Abstract
Cells expressing ricin B chain within the secretory pathway are significantly more resistant to intoxication by ricin holotoxin but not to other cytotoxins that exploit similar endocytic routes to the cytosol. Furthermore, cells expressing the related B chain of abrin are protected against both incoming abrin and ricin. These phenotypes can be correlated with the abilities of the respective B chains to form disulphide-linked A–B holotoxins, since abrin B chain forms heterodimers with either abrin or ricin A chains, whereas ricin B chain forms heterodimers with ricin A chain only. In the ricin B-expressing cells, this newly made lectin disappears with biphasic kinetics comprising a retention phase followed by slow turnover and disposal after disengagement from calnexin cycle components. Interference with ricin cytotoxicity occurs during the early retention phase when ricin B chain is associated with PDI (protein disulphide-isomerase). The data show that retrotranslocation of incoming toxin is impeded by PDI-catalysed formation of heterodimers between endogenous B and A chains derived from reduced holotoxin, thus proving that reduction of ricin occurs in the endoplasmic reticulum. In contrast with other toxins, ricin does not appear to require either proteolytic cleavage or unfolding for PDI-catalysed reduction.
Keywords: endoplasmic reticulum-associated protein degradation (ERAD), oxidoreductase, protein disulphide-isomerase, retrograde transport, ricin, toxin
Abbreviations: ATA, abrin isoform F A chain; ATB, abrin B chain; BFA, Brefeldin A; BMH, bis-maleimidohexane; CNX, calnexin; CST, castanospermine; CTA, cholera toxin A; DMEM, Dulbecco's modified Eagle's medium; DMM, deoxymannojirimycin; DTT, dithiothreitol; endo H, endoglycosidase H; ER, endoplasmic reticulum; FCS, foetal calf serum; NEM, N-ethylmaleimide; PDI, protein disulphide-isomerase; PE, Pseudomonas exotoxin A; RTA, ricin toxin A chain; RTB, ricin toxin B chain; SFM, serum-free medium; SLT, Shiga-like toxin 1; Tn, tunicamycin
INTRODUCTION
Ricin, a toxin purified from the seeds of the castor-oil plant Ricinus communis, is capable of killing mammalian cells by irreversibly removing a specific adenine residue from a highly conserved loop present in the 28 S rRNA of ribosomes [1]. Since this purine is required for binding the EF-2 ternary complex to the ribosome, the result is a cessation of protein elongation leading to a halt in protein synthesis and, ultimately, cell death. Structurally, ricin is composed of a glycosylated A chain, RTA (ricin toxin A chain; molecular mass 32 kDa), which bears the toxic rRNA-specific N-glycosidase activity, covalently linked by a reducible disulphide bond to a glycosylated D-galactose- and N-acetylgalactosamine-specific lectin, the B chain (RTB; molecular mass 34 kDa) [2]. When ricin is applied externally to mammalian cells, both cell internalization and membrane translocation steps are required for a toxic effect [3]. The former is initiated by RTB, which binds exposed cell-surface galactosyl residues. The bound toxin is subsequently endocytosed with a portion undergoing retrograde transport from the Golgi to the ER (endoplasmic reticulum) [4–6]. From the ER, either holotoxin or reduced RTA then enters the cytosol by exploiting the route of misfolded substrates for ER-associated proteolytic degradation [7–9]. For RTA to act, it must be reductively cleaved from RTB to release a steric block of the active site [10,11].
ER proteins have been implicated in the reduction of cholera toxin [12–14] and PE (Pseudomonas exotoxin A) [15], two ER-translocating toxins that are structurally and functionally distinct from ricin. Indeed proteolytically nicked A chains of cholera toxin can be reduced in vitro using mixtures of GSH and GSSG in the total absence of ER proteins [12–14]. However, for ricin, neither the site of reduction nor the identities of proteins involved in this modification have been formally demonstrated. It has been reported that a non-reducible, covalently coupled ricin holotoxin remains cytotoxic to mammalian cells, suggesting that both subunits may translocate to the cytosol where proteolysis is able to liberate a catalytic fragment [16]. In contrast, co-expression and targeting of ricin subunits to the ER lumen of transformed plant cells has strongly supported the model that only free RTA is competent for retrotranslocation from ER to the cytosol [17,18]. In such cells, the pronounced toxic effect observed when newly synthesized, glycosylated RTA is retrotranslocated across the plant cell ER can be mitigated when both RTA and RTB subunits are simultaneously expressed. In this situation, heterodimers are formed in the ER lumen that appear incapable of retrotranslocation but that are now competent for secretion [17].
It has been observed that expression of RTB alone in microinjected Xenopus laevis oocytes led not to its secretion but to intracellular entrapment in an endo H (endoglycosidase H)-sensitive form [19]. Similarly, RTB expressed in the secretory pathway of Saccharomyces cerevisiae remained intracellular [20]. In such systems, RTB, in the absence of its partner RTA, appears to be retained in the early endomembrane system, perhaps by long-lived interactions with molecular chaperones or other proteins.
We reasoned that if entrapment of ER-targeted RTB in the early secretory pathway occurred in mammalian cells also, its presence might interfere with toxicity from a subsequent challenge with holotoxin. We report here that the continual synthesis of RTB does indeed protect these cells from a subsequent ricin challenge, but not from other unrelated toxins known to traffic to the ER before their retrotranslocation. Interestingly, cell lines stably expressing the related ATB (abrin B chain) rather than RTB were protected against both incoming abrin and ricin. Low concentrations of DTT (dithiothreitol) disrupted resistance to ricin indicating a thiol-mediated process. The site of interaction of endogenous RTB and incoming ricin occurred in the ER lumen, and PDI (protein disulphide-isomerase) could be coimmunoprecipitated with endogenously expressed RTB. Furthermore, reduction of ricin by PDI could be recapitulated in vitro, confirming that ricin holotoxin, in the absence of further modifications such as proteolytic cleavage or unfolding, is a PDI substrate. The implications of these findings in relation to the reduction of ricin are discussed.
MATERIALS AND METHODS
Sources
Antibody against PDI was purchased from Stressgen Biotechnologies Corp. (Victoria, BC, Canada) and antibodies against ricin and abrin subunits were raised in rabbits by standard immunization. Radioisotopes were from Amersham Biosciences (Little Chalfont, Bucks., U.K.). RTB was purchased from Vector Laboratories (Burlingame, CA, U.S.A.), and ricin was from Sigma. Cross-linkers were obtained from Pierce (Cheshire, U.K.). Plasmids pIgplus and pDR2δEF1α were kindly provided by Awen Gallimore (IMM, Oxford, U.K.) and pLWRP62, which encodes mature human PDI carrying a His6-Met N-terminal tag, was a gift from L. Ruddock (Biocenter, Oulu, Finland).
Plasmid construction for expressing RTB and ATB in mammalian cells
To express B chains in the secretory pathway, a PCR-amplified DNA fragment encoding the CD33 signal peptide [21] was cloned into pDR2δEF1α followed by ligation of a PCR-amplified fragment of RTB-encoding DNA [22] or abrin-encoding DNA [23] to generate pJC877 and pJC878 respectively, in which expression is under the control of the mammalian EF1α promoter [24]. The predicted primary sequence of the encoded fusion protein comprises the CD33 signal peptide (amino acids 1–17), DKLAS (encoded by part of an original multiple cloning site), followed by residues encoding the relevant B chain.
Generation and maintenance of cell lines
HeLa cells, and stable cell lines derived from them, were maintained in DMEM/FCS (Dulbecco's modified Eagle's medium containing 10% foetal calf serum). For production of cell lines, 3×105 cells were seeded in the wells of 6-well tissue culture plates. On the following day, each well was incubated with 1 ml of SFM (serum-free medium) to which was added 30 μl of a preformed complex of 0.7 μg pJC877 DNA or pJC878 DNA and 3 μl LIPOFECTAMINE™ (Gibco) made in PBS (pH 7.4). After 6 h, the SFM mix was replaced with DMEM/FCS. Cells were grown for 2 days before incubation in medium containing hygromycin (200 μg/ml), and hygromycin-resistant colonies were cloned after limiting dilution. Stable cell lines expressing RTB or ATB were identified after immunoblotting of detergent extracts of candidate colonies using appropriate rabbit antisera. In parallel, cells were transfected with pMCEFlacZ [25], and a lacZ-expressing clone was chosen by β-galactosidase assay after clonal selection with 1 mg/ml G418. For growth rate estimates, cells were seeded at low density in 24-well tissue culture plates in 1 ml of DMEM/FCS, grown and counted at daily intervals after trypsin treatment.
Binding and uptake of toxin
Iodination of RTB was performed using IODO-BEADS iodination reagent (Pierce Biotech) following the manufacturer's recommendations. To monitor binding affinities, cells were plated in triplicate into 24-well dishes at a density of 0.5×106/well and incubated on ice for 2 h with binding buffer (PBS containing 20% FCS) containing increasing concentrations of [125I]RTB. Cells were then extensively washed with ice-cold PBS and removed for γ-counting. Scatchard analysis was performed on the data obtained. To monitor endocytosis, cells (0.5×106/well) were washed with PBS, placed on ice and overlaid with 100 ng/ml [125I]RTB in DMEM without FCS. Iodinated protein was allowed to bind for 15 min on ice followed by 15 min at 37 °C. Surface-bound RTB was removed by washing with ice-cold PBS containing 150 mM galactose, and incubation continued for 15 min on ice with SFM containing 0.3% (w/v) pronase (Sigma). After centrifugation, the radioactivity, corresponding to endocytosed RTB, was measured in cell pellets.
Cell treatments, pulse–chase and immunoprecipitations
Cells seeded at 0.5×106/well in 6-well plates and grown overnight were treated, where appropriate, with DMEM/FCS containing 10 μg/ml Tn (tunicamycin) (or 20 μM NaOH, the solvent carrier) for 6 h before sampling for immunoblots. In pulse–chase experiments, after washing twice with methionine/cysteine-free DMEM/FCS, incubation was continued for 30 min to deplete the intracellular pools of these amino acids. During this starvation period, cells could be treated with DMEM/FCS containing 10 μM clasto-lactacystin β-lactone (or 0.001% DMSO solvent carrier), 10 μg/ml BFA (Brefeldin A) (or 0.001% ethanol solvent carrier), 100 μg/ml CST (castanospermine), 2 mM DMM (deoxymannojirimycin) or various concentrations (0.25, 0.5 and 1 mM) of DTT. [35S]Promix was then added to 70 μCi/ml for 15 min when the cells were washed twice before incubation in DMEM/FCS, containing, where appropriate, clasto-lactacystin β-lactone, BFA, CST, DMM or DTT, so that these reagents were maintained throughout the experiment. At various time points, conditioned growth medium was collected, adjusted by the addition of protease inhibitors (Complete protease inhibitor cocktail; Roche, Basel, Switzerland), and stored on ice. Cell extracts were taken at the same time points by scraping into 1 ml of cold immunoprecipitation (IP) buffer (15 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.1% SDS and 2 mM NaN3 containing Complete protease inhibitors). Extracts and conditioned growth medium were clarified by centrifugation (10000 g, 4 min), and the clarified samples were tumbled with 50 μl of Pansorbin™ (Calbiochem, San Diego, CA, U.S.A.; Merck Biosciences, Poole, Dorset, U.K.) for 1 h. After centrifugation (10000 g, 1 min), precleared samples were immunoprecipitated overnight using 3 μl of specific antiserum against RTB or PDI, together with 50 μl of 0.1 mg/ml Protein A–Sepharose (Sigma) pre-equilibrated in IP buffer. Immunoprecipitates were washed twice with IP buffer, twice with high urea buffer (100 mM Tris/HCl, pH 7.5, 2 M urea, 200 mM NaCl, 1% Triton X-100 and 2 mM NaN3), once with high-salt buffer (20 mM Tris/HCl, pH 7.5, 500 mM NaCl, 1% Triton X-100 and 2 mM NaN3) and once with 10 mM Tris/HCl (pH 7.5), 50 mM NaCl and 2 mM NaN3 before PAGE. For endo H treatment, immunoprecipitates were suspended in 30 μl of 50 mM sodium citrate (pH 5.5) and 0.25% SDS and heated for 5 min at 95 °C. After pelleting the Sepharose beads, endo H was added to 1 m-unit/ml to the soluble fraction, and reactions were incubated overnight at 37 °C. For co-immunoprecipitations, cells were washed twice with PBS after the labelling period and incubated in 10 mM NEM (N-ethylmaleimide) for 10 min at room temperature (22–24 °C), or with 2 mM BMH (bis-maleimidohexane) (or DMSO solvent control), or a mixture of 2 mM BMH and 4 mM Tris[2-carboxyethyl]phosphine, or with 2 mM disuccinyl suberate as appropriate for 15 min at room temperature. After washing twice with PBS, cross-linkers BMH and disuccinyl suberate were quenched by incubation of the cells with 100 mM 2-mercaptoethanol or 10 mM Tris/HCl (pH 7.5) respectively for a further 15 min at room temperature. Cell extracts were then taken and treated as before.
Cytotoxicity assays
Cells were seeded at 1.5×104/well in a 96-well tissue culture plate, allowed to grow overnight and, after washing with PBS, were incubated for 4 h with 100 μl DMEM/FCS containing graded concentrations of ricin or other toxins, as indicated in the Figures. Subsequently, cells were washed twice with PBS and incubated in PBS containing 10 μCi/ml [35S]methionine for 90 min. After washing the cells twice with PBS, labelled proteins were precipitated with three washes in 5% (w/v) trichloroacetic acid, the wells were washed twice with PBS and the amount of radiolabel incorporated was determined after the addition of 200 μl of scintillation fluid, by scintillation counting in a Micro-Beta 1450 Trilux counter.
Reassociation of heterodimeric toxins
RTA (50 μg) or bacterially expressed ATA (abrin isoform F A chain; [23]) was mixed with 50 μg of purified RTB or plant-derived ATB [23] in a total volume of 1 ml of PBS containing 0.1 M lactose and 2% 2-mercaptoethanol. The mixture was dialysed overnight against 0.1 M lactose in PBS at 4 °C and then against PBS over three nights at 4 °C. Reassociated holotoxin was purified from free A chain on a 0.5 ml lactose–agarose column as described previously [26]. The appearance of A chain in the eluted fractions is diagnostic of a disulphide-bonded heterodimeric toxin.
Preparation of His-tagged recombinant PDI
Escherichia coli BL21(pLysS) transformed with pLWRP62 was grown to mid-exponential phase (A600 0.3) and expression of PDI was induced by the addition of isopropyl β-D-thiogalactoside to 1 mM. After 4 h incubation, cells were harvested, suspended in 20 mM Tris/HCl, pH 8.0 (Buffer A), lysed by sonication and, after removal of insoluble material by centrifugation, the aqueous phase was loaded on to a 6 ml Ni2+ column pre-equilibrated in Buffer A. After extensive washing with Buffer A containing 5 mM imidazole, bound proteins were eluted in Buffer A containing 20 mM imidazole. Fractions containing PDI were identified by immunoblotting, pooled, dialysed into 20 mM Tris/HCl (pH 7.3) and further purified on a Mono Q column, from where they were eluted with a 0–500 mM NaCl gradient. Fractions containing PDI were identified by SDS/PAGE and Coomassie Blue staining.
RESULTS
Expressed RTB is non-toxic to mammalian cells
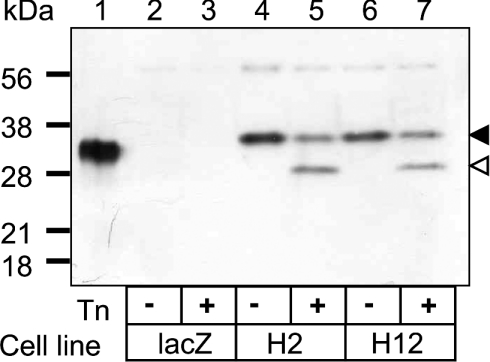
HeLa cells were transfected with pJC877 and, after hygromycin selection, colonies were screened for stable expression of RTB by immunoblotting of detergent-soluble cell extracts (Figure 1). A number of cell lines (Table 1) expressed a polypeptide of ∼36 kDa that cross-reacted with a rabbit antiserum specific for RTB; of these, two (HeLa/RTB-H2 and HeLa/RTB-H12) were selected for further study (Figure 1, lanes 4 and 6, black arrowhead). This polypeptide was approx. 2 kDa larger than native RTB purified from R. communis seeds (lane 1). Since RTB has two N-glycosylation sequons, this difference in mass may reflect differences in glycan processing between plant and animal systems. Alternatively, failure to cleave the CD33 signal peptide, if RTB were mistargeted, would result in a larger than expected polypeptide. To confirm correct ER targeting, cells were therefore pretreated with Tn for 6 h to produce a mature-sized, non-glycosylated RTB of 29 kDa (Figure 1, lanes 5 and 7, open arrowhead), the expected mass [22]. A significant proportion of RTB, representing the fraction made before the 6 h Tn pretreatment, remained fully glycosylated, suggesting a slow turnover of this protein. Since expression of this galactose-binding lectin did not affect either cell morphology (not shown) or growth rate (the population doubling times being 26.2, 25.2 and 24 h respectively for HeLa/LacZ, HeLa/RTB-H2 and HeLa/RTB-H12), we conclude that mammalian cells are tolerant to RTB sequestered within the endomembrane system.
Figure 1. RTB is expressed in HeLa cells without toxic effects.
Detergent-extracted proteins (10 μg) from vector-controlled HeLa cells expressing lacZ (lane 2), or from HeLa expressing ricin B (RTB) clone H2 (lane 4) or clone H12 (lane 6) together with corresponding Tn-treated samples (lanes 3, 5 and 7) were immunoblotted in parallel with RTB purified from R. communis seeds (lane 1). The black arrowhead represents the mass of expressed RTB and the open arrowhead represents the mass of mature, non-glycosylated RTB. Positions of migration of molecular-mass standards are displayed on the left.
Table 1. The protective effects of ER-targeted RTB and ATB expression against a 4 h challenge with ricin and abrin holotoxins.
| Protective effect of challenge (fold) | ||
|---|---|---|
| Cell line expressing RTB/ATB | Ricin | Abrin |
| RTB | ||
| RTB1 | 45 | 1.0 |
| RTB8 | 2.5 | 1.0 |
| RTB11 | 25 | 1.0 |
| RTB16 | 10.8 | 1.0 |
| H2 | 37.5 | 1.0 |
| H12 | 27.5 | 1.0 |
| ATB | ||
| ATB1 | 304 | 50 |
| ATB4 | 2.8 | 3.0 |
| ATB5 | 2.4 | 2.5 |
| ATB8 | 2.0 | 1.5 |
| ATB9 | 26.1 | 7.5 |
Expressed RTB protects cells against ricin
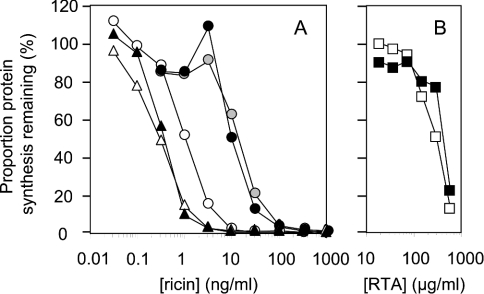
Control cells [HeLa cells expressing β-galactosidase (HeLa/LacZ), HeLa expressing hygromycin resistance and HeLa] and two RTB-expressing cell lines, HeLa/RTB-H2 and HeLa/RTB-H12, were treated with increasing concentrations of ricin and free RTA, and subsequent levels of protein synthesis were measured (Figure 2). Expression of RTB within the secretory pathway clearly protected against ricin challenge, with both cell lines being significantly more resistant to ricin intoxication compared with control cells (Figure 2A). In contrast, cells expressing ER-targeted RTB were not protected against free RTA added at doses known to permit fluid phase uptake along the productive pathway to the ER (Figure 2B) [27–29]. When cell line HeLa/RTB1, which protected 45-fold against ricin, was compared with cell line HeLa/RTB8, which protected only slightly against ricin (Table 1), the former was found to contain four times the amount of RTB at steady state compared with that found in the less resistant cell line (results not shown). Thus higher expression levels of RTB in the ER lead to greater protection from ricin challenge.
Figure 2. Expressed RTB protects cells against ricin.
(A) Non-transfected HeLa cells (○) and transfected HeLa cells expressing lacZ (▴), hygromycin (▵), RTB-H2 (•) or RTB-H12 (grey circles) were treated with ricin, and their ability to synthesize proteins subsequently was determined. (B) Non-transfected HeLa cells (□) and RTB-H2 cells (▪) were treated with recombinant RTA and their ability to synthesize proteins subsequently was determined.
Expressed RTB does not block cell-surface galactosyl residues or impair endocytosis
The protection against ricin provided by RTB in the endomembrane system might be explained by the secretion of functional galactose-binding RTB with subsequent blockade of cell-surface ricin-binding sites. Alternatively, intracellular binding of potential ricin receptors by the expressed RTB might interfere with their arrival on the cell surface to preclude holotoxin binding indirectly. To examine these, cells were incubated with dilutions of [125I]RTB at 0 °C to ensure binding but not uptake, and the amount of bound RTB was determined. Scatchard analysis revealed that there was no significant difference in the number of available surface binding sites or between the Kd values of control HeLa/LacZ cells and those expressing RTB (Table 2). These figures are comparable with previously determined values for holotoxin binding to HeLa cells [30]. Thus the interference with ricin cytotoxicity did not involve a competition for ricin receptors between secreted RTB and added ricin. Internalization of ricin was also followed to determine whether endocytosis had been deleteriously affected by the expression of RTB. After binding of 125I-labelled RTB to cells at 0 °C, the temperature was increased to 37 °C for 15 min to promote endocytosis, and the amount of [125I]RTB that entered the cells was monitored after washing the cell surface with lactose to remove surface-bound RTB. Similar amounts of labelled RTB entered control and RTB-expressing HeLa cell lines (Table 2).
Table 2. ER-targeted RTB blocks neither binding nor endocytosis of exogenously added RTB.
| Endocytosis assay | |||
|---|---|---|---|
| Scatchard analysis | |||
| Affinity (Kd, μM) | Binding sites/cell (×106) | Uptake of [125I]RTB (% relative to control cells) | |
| HeLa/LacZ | 1.17 (±0.04) | 91.9 (±9.5) | 100 |
| HeLa/RTB-H2 | 1.19 (±0.02) | 87.9 (±3.3) | 87.7 (±2.4) |
Control and RTB-expressing HeLa cells were also treated with Cy-3-labelled RTB and Cy-3-labelled E. coli SLT (Shiga-like toxin 1) B chain, to examine cell entry and routing by immunofluorescence microscopy. No obvious differences between the cell lines were observed (results not shown). We also examined any possible perturbation of the toxin uptake pathway indirectly, monitoring the cytotoxic potency of four other protein toxins, PE, SLT, diphtheria toxin and the structurally and functionally related type II ribosome-inactivating protein, abrin. PE, SLT, diphtheria toxin or abrin was added to control and RTB-expressing cells and ongoing protein synthesis was measured as described above and IC50 values were determined. There was no significant difference between the RTB-expressing cell lines and the control cell line for each toxin (results not shown). We conclude that the protective effect of expressed RTB against externally added ricin cannot be explained by events at the cell surface, or by interference with intracellular trafficking pathways or the translocation channels used by these toxins.
Formation of heterodimers explains the resistance to ricin
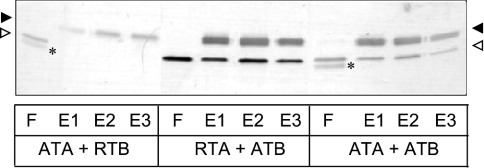
Strong protection was provided by ER-targeted RTB to incoming ricin, but no such protection was observed when these cell lines were challenged with abrin (Table 1). Abrin is a lectin with close structural and functional similarity to ricin, including identical receptor binding characteristics [31]. On performing disulphide reassociation experiments with purified A and B chains of ricin and abrin in vitro, we found that whereas ATA and ATB, and RTA and ATB, were able to associate to form disulphide-bonded holotoxins, the combination of ATA and RTB (as might potentially occur in the early secretory pathway of RTB-expressing cells treated with abrin) could not (Figure 3). This allowed us to predict that cells expressing ATB, a polypeptide that is competent to form disulphide bonds with both RTA and ATA, would show resistance to both holotoxins. Five ATB-expressing HeLa cell lines were therefore generated, and all of them exhibited significant resistance to both ricin and abrin holotoxins (Table 1). The level of resistance could again be correlated to the varied levels of ATB expression, as for RTB (results not shown). It was therefore deduced that in vivo, newly expressed ER-localized RTBs or ATBs interfered with incoming toxin by forming disulphide-bonded heterodimers with the newly reduced A chains. Intoxication was attenuated only with permitted combinations of subunits.
Figure 3. RTB and ATB form particular cross-heterodimers with toxin A chains in vitro.
Silver-stained reducing SDS/PAGE showing reassociations of combinations of toxin A chains (open arrowheads) and B chains (black arrowheads). After incubations (see the Materials and methods section), the mixtures were loaded on to immobilized α-lactose columns and bound B chains/disulphide-linked holotoxins were eluted with 100 mM lactose. In each case, F represents flowthrough; E1–E3 represent samples of the eluted fractions. ATA was from a bacterially expressed abrin F isoform; ATB was from an abrin A isoform purified from Abrus precatorius seeds; RTA was bacterially expressed [46]; RTB was purified from R. communis seeds. The asterisks denote a proteolytic breakdown product of ATA.
ER-targeted RTB is largely retained in the early secretory pathway
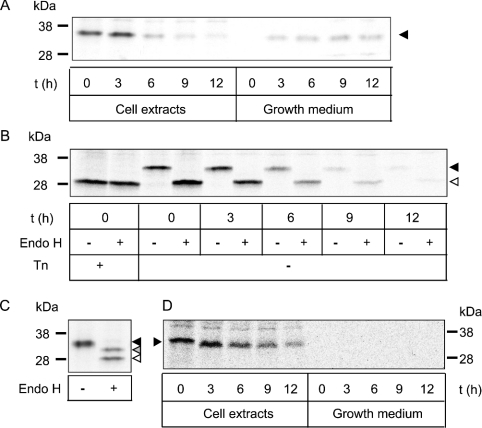
To determine where in the cell the newly made RTB was interfering with internalized toxin, we first analysed the stability of the expressed protein. Since nascent RTB was delivered into the endomembrane system, the possibility of its secretion was examined. Figure 4(A) shows that when cells were pulsed with radiolabelled methionine/cysteine for 15 min followed by a chase of up to 12 h, a small proportion of RTB was found in the medium. It was evident that the internal RTB remained sensitive to endo H, indicative of a glycoprotein trapped or cycling in the early secretory pathway (Figure 4B). In contrast, a form with partial endo H resistance was detected in the conditioned growth medium (Figure 4C), showing that RTB glycans could become terminally modified should transport through the Golgi occur. To test whether this material was secreted and not released from broken or dying cells, the cells were treated with BFA before and during pulse–chase analysis to redistribute Golgi contents to the ER (Figure 4D). The absence of RTB in the growth medium under these conditions shows that extracellular RTB has been genuinely secreted. We speculate that this unusual glycosylation pattern is that of a dimeric RTB that has a conformation permitting terminal glycosylation of only one of the four glycans present. Although non-reducing SDS/PAGE revealed that homodimers containing disulphide-linked RTB proteins did not exist in the medium (results not shown), it remained possible that non-covalently associated B chains do occur. However, it is clear that most of the intracellular RTB, being endo H-sensitive, must be contained in the early secretory pathway. It should be noted that indirect immunofluorescence of the RTB-expressing cells did not show any obvious staining difference between these and control HeLa cells. Cross-reaction of the anti-RTB antibodies with unrelated cellular proteins is a probable explanation for this (Figure 1 and later). Furthermore, at steady state, the presence of a small population of RTB in the relatively large volume of the ER lumen may preclude ready visualization.
Figure 4. Most of the RTB was retained in the early secretory pathway from where it disappeared with time.
(A) Pulse-labelled RTB (black arrowhead) from cell extracts and conditioned medium was immunoprecipitated after chase (t) for up to 12 h, and electrophoresed under reducing conditions. (B) Immunoprecipitates, as generated in (A), were incubated with or without endo H as described in the Materials and methods section. Such a treatment generated a faster migrating form (open arrowhead) that co-migrated with the Tn-treated samples. (C) RTB, recovered by immunoprecipitation from conditioned growth medium (black arrowhead), was treated with endo H to generate two faster migrating species (open and grey arrowheads). (D) Cells treated with BFA were lysed and RTB immunoprecipitated from cell and media fractions. Molecular-mass standards are shown on the left (A–C) or right (D).
Newly made RTB is subjected to ER quality control
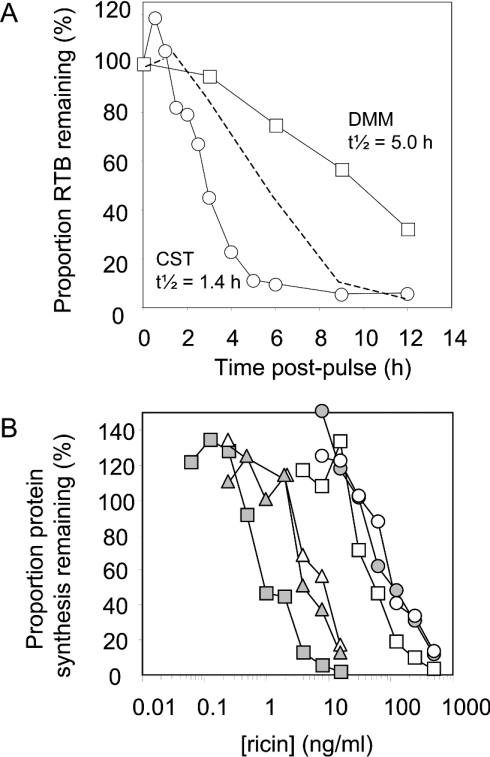
Pulse–chase analysis of cells expressing RTB and quantification of immunoprecipitates revealed biphasic kinetics of disappearance, with an approx. 2 h retention phase followed by gradual disposal also with a half-life of approx. 2 h (Figure 5, circles). Pulse-labelled RTB accumulated in the growth medium up to approx. 25% of the total by the 9 h chase point (results not shown), and then gradually disappeared from the medium by an unknown mechanism. Secretion was not the only reason for the loss of intracellular RTB, since addition of the specific proteasomal inhibitor clasto-lactacystin β-lactone significantly retarded the disappearance of intracellular material, increasing its half-life by more than 2-fold (Figure 5, squares) but had no effect on the proportion secreted (results not shown). This suggested that a fraction of RTB was mislocated to the cytosol where it was being targeted for degradation by cytosolic proteasomes. Since RTB is a glycoprotein, we tested whether this disposal involved an earlier engagement with the CNX (calnexin) cycle [32]. Treatment of cells with CST to inhibit glucosidases and thereby prevent entry into the cycle enhanced the disappearance of RTB, reducing its half-life compared with untreated controls (Figure 6A, circles). These results suggest that RTB normally interacts with CNX cycle components. To confirm this, cells were treated with DMM, an inhibitor of ER mannosidases, to extend interactions with CNX cycle proteins. In this situation, the half-life of pulse-labelled RTB was greatly extended (Figure 6A, squares). Blocking the entry of RTB into the cycle (by CST) did not, however, sensitize the cells to ricin (Figure 6B), as might have been predicted if interference with incoming holotoxin normally occurred when RTB was engaged with CNX cycle components. Consistent with this, the converse treatment to retain RTB in the CNX cycle using DMM did not result in an increased protection against ricin. Indeed, a slight sensitization was observed (Figure 6B). Together, these data suggest that although most RTB is eventually targeted to the CNX cycle for eventual disposal, the point at which it interferes with incoming ricin lies upstream, during the initial ER retention phase.
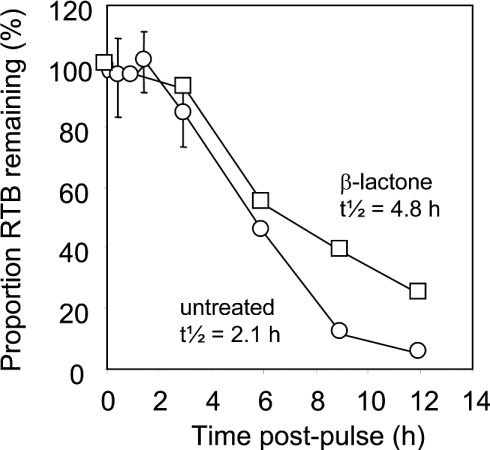
Figure 5. Expressed RTB was partially stabilized when proteasomes were inhibited.
The relative amount of label incorporated into RTB was quantified after electrophoresis using a PhosphorImager. Results are displayed graphically, plotting the proportion of RTB retained in cell extracts against the time of chase, for untreated cells (○) and cells treated with clasto-lactacystin β-lactone (□).
Figure 6. Disposal of RTB involved interaction with the CNX cycle in a manner that did not interfere with incoming ricin.
(A) Quantification of RTB in cell extracts after electrophoresis using a PhosphorImager. Results are displayed graphically, plotting the proportion of RTB retained in cell extracts against the time of chase, for cells treated with CST (○) or DMM (□), compared with untreated cells from Figure 5 (- - - -). (B) Effect of CST and DMM on cytotoxicity of ricin. HeLa cells (grey triangles) and HeLa/RTB-H2 cells (grey circles) were challenged for 4 h with increasing concentrations of ricin and their ability to incorporate [35S]methionine into proteins was determined and expressed as the proportion of remaining protein synthesis. In parallel, cells were treated with CST (▵, HeLa; ○, HeLa/RTB-H2) or DMM (grey squares, HeLa; □, HeLa/RB-H2) during the intoxication process.
Thiol exchange is implicated in the protection against ricin
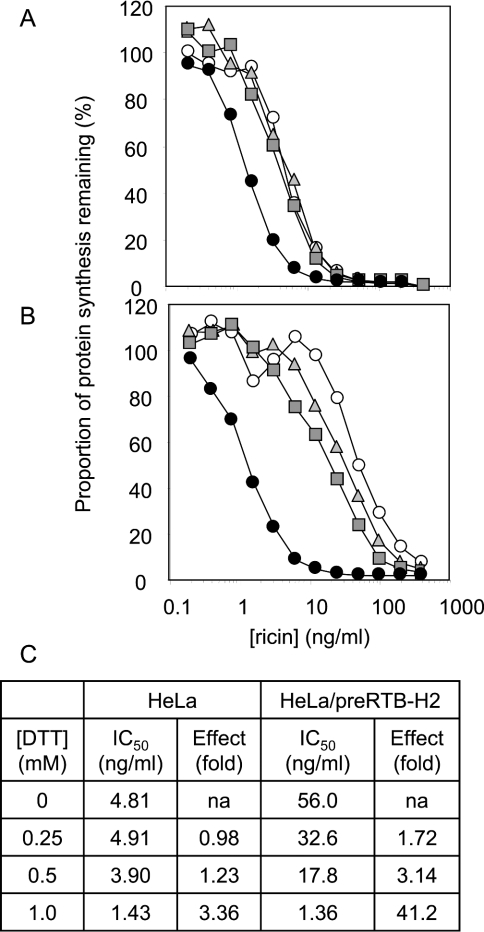
In the plant seed, where ricin is normally formed, RTB folds as part of a single-chain protein precursor comprising RTA and RTB domains separated by a small peptide linker. Subsequent processing into the heterodimeric toxin, the biologically active form, occurs after transport to storage vacuoles. To express functional RTB by itself would require the correct formation of four intra-chain disulphide bonds, leaving the most N-terminal cysteine residue (that normally couples with RTA) unpaired. This unpaired cysteine residue could be responsible for the relatively long early retention phase by acting as a thiol anchor to an ER chaperone. To test if thiol retention was involved in this relatively long first phase, HeLa cells (Figure 7A) and HeLa cells expressing RTB (Figure 7B) were treated with DTT, and their ability to cope with a ricin challenge was tested. Concentrations up to 0.5 mM DTT had little effect on the cytotoxicity of ricin towards HeLa cells, but at 1 mM DTT, the cells were rendered approx. 3.4-fold more sensitive to toxin (Figures 7A and 7C). Under these conditions, it is probable that reduction of ricin was more readily attained, facilitating release of RTA in the retrotranslocation compartment. Interestingly, very low levels of DTT (e.g. 0.25 mM) had a much more pronounced effect in sensitizing the normally ricin-resistant RTB-expressing cells to toxin, with 1 mM DTT restoring sensitivity to that shown by the control HeLa cells treated in an identical way. The outstanding approx. 12-fold difference in the effects of 1 mM DTT between HeLa and HeLa/RB-H2 cells was clearly related to the presence of expressed RTB, most probably by promoting its release from an anchor and thereby relieving a block on ricin reduction.
Figure 7. Disulphide bond reduction perturbs the protection provided by RTB against a ricin challenge.
HeLa cells (A) and HeLa/RTB-H2 (B) cells were treated with fresh medium (○) or with fresh medium containing 0.25 mM (grey triangles), 0.5 mM (grey squares) or 1 mM (•) DTT and challenged with increasing concentrations of ricin, and their ability to synthesize proteins subsequently was measured. (C) The concentrations of ricin required to reduce protein synthesis by 50% (IC50) and the effect (fold) of different DTT concentrations.
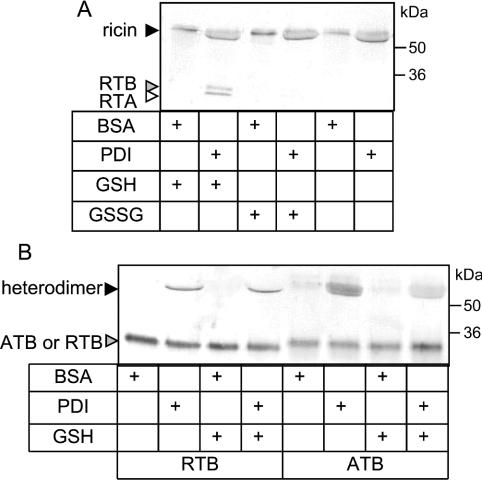
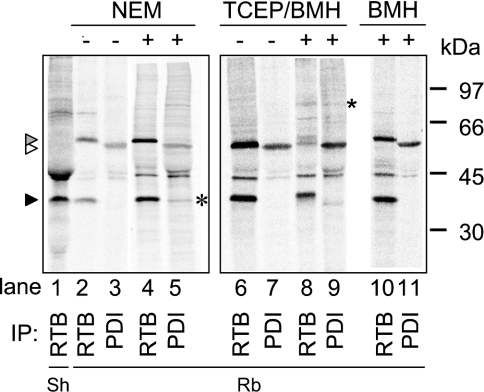
A member of the PDI family would make a good candidate for such an anchor. Indeed, we have demonstrated in vitro that PDI was not only sufficient to reduce unmodified holoricin in the presence of GSH (Figure 8A), but also to associate ricin subunits in the absence or presence of GSH (Figure 8B). Furthermore, formation of PDI-catalysed mixed heterodimers occurred in a solution containing RTA and ATB (Figure 8B) but not when RTB was present with ATA (results not shown), consistent with our earlier observations on the protective effects of toxin B chains expressed in the ER. To investigate whether interaction with PDI caused retention of RTB in vivo, we pulsed RTB-expressing cells for 15 min to follow its fate during the 2 h retention phase that precedes CNX cycle interactions and turnover. We then immunoprecipitated RTB and PDI under various conditions (Figure 9). In addition to the immunoselection of RTB (black arrowhead), rabbit anti-RTB antiserum cross-reacted with an irrelevant protein (molecular mass 58 kDa, grey arrowhead) that was not detectable using a sheep anti-RTB antibody (lane 1). A small fraction of the PDI pool is also radiolabelled during the short pulse, revealing a band migrating with the expected apparent molecular mass of 55 kDa (white arrowhead). Significantly, when cells were treated with 10 mM NEM to block disulphide exchange, a fraction of the labelled RTB could be co-immunoprecipitated with anti-PDI antibodies (asterisk, lane 5). The absence of a reciprocal co-precipitation of PDI using an anti-RTB antibody was not surprising, given the very large pool of unlabelled PDI present in the cells. Treatment of cells with the irreversible homobifunctional thiol-cross-linker BMH in the presence of Tris[2-carboxyethyl]phosphine, a reductant that does not interfere with the BMH reaction, resulted in the co-precipitation of a complex with an apparent molecular mass of ∼90 kDa (asterisk, lanes 8 and 9; compare with lanes 6 and 7), consistent with a cross-linked product of PDI (55 kDa) and mammalian-expressed RTB (36 kDa). This product cannot be co-immunoprecipitated after BMH treatment alone (lanes 10 and 11), confirming that the interaction between RTB and PDI is between bridged thiols that require reduction before cross-linking with BMH is possible. Taken together, these results support an in vivo interaction between newly synthesized RTB and PDI in the retention phase before the fate of RTB is determined by interactions with CNX cycle proteins.
Figure 8. PDI can dissociate and reassociate ricin subunits in vitro.
(A) Ricin (70 nM) was incubated with GSH (1 mM), GSSG (1 mM) or neither, in the presence of BSA (3.5 μM) or purified mammalian PDI (3.5 μM) at 30 °C for 30 min. Samples were analysed by non-reducing SDS/PAGE and immunoblotting with antibodies to RTA and RTB. (B) RTA (70 nM) was incubated with either RTB (70 nM) or ATB (70 nM) with or without GSH (1 mM) in the presence of BSA or purified mammalian PDI (3.5 μM) at 30 °C for 30 min. Samples were analysed by non-reducing SDS/PAGE and immunoblotting with antibodies to RTB and ATB.
Figure 9. PDI interacts with RTB.
Pulse-labelled cell extracts from untreated HeLa/RTB-H2 cells or cells treated with NEM or after cross-linking with BMH were immunoprecipitated (IP) using sheep antiserum (Sh) specific for RTB or rabbit antisera (Rb) raised against either RTB or PDI. TCEP, Tris[2-carboxyethyl]phosphine; black arrowhead, RTB; open arrowhead, PDI; grey arrowhead, an irrelevant crossreacting protein (58 kDa) that is immunoselected by the rabbit anti-RTB serum but not by the sheep anti-RTB serum; *, RTB co-immunoprecipitated with anti-PDI antibodies. Positions of molecular-mass standards are shown on the right.
DISCUSSION
In dissecting events during ricin uptake into cells, an unresolved question concerns the site of holotoxin reduction. We reasoned that since separated RTA and RTB can readily reassociate, the presence of excess RTB at the intracellular site of reduction in mammalian cells should interfere with ricin toxicity by acting as end-point receptors for any newly reduced RTA monomers. By identifying the molecules that interact with RTB at that time, we should determine some of the cell constituents that are important for the toxicity of ricin. To this end, we created cell lines expressing RTB directed to the ER via a mammalian signal peptide. Expression of such RTB significantly protected cells against a subsequent ricin challenge. Explanations such as secretion and re-binding of RTB at the cell surface to reduce the overall number of available surface binding sites, or interference with toxin routing, were ruled out. Endogenous RTB cannot interfere with the transport, reduction and/or retrotranslocation of incoming, plant-derived ricin by binding its glycans, because any galactose in the RTA glycan would be (non-substrate) β-1,3-linked [33], and RTB in the ricin used here, purified from R. communis, is endo H-sensitive [34] and will not contain terminal galactose.
The observed protection was specific to ricin, since there was no protection against other toxins, or indeed against abrin, a closely related toxin whose subunits can reassemble with those of ricin in certain combinations. We have shown that an RTB–ATA heterodimer does not form readily in vitro, suggesting that protection against incoming toxin, or the lack of it, depends on the capacity of RTB to form heterodimers. In contrast, when ATB was being expressed, such cells were protected against both ricin and abrin intoxication (but not other unrelated toxins), exactly as predicted by these rules of permissible mix and match, which we demonstrated here, and which confirmed an earlier study of hybrid toxins where the RTB–ATA combination was formed very inefficiently, possibly because of repulsion at the interface of these acidic subunits [35]. Since RTB and ATB exhibit identical sugar (receptor) binding ability towards HeLa cells [30], the specific protection against ricin cannot be explained by competitive binding to internalized receptors. We can therefore rule out the possibility that expressed RTB competes with incoming ricin but not with incoming abrin for receptors in the Golgi or elsewhere to prevent the holotoxins reaching the ER.
The critical reduction and association events occur in the ER. Newly made RTB was stable for approx. 2 h before gradually disappearing. While a proportion was secreted, the intracellular fraction remained sensitive to endo H, supporting localization within the early secretory pathway. Reagents blocking entry into or out of the CNX cycle showed that intracellular RTB remained within the ER lumen and that the gradual disappearance seen during kinetic analysis occurred after interactions with ER proteins involved in quality control. Cytosolic degradation of RTB was shown by a significant stabilization of the protein in cells when proteasomes were inhibited. The most plausible explanation for the extended ER residence of RTB and its eventual degradation is the absence of its cognate binding partner, RTA. As a solo subunit, RTB is probably scrutinized by the ER quality-control machinery, eventually leading to its disposal or eventual secretion.
So, how can the striking protection against ricin holotoxin provided by ER retention of RTB be explained? The large effects of low concentrations of DTT show that the interfering RTB is ER-localized by a thiol-mediated retention mechanism. Since there is a long RTB disposal phase involving interactions with the CNX cycle, a candidate partner for thiol-mediated interactions would be ERp57, the PDI homologue intimately associated with CNX [36,37]. However, inhibiting entry or exit of RTB into and from the CNX cycle did not affect the ability of RTB to protect against holotoxin and, hence, ERp57 cannot be involved. In vitro, we have demonstrated that ricin can be rapidly reduced under the same redox conditions that reduce cholera toxin [38], but only when PDI is present, and we have shown that PDI can also form disulphide bonds between toxin A and B chains under the same conditions. In a separate study, PDI reduced by thioredoxin reductase has been shown to cleave reductively ricin holotoxin in vitro, but no in vivo interaction was demonstrated beyond the participation of thioredoxin reductase [39]. A small amount of PDI has been reported at the surface of some cell types [40], but this has been shown not to reduce ricin holotoxin [41]. Similarly, PDI has been reported to occur in the cytosol [42]. Since there is no rationale for the cytosolic location of this protein, such a claim should be viewed with caution. The data we present demonstrate an ER location for both the thiol-mediated retention of newly made RTB and the reduction of incoming ricin. During the initial retention of RTB, we predict the formation of a disulphide bond between this subunit and PDI, since a thiol-specific homobifunctional cross-linking agent conjugates the two proteins only in the presence of a reductant. The purpose of this interaction is presumably to permit formation of the correct four disulphide bonds from the nine cysteine residues in the B-chain polypeptide.
Ricin holotoxin is not enzymically active [10,11] and must be reduced to liberate an active A chain. Since we show that reduction of ricin in the presence of GSH but in the absence of PDI is very slow and inefficient, RTA must become separated from holotoxin before reaching the reducing cytosol. Thus it is the retrotranslocation of RTA, rather than of the holotoxin, that must be hampered in RTB-expressing cells. RTB-expressing cells show no resistance to other ER-retrotranslocating toxins, including the closely related abrin and, hence, steric hindrance of the translocation channels would seem unlikely. From the known combinatorial properties of abrin and ricin subunits and the finding that newly made PDI-tethered RTB intercepts incoming ricin holotoxin but not incoming RTA, we deduce that the equilibrium formed between oxidized and reduced holotoxin is altered to favour re-oxidation of holotoxin-derived RTA with excess RTB. Therefore we propose that holotoxin reduction must occur in the vicinity of the PDI-anchored B chains to reform translocation-incompetent heterodimers immediately. It was not possible to detect these swapped subunit heterodimers by immunoprecipitation, even after metabolic labelling of the RTB subunit (results not shown), probably because the amounts of ricin reaching the ER are very small. The collective evidence shows that the ER and not Golgi or cytosol must be the site for ricin reduction and that this can normally be catalysed by PDI.
PDI has been implicated in the cell entry process of other, unrelated toxins that reach the ER lumen. Reductive release of the enzymic A1 chain of a proteolytically nicked CTA (cholera toxin A) has been shown to require the action of PDI in vitro [12,15]. Furthermore, for proteolytically nicked CTA, PDI has been proposed to act in vitro as a binding chaperone and unfoldase, as well as a thiol exchange protein [38], although this interpretation has been challenged [43]. In stark contrast with ricin, simple mixtures of GSSG and GSH, which approximate the ER redox conditions efficiently, reduce nicked CTA, indicating that PDI may simply augment its reduction [12–14]. Indeed, this may occur in the Golgi where cholera toxin reduction has been reported [44]. For PE, in vitro studies show that thermal or chemical unfolding of the proteolytically nicked holotoxin is required before PDI-catalysed reduction occurs [12,15]. For both cholera toxin and PE, the eventual fate of the non-enzymic portions remains unclear. The implication from the present study with ricin is that non-enzymic fragments would eventually be dislocated to the cytosol for proteasomal degradation. In contrast with these bacterial toxins, proteolytic cleavage and unfolding before holotoxin reduction have not been implicated in the entry process of ricin, and we further demonstrate in vitro that unmodified holotoxin is a PDI substrate. Despite these differences, the emerging mechanism of ricin action reveals a common feature with structurally and functionally distinct toxins that traffic to the ER, pinpointing a role for PDI. The mechanism by which PDI achieves concomitant reduction of ricin holotoxin and reformation of disulphide-linked heterodimers is not known. PDI is a multifunctional protein catalysing the formation, reduction and isomerization of disulphide bonds [45]. It has also been reported to act as a redox-dependent [38] and -independent binding chaperone [43]. The precise nature of the interaction between PDI and ricin/RTB will be the focus of a separate study.
Acknowledgments
We thank R. Freedman for a critical reading of this paper, L. Frigerio for helpful discussions and L. Ruddock for supplying pLWRP62. This work was supported by the Wellcome Trust (grant numbers 063058/Z/00/Z and 059738/Z/99/Z to L.M.R./J.M.L.) and Biotechnology and Biological Sciences Research Council (grant number 88/B16355 to J.M.L.).
References
- 1.Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- 2.Olsnes S., Pihl A. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry. 1973;12:3121–3126. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- 3.Lord J. M., Jolliffe N. A., Marsden C. J., Pateman S. C., Smith D. C., Spooner R. A., Watson P. D., Roberts L. M. Ricin: mechanisms of cytotoxicity. Toxicol. Rev. 2003;22:53–64. doi: 10.2165/00139709-200322010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Rapak A., Falnes P. O., Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida T., Chen C. C., Zhang M. S., Wu H. C. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin, and Pseudomonas toxin. Exp. Cell Res. 1991;192:389–395. doi: 10.1016/0014-4827(91)90056-z. [DOI] [PubMed] [Google Scholar]
- 6.Okimoto T., Seguchi T., Ono M., Nakayama Y., Funatsu G., Fujiwara T., Ikehara Y., Kuwano M. Brefeldin A protects ricin-induced cytotoxicity in human cancer KB cell line, but not in its resistant counterpart with altered Golgi structures. Cell Struct. Funct. 1993;18:241–251. doi: 10.1247/csf.18.241. [DOI] [PubMed] [Google Scholar]
- 7.Lord J. M., Roberts L. M. Toxin entry: retrograde transport through the secretory pathway. J. Cell Biol. 1998;140:733–736. doi: 10.1083/jcb.140.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesche J., Rapak A., Olsnes S. Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 1999;274:34443–34449. doi: 10.1074/jbc.274.48.34443. [DOI] [PubMed] [Google Scholar]
- 9.Falnes P. O., Sandvig K. Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 2000;12:407–413. doi: 10.1016/s0955-0674(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 10.Lewis M. S., Youle R. J. Ricin subunit association. Thermodynamics and the role of the disulfide bond in toxicity. J. Biol. Chem. 1986;261:11571–11577. [PubMed] [Google Scholar]
- 11.Wright H. T., Robertus J. D. The intersubunit disulfide bridge of ricin is essential for cytotoxicity. Arch. Biochem. Biophys. 1987;256:280–284. doi: 10.1016/0003-9861(87)90447-4. [DOI] [PubMed] [Google Scholar]
- 12.Orlandi P. A. Protein-disulfide isomerase-mediated reduction of the A subunit of cholera toxin in a human intestinal cell line. J. Biol. Chem. 1997;272:4591–4599. [PubMed] [Google Scholar]
- 13.Majoul I., Ferrari D., Soling H. D. Reduction of protein disulfide bonds in an oxidizing environment. The disulfide bridge of cholera toxin A-subunit is reduced in the endoplasmic reticulum. FEBS Lett. 1997;401:104–108. doi: 10.1016/s0014-5793(96)01447-0. [DOI] [PubMed] [Google Scholar]
- 14.Tsai B., Rapoport T. A. Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J. Cell Biol. 2002;159:207–216. doi: 10.1083/jcb.200207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee M. L., FitzGerald D. J. Reduction of furin-nicked Pseudomonas exotoxin A: an unfolding story. Biochemistry. 1999;38:16507–16513. doi: 10.1021/bi991308+. [DOI] [PubMed] [Google Scholar]
- 16.Mohanraj D., Ramakrishnan S. Cytotoxic effects of ricin without an interchain disulfide bond: genetic modification and chemical crosslinking studies. Biochim. Biophys. Acta. 1995;1243:399–406. doi: 10.1016/0304-4165(94)00166-u. [DOI] [PubMed] [Google Scholar]
- 17.Frigerio L., Vitale A., Lord J. M., Ceriotti A., Roberts L. M. Free ricin A chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts. J. Biol. Chem. 1998;273:14194–14199. doi: 10.1074/jbc.273.23.14194. [DOI] [PubMed] [Google Scholar]
- 18.Di Cola A., Frigerio L., Lord J. M., Ceriotti A., Roberts L. M. Ricin A chain without its partner B chain is degraded after retrotranslocation from the endoplasmic reticulum to the cytosol in plant cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14726–14731. doi: 10.1073/pnas.251386098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson P. T., Hussain K., Woodland H. R., Lord J. M., Roberts L. M. The effects of N-glycosylation on the lectin activity of recombinant ricin B chain. Carbohydr. Res. 1991;213:19–25. doi: 10.1016/s0008-6215(00)90594-9. [DOI] [PubMed] [Google Scholar]
- 20.Richardson P. T., Roberts L. M., Gould J. H., Lord J. M. The expression of functional ricin B-chain in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1988;950:385–394. doi: 10.1016/0167-4781(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 21.Hussain I., Powell D. J., Howlett D. R., Chapman G. A., Gilmour L., Murdock P. R., Tew D. G., Meek T. D., Chapman C., Schneider K., et al. ASP1 (BACE2) cleaves the amyloid precursor protein at the beta-secretase site. Mol. Cell. Neurosci. 2000;16:609–619. doi: 10.1006/mcne.2000.0884. [DOI] [PubMed] [Google Scholar]
- 22.Lamb F. I., Roberts L. M., Lord J. M. Nucleotide sequence of cloned cDNA coding for preproricin. Eur. J. Biochem. 1985;148:265–270. doi: 10.1111/j.1432-1033.1985.tb08834.x. [DOI] [PubMed] [Google Scholar]
- 23.Deeks E. D., Cook J. P., Day P. J., Smith D. C., Roberts L. M., Lord J. M. The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry. 2002;41:3405–3413. doi: 10.1021/bi011580v. [DOI] [PubMed] [Google Scholar]
- 24.Kim D. W., Uetsuki T., Kaziro Y., Yamaguchi N., Sugano S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene. 1990;91:217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 25.Marais R., Spooner R. A., Light Y., Martin J., Springer C. J. Gene-directed enzyme prodrug therapy with a mustard prodrug/carboxypeptidase G2 combination. Cancer Res. 1996;56:4735–4742. [PubMed] [Google Scholar]
- 26.Wales R., Richardson P. T., Roberts L. M., Woodland H. R., Lord J. M. Mutational analysis of the galactose binding ability of recombinant ricin B chain. J. Biol. Chem. 1991;266:19172–19179. [PubMed] [Google Scholar]
- 27.Sandvig K., van Deurs B. Selective modulation of the endocytic uptake of ricin and fluid phase markers without alteration in transferrin endocytosis. J. Biol. Chem. 1990;265:6382–6388. [PubMed] [Google Scholar]
- 28.Simpson J. C., Roberts L. M., Lord J. M. Free ricin A chain reaches an early compartment of the secretory pathway before it enters the cytosol. Exp. Cell Res. 1996;229:447–451. doi: 10.1006/excr.1996.0390. [DOI] [PubMed] [Google Scholar]
- 29.Svinth M., Steighardt J., Hernandez R., Suh J. K., Kelly C., Day P., Lord M., Girbes T., Robertus J. D. Differences in cytotoxicity of native and engineered RIPs can be used to assess their ability to reach the cytoplasm. Biochem. Biophys. Res. Commun. 1998;249:637–642. doi: 10.1006/bbrc.1998.9207. [DOI] [PubMed] [Google Scholar]
- 30.Sandvig K., Olsnes S., Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J. Biol. Chem. 1976;251:3977–3984. [PubMed] [Google Scholar]
- 31.Barbieri L., Battelli M. G., Stirpe F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta. 1993;1154:237–282. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 32.Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell. Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 33.Fitchette A. C., Cabanes-Macheteau M., Marvin L., Martin B., Satiat-Jeunemaitre B., Gomord V., Crooks K., Lerouge P., Faye L., Hawes C. Biosynthesis and immunolocalization of Lewis a-containing N-glycans in the plant cell. Plant Physiol. 1999;121:333–344. doi: 10.1104/pp.121.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lord J. M., Harley S. M. Ricinus communis agglutin B chain contains a fucosylated oligosaccharide side chain not present on ricin B chain. FEBS Lett. 1985;189:72–76. [Google Scholar]
- 35.Olsnes S., Pappenheimer A. M., Jr, Meren R. Lectins from Abrus precatorius and Ricinus communis. II. Hybrid toxins and their interaction with chain-specific antibodies. J. Immunol. 1974;113:842–847. [PubMed] [Google Scholar]
- 36.Ellgaard L., Helenius A. ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol. 2001;13:431–437. doi: 10.1016/s0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 37.Russell S. J., Ruddock L. W., Salo K. E., Oliver J. D., Roebuck Q. P., Llewellyn D. H., Roderick H. L., Koivunen P., Myllyharju J., High S. The primary substrate binding site in the b′ domain of ERp57 is adapted for ER lectin association. J. Biol. Chem. 2004;279:18861–18869. doi: 10.1074/jbc.M400575200. [DOI] [PubMed] [Google Scholar]
- 38.Tsai B., Rodighiero C., Lencer W. I., Rapoport T. A. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell (Cambridge, Mass.) 2001;104:937–948. doi: 10.1016/s0092-8674(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 39.Bellisola G., Fracasso G., Ippoliti R., Menestrina G., Rosen A., Solda S., Udali S., Tomazzolli R., Tridente G., Colombatti M. Reductive activation of ricin and ricin A-chain immunotoxins by protein disulfide isomerase and thioredoxin reductase. Biochem. Pharmacol. 2004;67:1721–1731. doi: 10.1016/j.bcp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Turano C., Coppari S., Altieri F., Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J. Cell. Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 41.Ryser H. J., Mandel R., Ghani F. Cell surface sulfhydryls are required for the cytotoxicity of diphtheria toxin but not of ricin in Chinese hamster ovary cells. J. Biol. Chem. 1991;266:18439–18442. [PubMed] [Google Scholar]
- 42.Wroblewski V. J., Masnyk M., Khambatta S. S., Becker G. W. Mechanisms involved in degradation of human insulin by cytosolic fractions of human, monkey, and rat liver. Diabetes. 1992;41:539–547. doi: 10.2337/diab.41.4.539. [DOI] [PubMed] [Google Scholar]
- 43.Lumb R. A., Bulleid N. J. Is protein disulfide isomerase a redox-dependent molecular chaperone? EMBO J. 2002;21:6763–6770. doi: 10.1093/emboj/cdf685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastiaens P. I., Majoul I. V., Verveer P. J., Soling H. D., Jovin T. M. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 1996;15:4246–4253. [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman R. B., Hirst T. R., Tuite M. F. Protein disulphide isomerase: building bridges in protein folding. Trends Biochem. Sci. 1994;19:331–336. doi: 10.1016/0968-0004(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 46.Day P. J., Ernst S. R., Frankel A. E., Monzingo A. F., Pascal J. M., Molina-Svinth M. C., Robertus J. D. Structure and activity of an active site substitution of ricin A chain. Biochemistry. 1996;35:11098–11103. doi: 10.1021/bi960880n. [DOI] [PubMed] [Google Scholar]