Abstract
Idiopathic pulmonary fibrosis (IPF) diagnosis is still the diagnosis of exclusion. Differentiating from other forms of interstitial lung diseases (ILDs) is essential, given the various therapeutic approaches. The IPF course is now unpredictable for individual patients, although some genetic factors and several biomarkers have already been associated with various IPF prognoses. Since its early stages, IPF may be asymptomatic, leading to a delayed diagnosis. The present review critically examines the recent literature on molecular biomarkers potentially useful in IPF diagnostics. The examined biomarkers are grouped into breath and sputum biomarkers, serologically assessed extracellular matrix neoepitope markers, and oxidative stress biomarkers in lung tissue. Fibroblasts and complete blood count have also gained recent interest in that respect. Although several biomarker candidates have been profiled, there has yet to be a single biomarker that proved specific to the IPF disease. Nevertheless, various IPF biomarkers have been used in preclinical and clinical trials to verify their predictive and monitoring potential.
Keywords: Idiopathic pulmonary fibrosis, Breath biomarker, Oxidative stress biomarker, Neoepitope, Sputum biomarker, Fibroblast
Highlights
-
•
Interstitial lung disease epidemiology origin is still unknown
-
•
Current diagnosis of idiopathic pulmonary fibrosis, IPF, is by symptoms
-
•
Determining breath and sputum biomarkers and neoepitopes helps diagnose IPF
-
•
Fibroblast activation protein-specific PET/CT are imaged for IPF diagnosis
-
•
Oxidative stress biomarkers are determined, and a complete blood count is performed in IPF diagnosis
1. Introduction
Interstitial lung diseases, ILDs, involve a heterogeneous set of over 200 conditions mainly affecting the interstitial compartment of the lung parenchyma. A pathologic buildup of inflammatory and mesenchymal cells is the primary abnormalities encountered [1,2]. Overproduction and abnormal growth of extracellular matrix, mainly collagen, causing fibrosing ILD, are the main reasons for these cellular variations. There are ∼199 per 100,000 individual cases of ILD in the United States [3]. The occurrence per 100,000 individuals is higher in females (219) than in males (180) [4]. Some ILDs may occur secondary to a known reason, including autoimmune diseases, such as lung inflammation, rheumatoid arthritis, sarcoidosis, scleroderma, and lupus. Those result from breathing in a foreign substance such as dust, fungus, or mold (hypersensitivity pneumonitis). Some drugs, including nitrofurantoin, sulfonamides, bleomycin, amiodarone, methotrexate, gold, infliximab, etanercept, and other chemotherapy medicines, as well as other factors like asbestos, coal dust, cotton dust, and silica dust (called occupational lung disease) cause ILD as well as infection and partial recovery from diseases like COVID-19, while others, the idiopathic interstitial pneumonia (IIP) cases, have no identifiable cause [5]. Idiopathic pulmonary fibrosis (IPF) is a pulmonary fibrosis of origin that remains unknown. It is one of the most aggressive forms of IIP, symptomized by chronic, developing fibrosis related to remodelation due to irregular deposition of collagen and extracellular matrix [6], developing respiratory failure, and high mortality [7]. The etiology of the disease is still unknown, and it primarily occurs in individuals ∼70–75 years old. Intrinsic and extrinsic risk factors could favor the disease's development in individuals with a genetic predisposition. However, the exact trigger mechanisms still need to be clarified, and the median survival from the diagnosis time is below four years [8].
2. Epidemiology
The most frequent disease of ILD is IPF. Its reported incidence rates vary considerably depending on the data collection method and diagnostic case definition. The disease is estimated to affect ∼3 million people globally [9]. The incidence of IPF is evidenced to increase [10]. A systematic review of the worldwide IPF incidence estimated a rate of 2.8–9.3 per 100,000 people per year in North America and Europe, with markedly lower rates in Asia and South America [7]. Within countries, regional variations may depend on exposure to environmental or occupational risk factors [[11], [12], [13], [14], [15]]. The total number of diagnosed prevalent cases of IPF in the US, Germany, Spain, France, Italy, and Japan in 2021 was 194,878. The highest diagnosed prevalent cases of IPF were accounted for in the US, with 94,736 cases. A recent analysis of the UK care data calculated a rise in the incidence of 78% from 2000 through 2012 and a doubling of cases, estimated at 38.8 per 100,000 people [15], with the substantial growing economic burden on global healthcare [16].
The major problem with IPF is mortality, which is high, and a reported median survival is from two to four years from diagnosis [17]. More recent reports show no improvement in survival [15,18,19]. Furthermore, the mortality rate is increasing. However, this may partially reflect the improvement of the diagnosis [13,20]. Currently, two antifibrotic drugs reduce disease progression, but their global impact on IPF survival is unclear. Early results from the open-label extension clinical trial of pirfenidone showed a median survival of 77.2 months on treatment [21]. IPF is a heterogeneous disease with diverse disease development [22], and its prediction is difficult.
3. Current methods of idiopathic pulmonary fibrosis diagnosis
An adequate diagnosis is crucial to help with prognosis and optimize the treatment selection with the recently developed two effective drugs for patients with IPF. The traditional approach is based on diagnosing precise clinical and laboratory evaluations.
Other IPF reasons involve chronic hypersensitivity pneumonitis caused by significant environmental exposures, connective tissue disease-related interstitial lung disease (by estimating the patient and their serum for indication of systemic autoimmunity), and drug toxicity [9]. Clinical assessment is responsible for eliminating these causes. Recognizing conditions in a patient's environment helps assess patients with suspected IPF. However, no confirmed survey can guarantee a complete clinical evaluation. Nevertheless, a thorough medical history should be considered, describing all possible environmental effects, extrapulmonary symptoms, and a relevant family medical history. An IPF diagnosis might be wrong if this history was neglected [9].
The chest high-resolution computed tomography (HRCT) is the most critical part of the preliminary assessment of patients with suspected IPF. Its result decides the subsequent methods of examination. The most typical HRCT procedure adopted for the assessment of diffuse lung disease involves a volumetric sampling of thin segments (typically ≤15 mm thick) combined with a high spatial frequency reconstruction algorithm [9].
Several characteristic syndromes are indicative of usual interstitial pneumonia (UIP). Among them are characteristic findings on HRCT, including honeycombing, traction bronchiectasis, and either-or traction bronchioles, fine mesh often associated with lung parenchymal opacification (GGO) [23]. There are four diagnostic categories described as "UIP pattern," "probable UIP pattern," "pattern indeterminate for UIP," and "alternative pattern." UIP is the distinctive radiological pattern of IPF. Honeycombing is a unique feature of UIP. If it is present, then an HRCT diagnosis of UIP is positive [23]. A pattern of UIP on HRCT precludes surgical lung biopsy for diagnostic purposes. Suppose we are dealing with usual interstitial pneumonia or non-usual interstitial pneumonia. In that case, a lung biopsy is suggested to support a positive IPF diagnosis [17].
However, thoracic radiologists have encountered numerous difficulties with HRCT. Most IPF patients do not reveal characteristic HRCT features of a UIP pattern. Moreover, surgical lung biopsy cannot be applied to many patients because of their age or other existing diseases. Therefore, several studies [19,24,25] have attempted to expand the range of HRCT features considered sufficiently precise to diagnose IPF and preclude the need for biopsy. However, biopsy remains an essential diagnostic tool for patients with suspected IPF if HRCT presentation is not typical of interstitial pneumonia. The current knowledge recommends taking tissue biopsies from more than one location in the lung. That is related to significant differences in the distribution and morphology of abnormalities [26]. Therefore, a way must be found to select these locations in a targeted manner. In most patients, video-assisted thoracic surgery is preferred, whose diagnostic advancement is comparable to thoracotomy [27].
For new individuals diagnosed with ILD for a reason apparently not known and clinically suspected to have IPF, the panel recommended serologic testing to aid in excluding connective tissue disease as a potential cause of ILD [28]. For all patients with newly diagnosed ILD, routine serologic tests should be performed. Serologic panels regularly comprise antinuclear antibodies (by immunofluorescence), C-reactive protein, erythrocyte sedimentation rate, myositis panel, rheumatoid factor, and anti-cyclic citrullinated peptide.
Nevertheless, further development of effective methods to diagnose IPF in the early stage and clinical patient samples is crucial in reducing mortality. Further research is needed to determine what other factors, such as biomarkers, might be needed to refine the diagnostic process further. Various indicators have been examined in this field, and their role in IPF patients has been established. However, there is still an urgent need to understand the mechanism of this pulmonary disease and develop novel indicators because only the evaluation of the whole spectrum of biomarkers can ensure an accurate diagnosis.
Although the pathogenesis of IPF is still unknown, abnormal extracellular matrix (ECM) remodeling can contribute to cellular changes and irreversible growth of collagenous scar tissue in the lung [29,30]. ECM-modifying enzymes, including MMP-7 and MMP-1 matrix metalloproteinases (MMPs), are promising predictive biomarkers, predominantly in a mixture, for IPF compared with chronic hypersensitivity pneumonitis [31]. Moreover, plasma surfactant protein D (SP-D), MMP-7, and osteopontin combination appeared particularly useful in distinguishing IPF from other interstitial pneumonia cases [32].
Over the last decade, the growing interest in MMPs has pushed the development of various devices based on many recognition methods and signal transductions to measure MMPs' enzymatic activity. Methods for profiling of MMPs include Western Blot. In that profiling, proteins are separated by gel electrophoresis, then exposed to MMP antibodies (zymography), where a substrate is polymerized with acrylamide in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, separating proteins by mass, and activity-based profiling [33]. In addition to these conventional techniques, various sensors based on electrochemistry [34,35], fluorescence [36,37], surface-enhanced Raman scattering (SERS) [38], etc., have continuously been devised for MMPs determination. Among them, sensors with molecularly imprinted polymers (MIPs) as recognizing units appeared promising for proteins' sensing and extracting from biological fluids [39], thus proving their applicability for MMPs' sensing. For example, a conducting MIP film deposited on an extended-gate field-effect transistor (EG-FET) transducer's gate was proposed to determine the MMP-1 epitopes [40]. Toward that, the Basic Local Alignment Search Tool (BLAST) software was used to select epitopes that were most suitable for imprinting. Two relatively short ones were selected from a group of MMP-1 epitopes. Potentiodynamic electropolymerization was used for simultaneous MIPs, templated with the epitopes of the MMP-1 biomarker, preparation, and deposition as thin films on electrodes. The resultant chemical sensors, featuring MIP film-recognizing units integrated with EG-FET transducers, determined these epitopes and the whole MMP-1 analyte. In another study, stable, non-aggregating peptide epitopes were simultaneously imprinted in conducting polymers and deposited on indium–tin–oxide (ITO) electrodes [41]. The MMP-1 was determined using cyclic voltammetry (CV) with the Fe(CN)63−/Fe(CN)64− redox probe present in the test solution, actuating the "gate effect." Finally, MMP-1, produced by A549 human lung (carcinoma), was accumulated in the culture medium, and then determined with MIP film-coated electrodes. Moreover, by peptide-imprinted electropolymerization, the same authors incorporated poly(triphenylamine rhodanine-3-acetic acid-co-3,4-ethoxylenedioxythiophene)s onto a continuous monolayer of molybdenum disulfide (MoS2) on the indium-tin-oxide (ITO) electrode for MMP-1 electrochemical determination in the A549 cell line culture medium [42].
Many reviews concern MMP biomarkers for IPF [[43], [44], [45]]. Moreover, many possible radiological and peripheral blood molecular biomarkers were identified and summarized elsewhere [46]. However, searching for new tools is still necessary for comprehensive clinical evaluation. The present review article demonstrates the potential diagnostic capability of other important biomarkers that have been documented in preclinical studies.
4. Novel biomarkers of idiopathic pulmonary fibrosis
4.1. Breath biomarkers
Breathomics has become a steadily expanding field focusing on the understanding of the nature of exhaled breath components, namely, carbon dioxide (CO2), molecular oxygen (O2), nitric oxide (NO), and other volatile organic compounds (VOCs), e.g., acetone, isoprene, ammonia, ethanol, and exhaled breath condensate (EBC).
The exhaled breath may be a source of information about the respiratory system. Therefore, it may represent an alternative source of novel biomarkers in IPF. Breath research has been conducted for many years [47], resulting, among others, in successfully transitioning from research to clinical practice use of NO in asthma [[48], [49], [50]]. NO is a free radical. It participates in many physiological and pathophysiological processes. For instance, NO may presumably increase the expression of pro-fibrotic mediators, including transforming growth factor beta-1 (TGF-β1), in lung fibrosis [51]. In patients with IPF, exhaled NO concentrations were higher than in healthy subjects (HSs) [52,53].
In clinical practice, one of the methods for NO determination in breath and then reporting is an air fractional exhaled NO test performed at a flow rate of 50 mL/s (FENO50). NO determined in this way is increased in the respiratory tract and decreased in the alveoli. Moreover, capturing FENO at different flow rates allows for the estimation of the NO concentration at the level of the alveolus (NOalv). Fractionated exhaled NO, determined using chemiluminescence, electrochemical, or laser analyzer, is a surrogate marker of airway inflammation [54]. However, the FENO50 test was inconsistent in IPF, with the NO concentration ranging from 20.8 to 31.5 ppb [52,55]. The upper reported NO limit in healthy adults ranged from 30.3 to 50.6 ppb, depending on age and gender [56].
Although FENO50 is unlikely to be used in IPF, NOalv may have the potential to be a clinical biomarker. High alveolar NO participates in a pathophysiological process in lung fibrosis. The meta-analysis suggested that NOalv is higher in IPF patients than in controls [49]. Average NOalv concentrations in IPF patients were 8.5 (±5.5) ppb, i.e., much higher than in HSs, equal to 4.4 (±2.2) ppb. In a recent study of 433 HSs, the reported upper NOalv limit was 3.88–3.93 ppb, depending on age [57]. An alternative role for NOalv may be disease monitoring, as NOalv concentrations reflect deteriorating lung function [58] and are inversely related to lung function parameters in fibrotic ILD, a phenomenon not encountered with FENO [59,60].
Exhaled breath condensate (EBC) accumulation is an appealing, non-invasive way to collect soluble components from the lower airways in the respiratory system. The correlation between EBC biomarkers and pulmonary inflammation has been widely reported [61,62]. EBC is a phase of condensed water vapor with tiny amounts of non-volatile and water-soluble volatile compounds [38,39]. Following collection, relevant analytes can be determined using portable sensors [63,64] or mass spectrometry [65].
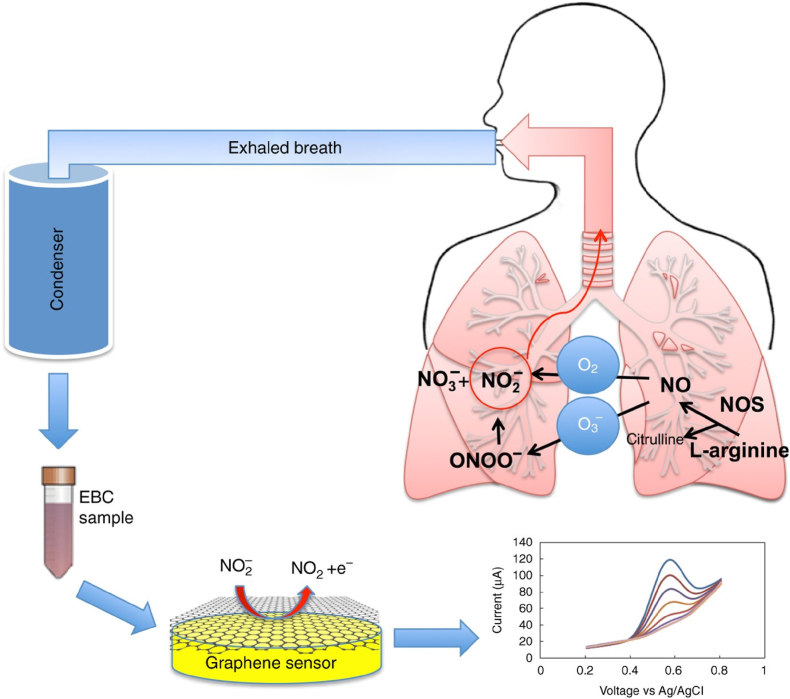
One of the portable nitrite sensors using EBC [Fig. 1] utilizes specific features of reduced graphene oxide [64]. That reveals fast electron transfer in electrolyte solutions, enabling highly sensitive electroanalytical determining of analytes with negligible fouling. This sensor was mounted in an electrochemical cell made of polydimethylsiloxane, which is needed for analyzing small EBC samples. The sensor determined nitrite at an overpotential as low as 0.70 V vs. Ag/AgCl with a 0.21 μA μM−1 cm−2 sensitivity in the 20–100 μM range and 0.10 μA μM−1 cm− 2 in the 100–1000 μM range, a low limit of detection of 0.83 μM in the EBC matrix.
Fig. 1.
The flowchart of collecting an exhaled breath condensate (EBC) sample, then electrochemical detection of the nitrite content. Reproduced from Ref. [64] under the terms of the Creative Commons CC BY license.
The subsequent studies provided information on the effect of storage and other critical analytical parameters, including the choice of the supporting electrolyte and pH on the EBC matrix [63] for determining nitrite using graphene oxide. For that, the nitrite concentration, electrochemically determined in freshly collected EBC samples, was compared with that of an aliquot of the samples frozen at −80 °C for one month. These determinations were compared with chemiluminescence sensing to validate the electrochemical determination method. The electrical properties of the EBC sample matrix were also studied with electrochemical impedance spectroscopy and electrical impedance [63].
Moreover, EBC can be a source of biomarkers implicated in the pathogenesis of IPF [55,66,67]. [Table 1] summarizes biomarkers determined from EBCs, the analytical methods used, and concentrations in IPF patients compared to HSs. Products of oxidative stress can be beneficial, one potential mechanism suggested in the fibrotic process [68]. Among others are hydrogen peroxide (H2O2), a reactive oxygen species, and 8-isoprostane, a prostaglandin-like (PG-like) substance, which were elevated in EBC in patients with IPF [67,69]. In 16 IPF patients, the mean H2O2 concentration was significantly higher than in 15 HSs, i.e., 0.36 vs. 0.16 μM. Similarly, in IPF patients, the mean 8-isoprostane concentration was significantly higher than in HSs, i.e., 74 vs. 33 pg/mL [67].
Table 1.
Biomarkers determined from exhaled breath condensates (EBCs) with the analytical method used and concentration in idiopathic pulmonary fibrosis (IPF) patients compared to healthy subjects (HSs).
| Biomarker | Analytical method | Concentration in IPF patients compared to healthy subjects | Reference |
|---|---|---|---|
| H2O2 | An enzymatic assay using horseradish peroxidase Spectrophotometric by horseradish peroxidase-catalyzed oxidation of tetramethylbenzidine |
Higher Higher |
[67] [70] |
| 8-Isoprostane | Enzyme immunoassay | Higher | [67] |
| Total nitrogen oxides (NOx) | Chemiluminescence NO analyzer | Higher | [70] |
| 3-Nitrosotyrosine | Enzyme immunoassay | Higher | [70] |
| Total proteins | A Quantipro BCA assay | Higher | [70] |
| Carbon monoxide | Chemiluminescence CO analyzer | Higher | [70] |
| 8-IsoPGF2α | Enzyme immunoassay | Higher | [69] |
| Ethane | GC-MS | Higher | [72] |
| Metallic elements | ICP-MS | Higher concentrations of chromium, nickel, and silicon and a lower concentration of cobalt, copper, iron, molybdenum, and selenium | [73] |
| IL-8 | ELISA | Lower | [71] |
Concentrations of many oxidative stress and inflammatory biomarkers, determined in EBC in 20 IPF patients, were higher than in the 20 HSs [70]. That is, the 3-nitrosotyrosine (3-NT) concentration was 2.5 ng/mL in the 0.7–8.9 ng/mL range in the patients, while 0.3 ng/mL in the 0.1–1.1 ng/mL range in the controls, significantly correlating with concentrations of another biomarker, counting 8-isoprostane, total nitrogen oxides (NOx), total proteins, carbon monoxide, and H2O2.
Moreover, EBC total protein concentrations in IPF patients were substantially higher than in controls. A mean EBC 8-isoprostane concentration in IPF patients was also higher than in controls, i.e., 0.2 ng/mL in the 0.1–0.4 range vs. 0.08 ng/mL in the 0.04–0.2 ng/mL range [70].
EBC concentrations of the 8-iso-prostaglandin-F2α (8-isoPGF2α) oxidative stress biomarker were measured in IPF patients and control individuals. Isoprostanes are generated by the peroxidation of unsaturated fatty acids free radicals [69]. The isoprostane 8-isoPGF2α is an isomer of PGF1 generated by the peroxidation of arachidonic acid. In IPF patients, 8-isoPGF2α concentrations were significantly higher than in control subjects, being 24.5 (±15.8) vs. 4.6 (±1.0) pg/mL at the statistical significance level, p < 0.05.
Another study [71] assessed the variation in the cytokine profile in EBC following a six-month treatment with pirfenidone in patients with IPF. The interleukin (IL), namely, IL-6, IL-8, IL-15, TNF-α, and VEGF-A concentrations in EBC, were determined with enzyme-linked immunosorbent assay (ELISA), then compared at baseline and after six months of pirfenidone treatment. At baseline, 29 patients with IPF and 13 controls were evaluated. The cytokine concentrations did not differ between the groups except for the IL-8 concentration, which was lower in patients with IPF than in controls (p = 0.005). After six months of treatment with pirfenidone, EBC analysis did not disclose any changes in the cytokine concentrations.
For patients with IPF and other ILD forms, concentrations of other oxidative stress markers, including ethane, in exhaled breath exceeded those in controls [72].
Another study found differences in the concentration of metallic elements in the EBC in patients with IPF and controls [73]. Metal workers dominate the IPF populace. Hence, one can speculate that metal dust may influence disease development [74]. Apparently, Ni, Cr, and Si concentrations in patients with IPF were higher than in controls, whereas Co, Fe, Cu, Se, and Mo concentrations were increased in controls.
Volatile organic compounds (VOCs) are another promising source of breath biomarkers in IPF. Advantageously, breath VOCs can escape from the blood or interstitial tissue unchanged before being detected in exhaled air. Therefore, they contain metabolites highly indicative of fibrosis.
VOCs in breath samples were determined in IPF patients and HSs [75,76]. One study used multi-capillary column ion mobility spectrometry to determine VOCs in 40 IPF patients and 55 HSs [76]. This method recognized 85 VOC peaks in IPF patients and HSs, with significant differences in five peaks ascribed to acetoin, p-cymene, ethylbenzene, isoprene, and an unknown compound. The p-cymene concentration was lower in IPF patients, while concentrations of other VOCs were higher in these patients. This study highlighted the possibility of the breathomic discovery of new biomarkers useful for diagnostics and prognostics. Dedicated diagnostic tools, like electronic noses (e-noses), which are more accessible in clinical assays, could be developed once specific biomarkers or patterns of biomarkers ("breathprints") are identified.
E-noses operate using several cross-reactive gas sensors that react to multiple VOC mixture compounds. Hence, the e-nose technology has been proposed as a diagnostic tool for clinical and inflammatory phenotyping and to predict response to therapy [77,78]. In ILD, studies on breathomics are limited [79]. One study in sarcoidosis showed that the breathprint in patients with untreated pulmonary sarcoidosis could be distinguished from HSs, but treated sarcoidosis patients could not be discriminated from HSs [79]. Another recently published study evaluated the ability of the e-nose to identify various ILD subgroups and to compare ILD with HSs and patients with chronic obstructive pulmonary disease (COPD) [80]. Moreover, this study showed an adequate distinction between patients with ILD and those with HSs and COPD patients.
Hopefully, breath biomarker discovery studies will broaden our understanding of IPF pathophysiology and improve the accuracy of diagnosis. Moreover, novel biomarkers may be able to distinguish specific required phenotypes if a clinically relevant breath biomarker of IPF emerges. Further breathomics studies are guaranteed. Longitudinal studies would be beneficial. These would considerably increase the data pool and allow for assessing individual reproducibility. That also provides the opportunity to evaluate better the influence of initiating treatment on biomarker levels.
4.2. Sputum biomarkers
Induced sputum, which attracted clinical interest in airway diseases, has been proposed as a non-invasive alternative in diagnosing IPF [81,82]. Diagnostic procedures, such as ELISA, appear promising, but in the case of sputum-scarce individuals, they can have some limitations in clinical utility. Sputum samples often contain various substances, including mucus, enzymes, and cellular debris, that can interfere with the ELISA. Moreover, sputum samples may contain components that cross-react with the antibodies used in the ELISA, resulting in nonspecific binding and false-positive results. Furthermore, sputum samples can vary in quality, consistency, and cellularity. For example, sputum may come from various lung lobes, which can affect the performance of the ELISA. Poor-quality samples may yield unreliable results or require additional processing steps to improve accuracy. Despite these drawbacks, ELISA remains a valuable tool for evaluating specific antigens or antibodies in sputum samples, particularly in the diagnosis of respiratory infections and diseases, including tuberculosis and pneumonia. However, it is crucial to consider these limitations and validate the results using alternative methods whenever possible.
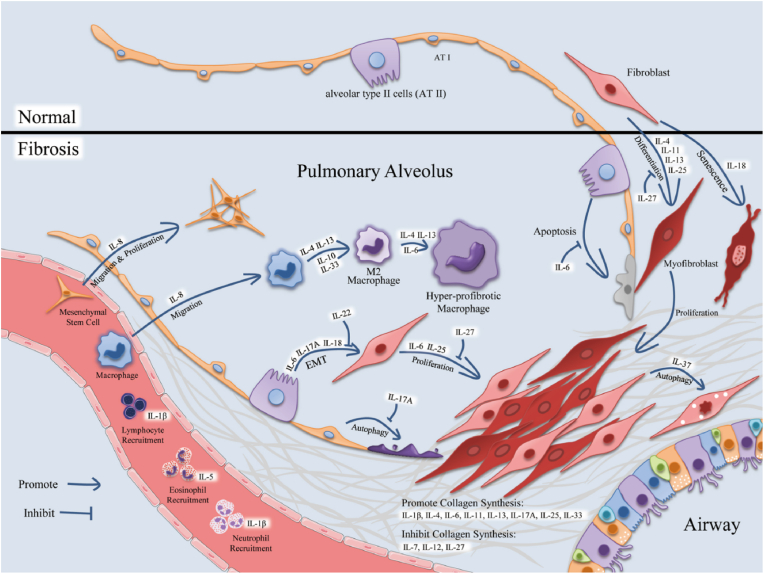
The ELISA multiplex was used for determining total sputum cell count as well as TGF-β, IGF-1, IGF-2, IGFBP-1, IGFBP-2, IGFBP-3, IL-8, IL-13, MMP-7, MMP-9, YKL-40, TNF-α, and KL-6 in the sputum supernatant of 15 patients with IPF, 32 patients with COPD, and 30 HSs [83]. ILs' functions are complex and diverse. Generally, they regulate the immune system by participating in innate immune responses, promoting the proliferation and differentiation of immune cells, and explicitly recruiting inflammatory cells. ILs can promote inflammation by regulating immune cell aggregation, affecting pulmonary fibrosis. Therefore, the level and function of ILs are altered in animal models of pulmonary fibrosis [Fig. 2] [84].
Fig. 2.
The flowchart of interleukins affection of the morphology and functions of various cells in idiopathic pulmonary fibrosis (IPF). Reproduced from Ref. [84] under the terms of the Creative Commons CC BY license.
Transforming growth factor beta (TGF-β), a critical factor of fibrosis, in the sputum supernatant from IPF patients was higher than in HSs [83]. Moreover, TGF-β gene expression was significantly raised in IPF patients compared to COPD patients. Furthermore, the research results confirmed the importance of MMP-7, an MMP specifically raised at gene and protein levels in sputum from IPF patients compared to COPD patients and HSs. While MMP-9 was increased in both IPF and COPD patients, the increased MMP-7 amount was remarkably limited to IPF patients compared to HSs. In addition, a Krebs von den Lungen 6 (KL-6) prognostic biomarker in sputum supernatant from IPF patients was significantly and specifically increased compared to HSs. The KL-6 concentration in sputum changes, related to the decrease in lung volume, may suggest that sputum is a medium more appropriate than serum to assess the epithelial and alveolar damage occurring in IPF patients. Moreover, serum insulin-like growth factor-binding protein-2 (IGFBP-2) was elevated in patients with IPF [85], particularly in sputum. Gene expression of IGFBP-2 in patients with IPF increased compared to HSs and patients with COPD.
Gene expression of interleukin-6 (IL-6) was higher than in both patients with HSs and COPD, though the protein concentration remained comparable to that measured in HSs [83]. However, the interleukin-8 (IL-8) concentration in IPF patients was significantly higher than in HSs both at the gene and protein levels, while, as for IL-6, the increase in patients with COPD was only seen at the protein level.
The exosome microRNA (miRNA) was determined in the sputum of IPF patients [86]. With a miRNA quantitative polymerase chain reaction array, there was a pronounced dysregulation of sputum exosomal miRNA levels between IPF patients and HSs; it was identified as a unique signature of three miRNAs. Interestingly, a correlation between one of the miRNA levels and the diffusing capacity of the lungs for carbon monoxide/alveolar volume was negative. These findings may thus lead to a deeper understanding of the identified sputum exosomal miRNAs' roles in IPF pathogenesis and thus open new routes for therapeutic approaches.
The total glutathione (GSH) concentration in the induced sputum of 16 non-smoking IPF patients was ca. four-fold lower than in 15 HSs, i.e., mean GSH was 1.4 (±0.34) vs. 5.8 (±0.98) μM [87]. Moreover, there was an inverse association between the GSH sputum concentration and disease severity, while a positive correlation between GSH and vital capacity. Apparently, the GSH determination in sputum might be supportive in assessing and monitoring IPF patients.
4.3. Extracellular matrix neoepitope markers
A hallmark of IPF is the accumulation and degradation of the lung's excess extracellular matrix (ECM). The ECM is a 3-D network of extracellular macromolecules and minerals, including collagen, enzymes, glycoproteins, and hydroxyapatite, providing structural and biochemical support to surrounding cells. Most extracellular collagen is degraded by a subset of MMPs, vis., MMP-1, MMP-3, MMP-7, MMP-8, MMP-13, MMP-14, MMP-16, and MMP-18 [88]. Extracellular collagen fragments generated by these MMPs are further digested by MMP-2 and MMP-9 [88]. In patients with IPF, rates of ECM protein synthesis significantly increased, and MMP-1, MMP-3, and MMP-7 have even been identified as putative prognostic serum biomarkers of the disease [89,90]. Each ECM protein cleavage by specific MMPs generates a unique neoepitope, i.e., an epitope the immune system has not encountered earlier. These neoepitopes are diagnostic and prognostic disease markers that are more accurate than their origin proteins [91].
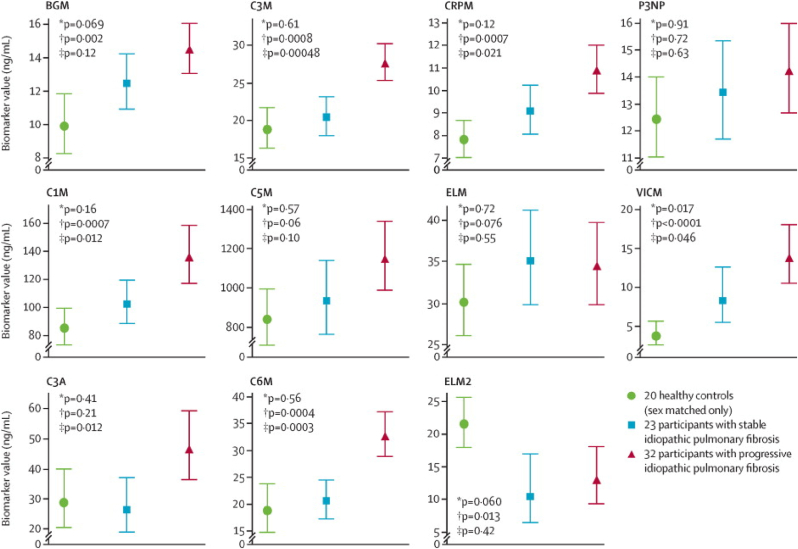
In the prospective observation of fibrosis in the lung clinical endpoints (PROFILE) study, serum samples of participants with IPF or idiopathic nonspecific interstitial pneumonia diagnosed were analyzed for a panel of new matrix metalloprotease (MMP)-degraded ECM proteins by the ELISA-based neoepitope assay [92]. PROFILE is a continuing multicenter, prospective, experimental cohort study of incident cases (within six months of presentation) of idiopathic fibrotic lung disease. Concentrations of 11 neoepitopes were measured in the discovery cohort of 55 IPF participants and a group of 20 healthy sex-matched (80% men) HSs with a mean age of 56.6 years [Fig. 3]. At baseline, levels of seven out of the matrix neoepitopes (BGM, C1M, C3M, C6M, CRPM, ELM2, and VICM) differed significantly between IPF patients and HSs. Concentrations of six neoepitopes (C1M, C3A, C3M, C6M, CRPM, and VICM) were meaningfully higher at baseline in individuals with progressive disease than in those with stable disease [Fig. 3]. In one particular case, namely with C3A, the biomarker value in stable IPF disease was even lower than in healthy subjects. That indicates that the C3A biomarker can serve as an analytical tool only for progressive disease.
Fig. 3.
Baseline comparison of neoepitope concentrations in the discovery cohort in healthy controls and idiopathic pulmonary fibrosis (IPF) suffering participants with stable or progressive disease [92]. Biomarkers: BGM: biglycan; C3M: MMP-9 mediated collagen type III degradation product; CRPM: C-reactive protein metabolite; P3NP: pro-collagen type III N-terminal peptide; C1M: collagen type-I degradation product; C5M:collagen type V degradation product; ELM: elastin degradation product; VICM: citrullinated and MMP-degraded vimentin biomarker; C3A: collagen type III degradation product; C6M: collagen type VI degradation product; ELM2: MMP-9,12 mediated elastin degradation product [92]. Reprinted with permission from Elsevier, copyright 2015 [92].
After analysis of the discovery cohort, eight neoepitopes, vis., BGM, C1M, C3A, C3M, C5M, C6M, CRPM, and VICM, were determined in a validation cohort of 134 IPF patients and 50 age-matched and sex-matched HSs (mean age 63.6 years, 80% men). Baseline C1M, C3M, C6M, and CRPM concentrations in participants with IPF appeared meaningly higher than in HSs (figure not shown). This study represents the first proof of concept that serial observation of ECM remodeling and the change in ECM characteristics may yield crucial prognostic insights for patients. Moreover, this analysis showed proof-of-principle that serial longitudinal determination of serum biomarkers provides essential information over and above single point-in-time determinations and could address the crucial unmet need in the field of IPF research for predictive biomarkers of molecular phenotype or therapeutic response demonstrated in subjects recruited to the study.
In another study, C3M, C6M, PRO-C3, and PRO-C6 were measured in 185 patients of the pulmonary fibrosis bio-marker (PFBIO) cohort with newly diagnosed IPF [93]. Biomarkers of the ECM remodeling were assessed in serum by specific competitive ELISA tests using monoclonal antibodies for neoepitopes. The study showed that neoepitope biomarkers of ECM remodeling assessed at the time of IPF diagnosis were associated with several indices of disease severity, short-term progression, and long-term mortality. High baseline levels of C3M, C6M, PRO-C3, and PRO-C6 were associated with more advanced disease at the time of diagnosis. Baseline levels of C6M and PRO-C3 were also associated with mortality over three years of follow-up.
Moreover, PRO-C3, C1M, and C3M were evaluated in another PFBIO cohort of 178 newly diagnosed IPF patients [94]. Increased baseline levels of type I and III collagen turnover biomarkers were associated with a higher risk of disease progression within 12 months compared to patients with a low baseline type I and III collagen turnover. Patients with progressive disease had serum levels of C1M and PRO-C3 higher than those with stable disease over one year. Furthermore, the authors demonstrated that longitudinal levels of type I and III collagen turnover could distinguish patients with progressive disease in a real-world cohort.
In subsequent research, a longitudinal study on the correlation between type VI collagen turnover and disease progression in IPF in a real-world cohort of 178 newly diagnosed IPF patients was presented [95]. This research assessed two serological biomarkers, namely, PRO-C6 and C6M, over time to investigate their relationship with the progression of IPF, providing insights into potential biomarkers for monitoring disease advancement. Blood samples and clinical data were collected from baseline, six-month, and 12-month visits. The biomarkers were measured by competitive ELISA using monoclonal antibodies. It appeared that patients with IPF exhibited significantly higher serum levels of PRO-C6 compared to those with stable disease over 12 months. Additionally, there was a tendency for higher C6M levels in patients with progressive disease compared to stable patients over the same time.
The subsequent study evaluated biomarkers of collagen synthesis for their effectiveness in IPF prediction in a separate cohort of people recruited into the PROFILE investigation [96]. Moreover, it compared these biomarkers to their collagen-equivalent degradation neoepitopes, allowing evaluation of the connection of synthesis to degradation during IPF development. The cohort of individuals recruited for this study represented a sub-group of 145 participants, predominantly (81%) men with a mean age of 71.7 years. None of them was tested in the previous PROFILE study on neoepitopes [92,96], and all had at least one year of follow-up when neoepitope analyses were performed.
For comparison, serum samples from patients were analyzed with ELISAs for the formation markers of pro-collagen type 1 N-terminal propeptide (P1NP), N-terminal propeptide of collagen type IIIα1 (PRO-C3), and C-terminal (endotrophin) of collagen type VIα3 (PRO-C6) [96]. Most likely, fibrogenesis in IPF results from an inequity of collagen formation and degradation. Therefore, paired markers of degradation for the same collagens were also determined, namely, type 1 (C1M), type 3 (C3M), and type 6 (C6M) and, for comparison with former results [7], the matrix molecule biglycan (BGM) and C reactive protein metabolite (CRPM).
At baseline, concentrations of PRO-C3 and PRO-C6, but not P1NP, were significantly elevated in patients with IPF compared with HSs. Collagen type 3, fibrillar collagen, is a crucial lung interstitial extracellular matrix component. Activated fibroblasts synthesize this collagen [97]. The results agreed with those of former investigations. They revealed that collagen type-3 formation is increased in patients with IPF and that concentrations of type 3 pro-collagen peptide in serum [18] and lavage fluid were high [9,22]. In the case of collagen type 3, the synthesis neoepitope (PRO-C3) was a more useful biomarker of disease development than the paired degradation biomarker, C3M. Moreover, variations in collagen type 6 indicated the disease development in IPF patients, presumably being a significant factor causing fibrosis. Collagen type 6 filaments are an integral part of the basement membrane and interstitial matrix of the lung interface. These filaments form a significant network for immobilizing collagen fibers, basement membranes, and cells. The outcomes supported this collagen role in fibrosis development. C1M, C3M, C6M, and CRPM neoepitope concentrations in the serum of individuals with IPF were significantly elevated compared with HSs at baseline. Moreover, BGM, C1M, C3M, and C6M concentrations differed between individuals with stable and progressive IPF, but these differences only became significant after six months. Interestingly, the C1M and C6M concentrations were markedly higher from 1 month onward [96].
Another study analyzed a serological marker of the N-terminal neoepitope generated by lysyl oxidase-like 2 (LOXL2) [95]. LOXL2 is associated with an unfavorable prognosis in cancer and IPF. Researchers developed an ELISA determining the LOXL2 neoepitope produced through the release of the signal peptide during LOXL2 maturation [98]. LOXL2 was determined in the serum of patients with breast, colorectal, lung, ovarian, pancreatic, prostate cancer, melanoma, and IPF, as well as HSs (n = 16). LOXL2 concentrations were markedly (p < 0.001–0.05) higher than in controls, in serum from patients with breast, colorectal, lung, ovarian, and pancreatic cancer (mean range of 49–84 ng/mL) but not in prostate cancer (mean of 36 ng/mL) and patients with malignant melanoma (41 ng/mL). Moreover, this serum concentration in IPF patients was higher than in HSs (mean of 76.5 vs. 46.8 ng/mL, p > 0.001).
4.4. Fibroblast activation protein-specific PET/CT imaging
Fibroblasts are the primary effector cells causing lung fibrogenesis in IPF patients [7]. Upon injury, fibroblast activation leads to their proliferation, migration, and remodeling of the lung extracellular matrix, resulting in progressive functional deterioration and, finally, lung damage [8]. Fibroblast activation protein-α (FAP) is a serine protease selectively expressed on activated stromal fibroblasts in the course of tissue remodeling; it is connected to pulmonary fibrosis [9,10]. Because in healthy tissues, FAP expression is relatively low, this protease has become an attractive molecular indicator for various disease diagnosis and treatment, including wound healing, epithelial cancers, and inflammatory condition [12]. Radiolabeled quinoline-based small-molecule fibroblast activation protein inhibitors (FAPIs) were used as positron emission tomography (PET) radiotracers to noninvasively and quantitatively evaluate FAP expression [11,13,17]. Importantly, murine and human fibroblast activation proteins share 89% sequence identity in preclinical studies, and the catalytic domain is maintained [15,16]. FAPI derivates strongly attract human and murine FAP, although murine affinity to FAP is lower than to human FAP [17]. FAPI PET studies have so far been focused on detecting cancer, considering that FAP is expressed in 90% of all epithelial cancers [18,20]. In comparison, the potential of FAPI PET for evaluating non-oncologic disease processes, including pulmonary fibrosis, remains relatively underexplored. In summary, the usefulness of the FAPI PET in assessing non-oncologic disease processes, including pulmonary fibrosis, is still poorly understood.
In one study, the possibility was increased of radiolabeled FAPI-46 with gallium-68 (68Ga-FAPI-46) for non-invasive diagnosing of pulmonary fibrosis activity in a well-established murine model of the disease [99]. Because fibroblast activation and FAP expression precede fibrogenesis [9,22], 68Ga-FAPI-46 appeared exceptionally suitable for detecting lung fibrosis and thoughtfully monitoring its development. 68Ga-FAPI-46 PET helped reveal lung damage in a model of bleomycin pulmonary fibrosis and detect increased fibrogenesis in a model of pulmonary fibrosis. Dynamic PET disclosed lung uptake of 68Ga-FAPI-46 in bleomycin-treated mice higher than saline controls, even a week after the damage when fibrosis, i.e., collagen accumulation, levels were relatively low. Notably, the 68Ga-FAPI-46 uptake in the lung of diseased animals from 7 to 14 days increased, in accord with the elevated FAP expression and extracellular matrix deposition found by histology in the pulmonary fibrosis model at 14 days [47].
Another analysis aimed to evaluate the imaging features of static and (or) dynamic 68Ga-FAPI PET/CT in many types of fibrotic ILD (fILD) and to demonstrate FAP expression of fILD changes. For that, FAP immunohistochemistry of human fILD biopsy samples and lung sections of genetically modified mice with IPF was accomplished [100].
A group of 15 patients, 56–80 years old (averaging 71.2), with various fILD subtypes, were examined with 68Ga-FAPI PET/CT. It was chosen from 1135 patients with suspected lung cancer examined between July 2018 and August 2019. As many as 1104 (97.3%) of them were examined by 2-deoxy-2-[fluorine-18]fluoro-d-glucose (18F-FDG) PET/CT and 31 (2.7%) by 68Ga-FAPI PET/CT, counting 15 patients with fILD examined retrospectively. Clinical examinations engaging 68Ga-FAPI PET/CT imaging in all these patients resulted in suspecting LC.
68Ga-FAPI PET/CT was applied to detect fibrotic areas and LC variations in fILD patients. The importance of 68Ga-FAPI PET/CT in managing fILD, especially as a conceivable predictor and an indicator of response to therapy, is still to be assessed. 68Ga-FAPI PET does not reveal enhanced glucose metabolism. Instead, it visualizes reactive fibroblasts (a significant player in fibrosis). Therefore, it may be more suitable than 18F-FDG PET for imaging fibrotic activity and evaluating therapy response. That is because 18F-FDG PET describes only the inflammatory component. A recent preliminary investigation in 21 patients with the fILD subtype systemic sclerosis-associated ILD demonstrated that tracer accumulation in 68Ga-FAPI PET/CT was related to disease development independently of established predictors of progression. Moreover, it showed that 68Ga-FAPI uptake decreased after antifibrosis treatment.
4.5. Fluorescence imaging of oxidative stress biomarkers
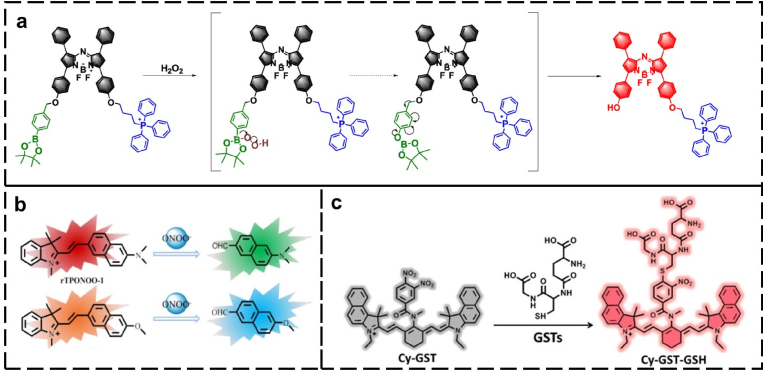
Fluorescence imaging is based on detecting electromagnetic radiation in the ultraviolet–visible-(near infrared) (UV–vis–NIR) range emitted by fluorophores [101]. Because of the advantages of high sensitivity, tunable selectivity, safety, and noninvasiveness, fluorescence imaging has found an application in visualizing vascular systems [102], lymphatic systems [103], various tumor tissues [104], and real-time blood flow [105]. Moreover, fluorescence imaging reveals enormous possibilities in facilitating the development of accurate therapeutic strategies for diseases, including imaging-guided cell therapy, drug delivery, and (or) release monitoring, and photodynamic therapy [[106], [107], [108], [109], [110]]. Many excellent activatable fluorescent probes [Fig. 4] have been utilized for evaluating IPF by detecting the fluctuation of biologically relevant markers, including NO, H2O2, peroxynitrate (ONOO−), glutathione S-transferase (GST), glutathione (GSH), and cyclooxygenase-2 (COX-2).
Fig. 4.
Fluorescent probes used to evaluate IPF by detecting the fluctuation of biologically relevant markers (a) H2O2 (Adapted with permission from Ref. [112]. Copyright 2021 American Chemical Society), (b) peroxynitrate (ONOO−) (Adapted with permission from Ref. [113]. Copyright 2019 American Chemical Society), and (c) glutathione S-transferase (GST) (Adapted with permission from Ref. [114]. Copyright 2019 American Chemical Society).
Mitochondria are the primary source of cellular H2O2 [111]. Mitochondrial-targeted fluorescent probes are tools for possible strategies to deeply understand the disease rate, progression mechanism, and early disease diagnosis and treatment. A straightforward and effective method involves introducing triphenylphosphonium mitochondrial-targeted groups into the fluorescent scaffold [112]. Mitochondrial H2O2 was selectively determined by combining the azo-NIR-fluorescent dye, 4-(bromomethyl) phenylboronic acid pinacol ester (an H2O2 response unit) with triphenylphosphonium cation (mitochondrial-targeted group) in one molecular probe. It showed slight fluorescence because of photoinduced electron transfer between azo-fluorescent dye and the H2O2 response unit. In the H2O2 presence, the NIR fluorescence was turned on thanks to the inhibition of photoinduced electron transfer. Next, variations in H2O2 concentration during fibrosis were successfully visualized by in vitro and in vivo fluorescence imaging, indicating the oxidative stress levels in fibroblasts.
Because the levels of oxidative stress in mitochondria are relevant to pulmonary fibrosis, the levels of reactive oxygen species, e.g., ONOO−, are inversely proportional to pulmonary function in IPF; thus, they may predict disease severity [113,115]. Nonetheless, the potential biological roles of ONOO− for the development of pulmonary fibrosis have not yet been fully understood. A ratiometric two-photon NIR-fluorescent probe, rTPONOO-1, with mitochondria-targeting abilities for sensitive and selective ONOO− detection with fast response, was devised [113]. A NIR-fluorescent probe was prepared via acedan (two-photon fluorescent dye) direct condensation with an indolium derivative. Its C C bond could selectively be cleaved by ONOO−, causing a blue emission wavelength shift. The resolution of two-photon imaging in frozen lung sections was high at penetration depths not exceeding 110 μm. Furthermore, the fluorescence ratio changes of rTPONOO-1 in bleomycin-treated mice linearly depended on the bleomycin concentration, allowing for predicting pulmonary fibrosis progression early.
GST is essential in promoting the formation of pulmonary fibrosis. It contributes to the defense of biomacromolecules from oxidative stress. To this end, it repairs the damage to the membrane phospholipids and inhibits the microsome peroxidation induction [116]. A cyanine-based NIR-fluorescent probe for detecting GST (Cy-GST) concentration fluctuations in cell and mouse models during the progression of pulmonary fibrosis was synthesized [114]. With the Cy-GST help, results demonstrated that the level of GSTs in the pulmonary fibrosis cell models is higher than in normal cells.
Gamma-glutamyl transpeptidase (GGT), a membrane-relevant enzyme, is crucial in many pathological and physiological processes regulating redox metabolism, cancers, diabetes, and drug resistance [117,118]. The abnormal state of oxidative stress is noteworthy, as it could increase the GGT concentration. GGT can balance the GSH metabolism and homeostasis in the cell, thus regulating oxidative stress created under pathological conditions. Therefore, researchers explored the relationship between GGT and IPF [Fig. 5] [119].
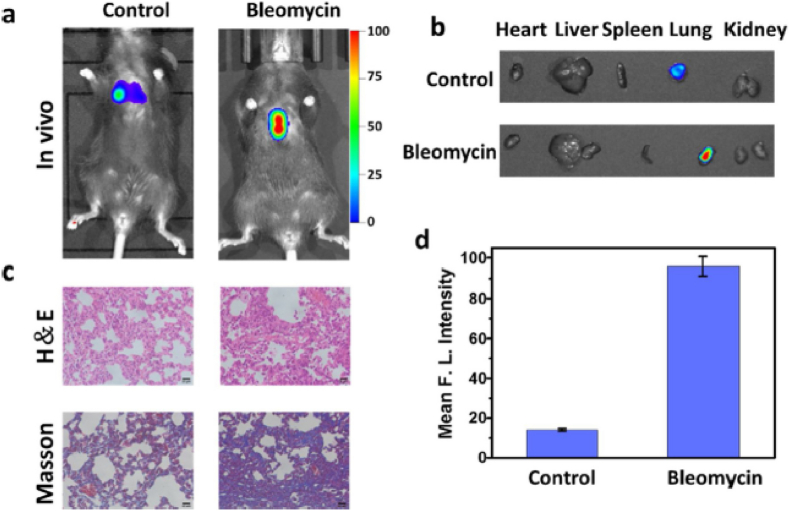
Fig. 5.
(a) Fluorescent images of GGT utilizing Cy-GGT of normal mice as the control group and mice with 28-day bleomycin stimulation as the bleomycin group [119]. (b) Pictures of isolated organs, vis., heart, kidney, liver, lung, and spleen [119]. (c) Routine (H&E) and Masson pathology of each group [119]. (d) Mean intensities of fluorescence images shown in panel (a) [119]. Reprinted with permission from Elsevier, copyright 2020 [119].
They devised a Cy-GGT NIR-fluorescent probe with 780-nm emission to disclose the GGT behavior in cell models and in vivo. By tracking the GGT level in living cell models and in vivo, the researchers evaluated diverse Cy-GGT roles in living systems in detail. Furthermore, Cy-GGT was applied to unravel the GGT enhancement in IPF cell and mice models, revealing the close relationship between GGT and IPF. More significantly, convincing and intuitive evidence was provided for changes in the GGT concentration during the progression of pulmonary fibrosis. That may open a new route for the precise clinical diagnosis of IPF. In the oxidative stress and pulmonary fibrosis cell models, the GGT concentration was higher than in normal cells. The researchers validated the GGT elevation in the mice models of pulmonary fibrosis to confirm further the underlying relation between GGT concentration and IPF extent.
The NO concentration upregulates in the alveoli of IPF patients and correlates with the severity of the disease. Accordingly, a dicyanomethylene-4H-chromene (DCM)-based fluorescent probe for NO (DCM−NO) was devised to detect IPF by determining variations in the NO concentration [120]. The DCM−NO probe is essentially nonfluorescent because of the molecular rotor rotation of the 4-(4-nitrophenyl)thiosemicarbazide moiety. After reacting with NO, the 4-(4-nitrophenyl)thiosemicarbazide sensing group is degraded to form the red-emitting DCM−NH2 fluorophore. The response time is short, and a Stokes shift is substantial for the DCM-NO probe. The probe's ability to detect IPF through monitoring the NO concentration changes was assessed. When DCM−NO was applied to detect the increased NO levels in pulmonary fibrosis cells, tissues, and mice models, its disease-to-normal contrast imaging results were excellent. Remarkably, the DCM−NO probe may be a powerful tool for IPF diagnosis.
Cyclooxygenase-2 (COX-2) is an inducible enzyme. It is closely related to pulmonary fibrosis [121,122]. A Cy-COX NIR-fluorescent probe is prepared by indomethacin (response group) and cyanine (NIR-fluorescent dye) covalently linking with hexanediamine. It successfully evaluated the organisms' level and image COX-2 [109]. An indomethacin molecule can occupy the hydrophobic molecular cavity of the COX-2 homodimer. Then, Cy-COX assumes an unfolded conformation to suppress the PET effect with turn-on fluorescence. The imaging of the pulmonary fibrosis mouse model showed that COX-2 was involved in alveolar cell inflammation and early pulmonary fibrosis through high expression.
4.6. Complete blood count
Complete blood count (CBC) can significantly affect the prediction of patients with various chronic lung diseases [[123], [124], [125]]. Recently, three significant pieces of research on ∼10,000 IPF patients and scleroderma-associated interstitial lung disease patients have disclosed elevated peripheral blood monocyte count as a biomarker of disease development and mortality [125,126]. Accordingly, another CBC parameter, vis., red cell distribution width (RDW), has been related to worse clinical outcomes in several chronic lung diseases, including IPF and chronic obstructive pulmonary disease [124,127]. Elevated RDW presumably symptomizes early hypoxemia [124,128]. The predictive role of CBC parameters, including monocyte count and RDW, was evaluated in two cohorts (derivation and validation) of individuals with IPF in a real-life clinical environment [124].
Overall, 489 patients (derivation cohort: N = 300, validation cohort: N = 189) were included in the analysis. The average age was 74 (73–75) years for the derivation cohort and 74 (72–75) years for the validation cohort [124]. IPF patients were predominantly men in the derivation (83.3%, N = 250) and validation cohort (78.8%, N = 149). Treatment-naive patients with IPF and available CBC were first enrolled for baseline analysis, then after 6 and 12 months of antifibrotic treatments.
Median values of CBC parameters were recorded. Patients were grouped into subgroups according to the median value of each CBC parameter in the derivation cohort (high and low). The Mann-Whitney test was executed to assess differences in a forced vital capacity percent predicted (FVC%pred) and diffusion lung capacity for CO percent predicted (DLCO%pred) between subgroups of patients split by the median value of CBC parameters.
The median monocyte count was 0.60 (0.57–0.62) and 0.52 (0.50–0.58) K/μL for the derivation and the validation cohort, respectively. The median RDW for the derivation cohort was 14.1% (13.9–14.3)%, and it was 13.7% (13.6–13.8)% for the validation cohort. Finally, median FVC%pred was 77.0% (75.0–79.8)% and 76.2% (71.7–80.8)%, while median DLCO%pred was 51.0% (47.1–53.8)% and 41.9% (40.3–44.9)% for the derivation and validation cohort, respectively. Evidently, patients in the group of high monocytes and RDW suffered from more progressive disease at baseline. That was because the median FVC%pred [75.0%, (71.3–76.7)%] and DLCO%pred [47.5%, (44.3–52.3)%] of the high monocyte group (≥0.60 K/μL) was meaningfully lower than that of the low monocyte group (<0.60 K/μL). Importantly, baseline median DLCO%pred [37.8%, (35.5–41.1)%] for patients in the high monocyte group of the validation cohort was significantly lower than that for patients in the low monocyte group [45.5%, (41.9–49.4)%, (p < 0.001)]. However, monocyte count and RDW were not associated with disease progression or a one-year antifibrotic treatment.
In the derivation cohort, individuals in the group with high monocyte count (≥0.60 K/μL) experienced a risk of all-cause mortality higher than that of the group with low monocyte count (<0.60 K/μL). Furthermore, the pooled analysis of the study population showed that the risk of death from any cause in patients with a baseline monocyte count ≥0.95 K/μl was higher than in patients with a baseline monocyte count <0.95 Κ/μl.
These real-life studies revealed that peripheral blood monocyte counts predicted all-cause mortality in the baseline cohort and a pooled group of highly characterized IPF patients [124]. Apparently, patients with elevated monocyte counts and RDW had more advanced disease at initial evaluation than those with low levels. Functional decline evaluated no high monocyte count or RDW relation to one-year disease development. Also, antifibrotic treatment did not affect monocyte count or RDW over a one-year follow-up.
5. Conclusions
No simple, rapid, and effective diagnostic tools for idiopathic pulmonary fibrosis (IPF) are available if high-resolution computed tomography (HRCT) or surgical lung biopsy is impossible. Identifying IPF-specific biomarkers would greatly help in performing the early IPF diagnosis. The need for dependable biomarkers is growing. The validation of valuable and precise predictive biomarkers could decrease diagnosis uncertainty and the use of invasive diagnostic procedures. Predictive and therapeutic biomarkers could help classify patients based on severity and disease progression for personalizing management. Furthermore, reliable biomarkers capable of predicting acute exacerbation could implement prevention actions and modify the prediction of those events. Moreover, new biomarkers may be able to distinguish some phenotypes existing within IPF. Several compounds are potentially valued as biomarkers in IPF. However, progress is undoubtedly required if a clinically relevant biomarker is to emerge in IPF. Further research is envisioned. Longitudinal investigations would be beneficial because they considerably increase the data pool and allow for individual reproducibility assessment. Moreover, they would enable better evaluation of the effect of early treatment on biomarker levels, an issue that may cofound cross-sectional studies. Pooling resources and collaborating within larger consortia could offer a valuable opportunity to compare markers across datasets and explore their combined potential in forming composite scores.
Declaration of competing interest
The authors declare no competing financial or personal interests that can influence the work presented in this paper.
Acknowledgment
The National Center for Research and Development (NCBR) of Poland financially supported the present research through Grant No. SensIPF PL-TW/VI/2/2019 to WK.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Wijsenbeek M, Kreuter M, Olson A, Fischer A, Bendstrup E, Wells CD, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin. 2019;35(11):2015–2024. doi: 10.1080/03007995.2019.1647040. [DOI] [PubMed] [Google Scholar]
- 2.Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022;400(10354):769–786. doi: 10.1016/S0140-6736(22)01052-2. [DOI] [PubMed] [Google Scholar]
- 3.Cerro Chiang G, Parimon T. Understanding interstitial lung diseases associated with connective tissue disease (CTD-ILD): genetics, cellular pathophysiology, and biologic Drivers. Int J Mol Sci. 2023;24(3):2405. doi: 10.3390/ijms24032405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeganathan N, Sathananthan M. The prevalence and burden of interstitial lung diseases in the USA. ERJ Open Res. 2022;8(1):630–2021. doi: 10.1183/23120541.00630-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Society A.T. Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000;161(2):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 7.Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic pulmonary fibrosis (IPF): an Overview. J Clin Med. 2018;7(8):201. doi: 10.3390/jcm7080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruwys S, Hein P, Humphries B, Black D. Drug discovery and development in idiopathic pulmonary fibrosis: challenges and opportunities. Drug Discov Today. 2020;25(12):2277–2283. doi: 10.1016/j.drudis.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Martinez FJ, Chisholm A, Collard HR, Flaherty KR, Myers J, Raghu G, et al. The diagnosis of idiopathic pulmonary fibrosis: current and future approaches. Lancet Respir Med. 2017;5(1):61–71. doi: 10.1016/S2213-2600(16)30325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795–806. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 11.Gribbin J, Hubbard RB, Le Jeune I, Smith CJP, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins RB, Burke N, Fell C, Dion G, Kolb M. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J. 2016;48(1):187–195. doi: 10.1183/13993003.01504-2015. [DOI] [PubMed] [Google Scholar]
- 13.Navaratnam V, Fleming KM, West J, Smith CJP, Jenkins RG, Fogarty A, et al. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax. 2011;66(6):462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2(7) doi: 10.1016/S2213-2600(14)70101-8. 566–72. [DOI] [PubMed] [Google Scholar]
- 15.Strongman H, Kausar I, Maher TM. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther. 2018;35(5):724–736. doi: 10.1007/s12325-018-0693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamantopoulos A, Wright E, Vlahopoulou K, Cornic L, Schoof N, Maher TM. The burden of Illness of idiopathic pulmonary fibrosis: a comprehensive evidence review. Pharmacoeconomics. 2018;36(7):779–807. doi: 10.1007/s40273-018-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaunisto J, Kelloniemi K, Sutinen E, Hodgson U, Piilonen A, Kaarteenaho R, et al. Re-evaluation of diagnostic parameters is crucial for obtaining accurate data on idiopathic pulmonary fibrosis. BMC Pulm Med. 2015;15(1):92. doi: 10.1186/s12890-015-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghu G, Lynch D, Godwin JD, Webb R, Colby TV, Leslie KO, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med. 2014;2(4):277–284. doi: 10.1016/S2213-2600(14)70011-6. [DOI] [PubMed] [Google Scholar]
- 20.Marshall DC, Salciccioli JD, Shea BS, Akuthota P. Trends in mortality from idiopathic pulmonary fibrosis in the European Union: an observational study of the WHO mortality database from 2001–2013. Eur Respir J. 2018;51(1) doi: 10.1183/13993003.01603-2017. [DOI] [PubMed] [Google Scholar]
- 21.Costabel U, Albera C, Lancaster LH, Lin CY, Hormel P, Hulter HN, et al. An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (RECAP) Respiration. 2017;94(5):408–415. doi: 10.1159/000479976. [DOI] [PubMed] [Google Scholar]
- 22.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 23.Behr J, Günther A, Bonella F, Dinkel J, Fink L, Geiser T, et al. S2K guideline for diagnosis of idiopathic pulmonary fibrosis. Respiration. 2021;100(3):238–271. doi: 10.1159/000512315. [DOI] [PubMed] [Google Scholar]
- 24.Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL, et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147(2):450–459. doi: 10.1378/chest.14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruden JF, Panse PM, Leslie KO, Tazelaar HD, Colby TV. UIP diagnosed at surgical lung biopsy, 2000-2009: HRCT patterns and proposed classification system. Am J Roentgenol. 2013;200(5):W458–67. doi: 10.2214/AJR.12.9437. [DOI] [PubMed] [Google Scholar]
- 26.Travis WD, Costabel U, Hansell DM, King TE, Jr., Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JD, Urschel JD, Cox G, Olak J, Young J.E., Kay J.M., et al. A randomized, controlled trial comparing thoracoscopy and limited thoracotomy for lung biopsy in interstitial lung disease. Ann Thorac Surg. 2000;70(5):1647–1650. doi: 10.1016/s0003-4975(00)01913-5. [DOI] [PubMed] [Google Scholar]
- 28.Thomson CC, Duggal A, Bice T, Lederer DJ, Wilson KC, Raghu G. 2018 Clinical practice guideline summary for Clinicians: diagnosis of idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2019;16(3):285–290. doi: 10.1513/AnnalsATS.201809-604CME. [DOI] [PubMed] [Google Scholar]
- 29.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, et al. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the National Heart, Lung, and Blood Institute. Am J Pathol. 2014;184(6):1643–1651. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124(11):4673–4677. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4) doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin Index Distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016;194(10):1242–1251. doi: 10.1164/rccm.201505-0862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang M. Matrix metalloproteinase profiling and their roles in disease. RSC Adv. 2023;13(9):6304–6316. doi: 10.1039/d2ra07005g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arévalo B, Ben Hassine A, Valverde A, Serafín V, Montero-Calle A, Raouafi N, et al. Electrochemical immunoplatform to assist in the diagnosis and classification of breast cancer through the determination of matrix-metalloproteinase-9. Talanta. 2021;225 doi: 10.1016/j.talanta.2020.122054. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Yuan Y, Zheng Y, Chai Y, Yuan R. An electrochemical peptide cleavage-based biosensor for matrix metalloproteinase-2 detection with exonuclease III-assisted cycling signal amplification. Chem Commun. 2016;52(35):5943–5945. doi: 10.1039/c6cc00928j. [DOI] [PubMed] [Google Scholar]
- 36.Chi C, Zhang Q, Mao Y, Kou D, Qiu J, Ye J, et al. Increased precision of orthotopic and metastatic breast cancer surgery guided by matrix metalloproteinase-activatable near-infrared fluorescence probes. Sci Rep. 2015;5 doi: 10.1038/srep14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Kim YP. Fluorescent and Bioluminescent Nanoprobes for in vitro and in vivo detection of matrix metalloproteinase activity. BMB Rep. 2015;48(6):313–318. doi: 10.5483/BMBRep.2015.48.6.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong T, Kong KV, Goh D, Olivo M, Yong KT. Sensitive surface-enhanced Raman scattering multiplexed detection of matrix metalloproteinase 2 and 7 cancer markers. Biomed Opt Express. 2015;6(6):2076–2087. doi: 10.1364/BOE.6.002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarman A, Kurbanoglu S, Zebger I, Scheller FW. Simple and robust: the claims of protein sensing by molecularly imprinted polymers. Sens Actuators B Chem. 2021;330 [Google Scholar]
- 40.Bartold K, Iskierko Z, Borowicz P, Noworyta K, Lin CY, Kalecki J, et al. Molecularly imprinted polymer-based extended-gate field-effect transistor (EG-FET) chemosensor for selective determination of matrix metalloproteinase-1 (MMP-1) protein. Biosens Bioelectron. 2022;208 doi: 10.1016/j.bios.2022.114203. [DOI] [PubMed] [Google Scholar]
- 41.Lee MH, Lin CC, Sharma PS, Thomas JL, Lin CY, Iskierko Z, et al. Peptide selection of MMP-1 for electrochemical sensing with epitope-imprinted poly(TPARA-co-EDOT)s. Biosensors. 2022;12(11):1018. doi: 10.3390/bios12111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MH, Lin CC, Kutner W, Thomas JL, Lin CY, Iskierko Z, et al. Peptide-imprinted conductive polymer on continuous monolayer molybdenum disulfide transferred electrodes for electrochemical sensing of Matrix Metalloproteinase-1 in lung cancer culture medium. Biosens Bioelectron X. 2023;13 [Google Scholar]
- 43.Guiot J, Moermans C, Henket M, Corhay JL, Louis R. Blood biomarkers in idiopathic pulmonary fibrosis. Lung. 2017;195(3):273–280. doi: 10.1007/s00408-017-9993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirchhain A, Poma N, Salvo P, Tedeschi L, Melai B, Vivaldi F, et al. Biosensors for measuring matrix metalloproteinases: an emerging research field. TrAC - Trends Anal Chem. 2019;110:35–50. [Google Scholar]
- 45.Stainer A, Faverio P, Busnelli S, Catalano M, Della Zoppa M, Marruchella A, et al. Molecular biomarkers in idiopathic pulmonary fibrosis: state of the Art and future Directions. Int J Mol Sci. 2021;22(12):6255. doi: 10.3390/ijms22126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somogyi V, Chaudhuri N, Torrisi SE, Kahn N, Müller V, Kreuter M. The therapy of idiopathic pulmonary fibrosis: what is next? Eur Respir Rev. 2019;28(153):190021. doi: 10.1183/16000617.0021-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163(7):1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 48.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayton C, Terrington D, Wilson AM, Chaudhuri N, Leonard C, Fowler SJ. Breath biomarkers in idiopathic pulmonary fibrosis: a systematic review. Respir Res. 2019;20(1):7. doi: 10.1186/s12931-019-0971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petsky HL, Kew KM, Turner C, Chang AB. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016;9(9) doi: 10.1002/14651858.CD011440.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu YC, Wang LF, Chien YW. Nitric oxide in the pathogenesis of diffuse pulmonary fibrosis. Free Radic Biol Med. 2007;42(5):599–607. doi: 10.1016/j.freeradbiomed.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Cameli P, Barbagli E, Rottoli P. Exhaled nitric oxide is not increased in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(1):39–40. [PubMed] [Google Scholar]
- 53.Zhao Y, Cui A, Wang F, Wang XJ, Chen X, Jin ML, et al. Characteristics of pulmonary inflammation in combined pulmonary fibrosis and emphysema. Chin Med J. 2012;125(17):3015–3021. [PubMed] [Google Scholar]
- 54.Wyszyńska M, Nitsze-Wierzba M, Czelakowska A, Kasperski J, Żywiec J, Skucha-Nowak M. An evidence-based review of application devices for nitric oxide concentration determination from exhaled air in the diagnosis of inflammation and treatment monitoring. Molecules. 2022;27(13):4279. doi: 10.3390/molecules27134279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furukawa K, Sugiura H, Matsunaga K, Ichikawa T, Koarai A, Hirano T, et al. Increase of nitrosative stress in patients with eosinophilic pneumonia. Respir Res. 2011;12(1):81. doi: 10.1186/1465-9921-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torén K, Murgia N, Schiöler L, Bake B, Olin AC. Reference values of fractional excretion of exhaled nitric oxide among non-smokers and current smokers. BMC Pulm Med. 2017;17(1):118. doi: 10.1186/s12890-017-0456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Högman M, Thornadtsson A, Liv P, Hua-Huy T, Dinh-Xuan AT, Tufvesson E, et al. Effects of growth and aging on the reference values of pulmonary nitric oxide dynamics in healthy subjects. J Breath Res. 2017;11(4) doi: 10.1088/1752-7163/aa7957. [DOI] [PubMed] [Google Scholar]
- 58.Kotecha J, Shulgina L, Sexton DW, Atkins CP, Wilson AM. Plasma vascular Endothelial growth factor concentration and alveolar nitric oxide as potential predictors of disease progression and mortality in idiopathic pulmonary fibrosis. J Clin Med. 2016;5(9):80. doi: 10.3390/jcm5090080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cameli P, Bargagli E, Bergantini L, d’Alessandro M, Pieroni M, Fontana GA, et al. Extended exhaled nitric oxide analysis in interstitial lung diseases: a systematic review. Int J Mol Sci. 2020;21(17):6187. doi: 10.3390/ijms21176187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guilleminault L, Saint-Hilaire A, Favelle O, Caille A, Boissinot E, Henriet AC, et al. Can exhaled nitric oxide differentiate causes of pulmonary fibrosis? Respir Med. 2013;107(11):1789–1796. doi: 10.1016/j.rmed.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 62.Van Berkel JJ, Dallinga JW, Möller GM, Godschalk RW, Moonen EJ, Wouters EF, et al. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir Med. 2010;104(4):557–563. doi: 10.1016/j.rmed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Gholizadeh A, Black K, Kipen H, Laumbach R, Gow A, Weisel C, et al. Detection of respiratory inflammation biomarkers in non-processed exhaled breath condensate samples using reduced graphene oxide. RSC Adv. 2022;12(55):35627–35638. doi: 10.1039/d2ra05764f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gholizadeh A, Voiry D, Weisel C, Gow A, Laumbach R, Kipen H, et al. Toward point-of-care management of chronic respiratory conditions: electrochemical sensing of nitrite content in exhaled breath condensate using reduced graphene oxide. Microsys Nanoeng. 2017;3(1) doi: 10.1038/micronano.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie ZZ, Morris JD, Mattingly SJ, Sutaria SR, Huang JP, Nantz MH, et al. Analysis of a Broad range of Carbonyl metabolites in exhaled breath by UHPLC-MS. Anal Chem. 2023;95(9):4344–4352. doi: 10.1021/acs.analchem.2c04604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ono E, Mita H, Taniguchi M, Higashi N, Tsuburai T, Miyazaki E, et al. Comparison of cysteinyl leukotriene concentrations between exhaled breath condensate and bronchoalveolar lavage fluid. Clin Exp Allergy. 2008;38(12):1866–1874. doi: 10.1111/j.1365-2222.2008.03108.x. [DOI] [PubMed] [Google Scholar]
- 67.Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, et al. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest. 2006;36(5):362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 68.Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med. 2009;103(9):1245–1256. doi: 10.1016/j.rmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu Y, Dobashi K, Sano T, Yamada M. ROCK activation in lung of idiopathic pulmonary fibrosis with oxidative stress. Int J Immunopathol Pharmacol. 2014;27(1):37–44. doi: 10.1177/039463201402700106. [DOI] [PubMed] [Google Scholar]
- 70.Chow S, Thomas PS, Malouf M, Yates DH. Exhaled breath condensate (EBC) biomarkers in pulmonary fibrosis. J Breath Res. 2012;6(1) doi: 10.1088/1752-7155/6/1/016004. [DOI] [PubMed] [Google Scholar]
- 71.Jaskiewicz K, Mycroft K, Maskey-Warzechowska M, Paralusz K, Siemiez N, Nejman-Gryz P, et al. Exhaled biomarkers in idiopathic pulmonary fibrosis—a six-month follow-up study in patients treated with pirfenidone. J Clin Med. 2020;9(8):2523. doi: 10.3390/jcm9082523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanoh S, Kobayashi H, Motoyoshi K. Exhaled ethane: an in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest. 2005;128(4):2387–2392. doi: 10.1378/chest.128.4.2387. [DOI] [PubMed] [Google Scholar]
- 73.Corradi M, Acampa O, Goldoni M, Adami E, Apostoli P, de Palma G, et al. Metallic elements in exhaled breath condensate of patients with interstitial lung diseases. J Breath Res. 2009;3(4) doi: 10.1088/1752-7155/3/4/046003. [DOI] [PubMed] [Google Scholar]
- 74.Hubbard R, Cooper M, Antoniak M, Venn A, Khan S, Johnston I, et al. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet. 2000;355(9202):466–467. doi: 10.1016/S0140-6736(00)82017-6. [DOI] [PubMed] [Google Scholar]
- 75.Mazzone PJ, Hammel J, Dweik R, Na J, Czich C, Laskowski D, et al. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax. 2007;62(7):565–568. doi: 10.1136/thx.2006.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamada YI, Yamada G, Otsuka M, Nishikiori H, Ikeda K, Umeda Y, et al. Volatile organic compounds in exhaled breath of idiopathic pulmonary fibrosis for discrimination from healthy subjects. Lung. 2017;195(2):247–254. doi: 10.1007/s00408-017-9979-3. [DOI] [PubMed] [Google Scholar]
- 77.de Vries R, Dagelet YWF, Spoor P, Snoey E, Jak PMC, Brinkman P, et al. Clinical and inflammatory phenotyping by breathomics in chronic airway diseases irrespective of the diagnostic label. Eur Respir J. 2018;51(1):1701817. doi: 10.1183/13993003.01817-2017. [DOI] [PubMed] [Google Scholar]
- 78.de Vries R, Muller M, van der Noort V, Theelen W, Schouten RD, Hummelink K, et al. Prediction of response to anti-PD-1 therapy in patients with non-small-cell lung cancer by electronic nose analysis of exhaled breath. Ann Oncol. 2019;30(10):1660–1666. doi: 10.1093/annonc/mdz279. [DOI] [PubMed] [Google Scholar]
- 79.Dragonieri S, Brinkman P, Mouw E, Zwinderman AH, Carratú P, Resta O, et al. An electronic nose discriminates exhaled breath of patients with untreated pulmonary sarcoidosis from controls. Respir Med. 2013;107(7):1073–1078. doi: 10.1016/j.rmed.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 80.Krauss E, Haberer J, Maurer O, Barreto G, Drakopanagiotakis F, Degen M, et al. Exploring the ability of electronic nose technology to recognize interstitial lung diseases (ILD) by non-invasive breath Screening of exhaled volatile compounds (VOC): a Pilot study from the European IPF registry (eurIPFreg) and Biobank. J Clin Med. 2019;8(10):1698. doi: 10.3390/jcm8101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antoniou KM, Alexandrakis M, Tzanakis N, Tsiligianni I, Tzortzaki EG, Siafakas NM, et al. Induced sputum versus bronchoalveolar lavage fluid in the evaluation of patients with idiopathic pulmonary fibrosis. Respiration. 2005;72(1):32–38. doi: 10.1159/000083398. [DOI] [PubMed] [Google Scholar]
- 82.Fireman E, Lerman Y. Induced sputum in interstitial lung diseases. Curr Opin Pulm Med. 2006;12(5):318–322. doi: 10.1097/01.mcp.0000239547.62949.82. [DOI] [PubMed] [Google Scholar]
- 83.Guiot J, Henket M, Corhay JL, Moermans C, Louis R. Sputum biomarkers in IPF: evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.She YX, Yu QY, Tang XX. Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov. 2021;7(1):52. doi: 10.1038/s41420-021-00437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guiot J, Bondue B, Henket M, Corhay JL, Louis R. Raised serum levels of IGFBP-1 and IGFBP-2 in idiopathic pulmonary fibrosis. BMC Pulm Med. 2016;16(1):86. doi: 10.1186/s12890-016-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Njock MS, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F, et al. Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax. 2019;74(3):309–312. doi: 10.1136/thoraxjnl-2018-211897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beeh KM, Beier J, Haas IC, Kornmann O, Micke P, Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2002;19(6):1119–1123. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 88.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(11):L709–21. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DePianto DJ, Chandriani S, Abbas AR, Jia G, N’Diaye EN, Caplazi P, et al. Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis. Thorax. 2015;70(1):48–56. doi: 10.1136/thoraxjnl-2013-204596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karsdal MA, Krarup H, Sand JMB, Christensen PB, Gerstoft J, Leeming DJ, et al. Review article: the efficacy of biomarkers in chronic fibroproliferative diseases – early diagnosis and prognosis, with liver fibrosis as an exemplar. Aliment Pharmacol Ther. 2014;40(3):233–249. doi: 10.1111/apt.12820. [DOI] [PubMed] [Google Scholar]
- 92.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;3(6):462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 93.Hoyer N, Jessen H, Prior TS, Sand JMB, Leeming DJ, Karsdal MA, et al. High turnover of types III and VI collagen in progressive idiopathic pulmonary fibrosis. Respirology. 2021;26(6):582–589. doi: 10.1111/resp.14056. [DOI] [PubMed] [Google Scholar]
- 94.Jessen H, Hoyer N, Prior TS, Frederiksen P, Karsdal MA, Leeming DJ, et al. Turnover of type I and III collagen predicts progression of idiopathic pulmonary fibrosis. Respiratory research. 2021;22(1):205. doi: 10.1186/s12931-021-01801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jessen H, Hoyer N, Prior TS, Frederiksen P, Rønnow SR, Karsdal MA, et al. Longitudinal serological assessment of type VI collagen turnover is related to progression in a real-world cohort of idiopathic pulmonary fibrosis. BMC Pulm Med. 2021;21(1):382. doi: 10.1186/s12890-021-01684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Organ LA, Duggan AR., Oballa E, Taggart SC, Simpson JK, Kang’ombe AR, et al. Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir Res. 2019;20(1):148. doi: 10.1186/s12931-019-1118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leeming DJ, Willumsen N, Sand JMB, Holm Nielsen S, Dasgupta B, Brodmerkel C, et al. A serological marker of the N-terminal neoepitope generated during LOXL2 maturation is elevated in patients with cancer or idiopathic pulmonary fibrosis. Biochem Biophys Rep. 2019;17:38–43. doi: 10.1016/j.bbrep.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosenkrans ZT, Massey CF, Bernau K, Ferreira CA, Jeffery JJ, Schulte JJ, et al. [(68) Ga]Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur J Nucl Med Mol Imaging. 2022;49(11):3705–3716. doi: 10.1007/s00259-022-05814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Röhrich M, Leitz D, Glatting FM, Wefers AK, Weinheimer O, Flechsig P, et al. Fibroblast activation protein-specific PET/CT imaging in fibrotic interstitial lung diseases and lung cancer: a Translational Exploratory study. J Nucl Med. 2022;63(1):127–133. doi: 10.2967/jnumed.121.261925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ximendes E, Benayas A, Jaque D, Marin R. Quo Vadis, Nanoparticle-Enabled in vivo fluorescence imaging? ACS Nano. 2021;15(2):1917–1941. doi: 10.1021/acsnano.0c08349. [DOI] [PubMed] [Google Scholar]
- 102.Zheng Z, Li D, Liu Z, Peng HQ, Sung HHY, Kwok RTK. Aggregation-induced Nonlinear Optical effects of AIEgen Nanocrystals for Ultradeep in vivo Bioimaging. Adv Mater. 2019;31(44) doi: 10.1002/adma.201904799. [DOI] [PubMed] [Google Scholar]
- 103.Nakajima Y, Asano K, Mukai K, Urai T, Okuwa M, Sugama J, et al. Near-infrared fluorescence imaging directly visualizes lymphatic Drainage Pathways and connections between Superficial and Deep lymphatic systems in the mouse Hindlimb. Sci Rep. 2018;8(1):7078. doi: 10.1038/s41598-018-25383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bai Y, Zhao J, Wang S, Lin T, Ye F, Zhao S. Carbon dots with Absorption red-Shifting for two-photon fluorescence imaging of tumor tissue pH and Synergistic Phototherapy. ACS Appl Mater Interfaces. 2021;13(30):35365–35375. doi: 10.1021/acsami.1c08076. [DOI] [PubMed] [Google Scholar]
- 105.Hong G, Zou Y, Antaris AL, Diao S, Wu D, Cheng K, et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat Commun. 2014;5:4206. doi: 10.1038/ncomms5206. [DOI] [PubMed] [Google Scholar]
- 106.Han HH, Tian H, Zang Y, Sedgwick AC, Li J, Sessler JL, et al. Small-molecule fluorescence-based probes for interrogating major organ diseases. Chem Soc Rev. 2021;50(17):9391–9429. doi: 10.1039/d0cs01183e. [DOI] [PubMed] [Google Scholar]
- 107.Jia Q, Zhao Z, Liang K, Nan F, Li Y, Wang J, et al. Recent advances and prospects of carbon dots in cancer nanotheranostics. Mater Chem Front. 2020;4(2):449–471. [Google Scholar]
- 108.Li X, An G, Wang Y, Liang D, Zhu Z, Tian L. Targeted migration of bone marrow mesenchymal stem cells inhibits silica-induced pulmonary fibrosis in rats. Stem Cell Res Ther. 2018;9(1):335. doi: 10.1186/s13287-018-1083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Z, Zou H, Zhao Z, Zhang P, Shan GG, Kwok RTK, et al. Tuning Organelle Specificity and photodynamic therapy Efficiency by molecular function Design. ACS Nano. 2019;13(10):11283–11293. doi: 10.1021/acsnano.9b04430. [DOI] [PubMed] [Google Scholar]
- 110.Xu W, Wang D, Tang BZ. NIR-II AIEgens: a Win-Win integration towards Bioapplications. Angew Chem, Int Ed. 2021;60(14):7476–7487. doi: 10.1002/anie.202005899. [DOI] [PubMed] [Google Scholar]
- 111.Wei Y, Liu Y, He Y, Wang Y. Mitochondria and lysosome-targetable fluorescent probes for hydrogen peroxide. J Mater Chem B. 2021;9(4):908–920. doi: 10.1039/d0tb02440f. [DOI] [PubMed] [Google Scholar]
- 112.Song X, Bai S, He N, Wang R, Xing Y, Lv C, et al. Real-time evaluation of hydrogen peroxide Injuries in pulmonary fibrosis mice models with a mitochondria-targeted near-infrared fluorescent probe. ACS Sens. 2021;6(3):1228–1239. doi: 10.1021/acssensors.0c02519. [DOI] [PubMed] [Google Scholar]
- 113.Zhan Z, Liu R, Chai L, Dai Y, Lv Y. Visualization of lung inflammation to pulmonary fibrosis via Peroxynitrite fluctuation. Anal Chem. 2019;91(17):11461–11466. doi: 10.1021/acs.analchem.9b02971. [DOI] [PubMed] [Google Scholar]
- 114.He N, Bai S, Huang Y, Xing Y, Chen L, Yu F, et al. Evaluation of glutathione S-transferase inhibition effects on idiopathic pulmonary fibrosis therapy with a near-infrared fluorescent probe in cell and mice models. Anal Chem. 2019;91(8):5424–5432. doi: 10.1021/acs.analchem.9b00713. [DOI] [PubMed] [Google Scholar]
- 115.Daniil ZD, Papageorgiou E, Koutsokera A, Kostikas K, Kiropoulos T, Papaioannou AI, et al. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2008;21(1):26–31. doi: 10.1016/j.pupt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 116.Strange RC, Jones PW, Fryer AA. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett. 2000;112–113:357–363. doi: 10.1016/s0378-4274(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 117.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express .gamma.-glutamyl transpeptidase. Biochemistry. 1993;32(24):6302–6306. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- 118.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of γ-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71(3):231–238. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 119.He N, Wang Y, Huang Y, Wang X, Chen L, Lv C. A near-infrared fluorescent probe for evaluating glutamyl transpeptidase fluctuation in idiopathic pulmonary fibrosis cell and mice models. Sens Actuators B Chem. 2020;322 [Google Scholar]
- 120.Xu F, Wang Q, Jiang L, Zhu F, Yang L, Zhang S, et al. Evaluation of nitric oxide fluctuation via a fast, responsive fluorescent probe in idiopathic pulmonary fibrosis cells and mice models. Anal Chem. 2022;94(9):4072–4077. doi: 10.1021/acs.analchem.1c05643. [DOI] [PubMed] [Google Scholar]
- 121.Feng F, Wang Z, Li R, Wu Q, Gu C, Xu Y, et al. Citrus alkaline extracts prevent fibroblast senescence to ameliorate pulmonary fibrosis via activation of COX-2. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108669. [DOI] [PubMed] [Google Scholar]
- 122.Robertson JA, Sauer D, Gold JA, Nonas SA. The role of cyclooxygenase-2 in Mechanical Ventilation–induced lung injury. Am J Respir Cell Mol Biol. 2012;47(3):387–394. doi: 10.1165/rcmb.2011-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karampitsakos T, Dimakou K, Papaioannou O, Chrysikos S, Kaponi M, Bouros D, et al. The role of increased red cell distribution width as a negative prognostic marker in patients with COPD. Pulm Pharmacol Ther. 2020;60 doi: 10.1016/j.pupt.2019.101877. [DOI] [PubMed] [Google Scholar]
- 124.Karampitsakos T, Torrisi S, Antoniou K, Manali E, Korbila I, Papaioannou O, et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with Idiopathic Pulmonary Fibrosis. Respir Res. 2021;22(1):140. doi: 10.1186/s12931-021-01725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scott MKD, Quinn K, Li Q, Carroll R, Warsinske H, Vallania F, et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. Lancet Respir Med. 2019;7(6):497–508. doi: 10.1016/S2213-2600(18)30508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teoh AKY, Jo HE, Chambers DC, Symons K, Walters EH, Goh NS, et al. Blood monocyte counts as a potential prognostic marker for idiopathic pulmonary fibrosis: analysis from the Australian IPF registry. Eur Respir J. 2020;55(4):1901855. doi: 10.1183/13993003.01855-2019. [DOI] [PubMed] [Google Scholar]
- 127.Nathan SD, Reffett T, Brown AW, Fischer CP, Shlobin OA, Ahmad S, et al. The red cell distribution width as a prognostic indicator in idiopathic pulmonary fibrosis. Chest. 2013;143(6):1692–1698. doi: 10.1378/chest.12-1368. [DOI] [PubMed] [Google Scholar]
- 128.Epstein D, Nasser R, Mashiach T, Azzam ZS, Berger G. Increased red cell distribution width: a novel predictor of adverse outcome in patients hospitalized due to acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2018;136:1–7. doi: 10.1016/j.rmed.2018.01.011. [DOI] [PubMed] [Google Scholar]