Abstract
Many organisms accumulate compatible solutes under environmental stress conditions. Cyanobacteria accumulate compatible solutes in response to increased external salinity, with tolerance increasing from Suc (sucrose) or trehalose to 2-O-(α-D-glucopyranosyl)-glycerol and glycinebetaine accumulating species. It is not clear how these different solutes influence salt tolerance. One possible explanation may be a differential ability of these solutes to stabilize membranes under stress conditions. We therefore performed drying experiments with liposomes in the presence of compatible solutes. Suc, trehalose and sorbitol protected liposomes from leakage of a soluble marker and from membrane fusion during drying and rehydration. 2-O-(α-D-glucopyranosyl)-glycerol was less effective and glycinebetaine showed hardly any effect. In combination with Suc, the latter two solutes showed improved protection. Lipid-phase transitions are known to contribute to solute leakage from liposomes. We determined phase transitions in dry membranes in the absence or presence of the solutes, using Fourier-transform infrared spectroscopy. The ability of the solutes to decrease the phase transition temperature corresponded closely to their ability to protect the liposomes against solute leakage. All solutes interacted with the phosphate in the lipid headgroups. The magnitude of the shift in the asymmetric P=O stretching vibration correlated closely with the lipid-phase transition temperature. This indicates that the degree of membrane protection afforded by the solutes is mainly determined by their ability to interact with the membrane lipids. However, this is not a determinant of cellular protection against salt stress, as the solutes show a reverse order when ranked with regard to protection against these stresses.
Keywords: compatible solutes, cyanobacteria, desiccation, model membranes, osmolytes, stress tolerance
Abbreviations: Bet, glycinebetaine; CF, carboxyfluorescein; EPC, egg phosphatidylcholine; FTIR, Fourier-transform infrared; GG, 2-O-(α-D-glucopyranosyl)-glycerol; Sor, sorbitol; Suc, sucrose; Tre, trehalose
INTRODUCTION
Many organisms have acquired mechanisms to adapt to a wide range of environmental conditions, including changes in external salinity. Basis for the salt acclimation is active extrusion of inorganic ions combined with the accumulation of compatible solutes. In additon, compatible solutes are synthesized in response to osmotic stress or low temperature (see [1,2] for reviews). For cyanobacteria, a close relationship between the principal osmoprotective compound of a species and its salt tolerance was found (see [3] for a review). Strains with low tolerance (up to 0.7 M NaCl) accumulate the disaccharides Suc (sucrose) or Tre (trehalose), strains with moderate salt tolerance (up to 1.8 M NaCl) accumulate the heteroside GG [2-O-(α-D-glucopyranosyl)-glycerol] and strains with high salt tolerance (up to 2.7 M NaCl) accumulate the amino acid derivates Bet (glycinebetaine) or glutamatebetaine [4]. These compounds are synthesized de novo. Additionally, uptake systems for these compounds are known, but until now only two of those systems transporting Bet [5] or GG, Suc and Tre [6] have been described in cyanobacteria.
Higher plants mainly accumulate Suc, Sor (sorbitol), Bet or proline in response to drought, salinity or low-temperature stress [2], but the accumulation of Tre has also been shown in some extremely drought-tolerant (resurrection) plants [7–9]. Transgenic plants expressing bacterial genes to synthesize the compatible solutes Tre or Bet showed increased salt, frost and drought tolerance despite their rather low internal concentrations (see [10,11] for reviews). Also, mutants of salt-sensitive cyanobacterial strains that accumulate GG [12] or mutant plants that accumulate glycerol [13] acquired increased salt tolerance.
Compatible solutes can act colligatively by increasing the osmotic potential and thereby improving the water status of the cells under osmotic stress conditions. There is good evidence from recent research that this is the mechanism by which the accumulation of Bet improves the salt tolerance of Escherichia coli cells [14]. In addition, osmolytes can stabilize macromolecular structures such as proteins by preferential exclusion from the hydration shell of proteins [15] and can also assist refolding of unfolded polypeptides by chaperone proteins [16].
Compatible solutes can also have specific protective effects by direct interaction with proteins or membrane lipids. This has been documented especially for sugars such as Suc and Tre for their ability to stabilize proteins and membranes during drying (see [17,18] for reviews).
The aim of the present study was a systematic comparison of the protective abilities of the compatible solutes known from cyanobacteria. In addition, we have included Sor in our studies, a linear sugar alcohol that can be used to make additional structure–function comparisons. Since in many cases membranes are the primary targets of cellular damage under stress conditions, we investigated the protective properties of the solutes during drying and rehydration of lipid vesicles and the interactions of the lipids with the solutes in the dry state. Membrane protection during drying poses especially stringent requirements on solutes [19] and might therefore reveal the protective potential of these substances. Since this has been investigated in some detail for Suc and Tre [18], we have a solid basis for comparing the effects of these disaccharides with those of the other solutes in our experimental system. Different stabilization effects of particular compounds during drying may help to explain the different salt resistance levels in relation to the accumulated compatible solute in cyanobacteria and shed light on the physicochemical basis of stress tolerance in these and other organisms.
EXPERIMENTAL
Materials
EPC (egg phosphatidylcholine) was purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.). CF (carboxyfluorescein) was obtained from Molecular Probes (Eugene, OR, U.S.A.) and was purified according to the procedure described in [20]. N-(7-nitro-2,1,3-benzoxadiazol-4-yl)-phosphatidylethanolamine and N-(lissamine rhodamine B sulphonyl)-dioleoylphosphatidylethanolamine were purchased from Molecular Probes. Suc, Tre and Bet were purchased from Sigma. Preparation of GG from salt-acclimated cells of Synechocystis sp. strain PCC 6803 and analysis of purity by HPLC have been described in [6].
Preparation of liposomes
EPC was dried from chloroform under a stream of N2 and stored under vacuum overnight to remove traces of solvent. Liposomes were prepared from hydrated lipids using a hand-held extruder with two layers of polycarbonate membranes with 100 nm pores ([21]; Avestin, Ottawa, Canada).
Leakage experiments
For leakage experiments, an appropriate amount of lipid was hydrated in 0.25 ml of 100 mM CF, 10 mM Tes and 0.1 mM EDTA (pH 7.4). After extrusion, the vesicles were passed through a NAP-5 column (Sephadex G-25; Amersham Biosciences) equilibrated in TEN buffer [10 mM Tes, 0.1 mM EDTA (pH 7.4) and 50 mM NaCl], to remove the CF not entrapped by the vesicles. The eluted samples were then diluted with TEN to a lipid concentration of approx. 10 mg/ml. Liposomes (40 μl) were mixed with an equal volume of concentrated solutions of sugars in TEN and 20 μl aliquots were applied to the wells of 60-well microplates. The plates were dried in desiccators at 28 °C and 0% relative humidity for 24 h in the dark. Damage to the liposomes was determined as CF leakage after rehydration as described in detail in [22]. Results are expressed as means±S.D. for three parallel samples. Where no error bars are visible, they are smaller than the symbols.
Fusion experiments
For liposome fusion experiments, two liposome samples were prepared in TEN. One sample contained 1 mol% each of N-(7-nitro-2,1,3-benzoxadiazol-4-yl)-phosphatidylethanolamine and N-(lissamine rhodamine B sulphonyl)-dioleoylphosphatidylethanolamine in EPC, the other sample contained only EPC. After extrusion, liposomes were combined in the ratio 1:9 (labelled/unlabelled), resulting in a lipid concentration of 10 mg/ml. Liposomes (40 μl) were mixed with an equal volume of concentrated solutions of sugars in TEN and 20 μl aliquots were applied to the inside of the caps of 1.5 ml microcentrifuge tubes. Samples were dried as described above. The samples were rehydrated by adding 1 ml of TEN buffer to each tube, and then quickly closing and inverting the tube. Membrane fusion was measured by resonance energy transfer [23] as described in [24,25]. Results are expressed as means±S.D. for three parallel samples. Where no error bars are visible, they are smaller than the symbols.
FTIR (Fourier-transform infrared) spectroscopy
Spectra were obtained from samples containing EPC liposomes and solutes in the ratio 1:2 (by wt). Liposomes were extruded in the presence of the solutes, so that the sugars were present on both sides of the membranes. Samples (50 μl) were spread on CaF2 windows and dried under the same conditions as described above. A window was then fixed in the vacuum chamber of a cuvette holder connected to a temperature control unit (Specac Eurotherm, Worthington, U.K.; [22]). The sample was first heated to 50 °C for 20 min under vacuum to remove residual moisture the lipid had taken up during sample handling. The effectiveness of this procedure was verified by the absence of a water band in the FTIR spectra at 1650 cm−1. The sample was then cooled to −30 °C, and after a 20 min equilibration the temperature was increased at a constant rate of 1 °C min−1. Spectra were recorded with a PerkinElmer GX 2000 FTIR spectrometer. After normalization of absorbance using the interactive abex routine, the peak frequencies of the CH2 symmetric stretch band, approx. 2850 cm−1, were determined by the automatic peak identification routine. Tm (gel to liquid-crystalline lipid-phase transition temperature) was estimated as the midpoint of the lipid-melting curve [26]. The phosphate asymmetric stretch vibrations in the 1300–1200 cm−1 region and the carbonyl stretch vibrations in the 1760–1700 cm−1 region were analysed after normalization of absorbance and baseline flattening, using the interactive abex and flat routines respectively. Spectral resolution in the phosphate asymmetric stretch region was enhanced by Fourier self-deconvolution applying a Lorentzian line shape, using the deconvolution routine contained in the Spectrum software [27]. The parameters used were γ=1.2 and length=20.
RESULTS
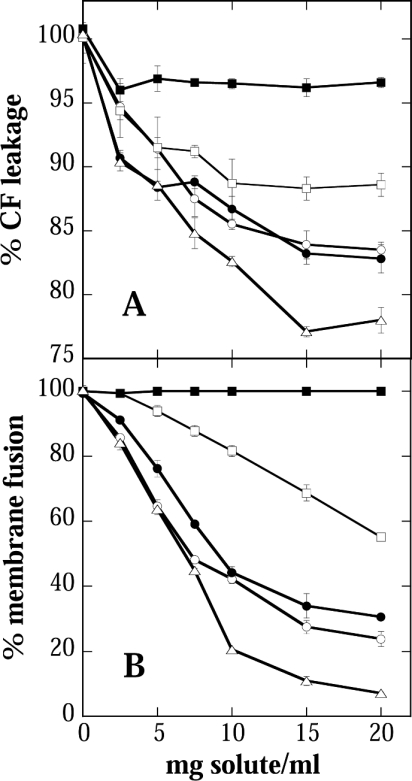
We investigated the ability of the compatible solutes Suc, Tre, GG, Bet and Sor to stabilize liposomes during drying and rehydration. Figure 1 shows that in the absence of any protective solute, liposomes lost their soluble content completely (Figure 1A) and suffered substantial fusion (Figure 1B). As expected from published data, both Suc and Tre protected the vesicles against damage. Due to the low concentrations of solutes employed (maximum solute-to-lipid mass ratio of 4) and the fact that they were present only on the outside of the vesicles, protection against leakage was only partial. However, differences between the solutes could be clearly distinguished. Suc and Tre were approximately equal in their ability to stabilize the liposomes, Sor was superior to these sugars, whereas GG showed a lower effectiveness and Bet showed hardly any protection.
Figure 1. Stability of liposomes after air drying in the presence of different solutes.
Phosphatidylcholine vesicles were dried at 0% relative humidity for 24 h in the presence of different concentrations of Suc (○), Tre (•), GG (□), Bet (▪) or Sor (▵). Liposome stability was assayed either as leakage of the soluble fluorescence marker CF from the vesicles (A) or as membrane fusion determined by a membrane lipid mixing assay (B).
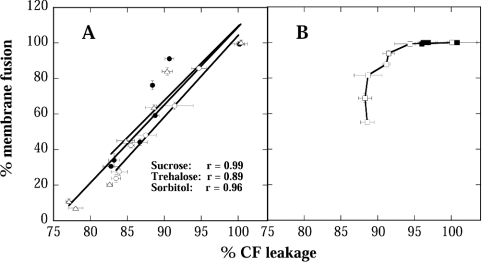
Since the fusion of liposomes during drying is one of the factors that can lead to leakage of soluble content, we investigated how far these two parameters were correlated. Figure 2 shows that for Suc, Tre and Sor, there was a clear linear correlation between leakage and fusion, indicating that the ability of these solutes to prevent leakage was strongly influenced by their ability to prevent fusion. For GG and Bet, on the other hand, no such correlation was evident. This was to be expected for Bet, because it did not show significant protection against either leakage or fusion. For GG, however, the results indicate that its ability to prevent fusion was higher than its ability to prevent leakage. This indicates that its inability to influence factors other than fusion, which contribute to leakage, limits the effectiveness of GG as a protectant.
Figure 2. Correlation between CF leakage and membrane fusion after drying and rehydration as shown in Figure 1.
The solid lines in (A) were fitted to the data by linear regression analysis and the correlation coefficients are shown. (B) Lines were used to connect the points, but no fitting was attempted. The symbols are the same as in Figure 1.
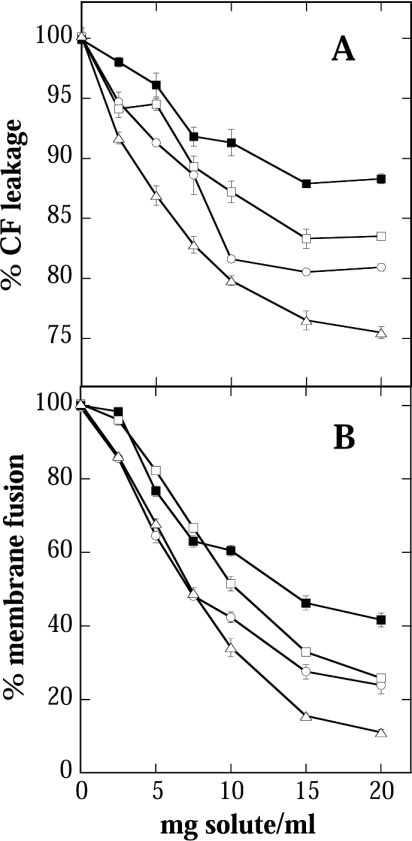
Solutes never occur in isolation in a cellular environment. It was therefore of interest to see whether combinations of compatible solutes would have significantly better effects than would result from purely additive action. Since Suc is ubiquitously present in all cells, we dried liposomes in mixtures of Suc and GG, Sor or Bet. It can be seen (Figure 3) that all mixtures prevented both leakage and fusion, albeit to different degrees. The relative effectiveness of the solutes, however, was not changed (cf. Figure 1), indicating that no strong synergistic effects occurred between Suc and the other solutes.
Figure 3. Effects of mixtures of different compatible solutes.
Stability of liposomes after air drying in the presence of Suc (○) or mixtures of Suc with GG (□), Bet (▪) or Sor (▵) at a 1:1 mass ratio. See Figure 1 for experimental details.
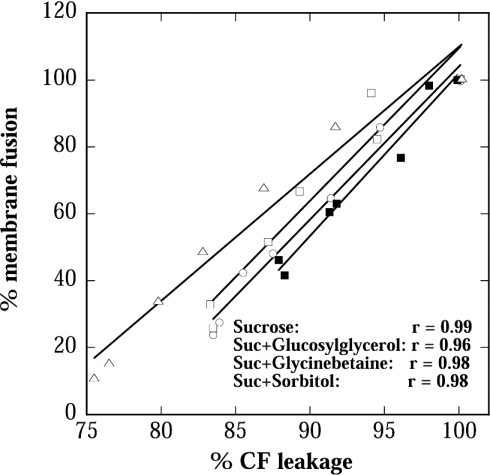
It is obvious from Figure 3(B) that the presence of Suc significantly improved protection against fusion, especially for Bet. Figure 4 shows that for all mixtures there was a linear correlation between leakage and fusion, indicating that the properties of Suc predominated over those of GG and Bet in the respective mixtures.
Figure 4. Correlation between CF leakage and membrane fusion after drying and rehydration as shown in Figure 3.
The solid lines were fitted to the data by linear regression analysis and the correlation coefficients are shown. The symbols are the same as in Figure 3.
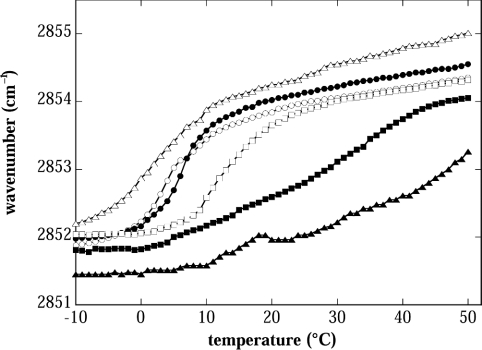
In addition to fusion, gel-to-liquid-crystalline lipid-phase transitions can also contribute to the leakage observed after drying and rehydration [18]. Therefore we have investigated the phase behaviour of dry liposomes in the absence or presence of the different osmolytes (Figure 5). All solutes induced a shift of Tm to lower temperatures. However, the highest shift was observed for Suc, Tre and Sor. It was smaller for GG and the smallest shift was observed for Bet. This is in accordance with the observed effectiveness of the solutes as protectants against leakage (Figure 1).
Figure 5. Lipid melting curves of dry EPC liposomes as determined by FTIR spectroscopy.
The wave number of the CH2 symmetric stretch vibration is plotted as a function of sample temperature. Tm was determined as the midpoint of each melting curve. The samples contained either purely EPC liposomes without any solutes (control, ▴: Tm=40 °C) or EPC liposomes in the presence of different solutes at a 1:2 mass ratio both inside and outside of the liposomes (Suc, ○: Tm=5 °C; Tre, •: Tm=6 °C; GG, □: Tm=15 °C; Bet, ▪: Tm=30 °C; Sor, ▵: Tm=4 °C).
There is good evidence that the reduction in Tm, which has been reported for several sugars, is the result of H-bonding interactions between the lipid headgroups and the carbohydrates in the dry state [18,28]. H bonding between OH groups of sugar and lipids can take place with the lipid carbonyl (C=O) and the phosphate (P=O) moieties. These possibilities were explored by FTIR spectroscopy.
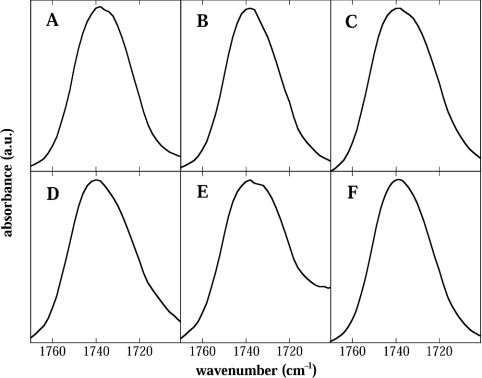
The C=O groups are situated at the interface between the hydrophobic hydrocarbon chains and the more hydrophilic headgroup region. The C=O band in FTIR spectra of hydrated diacyl lipids is split into at least two bands, with the upfield peak(s) due to non-hydrogen-bonded C=O groups and the downfield peak(s) due to hydrogen-bonded C=O groups [29,30]. The C=O peaks of dry EPC liposomes in the absence or presence of the compatible solutes are shown in Figure 6. None of the solutes resulted in a clear shift in the position of the C=O peak, compared with the control without any added solute. This indicates that the solutes do not penetrate this deeply into the membrane.
Figure 6. Infrared spectra in the carbonyl stretching region of dry liposomes.
Normalized C=O stretching band contours of pure EPC (A), and of EPC dried in the presence of Suc (B), Tre (C), GG (D), Bet (E) or Sor (F). All spectra were recorded at 50 °C. The peak maxima were located at 1738 cm−1 (A–C, E), 1740 cm−1 (D) and 1739 cm−1 (F).
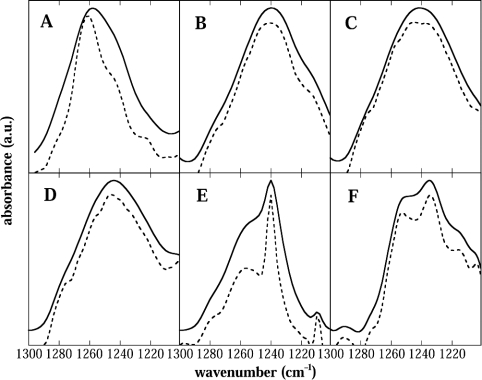
The P=O group of phospholipids is situated in the interfacial region of the membrane. The peak position of the asymmetrical stretching mode of P=O is sensitive to H bonding, shifting to lower wave numbers with increased H bonding [31,32]. The H bonding of Suc and Tre to dry lipids has been extensively studied in the past (see [18,28] for recent reviews). Figure 7 shows the expected shift of the P=O peak to lower wave numbers for Suc and Tre. After deconvolution, however, it could be seen that the P=O vibration in the presence of Tre was split into two peaks, whereas in the presence of Suc it remained as one peak. A similar, but even more pronounced splitting of the P=O peak was observed in the presence of Sor. The two peaks in the presence of Bet were not due to a splitting of the P=O peak. Rather, the absorbance band at 1240 cm−1 came from the Bet itself (results not shown), whereas the peak at 1256 cm−1 was due to the lipid P=O group. In contrast with Bet, the carbohydrates contribute no appreciable absorbance to this spectral region (cf. [33]). The P=O peak in the presence of GG had an intermediate position between those in the presence of Bet and Suc.
Figure 7. FTIR spectra of the phosphate asymmetric stretch region in dry EPC liposomes.
Normalized P=O stretching band contours (—) and contours after resolution enhancement through deconvolution (- - -) of pure EPC (A), and of EPC dried in the presence of Suc (B), Tre (C), GG (D), Bet (E) or Sor (F). All spectra were recorded at 50 °C. The peak maxima after deconvolution were located at 1262 cm−1 (A), 1239 cm−1 (B), 1242 and 1238 cm−1 (C), 1246 cm−1 (D), 1256 cm−1 (E), 1253 and 1235 cm−1 (F). The sharp peak at 1240 cm−1 in (E) is an absorbance band from Bet.
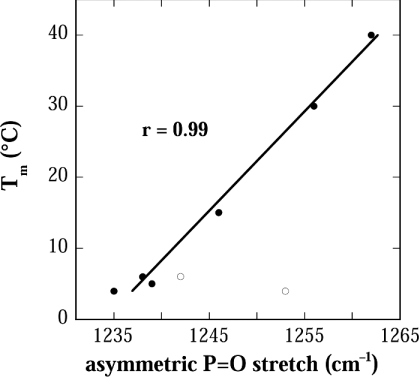
Between the different samples, the position of the P=O peak was highly variable (Figure 7), indicating large differences in the ability of these osmolytes to H bond to the lipid headgroups. Figure 8 shows that there was indeed a strong linear correlation between this ability to H bond to the lipid phosphate (as indicated by the P=O peak position) and the ability of the solutes to decrease the lipid-phase transition temperature (as indicated by Tm). The position of the second, upfield peak in the P=O spectra of Tre and Sor was not correlated with Tm. The reason for this heterogeneity in the H bonding between EPC and these two solutes and its functional significance are not clear at present.
Figure 8. Correlation between the position of the P=O peak and Tm.
The two open circles correspond to the position of the second upfield peak in the P=O spectra of Tre (Figure 7C) and Sor (Figure 7F). The line was fitted to the data by linear regression analysis. The regression coefficient (r) is indicated in the figure.
DISCUSSION
The protection of membranes against the stresses associated with drying requires two important properties from a solute: effective depression of the lipid-phase transition temperature and the ability to vitrify (see [18,34] for reviews).
The vitrification properties of solutes are commonly quantified by their calorimetric glass melting temperature, Tg. Solutes with a high Tg, such as Suc and Tre (approx. 50 and 100 °C respectively [24]) surround the membrane vesicles with a glassy matrix during drying at ambient temperature and thereby prevent close approach and fusion of liposomes [35]. This is clearly illustrated in Figure 1(B). Vesicle fusion is often accompanied by solute leakage [36,37] and therefore a close correlation exists between fusion and leakage during drying in the presence of these sugars (Figure 2). It is surprising in this regard that Sor showed better protection against fusion and leakage than the disaccharides (Figure 1), although its Tg has been reported between −5 and −9 °C [38,39]. However, Tg may not be the most important parameter in this regard. Tc is the critical temperature, where the dynamics of the molecules in a glass change from solid- to liquid-like. It has been suggested that a high Tc could contribute to the stability of dry specimens at temperatures significantly above Tg [40]. It has been shown that Tc for Sor is at 33 °C, approx. 40 °C above its Tg [41,42], whereas it is only 18 and 25 °C above Tg for Suc and Tre respectively [40]. This relatively high Tc may explain the good protection against fusion provided by Sor during drying at 28 °C.
There are, to the best of our knowledge, no data available for the glass transition behaviour of GG and Bet. However, visual inspection of the dry samples suggested that Bet at least partly crystallized during drying. This would be in agreement with the lack of protection against fusion by Bet. The fusion data would also suggest that GG has at least some glass-forming ability. The correlation analysis (Figure 2) indicated that the ability of GG to protect vesicles from leakage was not limited by its vitrification properties.
Mixtures of different carbohydrates have a Tg that is intermediate between that of the pure substances [24,39]. This is in agreement with our results on the effects on CF leakage and vesicle fusion of mixtures between Suc and Sor or GG (Figure 3). Interestingly, mixtures of Bet and Suc also showed some protection against leakage and fusion. Visual inspection confirmed that the mixtures did not crystallize. Incorporation of a non-glass-forming solute into a carbohydrate glass has been reported for mixtures of arbutin with Suc and Tre [24]. These mixtures also provided protection for liposomes against fusion during drying. The correlation analysis indicated that the effect of the mixture between Suc and Bet was clearly dominated by the superior ability of Suc to vitrify (Figure 4). It should be noted that in GG and Bet accumulating cyanobacteria minor amounts of Suc or Tre were also detected [4].
In addition to inducing fusion between liposomes, dehydration also leads to an increase in Tm. For EPC, Tm is increased from −5 °C in the fully hydrated state to approx. 40 °C in the dry state [22,43,44]. During rehydration at 28 °C, membranes therefore go from the gel to the liquid-crystalline state. Packing defects in the membranes during this transition in the presence of excess water lead to the leakage of soluble content, as probed by CF in the present study. Lyoprotectants such as Suc and Tre decrease Tm in the dry state and thereby decrease the leakage. Sor showed the same effect on Tm as Suc or Tre, whereas GG was less effective and Bet showed only a very small effect (Figure 5).
Evidence has been found in several studies that Suc and Tre decrease Tm in dry membranes by H bonding to the P=O group of phospholipids (see [18,34] for reviews). The same mechanism has also been suggested for the effects of fructans [22,44] and raffinose-family oligosaccharides [45]. In the present study, we have also detected H bonding of some of the solutes to the P=O group in dry EPC (Figure 7). The extent of H bonding, as indicated by the downfield shift in the P=O asymmetric stretching vibration, was strongly correlated with the Tm of the respective lipid membranes (Figure 8), indicating that this interaction determined the effectiveness of the solutes as lyoprotectants. Bet showed the weakest interaction with the P=O group, which may be due to the fact that it at least partially crystallized and, therefore, was not fully available for interactions with the membrane. It has, however, also been shown that Bet only very weakly interacts with the P=O group of lipids during freezing of liposomes, where no crystallization of Bet occurs [46]. Therefore this may be an inherent property of this molecule.
Interestingly, the P=O peak was split into two peaks in the presence of Tre and Sor. This could be clearly seen after resolution enhancement by deconvolution (Figure 7). For Sor, this split was already obvious in the original spectra. Although the effects of Tre have been extensively documented in the literature, this has not been reported before, presumably because of the lack of resolution enhancement in these studies (see e.g. [47,48]). A correlation with Tm, however, was only observed for the low-field peaks, whereas the high-field peaks, especially that of the P=O group in the presence of Sor, were clearly not correlated with Tm (Figure 8). Why Tre and Sor showed such heterogeneous interactions with the lipid P=O, and whether this has any functional implications, is not clear at present.
In addition to the P=O group, H bonding of solutes is also possible with the C=O group of diacyl lipids. This has been investigated in detail for a membrane-bound disaccharide [27]. In the present study, we found no evidence for interactions of the investigated solutes with the C=O group (Figure 6).
For the first time the ability of GG to protect membranes under stress conditions was tested in the present study and a significant stabilizing effect was found. Recently [49], it was found that GG also showed good thermostabilizing effects on isolated enzymes. Since GG belongs to the group of heterosides comprising a sugar and a polyol moiety, Sor as a polyol has been included in the study. Surprisingly, it showed slightly better membrane protection than the carbohydrates Tre and Suc, which are supposed to be particularly effective under these conditions. GG seems to share its protective properties with one of its constituent molecules, glycerol. Glycerol has been shown to be a good protectant for both biological [50–52] and model membranes [19] during freezing and thawing, but not during drying and rehydration [19]. However, even during freezing it prevented fusion to a smaller extent when compared with Suc or Tre. It also showed much weaker interactions with the lipid headgroup P=O [46]. The second constituent of GG, glucose, is also a poor glass former and it is not capable of preventing membrane fusion effectively during drying, but it shows good interaction with membranes and a large depression of Tm in the dry state [26]. In conclusion, we find that the effects of GG on membrane stability resemble those of glycerol more than those of glucose or Sor.
Regarding the stabilizing effect on membranes, a reverse order among the tested compatible solutes was found when compared with the ability to confer salt stress tolerance to cyanobacteria. This implies that membrane stability may not be the limiting factor for these cells under salt stress. In addition to purely osmotic effects, solutes such as Bet, but also Suc and Tre, have been shown to be extremely efficient in stabilizing soluble proteins [16,53] and peripherally bound membrane proteins [54–56] under stress conditions and this may also contribute to cellular salt stress tolerance. Cyanobacteria showing high tolerance to desiccation, on the other hand, accumulate Suc and Tre [57,58], which perfectly matches their membrane-protective properties.
References
- 1.Somero G. N. Adapting to water stress: convergence on common solutions. In: Somero G. N., Osmond C. B., Bolis C. L., editors. Water and Life. Berlin: Springer; 1992. pp. 3–18. [Google Scholar]
- 2.Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 3.Hagemann M., Schoor A., Mikkat S., Effmert U., Zuther E., Marin K., Fulda S., Vinnemeier J., Kunert A., Milkowski C., et al. The biochemistry and genetics of the synthesis of osmoprotective compounds in cyanobacteria. In: Ohren A., editor. Microbiology and Biogeochemistry of Hypersaline Environments. Boca Raton, FL: CRC Press; 1999. pp. 177–186. [Google Scholar]
- 4.Reed R. H., Borowitzka L. J., Mackay M. A., Chudek J. A., Foster R., Warr S. R. C., Moore D. J., Stewart W. D. P. Organic solute accumulation in osmotically stressed cyanobacteria. FEMS Microbiol. Rev. 1986;39:51–56. [Google Scholar]
- 5.Moore D. J., Reed R. H., Stewart W. D. P. A glycine betaine transport system in Aphanothece halophytica and other glycine betaine-synthesising cyanobacteria. Arch. Microbiol. 1987;147:399–405. [Google Scholar]
- 6.Mikkat S., Hagemann M., Schoor A. Active transport of glucosylglycerol is involved in salt adaptation of the cyanobacterium Synechocystis sp. strain PCC 6803. Microbiology. 1996;142:1725–1732. doi: 10.1099/13500872-142-7-1725. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi G., Gamba A., Limiroli R., Pozzi N., Elster R., Salamini F., Bartels D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol. Plant. 1993;87:223–226. [Google Scholar]
- 8.Drennan P. M., Smith M. T., Goldsworthy D., van Staden J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. J. Plant Physiol. 1993;142:493–496. [Google Scholar]
- 9.Iturriaga G., Gaff D. F., Zentella R. New desiccation-tolerant plants, including a grass, in the central high-lands of Mexico, accumulate trehalose. Aust. J. Bot. 2000;48:153–158. [Google Scholar]
- 10.Chen T. H. H., Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002;5:250–257. doi: 10.1016/s1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- 11.Nuccio M. L., Rhodes D., McNeil S. D., Hanson A. D. Metabolic engineering of plants for osmotic stress resistance. Curr. Opin. Plant Biol. 1999;2:128–134. doi: 10.1016/s1369-5266(99)80026-0. [DOI] [PubMed] [Google Scholar]
- 12.Ferjani A., Mustardy L., Sulpice R., Marin K., Suzuki I., Hagemann M., Murata N. Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol. 2003;131:1628–1637. doi: 10.1104/pp.102.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastmond P. J. Glycerol-insensitive Arabidopsis mutants: gli1 seedlings lack glycerol kinase, accumulate glycerol and are more resistent to abiotic stress. Plant J. 2004;37:617–625. doi: 10.1111/j.1365-313x.2003.01989.x. [DOI] [PubMed] [Google Scholar]
- 14.Cayley S., Record M. T. J. Roles of cytoplasmic osmolytes, water, and crowding in the response of Escherichia coli to osmotic stress: biophysical basis of osmoprotection by glycine betaine. Biochemistry. 2003;42:12596–12609. doi: 10.1021/bi0347297. [DOI] [PubMed] [Google Scholar]
- 15.Timasheff S. N. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu. Rev. Biophys. Biomol. Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- 16.Diamant S., Eliahu N., Rosenthal D., Goloubinoff P. Chemical chaperones regulate molecular chaperones in vitro and cells under combined salt and heat stresses. J. Biol. Chem. 2001;276:39586–39591. doi: 10.1074/jbc.M103081200. [DOI] [PubMed] [Google Scholar]
- 17.Crowe J. H., Crowe L. M., Carpenter J. F., Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver A. E., Hincha D. K., Crowe J. H. Looking beyond sugars: the role of amphiphilic solutes in preventing adventitious reactions in anhydrobiotes at low water contents. Comp. Biochem. Physiol. A. 2002;131:515–525. doi: 10.1016/s1095-6433(01)00514-1. [DOI] [PubMed] [Google Scholar]
- 19.Crowe J. H., Carpenter J. F., Crowe L. M., Anchordoguy T. J. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology. 1990;27:219–231. [Google Scholar]
- 20.Weinstein J. N., Ralston E., Leserman L. D., Klausner R. D., Dragsten P., Henkart P., Blumenthal R. Self-quenching of carboxyfluorescein fluorescence: uses in studying liposome stability and liposome-cell interaction. In: Gregoriadis G., editor. Liposome Technology, vol. 3. Boca Raton, FL: CRC Press; 1984. pp. 183–204. [Google Scholar]
- 21.MacDonald R. C., MacDonald R. I., Menco B. P. M., Takeshita K., Subbarao N. K., Hu L. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- 22.Hincha D. K., Zuther E., Hellwege E. M., Heyer A. G. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology. 2002;12:103–110. doi: 10.1093/glycob/12.2.103. [DOI] [PubMed] [Google Scholar]
- 23.Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- 24.Oliver A. E., Hincha D. K., Crowe L. M., Crowe J. H. Interactions of arbutin with dry and hydrated bilayers. Biochim. Biophys. Acta. 1998;1370:87–97. doi: 10.1016/s0005-2736(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 25.Hincha D. K., Oliver A. E., Crowe J. H. The effects of chloroplast lipids on the stability of liposomes during freezing and drying. Biochim. Biophys. Acta. 1998;1368:150–160. doi: 10.1016/s0005-2736(97)00204-6. [DOI] [PubMed] [Google Scholar]
- 26.Crowe J. H., Oliver A. E., Hoekstra F. A., Crowe L. M. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology. 1997;35:20–30. doi: 10.1006/cryo.1997.2020. [DOI] [PubMed] [Google Scholar]
- 27.Popova A. V., Hincha D. K. Intermolecular interactions in dry and rehydrated pure and mixed bilayers of phosphatidylcholine and digalactosyldiacylglycerol: a Fourier-transform infrared spectroscopy study. Biophys. J. 2003;85:1682–1690. doi: 10.1016/S0006-3495(03)74598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver A. E., Leprince O., Wolkers W. F., Hincha D. K., Heyer A. G., Crowe J. H. Non-disaccharide-based mechanisms of protection during drying. Cryobiology. 2001;43:151–167. doi: 10.1006/cryo.2001.2359. [DOI] [PubMed] [Google Scholar]
- 29.Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C=O-labeled phospholipids. Hydrogen bonding to carbonyl groups. Biochemistry. 1988;27:8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- 30.Lewis R. N. A. H., McElhaney R. N. Fourier transform infrared spectroscopy in the study of hydrated lipids and lipid bilayer membranes. In: Mantsch H. H., Chapman D., editors. Infrared Spectroscopy of Biomolecules. New York: Wiley-Liss; 1996. pp. 159–202. [Google Scholar]
- 31.Lewis R. N. A. H., McElhaney R. N. The structure and organization of phospholipid bilayers as revealed by infrared spectroscopy. Chem. Phys. Lipids. 1998;96:9–21. [Google Scholar]
- 32.Wong P. T. T., Mantsch H. H. High-pressure infrared spectroscopic evidence of water binding sites in 1,2-diacyl phospholipids. Chem. Phys. Lipids. 1988;46:213–224. [Google Scholar]
- 33.Wolkers W. F., Oliver A. E., Tablin F., Crowe J. H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004;339:1077–1085. doi: 10.1016/j.carres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Oliver A. E., Crowe L. M., Crowe J. H. Methods for dehydration tolerance: depression of the phase transition temperature in dry membranes and carbohydrate vitrification. Seed Sci. Res. 1998;8:211–221. [Google Scholar]
- 35.Sun W. Q., Leopold A. C., Crowe L. M., Crowe J. H. Stability of dry liposomes in sugar glasses. Biophys. J. 1996;70:1769–1776. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frolov V. A., Dunina-Barkovskaya A. Y., Samsonov A. V., Zimmerberg J. Membrane permeability changes at early stages of Influenza hemagglutinin-mediated fusion. Biophys. J. 2003;85:1725–1733. doi: 10.1016/S0006-3495(03)74602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller M., Katsov K., Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys. J. 2003;85:1611–1623. doi: 10.1016/S0006-3495(03)74592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angell C. A., Siell R. C., Sichina W. Viscosity-temperature function for sorbitol from combined viscosity and differential scanning calorimetry studies. J. Phys. Chem. 1982;86:1540–1542. [Google Scholar]
- 39.Orford P. D., Parker R., Ring S. G. Aspects of the glass transition behaviour of mixtures of carbohydrates of low molecular weight. Carbohydr. Res. 1990;196:11–18. doi: 10.1016/0008-6215(90)84102-z. [DOI] [PubMed] [Google Scholar]
- 40.Buitink J., van den Dries I. J., Hoekstra F. A., Alberda M., Hemminga M. A. High critical temperature above Tg may contribute to the stability of biological systems. Biophys. J. 2000;79:1119–1128. doi: 10.1016/S0006-3495(00)76365-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsujimi Y., Kobayashi M., Yagi T. Frequency and time-resolved spectroscopic study of liquid-glass transitions in D-sorbitol. Physica B. 1999;263:310–312. [Google Scholar]
- 42.Tsujimi Y., Kobayashi M., Furuta H., Yagi T. Ultra-slow relaxation dynamics in triglycine sulfate and D-sorbitol studied by time-resolved spectroscopy. J. Therm. Anal. Calorim. 1999;57:859–865. [Google Scholar]
- 43.Crowe J. H., Leslie S. B., Crowe L. M. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology. 1994;31:355–366. doi: 10.1006/cryo.1994.1043. [DOI] [PubMed] [Google Scholar]
- 44.Hincha D. K., Hellwege E. M., Heyer A. G., Crowe J. H. Plant fructans stabilize phosphatidylcholine liposomes during freeze-drying. Eur. J. Biochem. 2000;267:535–540. doi: 10.1046/j.1432-1327.2000.01028.x. [DOI] [PubMed] [Google Scholar]
- 45.Hincha D. K., Zuther E., Heyer A. G. The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochim. Biophys. Acta. 2003;1612:172–177. doi: 10.1016/s0005-2736(03)00116-0. [DOI] [PubMed] [Google Scholar]
- 46.Anchordoguy T. J., Rudolph A. S., Carpenter J. F., Crowe J. H. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987;24:324–331. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- 47.Crowe J. H., Hoekstra F. A., Nguyen K. H. N., Crowe L. M. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1996;1280:187–196. doi: 10.1016/0005-2736(95)00287-1. [DOI] [PubMed] [Google Scholar]
- 48.Tsvetkova N. M., Phillips B. L., Crowe L. M., Crowe J. H., Risbud S. H. Effect of sugars on headgroup mobility in freeze-dried dipalmitoylphosphatidylcholine bilayers: solid-state 31P NMR and FTIR studies. Biophys. J. 1998;75:2947–2955. doi: 10.1016/S0006-3495(98)77736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borges N., Ramos A., Raven N. D. A., Sharp R. J., Santos H. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles. 2002;6:209–216. doi: 10.1007/s007920100236. [DOI] [PubMed] [Google Scholar]
- 50.Hincha D. K., Heber U., Schmitt J. M. Antibodies against individual thylakoid membrane proteins as molecular probes to study chemical and mechanical freezing damage in vitro. Biochim. Biophys. Acta. 1985;809:337–344. [Google Scholar]
- 51.Lovelock J. E. Het mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim. Biophys. Acta. 1953;11:28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- 52.Santarius K. A. Freezing of isolated thylakoid membranes in complex media. VIII. Differential cryoprotection by sucrose, proline and glycerol. Physiol. Plant. 1992;84:87–93. [Google Scholar]
- 53.Santoro M. M., Liu Y., Khan S. M. A., Hou L.-X., Bolen D. W. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry. 1992;31:5278–5283. doi: 10.1021/bi00138a006. [DOI] [PubMed] [Google Scholar]
- 54.Hincha D. K. Release of two peripheral proteins from chloroplast thylakoid membranes in the presence of a Hofmeister series of chaotropic anions. Arch. Biochem. Biophys. 1998;358:385–390. doi: 10.1006/abbi.1998.0866. [DOI] [PubMed] [Google Scholar]
- 55.Murata N., Mohanty P. S., Hayashi H., Papageorgiou G. C. Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett. 1992;296:187–189. doi: 10.1016/0014-5793(92)80376-r. [DOI] [PubMed] [Google Scholar]
- 56.Papageorgiou G. C., Murata N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving Photosystem II complex. Photosynth. Res. 1995;44:243–252. doi: 10.1007/BF00048597. [DOI] [PubMed] [Google Scholar]
- 57.Hershkovitz N., Oren A., Cohen Y. Accumulation of trehalose and sucrose in cyanobacteria exposed to matric water stress. Appl. Environ. Microbiol. 1991;57:645–648. doi: 10.1128/aem.57.3.645-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Potts M. Desiccation tolerance of prokaryotes. Microbiol. Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]