Abstract
UK114, the goat liver tumour antigen, is a member of a widely distributed family of conserved low-molecular-mass proteins (YER057c/YjgF/UK114), the function of which is ill understood. To the various orthologues diverse functions have been ascribed, such as translation inhibition, regulation of purine repressor or calpain activation. Owing to a limited sequence similarity to Hsp90 (heat-shock protein 90), they have also been proposed to be molecular chaperones; however, this has never been tested. In the present paper, we report the cloning and characterization of the Drosophila orthologue, DUK114. In brief, DUK114 had no effect that would have qualified it as a calpain activator. In contrast, it proved to be a very potent molecular chaperone in in vitro assays. In a heat-aggregation test, it significantly decelerated the formation of citrate synthase aggregates. In a reverse assay, the recovery of the enzyme from urea- and heat-induced denatured states was accelerated almost 3-fold. On a molar basis, the chaperone activity of the 15-kDa DUK114 is comparable with that of Hsp90, the almost 6-times-larger archetypal molecular chaperone. In similar assays, DUK114 was ineffective with Drosophila calpain A or calpain B. To test for its chaperone activity in vivo, DUK114 was transfected into Schneider (S2) cells; after heat shock, the number of viable non-transfected cells started to increase after a lag time; in the presence of DUK114, cell proliferation started at once. Our work is the first experimental evidence that DUK114, and possibly other members of this family, are molecular chaperones.
Keywords: calpain activator, chaperone, DUK114, folding, heat shock, Schneider S2 cell
Abbreviations: BBCA, bovine brain calpain activator; GFP, green fluorescent protein; Hsp, heat-shock protein; Ni-NTA, Ni2+-nitrilotriacetate; SFM, serum-free medium
INTRODUCTION
UK114, the goat liver tumour antigen [1], is a member of the YER057c/YjgF/UK114 family of conserved low-molecular-mass proteins, which are widely distributed in prokaryotes and eukaryotes. These proteins are typically approx. 130 amino acids in length, and the family of over 200 orthologues can be divided into low-identity and high-identity groups, depending on the level of sequence similarity [2,3]. Although the ubiquity and evolutionary conservation of these proteins indicates some common and essential role, no such function has so far been demonstrated convincingly. Instead, a wide variety of distinct, often conflicting, functions for members of the high-identity group have been proposed. The Bacillus subtilis orthologue, YabJ, mediates the adenine-induced repression of PurA expression by the purine repressor, PurR [4]. In Salmonella typhimurium, deletion of YjgF causes complex metabolic phenotypes, which point to its involvement in isoleucine biosynthesis and in coupling the oxidative pentose phosphate pathway to thiamine synthesis [5]. In Saccharomyces cerevisiae, Mmf1p (YIL051c) is also involved in isoleucine biosynthesis, but it also promotes cell proliferation and contributes to mitochondrial genome stability and integrity [6,7]. The evolutionary conservation of its function is demonstrated by the fact that the human orthologue, p14.5, can rescue the phenotype associated with the deletion of its gene [6]. The expression of the rat orthologue, perchloric-acid-soluble protein PSP (p23 [8]), is induced during liver cell differentiation and is strongly suppressed in tumour cells. This protein inhibits protein synthesis in a cell-free translation system [9,10], possibly due to its RNase activity specific to single-stranded mRNA [11]. A similar function has also been proposed for the human orthologue, p14.5 [10].
Our interest was kindled by the observation that some of these proteins behave as activators of the Ca2+-activated cytoplasmic thiol-proteases, calpains. In mammals, 14 genes encode calpains, and the activators isolated from rat [12] and bovine [13] brain were found to be specific to the ubiquitous μ-calpain; their effect was a significant sensitization of calpain to Ca2+. Within the YER057c/YjgF/UK114 family, the calpain activator is closest in sequence to goat liver UK114. The calpain system in Drosophila, the object of our present studies, consists of four genes, two of which code for active enzymes: calpain A and calpain B [14]. Their requirement for Ca2+ for activation is in the millimolar range in vitro, much too high to be encountered in intact cells. Therefore an activator to reduce the Ca2+ requirement would be of great importance. To this end, we cloned and tested the fruitfly orthologue of the calpain activator DUK114; as documented in the Results section, no activating effect on Drosophila calpains could be detected.
A common function of YER057c/YjgF/UK114 proteins, and DUK114 in particular, nevertheless, is suggested by the conservation of their key structural features. The three-dimensional structure of B. subtilis YabJ [2], Escherichia coli YjgF [15], S. cerevisiae Hmf1p [16], Haemophilus influenzae HI0719 [3] and goat UK114 [17] determined by X-ray crystallography, ultracentrifugation and NMR all show a homotrimeric subunit arrangement. In each structure, a distinct cavity lined with invariant residues at the subunit interface has been noted, suggesting a common function. The clue to this possible function has been provided by previous observations that rat liver p23 [8] and goat liver UK114 [1] display a significant sequence similarity to a conserved region of Hsp90. In fact, these two have been suggested to play a role as molecular chaperones, a function also invoked later in the case of B. subtilis YabJ [4] and yeast Mmf1p [6]. The chaperone activity of any of these proteins has never been directly tested, although it received indirect support from the heat-responsive expression of Hrp12 in rat hepatocytes [18].
Based on these inferences, we have tested the chaperone activity of DUK114. We provide evidence, for the first time, that a member of this rather enigmatic family is a very potent molecular chaperone.
EXPERIMENTAL
Materials
Restriction enzymes were purchased from New England Biolabs. The pET22b expression vector and E. coli BL21(DE3) cells were obtained from Novagen. The Ni-NTA (Ni2+-nitrilotriacetate) resin was purchased from Qiagen. Drosophila Schneider (S2) cells and all reagents for maintaining them were purchased from Invitrogen. All other chemicals were from Sigma.
Construction of expression vectors
The DUK114 sequence was intensified by PCR from a Drosophila third instar larvae cDNA library and cloned into a pET22b expression vector resulting in DUK114-pET22b. The DUK114 was also cloned into Drosophila transfection vector pRmeGFP on the N-terminus of the GFP (green fluorescent protein) (DUK114-pRmeGFP). (The construction of the Drosophila transfection vector pRmeGFP was carried out through the modification of pRm-Ha3 vector. Ha3 was replaced with the GFP-coding sequence with multiple cloning sites on both ends). The latter construct produces DUK114 with an N-terminal GFP under the control of a metallothionein promoter.
Expression of recombinant DUK114 proteins in E. coli
The E. coli BL21(DE3) strain was transformed with the expression vectors using conventional techniques. Cells were grown in NZYM medium (1 g of NZ-amine®, 0.5 g of NaCl, 0.5 g of Bacto yeast extract, 0.1 g of casamino acids, 0.2 g of MgSO4·7H2O in 100 ml of water) containing carbenicillin (50 μg/ml) at 37 °C, 250 rev./min, until reaching a D600 of 0.5–0.7. Expression was induced at 30 °C for 3 h by 0.4 mM IPTG (isopropyl β-D-thiogalactoside). The culture was cooled on ice and centrifuged at 3000 g for 10 min at 4 °C. Cells were suspended in 50 mM NaH2PO4/Na2HPO4, pH 7.5, 1 M NaCl, 5 mM EDTA, 1% (v/v) Tween 20, 2% (v/v) glycerol, 20 mM imidazole, 1 mM PMSF, 5 mM benzamidine and 20 mM 2-mercaptoethanol (suspension buffer), and sonicated six times for 15 s at 16 μs (MSE sonicator) on ice. The lysate was centrifuged at 100000 g for 30 min at 4 °C. The supernatant was applied to a Ni-NTA–agarose column after the addition of MgCl2 to a final concentration of 5 mM. After washing with ten column volumes of washing buffer (suspension buffer without EDTA), calpain was eluted with a step gradient of washing buffer containing 40, 60, 80 and 250 mM imidazole. The protein was dialysed against calpain buffer (10 mM Hepes, 1 mM EDTA and 150 mM NaCl, pH 7.5) for further studies.
CD measurements
CD spectra were recorded in 10 mM Tris/HCl, pH 7.5, and 150 mM NaCl with or without 8 M urea in a 1-mm pathlength cuvette on a Jasco J-720 spectropolarimeter in continuous mode with 1 nm bandwidth, 8 s response time and 20 nm/min scan-speed. Each spectrum presented is the average of nine separate scans. The temperature was maintained at 25 °C with a Neslab RTE-111 circulating water bath.
Calpain activity measurements
Calpain activity was measured at room temperature (25 °C), at 0–12 mM free Ca2+ concentration for 30 min with calpain A and 5 min with calpain B and μ-calpain with or without DUK114. Calpains were either pre-incubated or not with DUK114 before adding Ca2+. DUK114 was added at a molar ratio from 1 to 10. Activity was measured with a caseinolytic assay as described in [19].
Citrate synthase assays [20,21]
Citrate synthase aggregation
To measure thermal aggregation of the native enzyme, 100 μM or 10 μM pig heart citrate synthase (Roche) was diluted 100-fold in 40 mM Hepes/KOH, pH 7.5, in thermostatically controlled cuvette at 43 °C in the presence or absence of the compound tested. Light scattering was measured in a Hitachi F-4500 spectrofluorimeter at 650 nm with 2.5 nm slit.
Citrate synthase renaturation (after heat denaturation)
Native enzyme (100 nM), diluted in the same 40 mM Hepes/KOH buffer, pH 7.5, was kept at 44 °C in an Eppendorf plastic tube for 15 min in the presence or absence of the compound tested. Renaturation was initiated by shifting the samples to 25 °C. Enzymic activity measurement is based on the reaction of the liberated acetyl CoA with Ellman's reagent. The absorbance of the new thiol compound was detectable at 412 nm for 1 min. The assay was performed at 25 °C and started by the addition of 100 μM acetyl CoA, 100 μM Ellman's reagent and 500 μM oxaloacetic acid.
Citrate synthase renaturation (after chemical denaturation)
Native enzyme (10 μM) was denatured in 50 mM Tris/HCl, pH 8.0, 2 mM EDTA and 8 M urea at 25 °C for 1.5 h. The renaturation was started by dilution of the enzyme to 100-fold into Hepes buffer, pH 8.0, 50 mM KCl, 5 mM potassium acetate and 10 mM ammonium sulphate, containing the compounds tested (at 10-fold higher concentrations). Enzymic activity was measured after various times as described above.
Renaturation of calpain A and B
Enzymes were denatured with 8 M urea, kept at 25 °C for 90 min, then dialysed against calpain buffer (supplemented with 5 mM 2-mercaptoethanol) for 3 h. Activity was measured every day for a period of 1 week.
RNA preparation
Drosophila Schneider (S2) cells were harvested by centrifugation at 3000 g for 6 min. Total RNA to be used for reverse transcription and PCR was prepared with TRIzol® reagent (Invitrogen) according to the manufacturer's instructions and assayed by conventional methods. Primers were designed for Hsp (heat-shock protein) 70, Hsp83 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as controls.
Schneider (S2) cells
Schneider (S2) cells were cultured in SFM (serum-free medium) supplemented with 20 mM L-glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin at 24 °C. For stable transfection of Schneider (S2) cells, the DUK114-pRmeGFP vector was co-transfected with a Bluescript puromycin vector in the presence of Cellfectin reagent (Invitrogen), following the instructions of the manufacturer. The stable cell line was reached after five passages. The puromycin concentration was 50 μg/ml. Stably transfected cells expressing DUK114–GFP were seeded on to glass coverslips in the growth medium and induced with 0.7 mM CuSO4 for 6 h in SFM containing puromycin. Cells were washed in PBS then fixed in 3% (w/v) paraformaldehyde for 10 min. Cells were mounted with Mowiol 4-88 (Calbiochem) supplemented with DAPI (4,6-diamidino-2-phenylindole) and analysed by a Leica DMLS fluorescent microscope equipped with a Spot RT colour digital camera (Diagnostic Instruments).
Heat-shock of D. melanogaster
Drosophila in glass vials was put into a water bath for 1 h at 37 °C. Then they were immediately, or after different times of recovery, homogenized in ice-cold calpain buffer supplemented with 1 mM PMSF, 5 mM benzamidine and 5 mM 2-mercaptoethanol.
Cell-growth determination
For determination of cell growth, the Sigma MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide]-based Cell Growth Determination Kit was used, according to the manufacturer's instruction.
Other methods
SDS/PAGE was carried out according to [22]. Immunoblotting was performed according to [23].
RESULTS
The EST (expressed sequence tag) clone GM01181, comprising the full-length cDNA of DUK114, was analysed. The ensuing amino acid sequence is aligned with that of BBCA (bovine brain calpain activator) in Figure 1. The two proteins exhibited 50% identity and 75% similarity. The DUK114 cDNA was cloned into a pET22b vector and expressed in E. coli as described in the Experimental section. Purification by metal-chelate chromatography yielded a practically homogeneous preparation, a single heavy band at about 14 kDa apparent molecular mass in SDS/PAGE (Figure 1, inset). The availability of plenty of pure protein allowed us to characterize DUK114 thoroughly for structural and functional properties.
Figure 1. Sequence comparison of DUK114 (AAM50212) and BBCA (P80601).
Sequence comparison of DUK114 and BBCA is shown in the left-hand panel. The identity (black boxes) of the two sequences is 50%, the overall similarity is 75%. The alignment was made by the ClustalW method [26]. The right-hand panel shows a SDS/polyacrylamide gel of recombinant purified DUK114 (lane B), with calibration protein mixture (lane A).
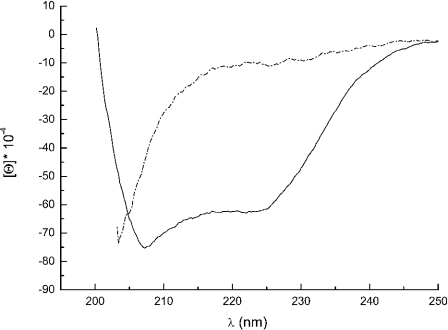
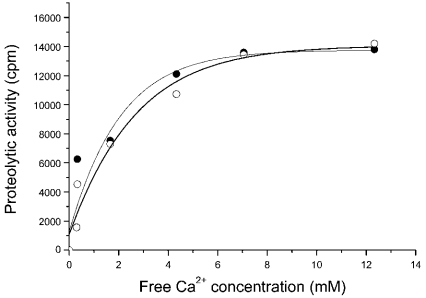
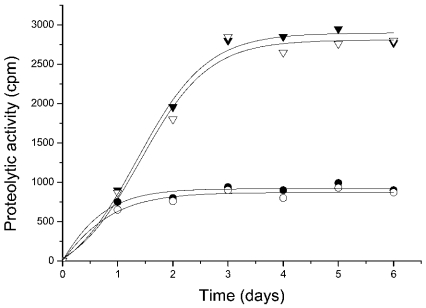
First, we checked whether the purified protein was a properly folded polypeptide. The CD spectrum (Figure 2) of DUK114 was characteristic of a mature protein, which disappeared if the protein was denatured in 8 M urea. Then we tested the protein for its ability to activate Drosophila calpains A and B, and μ-calpain. In brief, we could not detect any activating effect that would have manifested itself in an increase of Ca2+-sensitivity or maximal activity (Figure 3). Likewise, DUK114 did not influence the time course or extent of renaturation of calpains A and B after denaturation in urea (Figure 4). The addition of DUK114 during heat-denaturing of calpains A and B at 43 °C did not help to preserve activity (results not shown). DUK114 had no effect on the autolysis of calpain B and μ-calpain (results not shown). We conclude that DUK114 is not an activator for Drosophila calpain A or B.
Figure 2. Far UV CD spectra of DUK114 in buffer (solid line) and in 8 M urea (dashed/dotted line).
Molar ellipticity of DUK114 under normal (solid line) and denaturing conditions (dashed/dotted line). (Note that urea absorbance precluded recording over the whole wavelength region).
Figure 3. Ca2+-dependence of calpain B activity in the presence (•) and absence (○) of DUK114.
The molar ratio DUK114/Calpain B was 10. The final concentration of calpain B in the assay mixture was 9.6 μM. Overall [Ca2+]1/2=1.8 mM. Analogous curve-pairs were obtained with calpain A and μ-calpain, but [Ca2+]1/2 values were 3.4 mM and 200 μM respectively. Data are representative of three independent experiments.
Figure 4. Time course of renaturation of calpains A and B after urea-denaturation in the absence and presence of DUK114.
Radioactivity before denaturation for calpain A was 7700 c.p.m. and for calpain B, 3000 c.p.m. The DUK114/enzyme molar ratio was 10. Calpain A in the absence (▿) and presence (▾) of DUK114; calpain B in the absence (○) and presence (•) of DUK114. The recovery of activity was 36% for calpain A and 27% for calpain B. Data are representative of three independent experiments.
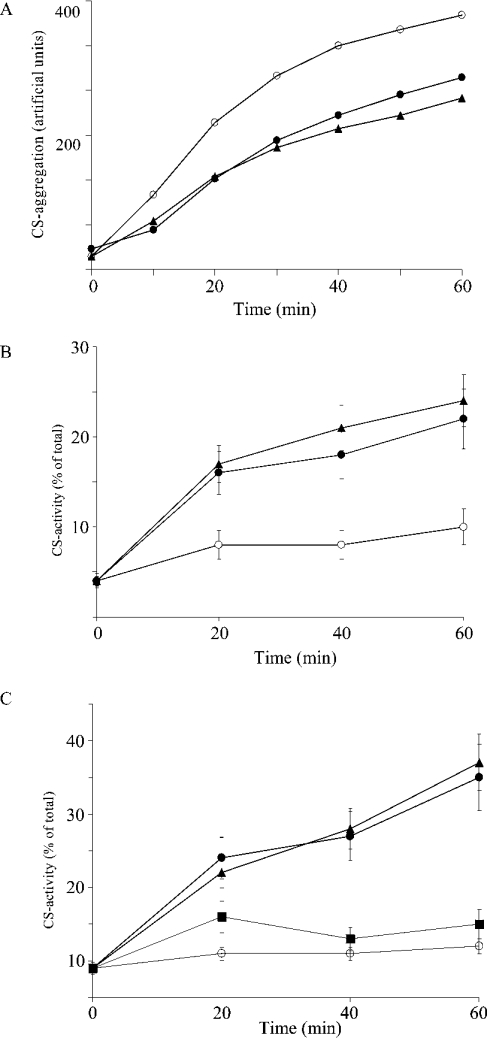
Since members of the UK114 family are also related to Hsps [13], we probed DUK114 in various conventional chaperone tests. Since heat-shock elements have been conserved during evolution, it was reasonable to assume that if DUK114 had chaperone activity, it would show up in a heterologous system. Therefore we challenged DUK114 in three different chaperone assays using citrate synthase as a target protein: (i) inhibition of heat-induced aggregation of citrate synthase (Figure 5A), (ii) promotion of temperature-drop-induced renaturation of heat-denatured citrate synthase (Figure 5B), and (iii) promotion of renaturation of urea-denatured citrate synthase (Figure 5C). As seen in Figure 5, DUK114 is a potent chaperone in all three assays, its effect being comparable with that of Hsp90, the archetype of a chaperone protein [24].
Figure 5. Chaperone tests on citrate synthase.
(A) Time course of citrate synthase (final concentration, 100 nM) aggregation without effector (○), in the presence of Hsp90 (1 μM, ▴) or DUK114 (1 μM, •). (B) Time course of citrate synthase (100 nM) renaturation after heat treatment without effector (○), in the presence of Hsp90 (1 μM, ▴) or DUK114 (1 μM, •). (C) Time course of citrate synthase (final concentration, 100 nM) renaturation from 8 M urea without effector (○), in the presence of BSA (1 μM, ▪), Hsp90 (1 μM, ▴) or DUK114 (1 μM, •). Data are representative of five independent experiments.
Encouraged by these results and led by the observation that Hsp90 is an ATP-binding chaperone [24], we tested the ATP binding of DUK114. Using various assays (affinity chromatography on ATP–agarose as well as ATP-driven affinity cleavage), we did not find any effects pointing towards an ATP binding by DUK114 (results not shown).
Next, we analysed the expression of the DUK114 message in adult fruitflies after heat shock (1 h at 37 °C) by reverse-transcription PCR, and found it to be constant, unaffected by heat-shock, just like the message for the Drosophila chaperone Hsp83. In contrast, the message of Hsp70 markedly increased on heat-shock, reached a maximum at 2–3 h, and considerably declined by 7 h (results not shown). DUK114 is presumably regulated at the protein level rather than at the mRNA level.
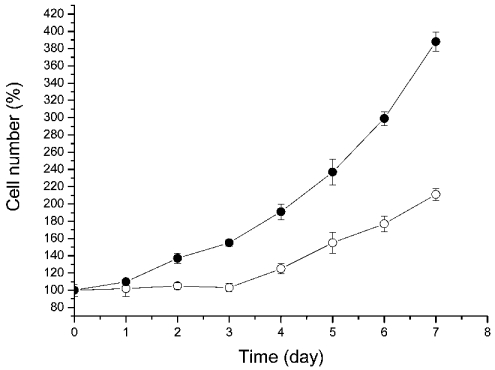
In an attempt to find chaperone-like activity for DUK114 in Drosophila, we transfected Drosophila Schneider (S2) cells with a DUK114–GFP construct. Microscopic examination of the cells revealed homogeneous intracellular distribution of the conjugate. Then cells were heat-shocked (1.5 h at 37 °C) and the cell number was recorded for 7 h. As seen in Figure 6, cells expressing DUK114 started to grow much earlier than did control cells not expressing DUK114. It seems that DUK114 protects some essential factor(s) of cell proliferation during heat shock.
Figure 6. Recovery of Drosophila Schneider (S2) cells after heat shock.
The heat treatment (1.5 h at 37 °C) ended at zero time. The cell number after heat shock was taken as 100%. ○, control cells; •, cells overexpressing the DUK114–GFP conjugate. Overexpression of GFP alone had no effect on cell growth. Data are representative of three independent experiments.
DISCUSSION
When we embarked on this work, we intended to find and characterize an activator protein for calpain A and/or calpain B from Drosophila. This expectation seemed to be well supported by data in the literature claiming that, in the high-identity group of the ubiquitous protein family YjgF/YER 057c/UK114, some members are calpain activators. In particular, the BBCA, found to be specific for μ-calpain [12], was almost identical in its sequence with UK114, a tumour antigen from goat liver [1], while being 50% identical with the Drosophila protein DUK114 (Figure 1). However, defying expectations, DUK114 gave no evidence of being a calpain activator: it failed to increase the Ca2+-sensitivity of Drosophila or mammalian calpains and did not assist renaturation of calpain A or B from urea-denaturation.
With regard to the biological function of members of the protein family in question, ironically, there have been plenty of suggestions (see the Introduction). One is based on the high sequence similarity with a segment of Hsp90 [1,8], and has led to speculations about a chaperone-like function [6]. This suggestion has not so far been tackled experimentally. Therefore we set out to examine DUK114 for chaperone activity. As documented in Figure 5, this lead proved to be the right one to follow. DUK114 was highly effective as a chaperone in conventional chaperone tests using mammalian citrate synthase as target, its efficiency was comparable with that of Hsp90, a classic chaperone [24]. Since our experiments showed that DUK114 is not an ATP-binding protein, we did not examine its effect in ATP-dependent refolding assays. The similarity of DUK114 to Hsp90 lies between the N- and C-terminal ATP-binding sites of Hsp90 [1,8,24], which gives further support for the ATP-independent function of DUK114.
As with Drosophila chaperone Hsp83, the DUK114 message is not affected by heat-shock. This is noteworthy in light of our observation that Drosophila Schneider (S2) cells transfected with GFP-tagged DUK114 recovered significantly faster after heat shock than did control cells not overexpressing DUK114. We do not know how, through what molecular interactions, DUK114 protects the cell machinery. Paradoxically, we can only say that the point of positive interference is very probably not in the calpain system. Incidentally, we have recently provided evidence that arrest of calpain activity by the specific inhibitor PD105606 retards the cell cycle at multiple points [25]. It should be clear that our present data cannot be regarded as contradictory to the latter finding. Besides the calpain system, there are many other levers to gear the cell-proliferation machinery.
In conclusion, we provided experimental evidence, the first in the literature, that DUK114 and possibly other members of this protein family are molecular chaperones.
Acknowledgments
We acknowledge the help and expertise of Dr Csaba Sőti (Department of Medical Chemistry, Semmelweis University, Budapest, Hungary) in the ATP-binding assays. This work was supported by grants from OTKA (Hungarian Research Fund) numbers. T-34255 and Ts-040723 to P. F., T-37357, EU6th Framework (FP6-506850) and Hungarian Ministry of Social Welfare (ETT-32/03) to P. C., P. T. acknowledges the support of a Bolyai Janos Scholarship.
References
- 1.Ceciliani F., Faotto L., Negri A., Colombo I., Berra B., Bartorelli A., Ronchi S. The primary structure of UK114 tumor antigen. FEBS Lett. 1996;393:147–150. doi: 10.1016/0014-5793(96)00850-2. [DOI] [PubMed] [Google Scholar]
- 2.Sinha S., Rappu P., Lange S. C., Mantsala P., Zalkin H., Smith J. L. Crystal structure of Bacillus subtilis YabJ, a purine regulatory protein and member of the highly conserved YjgF family. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13074–13079. doi: 10.1073/pnas.96.23.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons L., Bonander N., Eisenstein E., Gilson M., Kairys V., Orban J. Solution structure and functional ligand screening of HI0719, a highly conserved protein from bacteria to humans in the YjgF/YER057c/UK114 family. Biochemistry. 2003;42:80–89. doi: 10.1021/bi020541w. [DOI] [PubMed] [Google Scholar]
- 4.Rappu P., Shin B. S., Zalkin H., Mantsala P. A role for a highly conserved protein of unknown function in regulation of Bacillus subtilis purA by the purine repressor. J. Bacteriol. 1999;181:3810–3815. doi: 10.1128/jb.181.12.3810-3815.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enos-Berlage J. L., Langendorf M. J., Downs D. M. Complex metabolic phenotypes caused by a mutation in yjgF, encoding a member of the highly conserved YER057c/YjgF family of proteins. J. Bacteriol. 1998;180:6519–6528. doi: 10.1128/jb.180.24.6519-6528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxelmark E., Marchini A., Malanchi I., Magherini F., Jaquet L., Hajibagheri M. A., Blight K. J., Jauniaux J. C., Tommasino M. Mmf1p, a novel yeast mitochondrial protein conserved throughout evolution and involved in maintenance of the mitochondrial genome. Mol. Cell. Biol. 2000;20:7784–7797. doi: 10.1128/mcb.20.20.7784-7797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J. M., Yoshikawa H., Shirahige K. A member of the YER057c/yjgf/Uk114 family links isoleucine biosynthesis and intact mitochondria maintenance in Saccharomyces cerevisiae. Genes Cells. 2001;6:507–517. doi: 10.1046/j.1365-2443.2001.00443.x. [DOI] [PubMed] [Google Scholar]
- 8.Levy-Favatier F., Cuisset L., Nedelec B., Tichonicky L., Kruh J., Delpech M. Characterization, purification and cDNA cloning of a rat perchloric-acid-soluble 23-kDa protein present only in liver and kidney. Eur. J. Biochem. 1993;212:665–673. doi: 10.1111/j.1432-1033.1993.tb17704.x. [DOI] [PubMed] [Google Scholar]
- 9.Oka T., Tsuji H., Noda C., Sakai K., Hong Y. M., Suzuki I., Munoz S., Natori Y. Isolation and characterization of a novel perchloric acid-soluble protein inhibiting cell-free protein synthesis. J. Biol. Chem. 1995;270:30060–30067. doi: 10.1074/jbc.270.50.30060. [DOI] [PubMed] [Google Scholar]
- 10.Schmiedeknecht G., Kerkhoff C., Orso E., Stohr J., Aslanidis C., Nagy G. M., Knuechel R., Schmitz G. Isolation and characterization of a 14.5-kDa trichloroacetic-acid-soluble translational inhibitor protein from human monocytes that is upregulated upon cellular differentiation. Eur. J. Biochem. 1996;242:339–351. doi: 10.1111/j.1432-1033.1996.0339r.x. [DOI] [PubMed] [Google Scholar]
- 11.Morishita R., Kawagoshi A., Sawasaki T., Madin K., Ogasawara T., Oka T., Endo Y. Ribonuclease activity of rat liver perchloric acid-soluble protein, a potent inhibitor of protein synthesis. J. Biol. Chem. 1999;274:20688–20692. doi: 10.1074/jbc.274.29.20688. [DOI] [PubMed] [Google Scholar]
- 12.Melloni E., Michetti M., Salamino F., Sparatore B., Pontremoli S. Mechanism of action of a new component of the Ca2+-dependent proteolytic system in rat brain: the calpain activator. Biochem. Biophys. Res. Commun. 1998;249:583–588. doi: 10.1006/bbrc.1998.9200. [DOI] [PubMed] [Google Scholar]
- 13.Melloni E., Michetti M., Salamino F., Pontremoli S. Molecular and functional properties of a calpain activator protein specific for μ-isoforms. J. Biol. Chem. 1998;273:12827–12831. doi: 10.1074/jbc.273.21.12827. [DOI] [PubMed] [Google Scholar]
- 14.Jékely G., Friedrich P. Characterization of two recombinant Drosophila calpains: CALPA and a novel homolog, CALPB. J. Biol. Chem. 1999;274:23893–23900. doi: 10.1074/jbc.274.34.23893. [DOI] [PubMed] [Google Scholar]
- 15.Volz K. A test case for structure-based functional assignment: the 1.2 Å crystal structure of the YjgF gene product from Escherichia coli. Protein Sci. 1999;8:2428–2437. doi: 10.1110/ps.8.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deaconescu A. M., Roll-Mecak A., Bonanno J. B., Gerchman S. E., Kycia H., Studier F. W., Burley S. K. X-ray structure of Saccharomyces cerevisiae homologous mitochondrial matrix factor 1 (Hmf1) Proteins. 2002;48:431–436. doi: 10.1002/prot.10151. [DOI] [PubMed] [Google Scholar]
- 17.Deriu D., Briand C., Mistiniene E., Naktinis V., Grutter M. G. Structure and oligomeric state of the mammalian tumour-associated antigen UK114. Acta Crystallogr. Sect. D. Biol. Crystallogr. 2003;59:1676–1678. doi: 10.1107/s0907444903014306. [DOI] [PubMed] [Google Scholar]
- 18.Samuel S. J., Tzung S. P., Cohen S. A. Hrp12, a novel heat-responsive, tissue-specific, phosphorylated protein isolated from mouse liver. Hepatology. 1997;25:1213–1222. doi: 10.1002/hep.510250525. [DOI] [PubMed] [Google Scholar]
- 19.Pintér M., Stierandova A., Friedrich P. Purification and characterization of a Ca2+-activated thiol protease from Drosophila melanogaster. Biochemistry. 1992;31:8201–8206. doi: 10.1021/bi00150a012. [DOI] [PubMed] [Google Scholar]
- 20.Jacob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 21.Nardai G., Sass B., Eber J., Orosz G., Csermely P. Reactive cysteines of the 90-kDa heat shock protein, Hsp90. Arch. Biochem. Biophys. 2000;384:59–67. doi: 10.1006/abbi.2000.2075. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csermely P., Schnaider T., Sőti Cs., Prohászka Z., Nardai G. The 90-kDa molecular chaperone family: structure, function and clinical applications: a comprehensive review. Pharmacol. Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 25.Jánossy J., Ubezio P., Apáti A., Magócsi M., Tompa P., Friedrich P. Calpain as a multi-site regulator of cell cycle. Biochem. Pharmacol. 2004;67:1513–1521. doi: 10.1016/j.bcp.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Combet C., Blanchet C., Geourjon C., Deléage G. NPS@: network protein sequence analysis. Trends Biol. Sci. 2000;25:147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]