Abstract
Respiratory syncytial virus (RSV) is a substantial cause of morbidity and mortality globally. A candidate RSV prefusion (pre-F) stabilized subunit vaccine, DS-Cav1, has previously been shown to elicit potent and durable neutralizing activity in a phase 1 clinical trial in healthy adults. Here, we used fluorescently labeled probes and flow cytometry to evaluate the antigen specificity and phenotype of RSV F-specific B cells longitudinally following DS-Cav1 immunization. Peripheral blood mononuclear cells (PBMCs) collected at timepoints before the first immunization through the end of the trial at 44 weeks were assessed by flow cytometry. Our data demonstrate a rapid increase in the frequency of pre-F-specific IgG+ and IgA+ B cells following the first immunization, and a modest increase after a second immunization at week 12. Nearly all F-specific B cells downregulated CD21 and upregulated the proliferation marker CD71 after the first immunization, with less pronounced activation after the second immunization. Memory B cells (CD27+CD21+) specific for pre-F remained elevated above baseline at 44 weeks post-vaccination. DS-Cav1 vaccination also activated human metapneumovirus (HMPV) cross-reactive B cells capable of binding prefusion-stabilized HMPV F protein, and increased HMPV F-binding antibodies and neutralizing activity for HMPV in some participants. In summary, vaccination with RSV pre-F resulted in the expansion and activation of RSV and HMPV F-specific B cells that were maintained above baseline for at least 10 months and could contribute to long-term pneumovirus immunity.
One Sentence Summary:
A respiratory syncytial virus (RSV) pre-fusion (F) subunit vaccine elicited RSV F-specific and human metapneumovirus cross-reactive B cells in humans.
INTRODUCTION
Respiratory syncytial virus (RSV) remains a substantial cause of disease and mortality in both infants and the elderly. RSV is associated with nearly half of medically attended respiratory infections in children under a year of age and causes an estimated 33.1 million episodes of acute lower respiratory infections (LRTI), 3.2 million hospitalizations, and 118,200 deaths in children under five years of age annually (1). RSV infects about 5 to 10% of adults and is responsible for an estimated 10,000 deaths in older adults (greater than 64 years of age) each year (2, 3). Older adults with underlying heart and lung disease or compromised immune systems are at the greatest risk for RSV-related morbidity and mortality, with an annual impact that can be as large as influenza (4). A variety of RSV vaccine candidates are being tested clinically in pediatric and older adult high-risk groups, or in pregnant women to confer protection in infants following perinatal antibody transfer (5). The primary goal of most vaccines is the elicitation of antibodies targeting the RSV viral fusion glycoprotein (F) (6, 7). The effectiveness of antibodies targeting F has been demonstrated by the success of passive monoclonal antibody therapy in high-risk populations; further, higher F-specific antibodies correlate with lower susceptibility to infection and disease (8–12). Although a large-scale efficacy trial of an RSV F-based protein vaccine in pregnant women failed to meet prespecified efficacy endpoints, it illustrated that a two to three-fold rise in RSV neutralizing activity in pregnant women offers measurable protection against severe disease in infants (13). This suggests a greater boost from a stabilized prefusion F (pre-F) vaccine could result in more durable protection.
The F protein is required for fusion of the viral and host cell membranes, and pre-F has multiple structurally defined, relatively co-dominant antigenic sites (7, 14, 15). Although several of these antigenic sites (I-IV) are retained to different extents on the postfusion conformation (post-F), antibodies that bind sites Ø and V, which are unique to pre-F, possess the highest neutralizing potency (12, 16, 17). As a result, vaccines that preserve the structure of pre-F have substantially improved the quality of elicited antibodies compared to prior post-F or structurally undefined F-based vaccines (13, 18–24). The unprecedented ten-fold rise in neutralizing activity after pre-F vaccination was durable, with neutralizing activity remaining three- to five-fold above baseline titers up to a year post-vaccination (18, 21). Pre-F subunit vaccines are currently being tested in large-scale efficacy trials in pregnant women and older adults (Clinical Trial ID: NCT04605159, NCT04886596). Serum binding and neutralizing antibodies are the most standard readouts of immunogenicity in clinical trials. The specificity, phenotype, and function of vaccine-elicited memory B cells are not often evaluated and are less well understood. Memory B cells may become antibody-secreting cells (ASC), proliferate in the germinal center, or differentiate into long-lived plasma cells (LLPC) (25). Vaccination can bolster long-lived memory B cells and plasma cells, increasing recall capacity and conferring protection from future infections.
We recently published the longitudinal, full-trial analysis of serological readouts from the phase 1 clinical trial of DS-Cav1 (NCT03049488), a safe and well-tolerated pre-F subunit vaccine that increased neutralizing activity between 7.5- and 12.6-fold when administered at 50, 150, or 500 microgram doses with or without Alhydrogel adjuvant (alum) (18). In depth analyses of F-specific B cells in six trial participants demonstrated that DS-Cav1 boosted B cell lineages, many of which were present in multiple individuals, with specificity for all described antigenic sites on pre-F (12, 16, 17). Here, we further extended our analyses to define the impact of DS-Cav1 vaccination on F-specific memory B cell populations. Biotinylated pre-F and post-F proteins were conjugated to a streptavidin-labeled fluorophore and used to identify F-specific B cells. These capture probes were used in parallel with a multiparameter flow cytometry panel to determine the specificity and phenotype of responding B cells longitudinally for all 95 individuals enrolled in the trial. In addition to robustly eliciting and activating RSV F-specific B cells, DS-Cav1 vaccination activated human metapneumovirus (HMPV) cross-reactive B cells capable of binding HMPV F in its prefusion conformation. This was commensurate with an increase in HMPV neutralizing and F-binding antibodies in DS-Cav1 vaccinated individuals. We further compared the response of participants that received a single immunization to those that received a second immunization twelve weeks after the first, and found no differences in the long-term B cell memory response with a second immunization. Our results offer insight into B cell specificity and function after one or two immunizations of healthy, RSV-experienced adults with an RSV pre-F vaccine.
RESULTS
DS-Cav1 Vaccination Potently Elicited Pre-F Specific Memory B Cells.
Ninety-five participants were enrolled in the phase 1 trial of DS-Cav1, which was administered with or without alum at escalating doses (50, 150, or 500 μg) to healthy adults aged 18 to 50. Our full-trial serological analyses ruled out an adjuvant effect in this antigen-experienced population and identified only a marginal effect of dose and number of immunizations (18). We compared the response to vaccination between males and females and there was no difference (fig. S1). We were interested in further understanding the limited impact of the second immunization at week 12 on serum neutralization and undertook a longitudinal comparison of B cell responses in participants that received a single immunization at week 0 (33 of 95) to those that received immunizations at week 0 and week 12 (62 of 95). We used multiparameter flow cytometry, combining B cell surface markers with fluorescently-labeled RSV pre-F and post-F capture probes to evaluate the specificity, phenotype, and durability of F-specific memory B cells in peripheral blood mononuclear cells (PBMC) collected at weeks 0 (first immunization), 1, 2, 4, 12 (second immunization), 13, 14, 16, 28, and 44 (end of trial).
Immunoglobulin (Ig)G+ B cells were binned for specificity based on binding to the RSV F probes as having a strong preference for the pre-F probe (pre-F), having a strong preference for the post-F probe (post-F) or binding both probes (dual) (Fig. 1A, fig. S2). For most individuals, the frequency of F-specific B cells rose dramatically by week 2 (Fig. 1B). This rise was most pronounced in the pre-F and dual populations, which rose from 0.11 (±0.1)% to 2.5 (±1.8)% and 0.06 (±0.051)% to 1.1 (±1.2)%, respectively. The post-F population had a slight but statistically significant (p<0.0001) increase from 0.024 (±0.017)% to 0.13 (±0.12)%. We compared the change in the frequency of F-specific IgG+ B cells between weeks 12 and 14 for the 33 individuals that received a single immunization at week 0 and the 62 individuals who received a first immunization at week 0 and a second immunization at week 12. Although there was no change in the frequency of any of the probe-binding populations between weeks 12 and 14 in individuals immunized once, the frequency of pre-F and dual B cells significantly (p<0.0001) increased in individuals that received a second immunization (Fig. 1C). Regardless of the number of vaccinations received, pre-F and dual-probe-binding populations remained significantly (p<0.0001) above baseline at the end of the trial at week 44 (Fig. 1D). Assessing all ten timepoints longitudinally for individuals receiving one (Fig. 1E) and two (Fig. 1F) immunizations clearly illustrates the high prevalence of pre-F and dual IgG+ B cells for the first several weeks after vaccination, with a more modest boost in the frequency of those populations in individuals immunized again at week 12. Similar cross-sectional and longitudinal analyses of the frequency of probe-binding IgA+ B cell populations were performed and are presented in fig. S3A to D and fig. S3E and F, respectively. In summary, DS-Cav1 immunization substantially and durably increased the frequency of F probe-binding IgG+ and IgA+ B cells, particularly those capable of binding the pre-F probe, with a modest boost following the second immunization.
Figure 1. DS-Cav1 immunization increases the frequency of RSV F-specific B cells.
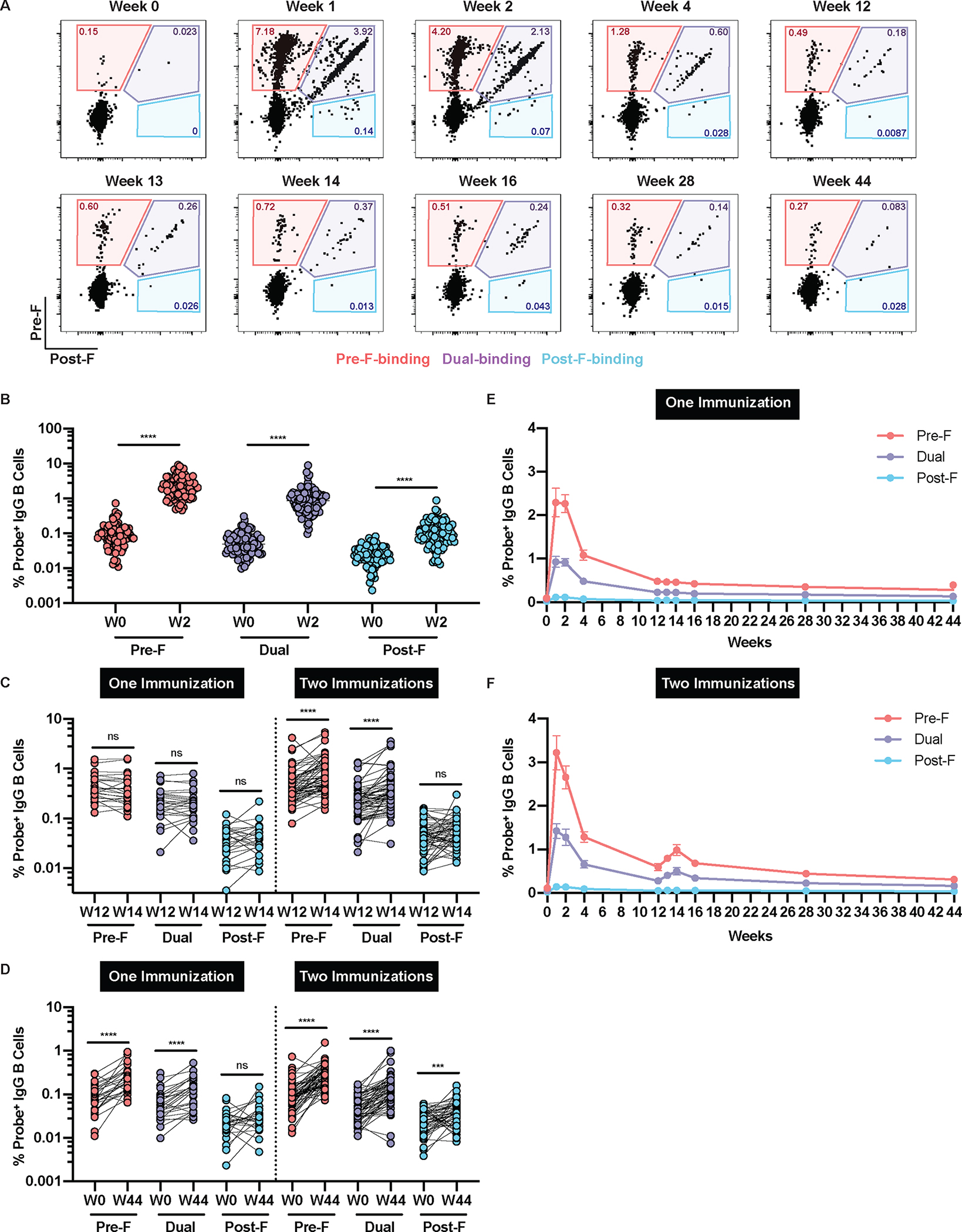
(A) Representative flow cytometry plots are shown from a single participant that received two immunizations showing probe-binding frequencies of RSV pre-F-binding (red), dual-binding (purple), and post-F-binding (blue) IgG+ B cells in PBMC. (B) The frequency of F-specific IgG+ B cells were quantified before and two weeks after the first immunization (n=95). The frequency of RSV F-specific IgG+ B cells were quantified in individuals who received either one (n=33) or two (n=62) vaccinations at (C) week 12 and week 14 and (D) week 0 and week 44. There were no statistical differences (by Mann-Whitney nonparametric t-test, p>0.05) in responses at W44 between individuals that received a single vaccination and those that received two vaccinations. Longitudinal frequencies of RSV probe-binding IgG+ B cells are shown for individuals who received (E) one (n=33) or (F) two (n=62) immunizations. Data are presented as mean ± standard error of the mean (SEM). Significance was determined using a Wilcoxon paired t-test; ***p<0.001 and ****p<0.0001. ns, not significant. For all of the data shown, cells were gated on mononuclear cells/singlets/live/CD19+/CD20+CD38−/IgD−/IgG+/CD27+.
We measured plasmablasts (PB), defined as cluster of differentiation (CD)38+CD20− (fig. S2), longitudinally, noting a sharp spike in frequency at one-week post-immunization that declined by week 2, and no increase following the second immunization at week 12 (fig. S4A). As expected, mobilization of plasmablasts at week 1 correlated well with the fold-change in serum neutralizing activity against RSV subtype A between weeks 0 and 4 (fig. S4B). We correlated frequencies of pre-F-, dual-, and post-F-specific memory IgG+ B cells (IgG+CD27+) to the week 0 to week 4 fold-change in serum neutralization, demonstrating that pre-F and dual-specificity B cells better correlated with the increase in neutralizing activity than those of the post-F specificity (fig. S4C). Our data demonstrate that mobilization of pre-F-specific plasmablasts and memory B cells coincide with the robust increase in neutralizing activity observed in the clinical trial.
DS-Cav1 vaccination activated pre-F and dual-binding memory B cells.
We next interrogated the phenotype of the pre-F and dual probe-binding IgG+ B cell populations at each of the ten longitudinal timepoints. Probe-binding memory IgG+ B cells (CD19+IgM−IgD−IgG+CD27+) were segregated into four populations; cells expressing CD21 in the absence of CD71 were defined as resting memory, whereas CD21−CD71+, CD21+CD71+, CD21−CD71− subsets were identified and all considered as activated memory cells (Fig. 2A). By week 1, coinciding with the peak frequency of probe-binding cells (Fig. 1E and F), 95.2% and 94.2% of the pre-F-binding and dual-binding B cell populations exhibited an activated phenotype, respectively, with the majority downregulating CD21 and upregulating CD71, which are hallmarks of B cell activation and proliferation (26, 27). Over time, pre-F-binding and dual-binding B cell populations shifted to more intermediate activated phenotypes (CD21+CD71+ and CD21−CD71−) and were predominantly resting memory B cells by week 12 (Fig. 2B). In individuals that received a second immunization at week 12, there was a shift toward higher proportions of activated cells at weeks 13 (59.4% and 55.7% for pre-F-binding and dual-binding, respectively), but this was not as pronounced as activation following the first immunization (Fig. 2C). Pre-F-binding and dual-binding IgA+ memory B cells were also assessed. Similarly, a second immunization with DS-Cav1 modestly increased the frequency of activated pre-F-binding and dual-binding memory IgA+ B cells at week 13, which was not observed in individuals who received only one immunization (fig. S5). In summary, DS-Cav1 vaccination rapidly and transiently activated pre-F and dual IgG+ and IgA+ B cells, particularly after the first immunization and with a more muted response after the second immunization.
Figure 2. DS-Cav1 immunization activates pre-F and dual IgG+ B cells.
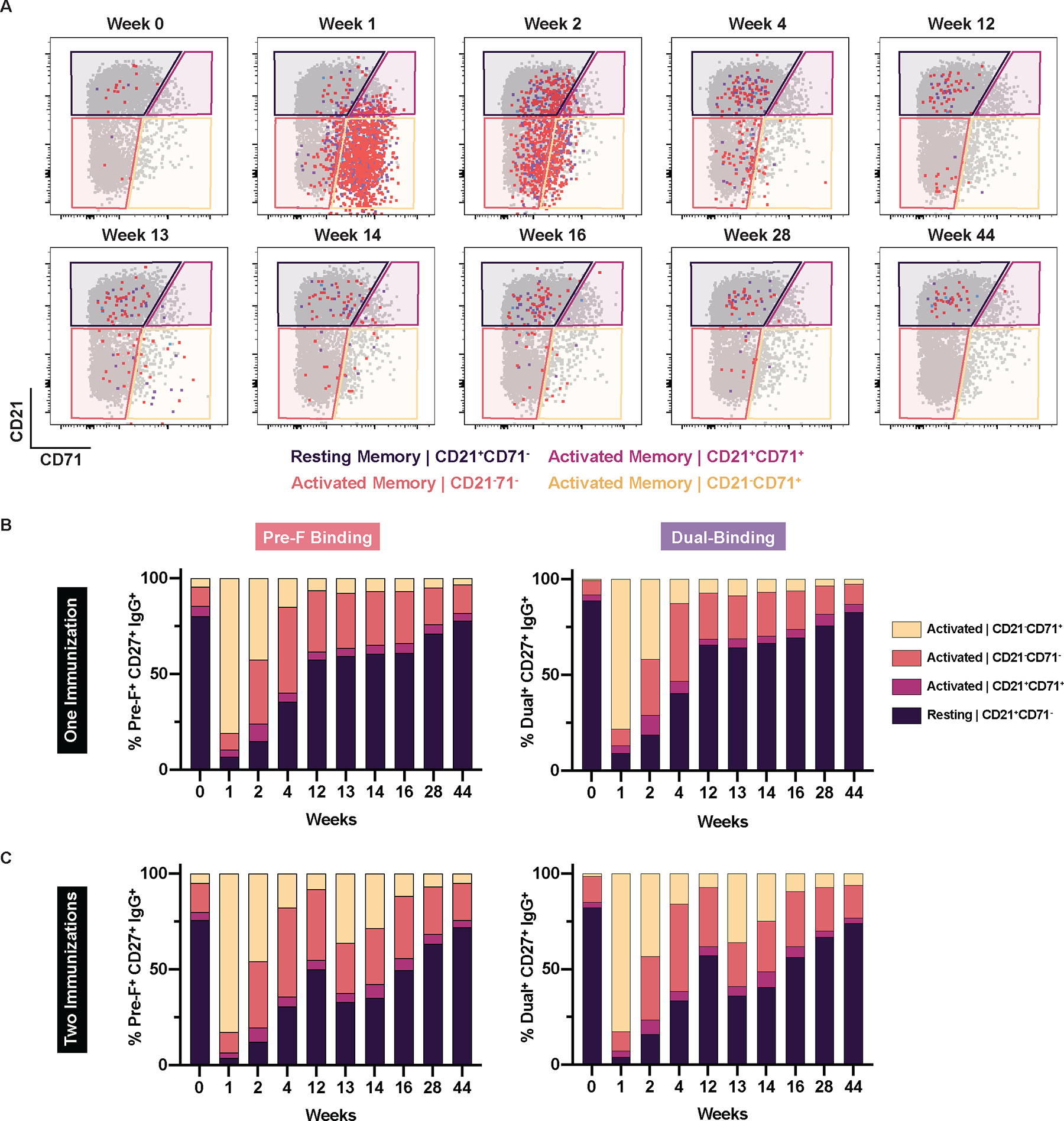
(A) Representative flow cytometry plots from a single participant that received two immunizations show RSV pre-F-binding (red), dual-binding (purple), and post-F-binding (blue) IgG+ B cells overlaid on top of total IgG+ memory B cells (gated on mononuclear cells/singlets/live/CD19+/CD20+CD38−/IgD−/IgG+/CD27+). The populations were defined based on their expression of CD21 (downregulated upon activation) and CD71 (proliferation marker). The frequencies of RSV pre-F-binding and dual-binding IgG+CD27+ B cells were quantified in individuals who received (B) one (n=33) or (C) two (n=62) immunizations. Probe-binding frequencies are normalized to 100%.
DS-Cav1 increased HMPV binding and neutralization and elicited HMPV cross-reactive B cells.
Cross-reactive antibodies have been identified based on structural homology between RSV F and the fusion protein of other pneumoviruses (28, 29). We hypothesized that immunization with DS-Cav1 would increase reactivity to the F protein of HMPV and improve HMPV neutralizing activity. We first evaluated serum antibodies against prefusion HMPV F (30) in 50 of the 95 participants at week 0, week 2, and week 4. HMPV pre-F binding increased by week 2, and slightly declined by week 4, but remained significantly above baseline (p=0.0012, Fig. 3A). Although responses were highly variable from participant to participant, serum neutralization of HMPV increased modestly but significantly in these 50 individuals between week 0 and week 2 (p=0.0001, Fig. 3B). We included prefusion HMPV F probes separately labeled with two different fluorochromes to identify and enumerate HMPV F-binding B cells for these 50 individuals. HMPV F-binding cells were further broken down into RSV F cross-reactive and non-cross-reactive populations (fig. S2). The week 0 frequency of HMPV pre-F probe binding IgG+ B cells was similar to the frequency of RSV pre-F probe binding cells (sum of pre-F and dual populations), indicating a similar resting memory B cell response to these two pathogens in healthy adults (Fig. 3C). Most cross-reactive B cells bound only the RSV pre-F probe, with only a small proportion binding both RSV pre-F and post-F probes (fig. S6).
Figure 3. DS-Cav1 immunization increases HMPV F-binding and neutralizing activity.
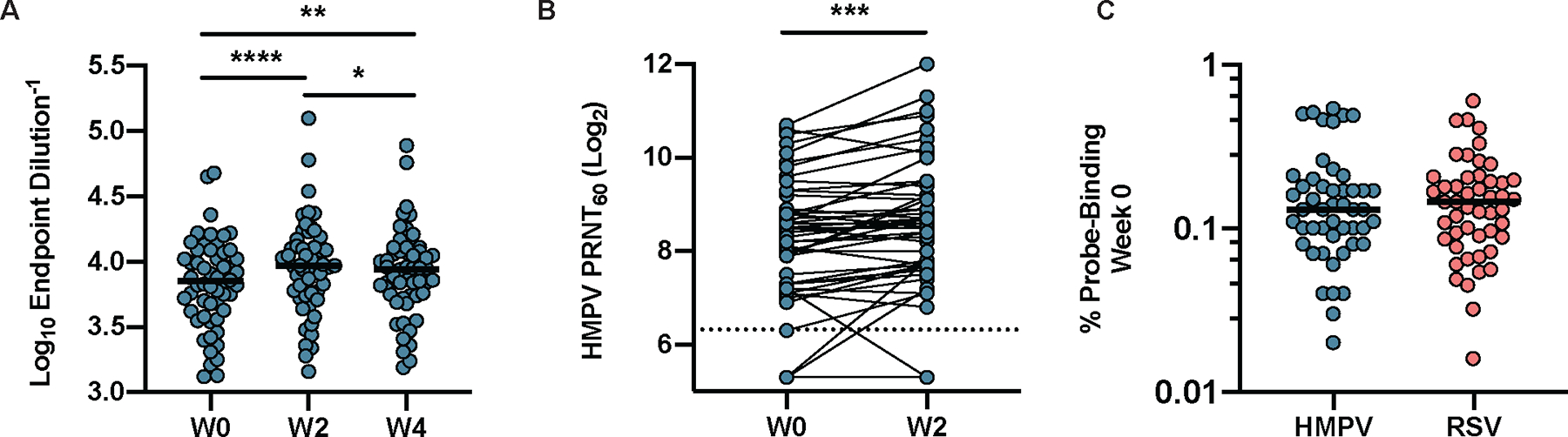
(A) HMPV pre-F-specific IgG in the serum was measured in 50 individuals at weeks 0, 2, and 4. (B) HMPV serum neutralizing activity measured at weeks 0 and 2 in the same 50 individuals. PRNT60, 60% plaque reduction neutralizing titer. The dashed line indicates the limit of detection. (C) The frequency of pre-existing (week 0) RSV pre-F (red) and HMPV pre-F (teal) probe-binding IgG+ B cells (n=50) is shown. Following gating on mononuclear cells/singlets/live/CD19+/CD20+CD38−/IgD−/IgG+/CD27+ B cells, the percentage binding either both HMPV pre-F probes, or the RSV pre-F probe was determined. Significance was calculated using a parametric paired t-test; *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. The horizontal bars in (A) and (C) indicate the median.
The frequency of HMPV F-binding IgG+ B cells was assessed longitudinally and demonstrated the capacity of DS-Cav1 immunization to recruit cross-reactive B cells regardless of the number of DS-Cav1 immunizations received (Fig. 4, A and B). Although cross-reactive B cells were present at a lower frequency than HMPV-only-binding B cells at baseline, specific elicitation of cross-reactive B cells led to a higher proportion of these cells at week 2 compared to B cells that bound only the HMPV F probes (Fig. 4C). A similar pattern was observed for IgA+ cross-reactive B cells at a lower frequency (fig. S7). The frequency of RSV pre-F binding B cells (sum of pre-F and dual populations) correlated with the frequency of HMPV F cross-reactive B cells at week 2 (Fig. 4D), indicating that responsiveness to DS-Cav1 is a predictor of the elicitation of cross-reactive B cells. We evaluated the phenotype of IgG+ B cells (Fig. 4E), comparing activation of cross-reactive B cells in individuals that received one or two DS-Cav1 immunizations. We found these cross-reactive cells to be as potently activated as RSV-F-specific B cells by week 1, with a similar kinetic transition back to resting memory based on whether a second immunization was administered (Fig. 4F, data file S1). This activation was restricted to the cross-reactive B cells, as no activation was observed for those that only bound the HMPV F probes (Fig. 4G, data file S1). Taken together, our serological and cellular results unequivocally demonstrate the ability of DS-Cav1 to activate cross-reactive immune responses to HMPV and RSV.
Figure 4. DS-Cav1 immunization activates pneumovirus cross-reactive B cells.
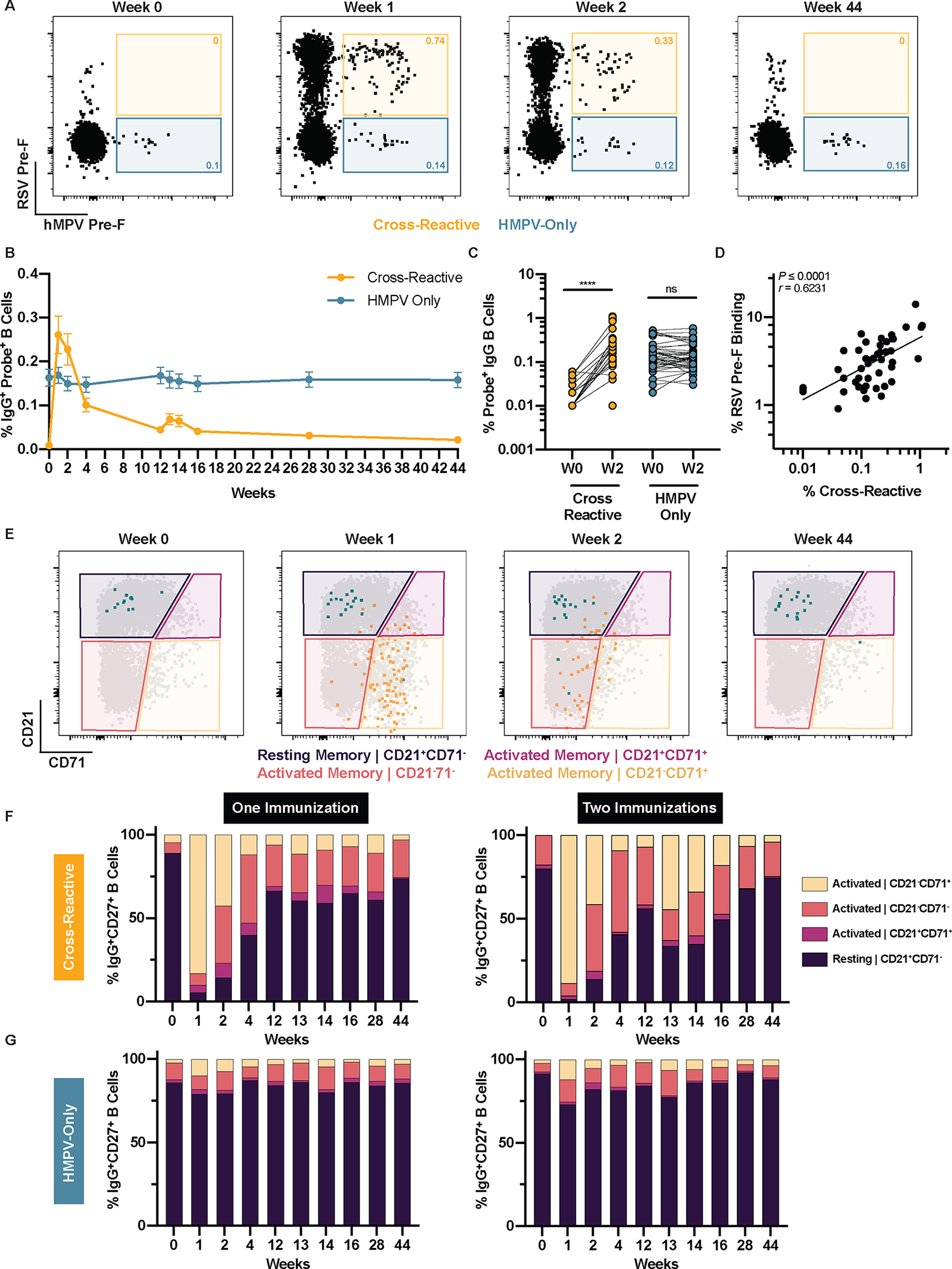
HMPV pre-F probe-binding IgG+ B cells were assessed in 50 of 95 participants. Memory B cells were gated (mononuclear cells/singlets/live/CD19+/CD20+CD38−/IgD−/IgG+/CD27+) and frequencies of probe-binding populations were determined. (A) Representative plots from a single participant show probe-binding frequencies of HMPV and RSV cross-reactive (yellow) and HMPV-specific (green) B cells at weeks 0, 1, 2, and 44. (B) Longitudinal probe-binding frequencies of cross-reactive and HMPV-only B cells are shown. Data are presented as mean ± SEM. (C) Probe-binding frequencies in cross-reactive and HMPV-specific B cells were measured at week 0 and week 2. Significance was calculated using a Wilcoxon paired t-test (pairs with zero probe-binding events at W0 were excluded). ****p<0.0001; ns, not significant. (D) The correlation of RSV pre-F-binding with HMPV F-cross-reactive probe-binding frequencies at week 2 is shown. (E) Representative flow plots from one participant show cross-reactive (yellow) and HMPV-specific (green) B cells overlaid on total memory B cells (IgG+CD27+). Phenotypes were defined based on expression of CD21 and CD71. The relative frequencies of IgG+CD27+ (F) cross-reactive and (G) HMPV-specific B cells are shown for individuals who received one (n=31) or two (n=19) immunizations. Probe-binding frequencies were normalized to 100.
DISCUSSION
Our longitudinal evaluation of memory B cell specificity and phenotype for all 95 individuals enrolled in the phase 1 VRC 317 clinical trial of the RSV pre-F subunit vaccine DS-Cav1 revealed the rapid and robust elicitation of IgG+ and IgA+ RSV F-specific B cells. Most elicited cells were specific to pre-F alone or of dual specificity. Although there was a modest boost in the frequency of post-F-binding B cells, it is most likely attributed to B cells capable of binding both probes, but with a strong preference for the post-F capture probe. These cells could be binned as post-F preferring given the heterogeneity of dual-binding cells. Despite natural waning and a transition back to resting memory phenotype, our results demonstrate that the frequency IgG+ RSV F-specific memory B cells remains elevated above the baseline at week 44 regardless of the number of vaccinations, emphasizing the durability of the memory B cell response garnered from DS-Cav1 vaccination.
Our results also demonstrated that an RSV pre-F antigen can elicit an HMPV cross-reactive B cell responses in humans. RSV and HMPV F cross-binding B cells were potently activated and readily detectable following DS-Cav1 vaccination. A prior trial of an RSV post-F vaccine candidate hinted at protection from illness caused by HMPV in older adults (24), yet HMPV-specific immunity was not directly measured. Interestingly, most cross-reactive B cells elicited by DS-Cav1 bound only RSV pre-F and not the post-F probe, hinting at a potential preference for antigenic sites Ø and V; alternatively, these data may suggest a preference for the region of site III that is in a groove between the tip of site II on one protomer and site IV on the adjacent protomer that is more accessible on pre-F than post-F. Therefore, exclusive pre-F-binding B cells are more likely to be cross-reactive than dual-binding B cells that recognize sites present on both pre-F and post-F conformations. Further studies of the antigenic site-specificity of cross-reactive B cell responses are underway. Given the elicitation of HMPV-specific antibodies and cross-reactive B cells demonstrated here, an RSV pre-F vaccine may provide increased protection against HMPV, particularly in older adults, which may be a point of interest in ongoing, large-scale pre-F subunit vaccine efficacy trials.
Most adults have had several previous encounters with RSV and thus experienced a robust recall of activated F-specific B cells following the first immunization. Pre-existing immunity, coupled with the relatively short interval between the first and second immunization, could also contribute to the paucity of plasmablasts mobilized by the second immunization. It is possible that waiting until most of the F-specific cells had returned to the resting memory phenotype at week 44 would have resulted in a more robust boost, which could be addressed in subsequent studies. This is consistent with a modest, transient boost in serum antibodies between weeks 12 and 14 (18). RSV F-specific B cells were less activated with the second immunization, expressing a more “intermediate” activated phenotype (CD21−CD71− and CD21+CD71+). Regardless of the number of vaccinations, F-specific memory B cells remained elevated above baseline at the last study time point (week 44). These results, along with the sustained elevation of serum neutralizing activity elicited in this phase 1 trial, demonstrate that pre-F subunit vaccines could be administered in a one-dose regimen (18). Generating a long-lasting memory response with one immunization is particularly advantageous for vaccination in pregnant women and in the elderly.
Antibody responses to RSV infection are particularly short-lived, and biological mechanisms for this have not been fully elucidated (31–33). One possible contributor could be the lack of a sustained LLPC population, as LLPC are responsible for secreting antibodies upon antigen re-exposure. The robust activation and recall of CD21-downregulated, F-specific memory B cells suggest that DS-Cav1 vaccination may elicit pre-F-specific B cells that can serve as precursors to LLPCs (27). Generating LLPCs specific for the most neutralization-sensitive sites on RSV F might confer an advantage for protection from disease in subsequent RSV infections in older adult populations and improve perinatal transfer of higher potency antibodies to infants after maternal immunization. Recent studies in influenza suggested that vaccine-elicited LLPCs declined within a year, but the pre-existing influenza-specific cells remained stable over time (34). Although this concept has yet to be explored in the context of RSV, it is possible that DS-Cav1 vaccination amplifies pre-existing immunity and provides a sustained improvement to the baseline response. Additional studies are warranted to compare the durability of LLPC elicited by DS-Cav1 to those elicited by RSV infection.
This first in human phase 1 clinical study has some limitations due to the small number and demographic makeup of the participants, precluding the assessment of vaccine efficacy. Healthy adults between 18 and 50 are not at risk for severe RSV disease and the study was not designed to detect infection. Thus, we are unable to relate our findings to prevention of infection or disease. Additionally, our analysis was also limited because it was confined to PBMC, and samples were not acquired from other tissues important for understanding B cell responses to vaccination. Sampling of lymph nodes shortly after vaccination would have enabled a better understanding of B cell and T follicular helper (TFH) cell responses in B cell follicles. Bone marrow sampling pre- and post-vaccination might have allowed us to discern if vaccination resulted in sustained elevation of RSV F-specific LLPC above baseline. The contribution of different populations of RSV-specific B cells (memory, activated memory, plasmablasts, LLPC) to the prevention of infection and disease is not known, and will be difficult to parse out due to the interconnection and coexistence of multiple subsets with some functional overlap. Despite these limitations, our data give insight into the responses might occur in the major target populations of RSV-experienced pregnant women and older adults, in which large scale trials are underway.
In summary, our evaluation of vaccine-elicited B cells affirms that a single vaccination is sufficient to elicit robust and durable RSV F-specific memory B cells in antigen-experienced individuals. The recruitment of HMPV cross-reactive B cells suggests that pre-F vaccination could potentially offer enhanced protection from disease attributed to HMPV, which remains to be evaluated in ongoing or future pre-F vaccine trials. The expansion and activation of RSV F-specific and HMPV cross-reactive B cells expressing an LLPC precursor phenotype could have a profound and long-term impact on pneumovirus-specific immunity. The durable B cell memory, along with the previously reported maintenance of potent neutralizing antibodies in the serum make pre-F subunit vaccines a promising candidate for pregnant women and the elderly.
MATERIALS AND METHODS
Study Design
VRC 317 (NCT03049488) was a randomized, open-label phase 1 clinical trial to evaluate the dose, safety, tolerability, and immunogenicity of a stabilized prefusion RSV F subunit protein vaccine (DS-Cav1). Participants received either one or two vaccinations with 50, 150, or 500 μg of DS-Cav1 and were randomly allocated 1:1 to receive the vaccine with or without alum, using permuted block randomization with block sizes of 4 or 6, chosen at random. Due to the dose-escalation design, participants were not randomly allocated between doses, and were assigned to a group upon electronic enrollment by a study clinician. Participants and investigators were not masked to group assignment. Primary study endpoints for the full trial have been reported (18) and demonstrated no effect of the inclusion of adjuvant, and a transient benefit of increased doses. Sample size and inclusion/exclusion criteria were determined based on primary trial endpoints. For the current exploratory analyses, we compared B cell responses in the 62 individuals that were vaccinated with DS-Cav1 at weeks 0 and 12 to the 33 individuals that received a single vaccination at week 0, and no samples were excluded from our analyses. Although some timepoints could not be assessed due to attrition (87 of 95 individuals remained enrolled through study completion), all samples collected were processed and included in these analyses. All human samples were collected under the Vaccine Research Center (VRC)/National Institutes of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) clinical protocol, in compliance with the NIH Institutional Review Board (IRB) approved procedures (IRB reference number 084981). All individuals met protocol eligibility criteria and agreed to participate in the study by signing the IRB-approved informed consent. All research studies with these samples were conducted by protecting the rights and privacy of all study participants.
Generation of RSV and HMPV probes
Avi-tagged RSV pre-F, RSV post-F, and HMPV pre-F probes were produced as previously described (30, 35). To generate a prefusion-stabilized HMPV F (pre-F) protein construct, amino acid residues 1–490 from HMPV F subtype A1 strain NL/1/00 were cloned into the mammalian expression vector pαH. Specific point substitutions were introduced at A113C, A185P, G294E, A339C, and H368N. Furthermore, the putative cleavage site 99RQSR102 was changed to the furin cleavage site 99RRRR102. The T4 fibritin trimerization motif (foldon) was added at the C-terminus followed by an HRV3C cleavage site, an 8xHis tag, and StrepTagII. Additionally, an AviTag version of the construct was made where the tags with a 6xHis tag and an AviTag trailing foldon. Using polyethyleneimine (PEI), Freestyle-293F cells (Thermo Fisher Scientific, CVCL_D603) grown in Freestyle 293 expression medium were transiently co-transfected at a 4:1 ratio of HMPV F and furin-expressing plasmids. After three hours, a final concentration of 0.1% (v/v) Pluronic F-68 (Gibco) was added to the cells. Six days post-transfection, secreted proteins were either purified over Ni-NTA Superflow resin (Qiagen; AviTag version) or Strep-Tactin Sepharose resin (IBA; StrepTagII version). Eluate from the affinity columns were concentrated and then purified by gel filtration using a Superose 6 Increase 10/300 column (GE Healthcare). All proteins were biotinylated using the BirA biotin-protein ligase reaction kit (Avidity) according to the manufacturer’s instructions. Bio-Layer Interferometry (Octet) was used to confirm biotinylation of the proteins by testing binding to streptavidin sensors. Retention of antigenicity was confirmed by testing the biotinylated RSV F against monoclonal antibodies D25 and palivizumab (expressed in-house), and biotinylated HMPV F against MPE8, MF14, MF17, 1025, ADI8992 (gifts from Jose Melero). Biotinylated proteins were conjugated using either streptavidin-labeled allophycocyanin (APC, Invitrogen), brilliant violet (BV) 421 (BD Biosciences), BV786 (BD Biosciences), or BV711 (BD Biosciences). Probe-conjugation reactions were prepared at a 3:1 molecular ratio of biotinylated protein to streptavidin. The labeled streptavidin conjugates were added in five separate increments every 20 minutes. Between each addition, the probes were incubated in the dark on a rotator at 4°C. Optimal titers were determined using PBMC samples collected from individuals enrolled in the VRC 317 clinical trial.
B cell phenotyping
Due to sample availability, a single vial of approximately 10 million PBMC was assessed for each individual at each timepoint. Frozen PBMCs from the VRC 317 clinical trial were thawed and stained for viability for 20 minutes at room temperature with the Fixable Blue Dead Cell Stain Kit diluted first in 50 μL of DMSO then at a final concentration of 1:400 in PBS (Thermo Fisher Scientific), followed by two washes then surface staining using anti-human IgA FITC (Miltenyi, S11–8E10, AB_1036156, 1:80), IgM PerCP-Cy5.5 (BD Biosciences, G20–127, AB_10611998, 1:50), CD8 BV510 (BioLegend, RPA-T8, AB_2561942, 1:640), CD3 BV510 (BioLegend, OKT3, AB_2561943, 1:160), CD56 BV510 (BioLegend, HCD56, AB_2561944, 1:160), CD14 BV510 (BioLegend, M5E2, AB_2561946, 1:320), CD27 BV605 (BioLegend, O323, AB_2561450, 1:80), CD11c BV650 (BioLegend, 3.9, AB_2562231. 1:400), CD72 BV711 (BD Biosciences, J4–117, AB_2741766, 1:640), CD19 ECD (Beckman Coulter, J3–119, AB_130854, 1:40), CD21 PE-Cy5 (BD Biosciences, B-ly4, AB_394028, 1:40), CD71 PE-Cy7 (BioLegend, CY1G4, AB_2563119, 1:1200), IgD BUV395 (BD Biosciences, IA6–2, AB_2738435, 1:100), CD38 BUV661 (BD Biosciences, HIT2, AB_2744377, 1:40), IgG Alexa 700 (BD Biosciences, G18–145, AB_10612406, 1:40), and CD20 APC-Cy7 (BioLegend, 2H7, AB_314262, 1:200) in addition to fluorescently-labeled RSV pre-F and RSV post-F probes for 20 minutes at room temperature. HMPV pre-F B cell probes were added into the panel for samples from the last 50 individuals analyzed. All samples were stained in Brilliant Stain Buffer (BD Biosciences). Samples were acquired on a Symphony A5 flow cytometer (BD Biosciences) and data were analyzed using FlowJo version 10 following the schematic presented in fig. S2.
HMPV Enzyme Linked Immunosorbent Assay
Flat bottom, 96-well plates (Thermo Fisher Scientific) were coated with 1 μg/mL of HMPV pre-F diluted in phosphate buffered saline (PBS) and incubated overnight at 4°C. For all the subsequent steps, plates were washed three times in PBS with 0.05% Tween 20 (PBS-T) between each step, all incubations were done at room temperature, and 5% milk in PBS was used as the buffer. The following day, plates were washed and blocked with 200 μL of buffer and incubated for one hour. Four-fold serial dilutions of the serum samples were performed in duplicate starting at 1:400 and added to the coated plates. After one hour, plates were washed and incubated with 100 μL of anti-IgG-horseradish peroxidase (HRP) (1:6000 dilution, Southern Biotech). Plates were developed by adding 100 μL of SureBlue (SeraCare) for seven minutes, followed by 100 μL of 0.501 M sulfuric acid. Plates were read using a SpectraMax Paradigm (Molecular Devices) at 450nm and 650nm. For endpoint calculation, plates were background subtracted and the endpoint was determined as 0.1 (10 times the background of no-serum control). To ensure consistency, all longitudinal time points for each participant were included in the same run, and monoclonal antibodies MPE8 (expressed in-house) and ADI8992 (a gift from Jose Melero) were included with each run.
HMPV Neutralization
Serum neutralization against A lineage HMPV (Strain CAN97–83) was measured using 60% plaque reduction neutralizing titer (PRNT60) assay. Serial 6-fold dilutions of serum samples (starting at 1:80 dilution) were prepared and incubated with live recombinant HMPV, at 37°C in 5% carbon dioxide incubator for 60 minutes, and then inoculated on 95% confluent Vero (CVCL_0059) cell monolayers in 24-well plates. Cells and serum samples were incubated at 37°C for 2 hours, washed with media, and then overlaid with 0.8% methylcellulose in Opti-MEM (Gibco) supplemented with 4% TrypLE Select. After 7 days of incubation at 37°C, the methylcellulose overlay was removed, and plates were fixed with 1 mL/well of ice cold 80% methanol overnight. Plaques were stained with hyperimmune rabbit anti-HMPV hyperimmune serum, affinity purified antibody peroxidase labeled anti-Rabbit Immunoglobulin G (both heavy and light chains, KPL), and TrueBlue substrate (KPL). Plaques were counted using an inverted microscope and 60% plaque reduction titers were calculated (36).
Statistical analysis
All data were analyzed using GraphPad Prism version 9.2.0. Statistical comparisons of cross-sectional and longitudinal samples from the same group of participants were performed using either paired or non-paired t-tests. For comparisons where data passed the Anderson-Darling normality test, a parametric paired t-test was used, for those that did not pass the test assumption, a non-parametric test was employed. Pearson correlation analysis was used for correlations. Details of statistical tests applied are listed in the figure legends.
Supplementary Material
Acknowledgements
We would like to thank Azad Kumar for RSV pre-F and post-F protein production, and the VRC317 Study Team (Somia P. Hickman, Pamela J. Costner, LasSonji A. Holman, Katherine Houser, Nina M. Berkowitz, Ingelise J. Gordon, Galina V. Yamshchikov, Martin R. Gaudinski, Thuy Nguyen, Anita Arthur, Jennifer Cunningham, Aba Eshun, Brenda Larkin, Floreliz Mendoza, Laura Novik, Jamie Saunders, Xiaolin Wang, William Whalen, Cristina Carter, Cynthia Starr Hendel, Sarah Plummer, Abidemi Ola, Alicia Widge, Maria C. Burgos Florez, Lam Le, Iris Pittman, Ro Shauna S. Rothwell, Olga Trofymenko, Olga Vasilenko, Preeti Apte, Renunda Hicks, Cora Trelles Cartagena, Pernell Williams, LaShawn Requilman, and Colin Tran) and Vaccine Research Center Production Program (Bob Lin, Robert Bailer, Richard M. Schwartz, Lisa A. Kueltzo, Kevin Carlton, Jason G. Gall, Shufeng Bai, Elizabeth Carey, Amy L. Chamberlain, Ya-chen Chang, Mingzhong Chen, Peifeng Chen, Jon Cooper, Colleen Fridley, Mridul Ghosh, Deepika Gollapudi, Janel Holland-Linn, Joe Horwitz, Althaf Hussain, Vera Ivleva, Florence Kaltovich, Kristin Leach, Christopher Lee, Amy Liu, Xun Liu, Slobodanka Manceva, Amritha Menon, Attila Nagy, Sarah O’Connell, Rahul Ragunathan, Jennifer Walters, and Zhong Zhao) for their support throughout the trial.
Funding
This work was supported by intramural funding from the National Institute of Allergy and Infectious Diseases: 1ZIAAI005129–06 and 1ZIAAI0003782–39
Footnotes
Competing interests: BSG and JSM are inventors on patents for the stabilization of the RSV F protein (WO2014160463A1, Prefusion RSV F proteins and their use). The other authors declared no competing interests.
Data and materials availability:
All data associated with this study are in the paper or supplementary materials. Reagents and materials associated with this study are available from the corresponding author (T.J.R.) after completion of a materials transfer agreement.
References and Notes
- 1.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang D-A, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lázaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccalà G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida L-M, Yu H, Zar HJ, Campbell H, Nair H, R. S. V. G. E. Network, Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K, Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE, Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352, 1749–1759 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Leaver BA, Smith BJ, Irving L, Johnson DF, Tong SYC, Hospitalisation, morbidity and outcomes associated with respiratory syncytial virus compared with influenza in adults of all ages. Influenza Other Respi. Viruses 16, 474–480 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, Mejias A, Karron RA, Simões EA, Knezevic I, Ramilo O, Piedra PA, Chu HY, Falsey AR, Nair H, Kragten-Tabatabaie L, Greenough A, Baraldi E, Papadopoulos NG, Vekemans J, Polack FP, Powell M, Satav A, Walsh EE, Stein RT, Graham BS, Bont LJ, Respiratory F Syncytial Virus Network, The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect. Dis. 18, e295–e311 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Magro M, Mas V, Chappell K, Vázquez M, Cano O, Luque D, Terrón MC, Melero JA, Palomo C, Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl. Acad. Sci. U. S. A. 109, 3089–3094 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS, Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340, 1113–1117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domachowske JB, Khan AA, Esser MT, Jensen K, Takas T, Villafana T, Dubovsky F, Griffin MP, Safety, Tolerability and Pharmacokinetics of MEDI8897, an Extended Half-life Single-dose Respiratory Syncytial Virus Prefusion F-targeting Monoclonal Antibody Administered as a Single Dose to Healthy Preterm Infants. Pediatr. Infect. Dis. J. 37, 886–892 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogovik AL, Carleton B, Solimano A, Goldman RD, Palivizumab for the prevention of respiratory syncytial virus infection. Can. Fam. Physician 56, 769–772 (2010). [PMC free article] [PubMed] [Google Scholar]
- 10.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 102, 531–537 (1998). [PubMed] [Google Scholar]
- 11.Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, Villafana T, DeVincenzo JP, Nirsevimab Study G, Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 383, 415–425 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang G-Y, Druz A, Georgiev IS, Rundlet EJ, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Nason MC, Capella C, Peeples ME, Ledgerwood JE, McLellan JS, Kwong PD, Graham BS, Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 7, 309ra162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simões EAF, Swamy GK, Agrawal S, Ahmed K, August A, Baqui AH, Calvert A, Chen J, Cho I, Cotton MF, Cutland CL, Englund JA, Fix A, Gonik B, Hammitt L, Heath PT, de Jesus JN, Jones CE, Khalil A, Kimberlin DW, Libster R, Llapur CJ, Lucero M, Pérez Marc G, Marshall HS, Masenya MS, Martinón-Torres F, Meece JK, Nolan TM, Osman A, Perrett KP, Plested JS, Richmond PC, Snape MD, Shakib JH, Shinde V, Stoney T, Thomas DN, Tita AT, Varner MW, Vatish M, Vrbicky K, Wen J, Zaman K, Zar HJ, Glenn GM, Fries LF, Prepare Study G, Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N. Engl. J. Med. 383, 426–439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLellan JS, Yang Y, Graham BS, Kwong PD, Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J. Virol. 85, 7788–7796 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLellan JS, Chen M, Chang J-S, Yang Y, Kim A, Graham BS, Kwong PD, Structure of a major antigenic site on the respiratory syncytial virus fusion glycoprotein in complex with neutralizing antibody 101F. J. Virol. 84, 12236–12244 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilman MSA, Castellanos CA, Chen M, Ngwuta JO, Goodwin E, Moin SM, Mas V, Melero JA, Wright PF, Graham BS, McLellan JS, Walker LM, Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci Immunol 1, eaaj1879 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhamedova M, Wrapp D, Shen CH, Gilman MSA, Ruckwardt TJ, Schramm CA, Ault L, Chang L, Derrien-Colemyn A, Lucas SAM, Ransier A, Darko S, Phung E, Wang L, Zhang Y, Rush SA, Madan B, Stewart-Jones GBE, Costner PJ, Holman LA, Hickman SP, Berkowitz NM, Doria-Rose NA, Morabito KM, DeKosky BJ, Gaudinski MR, Chen GL, Crank MC, Misasi J, Sullivan NJ, Douek DC, Kwong PD, Graham BS, McLellan JS, Mascola JR, Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses. Immunity 54, 769–780 e766 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruckwardt TJ, Morabito KM, Phung E, Crank MC, Costner PJ, Holman LA, Chang LA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, Gaudinski MR, Lin B, Bailer R, Chen M, Ortega-Villa AM, Nguyen T, Kumar A, Schwartz RM, Kueltzo LA, Stein JA, Carlton K, Gall JG, Nason MC, Mascola JR, Chen G, Graham BS, V. R. C. s. team, Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir. Med. 9, 1111–1120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroux-Roels G, De Boever F, Maes C, Nguyen TL-A, Baker S, Gonzalez Lopez A, Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 37, 2694–2703 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Walsh EE, Falsey AR, Scott DA, Gurtman A, Zareba AM, Jansen KU, Gruber WC, Dormitzer PR, Swanson KA, Radley D, Gomme E, Cooper D, Schmoele-Thoma B, Group CS, A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 225, 357–1366 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmoele-Thoma B, Falsey AR, Walsh EE, Swanson K, Zareba A, Cooper D, Gruber WC, Jansen KU, Radley D, Scott D, Dormitzer PR, 2755. Phase 1/2, first-in-human study of the safety, tolerability, and immunogenicity of an RSV prefusion F-based subunit vaccine candidate. Open Forum Infect. Dis. 6, S970–S970 (2019). [Google Scholar]
- 22.Aliprantis AO, Shaw CA, Griffin P, Farinola N, Railkar RA, Cao X, Liu W, Sachs JR, Swenson CJ, Lee H, Cox KS, Spellman DS, Winstead CJ, Smolenov I, Lai E, Zaks T, Espeseth AS, Panther L, A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccin. Immunother. 17, 1248–1261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams K, Bastian AR, Feldman RA, Omoruyi E, de Paepe E, Hendriks J, van Zeeburg H, Godeaux O, Langedijk JPM, Schuitemaker H, Sadoff J, Callendret B, Phase 1 Safety and Immunogenicity Study of a Respiratory Syncytial Virus Vaccine With an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in Adults Aged ≥60 Years. J. Infect. Dis. 222, 979–988 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, Takas T, Levin MJ, Falsey AR, An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J. Infect. Dis. 216, 1362–1370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palm A-KE, Henry C, Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 10, 1787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy AK, Oshansky CM, Elbein R, Thomas S, Lyon GM, Spiropoulou CF, Mehta AK, Thomas PG, Boyd SD, Ahmed R, Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 17, 1226–1234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau D, Lan LY-L, Andrews SF, Henry C, Rojas KT, Neu KE, Huang M, Huang Y, DeKosky B, Palm A-KE, Ippolito GC, Georgiou G, Wilson PC, Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Guarino B, Silacci C, Marcandalli J, Marsland BJ, Piralla A, Percivalle E, Sallusto F, Baldanti F, Lanzavecchia A, Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 501, 439–443 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Wen X, Mousa JJ, Bates JT, Lamb RA, Crowe JE Jr., Jardetzky TS, Structural basis for antibody cross-neutralization of respiratory syncytial virus and human metapneumovirus. Nat Microbiol 2, 16272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battles MB, Más V, Olmedillas E, Cano O, Vázquez M, Rodríguez L, Melero JA, McLellan JS, Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein. Nat. Commun. 8, 1528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Walsh EE, Long CE, Schnabel KC, Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163, 693–698 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Falsey AR, Singh HK, Walsh EE, Serum antibody decay in adults following natural respiratory syncytial virus infection. J. Med. Virol. 78, 1493–1497 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Sande CJ, Mutunga MN, Okiro EA, Medley GF, Cane PA, Nokes DJ, Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J. Med. Virol. 85, 2020–2025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis CW, Jackson KJL, McCausland MM, Darce J, Chang C, Linderman SL, Chennareddy C, Gerkin R, Brown SJ, Wrammert J, Mehta AK, Cheung WC, Boyd SD, Waller EK, Ahmed R, Influenza vaccine-induced human bone marrow plasma cells decline within a year after vaccination. Science 370, 237–241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, Holman LA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, Gaudinski MR, Kumar A, Chang LA, Moin SM, Hill JP, DiPiazza AT, Schwartz RM, Kueltzo L, Cooper JW, Chen P, Stein JA, Carlton K, Gall JG, Nason MC, Kwong PD, Chen GL, Mascola JR, McLellan JS, Ledgerwood JE, Graham BS, Team VRCS, A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365, 505–509 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Coates HV, Alling DW, Chanock RM, An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol 83, 299–313 (1966). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are in the paper or supplementary materials. Reagents and materials associated with this study are available from the corresponding author (T.J.R.) after completion of a materials transfer agreement.


