot J., Lory P. The α1I T-type calcium channel exhibits faster gating properties when overexpressed in neuroblastoma/glioma NG 108-15 cells. Eur. J. Neurosci. 2001;14:1678–1686. doi: 10.1046/j.0953-816x.2001.01796.x. [DOI] [PubMed] [Google Scholar]
- 44.Gendron F. P., Neary J. T., Theiss P. M., Sun G. Y., Gonzalez F. A., Weisman G. A. Mechanisms of P2×7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am. J. Physiol. Cell Physiol. 2003;284:C571–C581. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan M. D., Olschowka J. A., O'Banion M. K. Cyclooxygenase-1 behaves as a delayed response gene in PC12 cells differentiated by nerve growth factor. J. Biol. Chem. 1997;272:18534–18537. doi: 10.1074/jbc.272.30.18534. [DOI] [PubMed] [Google Scholar]
- 46.Lin H., Hikawa N., Takenaka T., Ishikawa Y. Interleukin-12 promotes neurite outgrowth in mouse sympathetic superior cervical ganglion neurons. Neurosci. Lett. 2000;278:129–132. doi: 10.1016/s0304-3940(99)00913-1. [DOI] [PubMed] [Google Scholar]
- 47.Tuttle J. B., Etheridge R., Creedon D. J. Receptor-mediated stimulation and inhibition of nerve growth factor secretion by vascular smooth muscle. Exp. Cell Res. 1993;208:350–361. doi: 10.1006/excr.1993.1256. [DOI] [PubMed] [Google Scholar]
- 48.Zhou A. W., Li W. X., Guo J., Du Y. C. Facilitation of AVP (4–8) on gene expression of BDNF and NGF in rat brain. Peptides. 1997;18:1179–1187. doi: 10.1016/s0196-9781(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 49.Affaitati A., Cardone L., Cristofaro T., Carlucci A., Ginsberg M. D., Varrone S., Gottesman M. E., Avvedimento E. V., Feliciello A. Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J. Biol. Chem. 2003;278:4286–4294. doi: 10.1074/jbc.M209941200. [DOI] [PubMed] [Google Scholar]
- 50.Kirschner P. B., Jenkins B. G., Schulz J. B., Finkelstein S. P., Matthews R. T., Rosen B. R., Beal M. F. NGF, BDNF and NT-5, but not NT-3 protect against MPP+ toxicity and oxidative stress in neonatal animals. Brain Res. 1996;713:178–185. doi: 10.1016/0006-8993(95)01513-2. [DOI] [PubMed] [Google Scholar]
- 51.Pak J. H., Huang F. L., Li J., Balschun D., Reymann K. G., Chiang C., Westphal H., Huang K. P. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11232–11237. doi: 10.1073/pnas.210184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNamara R. K., Huot R. L., Lenox R. H., Plotsky P. M. Postnatal maternal separation elevates the expression of the postsynaptic protein kinase C substrate RC3, but not presynaptic GAP-43, in the developing rat hippocampus. Dev. Neurosci. 2002;24:485–494. doi: 10.1159/000069359. [DOI] [PubMed] [Google Scholar]
- 53.Marsh H. N., Dubreuil C. I., Quevedo C., Lee A., Majdan M., Walsh G. S., Hausdirff S., Said F. A., Zoueva O., Kozlowski M., et al. SHP-1 negatively regulates neuronal survival by functioning as a TrkA phosphatase. J. Cell Biol. 2003;163:999–1010. doi: 10.1083/jcb.200309036. [DOI] [PMC free article] [PubMed] [Google Scholar]