Abstract
In Europe, hazelnuts (Corylus avellana) are a frequent cause of food allergies. Several important hazelnut allergens have been previously identified and characterized. Specific N-glycans are known to induce strong IgE responses of uncertain clinical relevance, but so far the allergenic potential of glycoproteins from hazelnut has not been investigated. The aim of the study was the molecular characterization of the glycosylated vicilin Cor a 11 from hazelnut and the analysis of its allergenic activity. Although MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS showed that one of two potential glycosylation sites of Cor a 11 was glycosylated, CD spectroscopy indicated that recombinant and natural Cor a 11 share similar secondary structures. Thus to analyse the impact of the glycan residues of Cor a 11 on IgE binding, the allergenic activity of natural glycosylated Cor a 11 and recombinant Cor a 11 was compared. In addition, the IgE sensitization pattern to recombinant Cor a 11, Cor a 1, Cor a 2 and Cor a 8 of 65 hazelnut allergic patients was determined in vitro. The prevalence of IgE reactivity to hazelnut vicilin Cor a 11 was below 50%. Basophil histamine-release assays were used to determine the allergenic activity of both natural and recombinant Cor a 11 in comparison with Cor a 1, a birch (Betula verrucosa) pollen-related major hazelnut allergen. Both forms of Cor a 11 induced mediator release from basophils to a similar extent, indicating that the hazelnut allergic patients had cross-linking IgE antibodies binding to the protein backbone and not to carbohydrate structures. In comparison to Cor a 1, a 10000-fold higher concentration of Cor a 11 was required to induce similar basophil mediator release. In conclusion, the hazelnut vicilin Cor a 11 is a minor allergen both in regard to prevalence and allergenic potency, whereas its glycan does not contribute to its allergenic activity.
Keywords: Cor a 11, cross-reactive carbohydrate determinants, glycoprotein, hazelnut (Corylus avellana) allergen, histamine release, vicilin-like (7 S) protein
Abbreviations: CCD, cross-reactive carbohydrate determinants; DBPCFC, double-blind placebo-controlled food challenge; LTP, lipid-transfer protein; MALDI–TOF MS, matrix-assisted laser-desorption ionization–time-of-flight MS; MMX, (Manα1-3)Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4GlcNAc; MMXF, (Manα1-3)Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc; MUXF, Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc; n, natural; OAS, oral allergy syndrome; r, recombinant; RACE, rapid amplification of cDNA ends; SPT, skin-prick testing
INTRODUCTION
Food allergies are caused by an inappropriate immune response to otherwise harmless proteins, resulting in elevated level of IgE antibodies, which are specific for food allergens. In the effector phase of the type I allergic reaction, IgE antibodies bound to receptors on the surface of mast cells and basophils are cross-linked by the allergen. This event leads to effector cell activation and results in the release of preformed mediators and de novo mediator synthesis. The physiological activity of these mediators determines the clinical symptoms of the allergic reaction.
Hazelnuts (Corylus avellana) are among the common tree nuts that cause allergic reactions [1]. Allergy to hazelnuts is often found in patients with tree pollen allergy. These patients frequently suffer from mild oral allergy syndrome (i.e. itching and swelling of the lips, tongue and throat) caused by cross-reactivity between tree pollen allergens and hazelnut proteins. Recently, the pollen-related hazelnut allergens Cor a 1.04 [2], a homologue of the major birch (Betula verrucosa) pollen allergen, Bet v 1, and Cor a 2 [3], a profilin, homologous with the birch pollen allergen Bet v 2, have been cloned and characterized. In a multicentre study performed in Denmark, Switzerland and Italy, Cor a 1.04 was identified as the major hazelnut allergen in 65 European patients sensitized to birch pollen whose food allergy to hazelnut was confirmed by a positive DBPCFC (double-blind placebo-controlled food challenge) [4]. In addition, severe allergic reactions to hazelnuts were reported in patients without tree pollen allergy. Two hazelnut allergens responsible for such severe reactions have been recently described: Cor a 8, an LTP (lipid-transfer protein) [5], and Cor a 9 [6], an 11 S globulin-like seed storage protein, which was identified as a legumin.
In respect to food allergy to other tree nuts, most tree nut allergens identified so far are seed storage proteins, such as legumins (11–13 S hexameric globulins with 30–40 kDa acidic and 17–20 kDa basic peptides), 2 S albumins (approx. 15 kDa, with 5–9 kDa subunits) and vicilins (7 S trimeric globulins comprising 45–53 kDa subunits) [7]. Several vicilins have been identified as major allergens, for example, Ara h 1 [8] from peanut (Arachis hypogaea), Jug r 2 [9] from walnut (Juglans regia) and Ana o 1 [10] from cashew nuts (Anacardium occidentale). Until now the allergenicity of the hazelnut vicilin has not been investigated. We have previously identified a 48-kDa glycoprotein from hazelnuts, initially characterized as minor allergen by Mueller et al. [11], as a member of the vicilin family (I. Lauer, U. Müller, S. Westphal, A. Conti, D. Haustein, S. Vieths and S. Scheurer, unpublished work).
Among the other known allergenic vicilins, Ara h 1 from peanut [12] was also shown to be glycosylated, whereas for Jug r 2 [9] from walnut only one unoccupied glycosylation site was described.
IgE responses to N-glycan moieties of glycoproteins [so-called CCDs (cross-reactive carbohydrate determinants)] are frequent, in particular in patients with plant allergies, but their clinical relevance is controversial. Some authors believe that IgE antibodies specific for CCD structures are generally of low biological activity and thus without clinical relevance [13]. To elicit an allergic reaction, not only specific binding of serum IgE to the allergen, but also cross-linking of cell-bound IgE is required. Depending on, for example, the affinity of the antibodies or the presence of irrelevant IgE, these two properties may not always correlate. Consequently, basophil or mast cell activation experiments are required to assess the allergenic potential of a molecule in vitro.
Examples for IgE-reactive glycoproteins are Api m 1 [14] from honeybee (Apis mellifera) venom, Cup a 1 [15] from cypress (Cupressus arizonica) pollen, Lyc e 2 [16] from tomato (Lycopersicon esculantum), Api g 5 [17] from celery (Apium graveolens), and Ara h 1 [12] from peanut. Relevant biological activity of IgE directed against CCDs was only verified for allergens with complex N-glycans containing α1,3-L-fucose and β1,2-D-xylose, such as Lyc e 2 from tomato [16] and Api g 5 from celery [17].
The aim of the present study was to characterize the hazelnut vicilin Cor a 11 at the molecular level, to analyse its glycan structure and to evaluate the contribution of the glycan moieties to its allergenic potential. In addition, we analysed the frequency of occurrence of specific IgE antibodies to recombinant (r)Cor a 1.04, rCor a 2 and rCor a 8, the most relevant hazelnut allergens in Europe, in a group of 65 adult patients with hazelnut allergy from Germany and Switzerland.
EXPERIMENTAL
Patients' sera
Serum samples were taken from a group of 65 patients with a positive case history of immediate-type reactions to hazelnuts. Most of the patients (n=39) were from Switzerland; the others were from Germany (Table 1). Only adults were included in the study; the age ranged from 18 to 68 years and 20% were male. Medical histories of the hazelnut allergic patients revealed 3 cases of systemic reactions (urticaria, nausea, dyspnea, anaphylaxis) after ingestion of hazelnuts, whereas all others suffered from an OAS (oral allergy syndrome). All hazelnut allergic patients were also allergic to birch pollen. All Swiss and 13 out of 26 German patients underwent SPT (skin-prick testing) with commercial hazelnut extract. In addition, all Swiss patients underwent hazelnut prick-to-prick tests. The clinical data of the majority of the Swiss patients have been reported elsewhere [18]. All underwent DBPCFC as described previously [18] and showed positive reactions.
Table 1. Clinical data of hazelnut allergic patients investigated in this study.
SPT positive, patients with positive SPT with hazelnut extract; prick-to-prick with native raw hazelnuts.
| CAP™ | ||||
|---|---|---|---|---|
| Hazelnut allergic patients | Class | n | SPT positive | Prick-to-prick positive |
| DBPCFC+Switzerland (n=39) | 0 | 9 | 30/39 | 28/39 |
| 1 | 6 | |||
| 2 | 14 | |||
| 3 | 10 | |||
| Germany (n=26) | 2 | 6 | 13/13 | − |
| 3 | 16 | |||
| 4 | 3 | |||
| 5 | 1 | |||
Preparation of allergen extract
An extract from raw hazelnuts was prepared by a low-temperature method as described by Vieths et al. [19].
Purification of natural (n)Cor a 11
To purify nCor a 11, freeze-dried hazelnut extract was dissolved to a protein concentration of 4 mg/ml and dialysed against starting buffer (50 mM Tris/HCl, pH 8). After filtration through a 0.45 μm filter (Sartorius, Göttingen, Germany) the protein solution was applied to a 20 ml HiPrep 16/10 DEAE FF column (Amersham Pharmacia Biotech, Uppsala, Sweden). Bound proteins were eluted with a linear salt gradient [50 mM Tris/HCl (pH 8)/1 M NaCl] at a flow rate of 5 ml/min. Further purification of the eluted fractions was performed by concanavalin A affinity chromatography (Amersham Pharmacia Biotech) in 20 mM Tris/HCl (pH 7.3)/0.5 M NaCl. Elution was carried out with α-D-methyl-mannoside (10, 25, 50 and 100 mM) (Sigma–Aldrich, Steinheim, Germany). Fractions (0.5 ml) were analysed by SDS/PAGE and immunoblotting.
N-terminal amino acid sequencing
Purified nCor a 11 was electroblotted on to a PVDF membrane. After staining with Coomassie Brillant Blue, the protein band was excised and analysed on an Applied Biosystems 492 Procise sequencer (Foster City, CA, U.S.A.) in pulse-liquid mode to determine the N-terminal partial sequence.
cDNA cloning
Total RNA was isolated from hazelnuts using the RNeasy Plant RNA mini kit (Qiagen, Hilden, Germany). The RNA was reverse-transcribed with the First Strand cDNA Synthesis kit (Amersham Pharmacia Biotech) using the NotI–d(T)18 oligonucleotide for priming. To obtain the complete coding region, the reverse transcription products were amplified using gene specific primers followed by a 5′-RACE (rapid amplification of cDNA ends). First, a degenerated 5′ primer (HN1deg: 5′-GAAGAGRATGAAAACCCTTATGTWTTT) was selected on the basis of a conserved region at the N-terminus of the protein. This conserved region was derived from the N-terminal amino acid sequence (SEEESYGEEQDXNPYVFSD) [11] of the 48-kDa hazelnut allergen and from a cDNA alignment with proteins similar to the hazelnut vicilin (sequences corresponding to the HN1deg primer are underlined). In combination with the oligo-(dT) primer, PCR was performed and the product subject to TOPO cloning (pCRII-TOPO vector, Invitrogen, Groningen, The Netherlands). Second, the leader sequence region was obtained by 5′-RACE. Subsequent sequencing resulted in definition of both the 5′- and 3′-ends of the reading frame.
For protein expression in Escherichia coli, the coding region without signal sequence was cloned into the pET100D vector containing a six-histidine tag using the pET Directional TOPO expression kit (Invitrogen). The cDNA was amplified using Vent DNA polymerase (New England Biolabs, Frankfurt am Main, Germany) and hazelnut cDNA together with the 3′ primer HN5 (5′-TCACCTAAAAGCACGACCGCCAC) and the 5′ primer HN7-CACC (5′-CACCAGTAGCGAGGAGGAAAGTTATGGGGAG), the latter extended by the sequence CACC to facilitate directional cloning. The PCR conditions were: 94 °C, 5 min, followed by 30 cycles of 94 °C, 1 min, 50 °C, 1 min, 72 °C, 1 min; then 5 min, 72 °C. DNA sequencing was performed using vector or gene-specific primers (MWG, Ebersberg, Germany).
Recombinant protein expression and purification
For expression of Cor a 11, the pET100D constructs were transformed in E. coli BL21 Star cells (Invitrogen) and protein synthesis was induced with 1 mM IPTG (isopropyl β-D-thiogalactoside) for 5 h at 37 °C. Bacteria were harvested by centrifugation (3000 g, 20 min, 4 °C) and stored at −80 °C. The pellet from a 1-litre bacterial culture was resuspended in lysis buffer (50 mM NaH2PO4, 500 mM NaCl and 2 mM imidazole, pH 8) and subjected to three freeze–thaw cycles using liquid nitrogen (frozen 3 times in liquid nitrogen). Cor a 11 was purified by metal-chelate affinity chromatography using a commercial kit (Qiagen) according to the manufacturers’ instructions. The protein content was determined using the Roti-Quant protein assay (Roth, Karlsruhe, Germany). Recombinant Cor a 1.04 (GenBank® accession no. AF136945) and Cor a 8 (AF329829) were cloned into the pET 15b or pET 16b vector respectively, both encoding for a N-terminal His tag, facilitating purification by metal-chelate affinity chromatography. Recombinant Cor a 2 (AF327622) was expressed as non-fusion protein and purified by poly(L-proline) affinity chromatography [3].
CD spectroscopy
CD spectra of natural and recombinant Cor a 11 were recorded using 0.17 mg/ml (5.2 μM) purified protein in 10 mM KH2PO4 buffer (pH 7.4) on a Jasco J-810 S spectropolarimeter (Jasco, Gross-Umstadt, Germany) at 20 °C with a step width of 0.2 nm and a band width of 1 nm. The spectral range was 185–255 nm at 50 nm/min. Ten scans were accumulated.
Analysis of N-linked glycans and peptides of nCor a 11 by MALDI–TOF MS
nCor a 11 (8 μg) was excised from a Coomassie Blue-stained SDS/PAGE gel after electrophoresis under reducing conditions and subjected to tryptic digestion as described elsewhere [20]. The extracted and dried peptides were dissolved in water/acetonitrile/trifluoroacetic acid (95:5:0.1, by vol.) and analysed by MALDI–TOF MS. Further preparation and MS analysis of N-glycans were performed according to Kolarich and Altmann [21].
Determination of specific IgE
Measurement of allergen-specific IgE was performed with the CAP™ FEIA system (Pharmacia Diagnostics, Uppsala, Sweden). IgE specific for carbohydrate structures was assayed by a glycan ELISA (CCD ELISA) as described previously [22]. For n Cor a 11 and r Cor a 11, 250 ng of protein/well was used as the solid-phase antigen instead of 1 μg/ml glycopeptide. Bound IgE was detected as described previously [22], except that streptavidin–horseradish peroxidase (Calbiochem, Bad Soden, Germany) and 3,3′,5,5′-tetramethylbenzidine (Merck, Darmstadt, Germany) as substrate were used. The absorption values of the sera were considered positive if the corrected values (corrected value=absorption−absorption of the negative control) were 3-fold higher than the negative control (A≥0.3).
Electrophoresis and immunoblotting
Purified n Cor a 11 and r Cor a 11 were separated by SDS/PAGE under reducing conditions. n Cor a 11 and r Cor a 11 (1.5 μg/cm), rCor a 1.04 (0.5 μg/cm), rCor a 2 (0.5 μg/cm) and rCor a 8 (0.5 μg/cm) were separated by Tricine SDS/PAGE. In contrast, rCor a 8 was treated under non-reducing conditions. Proteins were transferred on to nitrocellulose membranes (0.2 μm) by semi-dry electroblotting. The membrane was blocked in Tris-buffered saline/0.3% Tween 20 and incubated with 1:10 diluted patients' sera. Bound IgE antibodies were detected with the same antibody combination, as used in ELISA, followed by enhanced chemiluminescence (Pharmacia).
Basophil histamine release
Histamine release from stripped and passively sensitized basophils was performed as described elsewhere [23], with several modifications as described by Foetisch et al. [16]. Briefly, peripheral blood was drawn from non-allergic donors and peripheral blood mononuclear cells were isolated using Ficoll–Hypaque centrifugation. Receptor-bound IgE was stripped by lactic acid treatment, and the cells were re-sensitized with sera from allergic patients. Cells sensitized with a non-allergic serum served as negative control. Stimulation of the cells was performed according to the instructions of the manufacturer for the histamine assay kit (Immunotech, Marseille, France), with 10-fold dilutions of the allergens starting at 10 μg/ml. For testing, hazelnut extract, nCor a 11, rCor a 11, rCor a 1 and BSA (the last as a negative control) were used. The released histamine was measured by an enzyme immunoassay (Immunotech). After subtraction of the value caused by spontaneous release from the basophils, the allergen-induced histamine release was calculated as percentage of the total amount of histamine determined after lysis of the basophils by freezing and thawing of the cells twice. A histamine release of more than 10% was considered positive. All experiments were performed at least twice on separate days.
RESULTS
Molecular characterization of nCor a 11 and rCor a 11
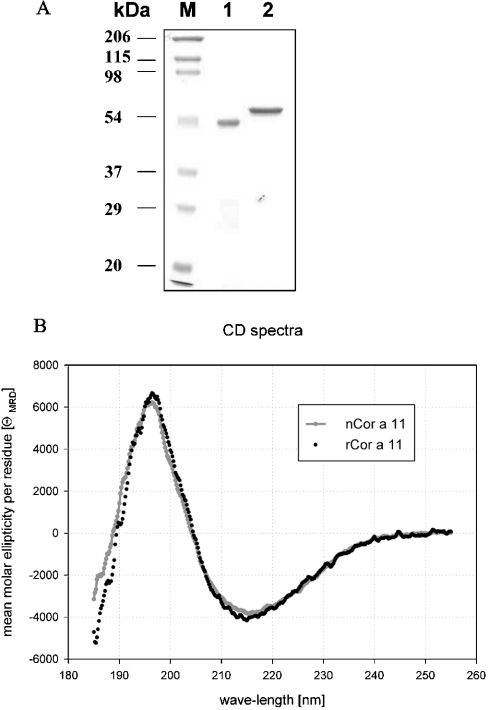
Hazelnut vicilin was purified from hazelnut extract by a two-step FPLC method. The protein was purified to approx. 98% (Figure 1A, lane 1). To verify the identity of the natural protein after purification, a N-terminal amino acid sequence analysis was performed after electrotransfer on to a PVDF membrane. The sequence of the 48-kDa band excised from the membrane was XXEESYGEEQDN. Of the ten amino acids that were clearly identified in our partial sequence, nine were identical with the sequence described by Müller et al. [11]. This degree of sequence identity clearly identified the protein as a vicilin. The observed sequence variation may result from the fact that two different hazelnut varieties were used in the present and previous study [11], which apparently contained a different distribution of dominant isoforms of the vicilin. The cDNA of Cor a 11 (GenBank® accession number AF441864) was identified as a 1341 bp open reading frame, encoding 401 amino acids of the mature protein with two potential N-glycosylation sites (Asn38 and Asn254) and a leader peptide of 46 amino acids. The protein was named Cor a 11 and submitted to the official database of the IUIS (International Union of Immunological Societies) Allergen Nomenclature Subcommittee [24]. Mature rCor a 11 showed amino acid sequence identities with different seed storage proteins, from sesame (Sesamum indicum) (Ses i 3: 57.6% identity), cashew nut (Ana o 1: 56.6% identity), walnut (Jug r 2: 49.7% identity) and peanut (Ara h 1: 34.9% identity).
Figure 1. Purification (A) and CD spectra (B) of nCor a 11 and rCor a 11.
(A) SDS/PAGE analysis of nCor a 11 (lane 1) and rCor a 11 (lane 2), with Brilliant Blue Coomassie staining. Molecular-mass markers (lane M). (B) CD spectra of nCor a 11 and rCor a 11.
The purities of both n Cor a 11 and r Cor a 11 are shown in Figure 1(A). rCor a 11 has an apparent molecular mass of approx. 55 kDa, originating from its additional N-terminal histidine tag and a vector-specific tag of 3.32 kDa.
The secondary structures of rCor a 11 and nCor a 11 were compared by CD spectroscopy. The two spectra showed a characteristic minimum at 215 nm and were nearly identical (Figure 1B). Both proteins appeared to be folded with α-helical and β-sheet structures, so it was concluded that rCor a 11 produced by E. coli had a secondary structure identical with that of nCor a 11. Neither glycosylation of nCor a 11 nor the N-terminal-fusion peptide of rCor a 11 seemed to have an effect on the secondary structure of the proteins.
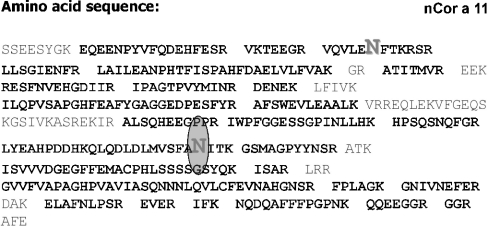
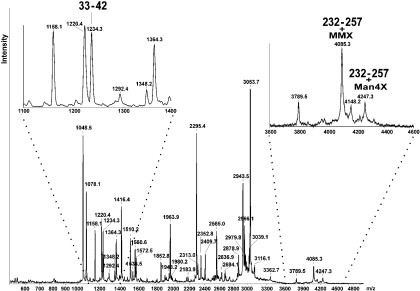
To analyse the two potential N-glycosylation sites, peptide mapping of nCor a 11 was performed. In Figure 2 the amino acid sequence of the vicilin and the two potential N-glycosylation sites (Asn38 and Asn254) are indicated. After trypsin digestion, peptide fragments were identified by MALDI–TOF MS on the basis of their calculated molecular mass. In total, 87% of the entire Cor a 11 amino acid sequence was covered by the identified peptides. The MS data indicated that only the second potential glycosylation site of Cor a 11 (Asn254) was actually glycosylated, whereas the first site (Asn38) was unoccupied (Figure 3 and Table 2).
Figure 2. Amino acid sequence of nCor a 11.
The tryptic peptides identified by MALDI–TOF MS are shown in bold. The two possible N-glycosylation sites are shown in large letters; only the second site (Asn254) is glycosylated.
Figure 3. Tryptic peptide map of nCor a 11 analysed by MALDI–TOF MS.
Inserts show a magnification of the m/z regions with the unglycosylated and glycosylated peptides, as described in Table 2.
Table 2. MALDI–TOF MS data of hazelnut vicilin.
Peptides and glycopeptides identified by MALDI–TOF MS giving a sequence coverage of 87%. Whereas the potential glycopeptide with Asn38 is not glycosylated, the glycopeptide with Asn254 could be identified in several forms. MC, missed cleavage; MSO, methionine sulphoxide; CysCAM, carboxyamidomethylcysteine; theo, theoretical; exp, experimental.
| Position | [MH]+theo | [MH]+exp | MC | Modification, residue | Peptide sequence |
|---|---|---|---|---|---|
| 9–25 | 2184.2 | 2183.9 | 0 | EQEENPYVFQDEHFESR | |
| 26–32 | 818.9 | 819.3 | 1 | VKTEEGR | |
| 33–42 | 1234.4 | 1234.3 | 1 | VQVLENFTKR | |
| 45–53 | 1049.2 | 1048.5* | 0 | LLSGIENFR | |
| 54–80 | 2965.5 | 2966.1 | 0 | LAILEANPHTFISPAHFDAELVLFVAK | |
| 83–89 | 808.0 | 808.0 | 0 | MSO, 87 | ATITMVR |
| 93–105 | 1572.7 | 1572.5 | 1 | RESFNVEHGDIIR | |
| 94–105 | 1416.5 | 1416.4 | 0 | ESFNVEHGDIIR | |
| 106–117 | 1348.6 | 1348.2 | 0 | MSO, 114 | IPAGTPVYMINR |
| 106–127 | 2565.0 | 2565.0 | 2 | MSO, 114 | IPAGTPVYMINRDENEKLFIVK |
| 118–122 | 634.6 | 634.8 | 0 | DENEK | |
| 128–154 | 2943.2 | 2943.5 | 0 | ILQPVSAPGHFEAFYGAGGEDPESFYR | |
| 155–166 | 1364.6 | 1364.3 | 0 | AFSWEVLEAALK | |
| 194–204 | 1221.3 | 1220.4† | 0 | ALSQHEEGPPR | |
| 205–221 | 1853.1 | 1852.8 | 0 | IWPFGGESSGPINLLHK | |
| 222–231 | 1158.2 | 1158.1 | HPSQSNQFGR | ||
| 232–257 | 4084.4 | 4085.3 | 1 | MSO, 249; MMX, 254 | |
| 4246.5 | 4247.3 | MSO, 249; Man4X, 254 | LYEAHPDDHKQLQDLDLMVSFANITK | ||
| 242–257 | 2862.1 | 2862.1 | 0 | MMX, 254 | QLQDLDLMVSFANITK |
| 2878.1 | 2878.9 | MSO, 249; MMX, 254 | |||
| 3024.2 | 3024.7 | M4X, 254 | |||
| 3040.2 | 3039.1 | MSO, 249; Man4X, 254 | |||
| 258–268 | 1219.3 | 1220.4† | 0 | MSO, 260 | GSMAGPYYNSR |
| 272–302 | 3363.7 | 3362.7 | 1 | CysCAM, 286 | ISVVVEGEGFFEMACPHLSSSSGSYQKISAR |
| 306–341 | 3788.3 | 3789.5 | 0 | CysCAM, 331 | GVVFVAPAGHPVAVIASQNNNLQVLCFEVNAHGNSR |
| 342–347 | 632.8 | 633.0 | 0 | FPLAGK | |
| 348–356 | 1078.2 | 1078.1 | 0 | GNIVNEFER | |
| 360–368 | 1047.2 | 1048.5* | 0 | ELAFNLPSR | |
| 369–372 | 532.6 | 533.0 | 0 | EVER | |
| 373–395 | 2683.9 | 2684.1 | 2 | IFKNQDQAFFFPGPNKQQEEGGR | |
| 376–388 | 1510.7 | 1510.2 | 0 | NQDQAFFFPGPNK | |
| 376–395 | 2295.4 | 2295.4 | 1 | NQDQAFFFPGPNKQQEEGGR | |
| 376–398 | 2565.7 | 2565.0 | 1 | NQDQAFFFPGPNKQQEEGGRGGR |
*/† Due to the low resolution of the instrument used, the m/z of the peptides 45–53/360–368 and 194–204/258–268 respectively overlapped and gave an average value. MALDI–Q(quadrupole)-TOF MS confirmed the presence of the individual peptides (results not shown).
The glycan map verified the presence of a xylosylated paucimannosidic N-glycan [74% MMX-type, 3% MMXF-type; where MMX is (Manα1-3)Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4GlcNAc and MMXF is (Manα1-3)Manα1-6(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc] as the main glycan structure of the natural hazelnut vicilin, which was in agreement with a previous study [11]. On this basis, it could be calculated that approx. 2% of the total mass of the hazelnut vicilin is due to the N-glycan.
Frequency of IgE binding indicates that Cor a 11 is a minor allergen in hazelnut
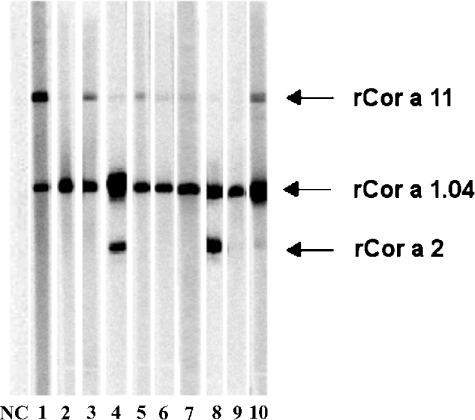
Immunoblot analyses were performed to determine the prevalence of IgE antibody reactivity to hazelnut vicilin using sera of 65 hazelnut allergic patients recruited in Germany and Switzerland, which both have a high population of birch trees. In addition, the IgE reactivity of Cor a 11 was compared with those of rCor a 1.04, rCor a 2 and rCor a 8, the last representing a hazelnut allergen not related to any occurring in pollens. Selected immunoblots are shown in Figure 4. According to the results of immunoblotting of 65 investigated patients, 98.5% had IgE to the major allergen, rCor a 1.04; rCor a 2 was recognized by IgE from 15.4% of the sera, whereas no reactivity was found towards rCor a 8 (LTP) (results not shown). r Cor a 11 and n Cor a 11 bound IgE from 43% and 47% of the serum samples respectively. rCor a 1.04 and rCor a 2 had strong IgE reactivity, resulting in intensive bands, whereas often a weaker signal for Cor a 11 was observed.
Figure 4. IgE reactivities to recombinant hazelnut allergens determined by Western blotting.
Separation of rCor a 1.04, rCor a 2 and rCor a 11 by SDS/PAGE (a selection of 10 out of 65 patients; NC, negative control). All sera shown were positive to rCor a 1.04, whereas patient 4 and 8 were also positive to rCor a 2. Four patients showed positive IgE reactivity to rCor a 11 (sera 1, 3, 5 and 10). Sera 3, 6, 8 and 10 were used in histamine-release assays. IgE binding to rCor a 8 was assayed in a separate experiment under non-reducing conditions and was negative for all patients (results not shown).
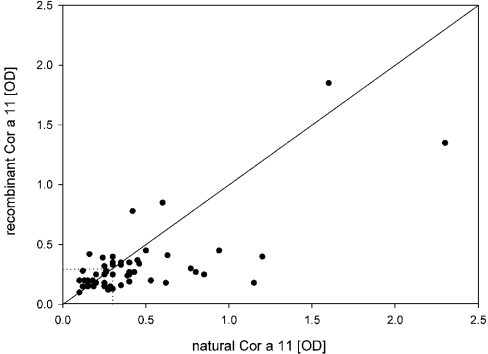
In addition, the IgE reactivity to nCor a 11 and rCor a 11 was analysed by ELISA and compared with the results of immunoblotting. nCor a 11 and rCor a 11 showed weak absorbances. The ELISA results of most sera were slightly above the threshold level with A between 0.3 and 0.5 (Figure 5). All patients' sera with a positive IgE reactivity in immunoblots were also positive in ELISA. Sera that showed the strongest reactivity in the immunoblot analysis gave also higher values in the ELISA. However, in comparison to rCor a 11, there was a tendency towards higher IgE binding of nCor a 11. The overall frequency of IgE binding to rCor a 11 (40% of the sera) and to the glycosylated nCor a 11 (47%) in ELISA was comparable with the results of immunoblotting. Therefore, the vicilin was classified as minor allergen.
Figure 5. IgE reactivity to nCor a 11 and rCor a 11 measured by ELISA.
The IgE-binding frequency was 47% for nCor a 11 and 40% for rCor a 11. Most values are just above the threshold between an A (‘OD’) of 0.3 and 0.5. There was a tendency for higher IgE binding to nCor a 11.
Immunoblot results of all 65 investigated sera revealed that all study participants were sensitized to at least one of the recombinant hazelnut allergens used in the present study. None of the patients was exclusively sensitized to Cor a 11. Even the 9 positive DBPCFC patients with a negative hazelnut CAP™ result (Table 1) showed IgE reactivity to at least one of the recombinant hazelnut allergens, indicating that, compared with extracts, a higher diagnostic sensitivity can be achieved with pure recombinant food allergens.
Basophil histamine release indicates that the allergenic activity of Cor a 11 does not depend on the glycan structure
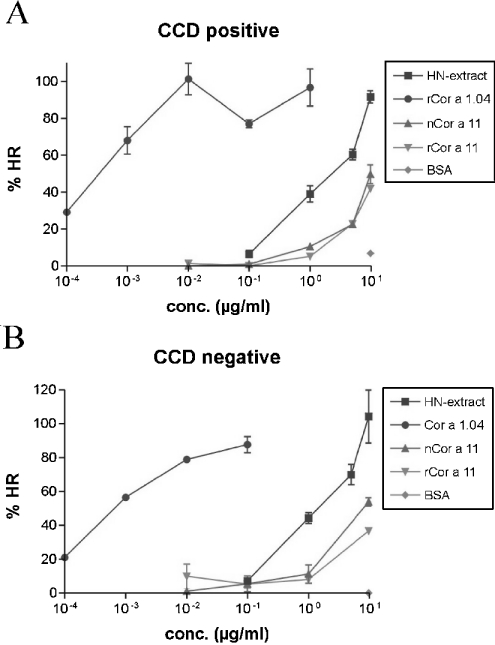
A functionally active allergen must contain at least two IgE epitopes for cross-linking the allergen-specific cell-bound IgE antibodies. Bridging of IgE on the surface leads to mast cell or basophil activation, which results in histamine release. In general, both carbohydrate as well as protein epitopes, or a combination of both, can lead to effector cell activation. To evaluate the contribution of the glycans to the allergenic activity in a functional assay, we performed histamine release with rCor a 11 and nCor a 11, using sera that were Cor a 11 positive according to the results of both immunoblotting and ELISA. Table 3 contains the corresponding ELISA values, immunoblot and CCD ELISA results of the sera which were tested in this assay, as well as a summary of the histamine-release data. In Table 3, the histaminerelease results are expressed as allergen concentration eliciting mediator release of 25% of the total histamine release from passively sensitized basophils. This 25% value was used to compare the biological activity of allergens from hazelnuts. Figure 6(A) shows the histamine release from stripped human basophils of a non-allergic subject that were passively sensitized with sera that contained IgE antibodies specific for carbohydrates as indicated by the results of the CCD ELISA (Table 3, serum 2). After stimulation with rCor a 11 and nCor a 11, histamine was released in a specific dose-dependent manner. Figure 6(A) shows that, in comparison with the natural molecule, the glycosylated nCor a 11 did not induce higher histamine release from basophils. The histamine release induced by both rCor a 11 and nCor a 11 was similar, indicating that the serum contained IgE antibodies that bound to the protein backbone and not to carbohydrate residues.
Table 3. Selected sera used in histamine release assays.
| Hazelnut extract | rCor a 1.04 | nCor a 11 | rCor a 11 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum number | Experiment… | HR† | HR | ELISA‡ | Blot | HR | ELISA | Blot | HR | CCD ELISA [22] |
| 1* | 0.2 | 1×10−4 | 0.8 | + | 8 | 0.3 | + | 6 | 0.48 | |
| 2* | 0.3 | 1×10−4 | 2.3 | ++ | 5 | 1.3 | ++ | 5 | 0.55 | |
| 3 | 0.3 | 1×10−4 | 0.5 | + | 2 | 0.4 | + | 3 | 0.15 | |
| 4 | 0.5 | 2×10−4 | 0.4 | + | 2 | 0.3 | + | 5 | 0 | |
| 5 | 0.3 | nd | 0.77 | ++ | 5 | 0.3 | + | 3 | 0.96 | |
| 6 | 0.2 | 8×10−4 | 0.43 | + | 10 | 0.3 | + | 10 | 0.78 | |
* DBPCFC positive.
† HR results are expressed as allergen concentration eliciting mediator release of 25% of the total histamine release from passively sensitised basophils in μg/ml. No histamine release was observed with the non-allergic control. Nd, not determined.
‡ Considered as positive with an absorbance of at least 0.3 (3-fold of the negative control).
Figure 6. Histamine-release results obtained with a CCD-positive serum (A) and a CCD-negative serum (B) from hazelnut allergic patients.
Hazelnut extract (HN-extract) and the major hazelnut allergen Cor a 1.04 were used as positive controls. BSA was used as negative control. The error bars represent the S.E.M. of single measurements repeated twice on different days (n=2). HR, histamine release.
Figure 6(B) shows the histamine release with a serum containing IgE antibodies specific for the protein backbone of Cor a 11 (Table 3, serum 4). Again, no difference in histamine release induced by rCor a 11 and nCor a 11 was observed. Both rCor a 11 and the glycosylated nCor a 11 had very similar allergenic activity.
In comparison with the major allergen Cor a 1.04, a 10000-fold higher concentration of vicilin was required to induce a similar mediator release, indicating that, in comparison with Cor a 11, the allergenic activity of the major allergen was several orders of magnitude higher. On the basis of total protein, a similar difference in allergenic activity was seen between Cor a 1.04 and hazelnut extract. This observation reflects the low abundance of natural Cor a 1.04 in hazelnut extract, since the major allergen represents less than 1% of the total protein of the extract.
DISCUSSION
In the present study, we identified the vicilin Cor a 11 as a new minor allergen in hazelnuts, analysed its glycan structure and the contribution of the glycan moiety to the allergenic activity of the protein. Analysis of a peptide map by MALDI–TOF MS revealed that nCor a 11 is glycosylated at one of the two potential glycosylation sites. The main glycan structure of the natural hazelnut vicilin published previously [11] was confirmed in the present study. We found that 74% of the glycans are MMX-type and 3% MMXF-type, which both represent typical N-glycan compounds in plants. β1-2-linked xylose and α1-3-linked fucose have been described as immunogenic structures [12], but the clinical relevance of such N-glycans as CCDs and IgE-binding epitopes is still under debate. By means of histamine-release assays, biological activity of anti-CCD IgE was recently found for the glycoproteins Lyc e 2 from tomato [16] in a subgroup of Lyc e 2-sensitized patients, and for Api g 5 from celery [17]. Both glycoproteins are able to activate basophils by cross-linking of cell-bound IgE. Histamine release was detected only with the natural glycosylated molecules containing MUXF- [Manα1-6-(Xylβ1-2)Manβ1-4GlcNAcβ1-4(Fucα1-3)GlcNAc] or MMXF-glycan structures, whereas the unglycosylated counterparts were inactive. Both allergens carry at least two CCD epitopes, the minimum required for cross-linking of receptor-bound IgE.
Similar to the design of the above mentioned studies, we compared the allergenic activity of non-glycosylated rCor a 11 and purified glycosylated hazelnut vicilin nCor a 11 using sera from hazelnut allergic patients from Central Europe. Even though the hazelnut vicilin carried only one potential carbohydrate epitope of the xylosylated paucimannosidic-type (74% MMX, 3% MMXF), cross-linking and subsequent histamine release should be possible if another epitope, either linear or conformational, exists on the protein backbone. Mediator release from basophils induced by both rCor a 11 and nCor a 11, using either CCD-positive or CCD-negative sera from hazelnut allergic patients, was similar. If the carbohydrates were biologically important IgE epitopes, we would expect that nCor a 11 would induce a higher histamine release than rCor a 11 if CCD-positive sera were used. However, our results clearly showed that the CCDs did not contribute to the allergenic activity of Cor a 11. This is in accordance with the results of our ELISA measurements; apart from a slight tendency for higher IgE binding to nCor a 11 in ELISA (Figure 5), the frequency of specific IgE antibodies detected with both rCor a 11 and nCor a 11 in our patient population was similar.
The major peanut allergen Ara h 1, which shows 35% sequence identity with the hazelnut vicilin, is also a glycoprotein with a single N-glycosylation site. N-glycan analysis of the peanut vicilin showed a plant-specific glycosylation of the MMX-type and no fucosylated species could be found [21]. The glycosylation of that allergen had no effect on its allergenic activity, although β1-2-linked xylose and α1-3-linked fucose of other glycoproteins were shown to be involved in IgE binding [12]. Both the major (MMX) and minor (MMXF) glycan structures of Cor a 11 contain an α1,3 mannose residue. In the case of Ara h 1 (MMX type), it was claimed that the presence of this mannose sterically hinders IgE binding. In contrast, glycosylated nApi g 5 from celery contains mainly the MUXF structure, without an α1,3 mannose residue, which is thought to represent the original immunogenic epitope, and showed biological activity [17].
To control the correct folding of both proteins after purification, CD spectroscopy was performed. Since the two protein spectra were nearly identical, we concluded that nCor a 11 and rCor a 11 share similar secondary structures and are suitable for further studies such as IgE-binding analysis and histamine-release assays.
In immunoblots and ELISA, the IgE-antibody reactivities to nCor a 11 and rCor a 11 of 65 hazelnut allergic patients were tested. With an IgE-binding frequency of 47% for the nCor a 11 and 43–40% for the rCor a 11, this allergen was classified as minor allergen.
Pastorello et al. [4] recently described a 47-kDa hazelnut allergen with the same partial N-terminal amino acid sequence as the hazelnut vicilin. This group also observed a weak signal for Cor a 11 in immunoblot analysis. On one hand, surprisingly, they described the hazelnut vicilin as a major allergen with an IgE-binding frequency of 84.2% in their patient population. On the other hand, the frequency of positive IgE responses to the 18-kDa hazelnut allergen (Cor a 1.04) and the 14.4-kDa allergen (Cor a 2) were comparable with our present study. We determined the prevalence of Cor a 1.04, Cor a 2 and Cor a 8 using standardized amounts of recombinant hazelnut allergens. In our present study, as in the study of Pastorello et al. [4], no sensitization to hazelnut LTP Cor a 8 could be detected in patients with pollen-related hazelnut allergy. However, in Mediterranean patients without birch pollen sensitization, LTP was the dominating hazelnut allergen, being responsible for a high rate of severe allergic reactions [4,5].
All vicilins showing significant sequence identity with the hazelnut vicilin, such as Ses i 3, Ana o 1, Jug r 2 and Ara h 1, were described as pollen-unrelated major allergens mainly in patient populations from the U.S.A. Beyer [25] compared the sequences of several 7 S globulins, such as Ses i 3, Ara h 1, Jug r 2, Ana o 1 and Cor a 11, at the amino acid level. Two known IgE-binding regions of Ara h 1 and Ana o 1 were compared with the corresponding sequence of the other vicilins. One IgE-binding epitope of the peanut allergen, Ara h 1, showed a degree of 66.6% similarity to Cor a 11, whereas the other one had 44.4% identity with Cor a 11, and 4 out of the 5 critical amino acids for IgE binding for Ara h 1 were identical. Due to the high degree of similarity of these partial sequences, we hypothesize that these might also be potential IgE epitopes of the hazelnut vicilin. IgE cross-reactivity between Ara h 1 and Cor a 11 would, for example be expected in peanut allergic subjects with concomitant hazelnut allergy. It is tempting to speculate that Cor a 11 would be a more relevant allergen in subjects allergic to hazelnut, as well as to peanuts or other tree nuts. Work is currently in progress to prove this hypothesis.
The relatively weak IgE reactivity of Cor a 11 observed in immunoblot and ELISA was also reflected by the histamine-release data. Compared with the major allergen, Cor a 1.04, a 10000-fold higher concentration of vicilin was required to induce a similar level of basophil activation. So far it is not clear if this level of biological activity is associated with a clinically relevant food allergy. However, according to the definition of the IUIS Allergen Nomenclature Subcommittee [24], allergens are exclusively defined by the ability to bind IgE and the frequency of IgE binding. We believe that biological activity data presented in this study should be taken into consideration for a revised definition of the terms ‘major allergen’ and ‘minor allergen’.
In conclusion, the vicilin Cor a 11 was identified as a minor allergen in hazelnut allergic patients from Central Europe in regard to both IgE binding frequency and allergenic potency. The glycans of Cor a 11 did not contribute to its allergenic activity.
Acknowledgments
We thank Dr Dirk Luettkopf for providing rCor a 2 (AF327622), Dr Sandra Westphal for guidance in cloning strategy and Professor Dr Iain Wilson for carefully reading the manuscript.
References
- 1.Tariq S. M., Stevens M., Matthes S., Rideout S., Twiselton R., Hide D. W. Cohort study of peanut and tree nut sensitization by age of 4 years. Br. Med. J. 1996;313:514–517. doi: 10.1136/bmj.313.7056.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luettkopf D., Mueller U., Skov P. S., Ballmer-Weber B. K., Wuethrich B., Skamstrup Hansen K., Paulsen L. K., Kastner M., Haustein D., Vieths S. Comparison of four variants of a major allergen in hazelnuts (Corylus avellana) Cor a 1.04 with the major hazel pollen allergen Cor a 1.01. Mol. Immunol. 2001;38:515–525. doi: 10.1016/s0161-5890(01)00087-6. [DOI] [PubMed] [Google Scholar]
- 3.Skamstrup Hansen K., Ballmer-Weber B. K., Luettkopf D., Skov P. S., Wüthrich B., Bindslev-Jensen C., Vieths S., Poulsen L. K. Roasted hazelnuts–allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy. 2003;58:132–138. doi: 10.1034/j.1398-9995.2003.23959.x. [DOI] [PubMed] [Google Scholar]
- 4.Pastorello E. A., Vieths S., Pravettoni V., Farioli L., Trambaioli C., Fortunato D., Luettkopf D., Calamari M., Ansaloni R., Scibilia J., et al. Identification of hazelnut major allergens in sensitive patients with positive double-blind, placebo-controlled food challenge results. J. Allergy Clin. Immunol. 2002;109:563–570. doi: 10.1067/mai.2002.121946. [DOI] [PubMed] [Google Scholar]
- 5.Schocker F., Luettkopf D., Scheurer S., Petersen A., Cistero-Bahima A., Enrique E., San Miguel-Moncin M., Akkerdaas J., Van Ree R., Vieths S., Becker W. M. Recombinant lipid transfer protein Cor a 8 from hazelnut: a new tool for in vitro diagnosis of potentially severe hazelnut allergy. J. Allergy Clin. Immunol. 2004;113:141–174. doi: 10.1016/j.jaci.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Beyer K., Grishina G., Bardina L., Grishin A., Sampson H. A. Identification of an 11 S globulin as a major hazelnut food allergen in hazelnut-induced systemic reactions. J. Allergy Clin. Immunol. 2002;110:517–523. doi: 10.1067/mai.2002.127434. [DOI] [PubMed] [Google Scholar]
- 7.Roux K. H., Teuber S. S., Sathe S. K. Tree nut allergens. Int. Arch. Allergy Immunol. 2003;131:234–244. doi: 10.1159/000072135. [DOI] [PubMed] [Google Scholar]
- 8.Burks A. W., Shin D., Cockwell G., Stanley J. S., Helm R., Bannon G. A. Mapping and mutational analysis of the IgE binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur. J. Biochem. 1997;245:334–339. doi: 10.1111/j.1432-1033.1997.t01-1-00334.x. [DOI] [PubMed] [Google Scholar]
- 9.Teuber S. S., Jarvis K. C., Dandekar A. M., Peterson W. R., Ansari A. A. Cloning and sequencing of a gene encoding a vicilin-like proprotein, Jug r 2, from english walnut kernel (Juglans regia), a major food allergen. J. Allergy Clin. Immunol. 1999;104:1311–1320. doi: 10.1016/s0091-6749(99)70029-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang F., Robotham J. M., Teuber S. S., Tawde P., Sathe S. K., Roux K. H. Ana o 1, a cashew (Anacardium occidentale) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002;110:160–166. doi: 10.1067/mai.2002.125208. [DOI] [PubMed] [Google Scholar]
- 11.Müller U., Lüttkopf D., Hoffmann A., Petersen A., Becker W. M., Schocker F., Niggeman B., Altmann F., Kolarich D., Haustein D., Vieths S. Allergens in native and roasted hazelnuts (Corylus avellana) and their cross-reactivity to pollen. Eur. Food Res. Technol. 2000;212:2–12. [Google Scholar]
- 12.Van Ree R., Cabanes-Macheteau M., Akkerdaas J., Milazzo J. P., Loutelier-Bourhis C., Rayon C., Villalba M., Koppelman S., Aalberse R. C., Rodriguez R., et al. β(1,2)-xylose and α(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 2000;275:11451–11458. doi: 10.1074/jbc.275.15.11451. [DOI] [PubMed] [Google Scholar]
- 13.Mari A., Iacovacci P., Afferni C., Barletta B., Tinghino R., Di Felice G. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergenic diseases. J. Allergy Clin. Immunol. 1999;103:1005–1011. doi: 10.1016/s0091-6749(99)70171-5. [DOI] [PubMed] [Google Scholar]
- 14.Tretter V., Altmann F., Kubelka V., Marz L., Becker W. M. Fucose α1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int. Arch. Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- 15.Alisi C., Afferni C., Iacovacci P., Barletta B., Tinghino R., Butteroni C., Puggioni E. M., Wilson I. B., Federico R., Schinina M. E., et al. Rapid isolation, characterization, and glycan analysis of Cup a 1, the major allergen of Arizona cypress (Cupressus arizonica) pollen. Allergy. 2001;56:978–984. doi: 10.1034/j.1398-9995.2001.103125.x. [DOI] [PubMed] [Google Scholar]
- 16.Foetisch K., Westphal S., Lauer I., Retzek M., Altmann F., Kolarich D., Scheurer S., Vieths S. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J. Allergy Clin. Immunol. 2003;111:889–896. doi: 10.1067/mai.2003.173. [DOI] [PubMed] [Google Scholar]
- 17.Bublin M., Radauer C., Wilson I. B., Kraft D., Scheiner O., Breiteneder H., Hoffmann-Sommergruber K. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003;17:1697–1699. doi: 10.1096/fj.02-0872fje. [DOI] [PubMed] [Google Scholar]
- 18.Ballmer-Weber B. K., Vieths S., Bucher C. H., Luettkopf D., Wuethrich B. Haselnussallergie: Validierung der diagnostischen Verfahren anhand der doppelblinden, plazebokontrollierten Nahrungsmittelprovokation. Allergologie. 2000;23:285–291. [Google Scholar]
- 19.Vieths S., Schoning B., Petersen A. Characterization of the 18-kDa apple allergen by two-dimensional immunoblotting and microsequencing. Int. Arch. Allergy Immunol. 1994;104:399–404. doi: 10.1159/000236698. [DOI] [PubMed] [Google Scholar]
- 20.Katayama H., Nagasu T., Oda Y. Improvement of in-gel digestion protocol for peptide mass fingerprinting by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2001;15:1416–1421. doi: 10.1002/rcm.379. [DOI] [PubMed] [Google Scholar]
- 21.Kolarich D., Altmann F. N-Glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electrophoretically separated nonmammalian proteins: application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal. Biochem. 2000;285:64–75. doi: 10.1006/abio.2000.4737. [DOI] [PubMed] [Google Scholar]
- 22.Foetisch K., Altmann F., Haustein D., Vieths S. Involvement of carbohydrate epitopes in the IgE response of celery-allergic patients. Int. Arch. Allergy Clin. Immunol. 1999;120:30–42. doi: 10.1159/000024217. [DOI] [PubMed] [Google Scholar]
- 23.Kleine Budde I., de Heer P. G., Van der Zee J. S., Aalberse R. C. The stripped basophil histamine release bioassay as a tool for the detection of allergen specific IgE in serum. Int. Arch. Allergy Immunol. 2001;126:277–285. doi: 10.1159/000049524. [DOI] [PubMed] [Google Scholar]
- 24.King T. P. Bull. WHO. 1994;72:796–806. [Google Scholar]
- 25.Beyer K. Characterization of allergenic food proteins. Curr. Opin. Allergy Immunol. 2003;3:189–197. doi: 10.1097/00130832-200306000-00007. [DOI] [PubMed] [Google Scholar]