Abstract
Introduction
Epilepsy surgery is the only curative treatment for patients with drug-resistant focal epilepsy. Stereoelectroencephalography (SEEG) is the gold standard to delineate the seizure-onset zone (SOZ). However, up to 40% of patients are subsequently not operated as no focal non-eloquent SOZ can be identified. The 5-SENSE Score is a 5-point score to predict whether a focal SOZ is likely to be identified by SEEG. This study aims to validate the 5-SENSE Score, improve score performance by incorporating auxiliary diagnostic methods and evaluate its concordance with expert decisions.
Methods and analysis
Non-interventional, observational, multicentre, prospective study including 200 patients with drug-resistant epilepsy aged ≥15 years undergoing SEEG for identification of a focal SOZ and 200 controls at 22 epilepsy surgery centres worldwide. The primary objective is to assess the diagnostic accuracy and generalisability of the 5-SENSE in predicting focality in SEEG in a prospective cohort. Secondary objectives are to optimise score performance by incorporating auxiliary diagnostic methods and to analyse concordance of the 5-SENSE Score with the expert decisions made in the multidisciplinary team discussion.
Ethics and dissemination
Prospective multicentre validation of the 5-SENSE score may lead to its implementation into clinical practice to assist clinicians in the difficult decision of whether to proceed with implantation. This study will be conducted in accordance with the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (2014). We plan to publish the study results in a peer-reviewed full-length original article and present its findings at scientific conferences.
Trial registration number
Keywords: EPILEPSY; EPILEPSY, SURGERY; NEUROPHYSIOLOGY; EEG
WHAT IS ALREADY KNOWN ON THIS TOPIC
Stereoelectroencephalography (SEEG) is the gold standard for the delineation of the seizure-onset zone (SOZ) in cases where the non-invasive presurgical evaluation provides a reasonable hypothesis to determine a focal epileptogenic zone but does not enable a direct surgical intervention. SEEG is associated with complication rates of 0.6%–4% and is cost-intensive and resource-intensive. Up to 40% of patients do not undergo subsequent resective surgery as no single, focal SOZ in non-eloquent cortex can be identified, or patients decide not to proceed with surgery (ie, satisfiable seizure control after radiofrequency thermocoagulation). The 5-SENSE Score is based on non-invasive presurgical data and was developed to predict whether a focal SOZ will be identified by SEEG.
WHAT THIS STUDY ADDS
Validation of the 5-SENSE Score in a prospective, international, multicentre validation study will prove its generalisability in a large-scale prospective cohort, allow incorporation of auxiliary diagnostics to improve score performance and confirm the added value of the score for clinical use.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
As SEEG is increasingly used worldwide, there is an urgent need to assess the utility of SEEG exploration. Implementation of the 5-SENSE Score in clinical practice will assist clinicians in this difficult decision-making. The 5-SENSE score will allow the identification of patients who are unlikely to benefit from SEEG and spare them the associated risks of this procedure.
Introduction
Epilepsy surgery is the only potentially curative treatment for patients with drug-resistant focal epilepsy1,3 with postoperative seizure freedom rates of up to 80%, depending on the epilepsy syndrome.1 4 Identification of the epileptogenic zone (EZ), defined as the area of the brain responsible for seizure initiation and organisation, and evaluation of its resectability are the major aims of presurgical evaluation.5
If non-invasive presurgical workup does not allow to proceed directly to surgery but does enable to formulate a reasonable hypothesis of the localisation of the EZ, stereoelectroencephalography (SEEG) is the gold standard to further delineate the EZ.6 SEEG is an invasive diagnostic procedure using stereotactically placed intracerebral EEG electrode recordings to localise the seizure-onset zone (SOZ), a surrogate marker for the EZ. It poses adverse event rates of 0.6%–4.0%,7,10 is strenuous for patients and physicians alike and is cost-intensive and resource-intensive. More importantly, up to 30%–40% of patients do not qualify for subsequent surgery, as no focal seizure generator can be identified or the SOZ is located in eloquent cortex.7,9 Therefore, accurate identification of patients who are unlikely to benefit from this invasive procedure is important.
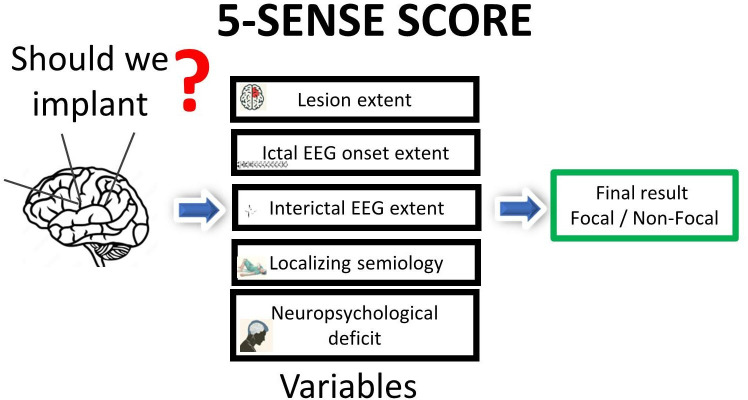
We developed an easily applicable score derived from non-invasive presurgical workup, the ‘5-SENSE Score’, to help predict whether a focal SOZ is likely to be identified by SEEG.11 By retrospective analysis of 128 patients who underwent SEEG at the Montreal Neurological Institute and Hospital, Montreal, Canada, five variables were identified to be predictive for focality. Based on these variables, a five-point score reflecting the main pillars of presurgical evaluation, the ‘5-SENSE Score’, was established. The score comprises the following five predictive variables: focal lesion on structural magnetic resonance imaging (S), absence of bilateral independent spikes in interictal scalp EEG (E), localising neuropsychological deficit (N), strongly localising semiology (S) and regional ictal scalp EEG onset (E) (figure 1). Score performance in the development cohort showed high sensitivity (83.3%; 95% CI 72.3% to 94.1%) and specificity (76.3%; 95% CI 66.7% to 85.8%), using a mean probability cut-off for focality of 37.6 (SD=3.5). An international multicentre validation study confirmed good diagnostic accuracy (specificity 76%; 95% CI 67.5% to 84.6%; sensitivity 52%; 95% CI 43.0% to 61.5%) of the 5-SENSE Score in a retrospective cohort study including 207 patients from 9 epilepsy centres in Europe (Paracelsus Medical University Hospital Salzburg, Austria; Masaryk University, Brno, Czech Republic; Carol Davila University of Medicine and Pharmacy Bucharest, Romania; Grenoble Institute of Neurosciences Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France) and North America (Dalhousie University and Hospital, Halifax, Canada; Massachusetts General Hospital, Boston; Montreal Neurological Institute and Hospital, Montreal, Canada; Northwestern University, Chicago, Illinois; University of Pittsburgh, Pittsburgh, Pennsylvania) (figure 1).
Figure 1. Scheme of the 5-SENSE Score. EEG, electroencephalography.
However, scores in medicine often show great performance in the original publication but suffer a significant decrease in diagnostic accuracy when applied to independent patient cohorts in clinical routine settings. Proof of score performance in a large-scale prospective setting is essential to demonstrate generalisability and clinical utility.12 13 Therefore, we now aim to validate the score in a large-scale international multicentre prospective validation study including more than 200 patients to demonstrate the prognostic value of the 5-SENSE Score in the prediction of focality in SEEG (objective #1). Auxiliary diagnostic methods (magnetic and electrical source imaging, fluorodeoxyglucose positron emission tomography (FDG-PET) and ictal hexamethylpropyleneamine oxime single-photon emission computerised tomography (SPECT))—currently not reflected in the score—are all now routinely used for non-invasive presurgical evaluation. We aim to evaluate, whether the incorporation of these auxiliary diagnostic methods might improve score performance and lead to an optimised ‘5-SENSE-Plus Score’ with increased diagnostic accuracy (objective #2). Last, we aim to assess the concordance of the 5-SENSE Score with the expert decisions regarding implantation, which is routinely made in the multidisciplinary team discussion (MTD) (objective #3). This is an essential step to prove generalisability and clinical utility of the 5-SENSE-Score to assist clinicians in identifying the important subset of patients where SEEG will reliably localise a circumscribed EZ. Such accurate prediction would decrease unnecessary invasive EEG procedures and spare patients’ associated risks. If successful, the necessary next step will be to perform a prospective multicentre clinical trial to confirm clinical utility and added value of the 5-SENSE Score in clinical practice.
Methods and analysis
Study design
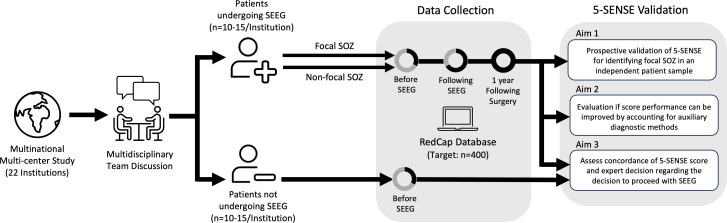
This study is a non-interventional, observational, multinational, multicentre, prospective validation study, performed from January 2024 onwards. The first 10–15 consecutive patients who are referred to SEEG and an equal number of patients discussed for SEEG with the decision against implantation for the purpose of localising a focal SOZ in each centre will be included. For inclusion and exclusion criteria, see table 1.
Table 1. Inclusion/exclusion criteria.
| Inclusion criteria: | Patients aged ≥15 years with no direct surgical approach who are discussed for invasive long-term video electroencephalography (EEG) monitoring with stereoelectroencephalography (SEEG) for presurgical evaluation of drug-resistant focal epilepsy at the multidisciplinary team discussion (MTD) |
| Availability of complete non-invasive presurgical workup (including high-resolution MRI according to the in centre epilepsy protocol, video-telemetry with the recording of a minimum of one habitual clinical and EEG seizure, available as original files for review) | |
| Exclusion criteria: | No telemetry/scalp EEG in centre |
| No protocol MRI in centre | |
| Subdural/GRID electrodes | |
| For objectives #1 and #2: primary hypothesis is already multifocal and the SEEG study is solely performed with the intention to perform treatment/intervention that is, to determine the optimal localisation of thermocoagulation or evaluate for responsive neurostimulation. | |
| For objective #3: patients with multifocal primary hypothesis may be included as “control group’ consisting of patients who are no direct surgical candidates and are discussed for SEEG, but no SEEG is attempted for identifying a focal SOZ following the MTD. |
SOZseizure-onset zone
Screening for inclusion will be performed at the MTD in which the decision to proceed with SEEG is made. Patients fulfilling inclusion criteria (table 1) will be invited to participate in the study and informed consent will be obtained (Supplement 1).
Data from routine non-invasive presurgical evaluation, SEEG and subsequent surgical approach (table 2) will be collected prospectively from patient charts by the local site investigators. Data will be deidentified and entered in a password-protected online database (RedCap) at designated time points (before SEEG, following SEEG, following surgery). As this is a non-interventional observational study, no additional diagnostic tests, interviews or follow-up visits will be scheduled because of the study. In the case of surgery following SEEG, we aim for additional data collection at follow-up 1 year after surgery (figure 2).
Table 2. Description of collected data.
| Demographic data | Age |
| Gender | |
| Handedness | |
| Age at epilepsy onset | |
| Duration of epilepsy | |
| Aetiology | |
| Structural Imaging (MRI epilepsy protocol) | Lesional/non-lesional |
| Type of lesion | |
| Extent of lesion | |
| Localisation of lesion | |
| Non-invasive long-term video-EEG monitoring | Interictal EEG |
| Ictal EEG | |
| Semiology | |
| Neuropsychology (NPSY) | Localising value of NPSY |
| Side of localisation | |
| Cognitive domain | |
| Auxiliary diagnostic methods | Fluordesoxyglucose positron emission tomography (FDG-ET) |
| Magnetic source imaging | |
| Electrical source imaging | |
| Ictal hexamethylpropyleneamine oxime versus single-photon emission computerised tomography | |
| Stereoelectroencephalography | Side of implantation |
| Localisation of implantation | |
| Number of electrodes | |
| Interictal EEG | |
| Ictal EEG | |
| Extent of identified seizure onset zone | |
| Epilepsy surgery | Type of surgery |
| Localisation of surgery | |
| Histology | |
| Outcome | Duration of follow-up (FU) |
| Outcome according to ILAE (International League Against Epilepsy) definition at last FU | |
| Outcome according to Engel classification at last FU |
EEGelectroencephalography
Figure 2. Study design of the prospective multicentre validation study of the 5-SENSE Score. SEEG, stereoelectroencephalography.
Patients who undergo SEEG will be divided according to the extent of the SOZ as identified by SEEG into focal (circumscribed) and non-focal SOZ. A focal SOZ was anatomically defined as sublobar or functionally including unilateral regions within the same anatomo-functional system. Seizure onset is defined as first sustainable SEEG signal change visually distinguishable from background activity within the context of a sustained rhythmic discharge and subsequent appearance of clinical signs, independent of the fast activity content.14 15 We will use the SOZ as a surrogate marker of the EZ.
Sample size
This is a multinational multicentre study including 22 tertiary epilepsy surgery centres in Asia, Australia, North America and Europe. In the context of validating a multivariable score, a classical power-based sample size calculation is not appropriate. Instead, since the focus is primarily on the resulting specificity (and sensitivity) for objective #1 (ie, the prospective validation), the sample size calculation is based on the aim of obtaining a prespecified precision of the two-sided 95% CI for that diagnostic accuracy measure. From our previous studies, the proportion of non-focal subjects in the cohort is expected to be at least 46% (development cohort: 80/128, retrospective validation cohort: 96/207).11 In this scenario, data on a total number of N=189 subjects (ie, focal plus non-focal subjects) must be collected in order to be able to estimate the specificity of the score to a precision of 10 percentage points. As for study objectives #1 and #2, only the subgroup of patients who undergo SEEG will be analysed, and an equal number of patients not undergoing SEEG for identification of a focal SOZ will be included as a control group for objective #3, the total sample size will be 380 patients (ie, patients undergoing SEEG as well as patients not undergoing SEEG), with 190 patients undergoing SEEG (according to the sample size calculation for objectives #1 and #2, see above). Since the attrition rate is expected to be small, we plan to recruit 400 patients in total (n=200 each in the SEEG and the control group).
Period studied and duration of the study
Following a 2-year recruitment period from Ethics Approval onwards (estimated period 01/2024–06/2026) data acquisition will last 24 months. In case of subsequent surgery, we aim for a follow-up period of 1 year after surgery.
Study objectives and data analysis
The primary aim of this large-scale, multinational, multicentre observational study is the validation of the 5-SENSE score in a prospective setting to demonstrate its generalisability and diagnostic accuracy which will be addressed by objective #1. Data from routine non-invasive presurgical evaluation (table 2) will be collected prospectively from patient charts by the local site investigators. Data will be deidentified and entered in a password-protected online database (RedCap) at designated time points (before SEEG, following SEEG, following surgery). Based on the collected data, the 5-SENSE score will be calculated for each patient by the principal investigators (BF, AA-R and GZ) following the decision of the MTD whether or not to pursue SEEG. The score result will not be available to the local site investigators and treating physicians to avoid bias in clinical decision-making. Most importantly, specificity, but also sensitivity, positive and negative predictive value and overall accuracy of the 5-SENSE Score for prediction of focality will be calculated.
Secondary aims are the optimisation of the 5-SENSE Score by incorporation of auxiliary diagnostics leading to the development of an advanced ‘5-SENSE-Plus’ Score (objective #2), as well as assessment of concordance of the 5-SENSE Score with the expert decisions of the MTD in all patients discussed for SEEG and thus its usefulness also in patients who are subsequently not implanted (objective #3). For objective #2, results from auxiliary diagnostic methods performed throughout presurgical evaluation (FDG-PET, electromagnetic source imaging (ESI/MSI), SPECT) will be collected and categorised as focal (or sublobar), lobar/unilateral regional, multifocal/multilobar unilateral, multifocal/multilobar bilateral and normal. For the development of a modified ‘5-SENSE-Plus’ Score, results from different auxiliary diagnostic methods will be merged into a single variable containing information from the different diagnostic modalities with the categories ‘focal’, ‘non-focal unilateral’, ‘non-focal bilateral’ and ‘normal/not specific’. Subsequently, a score development procedure will be performed using a combination of statistical modelling/variable selection, machine learning and expert opinion, analogously to the successfully established analysis pipeline that was applied for developing and validating the original 5-SENSE score.11
The aim of objective #3 is to assess the concordance of the 5-SENSE Score and the expert decisions made in the MTD regarding implantation and thus the potential of the 5-SENSE Score to reliably reproduce clinical decision-making. For this aim, it is essential to also include patients who are discussed for SEEG after being considered not direct candidates for resective surgery, but who are thereafter not implanted as the clinical expert assessment doubts focality. Therefore, for objective #3, also patients with the primary hypothesis of a multifocal/diffuse SOZ who are either not implanted or in whom SEEG was not performed to identify a focal SOZ, that is, implantation for radiofrequency thermocoagulation in case of, for example, periventricular nodular heterotopia or target identification for RNS in case of suspected bitemporal lobe epilepsy (see table 1, exclusion criteria) will be included as a ‘control group’. The result of the 5-SENSE Score, calculated for all patients (patients undergoing SEEG and the ‘control group’ not undergoing SEEG for identification of a focal SOZ), will be compared with the MTD decision regarding implantation, leading to four different scenarios (see table 3). One major limitation of objective #3 is that for patients not undergoing SEEG it remains unknown, whether a focal SOZ would have been identified by SEEG. Therefore, it is impossible to confirm whether the MTD decision or the 5-SENSE Score was correct. However, the results of objective #3 are expected to provide useful information for planning subsequent clinical trials that are targeted at providing robust evidence for implementing the score into clinical decision-making. In the present study, due to the non-interventional and observational design, no changes in the decision of whether to implant or not will be made based on the 5-SENSE Score.
Table 3. Objective #3 assessing concordance of the 5-SENSE Score with the expert decisions made in the multidisciplinary team discussion leading to four different scenarios.
| Multidisciplinary team meeting | 5-SENSE Score | Notes | ||
| + | Decision to perform SEEG | + | Points to a focal SOZ→ encourages SEEG | Concordant. Both MDT and 5-SENSE Score reach the same conclusion |
| + | Decision to perform SEEG | – | Points to non-focal SOZ→ against SEEG | We can assess if indeed the MTD decision or the 5 Sense score were concordant with the SEEG outcome |
| – | Decision against SEEG | – | Points to non-focal SOZ→ against SEEG | Concordant. Both MDT and 5-SENSE Score reach same conclusion |
| – | Decision against SEEG | + | Points to a focal SOZ→ encourages SEEG | No further conclusion can be drawn as we have no ground truth |
MDTmultidisciplinary team discussionSEEGstereoelectroencephalographySOZseizure-onset zone
Dissemination
Oversight
Confidentiality
Only data relevant to this study as outlined in this protocol will be collected by the research team. All the information collected during the research project will remain confidential to the extent required and provided by law. Patient data will be deidentified and coded. The code will be kept by the principal investigator at each participating site in a password-protected digital file behind the local firewall. The electronic data collected will be stored for a minimum of 7 years following completion of the study, then the digital files will be destroyed.
Informed consent
Informed consent of all participants will be collected at the time of referral to a multidisciplinary team discussion (MTD) for discussion of SEEG (supplement 1).
Dissemination plan
It is planned that the findings will result in at least 1–2 full-length peer-reviewed publications and that data will be presented at several international conferences and meetings. Meetings of interest in our field are the American Epilepsy Society Meeting, the International Epilepsy Congress and the European Epilepsy Conference as well as conferences focusing more broadly on general neurology. Whenever the study results are published or shared during scientific meetings or otherwise, it will not be possible to identify the participants.
Discussion
SEEG is increasingly used worldwide as the gold standard for the delineation of the SOZ in case of insufficient or inconclusive data from non-invasive presurgical workup.16 17 The reasons for that are manifold: classical surgically remediable epilepsy syndromes such as mesial temporal lobe epilepsy due to hippocampal sclerosis are decreasing and imaging and electrophysiological techniques render patients who were formally considered inoperable, candidates for epilepsy surgery.18 Furthermore, the advantages of SEEG compared with subdural techniques, such as the three-dimensional sampling of deep-seated structures and lower complication rates resulted in a shift from subdural intracerebral EEG recordings to SEEG in North American within the last decade.19 20 This leads to a challenging decision on whether to proceed with an invasive diagnostic procedure in patients with drug-resistant epilepsy. The 5-SENSE Score is a useful, easily applicable tool based on non-invasive diagnostic data that predicts whether a focal SOZ will unlikely be identified. Confirmation of diagnostic accuracy of the 5-SENSE Score in a large-scale prospective validation study is crucial not only for implementation of this diagnostic tool in clinical practice but also for application in future clinical trials in drug-resistant focal epilepsy populations.
Over the last decade, major advances in the use of auxiliary diagnostic methods for presurgical evaluation have been made. ESI/MSI techniques as well as FDG-PET are widely available and nowadays routinely used in specialised epilepsy centres performing SEEG. By incorporating of these auxiliary diagnostic methods, which were not available in the score development cohort, into an advanced ‘5-SENSE-Plus’ Score, diagnostic accuracy of the score might be further improved. Concordance of the 5-SENSE Score with the expert decisions regarding implantation made in an MTD, which is the current gold standard, could demonstrate the ability of the score to reliably reproduce clinical decision-making and prove generalisability and clinical utility also for patients who are subsequently not implanted.
The ‘5-SENSE Score’ and ‘5-SENSE-Plus Score’ are not intended to replace an MTD but may provide objective additional evidence supporting the challenging decision on whether to proceed with SEEG. This may lead to abstaining from an invasive diagnostic procedure in patients with a small likelihood of a focal EZ and spare them the risks associated with it.
If this research project is successful, we plan to conduct a prospective, multicentre, clinical trial to confirm the feasibility and the added value of the 5-SENSE Score in routine presurgical decision-making.
Acknowledgements
The authors express their gratitude to Tamir Avigdor for designing and creating figure 1.
Footnotes
Funding: PP is supported by an Emerging Leadership Investigator Grant from the Australian National Health and Medical Research Council (APP2017651), The University of Melbourne, Monash University, the Austin Medical Research Foundation and the Norman Beischer Medical Research Foundation. PP is supported by an Emerging Leadership Investigator Grant from the Australian National Health and Medical Research Council (APP2017651), The University of Melbourne, Monash University, the Austin Medical Research Foundation and the Norman Beischer Medical Research Foundation. PP is supported by an Emerging Leadership Investigator Grant from the Australian National Health and Medical Research Council (APP2017651), The University of Melbourne, Monash University, the Austin Medical Research Foundation and the Norman Beischer Medical Research Foundation.
Patient consent for publication: Not applicable.
Ethics approval: This study will be conducted in accordance with the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (2014), as well as with respect of the requirements set out in the applicable standard operation procedures of the Austrian Ethics Board and Duke University. The Duke Hospital Institutional Review Board as the lead ethics institution has approved the manuscript (REB- Pro00113692-INIT-1.1) as well as local Ethics Boards of all participating sites.
Provenance and peer review: Not commissioned; internally peer reviewed.
Contributor Information
Alexandra Astner-Rohracher, Email: alexandra.rohracher@gmail.com.
Alyssa Ho, Email: alyssa.m.ho@duke.edu.
John Archer, Email: hutton.j@unimelb.edu.au.
Fabrice Bartolomei, Email: fabrice.bartolomei@ap-hm.fr.
Milan Brazdil, Email: milan.brazdil@fnusa.cz.
Melita Cacic Hribljan, Email: melitacacic@yahoo.com.
James Castellano, Email: castellanojf@upmc.edu.
Irena Dolezalova, Email: irena.dolezalova@fnusa.cz.
Martin Ejler Fabricius, Email: martin.ejler.fabricius@regionh.dk.
Mercedes Garcés-Sanchez, Email: mergarces@gmail.com.
Kahina Hammam, Email: kahina.hammam@ap-hm.fr.
Akio Ikeda, Email: akio@kuhp.kyoto-u.ac.jp.
Kristin Ikeda, Email: Kristin.Ikeda@nshealth.ca.
Philippe Kahane, Email: philippe.kahane@univ-grenoble-alpes.fr.
Giridhar Kalamangalam, Email: giridhar.kalamangalam@neurology.ufl.edu.
Gudrun Kalss, Email: g.kalss@salk.at.
Mays Khweileh, Email: Mays.Khweileh@duke.edu.
Katsuya Kobayashi, Email: 31258a@kuhp.kyoto-u.ac.jp.
Patrick Kwan, Email: patrick.kwan@monash.edu.
Joshua Andrew Laing, Email: joshua.andrew.laing@gmail.com.
Markus Leitinger, Email: ma.leitinger@salk.at.
Samden Lhatoo, Email: samden.d.lhatoo@uth.tmc.edu.
Julia Makhalova, Email: julia.scholly@ap-hm.fr.
Aileen McGonigal, Email: a.mcgonigal@uq.edu.au.
Iona Mindruta, Email: ioanatmindruta@gmail.com.
Mary Margaret Mizera, Email: MaryMargaret.Mizera@nm.org.
Andrew Neal, Email: andrew.neal@monash.edu.
Irina Oane, Email: dr.popairina@gmail.com.
Prachi Parikh, Email: Prachi.parikh@duke.edu.
Piero Perucca, Email: piero.perucca@monash.edu.
Francesca Pizzo, Email: francesca.pizzo@ap-hm.fr.
Rodrigo Rocamora, Email: rrocamora@parcdesalutmar.cat.
Philippe Ryvlin, Email: philipperyvlin@gmail.com.
Victoria San Antonio Arce, Email: victoria.san.antonio@uniklinik-freiburg.de.
Stephan Schuele, Email: sschuele@nm.org.
Andreas Schulze-Bonhage, Email: andreas.schulze-bonhage@uniklinik-freiburg.de.
Ana Suller Marti, Email: ana.sullermarti@lhsc.on.ca.
Alexandra Urban, Email: urbana1@upmc.edu.
Vincente Villanueva, Email: vevillanuevah@yahoo.com.
Laura Vilella Bertran, Email: laura.vilella.bertran@psmar.cat.
Benjamin Whatley, Email: bwhatley@dal.ca.
Sandor Beniczky, Email: sbz@filadelfia.dk.
Eugen Trinka, Email: e.trinka@salk.at.
Georg Zimmermann, Email: georg.zimmermann@pmu.ac.at.
Birgit Frauscher, Email: birgit.frauscher@duke.edu.
References
- 1.Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med. 2017;377:1639–47. doi: 10.1056/NEJMoa1615335. [DOI] [PubMed] [Google Scholar]
- 3.Engel J, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: A review. JAMA. 2015;313:285–93. doi: 10.1001/jama.2014.17426. [DOI] [PubMed] [Google Scholar]
- 5.Vakharia VN, Duncan JS, Witt J-A, et al. Getting the best outcomes from epilepsy surgery. Ann Neurol. 2018;83:676–90. doi: 10.1002/ana.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frauscher B, Mansilla D, Abdallah C, et al. Learn how to interpret and use intracranial EEG findings. Epileptic Disord. 2024;26:1–59. doi: 10.1002/epd2.20190. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Martinez J, Bulacio J, Alexopoulos A, et al. Stereoelectroencephalography in the “difficult to localize” refractory focal epilepsy: early experience from a North American epilepsy center. Epilepsia. 2013;54:323–30. doi: 10.1111/j.1528-1167.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall JA, Khoo HM. Robotic-Assisted and Image-Guided MRI-Compatible Stereoelectroencephalography. Can J Neurol Sci. 2018;45:35–43. doi: 10.1017/cjn.2017.240. [DOI] [PubMed] [Google Scholar]
- 9.Cardinale F, Rizzi M, Vignati E, et al. Stereoelectroencephalography: retrospective analysis of 742 procedures in a single centre. Brain (Bacau) 2019;142:2688–704. doi: 10.1093/brain/awz196. [DOI] [PubMed] [Google Scholar]
- 10.Jehi L, Morita-Sherman M, Love TE, et al. Comparative Effectiveness of Stereotactic Electroencephalography Versus Subdural Grids in Epilepsy Surgery. Ann Neurol. 2021;90:927–39. doi: 10.1002/ana.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astner-Rohracher A, Zimmermann G, Avigdor T, et al. Development and Validation of the 5-SENSE Score to Predict Focality of the Seizure-Onset Zone as Assessed by Stereoelectroencephalography. JAMA Neurol. 2022;79:70–9. doi: 10.1001/jamaneurol.2021.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jehi L, Yardi R, Chagin K, et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: A retrospective analysis. Lancet Neurol. 2015;14:283–90. doi: 10.1016/S1474-4422(14)70325-4. [DOI] [PubMed] [Google Scholar]
- 13.Hadady L, Sperling MR, Alcala-Zermeno JL, et al. Prediction tools and risk stratification in epilepsy surgery. Epilepsia. 2024;65:414–21. doi: 10.1111/epi.17851. [DOI] [PubMed] [Google Scholar]
- 14.Spanedda F, Cendes F, Gotman J. Relations between EEG seizure morphology, interhemispheric spread, and mesial temporal atrophy in bitemporal epilepsy. Epilepsia. 1997;38:1300–14. doi: 10.1111/j.1528-1157.1997.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartolomei F, Lagarde S, Wendling F, et al. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia. 2017;58:1131–47. doi: 10.1111/epi.13791. [DOI] [PubMed] [Google Scholar]
- 16.Jayakar P, Gaillard WD, Tripathi M, et al. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55:507–18. doi: 10.1111/epi.12544. [DOI] [PubMed] [Google Scholar]
- 17.Isnard J, Taussig D, Bartolomei F, et al. French guidelines on stereoelectroencephalography (SEEG) Neurophysiol Clin. 2018;48:5–13. doi: 10.1016/j.neucli.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Jehi L, Friedman D, Carlson C, et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia. 2015;56:1526–33. doi: 10.1111/epi.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz JS, Abel TJ. Stereoelectroencephalography Versus Subdural Electrodes for Localization of the Epileptogenic Zone: What Is the Evidence? Neurotherapeutics. 2019;16:59–66. doi: 10.1007/s13311-018-00703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abou-Al-Shaar H, Brock AA, Kundu B, et al. Increased nationwide use of stereoencephalography for intracranial epilepsy electroencephalography recordings. J Clin Neurosci. 2018;53:132–4. doi: 10.1016/j.jocn.2018.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]