Abstract
Background
Myotonic dystrophy type 1 (DM1) is a rare neuromuscular disease caused by a CTG repeat expansion in the 3′ untranslated region of the DM1 protein kinase gene. Characteristic degenerative muscle symptoms include myotonia, atrophy, and weakness. We previously proposed an Musashi homolog 2 (MSI2)>miR-7>autophagy axis whereby MSI2 overexpression repressed miR-7 biogenesis that subsequently de-repressed muscle catabolism through excessive autophagy. Because the DM1 HSALR mouse model expressing expanded CUG repeats shows weak muscle-wasting phenotypes, we hypothesized that MSI2 overexpression was sufficient to promote muscle dysfunction in vivo.
Methods
By means of recombinant AAV murine MSI2 was overexpressed in neonates HSALR mice skeletal muscle to induce DM1-like phenotypes.
Results
Sustained overexpression of the murine MSI2 protein in HSALR neonates induced autophagic flux and expression of critical autophagy proteins, increased central nuclei and reduced myofibers area, and weakened muscle strength. Importantly, these changes were independent of MBNL1, MBNL2, and Celf1 protein levels, which remained unchanged upon Msi2 overexpression.
Conclusions
Globally, molecular, histological, and functional data from these experiments in the HSALR mouse model confirms the pathological role of MSI2 expression levels as an atrophy-associated component that impacts the characteristic muscle dysfunction symptoms in DM1 patients.
Keywords: Msi2, Myotonic dystrophy, Muscle atrophy, Adeno-associated virus, HSALR
Introduction
Myotonic dystrophy type 1 (DM1; OMIM #160900) is the most common muscular dystrophy in adults, with a worldwide disease prevalence between 1/8000 and 1/3000 [1], although a recent newborn screening program reported a higher frequency of the mutant allele (1/2100 [2]). DM1 is an autosomal dominant disease originating from the pathogenic expansion (>50) of the CTG trinucleotide microsatellite in the 3′-untranslated region of the DM1 protein kinase (DMPK) gene [3]. Consequently, mutant RNA carrying expanded CUG repeat becomes toxic by forming ribonuclear foci comprising secondary structures able to sequester RNA-binding proteins of the Muscleblind Like Splicing Regulator (MBNL) family [4]. MBNL1 plays a crucial role in regulating alternative splicing and polyadenylation sites in transcripts during development, causing a transition from fetal to adult patterns in muscle, while MBNL2 likely has a similar role in the brain [5,6]. In contrast, MBNL3 deficit was linked to age-associated pathologies observed in myotonic dystrophy [7]. While MBNLs are deprived of their normal function, the CUGBP Elav-Like Family Member 1 (CELF1) becomes activated and regulates splicing antagonizing MBNL activity [8]. This leads to a downstream cascade of mis-splicing events caused by MBNL sequestration and CELF activation, resulting in the maintenance of fetal splicing patterns in adult tissue for several critical muscle transcripts [9]. Consequently, some DM1 symptoms originate from expressing inappropriate muscle protein isoforms. For instance, myotonia and insulin resistance are linked to defects in CLCN1 [10] and INSR [11] regulation of inclusion of alternative exons, respectively, while heart conduction defects and cardiac arrhythmias are associated with SCN5A mis-splicing [12].
DM1 patients show multisystemic symptoms, although the most consistent feature is diseased muscles, known as myopathy, including progressive muscle wasting (atrophy), especially with a distal pattern, difficulty when relaxing a contracted muscle (myotonia), neuromuscular respiratory insufficiency, and dysphagia [13]. Globally, all these symptoms contribute to muscle dysfunction leading to reduced strength and endurance and thus to physical disability.
Multiple factors have been proposed to explain muscle loss, including AKT-GSK3β [14], TWEAK/Fn14 [15], AMPK/mTORC1 [16], and PKC [17] pathways [18]. Additionally, during the last few years, the pathological contribution of increased autophagy has gained relevance as a possible cause contributing to the imbalance of protein synthesis and degradation leading to muscle atrophy. Briefly, in a Drosophila model of the disease, hyperactivation of autophagy was confirmed concomitantly with reduced area of indirect flight muscles and reduced survival and motor capacity of model flies [19]. Genetic and chemical rescue of the pathway was sufficient to ameliorate molecular, histological, and functional phenotypes in Drosophila, murine and cellular models [20,21]. Dysregulation of autophagy linked to the alteration of molecular markers of muscle atrophy was also confirmed in DM1 muscle cells [19,22]. Additionally, a murine model that inducibly expresses 960 CUG shows histological alterations and muscle wasting [23]. A proteomic analysis of these DM1 model mouse muscle samples confirmed increased expression of proteins involved in autophagy, thus suggesting an imbalance between anabolic and catabolic pathways that regulate muscle maintenance.
Musashi homolog 2 (MSI2) is an RNA-binding protein member of the Musashi gene family, characterized by its function as an alternative splicing factor [24], mRNA translational regulator [25], and a cancer-driver gene in some tumors, including breast, brain, colon, lung, liver, pancreas, or leukemia [26]. Its function in these tumors relates to mRNA translation and stability [25]. In addition, Msi2 maintains stem cell self-renewal and promotes oncogenesis by enhancing cell proliferation [26]. We have recently demonstrated that MSI2 levels are increased in DM1 myoblasts and biopsy samples. Using different silencing strategies targeting MSI2, we observed that miR-7, whose biogenesis is inhibited by MSI2-Hu antigen R (HuR) complex [27], was enhanced, and atrophy-related phenotypes were restored in different DM1cell models [28]. Besides, it was demonstrated that miR-7 levels are reduced in human muscle biopsies and DM1 myotubes. Downregulation of miR-7 in control myotubes leads to DM1-like phenotypes, while restoration of normal miRNA levels in DM1 cells inhibits excessive autophagy [21]. Interestingly, miR-7 was described as a critical inhibitor of the autophagic pathway through the up-regulation of the mTOR signaling and direct translational repression of key autophagy genes, namely ATG7, ULK2, and ATG4A [29]. Additionally, miR-7 biogenesis is regulated by MSI2 and Hu antigen R (HuR). MSI2 binds through HuR to the conserved terminal loop of pri-miR-7-1, stabilizing the precursor miRNA and reducing the mature form [27].
Pivotal in DM1 research was the demonstration that human skeletal actin (HSA)-driven expression of long repeat (LR) CUG RNA in vivo (HSALR mice), independently of DMPK sequences, develop many characteristic DM1 symptoms, including myotonia, centrally located nuclei and variable muscle fiber size, and aberrant alternative splicing [30]. However, these mice do not show a clear and consistent muscle atrophy phenotype [31]. To demonstrate the involvement of specific proteins in the pathogenic mechanism of the disease, Li et al., 2020 recently used HSALR mice to overexpress Hnrnpa1 using recombinant adeno-associated viruses (rAAV). Hnrnpa1 was sufficient to boost DM1-like spliceopathy, thus demonstrating the pathogenic role of this protein in a DM1 context [32]. In a similar approach, independent authors promoted Staufen1 overexpression using recombinant adeno-associated virus (rAAV), which was sufficient to enhance progressive muscle wasting in this murine model [33].
We have previously observed that miR-7 levels in the skeletal muscle of HSALR do not differ from those in control mice [21]. Building off our in vitro results, we proposed that in vivo levels of Msi2 were critical to the muscle atrophy phenotype, potentially explaining HSALR failure to show overt muscle degeneration. Therefore, if miR-7 biogenesis is inhibited by Msi2 overexpression, reduced levels of the mature miR-7 will release repression of several autophagy-related genes, enhancing muscle catabolism beyond normal homeostasis and leading to muscle degradation. Considering this hypothesis, we report the results of overexpressing murine Msi2 in HSALR mice skeletal muscle and provide experimental evidence indicating the involvement of Msi2 to the muscle wasting phenotype in DM1.
Materials and methods
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from murine muscles was isolated using the miRNeasy Mini Kit (Qiagen, Germantown, Maryland, CA, USA, 217004). One microgram of RNA was digested with DNase I (Invitrogen, Carlsbad, CA, USA, 18068015) and reverse-transcribed with SuperScript II (Invitrogen, Carlsbad, CA, USA, 18064014) using random hexanucleotides (Sigma–Aldrich, San Luis, Missouri, USA, 11277081001). Mean of Gtf2b, Hprt1, and Gapdh expression were used as endogenous controls. 500 ng of mouse tissue cDNA was used as the template for RT-qPCR using miRCURY LNA SYBR® Green PCR Kit (Qiagen, Germantown, Maryland, USA, 1477.6). The primer sequences are listed in Supplementary Table 1. miRNA expression in murine muscles was quantified using specific miRCURY™-locked nucleic acid microRNA PCR primers (Qiagen, Germantown, Maryland, CA, USA, 339306) and was normalized to U1 snRNA. All procedures followed the corresponding manufacturer's recommendations. Expression levels were measured using an Applied Biosystems Quant Studio 5 Time PCR System. Expression relative to the endogenous gene and control group was calculated using the 2−ΔΔCt method.
Western blotting
For total protein extraction, quadriceps and gastrocnemius mouse muscles were homogenized in RIPA buffer (Fisher Scientific, Waltham, Massachusetts, USA, 10230544) supplemented with protease and phosphatase inhibitor cocktails (Roche Applied Science, Indianapolis, IN, USA, 4906837001). Total protein was quantified using a BCA assay kit (Thermo Scientific Pierce, Grand Island, NY, USA, 10741395) following the manufacturer's instructions. For specific protein detections, 20 μg of total protein were denatured for 5 min at 100 °C, electrophoresed on 12–15% SDS-PAGE gels, and transferred onto 0.45 μm nitrocellulose membranes (Cytiva, Little Chalfont, UK, GE10600002). Membranes were blocked with blocking solution [5% non-fat dried milk in PBS-T (8 mM Na2HPO4, 150 mM NaCl, 2 mM KH2PO4, 3 mM KCl, 0.05% Tween 20, pH 7.4)] for 1 h at room temperature (RT). Membranes were incubated overnight with blocking solution at 4 °C with the corresponding primary antibody: goat horseradish peroxidase (HRP)-conjugated anti-GAPDH (1:3500, Santa Cruz, Dallas, Texas, sc-365062), rabbit anti-ATG4A (1:1000, 108322), rabbit anti-LC3B (1:3000, 51520), rabbit anti-MSI2 antibody (1:1000, EP1305Y) or mouse anti-P62 (1:1000, 65416), all these from Abcam, Cambridge, UK. All primary antibodies, except anti-GAPDH were detected using goat HRP-conjugated anti-rabbit-IgG (1 h, 1:3500, Sigma–Aldrich, San Luis, Missouri, USA, A0545) or goat HRP-conjugated anti-mouse-IgG (1:5000, Sigma–Aldrich, San Luis, Missouri, USA, B7264) incubated for 1 h at RT. Images were acquired with an Amersham ImageQuant 800 and were quantified using ImageJ software (NIH).
Jess Simple Western system
V5-tag and Msi2 protein were quantified by automated Simple Western assays using the Jess system (ProteinSimple; Bio-Techne, Minneapolis, Minnesota, USA) following the manufacturer's recommendations. A final concentration of 0.4 mg/mL for V5, and 1.8 mg/mL for Msi2, of gastrocnemius or quadriceps muscle protein extract was mixed with 5 × master mix, heated at 95 °C for 5 min and kept on ice. 12–230 kDa Jess/Wes Separation Module (cat#SM-W004) was used, and 4 μl of each sample was loaded for 9 s. The incubation time of the primary and the secondary antibodies was 30 min. Rabbit anti-V5-Tag (Cell Signalling, Danvers, Massachusetts, USA, D3H8Q) was used at 1:20 dilution, and anti-Msi2 (Abcam, Cambridge, UK, 76148) at 1:250. Ready to use HRP-conjugated anti-rabbit antibody module (cat. #DM-001) was used as the secondary antibody. Msi2 antibody was prepared in antibody diluent 2 solution, and mouse anti-V5-Tag was prepared in 5% BSA. The RePlex Module was used to remove primary and secondary antibodies in a RePlex assay with total protein determination in a single run (cat. #RP-001). Total protein quantification was performed using the total protein detection module for chemiluminescence (cat. #DM-TP01).
rAAV generation
Gene synthesis, cloning, and production of rAAV vectors were carried out by the Viral Vector Production Unit from Universitat Autònoma de Barcelona (Barcelona, Spain). The construct designed for overexpressing isoform 4 of murine Msi2 (NP_001350124.1) was rAAV9-hDES-mMsi2-V5-T2A-Nanoluc. rAAV9-hDES-Nanoluc was used as a control. hDES refers to a human DESMIN promoter suitable for achieving gene expression in skeletal muscle [34]. The hDES-mMsi2-V5-T2A-Nanoluc construct was cloned into a pAAV plasmid containing the ITRs of the AAV9 genome. The resulting plasmid pAAV-hDES-mMsi2-V5-T2A-Nanoluc was used for AAV9 particle packaging. The sequence and description of the construct are shown in Supplementary Fig. 1. To generate the empty control rAAV9-hDES-Nanoluc, BamHI restriction sites were used to remove mMsi2-V5-T2A. All construct detail requests can be addressed to the corresponding author.
Animal experimentation and rAAV administration
Homozygous transgenic HSALR [30] mice were provided by Prof. C. Thornton (University of Rochester Medical Center, Rochester, NY, USA). Mice with the same genetic background (Friend Virus B: FVB) obtained from The Jackson Laboratory were used as controls. For systemic expression, P2–P3 HSALR male pups were injected once in the temporal vein with 35 μl of rAAV diluted in PBS using a 31G insulin needle. 15 mice received rAAV9-hDES-mMsi2-V5-T2A-Nanoluc (Msi2) and 10 rAAV9-hDES-Nanoluc (hDES). In both cases, 5 × 1011 vg were administered per animal. FVB (n = 8) and HSALR (n = 8) mice of the same age were injected with PBS 1x as controls. Mice were sacrificed six weeks after injection, and quadriceps and gastrocnemius were harvested. Each muscle was divided into three parts, two were snap frozen in liquid nitrogen for protein and RNA isolation, and the third was frozen in isopentane for histological analyses. Body weight was monitored weekly since they were weaned on day 21. For Msi2 and autophagic markers analyses, FVB and HSALR males of 1.5 and 4.5 months old were sacrificed, and quadriceps and gastrocnemius were harvested.
Forelimb grip strength test
The forelimb grip strength was measured on day 21 and before sacrifice, as previously described [20].
Histological analysis of mouse muscle samples
To quantify muscle fiber cross-sectional area (CSA), 10 μm-sections from gastrocnemius and quadriceps were obtained with a Leica CM 1510S cryostat. For muscle fiber CSA quantification, muscle sections were washed with PBS 1x and immunostained with Wheat Germ Agglutinin, Alexa Fluor™ 488 Conjugate (1:400, Biotium, Fremont, California, USA, 29022–1) for 20 min, washed three times with PBS 1x and finally mounted with VECTASHIELD® mounting medium containing 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (Vector Laboratories, London, UK, H-1200-10) to detect the nuclei. Images were taken in an LSM800 confocal microscope (Zeiss, Stuttgart, Germany) at 400x magnification. Around 5000 fibers were analyzed in each condition using Zeiss analysis software (ZEN).
Sections were stained with hematoxylin-eosin (H&E) and mounted with DPX (Sigma–Aldrich, San Luis, Missouri, USA, 06522) according to standard procedures to quantify central nuclei in muscle fibers. Images were taken at 100 × magnification with a Leica DM2500 microscope. For central nuclei quantification, 500 fibers were analyzed in each mouse using ImageJ software (NIH).
In vivo bioluminescence imaging
Mice infected with Msi2 (n = 4) or hDES, as control, (n = 4) rAAV9, or administered PBS (n = 3), as previously described, were injected intraperitoneally with 100 μl of 3.5 mM fluorofurimazine (Promega, Madison, Wisconsin, USA, CS320501) before sacrifice. Mice were anesthetized with isoflurane and 10 min post substrate injection, the animals were imaged every 5 min for 25 min at the IVIS Lumina X5 (PerkinElmer, Waltham, Massachusetts, USA). Data analysis was performed by drawing regions of interest in the images taken at the peak of bioluminescence emission.
Modelization of the muscle fibers' cross-sectional area
A generalized linear mixed model (GLMM) [35]) was fitted to the CSA of muscle fibers. Since this experiment consists of repeated measurements of subjects, a random effect term for each mouse was included in the model. Msi2-infected groups were considered as the fixed-effects parameter. Moreover, the CSA data of muscle fibers have a skewness that deviates from the normal distribution; for this reason, it was assumed a gamma distribution for the response variable and a logarithmic link function so that the logarithm of the mean CSA is linearly connected to the predictors in the model. As the ultimate purpose was to evaluate the difference in muscle fiber area of each treatment group with respect to hDES, three hypothesis tests were performed on the basis of the coefficients of the GLMM model.
Blood assays
When animals were sacrificed, the blood was collected by cardiac puncture exsanguination, and the samples were analyzed by Laboratorios Montoro Botella (Valencia, Spain). White blood cell differential count (basophils, segmented cells, stab cells, eosinophils, and lymphocytes) and biochemistry parameters (creatinine, total cholesterol, glucose, triglycerides, amylase, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and total bilirubin) were analyzed in the different experimental conditions.
Statistical analyses
In all molecular studies, for comparison of mean data, all parameters were assumed to follow a normal distribution. The samples were compared using unpaired t-test (α = 0.05), applying Welch's correction when necessary or multiple Student's t-test with Holm-Sidak correction for multiple comparisons. Sample size (n) is included in the captions.
The white blood differential cell count statistical analyses were performed applying multivariate analysis of variance (MANOVA) with Tukey HSD post hoc test.
Two-tailed Binomial test (α = 0.05) was used to compare the number of parameters with significant differences and no differences between PBS and hDES.
Results
HSALR model mice do not reproduce the alteration of the MSI2-miR-7-autophagy axis described in DM1 patients
Considering previously reported data [19,28], we hypothesized that dysregulation of the MSI2-miR-7-autophagy axis contributes to an imbalance between the synthesis and degradation of muscle proteins, thus leading to muscle atrophy and dysfunction. We first tested this hypothesis with the HSALR murine model of disease expressing 250 CTG repeats, which is widely used as it reproduces relevant disease phenotypes such as myotonia, centrally located nuclei in muscle fibers suggestive of an incomplete maturation of type 1 fibers in a DM1 context [36] and alternative splicing defects [30], but shows weak or no muscle atrophy phenotypes [30,33]. Therefore, MSI2 levels were analyzed in the quadriceps and gastrocnemius of age-matched HSALR and FVB controls of 1.5 and 4.5 months. The results showed that both protein and transcript Msi2 levels were similar among control and model mice independently of age [Fig. 1 A-C]. Two Msi2 immunoreactive bands corresponding to Msi2 isoforms (31 and 35 kDa) were revealed by Western blotting [Fig. 1A] whereas only one was detected by Jess Simple Western [Fig. 1B] due to the different composition of gels in capillaries, which makes the technique, although more precise and sensitive, with less resolution. Similar results were observed for miR-7 expression [Fig. 1D]. Finally, it was confirmed that the model mice did not reproduce the autophagy hyperactivation found in patients [18] by quantifying the levels of Atg7, which is a direct target of miR-7 [29], the LC3II/LC3I ratio and P62 proteins [Fig. 2].
Fig. 1.
Msi2 and miR-7 expressions were normal in HSALRmodel mice. A Quantification and representative Western blots of total Msi2 in protein extracts obtained from control (FVB, n = 6–8) and DM1 model mice (HSALR, n = 6–8) at 1.5 (blue bars) and 4.5 (red bars) months of age. Determination was performed in the quadriceps (qd) and gastrocnemius (gt). Gapdh was used as an endogenous control. Molecular weight sizes are given in kDa. B Relative Msi2 protein was quantified using Jess Simple Western technology in the same samples as in (A). A representative lane view shows the bands (upper panel) observed. Msi2 signal was normalized to total protein (lower panel). C and D Quantification by RT-qPCR of the relative expression of Msi2 (n = 6–8, panel C) and miR-7 (n = 6–8, panel D) in the same samples as in a. miR-7 quantification was relative to endogenous U1. Msi2 was referenced to Gtf2b, Gapdh, and Hprt1 expression. At least three independent experiments were carried out, and three technical replicates were performed from each biological sample in b and d. The bar graphs show the mean ± SEM; no significant differences were detected between FVB and HSALR according to multiple Student's t-test with Holm-Sidak correction for multiple comparisons.
Fig. 2.
HSALRmice do not reproduce excessive autophagy in skeletal muscle. A Quantification and B representative blots, with indication of molecular weight sizes to the right in kDa, of the immunodetection of total Msi2, LC3II/LC3I ratio, and total P62 protein in protein extracts obtained from control (FVB, n = 6–8) and DM1 model mice (HSALR, n = 6–8) at 1.5 (blue bars) and, 4.5 (red bars) months of age. Quantification was carried out in quadriceps and gastrocnemius. Gapdh was used as an endogenous control. The bar graphs show the mean ± SEM; no significant differences were detected between FVB and HSALR according to multiple Student's t-test with Holm-Sidak correction for multiple comparisons.
rAAV-infected mice overexpress murine Msi2 in the skeletal muscle
To demonstrate that Msi2 overexpression was sufficient to enhance disease-related muscle phenotypes, we overexpressed Msi2 in HSALR model mice. For that purpose, we generated recombinant adeno-associated virus (rAAV) particles encoding Msi2 under the control of the human DESMIN promoter [Fig. 3A][Supplementary Fig. 1]. The recombinant adeno-associated viral serotype 9 (rAAV9) pseudotype was selected to ensure a broad and sustained expression of the transgene in skeletal muscle after intravenous injection [37]. Specifically, murine Msi2 isoform 4 was chosen as it contains all described exons and its sequence is identical to the canonical isoform of MSI2 in humans. Msi2 was fused to the V5 tag for subsequent immunodetection. Nanoluciferase (Nanoluc) reporter was also included, separated from Msi2 by a T2A linker, which induces ribosomal skipping during translation [38], allowing both Msi2 and Nanoluc proteins to be synthesized from one transcript with two ORFs. This construct is hereafter referred to as Msi2. As a control, a similar construct was generated containing only Nanoluc controlled by the human DESMIN promoter. We refer to this construct as hDES. We thus injected Msi2 or hDES rAAV9 in the temporal vein of P2–P3 HSALR pups and collected samples six weeks post-infection. As a procedural control, model mice were treated with 1X PBS.
Fig. 3.
rAAV9 targets HSALRskeletal muscle. A Experimental design. Recombinant AAV serotype 9 expressing Msi2 (upper) and control constructs (lower) were injected intravenously in P2–P3 HSALR male pups. 42 days post-injection, mice were sacrificed, and quadriceps and gastrocnemius muscles were dissected. Abbreviations: hDES: Human DESMIN promoter; mMsi2: murine Msi2 isoform 4; V5: V5-tag; Nanoluc: nanoluc transgene; ITR: inverted terminal repeat sequences of 145 bp in length. T2A: Linker to allow both Msi2 and Nanoluc expression under the control of the hDES promoter. BIn vivo imaging after subcutaneous administration in HSALR mice treated with PBS (n = 3), or rAAV9-hDES (n = 4) or rAAV9-Msi2 (n = 4). C Quantification of total maximum flux in Radiance of fluorofurimazine after intraperitoneal administration in Msi2 and hDES-treated mice. D Quantification by RT-qPCR of Nanoluc transgene expression in quadriceps and gastrocnemius of control mice (FVB, n = 8), or HSALR treated with vehicle (PBS, n = 8), rAAV9-hDES (n = 10), or rAAV9-Msi2 (n = 15) viruses. Nanoluc was normalized to Gtf2b, Gapdh, and Hprt1 expression. At least three biological replicates were carried out in C and D, and three technical replicates were performed from each biological sample in D. Bars show the mean ± SEM. No significant differences were detected between hDES and Msi2 according to Student's t-test.
Prior to euthanasia, the Nanoluc substrate, fluorofurimazine, was injected to generate bright bioluminescent imaging. This experiment confirmed that the transgene was expressed in skeletal muscle in hDES and Msi2-infected mice. As expected, no signal was detected in mice injected with PBS [Fig. 3B]. The brightness generated by bioluminescence was quantified overtime at 5 min intervals, and no differences were detected between hDES and Msi2, confirming that rAAV expression was similar in both experimental groups [Fig. 3c]. These results were confirmed by quantification of Nanoluc transcripts in quadriceps and gastrocnemius that evidenced similar expression of the reporter in both hDES and Msi2 groups [Fig. 3D]. Blood serum was extracted from all experimental groups for biochemical analyses and white blood cell count [Supplementary Table 2]. Importantly, no differences were observed in any of the parameters analyzed between the PBS and hDES groups, which rules out a deleterious effect linked to rAAV infection per se. Alkaline phosphatase (AP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin levels are part of the biochemical determinations aimed at detecting hepatic affections [39]. When comparing hDES vs. Msi2, it was observed that AP and bilirubin were increased in Msi2-transduced animals, ALT remained unchanged, while AST was decreased. Low levels of this enzyme are associated with vitamin B6 deficiency [40]. Overall, the results suggest that Msi2 overexpression damages the liver, although the reduction in AST would require further testing before confirming liver disease. Additionally, it is important to note that AP levels have been shown to correlate positively with low muscle mass and have been proposed as a predictive marker of sarcopenia [41] while independent studies performed in patients with type 2 diabetes suggested a positive correlation between bilirubin levels and decreased skeletal muscle mass [42]. Finally, no significant changes were detected among the groups of interest in the white blood cell counts.
rAAV9-infected muscles express functional Msi2 protein
Once confirmed that AAV targeted the skeletal muscles in infected model mice, we addressed Msi2 levels in the quadriceps and gastrocnemius six weeks after treatment. It was confirmed that Msi2 remained stable in model mice treated with PBS when compared to controls (FVB). Additionally, data showed that protein expression increased by about 2-fold in quadriceps and 4-fold in gastrocnemius compared to model mice infected with empty rAAV (hDES). These differences were also significant at the mRNA level [Fig. 4A–C]. Unlike what was observed in other DM1 models or patients, we detected decreased Msi2 transcripts in quadriceps of model mice. Overall, we observed that there were no significant differences between the PBS and hDES groups [Supplementary Table 3], so in this and subsequent experiments the effect of Msi2 overexpression will be assessed by comparing Msi2 vs. hDES. Furthermore, after performing an overall analysis of the results obtained for the different parameters between the and hDES groups, we confirmed that there was a significantly greater number of unchanged parameters between both conditions (p = 0.0009 quadriceps; p = 0.0041 gastrocnemius), thus supporting the conclusion that non-specific effects by hDES were minoritarian. V5-tagged (rAAV-derived) Msi2 was immunodetected using Jess Simple Western technology [Fig. 4D], making Msi2-V5 expression in infected muscle fibers conspicuous. The expression of the HSALR transgene was quantified in all animals involved in the experiment denoting a great variability between muscles and animals [Supplementary Fig. 2]. Notably, expression variability was much more evident in gastrocnemius where differences between hDES and Msi2 groups resulted statistically significant.
Fig. 4.
Msi2 is overexpressed in skeletal muscle upon rAAV9 infection. A,B Western blotting quantification and representative blots of Msi2 levels in quadriceps and gastrocnemius from control and neonate DM1 model mice injected with PBS (FVB and PBS), rAAV9-hDES or rAAV9-Msi2. Protein levels were normalized to Gapdh. C Quantification by RT-qPCR of the relative expression of Msi2 in the same experimental groups as in a,b. D V5 protein was detected using Jess Simple Western technology. A representative lane view shows the bands immunodetected with an anti-V5 antibody (left panel) and total protein (right panel) used for internal normalization. e Msi2 targets: miR-7, Tgfbr1, and P21 in the indicated biological groups. miR-7 quantification was normalized to endogenous U1 levels and Msi2, Tgfbr1, and P21, were normalized to mean of Gtf2b, Gapdh and Hprt1 expression. In all cases, relative levels were normalized to hDES samples. p values were calculated using Student's t-test. In all cases comparisons were FVB vs. PBS and hDES vs. Msi2. Plots represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. FVB (n = 8), HSALR PBS (n = 8), HSALR rAAV9-hDES (n = 6–10) and HSALR rAAV9-Msi2 (n = 13–15).
Once overexpression was confirmed in Msi2-transduced mice, the protein's functionality was assessed by evaluating the expression of three MSI2 direct targets. We confirmed a 1.4-fold and 12-fold reduction in miR-7 levels in quadriceps and gastrocnemius, respectively, compared to the group treated with the empty vector, hDES [27]. Additionally, we quantified Tgfbr1 levels, as it was demonstrated to be regulated by MSI2 [43] and also P21 (also known as Cdkn1a), which, in contrast to TGFBR1, is inhibited by MSI2 [44]. Tgfbr1 levels increased between 30 and 40% in both analyzed muscles, while P21 levels remained unchanged upon sustained Msi2 expression [Fig. 4E].
Sustained Msi2 overexpression promotes autophagy independently of Mbnl, Celf1, and Hnrnpa1 proteins
We analyzed by RT-qPCR the expression of nine genes related to autophagy, myogenesis, and muscle atrophy and differentiation [Fig. 5 A-I]. Globally, we observed that these markers were not impaired in HSALR model mice. These results explain, at least partially, the limited muscle dysfunction in HSALR mice. These data were fully consistent with previous works in which HSALR mice do not reproduce the pathological increase in autophagy reported in patients and different DM1 models [19,23,28]. However, upon Msi2 overexpression in skeletal muscle, we observed dysregulation of Atg4, Atg7, Atrogin 1, Mrf4, and LC3II/LC3I in both analyzed muscles, indicating that Msi2 was sufficient to change muscle gene expression towards disease-like patterns. However, the effect of Msi2 overexpression on other analysed markers was milder as dysregulation was detected in one of two analyzed muscles. An exception was Mstn, which, besides being the only one of 9 genes altered in one of the muscles of the model mice, did not change upon Msi2 overexpression [Fig. 5I]. Consistently with our initial hypothesis, increased Msi2 expression in model mice was sufficient to activate autophagic flux as determined by the LC3II/LC3I ratio [45] [Fig. 5J,M]. Additionally, we assessed levels of P62, a scaffold protein that carries cargoes committed to lysosomal degradation to the autophagosome. Increased autophagic activity leads to P62 reduction as it is removed by autophagy itself [45]. A decrease in gastrocnemius of almost half was observed in model mice injected with rAAV-Msi2 compared to those treated with control construct hDES. However, no significant effect was detected in the quadriceps where, in fact, significantly lower P62 levels were detected in the disease model mice [Fig. 5K,N]. Finally, ATG7 levels were evaluated. This protein promotes phagophore growth by means of ATG8 lipidation through its E1-like enzymatic activity during the elongation and maturation steps of degradative autophagy [45]. We observed in quadriceps from Msi2 infected mice an almost 2-fold increase in the expression of Atg7 when compared to the hDES group [Fig. 5L,O]. Conversely, no effect was detected in gastrocnemius from the same mice.
Fig. 5.
Msi2-overexpressing mice show altered muscle homeostasis gene expression. A-I Quantification by RT-qPCR (2−ΔΔCt) of the indicated genes in samples obtained from quadriceps or gastrocnemius from control and model mice treated with PBS (FVB and PBS, respectively), rAAV9-hDES or with rAAV9-Msi2. All values are relative to hDES in each muscle (dashed line). Gene's expression is normalized to mean of Gapdh, Gtf2b, and Hprt1 expression. J-O Relative quantification and representative western blots, with indication of molecular weight sizes to the right in kDa of the LC3-II/LC3-I ratio, P62, and Atg7 protein in protein extracts obtained from the same biological groups as in a-i. Gapdh was used as an endogenous control. Plots represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 according to Student's t-test. In all cases comparisons were FVB vs. PBS and hDES vs. Msi2. FVB (n = 8), HSALR PBS (n = 8), HSALR rAAV9-hDES (n = 6–10) and HSALR rAAV9-Msi2 (n = 13–15).
To investigate whether Msi2 may contribute to the DM1 pathophysiology through already described splicing factors involved in the pathogenic mechanism of the disease Mbnl1, Mbnl2, Celf1, and Hnrnpa1, levels were assessed upon AAV infection [Fig. 6]. Notably, no effect was detected upon Msi2 modulation in the total protein levels of none of the splicing factors compared to hDES controls, thus suggesting that the atrophy promoting gene expression changes generated by increased Msi2 expression is independent of Mbnl, Celf1, and Hnrnpa1 protein level changes. In contrast, the results suggest that increased Msi2 expression's effects are mediated by the induction of proteins/genes involved in the ubiquitin-proteasome system and/or autophagy.
Fig. 6.
DM1 altered splicing factors remain unchanged upon rAAV-Msi2 overexpression. Relative protein expression levels in quadriceps and gastrocnemius of A Mbnl1, B Mbnl2, C Celf1, and D Hnrnpa1 in FVB and HSALR treated with PBS as vehicle or HSALR injected with empty rAAV9 as control (hDES) or rAAV9 expressing Msi2 (Msi2). Data was relative to hDES. Gapdh was the endogenous control. Plots represent mean ± SEM. No significant differences were detected according to Student's t-test. In all cases comparisons were FVB vs. PBS and hDES vs. Msi2. FVB (n = 8), HSALR PBS (n = 8), HSALR rAAV9-hDES (n = 6–10) and HSALR rAAV9-Msi2 (n = 13–15).
Increased Msi2 expression causes myopathy in HSALR skeletal muscle and alters muscle histology
After weaning, we functionally characterized the impact of Msi2 overexpression in a DM1 context. The body weight of the mice was measured at four time points prior to euthanasia. The results showed that there were no differences in weight between the model mice and the FVB controls nor when comparing the Msi2-overexpressing mice with their hDES control [Fig. 7A][Supplementary Table 4].
Fig. 7.
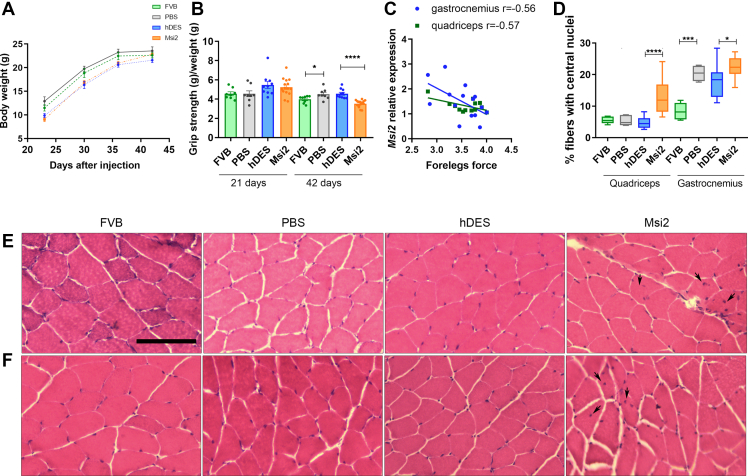
Msi2 overexpression enhances DM1-like phenotypes in HSALRmice. A Body weight measured at the indicated time points after the injection of age-matched controls and P2–P3 DM1 model mice treated with PBS (FVB and PBS, respectively), rAAV9-hDES, or rAAV9-Msi2. B Mice grip strength was normalized to mouse body weight at the indicated intermediate time point and before the sacrifice in the same biological groups described in a. Plots represent mean ± SEM. C Pearson's correlation between Msi2 relative expression and grip strength measured in rAAV9-Msi2 mice D Analysis of the percentage of muscle fibers with central nuclei in quadriceps and gastrocnemius muscles of the indicated experimental condition. The boxplots show the median with the interquartile range with the minimum and maximum values. Representative hematoxylin and eosin staining of E quadriceps and F gastrocnemius muscles from all four experimental groups. Arrows point to examples of centrally located nuclei in muscle fibers. Scale bar = 100 μm ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 according to Student's t-test. In all cases comparisons were FVB vs. PBS and hDES vs. Msi2; FVB (n = 8), HSALRPBS (n = 8), HSALR rAAV9-hDES (n = 10) and HSALR rAAV9-Msi2 (n = 15).
Foreleg strength was measured and ratioed to each individual's weight at 21 days of age and prior to sacrifice. The results showed that at the first studied time point, there were no differences between different groups. These results are in line with those published by Wei et al. 2018, where authors reported that young HSALR mice of 1 month of age did not display grip weakness [14]. However, in 42-day-old mice, a significant decrease in grip strength was observed in individuals infected with rAAV expressing Msi2 compared to those infected with empty rAAV as a control. Furthermore, since no differences in weight were observed at this age, the possibility that the lower relative strength of Msi2-overexpressing mice was due to lower body mass is ruled out [Fig. 7B]. Strikingly, HSALR model mice at the indicated age showed more grip strength than FVB controls, contrary to what we observed at 4.5 months of age [20]. Remarkably, we observed a significant negative correlation between Msi2 levels and forelegs force in quadriceps (r = −0.57, p = 0.04) and gastrocnemius (r = −0.56, p = 0.03) from mice overexpressing Msi2, highlighting the relevance of Msi2 dysregulation as a contributor to the muscle phenotype [Fig. 7C]. Hematoxylin and eosin staining was performed on quadriceps and gastrocnemius cross-sections. The overexpression of Msi2 in adult DM1 skeletal muscle supports that it negatively impacts muscle histology [Fig. 7D–F]. Indeed, quadriceps and gastrocnemius muscles from these mice displayed a significantly higher percentage of central nucleation of ∼3 and 1.25 fold, respectively, compared with hDES, which is consistent with histological features of skeletal muscle biopsy in DM1 [46]. Notably, there are no histopathological features at this age in quadriceps, other than occasional central nuclei.
Additionally, wheat germ agglutini (WGA) staining was performed in quadriceps and gastrocnemius sections from all the experimental groups to stain the plasma membrane necessary to measure muscle fibers' cross-sectional area (CSA) [Fig. 8A–C]. No differences were detected between the CSA of control mice (FVB) and disease model mice (PBS), reinforcing the weak myopathic phenotype of the HSALR model. Sustained Msi2 overexpression led to a significant 20 and 30% decrease in CSA in quadriceps and gastrocnemius, respectively [Fig. 8B and C].
Fig. 8.
Msi2 overexpression reduces area of muscular fibers. A Representative confocal images of WGA-stained (green) quadriceps and gastrocnemius sections from control and DM1 model mice treated with PBS (FVB and PBS, respectively) or DM1 mice injected with rAAV9-hDES or rAAV9-Msi2. Nuclei were counterstained with DAPI (blue). Scale bar = 50 μm. Quantification of the CSA of fibers from B quadriceps and C gastrocnemius muscles in the indicated biological groups. Tables indicate the minimum, maximum, and median values (in μm2) of the area of the fibers in the indicated conditions. C quadriceps and D gastrocnemius fiber CSA (μm2) displayed as a frequency distribution (% of total) of the same biological groups as in B, C. Values above the vertical dashed lines indicate the median area of each biological condition. Plots represent mean ± SEM. ∗p < 0.05, and ∗∗∗∗p < 0.0001 according to multiple Student's t-test. In all cases comparisons were FVB vs. PBS and hDES vs. Msi2; FVB (n = 6), HSALR, PBS (n = 8), HSALR rAAV9-hDES (n = 9) and HSALR rAAV9-Msi2 (n = 13–14). The number of fibers analyzed (n) in each condition is shown in B and C.
In agreement with these data, the frequency distribution of CSA showed an increased frequency of smaller fibers in mice overexpressing Msi2 concomitant with a decreased frequency of larger fibers when compared to DM1 model mice infected with the empty vector [Fig. 8D and E and Supplementary Fig. 3]. With this information, we performed a generalized linear mixed model assuming a gamma distribution of the fibers' area. In this model, the logarithm of the mean of the fiber area is linearly adjusted to each treatment. The model also takes into account the different areas that have been measured in the same mouse. Therefore, a random effect associated with the mouse is also considered. No significant differences were detected in the fibers area of the quadriceps, although a clear tendency to a reduction was observed in Msi2-expressing mice when compared to hDES (p = 0.054). However, in gastrocnemius, a 30% reduction in the fibers' area was detected (p < 0.00001; Supplementary Table 5) concomitantly with decreased mean of the CSA.
Altogether, our results show that sustained expression of Msi2 in a HSALR background is sufficient to boost functional and histological phenotypes that suggest increased myopathy.
Discussion
Myotonic dystrophy type 1 is a multisystemic disease with multiple characteristic symptoms at molecular (insulin resistance, alternative splicing defects, or calcium homeostasis dysregulation), histological (fiber size variability and central nucleation), and functional levels, including myotonia, muscle weakness, and atrophy [18]. The murine model HSALR displays myotonia and muscle weakness, centrally located nuclei in muscle fibers, and alternative splicing impairment [30,47]. However, its muscle atrophy phenotype is weak or absent, as reported here and independent studies [31,33].
Available data support that the increase in autophagy in DM1 contributes to the imbalance between protein synthesis and degradation that leads to the atrophic phenotype [19,48]. Specifically, we hypothesize that the disease-associated upregulation of MSI2 leads to a defect in miR-7 biogenesis [21,28]. This fact results in the derepression of autophagy mediated by the miR-7 decreased repressor activity on its targets, including ATG4, ATG7, and ULK2 [29]. In this work, we have shown that Msi2 protein levels remain unaltered in model mice concomitantly with control-like levels of autophagy activation state. However, in DM1 myotubes and DM1-patient's biopsies, miR-7 and MSI2 dysregulation were confirmed concurrently with evident markers of muscle atrophy and increased protein degradation [21,28].
By means of AAV infection, we discovered that sustained overexpression of murine Msi2 promotes a progressive myopathy characterized by molecular, histological, and functional deficits. Overall, we report that the overexpression of Msi2 in HSALR skeletal muscles exacerbates dystrophic and atrophic features by inhibiting miR-7 biogenesis concomitantly with activation of proteins and genes involved in the autophagic pathway. Thereby, Msi2 overexpressing mice recapitulate several histological abnormalities observed in the inducible CUG-960 model mice characterized by an overt muscle wasting phenotype accompanied by disruption of the balance between catabolic and anabolic pathways, including autophagy [23]. Furthermore, despite observing variability between individuals in quadriceps and gastrocnemius, the data obtained indicate that overexpression of Msi2 in HSALR muscle induces significant histological modifications, characterized by smaller myofibers and increased central nucleation leading to functional phenotypes such as weakened strength. At the molecular level, these phenotypes are accompanied by an increase of the autophagic activity marked by an increase of ATG7 and LC3-II/LC3-I ratio and decreased P62 accompanied by an induction of atrogenes expression. These results agree with previous reports demonstrating in HSALR mice under fasting conditions and in aged individuals the involvement of AMPK/mTORC1 deregulation in DM1 muscle pathophysiology [16]. Msi2 enhanced muscle dysfunction in the HSALR mouse model, but the extent to which these phenotypes might be overexpression-only effects vs DM1-dependent MSI2 overexpression effects could not be investigated because of lack of a Msi2 overexpression condition in the FVB genetic background.
Our results indicate that the pathological effect of Msi2 overexpression is independent of RNA-binding proteins well-known to be involved in disease pathogenesis, such as MBNL, CELF1, and HNRNPA [32]. These results are in line with those previously published, showing that miR-7 (whose biogenesis is directly regulated by MSI2) supplementation in DM1 muscle cells restored DM1-like phenotypes in an MBNL-independent manner [21]. However, in our previous work, we observed that targeting MSI2 through ASO gapmers in DM1 myotubes was sufficient to increase MBNL1 levels significantly [28], while in the current MSI2 upregulation was not sufficient to reduce MBNL1 levels in vivo. This suggests that either other factors contribute to the regulation of MBNL1 or that the levels of MSI2 overexpression achieved in our experiments are not high enough to reach autophagy levels that would have a significant impact on Mbnl1 degradation as it was reported that MBNL proteins were removed in an autophagy-dependent manner [20]. These results explain that HSALR model mice do reproduce the splicing defects characteristic of the disease and the phenotypes associated with these alterations but, in contrast, lack myopathy because catabolic degradation of proteins is insufficient to waste muscle significantly. Furthermore, results generated in vitro and in vivo models of the disease showed that CELF1 remains unchanged after both up- [Fig. 6C] and down-modulation of MSI2 [28]. These data suggest that the MSI2>miR-7>autophagy axis contributes to the muscle atrophy phenotype independently of the GSK3β/CELF1 pathway, which is dysregulated in DM1 and associated with muscle dysfunction [14]. Increased CELF1 and HNRNPA1 have been shown to exacerbate muscle dysfunction-related phenotypes in DM1 [32,49]. However, both proteins remained stable after MSI2 modulation. Taken together, it is possible that if all three factors contribute independently/additively to the atrophic phenotype, the worsening observed in rAAV9-Msi2-infected HSALR mice is not as potent as it might be if Celf1 and Hnrnpa1 expression were simultaneously promoted.
A certain degree of variability in Msi2 overexpression can be observed in muscle tissues and, consequently, in analyzed phenotypes [Fig. 4, Fig. 5]. Variability in the levels of the overexpressed protein and downstream effects are in line with those reported by Li et al., 2020 [32], where the authors used rAAV9 to overexpress the splicing regulatory factor HNRNPA1 and observed variability in the results among the analysed muscles, including gastrocnemius, and quadriceps. Since the authors report that transduced FVB control mice displayed similarly varied expression levels of overexpressed protein in different muscles compared to the patterns in transduced HSALR mice, this indicates that rAAV-mediated overexpression intrinsically fluctuates from muscle to muscle and interindividually. Indeed, it has been reported that rAAV9 shows myofiber-type preferences, which could be an underlying factor for mosaic transduction of skeletal muscle [50]. In this regard, it has been confirmed that HSALR mice show impaired myofiber-type distribution compared to unaffected controls [16]. This fact will likely favor heterogeneity in AAV infection, resulting in a wider dispersion of the values obtained for the different parameters studied. Additionally, [Supplementary Fig. 2] shows the considerable variability of the levels of CTG repeat-expressing transgene in HSALR model mice. Specifically, a significantly lower HSALR transgene expression in gastrocnemius in hDES mice suggests this muscle could be less sensitive to Msi2 levels because less toxic RNA is expressed. Consequently, we hypothesize that despite the higher Msi2 overexpression in gastrocnemius muscles, the lower additive effects by toxic RNA levels, leads to somewhat reduced overall overexpression phenotypes. In contrast, in quadriceps, where Msi2 levels relative to negative control hDES are lower than in gastrocnemius (1.7-fold vs. 3.8-fold, respectively), the opposite is true for toxic RNA amounts [Fig. 4]. Therefore, gene expression fluctuations stemming from the AAV overexpression method and the HSA transgene likely contribute to the variability observed in the results between muscles and animals. Overall, this issue has been addressed by conducting experiments with a sufficiently large number of individuals. A higher expression of Msi2 was detected in gastrocnemius concomitantly with a stronger reduction of miR-7 and CSA of the muscle fibers [Fig. 4, Fig. 8]. These results again support the association of MSI2 with the atrophic phenotype in a pathological context. We observed a negative correlation between force and Msi2 levels in mice injected with rAAV-Msi2 [Fig. 7C]. Interestingly, we have reported a similar relationship between MSI2 and ankle dorsiflexion strength in DM1 patients [28]. Morriss et al., 2018 characterized muscle atrophy in CUG960 mice, and as with HSALR overexpressing Msi2, the difference in muscle fiber size was more evident in smaller muscle fibers and the gastrocnemius than in the quadriceps [23]. However, our results suggest that more factors are contributing to this phenomenon as, overall, the enhancement of DM1-like was globally significant, but the potentiation of the pathological phenotypes was variable. It is also possible that further overexpression is necessary to induce stronger phenotypes.
In summary, our findings furnish compelling in vivo evidence demonstrating the association of levels of Msi2 overexpression with the progressive muscle degeneration observed in DM1. Thus, we propose a new axis: MSI2>miR-7>autophagy that contributes to the muscle atrophy phenotype together with other signaling cascades that were reported to contribute to DM1 muscle atrophy, including AKT/GSK3β [14], TWEAK/Fn14 [15], AMPK [16], or PKC [17]. Altogether, the MSI2>miR-7>autophagy axis could be explored as a novel target for therapeutic intervention.
Funding
This work was possible by research grants RTI2018-094599-B-100 from the Ministerio de Ciencia e Innovación-Agencia Estatal de Investigación, which included funds from the European Regional Development Fund (ERDF), and PROMETEO/2020/081 from the Generalitat Valenciana to R.A. Additional funding was from Instituto de Salud Carlos III, Ministry of Science and Innovation, PI21/00311 to M.P-A. M.S.-A. thanks the support of the Conselleria d’Educació, Investigació and Cultura i Esport (Generalitat Valenciana) as predoctoral (ACIF/2018/071) grant. N.M. was supported by a predoctoral fellowship (PRE2019-090622) from the Ministerio de Ciencia e Innovación-Agencia Estatal de Investigación. A.B. is grateful for the support of the Instituto de Salud Carlos III, Ministry of Science and Innovation, as a Sara Borrell postdoctoral grantee (CD21/00031). Part of the equipment employed in this work has been funded by Generalitat Valenciana and co-financed with ERDF funds (OP ERDF of Comunitat Valenciana2014-2020).
Data availability
All data supporting the findings of this study are available. Raw data are available on request.
Author contributions
R.A. provided the conceptual framework for the study. R.A., A.B., T.S., and M.P-A. conceived and supervised the experiments. R.A. and A.B. administered the project. M.P-A and R.A. obtained funds. N.M. and M.S.-A., performed the experiments and analyzed data. A.B. wrote the original article. R.A., M.S-A., and N.M. participated in the review and editing of the manuscript.
Ethics approval
Mouse handling and experimental procedures conformed to the European law regarding laboratory animal care and experimentation (2003/65/CE) and were approved by Conselleria de Agricultura, Generalitat Valenciana (reference number 2021/VSC/PEA/0019 and 2021/VSC/PEA/0155).
Declaration of competing interest
M.S-A., R.A., and A.B. are inventors in patent PCT/EP2022/054129.
Acknowledgements
We acknowledge the Bioinformatics and Biostatistics Unit of INCLIVA Biomedical Research Institute for its advice and support on the statistical analyses of our data.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2023.100667.
Contributor Information
Maria Sabater-Arcis, Email: maria.sabater@uv.es.
Nerea Moreno, Email: Nerea.moreno@uv.es.
Teresa Sevilla, Email: sevilla_ter@gva.es.
Manuel Perez Alonso, Email: manuel.perez@uv.es.
Ariadna Bargiela, Email: ariadna_bargiela@iislafe.es.
Ruben Artero, Email: ruben.artero@uv.es.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Ashizawa T, Gagnon C, Groh WJ, Gutmann L, Johnson NE, Meola G, et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol Clin Pract. 2018;8(6):507–520. doi: 10.1212/CPJ.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson NE, Butterfield RJ, Mayne K, Newcomb T, Imburgia C, Dunn D, et al. Population-based prevalence of myotonic dystrophy type 1 using genetic analysis of state-wide blood screening program. Neurology. 2021;96(7):e1045–53. doi: 10.1212/WNL.0000000000011425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3’ end of a transcript encoding a protein kinase family member. Cell. 1992;6(2):385. doi: 10.1016/0092-8674(92)90418-c. [DOI] [PubMed] [Google Scholar]
- 4.Yuan Y, Compton SA, Sobczak K, Stenberg MG, Thornton CA, Griffith JD, et al. Muscleblind-like 1 interacts with RNA hairpins in splicing target and pathogenic RNAs. Nucleic Acids Res. 2007;35(16):5474–5486. doi: 10.1093/nar/gkm601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konieczny P, Stepniak-Konieczna E, Sobczak K. MBNL proteins and their target RNAs, interaction and splicing regulation. Nucleic Acids Res. 2014;42(17):10873-87. doi: 10.1093/nar/gku767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batra R, Manchanda M, Swanson MS. Global insights into alternative polyadenylation regulation. RNA Biol. 2015;12(6):597–602. doi: 10.1080/15476286.2015.1040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Dixon DM, Dansithong W, Abdallah WF, Roos KP, Jordan MC, et al. Muscleblind-like 3 deficit results in a spectrum of age-associated pathologies observed in myotonic dystrophy. Sci Rep. 2016;6:30999. doi: 10.1038/srep30999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ET, Ward AJ, Cherone JM, Giudice J, Wang TT, Treacy DJ, et al. Antagonistic regulation of mRNA expression and splicing by CELF and MBNL proteins. Genome Res. 2015;25(6):858–871. doi: 10.1101/gr.184390.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Costa JM, Llamusi MB, Garcia-Lopez A, Artero R. Alternative splicing regulation by Muscleblind proteins: from development to disease. Biol Rev Camb Phil Soc. 2011;86(4):947–958. doi: 10.1111/j.1469-185X.2011.00180.x. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler TM, Lueck JD, Swanson MS, Dirksen RT, Thornton CA. Correction of ClC-1 splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy. J Clin Invest. 2007;117(12):3952–3957. doi: 10.1172/JCI33355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savkur RS, Philips AV, Cooper TA. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29(1):40–47. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 12.Pang PD, Alsina KM, Cao S, Koushik AB, Wehrens XHT, Cooper TA. CRISPR‐Mediated expression of the fetal Scn5a isoform in adult mice causes conduction defects and arrhythmias. J Am Heart Assoc. 2018;7(19):e010393 doi: 10.1161/JAHA.118.010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton CA. Myotonic dystrophy. Neurol Clin. 2014;32(3):705–719. doi: 10.1016/j.ncl.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei C, Stock L, Valanejad L, Zalewski ZA, Karns R, Puymirat J, et al. Correction of GSK3β at young age prevents muscle pathology in mice with myotonic dystrophy type 1. FASEB J. 2018;32(4):2073–2085. doi: 10.1096/fj.201700700R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadava RS, Foff EP, Yu Q, Gladman JT, Kim YK, Bhatt KS, et al. TWEAK/Fn14, a pathway and novel therapeutic target in myotonic dystrophy. Hum Mol Genet. 2015;24(7):2035–2048. doi: 10.1093/hmg/ddu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brockhoff M, Rion N, Chojnowska K, Wiktorowicz T, Eickhorst C, Erne B, et al. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J Clin Invest. 2017;127(2):549–563. doi: 10.1172/JCI89616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuyumcu-Martinez NM, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28(1):68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozimski LL, Sabater‐Arcis M, Bargiela A, Artero R. The hallmarks of myotonic dystrophy type 1 muscle dysfunction. Biol Rev Camb Philos Soc. 2021;96(2):716–730. doi: 10.1111/brv.12674. [DOI] [PubMed] [Google Scholar]
- 19.Bargiela A, Cerro-Herreros E, Fernandez-Costa JM, Vilchez JJ, Llamusi B, Artero R. Increased autophagy and apoptosis contribute to muscle atrophy in a myotonic dystrophy type 1 Drosophila model. Dis Model Mech. 2015;8(7):679–690. doi: 10.1242/dmm.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bargiela A, Sabater-Arcis M, Espinosa-Espinosa J, Zulaica M, Lopez de Munain A, Artero R. Increased Muscleblind levels by chloroquine treatment improve myotonic dystrophy type 1 phenotypes in in vitro and in vivo models. Proc Natl Acad Sci USA. 2019;116(50):25203–25213. doi: 10.1073/pnas.1820297116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabater-Arcis M, Bargiela A, Furling D, Artero R. miR-7 restores phenotypes in myotonic dystrophy muscle cells by repressing hyperactivated autophagy. Mol Ther Nucleic Acid. 2020;19:278–292. doi: 10.1016/j.omtn.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loro E, Rinaldi F, Malena A, Masiero E, Novelli G, Angelini C, et al. Normal myogenesis and increased apoptosis in myotonic dystrophy type-1 muscle cells. Cell Death Differ. 2010;17(8):1315–1324. doi: 10.1038/cdd.2010.33. [DOI] [PubMed] [Google Scholar]
- 23.Morriss GR, Rajapakshe K, Huang S, Coarfa C, Cooper TA. Mechanisms of skeletal muscle wasting in a mouse model for myotonic dystrophy type 1. Hum Mol Genet. 2018;27(16):2789–2804. doi: 10.1093/hmg/ddy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matalkah F, Jeong B, Sheridan M, Horstick E, Ramamurthy V, Stoilov P. The Musashi proteins direct post-transcriptional control of protein expression and alternate exon splicing in vertebrate photoreceptors. Commun Biol. 2022;5(1):1011. doi: 10.1038/s42003-022-03990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cragle CE, MacNicol MC, Byrum SD, Hardy LL, Mackintosh SG, Richardson WA, et al. Musashi interaction with poly(A)-binding protein is required for activation of target mRNA translation. J Biol Chem. 2019;294(28):10969–10986. doi: 10.1074/jbc.RA119.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudinov AE, Karanicolas J, Golemis EA, Boumber Y. Musashi RNA-binding proteins as cancer drivers and novel therapeutic targets. Clin Cancer Res. 2017;23(9):2143–2153. doi: 10.1158/1078-0432.CCR-16-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhury NR, de Lima Alves F, de Andres-Aguayo L, Graf T, Caceres JF, Rappsilber J., et al. Tissue-specific control of brain-enriched miR-7 biogenesis. Genes Dev. 2013;27(1):24–38. doi: 10.1101/gad.199190.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabater-Arcis M, Bargiela A, Moreno N, Poyatos-Garcia J, Vilchez JJ, Artero R. Musashi-2 contributes to myotonic dystrophy muscle dysfunction by promoting excessive autophagy through miR-7 biogenesis repression. Mol Ther Nucleic Acids. 2021;25:652–667. doi: 10.1016/j.omtn.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu DN, Jiang MJ, Mei Z, Dai JJ, Dai CY, Fang C, et al. microRNA-7 impairs autophagy-derived pools of glucose to suppress pancreatic cancer progression. Cancer Lett. 2017;400:69–78. doi: 10.1016/j.canlet.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Mankodi A, Logigian E, Callahan L, McClain C, White R, Henderson D, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289(5485):1769–1773. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 31.Bargiela A, Ten-Esteve A, Martí-Bonmatí L, Sevilla T, Perez Alonso M, Artero R. Quantitative magnetic resonance imaging assessment of muscle composition in myotonic dystrophy mice. Sci Rep. 2023;13(1):503. doi: 10.1038/s41598-023-27661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Zhuang Y, Batra R, Thomas JD, Li M, Nutter CA, et al. HNRNPA1-induced spliceopathy in a transgenic mouse model of myotonic dystrophy. Proc Natl Acad Sci USA. 2020;117(10):5472–5477. doi: 10.1073/pnas.1907297117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford Parks TE, Marcellus KA, Péladeau C, Jasmin BJ, Ravel-Chapuis A. Overexpression of Staufen1 in DM1 mouse skeletal muscle exacerbates dystrophic and atrophic features. Hum Mol Genet. 2020;29(13):2185–2199. doi: 10.1093/hmg/ddaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacak CA, Sakai Y, Thattaliyath BD, Mah CS, Byrne BJ. Tissue specific promoters improve specificity of AAV9 mediated transgene expression following intra-vascular gene delivery in neonatal mice. Genet Vaccines Ther. 2008;6:13. doi: 10.1186/1479-0556-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faraway JJ. Chapman and Hall/CRC; 2016. Extending the linear model with R. [Google Scholar]
- 36.Pisani V, Panico MB, Terracciano C, Bonifazi E, Meola G, Novelli G, et al. Preferential central nucleation of type 2 myofibers is an invariable feature of myotonic dystrophy type 2. Muscle Nerve. 2008;38(5):1405–1411. doi: 10.1002/mus.21122. [DOI] [PubMed] [Google Scholar]
- 37.Muraine L, Bensalah M, Dhiab J, Cordova G, Arandel L, Marhic A, et al. Transduction efficiency of adeno-associated virus serotypes after local injection in mouse and human skeletal muscle. Hum Gene Ther. 2020;31(3-4):233–240. doi: 10.1089/hum.2019.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis JE, Brameld JM, Hill P, Barrett P, Fjp Ebling, Jethwa PH. The use of a viral 2A sequence for the simultaneous over-expression of both the vgf gene and enhanced green fluorescent protein (eGFP) in vitro and in vivo. J Neurosci Methods. 2015;256:22–29. doi: 10.1016/j.jneumeth.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lala V, Zubair M, Minter DA. StatPearls Publishing; Treasure Island (FL): 2023. Liver function tests. StatPearls [Internet] PMID: 29494096. [PubMed] [Google Scholar]
- 40.Ono K, Ono T, Matsumata T. The pathogenesis of decreased aspartate aminotransferase and alanine aminotransferase activity in the plasma of hemodialysis patients: the role of vitamin B6 deficiency. Clin Nephrol. 1995;43(6):405–408. [PubMed] [Google Scholar]
- 41.Lee JH, Cho AR, Lee YJ. Relationship between serum alkaline phosphatase and low muscle mass index among Korean adults: a nationwide population-based study. Biomolecules. 2021;11(6):842. doi: 10.3390/biom11060842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoguchi Y, Inoguchi T, Eto T, Masakado M, Suehiro S, Yamauchi T, et al. Relationship between serum indirect bilirubin levels and skeletal muscle mass in older male and female patients with type 2 diabetes. PLoS One. 2022;17(11) doi: 10.1371/journal.pone.0276976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SM, Deering RP, Lu Y, Tivnan P, Lianoglou S, Al-Shahrour F, et al. Musashi-2 controls cell fate, lineage bias, and TGF-β signaling in HSCs. J Exp Med. 2014;211(1):71–87. doi: 10.1084/jem.20130736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Sheng W, Jia C, Shi X, Cao R, Wang G, et al. Musashi2 promotes the progression of pancreatic cancer through a novel ISYNA1‐p21/ZEB‐1 pathway. J Cell Mol Med. 2020;24(18):10560–10572. doi: 10.1111/jcmm.15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Arozena AA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vihola A, Bassez G, Meola G, Zhang S, Haapasalo H, Paetau A, et al. Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology. 2003;60(11):1854–1857. doi: 10.1212/01.wnl.0000065898.61358.09. [DOI] [PubMed] [Google Scholar]
- 47.Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;10(1):35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 48.Beffy P, Del Carratore R, Masini M, Furling D, Puymirat J, Masiello P, et al. Altered signal transduction pathways and induction of autophagy in human myotonic dystrophy type 1 myoblasts. Int J Biochem Cell Biol. 2010;42(12):1973–1983. doi: 10.1016/j.biocel.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Ward AJ, Rimer M, Killian JM, Dowling JJ, Cooper TA. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum Mol Genet 2010;19(18):3614-22. [DOI] [PMC free article] [PubMed]
- 50.Riaz M, Raz Y, Moloney EB, van Putten M, Krom YD, van der Maarel SM., et al. Differential myofiber-type transduction preference of adeno-associated virus serotypes 6 and 9. Skelet Muscle. 2015;5:37. doi: 10.1186/s13395-015-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available. Raw data are available on request.